Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
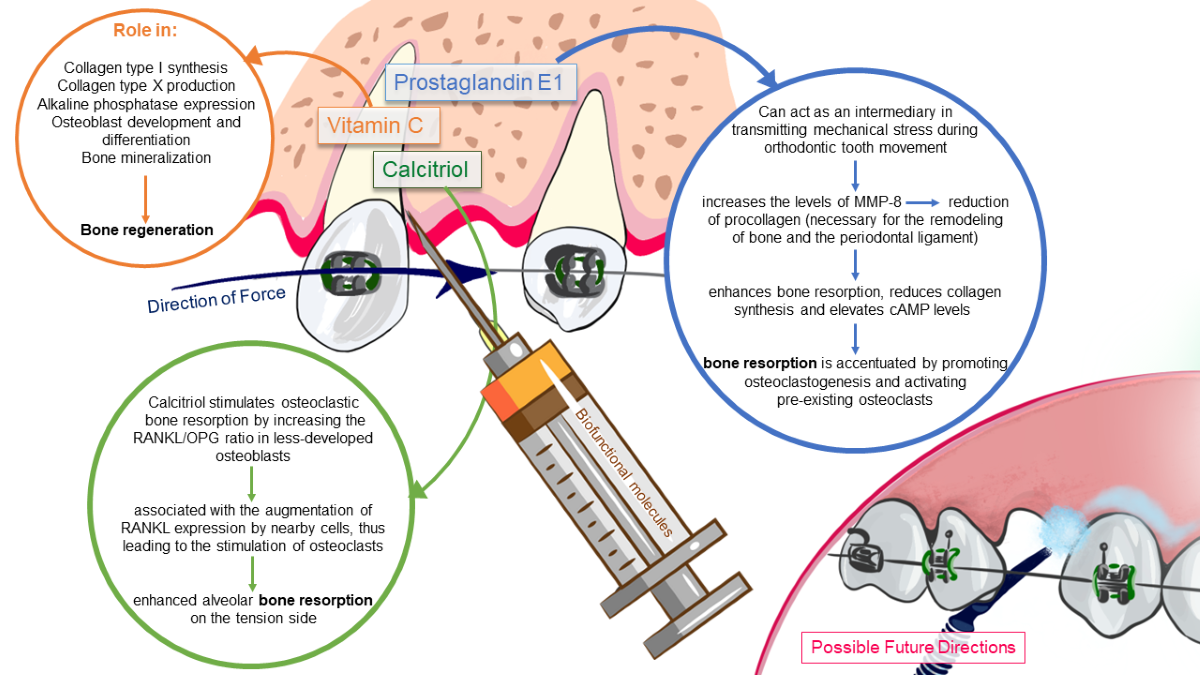
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Human Studies
3.1.1. Biofunctional Molecules Involved in Enhancing OTM
3.1.1.1. Calcitriol
3.1.1.2. Prostaglandins
3.1.1.3. Vitamin C
3.1.1.4. Recombinant human Relaxin
| Authors, year | Biofunctional molecules | Description of groups, dosage | Side effects | Outcome |
|---|---|---|---|---|
| 1.AlHasani et al., 2021 | Calcitriol | EG: OTM + 25pg/0.2ml Vitamin D3 diluted in DMSO CG: OTM + no intervention |
The intervention did not result in additional root resorption and did not adversely affect the integrity of the alveolar bone (p > 0.05 in canine root resorption evaluations between the experimental and control sides) | The local injection of vitamin D3 improved canine retraction and led to a more favorable periodontal response. The study validated and referenced previous human studies demonstrating a 51% acceleration in tooth movement on the experimental sides. |
| 2.Varughese et al., 2019 | Calcitriol | EG: OTM + 50pg per 0.2mL of calcitriol (1,25 DHC) gel CG: OTM + 0.2mL placebo gel |
The findings indicated a statistically significant decline in cancellous bone density on the experimental side in comparison to the control side. There also was a significant overall decline in bone density of the cortical bones during OTM, on both experimental and control sides. | Quantitative measurement of canine distalization revealed a highly significant difference (p < 0.001) between the experimental and control sides over a period of three months. During the second and third months, there was a notable increase in the rate of canine movement on the experimental side, which was statistically significant. |
| 3.IosubCiur et al., 2016 | Calcitriol | EG: OTM + 42 pg/mL - 0.2 mL of vitamin D3 diluted in DMSO CG: OTM + no intervention |
There was no evidence of root resorption observed three months after the initial treatment with vitamin D3, as assessed by cone-beam CT examination | The mean rate of tooth displacement was higher in the experimental side compared to the control side. The experimental teeth exhibited an average of 70% more pronounced tooth movement compared to the control teeth. The disparities between the two quadrants (control and experimental) exhibited statistical significance (p<0.0313). |
| 4.AlHasani et al., 2011 | Calcitriol | EG1: OTM + 15 pg calcitriol diluted in 0.2 mL DMSO EG2: OTM + 25 pg calcitriol diluted in 0.2 mL DMSO EG3: OTM + 40 pg calcitriol diluted in 0.2 mL DMSO CG: OTM + placebo (0.2 mL DMSO) |
The periapical radiographs revealed no detrimental impact of calcitriol on the periodontium. | The study found that, when calcitriol is injected locally, its effectiveness and cost efficiency in humans followed a dose-dependent pattern. Specifically, a dose of 25 pg of calcitriol resulted in a 51% faster rate of experimental canine movement compared to the control group. Additionally, doses of 15 pg and 40 pg each led to approximately a 10% acceleration in orthodontic tooth movement. |
| 5.Patil et al., 2005 | Prostaglandin E1 | EG: OTM + 1g PGE1/ 1ml lignocaine CG: OTM + placebo (lignocaine only) |
No adverse effects were noticed, neither macroscopically nor through X-ray imaging, in the area where PGE1 was injected during the experimental study and the two-year follow-up period of active orthodontic treatment. | The observed data clearly demonstrated a substantial enhancement in orthodontic tooth displacement on the side injected with PGE1, compared to the control side. The ratio of the intervention side to the control side was 1.7/1, as seen during a period of one month. |
| 6.Yamasaki et al., 1984 | Prostaglandin E1 | EG: OTM + 10 µg PGE1 CG: OTM + placebo (lidocaine only) |
No adverse effects were observed in the surrounding tissues when examined macroscopically and radiographically. | Local administration of 10 µg of PGE1, in the gingiva adjacent to the orthodontically treated teeth resulted in a significant increase in the rate of tooth movement compared to the control side. The distal canine movement rate was nearly 1.6 times higher on the side where PGE1 injections were administered compared to the side where the vehicle was injected. This difference was statistically significant (p< 0.05). |
| 7.Yussif et al., 2018 | Vitamin C | EG: OTM + vitamin C with a dosage calculated for a single tooth* CG: OTM + no intervention |
There was no evidence of bone or root resorption in the teeth that were treated after receiving a vitamin C injection. | The intervention group showed a clinically significant improvement in movement rate (2-2.5 mm), while the control group had a lower rate (0.5-1.5 mm). The p-value was found to be p<0.003. The results implied that locally administered vitamin C is a powerful catalyst for eruption that has the benefit of preserving the integrity of the periodontium. |
| 8.McGorray et al., 2012 | Recombinant human Relaxin | EG: OTM + 50 mg Relaxin divided in 2 injections of 0.1 mL CG: OTM + placebo vehicle |
None of the subjects exhibited any presence of anti-Relaxin antibodies at any given period and no side effects were noted. | There were no statistically significant differences in tooth displacement between the experimental and the control group. |
3.1.2. Risk of Bias Assessments
3.2. Animal Studies
3.2.1. Prostaglandin E1 and E2
3.2.2. Growth Factors
3.2.3. RANKL and RANKL expression plasmid
3.2.4. Calcitriol
3.2.5. PTH
3.2.6. Other Biosubstances
3.2.7. Risk of Bias Assessment
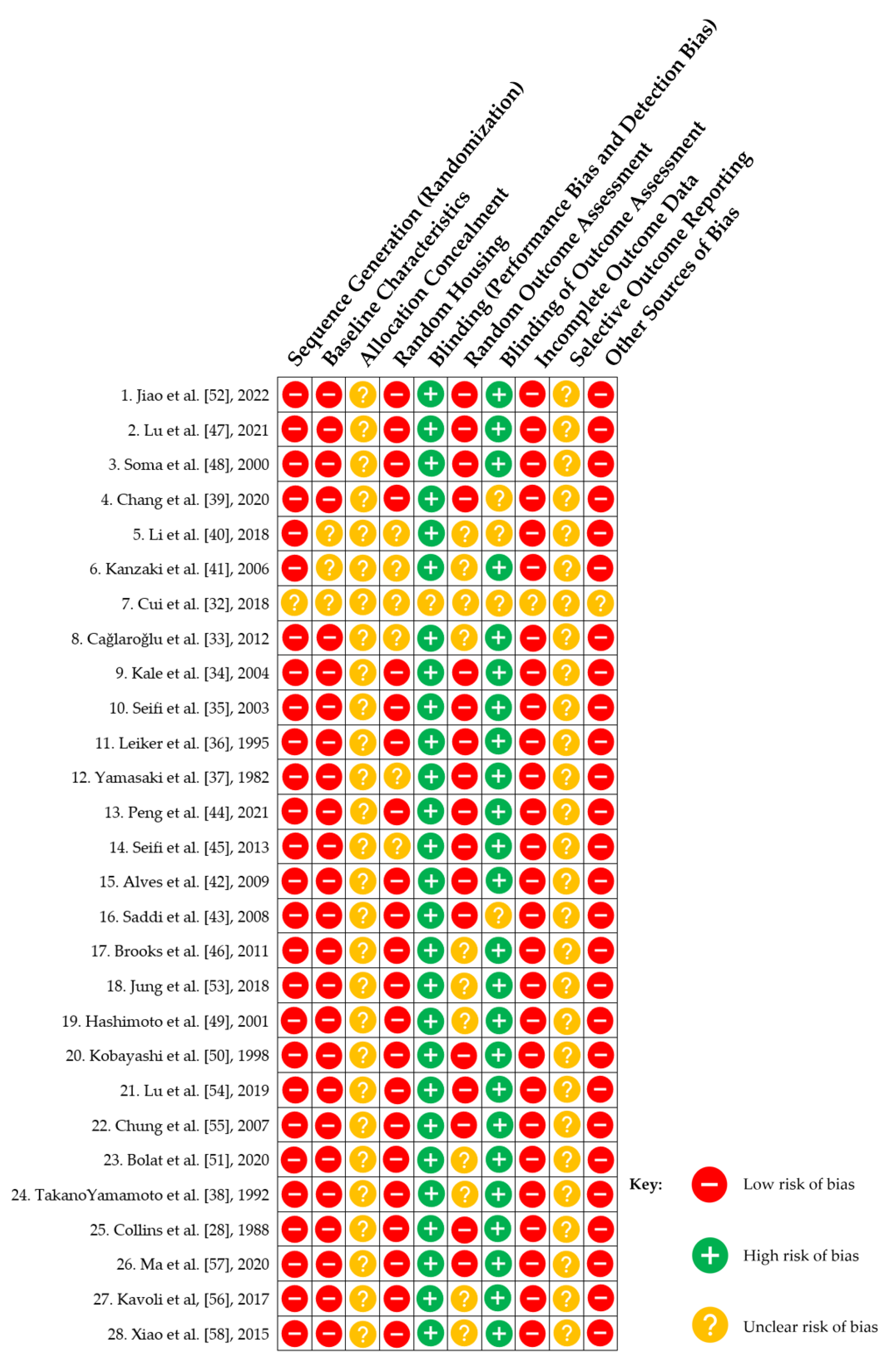
4. Discussion and Perspectives for Enhancing OTM
4.1. Biomolecules Involved in OTM
4.2. Pharmacokinetics and Experimental Properties of the Investigated Pharmacological Substances
4.3. Future Research Perspectives and Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abroms, L. Public Health in the Era of Social Media. Am. J. Public Health 2019, 109, S130–S131. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.; Patnaik, D. Social Media and Its Effects on Beauty. In. 2020; ISBN 978-1-83962-447-6. [Google Scholar]
- Dipalma, G.; Patano, A.; Ferrara, I.; Viapiano, F.; Netti, A.; Ceci, S.; Azzollini, D.; Ciocia, A.M.; Malcangi, G.; Inchingolo, A.D.; et al. Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy. Appl. Sci. 2023, 13, 9759. [Google Scholar] [CrossRef]
- Ozkan, T.; Arici, S.; Özkan, E. Acceleration of Orthodontic Tooth Movement: An Overview. Anadolu Klin. Tıp Bilim. Derg. 2018, 23, 121–128. [Google Scholar] [CrossRef]
- Arqub, S.A.; Gandhi, V.; Iverson, M.G.; Ahmed, M.; Kuo, C.-L.; Mu, J.; Dutra, E.; Uribe, F. The Effect of the Local Administration of Biological Substances on the Rate of Orthodontic Tooth Movement: A Systematic Review of Human Studies. Prog. Orthod. 2021, 22, 5. [Google Scholar] [CrossRef]
- Cellular, Molecular, and Tissue-Level Reactions to Orthodontic Force - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16627171/ (accessed on 23 December 2023).
