1. Introduction
Normal development in children depends on adequate thyroid function. This central role of thyroid hormones is best documented for neonates affected by congenital hypothyroidism, either due to the absence of the thyroid gland or due to defects in thyroid hormone synthesis. These children suffer from severe motor, verbal, cognitive, and somatic developmental delay [
1]. When detected early enough through the newborn screening and treated with high doses of thyroid hormone, these newborns will have a normal neurodevelopmental and somatic outcome [
2].
The regulation of thyroid function is dependent on a negative feedback loop involving the secretion of thyroid stimulating hormone (TSH) by the pituitary gland. Consequently, children with congenital hypothyroidism and reduced thyroid hormone production are diagnosed based on elevated plasma TSH levels [
3]. However, in addition to congenital hypothyroidism, which is associated with elevated TSH levels, there are several other rare conditions that affect neurodevelopment through a defect within the thyroid system, despite normal TSH levels. In these instances, the thyroid gland produces thyroid hormone in a normal manner, yet the subsequent
metabolism,
transport, or
receptor function may be disrupted (
Figure 1) [
4].
1.1. Thyroid Hormone Metabolism
The primary hormone secreted by the thyroid gland is thyroxine (T4), which is a prohormone that must be metabolized to the active hormone, triiodothyronine (T3) by deiodination by two enzymes, the deiodinases 1 and 2 (DIO1 and DIO2), which are members of the selenoprotein family [
5]. T3 can also be synthesized directly at tyrosine-residues of thyroglobulin in the thyroid gland. The majority of plasma T3 is produced in the liver and kidneys, where T4 is taken up to be deiodinated
via DIO1 to T3, which is then re-secreted into the circulation. Deiodination of T4 can already take place in the thyroid gland. Thus, under physiological conditions, direct T3 output from the thyroid accounts for up to 20% of the plasma T3 (
Figure 1) [
6]. In addition, T4 can also be deiodinated to active T3 in the target cells themselves, where T4 is locally activated by DIO2 [
5].
A specific rare disease has been identified that affects T4-metabolism, namely SECISBP2 deficiency, which affects the Selenocysteine Insertion Sequence-Binding Protein 2. SECISBP2 is a common cofactor for the synthesis of selenoenzymes, including deiodinases [
7]. Mutations in the
SECISBP2 gene reduce the deiodination of T4 to T3, resulting in mildly elevated plasma T4 and low-normal T3, while TSH is in the normal range. The clinical phenotype of the patients partially mimics hypothyroidism, including developmental delay and cognitive dysfunction [
8]. Most likely, clinical symptoms result from the reduced target cell deiodination of T4 and/or the lower available T3 plasma levels. However, it is possible that dysfunction of other selenoenzymes may also contribute to the phenotype of the patients.
1.2. Thyroid Hormone Transport
Besides activation by deiodination, thyroid hormones need to be transported into the liver and kidney to be metabolized or into the target cells, like neurons to execute their physiological function. This transport is complex, and until now, we know 15 transport proteins for transport of T3 or T4, and different target cells show particular patterns of transporter expression [
9]. Thus, a defect in a given transporter protein will result in a specific phenotype in particular target cells depending on the particular expression of their transporter proteins. For example, it seems that the lack of the MCT8 transporter, which is the best known and studied thyroid hormone transporter so far, cannot be compensated for in the CNS. This leads to a rare disease with severe developmental delay and a movement disorder called Allan-Herndon-Dudley Syndrome (AHDS) [
10,
11]. In contrast, other organs such as the brain, the heart, or the liver are not hypothyroid in AHDS patients, most likely due to the expression of further thyroid hormone transporters which compensate for the MCT8 deficiency. The MCT8 defect results in a disbalanced T4 metabolism resulting in elevated T3 and rather low T4 plasma concentrations. Thus, besides a hypothyroid brain, the elevated plasma T3 can cause a state of peripheral hyperthyroidism of the liver and heart; although the exact molecular pathophysiological of these plasma alterations are unknown.
1.3. Thyroid Hormone Receptors
Within the target cells, T3 functions as a transcription regulator because the thyroid hormone receptor (THR) is a transcription factor that can either be activated or inhibited by the ligand T3, depending on the involved cofactors [
12]. Two different THRs are known, the THRα and THRβ, which are coded by two different genes,
THRA and
THRB respectively. While
THRA is expressed in many tissues and mainly in the CNS,
THRB is preferentially expressed in the pituitary and thus involved in the negative feedback control of plasma thyroid hormone concentration
via TSH. In the case of a
THRB defect, patients have an impaired sensing of plasma thyroid hormone by the pituitary with the consequence of elevated TSH and consequently elevated T4 and T3. Clinical symptoms can arise through the overstimulation of the unaffected THRα by the elevated plasma hormones leading to hyperthyroid symptoms [
13]. In contrast, a genetic defect of
THRA does not result in elevated TSH since THRα is not involved in the feedback loop; instead, a local defect of THRα function within the target cells results in a local hypothyroid state that causes clinical symptoms resembling those of congenital hypothyroidism, e.g. developmental delay and cognitive dysfunction [
14,
15]. In addition, the defect in THRα function within the metabolizing organs like the liver and kidney results in a particular pattern of mildly elevated or high-normal T3 and inversely of mildly decreased or low-normal T4 [
16,
17]; again the exact mechanism of this plasma hormone changes is not known so far.
Thus, while congenital hypothyroidism can be easily diagnosed by an elevated TSH, which is already established in newborn-screening programs [
1], the three other genetic defects affecting thyroid metabolism, thyroid hormone transport and thyroid hormone receptor function cannot be diagnosed based on TSH. In severe cases of these rare diseases, the measurement of T4 and T3 would allow recognizing the elevated T4 in SECISBP2 deficiency and elevated T3 in MCT8 deficiency, but in many cases especially with THRα deficiency and mild cases of MCT8 and SECISBP2 deficiencies, the T4 and T3 values are within the normal range and the diagnosis can be missed. However, in all three diseases the plasma hormone levels deviate in a reciprocal manner so that the ratio of T3 and T4 tends to be more deviating from the normal range than the single hormone levels alone; thus, the T3/T4 ratio could be of diagnostic value.
Figure 1.
Thyroid hormone production, metabolism and action on target cells. T4 is produced exclusively in the thyroid by iodination of thyroglobulin (Tg) by the thyroid peroxidase (TPO), which is the sole source of plasma T4. Plasma T4 can be further deiodinated in peripheral tissues by deiodinases 1 and 2 (DIO 1+2) to T3, which is then red-secreted into the circulation and represents 80% of plasma T3, while 20% of plasma T3 is produced primarily in the thyroid. The actual ratio of plasma T3 to T4 is therefore the result of variable primary production in the thyroid and tightly regulated peripheral metabolism of T4 to T3 and its re-secretion. In the target cells, only the active hormone T3 binds the thyroid hormone receptors alpha and beta. To exert this final T3 effect, T3 can be transported directly from the plasma to the target cell, or it can be produced locally in the target cell from T4 by deiodinase 2. In the pituitary, the T3 effect leads to a down-regulation of TSH production, which is the key step in the negative thyroid-pituitary feedback loop that keeps thyroid hormone levels stable in the plasma [
5,
17].
Figure 1.
Thyroid hormone production, metabolism and action on target cells. T4 is produced exclusively in the thyroid by iodination of thyroglobulin (Tg) by the thyroid peroxidase (TPO), which is the sole source of plasma T4. Plasma T4 can be further deiodinated in peripheral tissues by deiodinases 1 and 2 (DIO 1+2) to T3, which is then red-secreted into the circulation and represents 80% of plasma T3, while 20% of plasma T3 is produced primarily in the thyroid. The actual ratio of plasma T3 to T4 is therefore the result of variable primary production in the thyroid and tightly regulated peripheral metabolism of T4 to T3 and its re-secretion. In the target cells, only the active hormone T3 binds the thyroid hormone receptors alpha and beta. To exert this final T3 effect, T3 can be transported directly from the plasma to the target cell, or it can be produced locally in the target cell from T4 by deiodinase 2. In the pituitary, the T3 effect leads to a down-regulation of TSH production, which is the key step in the negative thyroid-pituitary feedback loop that keeps thyroid hormone levels stable in the plasma [
5,
17].

