1. Introduction
Adjuvant therapy, including immune checkpoint inhibitors targeting programmed cell death 1 (PD-1) or cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) proteins, and targeted therapy with BRAF plus MEK inhibitors, has improved the clinical prognosis for postoperative melanoma patients at high risk of recurrence. In the CheckMate 238 trial, the nivolumab group treated with 3 mg/kg once every 2 weeks for 1 year demonstrated a 4-year recurrence-free survival (RFS) rate of 51.7% and a 4-year overall survival (OS) of 77.9%, compared to 76.6% for ipilimumab in resected stage III-IV melanoma [
1]. The EORTC 1325-MG/KEYNOTE-054 study showed that pembrolizumab treatment with 200 mg every 3 weeks for up to 18 doses or until disease recurrence or unacceptable toxicity resulted in a 3.5-year RFS rate of 59.8 - 61.4%, compared to 41.4 - 44.1% in the placebo group [
2]. In the KEYNOTE-716 Study, pembrolizumab achieved an 84.4% distant metastasis-free survival compared to 74.7% in the placebo group for Stage IIB or IIC disease [
3]. The COMBI-AD trial demonstrated that combined therapy with dabrafenib plus trametinib yielded a 5-year RFS rate of 52%, compared to 36% in the placebo group for resected stage III melanoma [
4]. However, as the incidence of mucosal and acral melanoma is higher in Asian cohorts, including Japanese populations (mucosal, 20 - 30%; acral type, 42 - 65% of all melanoma types), than in Caucasians (mucosal, 1 - 2%; acral melanoma, 1 - 1.5% of all melanoma cases) [
5], a small number of acral and mucosal melanoma cases were included in clinical trials.
Thus, this study aims to analyze the 3-year RFS and OS in 120 melanoma cases with stage IIC/III/IV in the adjuvant setting, treated with anti-PD-1 Abs or the combination of dabrafenib plus trametinib. The purpose is to evaluate the long-term effectiveness of adjuvant anti-PD-1 Abs or dabrafenib plus trametinib in a Japanese cohort, including more acral and mucosal patients.
2. Materials and Methods
2.1. Ethics
The protocol was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (2020-1-811), and by the ethics committees of each participating institution.
2.2. Patients
From January 2019 to September 2020, we retrospectively identified 120 patients who had been treated with anti-PD-1 (nivolumab or pembrolizumab) or BRAF plus MEK inhibitors (dabrafenib plus trametinib) in the adjuvant setting, as previously reported [
6,
7].
Patients had histologically confirmed cutaneous, acral, or mucosal melanoma and were categorized as either stage IIC or stage IIIA-C or had resectable IV disease according to the eighth edition of the Cancer Staging Manual of the American Joint Committee on Cancer (AJCC-8th). Additionally, histologic subtypes of cutaneous melanoma were evaluated using both Clark’s histological classification and the World Health Organization (WHO) classification, which categorizes them based on the histopathologic degree of cumulative solar damage (CSD) of the surrounding skin. In stage IV disease, adjuvant therapy was initiated after complete resection of distant or recurrent lesions.
2.3. Procedure
Patients with BRAF-wild type melanoma were treated with anti-PD-1 inhibitors (pembrolizumab or nivolumab). Nivolumab was administered intravenously at a dose of 240 mg every two weeks or 480 mg every four weeks, while pembrolizumab was given at a dose of 200 mg every three weeks for 12 months. Patients with BRAF-mutant melanoma received treatment with either anti-PD-1 inhibitors or dabrafenib plus trametinib combined therapy. Adjuvant therapy was continued for 12 months or until disease recurrence or unacceptable toxicity occurred. Withdrawal of the agents was approved for severe adverse events by the decision of the physician.
2.4. Assessment
The primary endpoint was the 3-year relapse-free survival (RFS) rate, defined as the time from adjuvant therapy induction to disease recurrence or the last date of examination. The secondary endpoint was the 3-year overall survival (OS), defined as the time from adjuvant therapy induction to the patient’s death from any cause. All disease recurrence analyses were based on investigator assessment. Periodic checkups and imaging, using computed tomography (CT), magnetic resonance imaging (MRI), or both, were performed for the 3-year follow-up.