- Roberts, W.E.; Graber, T.M.; Vanarsdall, R.L. Orthodontics: Current Principles and Techniques. Bone Physiol. Metab. Biomech. Orthod. Pract. 1994, 221–292. [Google Scholar]
- Andrade, I.; Taddei, S.R.A.; Souza, P.E.A. Inflammation and Tooth Movement: The Role of Cytokines, Chemokines, and Growth Factors. Semin. Orthod. 2012, 18, 257–269. [Google Scholar] [CrossRef]
- Patil, A.K.; Shetty, A.S.; Setty, S.; Thakur, S. Understanding the Advances in Biology of Orthodontic Tooth Movement for Improved Ortho-Perio Interdisciplinary Approach. J. Indian Soc. Periodontol. 2013, 17, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Mavreas, D.; Athanasiou, A.E. Factors Affecting the Duration of Orthodontic Treatment: A Systematic Review. Eur. J. Orthod. 2008, 30, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, K.J.; Brook, K.J.; Thomson, W.M.; Harding, W.J. Factors Influencing Treatment Time in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2006, 129, 230–238. [Google Scholar] [CrossRef]
- Melo, A.C.E.D.O.; Carneiro, L.O.T.; Pontes, L.F.; Cecim, R.L.; Mattos, J.N.R.D.; Normando, D. Factors Related to Orthodontic Treatment Time in Adult Patients. Dent. Press J. Orthod. 2013, 18, 59–63. [Google Scholar] [CrossRef]
- Montero Jiménez, O.G.; Dib Kanán, A.; Dipp Velázquez, F.A.; Aristizábal Pérez, J.F.; Moyaho Bernal, M.; de los, Á.; Salas Orozco, M.F.; Casillas Santana, M.A. Use of Hydrogels to Regulate Orthodontic Tooth Movement in Animal Models: A Systematic Review. Appl. Sci. 2022, 12, 6683. [Google Scholar] [CrossRef]
- Lin, Y.; Fu, M.L.; Harb, I.; Ma, L.X.; Tran, S.D. Functional Biomaterials for Local Control of Orthodontic Tooth Movement. J. Funct. Biomater. 2023, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- JabRef Bibliography Management. Available online: https://docs.jabref.org/ (accessed on 23 December 2023).
- Chapter 8: Assessing Risk of Bias in a Randomized Trial. Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 23 December 2023).
- Al-Hasani, N.; Albustani, A. Effect of Accelerated Canine Retraction by Vitamin D3 Local Administration on Apical Root Resorption, Alveolar Bone Integrity and Chairside Time A Prospective Clinical Study. Int. Med. J. 1994 2021, 28, 654–657. [Google Scholar]
- Varughese, S.T.; Shamanna, P.U.; Goyal, N.; Thomas, B.S.; Lakshmanan, L.; Pulikkottil, V.J.; Ahmed, M.G. Effect of Vitamin D on Canine Distalization and Alveolar Bone Density Using Multi-Slice Spiral CT: A Randomized Controlled Trial. J. Contemp. Dent. Pract. 2019, 20, 1430–1435. [Google Scholar]
- Iosub Ciur, M.-D.; Zetu, I.N.; Haba, D.; Viennot, S.; Bourgeois, D.; Andrian, S. Evaluation of the Influence of Local Administration of Vitamin D on the Rate of Orthodontic Tooth Movement. Rev. Med. Chir. Soc. Med. Nat. Iasi 2016, 120, 694–699. [Google Scholar] [PubMed]
- Al-Hasani, N.; Albustani, A.; Ghareeb, M.; Hussain, S. Clinical Efficacy of Locally Injected Calcitriol in Orthodontic Tooth Movement. Int. J. Pharm. Pharm. Sci. 2011, 3, 139–143. [Google Scholar]
- Patil, A.K.; Keluskar, K.M.; Gaitonde, S.D. The Clinical Application of Prostaglandin E1 on Orthodontic Tooth Movement - A Clinical Trial. J. Indian Orthod. Soc. 2005, 39, 91–98. [Google Scholar] [CrossRef]
- Yamasaki, K.; Shibata, Y.; Imai, S.; Tani, Y.; Shibasaki, Y.; Fukuhara, T. Clinical Application of Prostaglandin E1 (PGE1) upon Orthodontic Tooth Movement. Am. J. Orthod. 1984, 85, 508–518. [Google Scholar] [CrossRef]
- Efficacy and Safety of Locally Injectable Vitamin C on Accelerating the Orthodontic Movement of Maxillary Canine Impaction (Oral Mesotherapy Technique): Prospective Study | Request PDF. ResearchGate. [CrossRef]
- McGorray, S.P.; Dolce, C.; Kramer, S.; Stewart, D.; Wheeler, T.T. A Randomized, Placebo-Controlled Clinical Trial on the Effects of Recombinant Human Relaxin on Tooth Movement and Short-Term Stability. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2012, 141, 196–203. [Google Scholar] [CrossRef]
- Graber, L.; Vanarsdall, R.; Vig, K. Bone Physiology, Metabolism, and Biomechanics in Orthodontic Practice. 2011. [Google Scholar]
- Turner, A.G.; Anderson, P.H.; Morris, H.A. Vitamin D and Bone Health. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 65–72. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts: What Do They Do and How Do They Do It? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.K.; Sinclair, P.M. The Local Use of Vitamin D to Increase the Rate of Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 1988, 94, 278–284. [Google Scholar] [CrossRef]
- Effects of Matrix Metalloproteinase Inhibitors on Bone Resorption and Orthodontic Tooth Movement - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12939351/ (accessed on 23 December 2023).
- Yussif, N.M.; Abdul Aziz, M.A.; Abdel Rahman, A.R. Evaluation of the Anti-Inflammatory Effect of Locally Delivered Vitamin C in the Treatment of Persistent Gingival Inflammation: Clinical and Histopathological Study. J. Nutr. Metab. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- The Roles and Mechanisms of Actions of Vitamin C in Bone: New Developments - PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26358868/ (accessed on 23 December 2023).