To date, changes in the T3/T4 ratio have only been described in a few cases and in small cohorts, e.g. in patients with hyper- and hypothyroidism, in children with MCT8 deficiency [
18], in children with congenital hypothyroidism treated with L-thyroxin, and in relation to the iodine status in children [
19,
20,
21], but no normal values were indicated. However, the ratio of T3 to T4 has not been evaluated as a tool in the clinical diagnostic process, because of the lack of age-dependent reference data for the T3/T4 ratio in the form of pediatric centile charts. Therefore, we set out to establish reference data for the T3/T4 ratio to enable early diagnosis of children, who are affected by motor, language, and cognitive developmental delay as well as by muscular hypotonia due to
SECISBP2,
SLC16A2, or
THRA gene defects. In addition, we tested the discriminatory power of these centile charts to single out the genetic defects mentioned above by using published and our own patient hormone levels (Table A1).
For this purpose, we chose the levels of the free, non protein-bound thyroid hormone levels because
(i) these are the most commonly used parameters in clinical practice and
(ii) the fT3 and fT4 levels were available from two large German child health surveys; the “LIFE” and “KiGGS” studies [
22,
23]. We show here that the percentiles for the fT3/fT4 ratio do show an age- and sex-specific normal range and that the individual fT3/fT4 ratio values of patients affected by a
SECISBP2,
SLC16A2, or
THRA gene defects are outside the normal range. Therefore, we suggest the use of these fT3/fT4 ratio percentiles as a simple tool for the clinical differential diagnosis in patients with motor and cognitive developmental delay.
2. Results
To establish the fT3/fT4 ratio as a clinically applicable serum/plasma-derived parameter for the differential diagnosis of children with developmental delay, we generated fT3/fT4 percentiles based on fT3 and fT4 measurements obtained in two large German cohorts of children, adolescents and young adults. The combined data set represented n=23,522 data points from individuals aged from birth to 29 years (
Figure A1).
2.1. Centile charts for TSH, fT3, fT4, and fT3/fT4 ratio
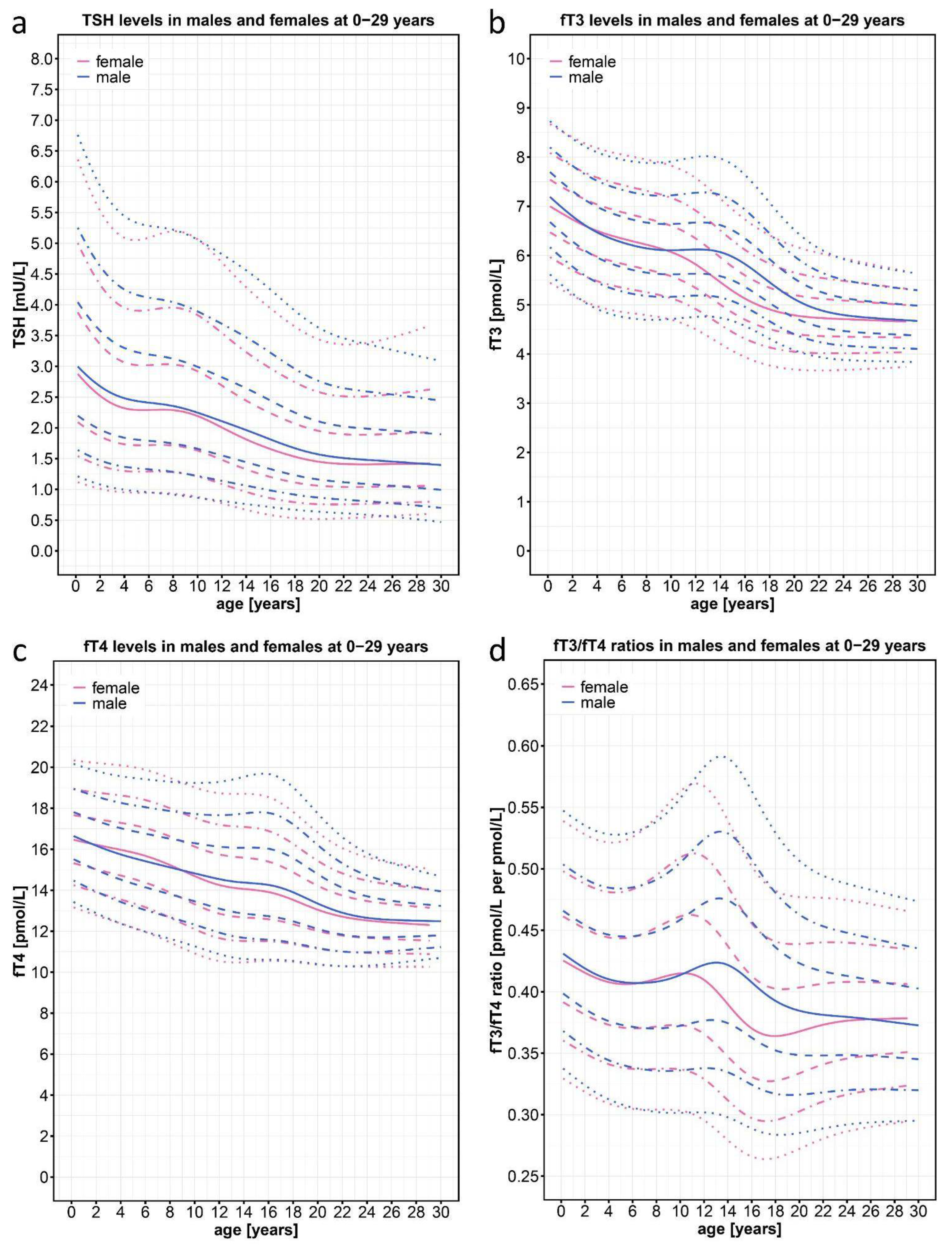
Consistent with previously published data, the TSH percentiles decreased constantly in both sexes over the entire range of 0-29 years of age (
Figure 2a). Consistent with the decrease of TSH levels and also consistent with published data [
21,
23], the fT3 and fT4 curves of our two cohorts show a downward slope with increasing age (
Figure 2b,c). In both cohorts, thyroid hormone levels were highest in the neonatal period and then declined, reaching a plateau at around 22 years of age. Interestingly, fT3 levels at birth (50
th percentile, 7.05 pmol/L) declined by 33% to the lowest level in adulthood (50
th percentile, 4.7 pmol/L), whereas fT4 levels declined by only 25% from the highest level (50
th percentile, 16.5 pmol/L) to the lowest level (50
th percentile, 12.5 pmol/L). Around the age of pubertal onset, the decline in both hormones was slowed, with a stronger effect on fT3 than on fT4, and most pronounced on fT3 in boys who remained on a plateau between the 8 and 14 years of age.
Given the parallel decline of fT3 and fT4 values, one would expect a more even age distribution of the fT3/fT4 ratio. However, we saw a clear age- and sex-dependent course (
Figure 2d) with higher values at birth and lower values in adulthood. Interestingly the fT3/fT4 ratio peaked at 11.2 years in girls and at 13.3 years in boys, roughly corresponding with the difference in the onset of puberty in both sexes. The overall range of the fT3/fT4 ratio from the highest 97
th percentile value in 13.3-year-old boys to the lowest 3
rd percentile value in 17-year-old girls was 0.59 to 0.27. Thus, to assess the individual fT3/fT4 ratio in the context of clinical diagnosis, it is necessary to use percentiles that reflect this wide range of normal values at different ages and in different sexes.
Figure 2.
Normal percentiles of TSH, fT3, fT4, and the fT3/fT4 ratio derived from n=23,522 data points of individuals (n=11,325 female; n=12,197 male) between 0 and 29 years of age from the joint KiGGS baseline study, KiGGS Wave 2, and LIFE studies that were based on the general pediatric and young adult population of Germany. The colored lines depict the 97
th, 90
th, 75
th, 50
th, 25
th, 10
th, and 3
rd percentiles, pink color represents female and blue color male patients. NB Point clouds for the fT3/fT4 centile charts are depicted on
Figure 1A.
Figure 2.
Normal percentiles of TSH, fT3, fT4, and the fT3/fT4 ratio derived from n=23,522 data points of individuals (n=11,325 female; n=12,197 male) between 0 and 29 years of age from the joint KiGGS baseline study, KiGGS Wave 2, and LIFE studies that were based on the general pediatric and young adult population of Germany. The colored lines depict the 97
th, 90
th, 75
th, 50
th, 25
th, 10
th, and 3
rd percentiles, pink color represents female and blue color male patients. NB Point clouds for the fT3/fT4 centile charts are depicted on
Figure 1A.