2.5. Statistical Analysis
The RFS rate was estimated for each group using the Kaplan-Meier method. The log-rank test was used to compare survival between groups. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated using Cox’s proportional hazards model in the univariate analysis. Cox’s proportional hazards models were also utilized for multivariate analyses. The significance level for all tests was set at a two-sided α = 0.05. The significance level for the log-rank test was also set at a two-sided α = 0.05. All statistical analyses were performed with JMP ver.16 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Demographic Data
Briefly, in the anti-PD-1 inhibitor group, one (1%) patient had stage IIC, 3 (3%) had stage IIIA, 12 (13%) had stage IIIB, 46 (52%) had stage IIIC, and 3 (3%) had stage IIID, while in the dabrafenib plus trametinib combined therapies group, 1 (3%) patient had stage IIIA, 6 (20%) had stage IIIB, 17 (57%) had stage IIIC, and 3 (10%) had stage IIID. Before adjuvant therapy, 26 patients with cutaneous and acral melanoma at stage III underwent complete lymph node dissection (CLND), while 65 patients did not.
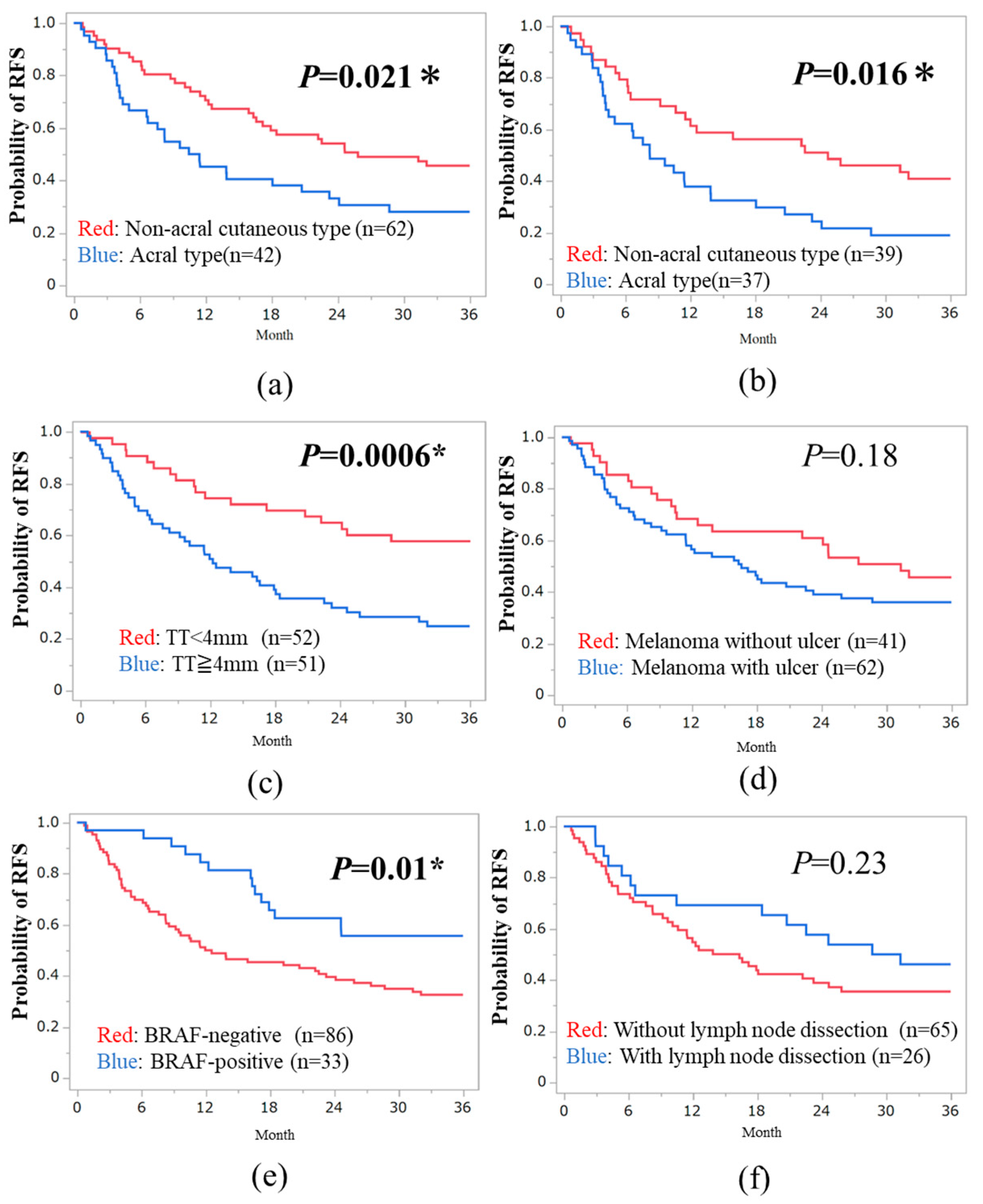
3.2. Three-Year RFS in Subtypes of Melanoma
The median relapse-free survival (RFS) for the 120 patients was 18.4 months, ranging from 0.69 to 36 months. The 3-year RFS rate was 35.8% (43/120 cases). In subtype-specific analysis, the 3-year RFS rate was 26.2% (11/42 cases) for acral melanoma, 44% (11/25 