- Cui, J.-J.; Wang, X.-X.; Wang, Y.; Chen, P.-P.; Ma, D.; Zhang, J. [Effect of akebiasaponin D with different concentrations on orthodontic tooth movement in rats]. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2018, 27, 129–134. [Google Scholar]
- Cağlaroğlu, M.; Erdem, A. Histopathologic Investigation of the Effects of Prostaglandin E2 Administered by Different Methods on Tooth Movement and Bone Metabolism. Korean J. Orthod. 2012, 42, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Kocadereli, I.; Atilla, P.; Aşan, E. Comparison of the Effects of 1,25 Dihydroxycholecalciferol and Prostaglandin E2 on Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2004, 125, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Eslami, B.; Saffar, A.S. The Effect of Prostaglandin E2 and Calcium Gluconate on Orthodontic Tooth Movement and Root Resorption in Rats. Eur. J. Orthod. 2003, 25, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Leiker, B.J.; Nanda, R.S.; Currier, G.F.; Howes, R.I.; Sinha, P.K. The Effects of Exogenous Prostaglandins on Orthodontic Tooth Movement in Rats. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 1995, 108, 380–388. [Google Scholar] [CrossRef]
- Yamasaki, K.; Shibata, Y.; Fukuhara, T. The Effect of Prostaglandins on Experimental Tooth Movement in Monkeys (Macaca Fuscata). J. Dent. Res. 1982, 61, 1444–1446. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Kawakami, M.; Yamashiro, T. Effect of Age on the Rate of Tooth Movement in Combination with Local Use of 1,25(OH)2D3 and Mechanical Force in the Rat. J. Dent. Res. 1992, 71, 1487–1492. [Google Scholar] [CrossRef]
- Chang, J.H.; Chen, P.-J.; Arul, M.R.; Dutra, E.H.; Nanda, R.; Kumbar, S.G.; Yadav, S. Injectable RANKL Sustained Release Formulations to Accelerate Orthodontic Tooth Movement. Eur. J. Orthod. 2020, 42, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chung, C.J.; Hwang, C.-J.; Lee, K.-J. Local Injection of RANKL Facilitates Tooth Movement and Alveolar Bone Remodelling. Oral Dis. 2019, 25, 550–560. [Google Scholar] [CrossRef]
- Kanzaki, H.; Chiba, M.; Arai, K.; Takahashi, I.; Haruyama, N.; Nishimura, M.; Mitani, H. Local RANKL Gene Transfer to the Periodontal Tissue Accelerates Orthodontic Tooth Movement. Gene Ther. 2006, 13, 678–685. [Google Scholar] [CrossRef]
- Alves, J.B.; Ferreira, C.L.; Martins, A.F.; Silva, G.A.B.; Alves, G.D.; Paulino, T.P.; Ciancaglini, P.; Thedei, G.; Napimoga, M.H. Local Delivery of EGF-Liposome Mediated Bone Modeling in Orthodontic Tooth Movement by Increasing RANKL Expression. Life Sci. 2009, 85, 693–699. [Google Scholar] [CrossRef]
- Saddi, K.R.G.C.; Alves, G.D.; Paulino, T.P.; Ciancaglini, P.; Alves, J.B. Epidermal Growth Factor in Liposomes May Enhance Osteoclast Recruitment during Tooth Movement in Rats. Angle Orthod. 2008, 78, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-X.; Guan, X.-Y.; Li, G.-H.; Zhong, J.-L.; Song, J.-K.; Xiao, L.-L.; Jin, S.-H.; Liu, J.-G. Recombinant Human Insulin-like Growth Factor-1 Promotes Osteoclast Formation and Accelerates Orthodontic Tooth Movement in Rats. J. Appl. Oral Sci. Rev. FOB 2021, 29, e20200791. [Google Scholar] [CrossRef]
- Seifi, M.; Badiee, M.R.; Abdolazimi, Z.; Amdjadi, P. Effect of Basic Fibroblast Growth Factor on Orthodontic Tooth Movement in Rats. Cell J. Yakhteh 2013, 15, 230–237. [Google Scholar]
- Brooks, P.J.; Heckler, A.F.; Wei, K.; Gong, S.-G. M-CSF Accelerates Orthodontic Tooth Movement by Targeting Preosteoclasts in Mice. Angle Orthod. 2011, 81, 277–283. [Google Scholar] [CrossRef]
- Lu, W.; Li, X.; Yang, Y.; Yi, J.; Xie, L.; Zhao, Z.; Li, Y. PTH/PTHrP in Controlled Release Hydrogel Enhances Orthodontic Tooth Movement by Regulating Periodontal Bone Remodaling. J. Periodontal Res. 2021, 56, 885–896. [Google Scholar] [CrossRef]
- Local and Chronic Application of PTH Accelerates Tooth Movement in Rats - S. Soma, S. Matsumoto, Y. Higuchi, T. Takano-Yamamoto, K. Yamashita, K. Kurisu, M. Iwamoto, 2000. Available online: https://journals.sagepub.com/doi/10.1177/00220345000790091301 (accessed on 16 April 2024).
- Hashimoto, F.; Kobayashi, Y.; Mataki, S.; Kobayashi, K.; Kato, Y.; Sakai, H. Administration of Osteocalcin Accelerates Orthodontic Tooth Movement Induced by a Closed Coil Spring in Rats. Eur. J. Orthod. 2001, 23, 535–545. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Takagi, H.; Sakai, H.; Hashimoto, F.; Mataki, S.; Kobayashi, K.; Kato, Y. Effects of Local Administration of Osteocalcin on Experimental Tooth Movement. Angle Orthod. 1998, 68, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Esenlik, E.; Öncü, M.; Özgöçmen, M.; Avunduk, M.C.; Yüksel, Ö. Evaluation of the Effects of Vitamins C and E on Experimental Orthodontic Tooth Movement. J. Dent. Res. Dent. Clin. Dent. Prospects 2020, 14, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Wang, J.; Yu, W.; Zhang, K.; Zhang, N.; Cao, L.; Jiang, X.; Bai, Y. Biocompatible Reduced Graphene Oxide Stimulated BMSCs Induce Acceleration of Bone Remodeling and Orthodontic Tooth Movement through Promotion on Osteoclastogenesis and Angiogenesis. Bioact. Mater. 2022, 15, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-Y.; Ahn, S.-J.; Oh, S.-W.; Kim, K.-A.; Park, K.-H.; Park, Y.-G. Effects of Transmucosal Thyroxine Administration on the Tooth Movement in an Animal Model. APOS Trends Orthod. 2018, 8, 64–70. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, X.; Firth, F.; Mei, L.; Yi, J.; Gong, C.; Li, H.; Zheng, W.; Li, Y. Sclerostin Injection Enhances Orthodontic Tooth Movement in Rats. Arch. Oral Biol. 2019, 99, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Baik, H.-S.; Soma, K. Bone Formation and Tooth Movement Are Synergistically Enhanced by Administration of EP4 Agonist. Am. J. Orthod. Dentofac. Orthop. Off. Publ. Am. Assoc. Orthod. Its Const. Soc. Am. Board Orthod. 2007, 132, 427.e13-20. [Google Scholar] [CrossRef] [PubMed]
- Kavoli, S.; Mirzaie, M.; Feizi, F.; Rakhshan, V.; Arash, V.; Bijani, A. Local Injection of Carrageenan Accelerates Orthodontic Tooth Movement: A Preliminary Experimental Animal Study. Int. Orthod. 2017, 15, 588–599. [Google Scholar] [CrossRef]
- Ma, D.; Wang, X.; Ren, X.; Bu, J.; Zheng, D.; Zhang, J. Asperosaponin VI Injection Enhances Orthodontic Tooth Movement in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922372. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-Q.; Wang, H.-T.; Li, Y.-L.; Zeng, Q.; Zhou, E.; Ni, X.; Huan, Z.-P. The Effects of Dried Root Aqueous Extract of Salvia Miltiorrhiza and Its Major Ingredient in Acceleration of Orthodontic Tooth Movement in Rat. Iran. J. Basic Med. Sci. 2015, 18, 1044–1049. [Google Scholar]
- Kumegawa, M.; Ikeda, E.; Tanaka, S.; Haneji, T.; Yora, T.; Sakagishi, Y.; Minami, N.; Hiramatsu, M. The Effects of Prostaglandin E2, Parathyroid Hormone, 1,25 Dihydroxycholecalciferol, and Cyclic Nucleotide Analogs on Alkaline Phosphatase Activity in Osteoblastic Cells. Calcif. Tissue Int. 1984, 36, 72–76. [Google Scholar] [CrossRef]
- Agarwal, S.; Chaubey, K.K.; Chaubey, A.; Agarwal, V.; Madan, E.; Agarwal, M.C. Clinical Efficacy of Subgingivally Delivered Simvastatin Gel in Chronic Periodontitis Patients. J. Indian Soc. Periodontol. 2016, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lv, J.; Yang, Y.; Cheng, G.; Guo, S.; Liu, C.; Ding, Y. Advances of Hydrogel Therapy in Periodontal Regeneration-A Materials Perspective Review. Gels Basel Switz. 2022, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online | Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/ (accessed on 17 June 2024).
