Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
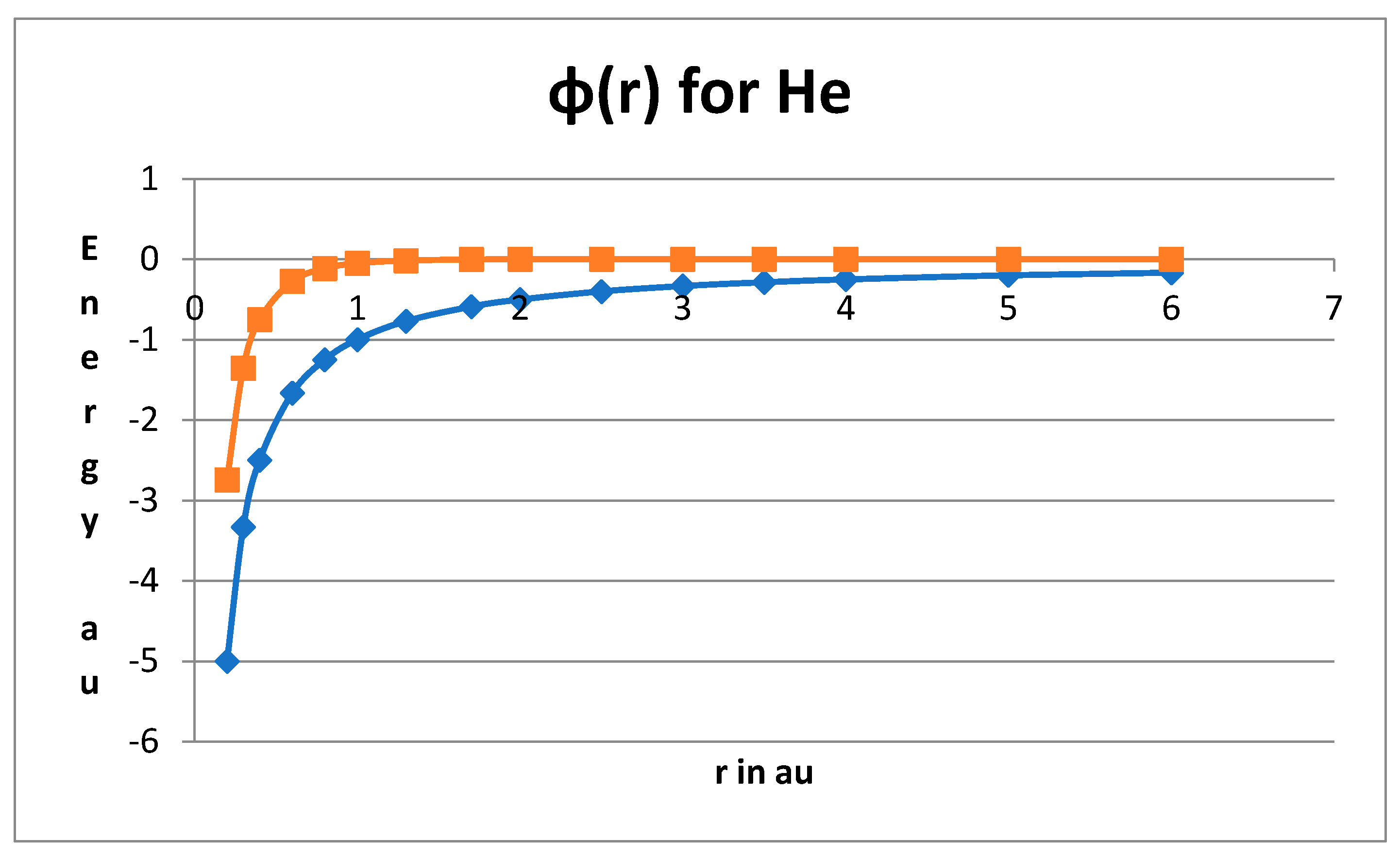
Understanding the Quantum-Mechanical Hydrogen Atom
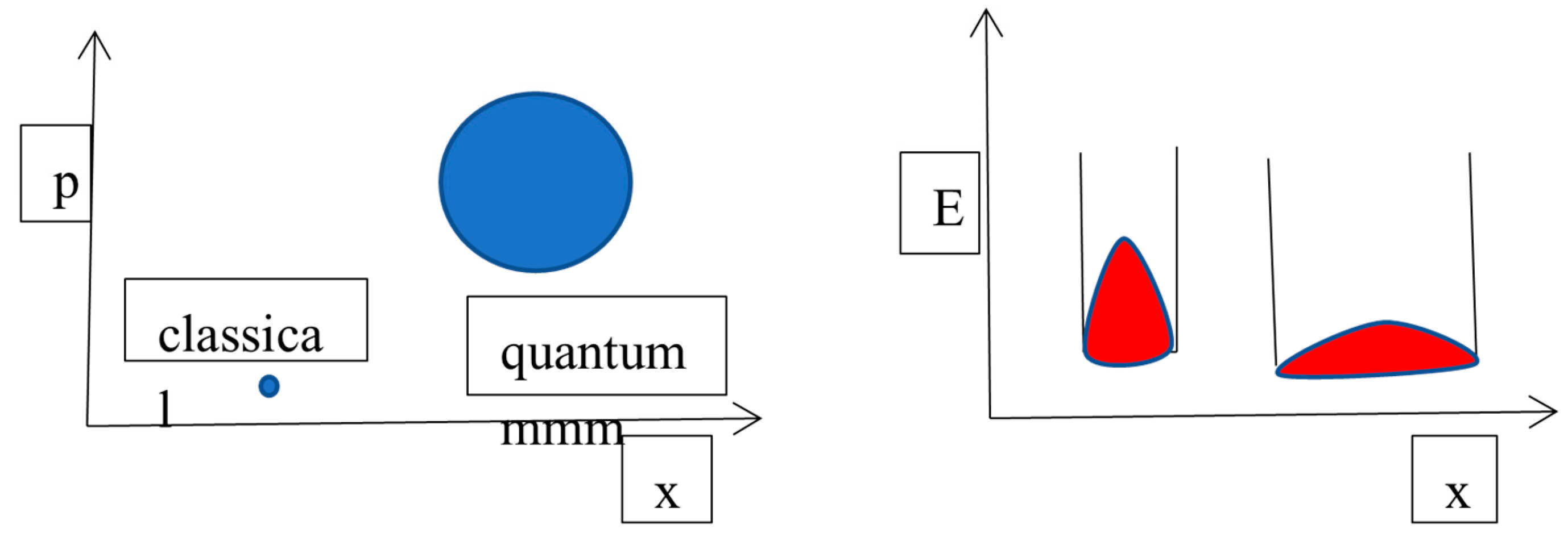
The Key Role of Quantum Diffusivity
The Origin of Periodic Atomic Properties - The Shell Structure of Hydrogenic Atoms
Hydrogenic Atoms