2.2. fT3/fT4-Ratios of Different Patient Cohorts
Next, we tested the percentiles for the fT3/fT4 ratio for their discriminative power between pathologies that were either related to the thyroid system (e.g., in patients with mutations in THRA, SLC16A2, or SECISBP2) or not (e.g., in patients with cerebral palsy).
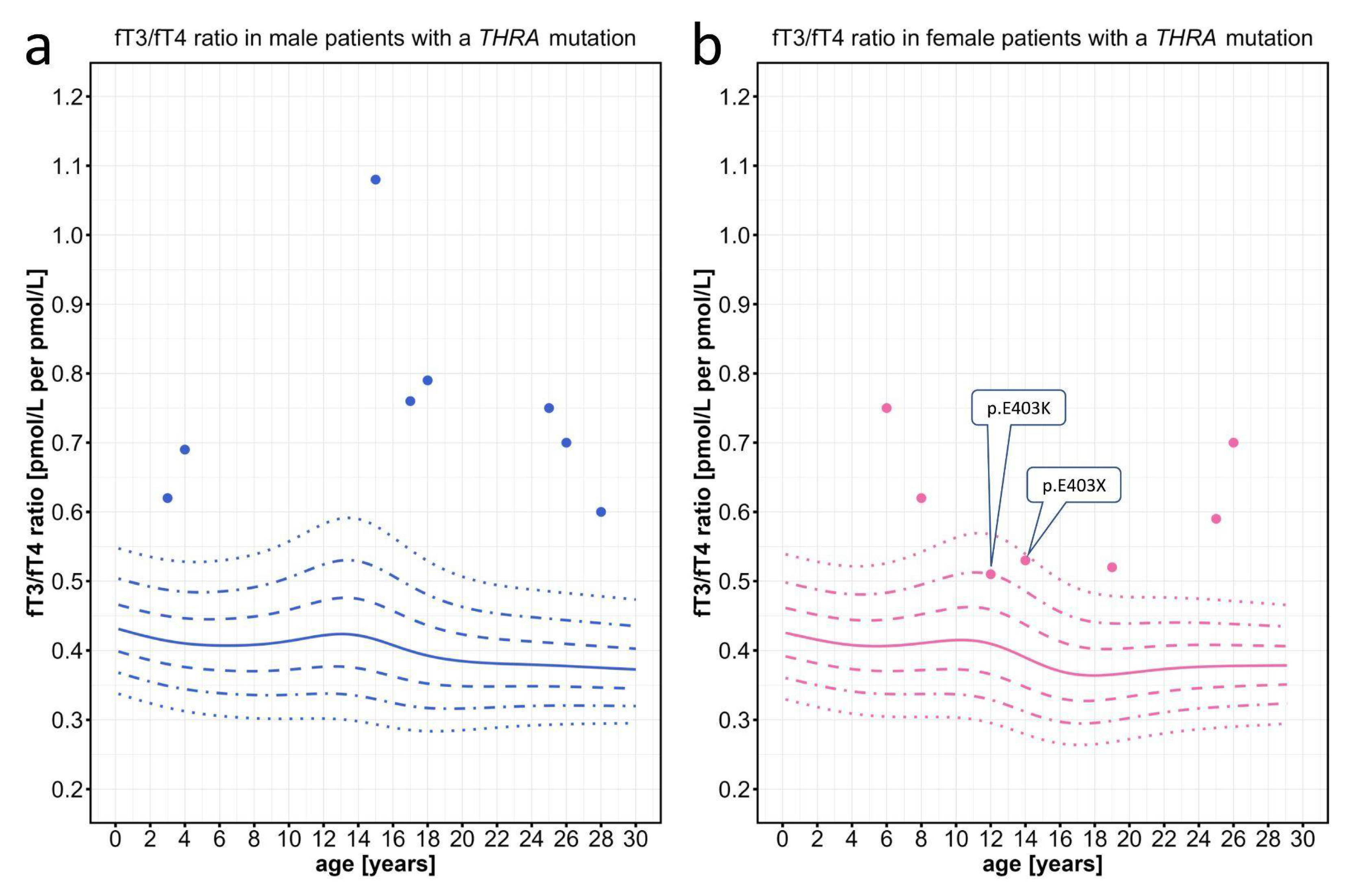
We first evaluated data from patients with
THRA mutations, which had been extracted from the literature. While the individual fT3 and fT4 values were mostly within the normal range, the fT3/fT4 ratio was above the 97
th percentile in most patients. In only two particular patients with a mutation in the most C-terminal amino acid residue 403 of THRα, the fT3/fT4 ratio was lower but still at or above the 90
th percentile (
Figure 3).
In patients with
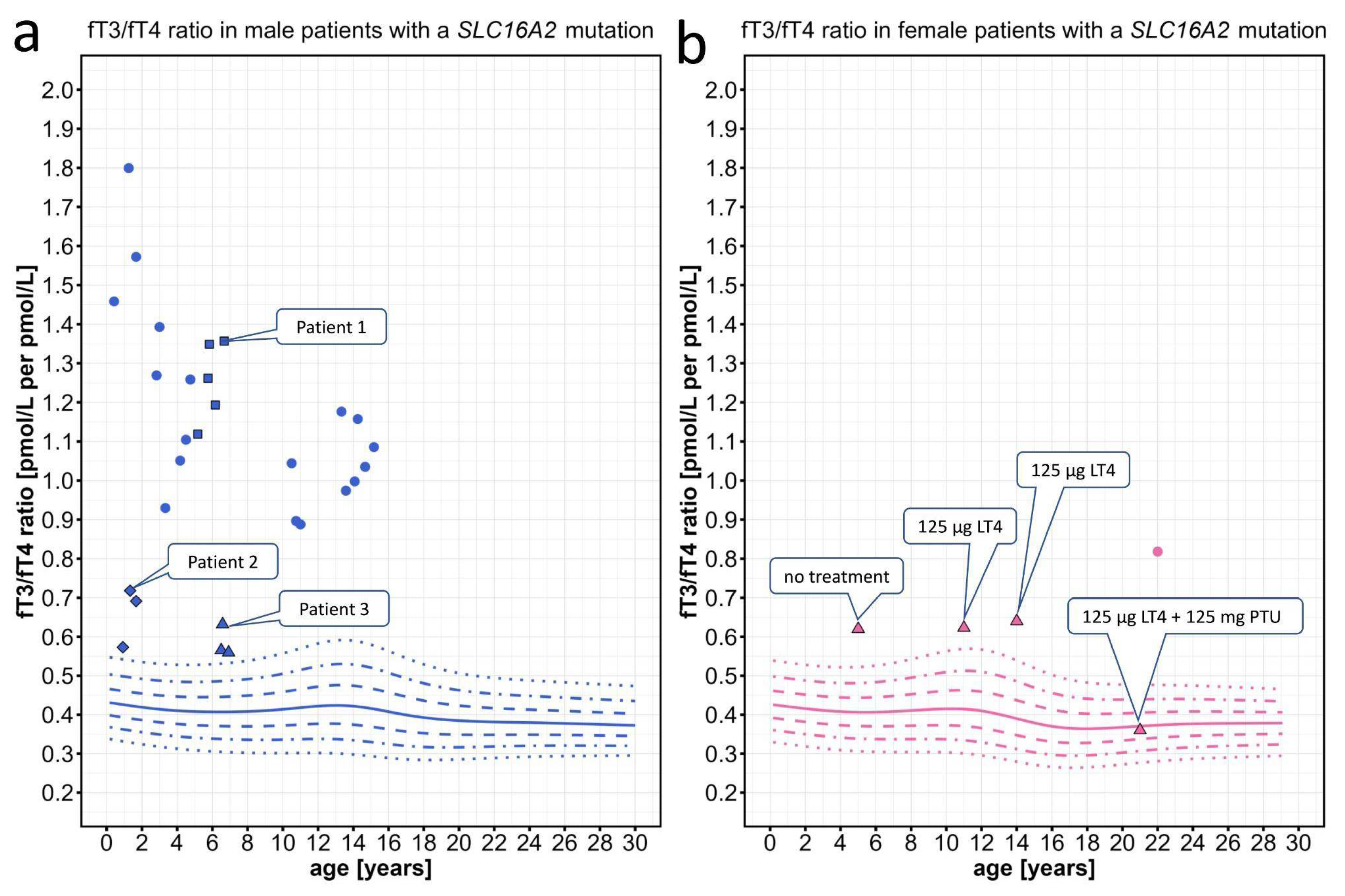
MCT8 deficiency and proven
SLC16A2 mutations
, the fT3/fT4 ratios were clearly located above the 97
th percentile (
Figure 4a). Partly, this could be expected since MCT8-deficient patients tend to have high fT3 plasma concentrations. However, this increase of the fT3/fT4 ratio was also seen in two very mildly affected patients, in whom AHDS would not have been the first differential diagnosis and in whom a
SLC16A2 mutation could have easily been missed due to their mild phenotype and partially normal fT3 values. Interestingly repeated measurements of the mildly affected patients all clustered around a similar fT3/fT4 ratio and were located only mildly above the 97
th percentile (
Figure 4a). To more fully illustrate the wide range of neurodevelopmental phenotypes observed in MCT8-deficient patients, who have all been shown to exhibit an elevated fT3/fT4 ratio, we have included a detailed description of the clinical phenotypes, accompanied by video sequences for one patient with a severe phenotype (Patient 1), one patient with a milder phenotype (Patient 2), and one patient with a very mild manifestation of the disease (Patient 3) (see section 4.1.5).