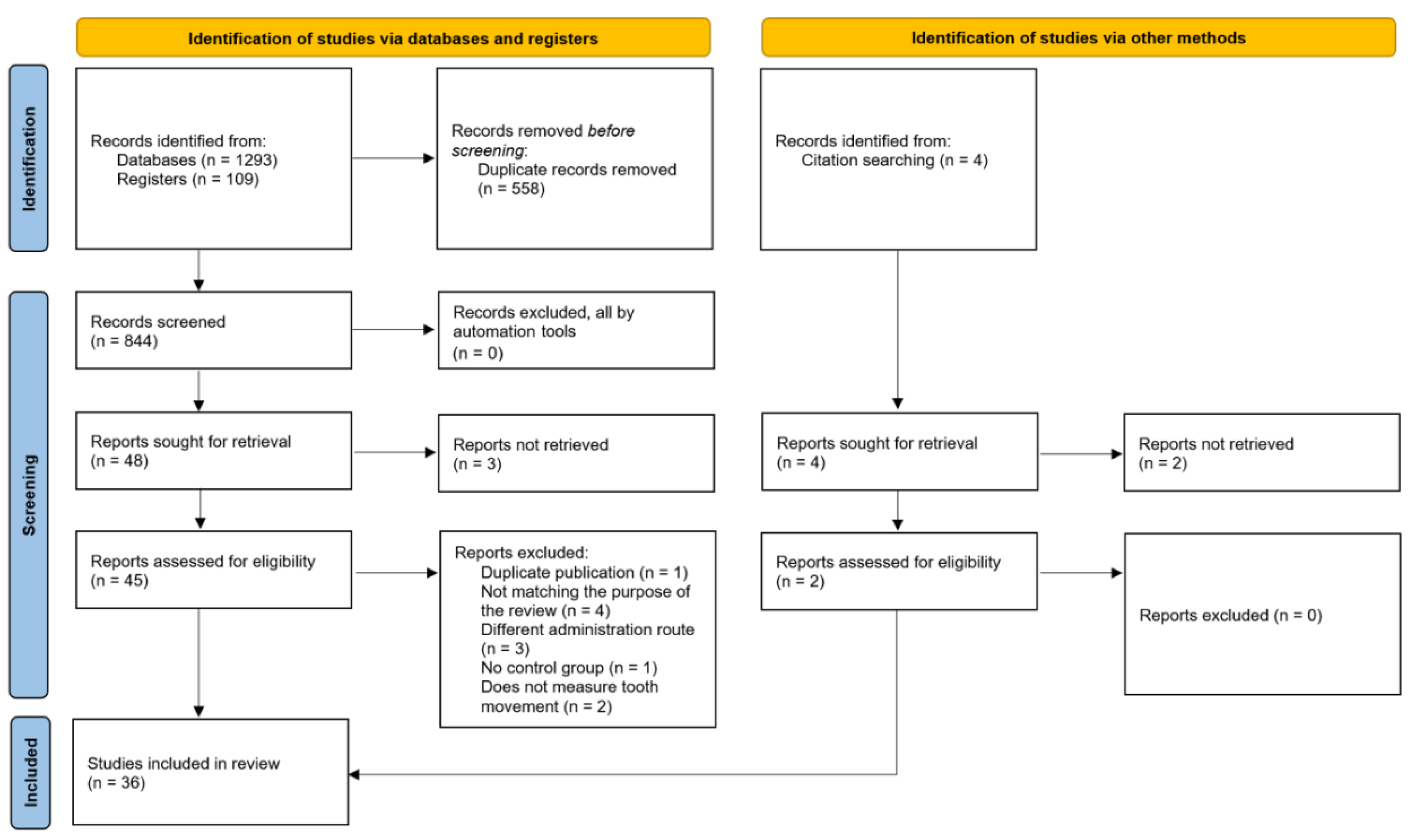
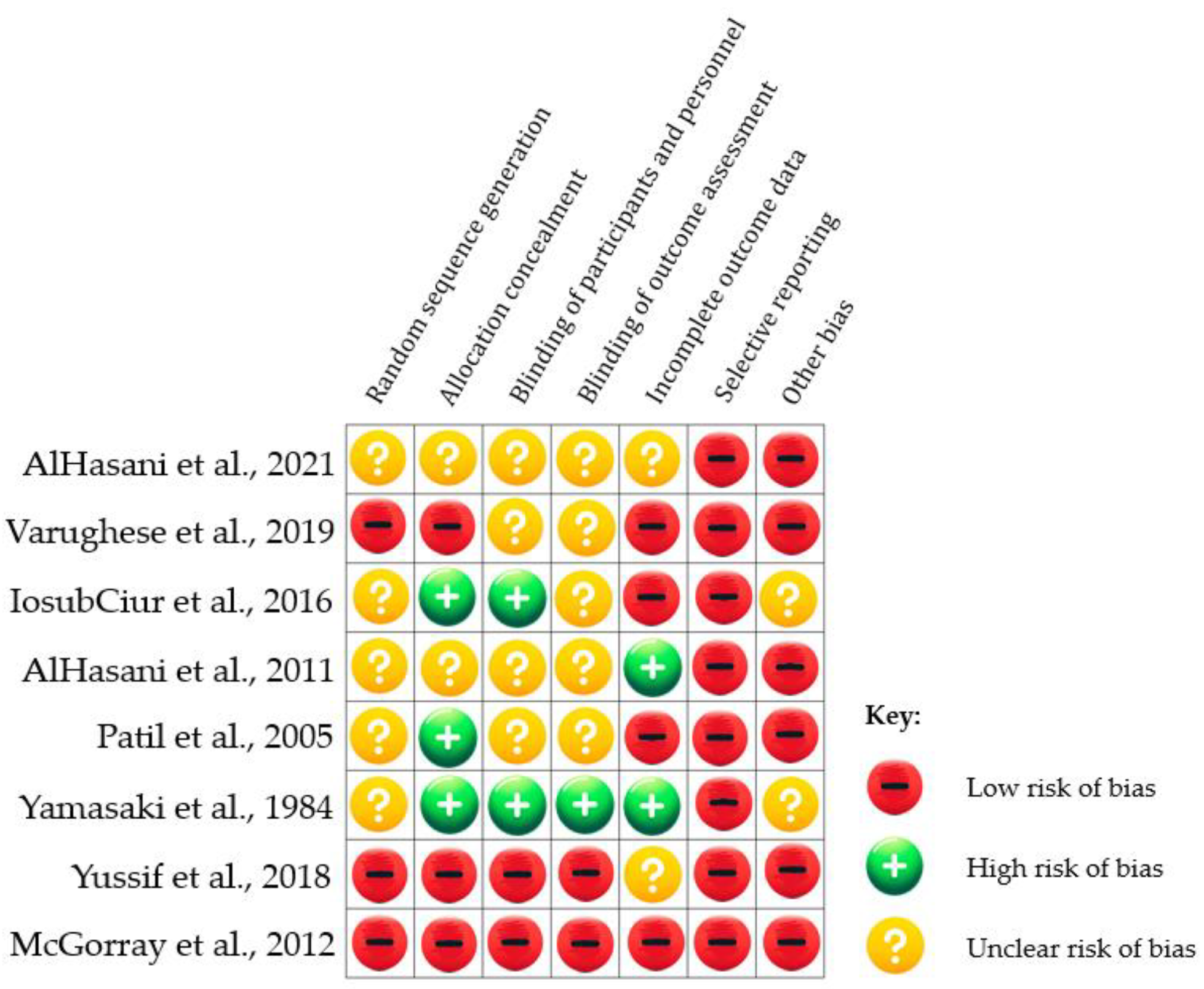
| Domain | Inclusion criteria | Exclusion criteria | |
|---|---|---|---|
| Population | Healthy human participants, undergoing any type of active OTM. Animal studies, with healthy subjects undergoing any type of active OTM. |
Studies where participants suffered from systemic or syndromic conditions, or where these conditions were induced. |
|
| Intervention | Local or topical administration of pharmacological agents capable to accelerate the rate of OTM | Studies where the aim was to inhibit OTM or enhance post orthodontic retention. | |
| Studies where, in order to manufacture the substance that could accelerate OTM, other invasive medical procedures, such as phlebotomy, were required (e.g., Platelet-rich plasma) |
|||
| The use of biomaterials and biosubstances that targeted other aspects of the orthodontic treatment such as reducing the associated pain | |||
| Comparison | Placebo intervention or no intervention | The use of a different substance or a different type of intervention for accelerating OTM (e.g., surgical interventions, low-level energy laser, vibration) | |
| Different dosages of the studied substance or different substances as long as there was a control group | |||
| Outcome | Quantitative data regarding the amount of tooth movement measured by various methods | ||
| If possible, data also regarding the status of the root and surrounding tissues (PDL, alveolar bone) | |||
| Study design | Experimental prospective controlled studies (randomized and non-randomized) | Non-comparative studies | |
| Clinical Trials | In vitro only or ex vivo studies | ||
| Case reports, reviews, systematic reviews, meta-analyses, case series, opinion articles, letters from editors | |||
| Database/Register | Keywords and MeSH terms |
|---|---|
| MEDLINE | ("tooth movement"[Title/Abstract] OR orthodontic*[Title/Abstract]) AND (pharmacological[Title/Abstract] OR drug*[Title/Abstract] OR prostaglandin*[Title/Abstract] OR vitamin[Title/Abstract] OR hormone[Title/Abstract] OR calcitriol[Title/Abstract]) AND (administration[Title/Abstract] OR local*[Title/Abstract] OR topical*[Title/Abstract]) NOT pain[Title/Abstract]) |
| ProQUEST | Abstract ((("tooth movement" OR orthodontics OR "orthodontic treatment") AND (pharmacological OR drug OR prostaglandin OR vitamin OR hormone OR substance) AND (administration OR use OR local OR topical) NOT pain)) OR summary(("tooth movement" OR orthodontics OR "orthodontic treatment") AND (pharmacological OR drug OR prostaglandin OR vitamin OR hormone OR substance) AND (administration OR use OR local OR topical) NOT pain))) |
| Web of Science core collection | (("tooth movement" OR orthodontic OR orthodontics) AND (pharmacological OR drug OR drugs OR substance OR prostaglandin OR prostaglandins OR vitamin OR hormone OR calcitriol) AND (administration OR local OR locally OR topical OR topically) NOT pain)) |
| Embase | (orthodontics:ti,ab,kw OR 'tooth movement':ti,ab,kw OR otm:ti,ab,kw) AND (prostaglandin:ti,ab,kw OR prostaglandins:ti,ab,kw OR vitamin:ti,ab,kw OR 'vitamin d':ti,ab,kw OR 'vitamin e':ti,ab,kw OR relaxin:ti,ab,kw OR hormone:ti,ab,kw OR calcitonin:ti,ab,kw OR calcitriol:ti,ab,kw OR pharmacological:ti,ab,kw OR drug:ti,ab,kw OR drugs:ti,ab,kw) AND (administration:ti,ab,kw OR local:ti,ab,kw OR topical:ti,ab,kw OR injection:ti,ab,kw) |
| Science direct | (((tooth AND movement) OR orthodontic) AND (pharmacological OR drug) AND (administration OR local OR topical))) |
| (((tooth AND movement) OR orthodontic) AND (prostaglandin OR prostaglandins) AND (administration OR local OR topical)) | |
| (((tooth AND movement) OR orthodontic) AND (hormone OR vitamin OR biological) AND (administration OR local OR topical))) | |
| ((tooth AND movement) AND orthodontics) AND (administration OR local OR topical)) | |
| Scopus | TITLE-ABS (("tooth movement" OR orthodont*) AND (pharmacol* OR drug* OR substance OR prostaglandin* OR vitamin OR hormone OR calcitriol) AND (administr* OR local* OR topical*) AND NOT pain) |
| TITLE-ABS (("tooth movement" OR orthodontic OR orthodontics) AND (pharmacological OR drug OR drugs OR substance OR prostaglandin OR prostaglandins OR vitamin OR hormone OR calcitriol) AND (administration OR local OR locally OR topical OR topically) AND NOT pain) | |
| Cochrane CENTRAL | tooth movement orthodontic local administration NOT pain |
| Clinicaltrials.gov | condition or disease:”tooth movement” other terms: orthodontic OR orthodontics |
| Authors, year, study design | Population, age, (sample) | Study duration | Biofunctional molecules | Administration path | Applied force (g), movement | Frequency of administration |
|---|---|---|---|---|---|---|
| 1. AlHasani et al., 2021 [17], S-M | Patients who required bilateral maxillary 1st premolars extraction, 18 to 35 years of age, (17 patients – 17 dental arches) | 3 to 6 months | Vitamin D3 – 1,25 dihydroxycholecalciferol (1,25 DHC) (calcitriol – ampoules (Calcitriol, Mibe, Germany) diluted in DMSO |
Injected locally into the distal periodontal sulcus of the canine | 200 g, maxillary canine retraction (distalization) | Administration on each orthodontic visit (every three weeks) until extraction space closure, starting right before force application |
| 2. Varughese et al., 2019 [18], S-M | Patients indicated for bilateral maxillary first premolar extraction, and minimum 5 mm of extraction space, 15 to 30 years of age, (15 patients – 15 dental arches) | 3 months | VITAMIN D - 1,25 dihydroxycholecalciferol (1,25 DHC) (calcitriol) | Local periodontal injection given distal to maxillary canine | 150 g per side, maxillary canine retraction (distalization) with TPA as anchorage reinforcement | One monthly administration, starting right before force application |
| 3. IosubCiur et al., 2016 [19], S-M | Patients who required bilateral extractions of the first premolars and need of bilateral distalization of canines, 13 to 34 years of age, (4 patients – 6 dental arches) | 3 weeks admin-istration, 3-month study | Vitamin D3 (calcitriol - Decostriol®, Mibe Jena, Germany). Diluted in DMSO | Intraligamentary injection | 150 g per side, canine retraction (distalization) | Administration once a week, for 3 weeks |
| 4. AlHasani et al., 2011 [20], S-M | Patients who required bilateral maxillary 1st premolars extraction and bilateral maxillary canines retraction, 17 to 28 years of age, (15 patients – 15 dental arches) | 3 weeks administration, 5 weeks measurement, 6 months study | Vitamin D3 (calcitriol - (Calcitriol, Mibe, Germany diluted in DMSO) | Injected into the PDL on the distal side of canines | 150 g per side, maxillary canine retraction (distalization) with TPA, ligation and stoppers as anchorage reinforcement | Administration once a week, repeated three times for every subject (at 1st, 2nd and 3rd visit) |
| 5. Patil et al., 2005 [21], S-M | Patients that had undergone first maxillary premolar extraction, 13 to 25 years of age, (14 patients – 14 dental arches) | 60 days (2 months) | Prostaglandin E1 (PGE1) | Injection by local infiltration in the vestibular area at the upper canine region | 150 per side, maxillary canines retraction (distalization) with TPA, molar stops and in 2 cases HG as anchorage reinforcement | Administration on the 1st day, 6th day and 17th day of the start of individual canine retraction |
| 6. Yamasaki et al., 1984 [22], S-M | Patients who required first premolar extraction, 10 to 26 years of age, (25 patients – 29 dental arches) |
Phase 1: up to 33 days Phase 2: up to 21 days Phase 3: up to 4.8 months |
Prostaglandin E1 (PGE1 - CD, One Pharmaceutical Company, Ltd., Osaka, Japan) | Injections in the submucosal area of the buccal side of the right first premolar, maxillary and/or mandibular canines | Phase 1: 100 g per side, buccal movement of first maxillary premolars Phase 2 and 3: 150 per side, maxillary and/or mandibular canine retraction (distalization) with different methods used as anchorage reinforcement |
Phase 1 and 2 – administration at various intervals, starting from day 0, with a total injection number between 3 and 5 Phase 3 – administration starting at day 0 and proceeding at approximately 10 days intervals, until space closure (1.5 – 4.8 months) |
