Preprint
Article
Emergency Awake Laparotomy Using Neuraxial Anaesthesia: A Case Series and Literature Review
Altmetrics
Downloads
155
Views
71
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
02 July 2024
Posted:
02 July 2024
You are already at the latest version
Alerts
Abstract
Introduction
Emergency laparotomy is a surgical procedure associated with significantly higher mortality rates compared to elective surgeries. Awake laparotomy under neuraxial anaesthesia has recently emerged as a promising approach in abdominal surgery to improve patient outcomes. This study aims to evaluate the feasibility and potential benefits of using lumbar spinal anaesthesia as the primary anaesthetic technique in emergency laparotomies.
Methods
We conducted a case series involving 16 patients who underwent emergency laparotomy for bowel ischemia, perforation, or occlusion. Lumbar spinal anaesthesia was employed as the main anaes-thetic technique. We analysed patient demographics, clinical characteristics, intraoperative details, and postoperative outcomes. The primary outcome measures included the adequacy of postoper-ative pain control, the incidence of postoperative complications, and mortality rates.
Results
Among the 16 patients, adequate postoperative pain control was achieved, with only 2 patients requiring additional analgesia. Postoperative complications, including sepsis, wound dehiscence, and pneumonia, were observed in 7 patients (44%). The observed mortality rate was relatively low at 6% (1 patient). Notably, conversion to general anaesthesia was not necessary in any of the cases, and no early readmissions were reported.
Discussion
Our findings highlight the feasibility and potential benefits of using neuraxial anaesthesia in emergency laparotomies. The observed low mortality rate and the avoidance of conversion to general anaesthesia suggest that lumbar spinal anaesthesia may be a useful alternative in emer-gency settings. However, the occurrence of postoperative complications in 44% of patients indi-cates the need for cautious patient selection and close monitoring. Further research with larger sample sizes is warranted to fully elucidate the efficacy, safety, and potential impact of this tech-nique on patient outcomes in emergency laparotomies.
Keywords:
Subject: Medicine and Pharmacology - Anesthesiology and Pain Medicine
1. Introduction
Emergency laparotomy is a surgical procedure to manage life-threatening conditions affecting the abdominal cavity. This surgery is frequently associated with a mortality rate that is ten times higher than elective surgeries [1]. Different factors can affect the outcome of emergency laparotomy such as age, ASA status, duration of symptoms, comorbidities, presence of sepsis and organ dysfunctions [2]. The implementation of Early Recovery After Surgery (ERAS) protocols has led to a significant reduction in morbidity and hospital length of stay for patients undergoing elective surgery [3]. Unfortunately, these pathways are still evolving in emergency surgery and more evidence is needed to improve the outcomes in this heterogeneous group of patients [4]. In emergency surgery, the choice of anaesthesia is crucial for reducing mortality rates. For example, the use of ketamine and dexmedetomidine during general anaesthesia can help improve the outcome of elderly patients in emergency surgery [5].
Very little has been published on the potential use of regional anaesthesia. Awake laparotomies using neuraxial anaesthesia could be an innovative alternative to general anaesthesia for emergency abdominal surgery. In fact, even if abdominal surgeries have been traditionally performed under general anaesthesia, neuraxial anaesthesia has been reported as a possible feasible solution to manage high risk patients during emergency surgery [6]. This technique was employed during the COVID-19 pandemic to help prevent aerosolizing the virus during induction of general anaesthesia and the results demonstrated reduced complications and enhanced recovery [7,8]. In fact, COVID-19 pandemic had a significant impact on anaesthesia practice in various fields [9]. Though these studies are interesting, further studies are needed to fully understand the safety and potential benefits of this method, as an alternative to general anaesthesia in emergency surgeries. In this report, we describe our experience using lumbar spinal anaesthesia on a group of patients that underwent emergency laparotomy for bowel ischaemia, perforation with acute peritonitis, or occlusion.