As a proof of concept, we also discovered an elevated fT3/fT4 ratio in two female MCT8-deficient patients with a heterozygous
SLC16A2 mutation with skewed X-inactivation. Both female patients were mildly affected, mainly by a delay of language development. In one of these female patients, treatment with levothyroxine in combination with the deiodinase inhibitor 6-n-propyl-2 thiouracil (PTU) [
24] normalized the fT3/fT4 ratio, possibly due to the combination of inhibition of T4-to-T3 deiodination and increase of T4 levels by supplementation (
Figure 4b).
Figure 4.
Individual data points for the fT3/fT4 ratio plotted on the percentiles for male and female patients with SLC16A2 mutations. Information about the individual patients can be found on Table A1. (a) We present repeated measurements for two mildly affected male patients (diamond, patient 2; triangle, patient 3) and one severely affected male patient (squares, patient 1). (b) Triangles represent a female patient under treatment with levothyroxine (LT4) and later addition of 6-n-propyl-2 thiouracil (PTU), which normalized the fT3/fT4 ratio. The colored lines depict the 97th, 90th, 75th, 50th, 25th, 10th, and 3rd percentiles.
Figure 4.
Individual data points for the fT3/fT4 ratio plotted on the percentiles for male and female patients with SLC16A2 mutations. Information about the individual patients can be found on Table A1. (a) We present repeated measurements for two mildly affected male patients (diamond, patient 2; triangle, patient 3) and one severely affected male patient (squares, patient 1). (b) Triangles represent a female patient under treatment with levothyroxine (LT4) and later addition of 6-n-propyl-2 thiouracil (PTU), which normalized the fT3/fT4 ratio. The colored lines depict the 97th, 90th, 75th, 50th, 25th, 10th, and 3rd percentiles.
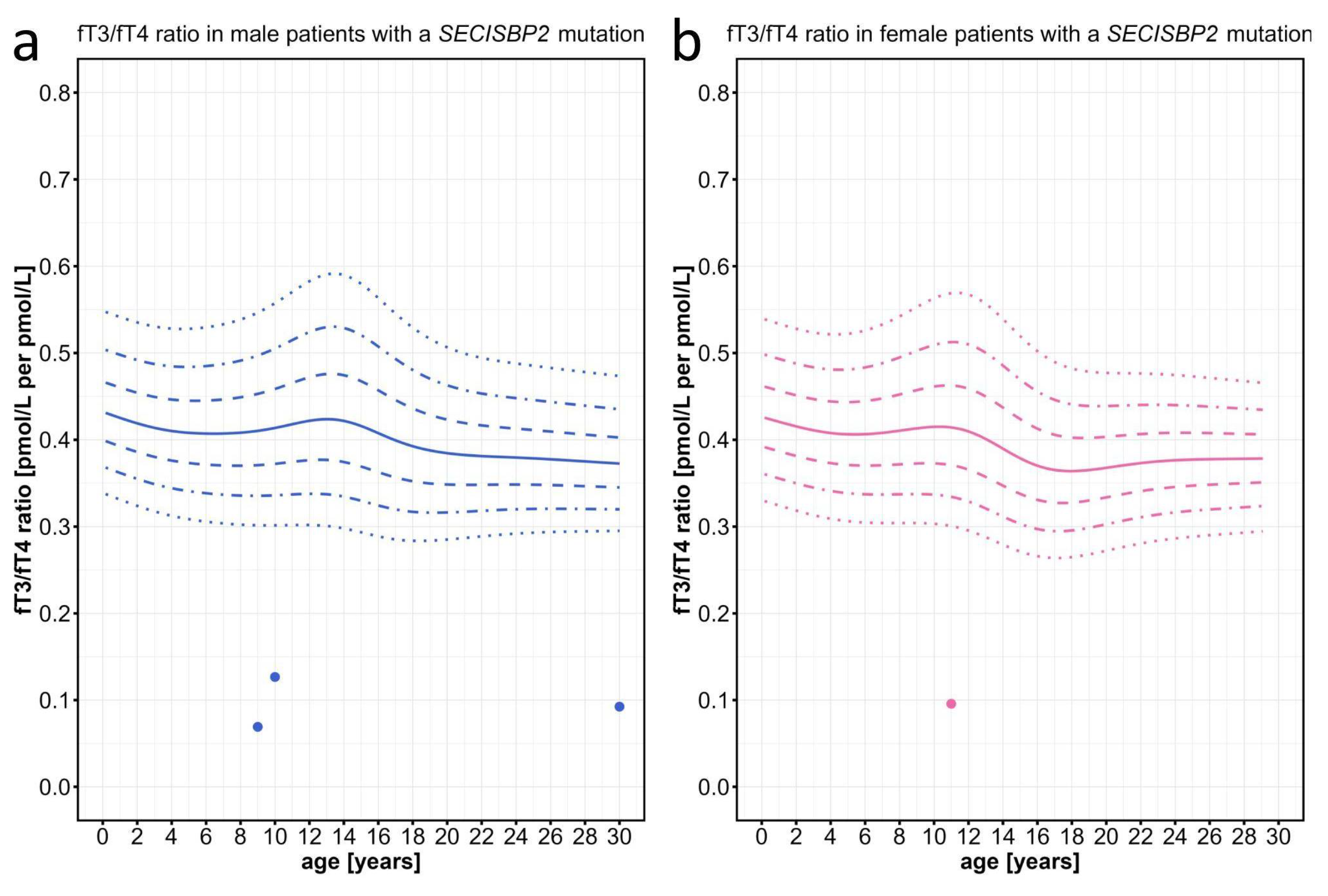
As expected by the already described rather elevated T4 and low-normal T3 values in patients with SECISBP2 defects, we found the fT3/fT4 ratios of these particular patients to be located clearly below the 3
rd percentile (
Figure 5).
