Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
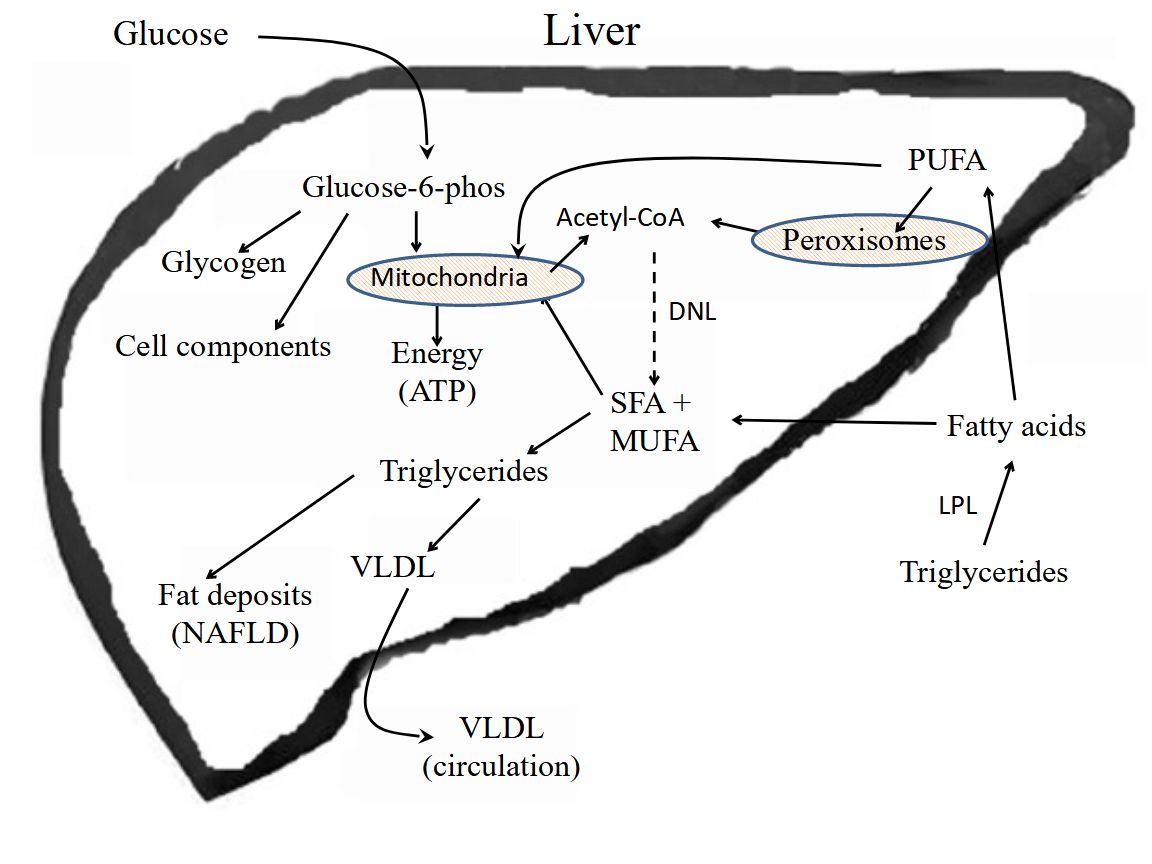
Introduction
Saturated Fats and Inflammation
Obesity and Its Associated Disorders
Conclusion
Contributions and Conflicts of Interest
Conflicts of interest
References
- Kannel WB, Dawber TR, Kagan A, Revostskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med 1961; 55: 33–50.
- Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation 1992; 86: 1046–60. [CrossRef]
- Schatz IJ, Kamal M, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. The Lancet 2001; 358: 351–355. [CrossRef]
- Kirihara Y, Hamazaki K, Hamazaki T, Ogushi Y, Tsuji H, Shirasaki S. The Relationship between Total Blood Cholesterol Levels and All-cause Mortality in Fukui City, and Meta-analysis of This Relationship in Japan. J Lipid Nutr 2008; 17: 67–78. [CrossRef]
- Yi S-W, Yi J-J, Ohrr H. Total cholesterol and all-cause mortality by sex and age: a prospective cohort study among 12.8 million adults. Sci Rep 2019; 9: 1596–1596. [CrossRef]
- Keys A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease. Harvard University Press: Cambridge, MA, 1980.