cases) for high-CSD, 43.2% (16/37 cases) for low-CSD, 33.3% (4/12 cases) for mucosal melanoma, and 50% (2/4 cases) for unknown primary sites. Non-acral cutaneous type (low- and high-CSD types) treated with anti-PD-1 Abs or dabrafenib plus trametinib combined therapy showed significantly better RFS compared to acral melanoma (HR, 0.56; 95% CI: 0.34 - 0.92;
P = 0.021,
Figure 1a). Additionally, significant differences were observed between acral and non-acral melanoma in the anti-PD1 Abs cohort (HR, 0.52; 95% CI, 0.30 - 0.89;
P = 0.016,
Figure 1b). Mucosal melanoma did not exhibit a poorer prognosis compared to non-acral melanoma, consistent with the previous 2-year analysis (
P = 0.46).
We divided both cutaneous and acral melanoma patients (n = 104) into two groups based on baseline or treatment-related factors. Patients with thin tumor thickness (TT) <4 mm showed significantly better RFS than those with thick TT≥ 4mm (median TT value: 4 mm; HR, 0.39; 95% CI, 0.23 - 0.68;
P = 0.0006,
Figure 1c). There was no significant difference between tumor ulcer-positive and -negative cases (
P = 0.18,
Figure 1d). BRAF-positive cases had a better prognosis than BRAF-negative cases (HR, 0.47; 95% CI, 0.26 - 0.85;
P = 0.01,
Figure 1e). CLND was not associated with RFS in stage III melanoma (
P = 0.23,
Figure 1f).
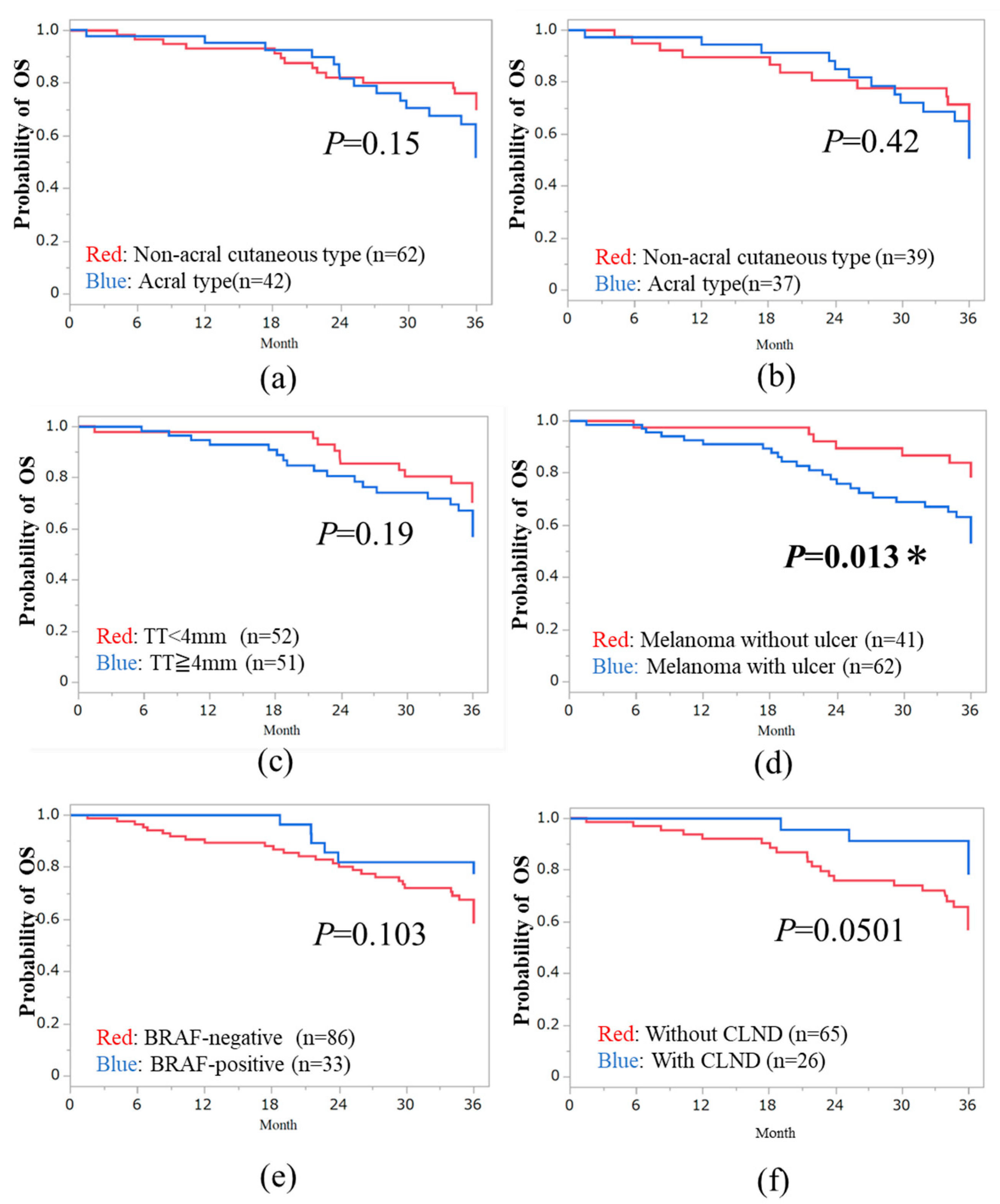
3.3. Three-Year OS in Subtypes of Melanoma