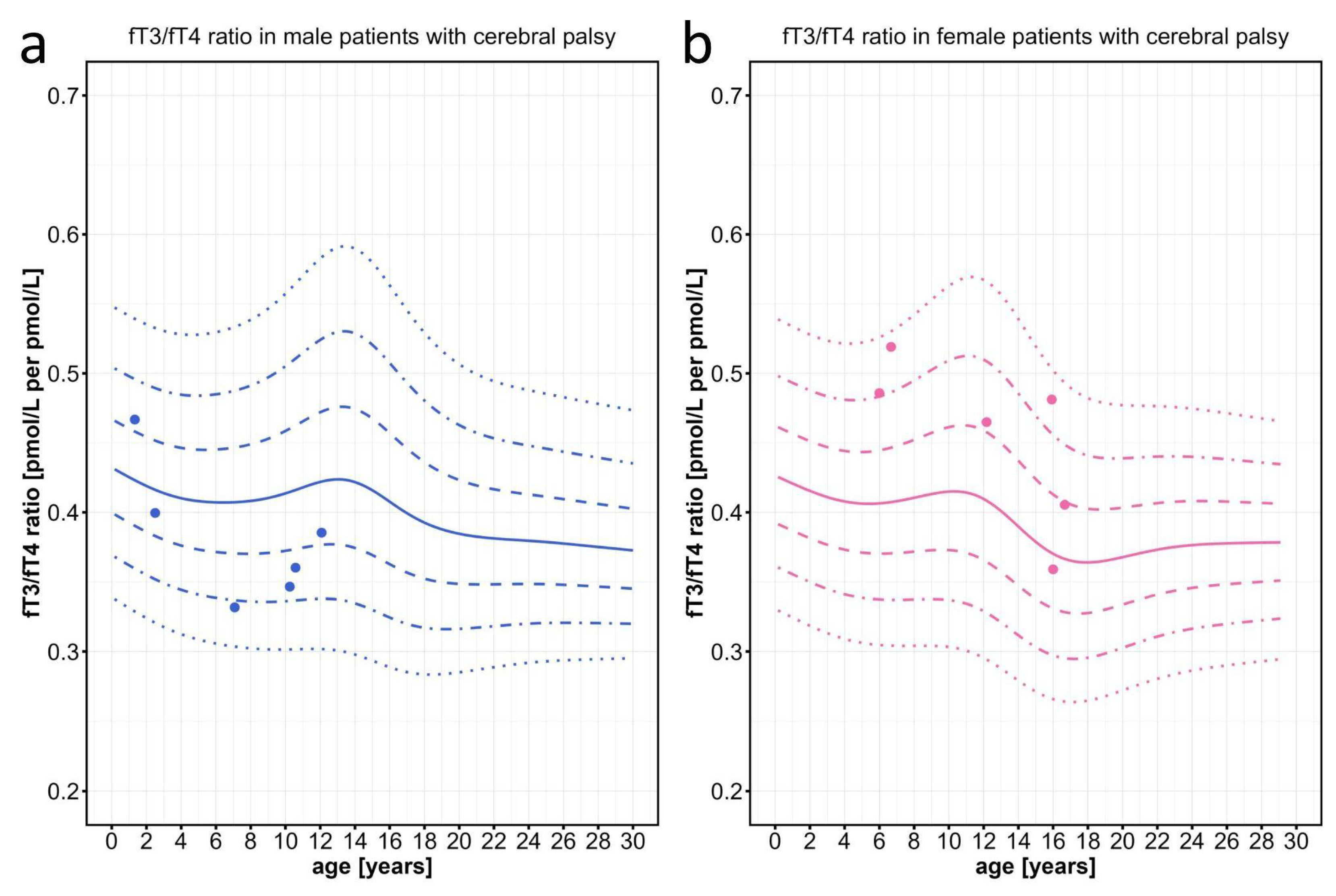
Finally, as a negative control, we tested the fT3/fT4 ratios of a cohort of children who were severely affected by cerebral palsy (CP) due to perinatal brain damage. Patients of this control cohort are also affected by severe symptoms including spasticity, dystonia, and weight loss in addition to developmental delay, which partially overlaps with the symptoms seen in MCT8 deficient patients. All values of these children were clearly within the normal range, irrespective of age or sex (
Figure 6).
3. Discussion
Primary congenital hypothyroidism is easily diagnosed based on elevated TSH and low T3 and T4 levels [
3]. Since the discovery of the various forms of thyroid hormone resistance, it has been shown that these rare disorders often have normal TSH, T3 and T4 levels and therefore likely to be massively underdiagnosed [
25]. Patients with these disorders have developmental and cognitive deficits that can be as severe as those associated with congenital hypothyroidism.
Despite normal absolute T3 and T4 levels, the ratio of the two hormones appears to be altered in these thyroid hormone-resistant disorders. However, the lack of normal age- and sex-specific values for the fT3/fT4 ratio has hampered the establishment of this serum/plasma parameter for screening and differential diagnosis of developmental delay.
Here we report the successful establishment of age- and sex-specific normal values for the fT3/fT4 ratio. Based on two large German population-based cohorts from the "KiGGS" and "LIFE" studies, more than 23,000 data sets were available to derive the percentile ranges for fT3, fT4, TSH and the fT3/fT4 ratio. The large data set of fT3/fT4 ratios is normally distributed. From birth, the mean values decrease until they show a temporary upward trend around the onset of puberty, with a significant difference between boys and girls. Therefore, the use of age- and sex-specific percentiles is mandatory for evaluation and clinical judgment.
When exploring the use of the newly generated fT3/fT4 percentiles for the diagnosis of three thyroid hormone resistance diseases, e.g. THRα-, MCT8- and SECISBP2-deficiency, we saw a clear separation of the individual patient fT3/fT4 values from the normal percentile ranges. Even patients with THRα- and MCT8-deficiency with entirely normal absolute T3 and T4 values were found to have elevated fT3/fT4 ratios above the 97th percentile. Only two patients with the most C-terminal mutations in residue 403 of the THRA gene were found to have lower fT3/fT4 ratios, which might point to a particular genotype-phenotype correlation concerning the impact of THRα-defects on T4 metabolism.
It seems reasonable to use the fT3/fT4 ratio as a further parameter for the differential diagnostic process of children with developmental delay but normal TSH levels. Despite the expectation that an increasing number of these children will be diagnosed by the thorough and early implementation of whole exome sequencing (WES) [
26,
27], the fT3/fT4 ratio will help to interpret variants of unknown significance (VUS) in
SLC16A2 or
THRA, and may direct the search towards non-coding areas of the genome that represent around 60% of cases [
28].
This is especially true due to the therapeutic options that are available and that will be developed in the near future for these thyroid hormone resistance diseases. Although the whole spectrum of symptoms cannot entirely be compensated, the treatment of THRα- and SECISBP2-defects with additional LT4 was shown to improve some clinical features like the growth delay [
29,
30,
31]. Based on the experience with congenital hypothyroidism, treatment needs to be established very early during development to improve cognitive and motor outcome. Our results with the finding of an altered fT3/fT4 ratio already in very young children (the youngest child tested was 0.9 years old), opens the possibility that the fT3/fT4 ratio might be a tool to detect these diseases already in the neonatal period; likely as a second tier test after initial genetic newborn screening [
32].
Our centile charts of the fT3/fT4 ratio are based on SI units (pmol/L). Unfortunately, there is a wide range of units used for hormone measurements, which might be confusing and might hamper the proper calculation of the fT3/fT4 ratio for an individual patient. Therefore we provide a web page (
http://www.thyroid-hormone-ratio.org) that allows entering the hormone measurements in a wide range of units and provides a printout that visualizes the individuals' TSH, fT3, and fT4 measurements, as well as the fT3/fT4 ratio within the age- and sex-adapted centile chart.
Presently, the molecular pathophysiology of the increased fT3/fT4 ratio is unknown. Our charts clearly suggest an age- and sex-dependent regulation. The regulator might be located at the primary hormone production in the thyroid gland or may be the consequence of different deiodination rates in those organs that contribute to the secondary T3 production from T4, such as liver, kidney, and muscle [
5,
17]. A recent metastudy of genome-wide associations described 13 gene loci that contribute to the fT3/fT4 ratio in the plasma, including the two deiodinase gene loci
DIO1 and
DIO2. However, the contributions of all these loci together, only explain a few percent of the variance [
33]. In addition, the (patho)physiological relevance of the fT3/fT4 ratio remains unclear, because organ cells that are the targets of thyroid hormone are able to generate their own T3 locally through intracellular deiodination of T4 (
Figure 1).
4. Materials and Methods
4.1. Description of the Study Samples
4.1.1 KiGSS Baseline Study (Robert Koch Institute, Berlin)
The KiGGS baseline study (German Health Interview and Examination Survey for Children and Adolescents) was the first nationwide study to survey health in children between 0-17 years and was performed between 2003-2006 by the Robert Koch Institute (RKI) of Berlin [
22]. It included n=17,641 children from 167 communities in Germany. The study acquired representative data on physical and mental health status (including thyroid health), health behavior and determinants, which were based on physical and laboratory investigations and interviews. The data were adjusted to exclude individuals with potential thyroid disease, e.g. individuals with TSH plasma levels of >10 mU/L or medication with an influence on thyroid function. In n=12,836 individuals between 3-17 years, laboratory investigations with regard to thyroid health were performed and included plasma values for TSH, fT3, fT4, as well as thyroid volume by ultrasound, and the iodine/creatinine quotient in the urine as a marker for iodine supply [
21,
34,
35,
36].