| 7. Yussif et al., 2018 [23], EG+CG | Patients with unilateral palatally positioned permanent canines, 15 to 40 years of age (12 patients) | 12 months | Vitamin C | Submucosal injection (parallel to the mucosal surface within papillary connective tissue) | Impacted canine traction by elastic power chain activated every 2 weeks and alignment of said canine after its appearance in the buccal cavity | Administration repeated every 2 weeks until canine alignment |
| 8. McGorray et al., 2012 [24], EG+CG | Patients needing minor incisor alignment of at least the maxillary incisors, 18 to 40 years of age, (39 patients) | 8 weeks of tooth movement + 4 weeks of retention (total 3 months) | Recombinant human Relaxin | Injection to the gingival tissue to a depth of approximately 2 mm (lingual and buccal side) | Anteroposterior movement of 2 mm via a series of 4 maxillary aligners with 0.5 mm programmed of anteroposterior movement for the selected central incisor (only crown tipping) | Once, immediately after establishing the OTM model via 2 injections |
| Authors, year, study design | Species, age, (sample), study duration | Substance w/o trade name, administration path | Applied force, movement | Frequency of administration, description of groups, dosage | Outcomes – amount of OTM for EG compared to CG, %, p-value |
|---|---|---|---|---|---|
| 1. Jiao et al. [52], 2022, EG + CG | Mi (mice), 8 week old male, (12), 10 days |
Biocompatible reduced graphene oxide (biomaterial), buccal submucous local injection around the {...} molar | 20 g, left first upper molar mesialization to incisor | Once, immediately after establishing the OTM model EG: OTM + 20 μL 10 mg/mL GOG solution CG: OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % - n.a. p < 0.05 |
| 2. Lu et al. [47], 2021, EG + CG | WR (rats), 7 week old male, (40), 14 days |
Parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP), injected into a micro-perforation (MOP) with a diameter of 0.2 mm on the alveolar bone approximately 3 mm mesial to the maxillary first molar and 2 mm below the gingival margin | 40 g, mesialization of upper first molars to incisors | Once, immediately applied after establishing the OTM model EG: OTM + PTH/PTHrP (R&D Systems) with the concentration of 1.25 μg/kg carried by PECE hydrogel CG: OTM + PBS/no intervention (gA: PTH/PBS gB: PTHrP/PBS gC: PTH/no intervention gD: PTHrP/no intervention gE(relapse group): PTH/PBS) |
amount of OTM - PTH mean value 0.78 ± SD 0.06 mm PTHrP mean value 0.81 ± SD 0.04 mm no MOP group (mean value 0.46 ± SD 0.05 mm) MOP group (mean value 0.51 ± SD 0.04 mm) % - n.a p < 0.005 |
| 3. Soma et al. [48], 2000, EG+CG | WR (rats), male, (56), 12 days |
Parathyroid hormone (PTH), local injection of PTH-MC (slow release formula) into the subperiosteum in the mesio-palatal region of the maxillary first molar | 30 g, mesialization of the right maxillary first molar to incisors | The first injection was made immediately after wire placement, and carried on every other day CG1: OTM + no intervention EG2: OTM + vehicle dissolved in MC gel EG3: OTM + 0.1 ug PTH dissolved in MC gel EG4: OTM + 1 ug PTH dissolved in MC gel EG5: OTM + 1 ug PTH dissolved in 0.9% saline EG6: OTM + systemic injection EG7: no OTM + 1 ug PTH dissolved in MC gel |
amount of OTM - PTH-MC injection at 1 μg/400 g body weight caused a 1.6-fold increase in the rate of tooth movement with a mean value 0.54 +/- SD 0.08 mm in the control group % - 1.6-fold increase p <0.05 compared with vehicle in MC, p <0.01 compared with PTH in saline. |
| 4. Chang et al. [39], 2020, EG + CG | WR (rats), 15 week old male, (24), 14 days |
Receptor activator of nuclear factor kappa-B ligand (RANKL) formulated in microspheres (RANKL formulation with PLGA-aqueous hydroxyethyl cellulose microspheres – 1.5 mg/4.5 μl), injected palatal to the left maxillary first molar (flapless osteoperforation with a ¼ round bur and high-speed handpiece on the mesiopalatal of the left first maxillary molar; then sealed the gingiva with cyanoacrylate tissue adhesive) | 5–8 g, left first upper molar mesialization to incisors | Once, immediately after establishing the OTM model EG: OTM + 1 μg RANKL - 1 mg microsphere in 3 μl 10 per cent HEC gel, PCG: OTM + placebo microspheres (1 mg microsphere in 3 μl 10 per cent HEC gel) CG: OTM + no intervention |
amount of OTM - RANKL formulation mean value 0.55 ± SD 0.25 mm Placebo formulation mean value 0.32 ± SD 0.1 mm No formulation mean value 0.24 ± SD 0.05 mm % - 129.2 % more tooth movement than no formulation and 71.8 % more than placebo formulation p< 0.05 |
| 5. Li et al. [40], 2018, EG + CG | Mi (mice), 6 week old male, (60), 42 days |
Receptor activator of nuclear factor kappa B ligand (RANKL), injections subperiosteally administered to the buccal premaxillary bone [...] under anaesthesia | 35 g, reciprocal force: buccal movement of upper incisors | Daily injections for 14 days EG: OTM + 0.04-μg/g (body weight) RANKL (Peprotech, Rocky Hill, NJ, USA) dissolved in 10-μl phosphate-buffered saline (PBS) CG: OTM + PBS |
amount of OTM - control (0.52 ± 0.06 mm) and experimental (0.49 ± 0.07 mm) groups on day 7 (no significant difference) control group 0.79 ± 0.12 mm, experimental group 1.15 ± 0.27 mm on day 14 control group 1.07 ± 0.12 mm, experimental group 1.55 ± 0.22 mm on day 21 % - n.a. p>0.05 on day 7 p<0.05 on day 14 p<0.01 on day 21 |
| 6. Kanzaki et al. [41], 2006, EG+CG / S-M | WR (rats), 6 week old male, (25), 21 days |
RANKL expression plasmid, injected into the subperiosteal area, adjacent to the upper right first molar |
g n.a., reciprocal force: palatal movement of first upper molars | Every 3 days until day 17 EG: HVJ envelope vector containing pcDNA-mRANKL 5-μl on the right side and PBS 5-μl on the contralateral side MG: OTM + mock vector solution 5-μl CG: no OTM + no intervention |
amount of OTM - RANKL transfection side was 0.63+/-0.06 mm at 21 days control side 0.48+/-0.05 mm at 21 days % - per cent acceleration of TM (the RANKL transfection side/ the control side) was 173.1, 137.8, 135.8 and 131.6% at days 3, 7, 14 and 21, respectively p<0.05 on day 3 and at the end of the experiment |
| 7. Cui et al. [32], 2018 EG + CG | WR (rats), 6 week old female, (40), 28 days |
Akebiasaponin D (Chinese herbal extract) and PGE 2, local injection | 40 g, mesialization of first upper molars | Once, immediately after establishing the OTM model EG1: OTM + 5 mg/kg ASD EG2: OTM + 10mg/kg ASD EG3: OTM + 25ug/kg PGE2 CG: OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % n.a. p<0.05 PGE2 on day 3 p<0.05 10mg/kg ASD group and 25ug/kg PGE2 group on day 7 p<0.05 in all three groups compared to the control group on days 14, 21, 28 However, there was no significant difference (P>0.05) between 10mg/kg ASD group and 25ug/kg PGE2 group |
| 8. Caǧlaroǧlu et al. [33], 2012, EG + CG | NZR (rabbits), adult male, (45), 21 days |
Prostaglandin E2 (PGE2), [PGE2 powder (p5640) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and prepared as a 1 mg/mL stock solution by dissolving in ethanol] submucosal - immediately distal to the maxillary incisors, or intra ligamentous route - PDL surrounding the maxillary incisors, by using an insulin syringe or intraligamentous injector | 20 g, reciprocal force: buccal movement of upper incisors | On days 0, 1, 3, 7, and 14, thereafter (total dose = 1.2 μg) EG1(gIII): OTM + PGE2 (10 μg/mL) 0.06-ml intravenous EG2(gIV): OTM + PGE2 (10 μg/mL) 0.06-ml submucosal EG3(gV): OTM + PGE2 (10 μg/mL) 0.06-ml intraligamenter CG1(NC-gI): no OTM + no intervention CG2(PC-gII): OTM + no intervention |
amount of OTM EG1(gIII) a mean value 2.87 +/- SD 0.18 mm (min. 2.52 – max 3.11 mm) EG2(gIV) a mean value 3.08 +/- SD 0.22 mm (min. 2.74 – max 3.40 mm) EG3(gV) a mean value 4.54 +/- SD 0.31 mm (min 3.98 – max 4.84 mm) CG2(PC-gII) a mean value 2.87 +/- SD 0.18 mm (min 2.52 – max 3.11 mm) % n.a. EG1(gIII) p>0.05 EG2(gIV) p<0.001 EG3(gV) p<0.001 |
| 9. Kale et al. [34], 2004, different EG + CG | SPR (rats), 6 week old male, (37), 9 days |
Prostaglandin E2 (PGE2, Sigma-Aldrich, St Louis, Mo) and calcitriol – 1,25-dihydroxycholecalciferol (1,25-DHCC, Roche, Basel, Switzerland), injected to the gingiva distal to the upper incisors |
20 g, reciprocal force: buccal movement of upper incisors | 1,25 DHCC on days 0, 3 and 6 and PGE2 once, immediately after establishing the OTM model EG1(gIV): OTM + 1,25 DHCC (20 μL of 10⁻10mol/L EG2(gV): OTM + PGE2 0.1 μg - 0.1 mL CG1(gI): no OTM + no intervention CG2(gII): OTM + no intervention CG3(gIII): OTM + dimethyl sulfoxide [DMSO] 20-μL (the vehicle of 1,25-DHCC) |