The median overall survival (OS) was 36.0 months (range, 1.5₋36 months). The 3-year OS rates for acral, high-CSD, low-CSD, mucosal, and unknown primary types were 47.6% (20/42 cases), 60% (15/25 cases), 64.9% (24/37 cases), 33.3% (4/12 cases), and 50% (2/4 cases), respectively. The 3-year OS based on the AJCC-8th staging was 75% for stage IIIA, 82.9% for stage IIIB, 54.1% for stage IIIC, and 75% for stage IIID disease in both acral and non-acral cutaneous melanomas. We analyzed the OS between acral and non-acral melanoma, and there were no significant differences (
P >0.05,
Figure 2a,b). Mucosal type did not exhibit a poorer prognosis compared to non-acral melanoma in terms of OS (
P >0.05). Regarding baseline or treatment-related factors, patients with tumor ulceration had a significantly better prognosis in terms of OS than those without tumor ulceration (HR, 0.39; 95% CI: 0.17 - 0.85;
P = 0.013,
Figure 2d). There were no significant differences in median TT, tumor ulceration, BRAF status, and CLND (
P >0.05,
Figure 2c,e,f).
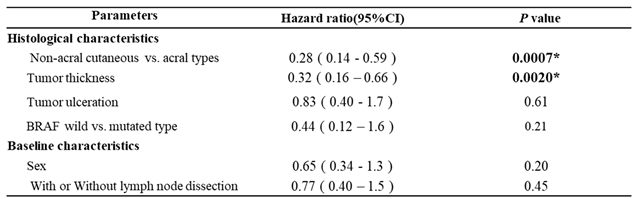
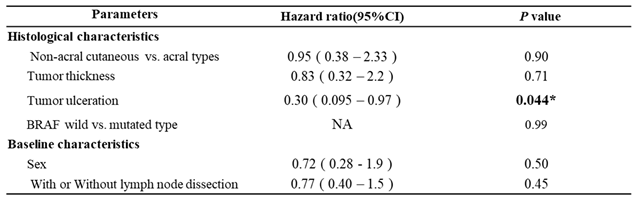
3.4. Multivariate Analysis of the Prognostic Factors in Both RFS and OS
Among 104 acral and non-acral cutaneous melanoma cases, we evaluated histological characteristics (acral type, TT, ulceration, and BRAF status), baseline characteristics (gender and age), and treatment options for multivariate analysis to identify relevant prognostic factors for RFS or OS. Moreover, we classified 104 patients into the anti-PD-1 Abs’ or dabrafenib plus trametinib combined therapies’ cohort.