4.1.2 KiGGS Wave 2 Study (Robert Koch Institute, Berlin)
The second follow-up study (KiGGS Wave 2) was again conducted as a health examination and interview survey between 2014-2017, with study participants now between 10-31 years of age and living in the same recruitment areas as for the baseline study (n=10,853) [
22]. Many of them had also participated in the first follow-up (KiGGS Wave 1) interviews. With KiGGS Wave 2 a new cross-sectional interview and examination survey was also realized. In n=6,465 participants, further physical and laboratory investigations were done, from n=2,971 study participants we have laboratory values reflecting thyroid health (TSH, fT3, fT4). For n=2,643 study participants we have the respective laboratory measurements for the age group between 18 and 29 years.
4.1.3. LIFE Child Study (University Leipzig)
The longitudinal epidemiological LIFE Child Study is part of the LIFE Research Center for Civilization Diseases and has collected developmental data on infants, children, and adolescents since 2011, primarily focusing on the so-called civilization diseases [
37] with a special focus on thyroid health [
23,
38]. For our centile chart calculation, we used TSH, fT3, and fT4 values from children aged between 0-6 years using n=5,413 data points from n=2,287 individuals. Within this time period, children were sampled between 1 and 8 times (mean=2.3, SD=1.51).
4.1.4. Measurement of Thyroid Hormone Values
LIFE Child Study: After an overnight fast, venous blood was taken in the morning. TSH, fT3, and fT4 values were measured by electrochemiluminescence assays using a Cobas 601 or 801 module (Roche Diagnostics, Mannheim, Germany). The mean inter-assay coefficient of variation for the three measured biomarkers ranged from 2.25% to 3.11%; the mean deviation from the target value varied between 3.33% and 4.82% [
23].
KiGGS Study: For the
KiGGS baseline study, venous blood was drawn after various (non-standard) periods of fasting. TSH, fT3, and fT4 values were measured using an electrochemiluminescence binding assay (Elecsys 2010, Roche Diagnostics, Mannheim, Germany). The interserial variation coefficient over the entire study period was 3.9%, 5.9%, and 5.3% retrospectively [
39]. For the
KiGGS Wave 2 study, TSH, fT3, and fT4 values were measured in the Central Epidemiological Laboratory of the Robert Koch Institute, Berlin using an immunoassay on the Architect-Analyzer CI 8200 (Abbott, Abbott Park, North Chicago, Illinois, USA).
4.1.5. Case Histories
Patients provided written informed consent for participation in the study and for publication of patient images. Ethical approval for the study was obtained from the Institutional Review Board of Charité (EA2/026/20). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
The MCT8-deficient male patient 1 (
Figure 4, blue squares) was born at term after a normal pregnancy. In the first months of life, he was severely floppy, triggering a two-year diagnostic odyssey. Trio-exom sequencing revealed a hemizygous 1 bp insertion in
SLC16A2 (c.1235dupG; p.L413Pfs*25) causing a frameshift. The patient developed a severe phenotype of the AHDS with muscular hypotonia progressing to hypertonia of the extremities (spastic-dystonic) with persistent axial hypotonia, severe global developmental delay (unstable head control, inability to sit at 6 years of age), intellectual disability, dysphagia, and severe underweight requiring a permanent gastrostomy tube (Video 1). Repeated measurements showed a highly elevated fT3/fT4 ratio.
The MCT8-deficient male patient 2 (
Figure 4, blue diamonds) was born at term
via Cesarean section after a normal pregnancy. During infancy, the patient showed mild hypotonia and developmental delay with head control at 10 months, free sitting at 12 months, and pulling to a standing position at 18 months. Later, he developed dystonia with "involuntary movements and pulling of the arms and legs" and hypersalivation (dysphagia) (Video 2). Intellectual disability was suspected. Trio-exome sequencing revealed a novel
SLC16A2 missense mutation (c.1399G>A; p.G467S) that was not listed in gnomAD. In this mildly affected patient, the fT3 was only slightly elevated.
The MCT8-deficient male patient 3 (
Figure 4, blue triangles) was born at term after a normal pregnancy. In the second year of life, he presented with mild developmental delay with the ability to walk unaided at 17 months and to speak his first words at 19 months. At the age of 6 years, he was also diagnosed with mild intellectual disability and intermittent "twisting/cramping of the hands" (suspected action-specific dystonia) (Video 3). Trio-exome sequencing discovered a highly conserved
SLC16A2 missense variant (c.1378A>T; p.I460F) that was not listed in gnomAD. In this patient with a very mild phenotype of AHDS, the elevated fT3 was only slightly above the 97
th percentile.
4.2. Statistical Methods
4.2.1. Raw Data Cleaning
Raw data were cleaned by removing individuals with a history of thyroid disease, TSH levels above 10 mU/L, medication with an influence on the thyroid hormone metabolism (e.g. L-thyroxine, iodine), and individuals with an increased iodine excretion above 1.08 mg/d.
4.2.2. Generating the Centile Charts
The centile charts were generated with the R package 'childsds' (Data and Methods Around Reference Values in Pediatrics) v0.8.0 by Mandy Vogel [
40]. The package allows the generation of centile charts from various cohort measurements, store these as standards and use these standards to calculate individual SDS values. The distribution parameters were estimated assuming a BCPEo (Box-COX power exponential) distribution. The used algorithm applied the gamlss function from the 'gamlss' R package [
41]. The linear regression plots were generated with the geom_smooth(method=lm) function of the 'ggplot2' R package [
42].
5. Conclusions
Thyroid hormone resistance due to MCT8, THRα, and SECISBP2 defects leads to severe developmental delay like congenital hypothyroidism but is characterized by normal TSH and partially normal T3 and T4 levels. This often delays the diagnosis. As there are therapeutic options, a diagnosis should be made as early as possible. Therefore, in the differential diagnosis of children with developmental delay, we recommend not only determining TSH as the standard parameter for normal thyroid function, but also measuring fT4 and fT3 levels to determine the fT3/fT4 ratio. While our data suggest that the fT3/fT4 ratio is pathognomonic from an early age, further studies are needed to establish the fT3/fT4 ratio as a possible neonatal screening parameter.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1,
Video_S1.mov: Video recording of patient 1 with a severe phenotype at 6 years of age.
Video_S2.mov: Video recording of patient 2 with a mild phenotype at 1.5 years of age.
Video_S3.mov: Video recording of patient 3 with a very mild phenotype at 6 years of age.
Author Contributions
Conceptualization, HK and MS; methodology, MV and MS; software, DS, MV, and MS; validation, NMW, HK and MS; formal analysis, MS; investigation, RT, MT, JK; resources, RT, MT, JK, MV; data curation, RT, MT, MV; clinical care of the patients, NMW, HK, and MS; writing—original draft preparation, NMW, HK, MS; writing—review and editing, RT, MT, JK, DS and MV; visualization, MS; supervision, HK and MS; project administration, HK and MS; funding acquisition, NMW, HK and MS. All authors have read and agreed to the published version of the manuscript.
Funding
NMW was supported by the Deutsche Forschungsgemeinschaft (DFG) Research Unit 2841 “Beyond the Exome”, and she is participant in the BIH Charité Junior Clinician Scientist Program for Rare funded by the Charité - Universitätsmedizin Berlin, the Berlin Institute of Health at the Charité (BIH), and the Alliance4Rare, and the Berliner Sparkassenstiftung Medizin. HK und MS were supported by the DFG TRR 296 "Local control of TH action (LocoTact)", project P06, and MS was supported by the DFG under Germany’s Excellence Strategy (EXC-2049-390688087) via the NeuroCure consortium at Charité - Universitätsmedizin Berlin.
Institutional Review Board Statement
Ethical approval for the study was obtained from the Institutional Review Board of Charité - Universitätsmedizin Berlin, Campus Virchow Klinikum in the year 2020 (EA2/026/20). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Informed Consent Statement
Patient or their parents and guardians provided written informed consent for participation at the study and for publication of their data and images (videos).
Data Availability Statement
KiGGS data from the Robert Koch Institute, Berlin: The authors confirm that some access restrictions apply to the data underlying the findings. The data set cannot be made publicly available because informed consent from study participants did not cover public deposition of data. However, the minimal data set underlying the findings is archived in the Research Data Centre at the Robert Koch Institute (RKI) and can be accessed by researchers on reasonable request. On-site access to the data set is possible at the Secure Data Center of the RKI’s Research Data Centre. Requests should be submitted to the Research Data Centre, Robert Koch Institute, Berlin, Germany at fdz@rki.de. Data of the LIFE child study, University of Leipzig: The dataset presented in this article cannot be shared publicly because of ethical and legal restrictions. The LIFE Child study is a study collecting potentially sensitive information. Publishing data is not covered by the informed consent provided by the study participants. Furthermore, the data protection concept of LIFE requires all (external as well as internal) researchers interested in accessing data to sign a project agreement. Researchers interested in accessing data from the LIFE Child study may contact the study by writing to forschungsdaten@medizin.uni-leipzig.de.