amount of OTM CG2(gII) mean 1.72 +/- SD 0.06 mm CG3(gIII) mean 1.72 +/- SD 0.06 mm EG1(gIV) mean 2.11 +/- SD 0.0.04 mm EG2(gV) mean 2.16 +/- SD 0.06 mm % n.a. p<0.001 between CG1/CG2/CG3 groups and both EG1/Eg2 groups p> between EG1 and EG2 |
| 10. Seifi et al. [35], 2003, | WR (rats), 8 week old male, (24), 21 days |
Prostaglandin E2 (PGE2) alone and with calcium gluconate (Ca), submucosally injected at the mesiobuccal mucosa of the right first molars | 60 g, mesialization of right maxillary first molar to incisors | Administration frequency - twice: on days 0 and 7 EG1(g3): OTM + 0.1 ml of 1 mg/ml PGE2 dissolved in 1 per cent lidocaine EG2(g4): OTM + PGE2 same dosage + 10% Ca (200 mg/kg) injected intra-peritoneally PCG(g2 – right side): OTM + 0.1 ml distilled water NCG (g1 – left side): no OTM + no intervention |
amount of OTM PCG mean 0.2162 +/- SD 0.0995 and range 0.14-0.45 mm EG1 mean 0.4700 +/- SD 0.2799 and range 0.20-0.91 mm EG2 mean 0.4012 +/- SD 0.1007 and range 0.29-0.57 mm % n.a. p<0.05 for EG1 compared to the control group |
| 11. Leiker et al. [36], 1995, EG+CG | SDR (rats), 8 week old male, (132), G1: 2 week / G2: 4 week |
Prostaglandin E2 (PGE2), injected in the mesiolingual gingiva of each maxillary first molar | 60 g, mesialization of first maxillary molar to incisors | G1a: single (1) injection G1b: 2 injections once a week (total 2) G2a: single (1) injection G2b: 4 injections once a week (total 4) EG: OTM + subgroups were further divided into four concentration subgroups (0.1, 1.0, 5.0, and 10.0 ug of PGE2) CG: OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % n.a. p<0.05 in all PGE2 groups compared to their respective non-PGE2 groups – overall at p < 0.0001 |
| 12. Yamasaki et al. [37], 1982, S-M | Macaca Fukasata (monkey), 5 year old female, (2), Exp1: 18 days, Exp2: 28 days |
Prostaglandin E1 and prostaglandin E2 (PGE1 No. G 511* and PGE2 No. G 512* *Ono Company, Ltd., Osaka, Japan - dissolved in saline at a concentration of 160 /ug/ml.), injected in the submucosal area of the distal side of the upper right canine | 100 g, distalization of upper right maxillary canine to second premolar | Exp1: days 0, 1, 5, 9, 12 and 15 EG: OTM + 0.25 ml of PGE2-CD (40 ug) CG: OTM + PBS Exp2: rate not specified; Eside and Cside reversed on day 14 EG: OTM + PGE1-CD CG: OTM + PBS |
amount of OTM Exp1 0.5 mm TM day 1, control 0.5 mm 1.3 mm TM day 5, control 0.5 mm 2.5 mm TM day 15, control 1.2 mm Exp2 ”almost double amount” of TM compared to the control side for the first two weeks ”TM results were reversed” in the second two-week period, and ”by the third week … both sides had the same amount of movement” switched sides on day 14 ”caused almost double the rate of tooth movement compared to the vehicle-injected side” % n.a. p-value n.a. |
| 13. Peng et al. [44], 2021, EG + CG | SDR (rats), 6-8 weeks old male, (80), 14 days |
Recombinant human insulin-like growth factor-1 (Pepro-Tech, USA), (injection) in the lateral buccal mucosa of the left maxillary first molar | 50 g, left first upper molar mesialization to incisors | Every 2 days EG: OTM + 400 ng rhIGF-1 (Pepro-Tech, USA) CG: OTM + PBS |
amount of OTM CG 0.040±0.040 mm EG 0.076±0.045 mm day 1 CG 0.164±0.010 mm EG 0.222±0.010 mm day 4 CG 0.267±0.013 mm EG 0.369±0.007 mm day 7 CG 0.339±0.015 mm EG 0.481±0.008 mm day 10 CG 0.410±0.014 mm EG 0.581±0.009 mm day 14 % n.a. p<0.01 from day 4 to day 14 |
| 14. Seifi et al. [45], 2013, EG + CG | WR (rats), 4 months old male, (50), 21 days |
Basic fibroblast growth factor [(bFGF) - Royan Institute] (cytokine), injection into the buccal vestibular mucosa next to the mesial root of the first molar | 60 g, mesialization of right first upper molar to incisor | Once, immediately after establishing the OTM model EG(A): OTM + 0.02 cc of 10 ng bFGF (Royan Institute) EG(B): OTM + 0.02 cc of 100 ng bFGF EG(C): OTM + 0.02 cc of 1000 ng bFGF PCG(D): OTM + PBS NCG(E): no OTM + no intervention |
amount of OTM EG(A) mean value 0.5333 mm EG(B) mean value 0.6633 mm EG(C) mean value 0.7700 mm PCG(D) mean value 0.2550 mm NCG(E) mean value 0.0217 mm % n.a. p<0.05 in all EG compared to PCG p<0.05 in EG(C) compared to EG(A) |
| 15. Alves et al. [42], 2009, EG+CG | HR (rats), male, (96), 21 days |
Epidermal growth factor (EGF-liposomes), injected in the mucosa adjacent to the appliance of the left side | 20 g, mesialization of left maxillary first molars to incisors | Once, immediately applied after establishing the OTM model, then daily PBS EG1(G2): OTM + empty liposomes in 10 μL of PBS solution EG2(G3): OTM + vesicles containing 0.5 ng/μL of EGF with ~100 nm of diameter in 10 μL of PBS solution (20 ng of EGF) EG3(G4): OTM + 20 ng of EGF– liposomes in 10 μL of PBS solution CG(G1): OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % n.a. p < 0.05 in all four periods analyzed, animals that received an injection of EGF–liposome solution showed increased tooth movement (p<0.05) as compared to the 3 groups |
| 16. Saddi et al. [43], 2008, EG+CG | HR (rats), male, (32), 5 days |
Epidermal growth factor (EGF - SOLUBLE), injected into the region of the root furcation of the left first molar | Insertion of elastic band between first and second molars, mesialization of the left maxillary first molar | Once, immediately applied after establishing the OTM model EG1(GI): OTM + 20 ng EGFliposomes in 10 uL PBS solution CG1(GII): OTM + liposomes in 10 uL PBS solution EG2(GIII): OTM + 20 ng EGF in 10 uL PBS solution CG2(GIV): OTM + 10 uL PBS solution |
amount of OTM EG1(GI) 0.55 mm CG1(GII) 0.26 mm EG2(GIII) 0.42 mm CG2(GIV) 0.26 mm % n.a. p<0.05 for both experimental groups compared to control |
| 17. Brooks et al. [46], 2011, EG+CG | Mi (mice), 10 week old male, (48), 6 days |
Macrophage colony-stimulating factor (M-CSF - Calbiochem, Gibbstown, N)) - an early osteoclast recruitment/differentiation factor, injected sub-periosteally into the distopalatal root of the right maxillary first molar |
12 (cN), mesialization of right maxillary first molars to incisors | Once, immediately applied after establishing the OTM model EG1(low): OTM + 0.1 mg/kg doses of recombinant mouse M-CSF (Calbiochem, Gibbstown, NJ) EG2(high): OTM + 1 mg/kg doses of recombinant mouse M-CSF (Calbiochem, Gibbstown, NJ) PCG: OTM + PBS NCG: no OTM |
amount of OTM PCG mean value 72 +/- 8.4 um day 2 (lag after tipping phase) PCG mean value 193 +/- 5.7 um day 6 (linear movement phase) NCG mean value 0 +/- 2.0 um day 2 NCG mean value 0 +/- 1.0 um day 6 EG1 (low) mean value 64 +/- 8.9 um day 2 EG1 (low) mean value 220 +/- 18.3 um day 6 EG2 (high) mean value 68 +/- 8.4 um day 2 EG2 (high) mean value 200 +/- 14.1 um day 6 % 14% increase after 6 days of treatment p<0.05 on day 6 for EG1(low dose) compared to PCG p>0.05 on day 6 for EG2(high dose) compared to PCG |
| 18. Jung et al. [53], 2018, EG + CG | Beagles (dogs), 18-24 months old male, (8), 4 weeks |
Exogenous thyroxine, transmucosal administration (via tablets bonded on the orthodontic appliance) | 100 (cN), mesialization of upper second premolars to canine and distalization of lower second premolars to fourth premolar and first molar | Starting from the application of the OTM model, tablets changed once a week EG: OTM + one dose of Thyroxine with a rate of dissolution of 18.69% after 24h. CG: OTM + no intervention |
amount of OTM EG – maxilla mean value 0.19 +/- SD 0.08 mm /week CG – maxilla mean value 0.18 +/- SD 0.10 mm /week EG – mandible mean value 0.20 +/- SD 0.08 mm /week CG – mandible mean value 0.16 +/- SD 0.07 mm /week % n.a. p>0.05 |
| 19. Hashimoto et al. [49], 2001, EG+CG | WR (rats), 5 week old male, (88), 10 days |
Osteocalcin (OC - a major noncollagenous bone matrix protein – purified rat OC), injected in the palatal subperiosteum adjacent to the furcation of the maxillary right first molar | 30 g, mesialization of the right maxillary first molar to incisor | The first administration was given on the day that the orthodontic appliance was inserted and repeated daily until day 9. EG1: OTM + 0.1 μg OC EG2: OTM + 1 μg OC EG3: OTM + 10 μg OC CG1: OTM + PBS CG2: OTM + albumin CG3: OTM + no intervention |
amount of OTM - n.a. (values presented in the article as an image) % EG1 147% EG2 152% EG3 121% TM Compared to CG1 p<0.05 EG1 and EG2 compared to CG1 and CG3 on day 10 |