In the anti-PD-1 Abs’ cohort, non-acral cutaneous type (HR, 0.33; 95% CI, 0.17 - 0.64;
P = 0.0010) and median tumor thickness (HR, 0.36; 95% CI, 0.19 - 0.69;
P = 0.0022) showed statistically significant differences in RFS (
Table 1), while in the combined therapies’ cohort, there were no significant factors (P >0.05, supplemental
Table 1). Only tumor ulceration was associated with OS in the anti-PD-1 Abs’ cohort (HR, 0.30; 95% CI, 0.095 - 0.97;
P = 0.0044,
Table 2). There was no significant difference in the dabrafenib plus trametinib combined therapies’ cohort (P >0.05, supplemental
Table 2).
4. Discussion
Acral melanoma exhibits a higher incidence in Asian countries, including Japan, compared to European countries and the U.S. (incidence rate: 1.0 - 1.5% vs. 42 - 65%) [
5]. Acral melanoma is characterized by higher rates of BRAF-wild type, lower tumor mutation burden, and a lack of ultraviolet (UV)-related mutational signatures compared to non-acral cutaneous melanoma [
8]. The numbers, localization, and phenotypes of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment are well-known indicators reflecting response to immunotherapy [
9,
10]. CD8+ T cells’ infiltration has been identified in multiple studies as an important prognostic factor for clinical outcomes and predictive value for response to immunotherapy [
11]. Total TIL numbers, including CD8+ lymphocytes, were significantly lower in both acral and mucosal types than in non-acral cutaneous types based on specimens from 137 patients [
12]. Additionally, acral melanoma was characterized by fewer NK cells and an almost complete absence of γδ T cells in the TILs [
13]. Since NK cells express cytotoxic proteins commonly found in CD8+ T cells that directly kill target cells [
14], acral melanoma may have an immunosuppressed environment compared to non-acral cutaneous melanoma.
In our 3-year analysis, we demonstrated that acral melanoma had a poorer prognosis compared to non-acral cutaneous melanoma in terms of RFS in the adjuvant setting (P <0.005, log-rank analysis). Significant differences were observed in TT and BRAF status at the 3-year RFS. In our cohort, most patients with BRAF-mutated melanoma opted for combination therapy with BRAF plus MEK inhibitors. In the Asian population, immune checkpoint inhibitors have shown lower responsiveness to advanced melanoma in several reports compared to clinical trials from Caucasians. Therefore, dermatologists or oncologists in Japan tend to administer BRAF+MEK inhibitors to BRAF-mutated cases [
15,
16]. In the ONO-4538-08 study, the 5-year OS rate was 66.7% for patients with superficial spreading melanoma (SSM) type, 14.3% for acral lentiginous melanoma (ALM) type, and 16.7% for mucosal type in the Japanese advanced melanoma cohort [
17]. Other histological characteristics (mucosal type, tumor ulceration), baseline characteristics (sex and age) did not show significant differences in RFS (
P>0.05). In multivariate analysis, acral melanoma and TT showed significant differences among 104 cutaneous and acral melanoma cases. We demonstrated that acral melanoma was one of the independent prognostic factors at 3-year RFS, as well as at 2-year RFS. Since patients treated with a combination of BRAF+MEK inhibitors had better RFS than those treated with anti-PD-1 inhibitors, non-acral types with higher BRAF mutation were preferred for the combination therapy, while acral melanoma with lower BRAF mutation received anti-PD-1 inhibitors.
From 1948 patients with cutaneous melanoma, the 5-year melanoma-specific survival (MSS) for stage IIIA-C was 89%, 81%, and 64%, respectively [
18]. In the Japanese Melanoma Study (JMS) using AJCC-8
th criteria, the 3-year disease-specific survival (DSS) was 92.3% for stage IIIA, 82.5% for stage IIIB, 67.1% for stage IIIC, and 38.5% for stage IIID disease in Asian populations [
19]. Notably, these data included 3,097 patients from 2005 to 2017. Our 3-year OS results were 75% for stage IIIA, 82.9% for stage IIIB, 54.1% for stage IIIC, and 75% for stage IIID disease in both acral and non-acral cutaneous melanoma. Unexpectedly, postoperative adjuvant therapy was not shown to be superior to previous literature. We suspect that the difference in sample size and melanoma subtype in our cohort had an impact on the results. Additionally, our data included patients who died from any cause, so we could not accurately assess the effectiveness of adjuvant therapy in stage III. In our cohort, patients treated with CLND tended to show better OS compared to those without CLND in the univariate analysis (
P = 0.051).