Acknowledgments
We thank the participating patients and their families. We furthermore thank Ursula Kuhnle, Michael Zech and Ján Necpál for providing thyroid hormone values of female patients with a SLC16A2 variant and Sofia Petrova for providing the thyroid hormone values of male patients with typical AHDS. The authors are grateful to Roche for an unrestricted research grant to finance the measurements of fT3, fT4, and TSH within this LIFE Child study.
Conflicts of Interest
NMW took part in a consultation with Primus Consulting Group GmbH, advised the movie theater film production company Hellinger-Doll and was paid by Biogen for a congress presentation. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Table A1: Tabulated individual patient data (sex, age, cohort, mutation, disease severity) as well as fT3, fT4, and fT3/fT4 data.
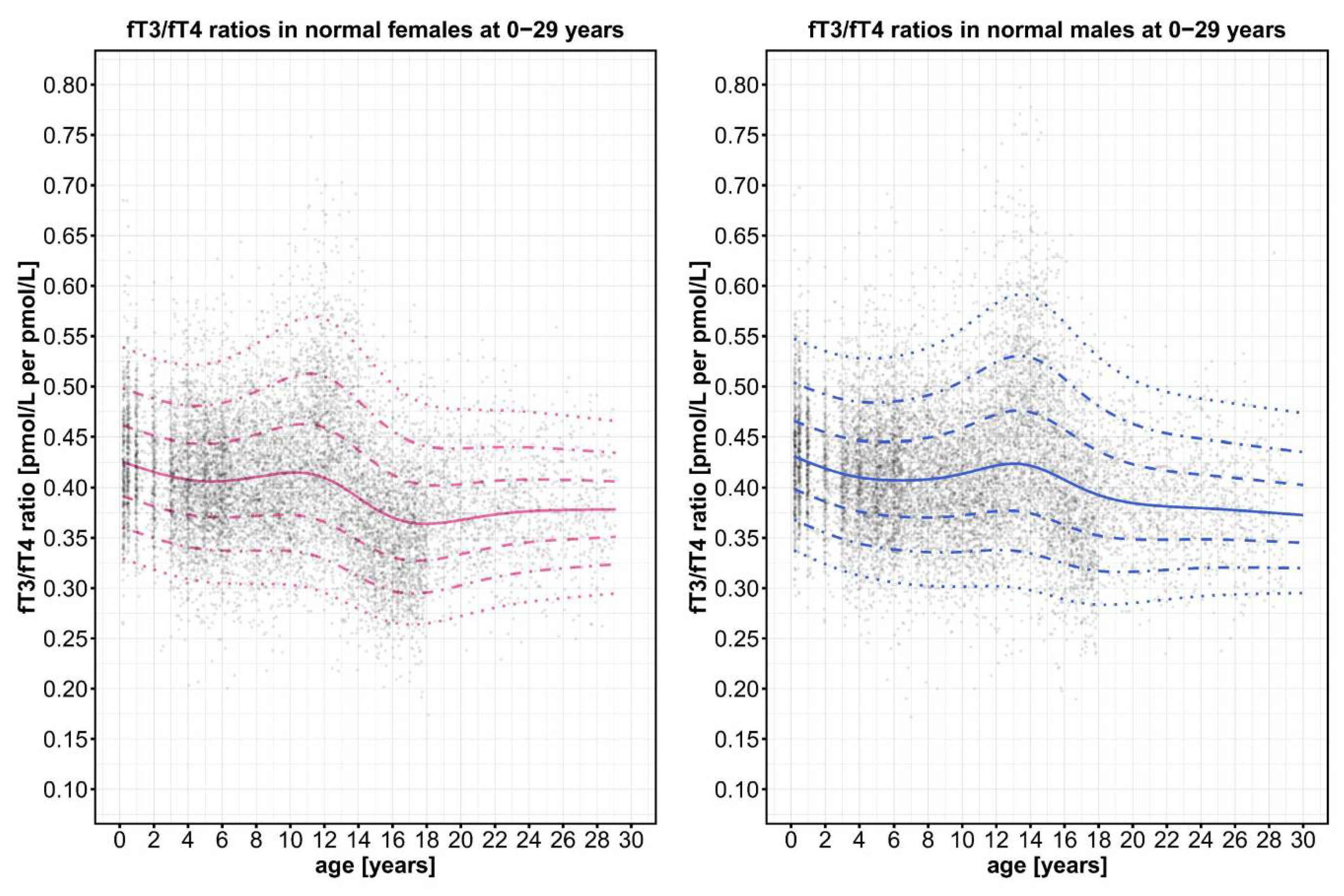
Figure A1.
Distribution of the point clouds for the fT3/fT4 ratios of all male and female control individuals.
Figure A1.
Distribution of the point clouds for the fT3/fT4 ratios of all male and female control individuals.
References
- van Trotsenburg, P.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [CrossRef]
- Aleksander, P.E.; Brückner-Spieler, M.; Stoehr, A.-M.; Lankes, E.; Kühnen, P.; Schnabel, D.; Ernert, A.; Stäblein, W.; Craig, M.E.; Blankenstein, O.; et al. Mean High-Dose l-Thyroxine Treatment Is Efficient and Safe to Achieve a Normal IQ in Young Adult Patients With Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2018, 103, 1459–1469. [CrossRef]
- Grüters, A.; Krude, H. Detection and Treatment of Congenital Hypothyroidism. Nat. Rev. Endocrinol. 2011, 8, 104–113. [CrossRef]
- Refetoff, S.; Dumitrescu, A.M. Syndromes of Reduced Sensitivity to Thyroid Hormone: Genetic Defects in Hormone Receptors, Cell Transporters and Deiodination. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 277–305. [CrossRef]
- Köhrle, J.; Frädrich, C. Deiodinases Control Local Cellular and Systemic Thyroid Hormone Availability. Free Radic. Biol. Med. 2022, 193, 59–79. [CrossRef]
- Laurberg, P. Mechanisms Governing the Relative Proportions of Thyroxine and 3,5,3′-Triiodothyronine in Thyroid Secretion. Metabolism 1984, 33, 379–392. [CrossRef]
- Dumitrescu, A.M.; Liao, X.-H.; Abdullah, M.S.Y.; Lado-Abeal, J.; Majed, F.A.; Moeller, L.C.; Boran, G.; Schomburg, L.; Weiss, R.E.; Refetoff, S. Mutations in SECISBP2 Result in Abnormal Thyroid Hormone Metabolism. Nat. Genet. 2005, 37, 1247–1252. [CrossRef]
- Fu, J.; Korwutthikulrangsri, M.; Gönç, E.N.; Sillers, L.; Liao, X.-H.; Alikaşifoğlu, A.; Kandemir, N.; Menucci, M.B.; Burman, K.D.; Weiss, R.E.; et al. Clinical and Molecular Analysis in 2 Families With Novel Compound Heterozygous SBP2 (SECISBP2) Mutations. J. Clin. Endocrinol. Metab. 2020, 105, e6–e11. [CrossRef]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, 146–201. [CrossRef]
- Friesema, E.C.; Grueters, A.; Biebermann, H.; Krude, H.; von Moers, A.; Reeser, M.; Barrett, T.G.; Mancilla, E.E.; Svensson, J.; Kester, M.H.; et al. Association between Mutations in a Thyroid Hormone Transporter and Severe X-Linked Psychomotor Retardation. The Lancet 2004, 364, 1435–1437. [CrossRef]
- van Geest, F.S.; Groeneweg, S.; Visser, W.E. Monocarboxylate Transporter 8 Deficiency: Update on Clinical Characteristics and Treatment. Endocrine 2021, 71, 689–695. [CrossRef]
- Vella, K.R.; Hollenberg, A.N. The Actions of Thyroid Hormone Signaling in the Nucleus. Mol. Cell. Endocrinol. 2017, 458, 127–135. [CrossRef]
- Pappa, T.; Refetoff, S. Resistance to Thyroid Hormone Beta: A Focused Review. Front. Endocrinol. 2021, 12. [CrossRef]
- Bochukova, E.; Schoenmakers, N.; Agostini, M.; Schoenmakers, E.; Rajanayagam, O.; Keogh, J.M.; Henning, E.; Reinemund, J.; Gevers, E.; Sarri, M.; et al. A Mutation in the Thyroid Hormone Receptor Alpha Gene. N. Engl. J. Med. 2012, 366, 243–249. [CrossRef]
- Dahll, L.K.; Westbye, A.B.; Vinorum, K.; Sejersted, Y.; Barøy, T.; Thorsby, P.M.; Hammerstad, S.S. Clinical and Biochemical Characteristics of Untreated Adult Patients With Resistance to Thyroid Hormone Alpha. J. Endocr. Soc. 2023, 7, bvad089. [CrossRef]
- Moran, C.; Chatterjee, K. Resistance to Thyroid Hormone Due to Defective Thyroid Receptor Alpha. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 647–657. [CrossRef]
- Bianco, A.C.; Dumitrescu, A.; Gereben, B.; Ribeiro, M.O.; Fonseca, T.L.; Fernandes, G.W.; Bocco, B.M.L.C. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr. Rev. 2019, 40, 1000–1047. [CrossRef]
- Remerand, G.; Boespflug-Tanguy, O.; Tonduti, D.; Touraine, R.; Rodriguez, D.; Curie, A.; Perreton, N.; Des Portes, V.; Sarret, C.; Group, R.S. Expanding the Phenotypic Spectrum of Allan–Herndon–Dudley Syndrome in Patients with SLC16A2 Mutations. Dev. Med. Child Neurol. 2019, 61, 1439–1447. [CrossRef]
- Mortoglou, A.; Candiloros, H. The Serum Triiodothyronine to Thyroxine (T3/T4) Ratio in Various Thyroid Disorders and after Levothyroxine Replacement Therapy. Horm. Athens Greece 2004, 3, 120–126. [CrossRef]