2. Materials and Methods
We present a case series of 16 patients who underwent emergency laparotomy for bowel ischaemia, perforation or occlusion between March 2023 and February 2024. Data were collected prospectively, and the report conformed to the ethical standards of the Declaration of Helsinki. All patients provided written consent to authorize the use and disclosure of their health information for the publication of these anonymized data. No ethical approval was required for this case series, as our institution’s guidelines do not mandate ethical approval for reporting individual cases or case series.
Inclusion and Exclusion Criteria for Neuraxial Anaesthesia (NA)
Patients were included in this study according to the following criteria: emergency laparotomy in patients ≥ 18 years old. Any range of Body Mass Index (BMI) and all ASA scores were accepted. Pregnancy, contraindications to spinal anaesthesia such as severe spinal deformity or disease, metastatic spinal disease, coagulopathy, concurrent use of anticoagulants or refusal to undergo neuraxial anaesthesia (NA) were also exclusion criteria for the study.
Data Collection
Electronic medical records were used to collect demographic and clinical data. Pre- and intra-operative variables such as age, gender, BMI, comorbidities, surgical diagnosis, previous abdominal surgery, length of hospital stay, intra- and post-operative complications and duration of surgery were collected. Pain intensity was evaluated postoperatively at 12, 24, 48 and 72 hours after surgery with the numeric rating scale (NRS). In patients with peritonitis, the Mannheim Peritonitis Index was calculated to evaluate the mortality risk [10]. This score is calculated from 8 different variables such as presence of organ failure, diffuse peritonitis, age >50 years, gender, malignancy, preoperative duration of peritonitis and type of abdominal exudate. The maximum total score is 47 and a value > 26 is frequently used as a cut-off value to identify patients at increased risk of mortality.
Neuraxial Anaesthesia: Technical Procedures
Patients were in the sitting or lateral position depending on patients’ condition to perform NA. Monitoring during and after the surgery and the recovery phase were conducted according to international recommendations [11]. In patients with significant abdominal pain due to peritonitis, a light sedation with ketamine 0.15-0.3mg/kg/h and midazolam 2mg was performed as procedural sedation during spinal anaesthesia. Spinal blocks were performed at the L2-L3 level. In 4 patients, an epidural catheter was inserted at the T9-T10 level for postoperative analgesia. This approach was selected for several reasons. Firstly, due to the anticipated long duration of the surgery, continuous pain management was necessary to ensure patient comfort. Secondly, some of these patients were considered at higher risk due to underlying health conditions or advanced age or limitations on the use of rescue medication due to multiple allergies. Additionally, the use of an epidural catheter aimed to mitigate the hemodynamic impact typically associated with spinal anaesthesia.
For all NA, a bolus of 0.5% hyperbaric Bupivacaine 8-10mg + Dexmedetomidine 10 μg + Morphine 100 μg was injected into the subarachnoid space 5-10 minutes before surgical incision. After spinal injection, patients were placed in 10-15° Trendelenburg position for a few minutes to increase the spread of the injected spinal solution. When the block reached the T4 level (confirmed by pinprick) the Trendelenburg position was removed, and the patient was considered ready for surgery. Prior to surgical incision, an additional pinprick evaluation at the T4 level was used to assess the adequate level of NA in all patients. All the patients received ondansetron 0.1mg/Kg IV to reduce postoperative nausea and vomiting (PONV) and reduce the incidence of hypotension after spinal anaesthesia [12]. In all patients, sedation was performed during surgery with small bolus injections of intravenous midazolam, up to 5mg, and ketamine 0.15-0.3mg/kg/h. Intraoperative fluid infusion was based on goal-directed fluid therapy [13,14].
Statistical Analysis
Due to this study design, only a descriptive statistical analysis was conducted. Continuous variables are reported as median and interquartile range while categorical data as relative number and percentage. The Shapiro–Wilk test was used to evaluate data distribution. Friedman test with Dunn’s correction for multiple comparisons was used to evaluate non-normal distributed repeated measurements. R software v4.2.2 (R Foundation for Statistical Computing, Vienna, Austria, Austria, www.r-project.org) was used for the analyses.