5. Conclusions
This study suggests that both acral and mucosal types in the adjuvant setting are less effective than non-acral cutaneous melanoma at the 3-year RFS. We retrospectively analyzed the 3-year RFS and OS in real-world melanoma patients in the adjuvant setting; however, this was not a large cohort study in the Asian population. It is difficult to evaluate the efficacy of adjuvant anti-PD-1 or dabrafenib plus trametinib combination therapy on OS compared with conventional adjuvant therapy or observation. Further investigation was needed with a large sample size and low bias.
Author Contributions
Y.M. designed the study, developed the main conceptual ideas, and outlined the manuscript. Y.A. and T.F. assisted in interpreting the results and revised the manuscript. K.Y., K.H., M.S., F.S., I.T., M.T., S.I., U.H., M.S., Y.Y., Y.K., A.R., O.K., and H.A. collected the data. Y.M. wrote the manuscript with assistance from Y.A. and T.F. All authors discussed the results and provided comments on the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Tohoku University Hospital, and the study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine, Sendai, Japan (permit no. 2020-1-811).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, T.F. The data are not publicly available due to containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Ascierto, P.A.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2302. [Google Scholar]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Chiarion Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N Engl J Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in Acral and Mucosal Melanoma: Current Status and Future Directions. Frontiers in immunology. 2021, 12, 680407. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Fujisawa, Y.; Yoshino, K.; Uchi, H.; Matsushita, S.; et al. Adjuvant Anti-PD-1 Antibody Therapy for Advanced Melanoma: A Multicentre Study of 78 Japanese Cases. Acta Derm Venereol. 2022, 102, adv00756. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Shoichiro, I.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Postoperative adjuvant therapy for 120 patients with melanoma, including acral and mucosal subtypes: a multicentre, observational study of 2-year follow-up results. The British journal of dermatology. 2023, 189, 476–478. [Google Scholar] [CrossRef] [PubMed]
- Dugan, M.M.; Perez, M.C.; Karapetyan, L.; Zager, J.S. Management of acral lentiginous melanoma: current updates and future directions. Frontiers in oncology. 2024, 14, 1323933. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nature reviews. Clinical oncology. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.A.; Amir, E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. British journal of cancer. 2017, 117, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Maibach, F.; Sadozai, H.; Seyed Jafari, S.M.; Hunger, R.E.; Schenk, M. Tumor-Infiltrating Lymphocytes and Their Prognostic Value in Cutaneous Melanoma. Frontiers in immunology. 2020, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Zhenjie, Z.; Oya, K.; Tanaka, R.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujisawa, Y. Poor Lymphocyte Infiltration to Primary Tumors in Acral Lentiginous Melanoma and Mucosal Melanoma Compared to Cutaneous Melanoma. Frontiers in oncology. 2020, 10, 524700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Smalley, I.; Chen, Z.; Wu, J.Y.; Phadke, M.S.; Teer, J.K.; Nguyen, T.; Karreth, F.A.; Koomen, J.M.; Sarnaik, A.A.; et al. Single-cell Characterization of the Cellular Landscape of Acral Melanoma Identifies Novel Targets for Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2022, 28, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Topham, N.J.; Hewitt, E.W. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 2009, 128, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Izumi, T.; Doi, R.; Kamimura, A.; Takai, S.; Teramoto, Y.; Nakamura, Y. Immune checkpoint inhibitor-based therapy for advanced acral and mucosal melanoma. Experimental dermatology. 2023, 32, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, K.; Ito, T.; Yoshikawa, S.; Yoshino, K.; Kiniwa, Y.; Ohe, S.; Isei, T.; Takenouchi, T.; Kato, H.; Mizuhashi, S.; et al. Systemic therapy for Asian patients with advanced BRAF V600-mutant melanoma in a real-world setting: A multi-center retrospective study in Japan (B-CHECK-RWD study). Cancer medicine. 2023, 12, 17967–17980. [Google Scholar] [CrossRef] [PubMed]
- Uhara, H.; Kiyohara, Y.; Uehara, J.; Fujisawa, Y.; Takenouchi, T.; Otsuka, M.; Uchi, H.; Fukushima, S.; Minami, H.; Hatsumichi, M.; et al. Five-year survival with nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. The Journal of dermatology. 2021, 48, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kanaki, T.; Stang, A.; Gutzmer, R.; Zimmer, L.; Chorti, E.; Sucker, A.; Ugurel, S.; Hadaschik, E.; Gräger, N.S.; Satzger, I.; et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. European journal of cancer (Oxford, England : 1990). 2019, 119, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Classification of 3097 patients from the Japanese melanoma study database using the American joint committee on cancer eighth edition cancer staging system. Journal of dermatological science. 2019, 94, 284–289. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).