- Sydlik, C.; Dubinski, I.; Bechtold, S.; Schmidt, H. Free Triiodothyronine/Free Thyroxine Ratio in Children with Congenital Hypothyroidism. Endocr. Connect. 2022, 11. [CrossRef]
- Johner, S.A.; Thamm, M.; Stehle, P.; Nöthlings, U.; Kriener, E.; Völzke, H.; Gärtner, R.; Remer, T. Interrelations Between Thyrotropin Levels and Iodine Status in Thyroid-Healthy Children. Thyroid® 2014, 24, 1071–1079. [CrossRef]
- Mauz, E.; Lange, M.; Houben, R.; Hoffmann, R.; Allen, J.; Gößwald, A.; Hölling, H.; Lampert, T.; Lange, C.; Poethko-Müller, C.; et al. Cohort Profile: KiGGS Cohort Longitudinal Study on the Health of Children, Adolescents and Young Adults in Germany. Int. J. Epidemiol. 2019, 1–12. [CrossRef]
- Surup, H.; Vogel, M.; Koerner, A.; Hiemisch, A.; Oelkers, L.; Willenberg, A.; Kiess, W.; Kratzsch, J. Pediatric Reference Intervals for Thyrotropin, Free Triiodothyronine, and Free Thyroxine and the Relevance of Body Mass Index and Puberty in Measurement Interpretation. Thyroid® 2021, 31, 1192–1202. [CrossRef]
- Wémeau, J.L.; Pigeyre, M.; Proust-Lemoine, E.; d’Herbomez, M.; Gottrand, F.; Jansen, J.; Visser, T.J.; Ladsous, M. Beneficial Effects of Propylthiouracil plus L-Thyroxine Treatment in a Patient with a Mutation in MCT8. J. Clin. Endocrinol. Metab. 2008, 93, 2084–2088. [CrossRef]
- Tagami, T. An Overview of Thyroid Function Tests in Subjects with Resistance to Thyroid Hormone and Related Disorders. Endocr. J. 2021, 68, 509–517. [CrossRef]
- Jansen, S.; Vissers, L.E.L.M.; de Vries, B.B.A. The Genetics of Intellectual Disability. Brain Sci. 2023, 13, 231. [CrossRef]
- Kernohan, K.D.; Boycott, K.M. The Expanding Diagnostic Toolbox for Rare Genetic Diseases. Nat. Rev. Genet. 2024, 1–15. [CrossRef]
- Rillig, F.; Grüters, A.; Schramm, C.; Krude, H. The Interdisciplinary Diagnosis of Rare Diseases. Dtsch. Arzteblatt Int. 2022, 119, 469–475. [CrossRef]
- van Gucht, A.L.M.; Moran, C.; Meima, M.E.; Visser, W.E.; Chatterjee, K.; Visser, T.J.; Peeters, R.P. Resistance to Thyroid Hormone Due to Heterozygous Mutations in Thyroid Hormone Receptor Alpha. Curr. Top. Dev. Biol. 2017, 125, 337–355. [CrossRef]
- Hamajima, T.; Mushimoto, Y.; Kobayashi, H.; Saito, Y.; Onigata, K. Novel Compound Heterozygous Mutations in the SBP2 Gene: Characteristic Clinical Manifestations and the Implications of GH and Triiodothyronine in Longitudinal Bone Growth and Maturation. Eur. J. Endocrinol. 2012, 166, 757–764. [CrossRef]
- Wilpert, N.-M.; Tonduti, D.; Vaia, Y.; Krude, H.; Sarret, C.; Schuelke, M. Establishing Patient-Centered Outcomes for MCT8 Deficiency: Stakeholder Engagement and Systematic Literature Review. Neuropsychiatr. Dis. Treat. 2023, 19, 2195–2216. [CrossRef]
- Watson, M.S.; Lloyd-Puryear, M.A.; Howell, R.R. The Progress and Future of US Newborn Screening. Int. J. Neonatal Screen. 2022, 8, 41. [CrossRef]
- Sterenborg, R.B.T.M.; Steinbrenner, I.; Li, Y.; Bujnis, M.N.; Naito, T.; Marouli, E.; Galesloot, T.E.; Babajide, O.; Andreasen, L.; Astrup, A.; et al. Multi-Trait Analysis Characterizes the Genetics of Thyroid Function and Identifies Causal Associations with Clinical Implications. Nat. Commun. 2024, 15, 888. [CrossRef]
- Thamm, M.; Ellert, U.; Thierfelder, W.; Liesenkötter, K.-P.; Völzke, H. Jodversorgung in Deutschland. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2007, 50, 744–749. [CrossRef]
- Albrecht, D.; Ittermann, T.; Thamm, M.; Grabe, H.-J.; Bahls, M.; Völzke, H. The Association between Thyroid Function Biomarkers and Attention Deficit Hyperactivity Disorder. Sci. Rep. 2020, 10, 18285. [CrossRef]
- Thamm, R.; Liesenkötter, K.-P.; Thamm, M. Jodversorgung von Kindern Und Jugendlichen in Deutschland - Querschnittsergebnisse Aus KiGGS Welle 2 Und Trends. Pädiatr. Prax. 2024, 100, 18–28.
- Quante, M.; Hesse, M.; Döhnert, M.; Fuchs, M.; Hirsch, C.; Sergeyev, E.; Casprzig, N.; Geserick, M.; Naumann, S.; Koch, C.; et al. The LIFE Child Study: A Life Course Approach to Disease and Health. BMC Public Health 2012, 12, 1021. [CrossRef]
- Wallborn, T.; Vogel, M.; Kneuer, A.; Thamm, M.; Dittrich, K.; Kiess, W.; Kratzsch, J. Spot Urine Iodine Levels below the WHO Recommendation Are Not Related to Impaired Thyroid Function in Healthy Children and Adolescents. Eur. J. Nutr. 2021, 60, 493–502. [CrossRef]
- Thierfelder, W.; Dortschy, R.; Hintzpeter, B.; Kahl, H.; Scheidt-Nave, C. [Biochemical measures in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 757–770. [CrossRef]
- Vogel, M. Package ‘Childsds’, Data and Methods Around Reference Values in Pediatrics. 2022, Version 0.8.0, doi:https://cran.r-project.org/web/packages/childsds/childsds.pdf.
- Rigby, R.A.; Stasinopoulos, D.M. Generalized Additive Models for Location, Scale and Shape. J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 507–554, . [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis.; Springer-Verlag New York, 2016; ISBN 978-3-319-24277-4.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).