3. Results
Sixteen patients were included in this study. Demographic and clinical variables are reported in Table 1. Ten patients were female (62.5%) while 6 patients (37.5%) were male. The median age was 75 years-old (65-84.5 IQR) and median BMI was 22.8 Kg/m2 (20.8-26.6 IQR). Five patients (31.2%) were classified as ASA 2, 8 patients (50%) as ASA 3, and the remaining 3 patients (18.8%) as ASA 4. A significant number of patients had comorbidities, including hypertension (8 patients), COPD (6 patients), and diabetes type II (4 patients). Eight patients (50%) underwent a previous abdominal surgery before emergency laparotomy, while the other 8 patients (50%) had no previous abdominal surgery. The diagnoses leading to emergency laparotomy included bowel perforations, volvulus, and bowel obstructions due to adhesions or hernias. Specific surgical interventions ranged from segmental resections, such as sigmoid colectomy and ileal resection, to more complex procedures like subtotal colectomy and Hartmann procedures. The median length of hospital stay was 11 days (8-25 IQR) and the median duration of surgery was 119 minutes (80-140 IQR). Notably, neuraxial anaesthesia (NA) was used as the primary anaesthetic technique in all cases, while in 4 patients (25%), an epidural catheter was placed to provide continuous pain management.
An adequate control of pain was obtained during the post operative period, from 12 to 72 hours after surgery. A gradual increase in NRS values was found from 12 to 72 hours after surgery, and though the observed trend was statistically significant (p=0.014), median NRS values were below the cut-off for the use of analgesic rescue medications (Figure 1). Only 2 patients (12%) required postoperative intravenous administration of rescue medications (paracetamol or ketorolac) for postoperative analgesia. No intraoperative hypotension and no complications (nausea, vomiting, coughing or discomfort) were observed. Only 1 patient (6%) required atropine injection for a transient bradycardia. Surgical relaxation was reported as adequate by most (92%) of the operating surgeons.
Six patients (37.5%) had postoperative complications. Three patients (19%%) developed sepsis and septic shock and 2 patients (12%) developed a surgical wound dehiscence requiring reoperation. One patient (6%) developed postoperative pneumonia. Three patients (19%) required postoperative ICU admission. One of them required admission to the ICU postoperatively for a few hours, without the need of mechanical ventilation or vasopressors. One patient was admitted to the ICU with a temporary open abdomen due to bowel ischemia, necessitating a second surgery. Subsequently, this patient was intubated in the ICU without complications, and the second surgery was performed 48 hours later. The third patient was admitted to the ICU with septic shock caused by anastomotic dehiscence five days after surgery, necessitating mechanical ventilation and infusion of vasopressors. Unfortunately, this patient died in the ICU 9 days after surgery. In summary, fifteen patients (94%) were discharged without postoperative symptoms. None of the patients reported post dural puncture headache and no neurological sequelae were observed throughout the post-operative period until discharge. The observed median Mannheim peritonitis index was 25 (21-37 IQR) in 9 patients (56%) with peritonitis; median estimated mortality was 26% (16-64 IQR). Regardless of the predicted mortality, the observed mortality rate was 6%.
4. Discussion
This study suggests the feasibility of lumbar spinal anaesthesia as the main anaesthetic technique for emergency laparotomy in patients with bowel ischaemia, perforation, or occlusion. In fact, our data show that spinal anaesthesia was technically feasible and associated with good intra- and post-operative outcomes. Adequate pain control was achieved during the postoperative period, with minimal need for rescue analgesia. Furthermore, the absence of intraoperative complications and the low incidence of adverse events highlight the safety profile of NA in emergency laparotomy. In high-risk and older patients, the need for rescue analgesia in the postoperative period can pose several significant challenges. These patients often have multiple comorbidities that increase the risks of complications and adverse effects such as hemodynamic instability, gastrointestinal disturbances, renal impairment and postoperative cognitive dysfunction or delirium [15]. Additionally, older patients frequently exhibit altered pharmacokinetics and pharmacodynamics, making it difficult to predict the efficacy and safety of standard dosages of analgesic medications [16]. These complications can prolong hospital stays and recovery times, adding to the overall burden on both the patient and the healthcare system [17].