| 20. Kobayashi et al. [50], 1998, EG+CG | WR (rats), 5 week old male, (33), 4 days |
Osteocalcin (OC - a major noncollagenous bone matrix protein - purified rat OC described in this article), injected into the submucosal palatal area corresonding to the root furcation of the maxillary right first molar | Insertion of elastic band between first and second molars, mesialization of the right maxillary first molar | Injection was repeated once a day from day 0 to 3 EG1: OTM + 0.01 ug purified osteocalcin / 20 ul PBS EG2: OTM + 0.1 ug purified osteocalcin / 20 ul PBS EG3: OTM + 1 ug purified osteocalcin / 20 ul PBS EG4: OTM + 10 ug purified osteocalcin / 20 ul PBS CG: OTM + PBS |
amount of OTM maximal amount of OTM observed in EG3 (1ug OC) % n.a. p<0.05 for EG3 (1ug OC) compared to CG on all 4 days p<0.05 for EG4 (10ug OC) starting on day 3 p<0.05 for EG2,3,4 on day 4 |
| 21. Lu et al. [54], 2019, S-M | WR (rats), 6 week old male, (48), 14 days |
Sclerostin protein (R&D systems, MN, USA) carried by PECE hydrogel, local injection at the compression side in the alveolar bone, approximately 4mm mesial from the maxillary first molar | 50 g, randomly left or right first upper molar mesialization to incisors | Once, immediately after establishing the OTM model ES1: OTM + 0.1 ml 0.8 μg/kg sclerostin protein carried by hydrogel (R&D systems, MN, USA) ES2: OTM + 0.1 ml 4 μg/kg same substance ES3: OTM + 0.1 ml 20 μg/kg same substance CS: OTM + PBS |
amount of OTM ES1: mean value 0.58 ± SD 0.07 mm ES2: mean value 0.65 ± SD 0.06 mm ES3: mean value 0.72 ± SD 0.04 mm % n.a. p<0.01 for ES2,ES3 compared to ES1 and CS |
| 22. Chung et al. [55], 2007, EG+CG / S-M | SDR (rats), 6-7 week old male, (25), 7 days |
A specific EP4 agonist (ONO-AE1-329), a drug that binds to the EP4 receptor to mimic the actions of ligand binding, injected locally into the distopalatal mucosa region of the right first molar under diethyl ether anesthesia | 6,5 (cN), reciprocal force: mesiodistal movement of the right maxillary first, second and third molar | The first injection was made on day 0; then injections were given twice a day at 7-hour intervals starting from day 3 until the rats were killed on the evening of day 7 (total of 10 injections). CG1 (EA - EP4 agonist): no OTM + 250 uL ONO-AE1-329 CG2 (V - vehicle): no OTM + vehicle EG1 (TMEA - tooth movement and EP4 agonist): OTM + 250 uL ONO-AE1-329 EG2 (TMV - tooth movement and vehicle): OTM + vehicle |
amount of OTM n.a. % - n.a. (values presented in the article as an image) p<0.05 for EG3 compared to EG4 |
| 23. Bolat et al. [51], 2020, EG+CG | WAR (rats), 6-8 week old male, (51), 18 days |
Vitamin C and Vitamin E, local injections were performed with a microsyringe into the periodontal area of the maxillary first molar | 50 g, mesialization of maxillary first molar to incisors | Administration every 3 days EG1: OTM + locally injected Vitamin C 20 μL EG2: OTM + locally injected Vitamin E 20 μL EG3: OTM + intraperitoneal 150 mg/kg vitamin C EG4: OTM + intraperitoneal 150 mg/kg vitamin E CG: OTM + no intervention |
amount of OTM In this experimental study, the application of systemic or local vitamin C and E did not affect the orthodontic tooth movement rate. % n.a. p<0.05 (The osteoblastic activity was considerably raised in all the vitamin groups. Additionally, the groups treated with systemic vitamins showed a significant increase in the quantity of collagen fibers on the tension side compared to the control group using the appliance.) |
| 24. TakanoYamamoto et al. [38], 1992, EG+CG | WR (rats), young 7 week old male (10) and adult 28 week old male (30) (total 40), 21 days |
1,25(OH)2D3 –Calcitriol (Sunstar Co., Osaka, and Japan Roche Co., Tokyo) – active form of vitamin D, injected locally into the submucosal palatal area of the root bifurcation of the right first molar |
5 to 20 g, buccal movement of the first upper molar | Every 3 days (young)EG: OTM + 1,25-(OH)2D3 20 μL of 10⁻10mol/L (young)CG: OTM + PBS (adult)NCG1: no OTM + PBS (adult)PCG1: no OTM + 1,25-(OH)2D3 20 μL of 10⁻10mol/L (adult)PCG2: OTM + PBS (adult)EG4: OTM + 1,25-(OH)2D3 20 μL of 10⁻10mol/L (adult)EG5: OTM + 1,25-(OH)2D3 20 μL of 10⁻8mol/L |
amount of OTM - young – 0.5 mm adult – 1.2 mm % for (young)EG - increased to 126% of that in PBS-injected control rats on day 20 for (adult)EG4 - increased to 245% compared to PBS by the end of the experiment for (adult)EG5 – increased to 154% compared to PBS by the end of the experiment *p<0.05 (young)EG - starting from day 15 to day 20 as compared with the rats which were injected with PBS |
| 25. Collins et al. [28], 1988, EG+CG / S-M | Cats, young adult, (10), 22 days |
Calcitriol – (1,25-dihydroxycholecalciferol – a vitamin D metabolite), intraligamentar injection into the distal portion of the periodontal ligament of canine teeth | 80 g, distalization of upper left canines to the third premolars | The first injection was made immediately after establishing the OTM model, then repeated once a week EG: OTM + 0.1 ml of DMSO containing 50 ug/ml of 1,25D CG: OTM + 0.1 ml of DMSO only |
amount of OTM - n.a. (values presented in the article as an image) % EG teeth moved 60% further than their matched control after 21 days (also divided by weeks) p < 0.05 |
| 26. Ma et al. [57], 2020, EG+CG | SDR (rats), 8 week old female, (64), 14 days |
Asperosaponin VI (ASA VI - chemical constituent isolated from Dipsacus asper Wall), injected into buccal submucoperiosteal of first upper molars | 40 g, mesialization of bilateral upper first molars to incisors | Once a day, every day during the orthodontic tooth movement period EG: OTM + 10 mg/kg ASA VI solution CG: OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % EG - approximately 1.2 times greater at day 3, 1.44 times greater at day 7, and 1.54 times greater at day 14. A significant difference was found between the ASA VI group and the control group on day 7 and day 14 p<0.05 |
| 27. Kavoli et al, [56], 2017, EG+CG | WR (rats), 4 months old, (28), 21 days |
Carrageenan (CGN - carrageenan 1% Sigma-Aldrich, St. Louis, Missouri, USA), a common food additive, injected into the mucosa of the buccal vestibule adjacent to the mesial root of the left first molar |
20 g, mesialization of left upper first molars to incisors | Once, immediately applied after establishing the OTM model EG: OTM + 40 mL carrageenan 1% (Sigma-Aldrich, St. Louis, Missouri, USA) CG: OTM + PBS |
amount of OTM - n.a. (values presented in the article as an image) % injection of carrageenan can speed up tooth movement by about 58% and increase the presence of osteoclasts by 40%, after 21 days; local injection of carrageenan is a method capable of accelerating orthodontic tooth movement by about 1.6-fold p = 0.053 |
| 28. Xiao et al. [58], 2015, EG+CG | SDR (rats), 6-8 week old male, (150), 30 days |
Aqueous extract of S. miltiorrhiza (ESM - Danshensu - content>98%; Nanjing Zelang pharmaceutical Technology Co., Ltd., Jiangsu, China; No.: ZL201104162;), injected into the buccal vestibular mucosa of first molar of left maxilla |
40 g, mesialization of left upper first molars to incisors | Once a day, every day CG: OTM + 0.5 ml/kg PBS EG1: OTM + 0.5 ml/kg ESM, which was equivalent to 0.75 g/kg of crude drugs) EG2: OTM + 0.5 ml/kg Danshensu, which as equivalent to 250mg/kg of body weight EG3: OTM + 0.5 ml/kg Danshensu, which as equivalent to 500mg/kg of body weight EG4: OTM + 0.5 ml/kg Danshensu, which as equivalent to 750mg/kg of body weight |
amount of OTM - n.a. (values presented in the article as an image) % n.a. p- from day 10 EG1 p<0.01, from day 20 also EG2, EG3 and EG4 p<0.05. |
| PGE1 | PGE2 | Calcitriol | PTH | Exogenous thyroxine | Vitamin C | |
|---|---|---|---|---|---|---|
| Synonyms | Alprostadil, Prostaglandin E1 | Dinoprostone, Prostaglandin E2 | 1-alpha-25-Dihydroxyvitamin D3 | Parathormone, Parathyroid hormone | L-T4, Levothyroxin, Thyroxine | Ascorbate, Ascorbic acid |
| Chemical formula | C20H34O5 | C20H32O5 | C27H44O3 | C408H674N126O126S2 | C15H11I4NO4 | C6H8O6 |
| Type and weight | Small molecule, Average: 354.487Monoisotopic: 354.240624195 | Small molecule, Average: 352.4651Monoisotopic: 352.224974134 | Small molecule, Average: 416.6365Monoisotopic: 416.329045274 | Single-chain polypeptide (mature protein), Protein average weight: 9420.0 Da | Small molecule, Average: 776.87Monoisotopic: 776.686681525 | Small molecule, Average: 176.1241Monoisotopic: 176.032087988 |