- Hegsted DM, McGandy RB, Myers ML, Stare FJ. Quantitative effects of dietary fat on serum cholesterol in man. Am J Clin Nutr 1965; 17: 281–95. [CrossRef]
- Malmros H, Wigand G. The effect on serum-cholesterol of diets containing different fats. Lancet 1957; 273: 1–7. [CrossRef]
- Bronte-Stewart B, Antonis A, Eales L, Brock JF. Effects of feeding different fats on serum-cholesterol level. Lancet 1956; 270: 521–6.
- Keys A, Anderson JT, Grande F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957; 273: 959–66.
- Lawrence GD. Dietary fats and health: Dietary recommendations in the context of scientific evidence. Adv Nutr 2013; 4: 294–302. [CrossRef]
- Lawrence GD. Perspective: The Saturated Fat-Unsaturated Oil Dilemma: Relations of Dietary Fatty Acids and Serum Cholesterol, Atherosclerosis, Inflammation, Cancer, and All-Cause Mortality. Adv Nutr 2021; 12: 647–656.
- Le Jossic-Corcos C, Gonthier C, Zaghini I, Logette E, Shechter I, Bournot P. Hepatic farnesyl diphosphate synthase expression is suppressed by polyunsaturated fatty acids. Biochem J 2005; 385: 787–794. [CrossRef]
- Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 2005; 135: 2075–2078. [CrossRef]
- Zinöcker MK, Svendsen K, Dankel SN. The homeoviscous adaptation to dietary lipids (HADL) model explains controversies over saturated fat, cholesterol, and cardiovascular disease risk. Am J Clin Nutr 2021; 113: 277–289. [CrossRef]
- Strand E, Lysne V, Grinna ML, Bohov P, Svardal A, Nygard O et al. Short-Term Activation of Peroxisome Proliferator-Activated Receptors alpha and gamma Induces Tissue-Specific Effects on Lipid Metabolism and Fatty Acid Composition in Male Wistar Rats. PPAR Res 2019; 2019: 8047627.
- Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol 2021; 18: 809–823. [CrossRef]
- Multiple Risk Factor Intervention Trial Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. Jama 1982; 248: 1465–77.
- Multiple Risk Factor Intervention Trial Research Group. Mortality rates after 10.5 years for participants in the Multiple Risk Factor Intervention Trial. Findings related to a priori hypotheses of the trial. The Multiple Risk Factor Intervention Trial Research Group. Jama 1990; 263: 1795–801.
- Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010; 91: 535–46. [CrossRef]
- Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010; 7: e1000252. [CrossRef]
- Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009; 89: 1425–32. [CrossRef]
- Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr 2010; 104: 1586–600.
- Carta G, Murru E, Banni S, Manca C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front Physiol 2017; 8: 902. [CrossRef]
- Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol 2007; 2: 229–237. [CrossRef]
- Cunnane SC, Ryan MA, Nadeau CR, Bazinet RP, Musa-Veloso K, McCloy U. Why is carbon from some polyunsaturates extensively recycled into lipid synthesis? Lipids 2003; 38: 477–84.
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Inv 2006; 116: 3015–25. [CrossRef]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol 2007; 27: 84–91.
- Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F et al. Fatty acids do not pay the toll: Effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis 2012; 11: 175. [CrossRef]
- Maeshima N, Fernandez RC. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol 2013; 3: 3. [CrossRef]
- Dimitrov I, Stankova T, Angelova P, Boyadjiev N, Georgieva K, Dimov I et al. Diet-Induced Early Inflammatory Response of Visceral Adipose Tissue in Healthy Male Wistar Rats. Nutrients 2024; 16: 1184. [CrossRef]
- Wong SW, Kwon M-J, Choi AMK, Kim H-P, Nakahira K, Hwang DH. Fatty acids modulate toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem 2009; 284: 27384–27392. [CrossRef]
- Yaribeygi H, Bo S, Ruscica M, Sahebkar A. Ceramides and diabetes mellitus: an update on the potential molecular relationships. Diabet Med 2020; 37: 11–19. [CrossRef]
- Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008; 47: 348–380. [CrossRef]
- Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 2011; 668 Suppl 1: S50-58. [CrossRef]
- Levental KR, Malmberg E, Symons JL, Fan Y-Y, Chapkin RS, Ernst R et al. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat Commun 2020; 11: 1339. [CrossRef]
- Mortensen MS, Ruiz J, Watts JL. Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis. Cells 2023; 12: 804. [CrossRef]
- G. D. Lawrence. Effect of dietary lipids on adjuvant-induced arthritis in rats. Nutr Res 1990; 10: 283–290. [CrossRef]
- Denko CW. Modification of adjuvant inflammation in rats deficient in essential fatty acids. Agents Actions 1976; 6: 636–641. [CrossRef]
- Chinn KS, Welsch DJ, Salsgiver WJ, Mehta A, Raz A, Obukowicz MG. Modulation of adjuvant-induced arthritis by dietary arachidonic acid in essential fatty acid-deficient rats. Lipids 1997; 32: 979–988. [CrossRef]
- Prickett JD, Trentham DE, Robinson DR. Dietary fish oil augments the induction of arthritis in rats immunized with type II collagen. J Immunol 1984; 132: 725–9. [CrossRef]
- Prickett JD, Robinson DR, Bloch KJ. Enhanced production of IgE and IgG antibodies associated with a diet enriched in eicosapentaenoic acid. Immunol 1982; 46: 819–826.