Spinal anaesthesia has many potential advantages over general anaesthesia. These advantages include a rapid onset, better suppression of stress response with reduced negative cardiocirculatory effects, deep sensory and motor block, avoidance of tracheal intubation and decreased need for postoperative analgesics [18]. All these advantages are particularly useful in elderly and critically ill patients undergoing emergency surgery. Moreover, neuraxial anaesthesia is associated with faster recovery of gastrointestinal transit after surgery, reduced incidence of PONV, decreased intraoperative blood loss and earlier mobilization of patients, with a resultant global cost reduction [3,19]. This approach not only enhances patient comfort and recovery but can also optimizes the utilization of medical resources in countries with limited healthcare resources or in areas with restricted access to medical facilities and supplies.
Locoregional anaesthesia is also associated with reduced postoperative pulmonary and neurocognitive complications and reduced postoperative intensive care admission [20]. By avoiding the use of volatile anaesthetics and minimizing systemic opioid exposure, patients may experience shorter hospital stays and improved overall outcomes [21]. Consequently, neuraxial anaesthesia has been suggested as an alternative to general anaesthesia in high-risk surgical patients undergoing elective abdominal surgery [22]. Indeed, recent articles show the use of spinal anaesthesia or continuous spinal anaesthesia at the thoracic level for various surgeries including laparoscopy [23,24,25,26,27]. However, spinal anaesthesia or continuous spinal anaesthesia was very rarely used for emergency surgery. A significant increase in the use of neuraxial anaesthesia for abdominal surgery was observed only during the COVID-19 pandemic to reduce droplet spread during airway manipulation. For this reason, the Royal College of Anaesthetists promoted the use of regional anaesthesia during the pandemic [28].
Moreover, regional anaesthesia has been recently suggested for postoperative pain management in transplant surgery [29]. Unfortunately, the adoption of this practice often depends on the specific transplant center or the discretion of individual practitioners. The use of combined spinal-epidural anaesthesia was recently suggested in a case report of robotic liver resection for hepatocellular carcinoma in a patient with severe comorbidities [30].
Emergency laparotomy is a surgical procedure performed for specific life-threatening situations such as bowel perforation, intestinal obstruction, traumatic injuries, or acute abdominal pain of uncertain origin. Unlike elective procedures, emergency laparotomy requires immediate intervention to prevent further complications. Different critical elements should be considered while treating patients undergoing emergency laparotomy and a comprehensive management of pre-, intra-, and postoperative care are essential steps [31].
In 2013, the first case report of the use of spinal anaesthesia for urgent laparotomy in a patient with severe myasthenia gravis was published [32]. The patient required urgent laparotomy for ileal perforation due to a 2.5-cm foreign body in the terminal ileum. After spinal anaesthesia at the L2-L3 level with 8 mg of 0.5% hyperbaric bupivacaine and 20μg of fentanyl, the patient underwent a 15-cm ileectomy with mechanical ileocecal anastomosis. No adverse respiratory events or hemodynamic instability was observed, and the patient was successfully discharged 12 days after surgery. Romanzi et al., [6] recently reported the feasible use of neuraxial anaesthesia in patients undergoing awake laparotomy. The authors included 43 patients requiring urgent abdominal surgery and 27 cases of elective abdominal surgery. Neuraxial anaesthesia was performed via combined spinal epidural (CSE) or spinal anaesthesia (SA) in 35.7% and 30% of patients respectively, while 34.3% underwent epidural anaesthesia (EA). Hyperbaric bupivacaine (10mg at 0.5%) and morphine sulphate (100-150mcg) were injected in the subarachnoid space. Sedation was necessary in 24.3% of patients during surgery and 5.7% required a conversion to general anaesthesia. Unfortunately, the authors did not report the level of spinal injection nor the level to which the anaesthetic arrived after allowing for spreading in the cranial direction. Consequently, no direct comparisons with our data are possible. In 2020, the same authors published another article during the first wave of the COVID-19 pandemic, including thirteen high risk (ASA score ≥ 3) patients who required emergency laparotomy [7]. Surgery was performed under different anaesthetic management including CSE, SA or EA. SA was mainly performed at L2-L3 or L3-L4 levels with hyperbaric 0.5% bupivacaine and morphine sulphate. Most of the included patients underwent CSE along with additional sedation. The authors reported good intra- and post-operative outcomes supporting the possible use of regional anaesthesia for awake laparotomy. Farda et al., [33] reported the use of SA for emergency laparotomy in Kabul, Afghanistan, in a place characterized by challenging conditions and healthcare limited resources. This article represents the most significant publication on the topic, since 196 patients underwent emergency laparotomy with SA at the L2-L3 or L3-L4 level. The authors used 15 mg of bupivacaine, injected in the subarachnoid space without other adjuvants such as morphine or fentanyl. However, a high incidence of hypotension (12.7%) was reported.