| Background | Alprostadil is a synthetic form of prostaglandin E1, a potent vasodilator for treating erectile dysfunction by promoting smooth muscle relaxation; it can also be used in neonatal patients with congenital heart defects, causing vasodilation and increased blood flow. | Significant impact on labor; It has stimulating effects on osteoblasts to secrete substances that promote bone resorption by osteoclasts; as a prescription substance, it is used as a vaginal capsule to prepare/induce labor. | Calcitriol is a potent vitamin D metabolite produced through UV light exposure; as a prescription substance it is used for treating secondary hyperparathyroidism, metabolic bone disease, hypocalcemia, osteoporosis, and mild to moderate plaque psoriasis in adults, administered orally and intravenously. | Used to treat hypocalcemia resulting from hypoparathyroidism, PTH is an analog of human parathyroid hormone. | Oral levothyroxine is a synthetic hormone that maintains normal T4 levels in hypothyroidism. Thyroid hormones, T4 and T3, have a potency of approximately 1:4 and have a strong effect on the cardiac system, making their status closely linked to heart rate, cardiac output, and systemic vascular resistance. | Vitamin C is a vitamin that is used to treat vitamin C deficiency, scurvy, prolonged wound and bone healing, urinary acidity, and as an overall antioxidant. It is also thought function as a reducing agent and coenzyme in various metabolic pathways and is considered an antioxidant. |
| Mechanism of action | Alprostadil is a smooth muscle relaxant used in neonatal patients with ductus arteriosus patency to prevent or reverse functional closure of the ductus arteriosus, increasing blood flow. In adult men it relaxes the trabecular smooth muscle of the corpora cavernosa and cavernosal arteries, leading to swelling, elongation, and rigidity due to the corporal veno-occlusive mechanism. | Dinoprostone, when administered intravaginally, stimulates the myometrium of the gravid uterus, similar to labor contractions, resulting in the evacuation of conception products. Its exact mechanism is unknown, but it may regulate calcium transport and intracellular concentrations. It also produces local cervical effects, possibly due to collagen degradation. | Calcitriol, as a psoriasis tratment, has antiproliferative effects on keratinocytes and promotes epidermal cell differentiation. Its anticarcinogenic action is linked to cellular vitamin D receptor (VDR) levels, which activate or suppress target gene transcription. VDRs, expressed in monocytes but induced after T and B cell activation, also mediate calcitriol's immunomodulating action. It also enhances the activity of certain vitamin D-receptor positive immune cells and sensitivity to cytokines generated by immune cells. | rhPTH's biological actions are mediated by binding to two high-affinity cell-surface receptors for its N-terminal and C-terminal regions, which are essential for normal bone metabolism. The N-terminal region is responsible for parathyroid hormone's bone building effects, while the C-terminal region regulates N-terminal fragment activity, demonstrating its antiresorptive activity. | Thyroid hormone boosts body cell metabolism, aiding in growth and development of tissues like bones and brain. In adults, it maintains brain function, food metabolism, and body temperature. | Ascorbic acid is essential for collagen formation and tissue repair in humans, and is reversibly oxidized to dehydroascorbic acid. It is involved in tyrosine metabolism, folic acid conversion, carbohydrate metabolism, iron metabolism, resistance to infections, and cellular respiration. |
| Absorption | Patients who received 20 μg of alprostadil intracavernously showed increased systemic plasma concentrations with a tmax and AUC of 4.8 min and 173 pg⋅min/mL, respectively. The absolute bioavailability of alprostadil from systemic exposure was estimated to be around 98% compared to a short-term intravenous infusion. | Absorbed at 0.3 mg per hour for 12 hours while the system is in place. | Intestinally absorbed, has a mean serum concentration of 60.0±4.4 pg/mL at 2 hours. Its peak plasma concentrations are reached within 3 to 6 hours, with an oral bioavailability of 70.6±5.8% in healthy patients. | For dosages of 100 micrograms, the absolute bioavailability following subcutaneous abdominal injection is 55%. | T4 absorption from the gastrointestinal tract is 40% to 80%, with most absorbed from the jejunum and upper ileum. Absorption is influenced by fasting, malabsorption syndromes, certain foods, age, and drugs like bile acide sequestrants and minerals. |
70% to 90% |
| Volume of distribution | n.a. | n.a. | Calcitriol distribution volume was 0.49±0.14 L/kg in healthy male individuals after intravenous treatment. There is evidence that calcitriol is transmitted into human milk at low quantities (2.2±0.1 pg/mL) in mothers. Calcitriol from maternal circulation may enter fetal circulation. | Following intravenous injection, the volume of distribution at steady-state is roughly 5.4 liters with an interpatient range of roughly 40%. | n.a. | n.a. |
| Protein binding | Mostly to albumin (81%) and, somewhat less for alpha-globulin IV-4 fraction (55%) | 73%, to albumin | 99,9%, to an alpha-globulin vitamin D binding protein | n.a. | 99% bound to plasma proteins (thyroxine-binding globulin, thyroxine-binding prealbumin and albumin) | 25% |
| Metabolism | Rapidly metabolized with a smaller portion absorbed in the systemic circulation and distributed throughout the body, except for the central nervous system. 60-90% of the circulating substance may be metabolized in the lungs. | PGE2's rapid metabolism occurs primarily in local tissues, with systemic absorption cleared mainly in maternal lungs and subsequently in the liver and kidneys. | Calcitriol metabolism involves two pathways: 24-hydroxylase activity in kidneys and target tissues like intestines, producing calcitroic acid, and stepwise hydroxylation of carbon-26 and carbon-23, resulting in 1a,25R(OH)2-26,23S-lactone D3, the major human metabolite. | PTH is primarily metabolized in the liver. Amino terminal fragments are metabolized in the liver, while carboxyl terminal groups are transported to the kidney for metabolism. Only 30% of circulating hormone is unfragmented. | 70% of secreted T4 is deiodinated to T3 and reverse triiodothyronine, with 80% circulating T3 derived from peripheral T4; liver, kidney, and other tissues are major sites of degradation. | Hepatic; ascorbic acid undergoes reversible oxidation to dehydroascorbic acid, two active forms in body fluids, and some is metabolized to inactive compounds like ascorbic acid-2-sulfate and oxalic acid. |
| Route of elimination | Metabolites are primarily excreted by the kidney within 24 hours of administration, with 88% and 12% excreted through urine and feces over 72 hours. Alprostadil and its metabolites are not retained in tissues. | Kidneys | 27% and 7% of radioactivity appears in feces and urine, respectively within 24 hours; calcitriol undergoes enterohepatic recycling and biliary excretion. | The kidney filters carboxy-terminal fragments, which are then fragmented during tubular reuptake. | Thyroid hormones are primarily eliminated by kidneys, with some remaining in the colon and 20% in the stool, with urinary excretion decreasing with age. | n.a. |
| Half-life | 5-10 minutes | < 5 minutes | 5-8 hours | 1.5 hours | 6-7 days | 16 days |
| Clearance | Total body clearance 115L/min after intravenous administration (20 μg) | n.a. | The metabolic clearance rate in healthy males was 23.5±4.34 ml/min, while in male patients with uraemia, it was 10.1±1.35 ml/min, and in pediatric patients, 15.3 mL/hr/kg. | n.a. | n.a. | n.a. |
| Toxicity | Oral LD50 in mice and rats is 186 mg/kg and 228 mg/kg, respectively. | Oral, mouse: LD50 = 750 mg/kg; Oral, rat: LD50 = 500 mg/kg. | LD50 (oral, rat) = 620 μg/kg; LD50 (intraperitoneal, rat) > 5 mg/kg | n.a. | LD50=20 mg/kg (orally in rat) | n.a. |
| State | Solid | Solid | Solid | Solid | Solid | Solid |
| Melting point (°C) | 115-116 °C | 67 °C | 113-114 °C | n.a. | 235.5 °C | 191 °C |
| Water solubility | 26.7 mg/L | 58.1 mg/L | Insoluble | n.a. | 0.105 mg/mL | 4E+005 mg/L (at 40 °C) |
| logP | 3.20 | 2.82 | 5 | n.a. | 4 | -1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