The Aufbau Model - An Empirical Mean-Field Model of Atoms
Simple Representation of Screening in an Atom
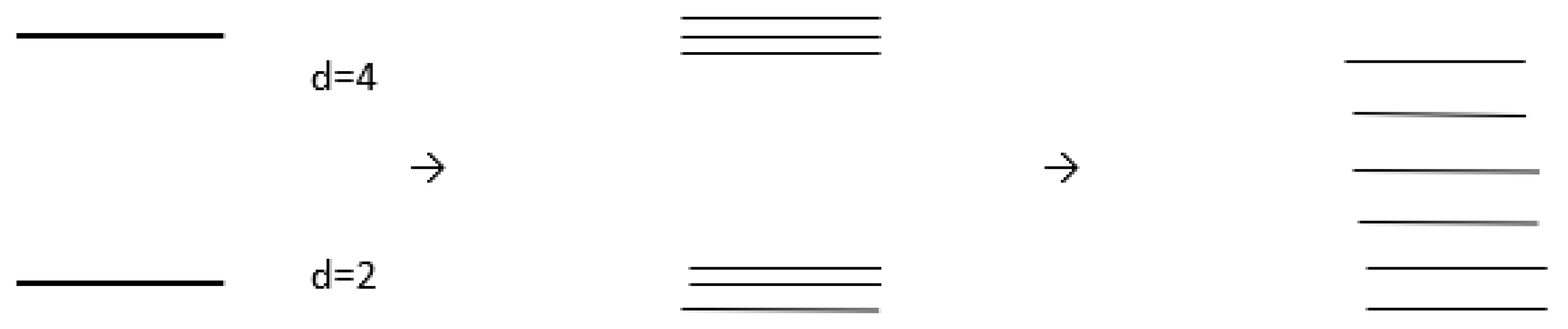
Example of Coupling between Degeneracy and Reactivity
The Normal Aufbau Rules for Orbital Population
- Minimize among as yet not fully occupied subshells;
- Minimize among the available subshells all of the same minimal -value.