We used neuraxial anaesthesia in patients with acute abdominal pathology requiring urgent surgical intervention (bowel ischaemia, perforation with acute peritonitis, or occlusion). No patient required conversion to general anaesthesia. We decided to perform SA with low-dose hyperbaric bupivacaine (8-10mg) because it has a more predictable cephalad spread during Trendelenburg position after spinal injection [34]. Moreover, using the Trendelenburg position immediately after injection is advantageous because it ensures venous return, thereby maintaining cardiac output and blood pressure. We decided to add dexmedetomidine as an adjuvant since it can shorten the onset time of spinal anaesthesia, prolong the block duration, and decrease the occurrence of shivering [35,36]. None of the patients experienced nausea and vomiting during surgery, probably due to the administration of pre-emptive antiemetics and the lack of postoperative opioids. In four patients a thoracic epidural catheter was combined with subarachnoid anaesthesia. In the first patient of our case series, the epidural catheter was added as a rescue therapy to potentially manage intra- and post-operative pain, due to a lack of sufficient experience in managing emergency laparotomy with spinal anaesthesia alone. In two patients, the epidural catheter was inserted due to the presence of significant comorbidities requiring lower doses of local anaesthetic in the subarachnoid space, or due to the expected surgical complexity with potentially intense postoperative pain. In the fourth patient, an epidural catheter was inserted to enhance postoperative pain management, given the limitations on the use of rescue medication due to multiple allergies, including non-steroidal anti-inflammatory drugs.
CSA was used by Pereira et al. [37] and Niraj et al. [38] for emergency laparotomy. The authors injected small amounts of 0.5% hyperbaric bupivacaine followed by small doses of 0.5 % isobaric levo-bupivacaine until a T6 dermatomal block was reached. Even if this technique was considered feasible and effective, a high risk of conversion to general anaesthesia due to accidental displacement of the spinal catheter was reported.
One of the most prevalent indications for considering regional anaesthesia is the respiratory function of the patient. Patients with significant underlying respiratory disease have a greater risk of prolonged post-operative ventilation following general anaesthesia [32]. Almost 70% of our patients were classified as ASA 3 or 4 due to multiple comorbidities and COPD was the most common comorbidity.
A recent publication showed that thoracic epidural anaesthesia could be another feasible option for patients with severe pulmonary disease requiring an awake emergency laparotomy for bowel ischaemia in the absence of postoperative intensive care monitoring [39]. Though this is certainly an interesting alternative to general anaesthesia and NA, the article is only a single patient case study.
Our preliminary data suggest a possible role of SA as the main anaesthetic technique for emergency laparotomy, though this topic necessitates careful considerations. Such considerations include patient selection criteria, anatomical difficulties, procedural feasibility, standardized protocols, and anaesthesia provider expertise. Consequently, the safety and efficacy of spinal anaesthesia in emergency awake laparotomy require further investigation through well-designed prospective studies with standardized protocols and comparative analyses against traditional techniques. Moreover, further studies with long-term follow-up are needed to better evaluate the feasibility of SA in emergency laparotomy and to confirm these preliminary findings.