- Marchix J, Choque B, Kouba M, Fautrel A, Catheline D, Legrand P. Excessive dietary linoleic acid induces proinflammatory markers in rats. J Nutr Biochem 2015; 26: 1434–1441. [CrossRef]
- Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids 2010; 82: 315–318.
- Cunnane SC. Problems with essential fatty acids: time for a new paradigm? Prog Lipid Res 2003; 42: 544–568.
- Raatz SK, Conrad Z, Jahns L. Trends in linoleic acid intake in the United States adult population: NHANES 1999-2014. Prostaglandins Leukot Essent Fatty Acids 2018; 133: 23–28. [CrossRef]
- Twarda-Clapa A, Olczak A, Białkowska AM, Koziołkiewicz M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022; 11: 1312. [CrossRef]
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017; 127: 1–4. [CrossRef]
- Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients 2013; 5: 2019–2027. [CrossRef]
- Adeva-Andany MM, Domínguez-Montero A, Adeva-Contreras L, Fernández-Fernández C, Carneiro-Freire N, González-Lucán M. Body Fat Distribution Contributes to Defining the Relationship between Insulin Resistance and Obesity in Human Diseases. Curr Diabetes Rev 2024; 20: e160823219824. [CrossRef]
- Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016; 125: 259–266. [CrossRef]
- Collins SM, Broadney MM, Ghane N, Davis EK, Jaramillo M, Shank LM et al. Free Fatty Acids as an Indicator of the Nonfasted State in Children. Pediatrics 2019; 143: e20183896. [CrossRef]
- Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab 2012; 97: 3302–3309. [CrossRef]
- Chaaba R, Bouaziz A, Ben Amor A, Mnif W, Hammami M, Mehri S. Fatty Acid Profile and Genetic Variants of Proteins Involved in Fatty Acid Metabolism Could Be Considered as Disease Predictor. Diagnostics (Basel) 2023; 13: 979. [CrossRef]
- Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab 2021; 50: 101238. [CrossRef]
- Kato K, Takamura T, Takeshita Y, Ryu Y, Misu H, Ota T et al. Ectopic fat accumulation and distant organ-specific insulin resistance in Japanese people with nonalcoholic fatty liver disease. PLoS One 2014; 9: e92170. [CrossRef]
- Shigiyama F, Kumashiro N, Furukawa Y, Funayama T, Takeno K, Wakui N et al. Characteristics of hepatic insulin-sensitive nonalcoholic fatty liver disease. Hepatol Commun 2017; 1: 634–647. [CrossRef]
- Fernandes Silva L, Vangipurapu J, Oravilahti A, Männistö V, Laakso M. Plasma Metabolite Signatures in Male Carriers of Genetic Variants Associated with Non-Alcoholic Fatty Liver Disease. Metabolites 2023; 13: 267. [CrossRef]
- Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest 2017; 127: 55–64.
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol 2010; 688: 1–23. [CrossRef]
- Krauss RM, Kris-Etherton PM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: NO. Am J Clin Nutr 2020; 112: 19-24. [CrossRef]
- Kris-Etherton PM, Krauss RM. Public health guidelines should recommend reducing saturated fat consumption as much as possible: YES. Am J Clin Nutr 2020; 112: 13–18. [CrossRef]
- Jaganjac M, Zarkovic N. Lipid Peroxidation Linking Diabetes and Cancer: The Importance of 4-Hydroxynonenal. Antioxid Redox Signal 2022; 37: 1222–1233. [CrossRef]
- Mercola J, D’Adamo CR. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023; 15: 3129. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