- The diffusivity of the quantum states of electrons;
- The good (but not perfect) validity of a mean-field model of the atoms with independent electrons in a spherically symmetric potential of screened Coulomb type.
The Periodic Table According to Our Quantum Aufbau Model
Chemical Bonding Mechanisms
- Redistribution of electrons among atoms to form molecules composed of stable (inert gas like) atomic ions held together by Coulomb attraction between unlike charges. (Ionic bonding)
- Achieving ground state non-degeneracy by removal of dynamical constraints to relax the “decomposability” of the electron dynamics and facilitate delocalization of electron motion over bonded atoms. (Covalent bonding)
Ionic Bonding
Covalent Bonding
The Interatomic Repulsion - Equilibrium Geometries of Molecules
Interatomic Electrostatic Interactions
The Pauli Repulsion
- Rising orbital energies (or kinetic energy) when electrons of equal spin are physically coincident;
- Steric repulsion (or excluded volume) when geometrically separated electrons of equal spin approach.
- 3.
- Atomic space contraction - Basis function overlap correction.
Conclusions
Acknowledgement
Conflicts of interest
References
- Kaji, M.D.I. Mendeleev’s Concept of Chemical Elements and The principles of Chemistry. Bull. Hist. Chem. 2002, 27, 4–16. [Google Scholar]
- Scerri, E.R. The Periodic Table—A Very Short Introduction, 2nd ed.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Schwarz, W.H.E.; Rich, R.L. Theoretical basis and Correct Explanation of the Periodic System: Review and Update. Journal of Chemical Education 2010, 87, 435–443. [Google Scholar] [CrossRef]
- Thomson, J.J. On the Structure of the Atom: An investigation of the stability and periods of oscillation of a number of corpuscles arranged at equal intervals around the Circumference of a Circle: With Application to the theory of Atomic Structure. Phil. Mag. Ser. 1904, 6, 237–265. [Google Scholar] [CrossRef]
- Rutherford, E. The Scattering of α and β Particles by Matter and the Structure of the Atom. Philos. Mag. 1911, 21, 669–688. [Google Scholar] [CrossRef]
- Lewis, G.N. The Atom and the Molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Langmuir, I. The Arrangement of Electrons in Atoms and Molecules. J. Am. Chem. Soc. 1919, 41, 868–934. [Google Scholar] [CrossRef]
- Lewis, G.N. Valence and the Structure of Atoms and Molecules; American Chemical Society Monograph Series: New York, NY, USA, 1923. [Google Scholar]
- Zhao, L.; Hermann, M.; Schwarz, W.H.E.; Frenking, G. The Lewis Pair Bonding Model: The physical background a century later. Nat. Rev. Chem. 2019, 3, 35–47. [Google Scholar] [CrossRef]
- Langmuir, I. Types of Valence. Science 1921, 54, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sidgwick, N.V.; Powell, H.M. Bakerian Lecture: Stereochemical Types and Valency Groups. Proc. Roy. Soc. A. 1940, 176, 153–180. [Google Scholar]
- Gillespie, R.J.; Nyholm, R.S. Inorganic Stereochemistry. Q. Rev. Chem. Soc. 1957, 11, 339. [Google Scholar] [CrossRef]
- Gillespie, R.J. Fifty Years of the VSEPR Model. Coord. Chem. Rev. 2008, 252, 1315–1327. [Google Scholar] [CrossRef]
- Pauling, L. The Shared-Electron Chemical Bond. Proc. Natl. Acad. Sci. USA 1928, 14, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The Nature of the Chemical Bond. II. The One-Electron Bond and the Three-Electron Bond. J. Am. Chem. Soc. 1931, 53, 3225–3237. [Google Scholar] [CrossRef]
- Pauling, L.; Wheland, G.W. The Nature of the Chemical Bond. V. The Quantum-Mechanical Calculation of the Resonance Energy of Benzene and Naphthalene and the Hydrocarbon Free Radicals. J. Chem. Phys. 1933, 1, 362–374. [Google Scholar] [CrossRef]