Despite the insights provided by this study, several limitations warrant consideration. First, the small sample size and single-centre nature of the study may limit the generalizability of these findings. Second, the absence of a control group receiving general anaesthesia precludes direct comparisons of outcomes between different anaesthesia modalities. Moreover, the lack of long-term follow-up limits the comprehensive assessment of long-term outcomes following emergency laparotomy under SA. Furthermore, while lumbar SA shows promise as an alternative anaesthetic technique, its applicability may be constrained by patient-specific factors, anatomical considerations, and the expertise of anaesthesia providers as previously reported [40]. In addition, the exclusion of patients with severe spinal deformity or disease and specific contraindications to SA may introduce selection bias and limit the external validity of this study. Finally, another significant limitation of this study is the lack of a comprehensive anaesthetic risk assessment using validated scoring systems such as the Portsmouth Physiologic and Operative Severity Score for the Enumeration of Mortality and Morbidity (P-POSSUM) [41], the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) [42], or the Charleston Comorbidity Index 2 [43]. Unfortunately, the quantification of these scores was not feasible due to missing variables.
5. Conclusions
Our preliminary data support the use of lumbar spinal anaesthesia as a viable alternative to general anaesthesia for emergency laparotomy in patients with bowel ischemia, perforation with acute peritonitis, or obstruction. No intraoperative complications were observed, and an adequate control of pain was achieved during the post-operative period. None of the patients reported post dural puncture headache and no neurological sequelae were observed during the post-operative period. Future prospective studies with adequate sample size are needed to confirm these preliminary findings and to elucidate optimal patient selection criteria and procedural protocols for maximizing the benefits of awake laparotomy under SA.
Author Contributions
Conceptualization, T.R., M.L.G.L. and R.M.C.; methodology, formal analysis, M.L.G.L.; data curation, M.L.G.L.; writing—original draft preparation, M.L.G.L.; writing—review and editing, G.C, D.M.A, G.V, G.C., P.C., M.M., R.M.C.; visualization, R.M.C.; supervision, M.M., G.V; All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. No ethical approval was re-quired for this case series, as our institution’s guidelines do not mandate ethical approval for reporting individual cases or case series.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aggarwal, G.; Scott, M.; Peden, C.J. Emergency Laparotomy, Anesthesiol. Clin. 40 (2022) 199–211. [CrossRef]
- Ahmed, A.; Azim, A. Emergency Laparotomies: Causes, Pathophysiology, and Outcomes, Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 24 (2020) S183–S189. [CrossRef]
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.B.W.; Fearon, K.C.H.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; Miller, T.; Radtke, F.F.; Garces, T.R.; Schricker, T.; Scott, M.J.; Thacker, J.K.; Ytrebø, L.M.; Carli, F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice, Acta Anaesthesiol. Scand. 60 (2016) 289–334. [CrossRef]
- Paduraru, M.; Ponchietti, L.; Casas, I.M.; Svenningsen, P.; Zago, M. Enhanced Recovery after Emergency Surgery: A Systematic Review. Bull. Emerg. Trauma 2017, 5, 70–78. [Google Scholar] [PubMed]