- Heitler, W.; London, F. Wechselwirkung neutraler Atome und homöopolare Bindung nach den Quantenmechanik. Z. Phys. 1927, 44, 455–472. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Helgaker, T.; Jörgensen, P.; Olsen, J. Molecular Electronic Structure Theory; Wiley: Chichester, UK, 2000. [Google Scholar]
- Zhao, L.; Pan, S.; Holzmann, N.; Schwerdtfeger, P.; Frenking, G. Chemical Bonding and Bonding Models of Main-Group Compounds. Chem. Rev. 2019, 119, 8781–8845. [Google Scholar] [CrossRef] [PubMed]
- Frenking, G.; Shaik, S. The Chemical Bond-Fundamental Aspects of Chemical Bonding; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Burdett, J.K. Chemical Bonds: A Dialog; Wiley: Chichester, England, 1997. [Google Scholar]
- Ruedenberg, K. Physical Nature of Chemical Bond. Rev. Mod. Phys. 1962, 34, 326–376. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: USA, 1994. [Google Scholar]
- Grinter, R. The Quantum in Chemistry: An Experimentalist’s View; Wiley: Chichester, England, 2005. [Google Scholar]
- Keeler, J.; Wothers, P. Chemical Structure and Reactivity: An integrated approach, 2nd ed.; Oxford University Press: Oxford, 2014. [Google Scholar]
- Kragh, H.S. Niels Bohr and the Quantum Atom—The Bohr Model of Atomic Structure 1913-1925; Oxford University Press: Oxford, 2012. [Google Scholar]
- Scerri, E.S. The Periodic Table. Its Story and Its Significance; Oxford University Press: Oxford, 2007. [Google Scholar]
- Nordholm, S. From Electronegativity towards Reactivity—Searching for a Measure of Atomic Reactivity. Molecules 2021, 26, 3680–3704. [Google Scholar] [CrossRef] [PubMed]
- Madelung, E. Die Mathematische Hilfsmittel der Physikers, 3rd ed.; Springer: Berlin, 1936; p. 359. [Google Scholar]
- Feynman, R.P.; Leighton, R.B.; Sands, M. The Feynman Lectures on Physics, Quantum Mechanics; Addison-Wesley Publishing Company: Reading, MA, USA, 1965; Volume III, pp. 10-1–10-12. [Google Scholar]
- Hellmann, H. Zur Rolle der Kinetischen Elektronenergie für die zwischen Atomäre Kräfte. Z. Phys. 1933, 85, 180–190. [Google Scholar] [CrossRef]
- Nordholm, S. Analysis of Covalent Bonding by Non-Ergodic Thomas-Fermi Theory. J. Chem. Phys. 1987, 86, 363–369. [Google Scholar] [CrossRef]
- Nordholm, S.; Bäck, A.; Bacskay, G.B. The mechanism of Covalent Bonding: Analysis within the Hückel Model of Electronic Structure. J. Chem. Ed. 2007, 84, 1201–1203. [Google Scholar] [CrossRef]
- Nordholm, S.; Eek, W. Ergodicity and Rapid Electron Delocalization—The Dynamical Mechanism of Atomic Reactivity and Covalent Bonding. Int. J. Quant. Chem. 2011, 111, 2072–2088. [Google Scholar] [CrossRef]
- Nordholm, S.; Bacskay, G.B. The Role of Quantum Dynamics in Covalent Bonding—A Comparison of Thomas-Fermi and Hückel Models. In Advances in Quantum Theory; Cotaescu, I.I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 107–152. [Google Scholar]
- Nordholm, S.; Bacskay, G.B. The Basics of Covalent Bonding in Terms of Energy and Dynamics. Molecules 2020, 25, 2667–2701. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, S. Analysis of Bonding by Quantum Chemistry—Resolving Delocalization Stabilization in a Mechanistic Basis and New Hückel Model. J. Phys. Chem. A 2023, 127, 3449–3471. [Google Scholar] [CrossRef]
- Esterhuysen, C.; Frenking, G. The Nature of the Chemical Bond Revisited. An Energy Partitioning Analysis of Diatomic Molecules E2 (E=N-Bi, F-I), CO and BF. Theor. Chem. Acc. 2004, 111, 381–389. [Google Scholar] [CrossRef]
- Levine, D.S.; Head-Gordon, M. Clarifying the quantum mechanical origin of the covalent bond. Nat. Commun. 2020, 11, 4893–4900. [Google Scholar] [CrossRef]
- Bacskay, G.B. Orbital Contraction and Covalent Bonding. J. Chem. Phys. 2022, 156, 204122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