- Ghazaly, H.F.; Hemaida, T.S.; Zaher, Z.Z.; Elkhodary, O.M.; Hammad, S.S. A pre-anesthetic bolus of ketamine versus dexmedetomidine for prevention of postoperative delirium in elderly patients undergoing emergency surgery: a randomized, double-blinded, placebo-controlled study, BMC Anesthesiol. 23 (2023) 407. [CrossRef]
- Romanzi, A.; Dragani, T.A.; Adorni, A.; Colombo, M.; Farro, A.; Maspero, M.; Zamburlini, B.; Vannelli, A. Neuraxial anesthesia for abdominal surgery, beyond the pandemic: a feasibility pilot study of 70 patients in a suburban hospital, Updat. Surg. 75 (2023) 1691–1697. [CrossRef]
- Romanzi, A.; Boleso, N.; Di Palma, G.; La Regina, D.; Mongelli, F.; Milanesi, M.; Putortì, A.; Rossi, F.; Scolaro, R.; Zanardo, M.; Vannelli, A. Awake Major Abdominal Surgeries in the COVID-19 Era, Pain Res. Manag. 2021 (2021) 8763429. [CrossRef]
- Romanzi, A.; Galletti, M.; Macchi, L.; Putortì, A.; Rossi, F.; Scolaro, R.; Vannelli, A. Awake laparotomy: is locoregional anesthesia a functional option for major abdominal surgeries in the COVID-19 era?, Eur. Rev. Med. Pharmacol. Sci. 24 (2020) 5162–5166. [CrossRef]
- Bianco, G.L.; Papa, A.; Schatman, M.E.; Tinnirello, A.; Terranova, G.; Leoni, M.L.G.; Shapiro, H.; Mercadante, S. Practical Advices for Treating Chronic Pain in the Time of COVID-19: A Narrative Review Focusing on Interventional Techniques, J. Clin. Med. 10 (2021) 2303. [CrossRef]
- Pathak, A.A.; Agrawal, V.; Sharma, N.; Kumar, K.; Bagla, C.; Fouzdar, A. Prediction of mortality in secondary peritonitis: a prospective study comparing p-POSSUM, Mannheim Peritonitis Index, and Jabalpur Peritonitis Index, Perioper. Med. Lond. Engl. 12 (2023) 65. [CrossRef]
- Gelb, A.W.; Morriss, W.W.; Johnson, W.; Merry, A.F. International Standards for a Safe Practice of Anesthesia Workgroup, World Health Organization-World Federation of Societies of Anaesthesiologists (WHO-WFSA) International Standards for a Safe Practice of Anesthesia, Can. J. Anaesth. J. Can. Anesth. 65 (2018) 698–708. [CrossRef]
- Hou, X.-M.; Chen, Y.-J.; Lai, L.; Liu, K.; Shen, Q.-H. Ondansetron Reduces the Incidence of Hypotension after Spinal Anaesthesia: A Systematic Review and Meta-Analysis, Pharm. Basel Switz. 15 (2022) 1588. [CrossRef]
- Kendrick, J.B.; Kaye, A.D.; Tong, Y.; Belani, K.; Urman, R.D.; Hoffman, C.; Liu, H. Goal-directed fluid therapy in the perioperative setting, J. Anaesthesiol. Clin. Pharmacol. 35 (2019) S29–S34. [CrossRef]
- Pavlovic, G.; Diaper, J.; Ellenberger, C.; Frei, A.; Bendjelid, K.; Bonhomme, F.; Licker, M. Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial, J. Clin. Monit. Comput. 30 (2016) 87–99. [CrossRef]
- Shellito, A.D.; Dworsky, J.Q.; Kirkland, P.J.; Rosenthal, R.A.; Sarkisian, C.A.; Ko, C.Y.; Russell, M.M. Perioperative Pain Management Issues Unique to Older Adults Undergoing Surgery: A Narrative Review, Ann. Surg. Open 2 (2021) e072. [CrossRef]
- McLachlan, A.J.; Bath, S.; Naganathan, V.; Hilmer, S.N.; Le Couteur, D.G.; Gibson, S.J.; Blyth, F.M. Clinical pharmacology of analgesic medicines in older people: impact of frailty and cognitive impairment, Br. J. Clin. Pharmacol. 71 (2011) 351–364. [CrossRef]
- Cohen, M.E.; Bilimoria, K.Y.; Ko, C.Y.; Richards, K.; Hall, B.L. Variability in length of stay after colorectal surgery: assessment of 182 hospitals in the national surgical quality improvement program, Ann. Surg. 250 (2009) 901–907. [CrossRef]
- Kettner, S.C.; Willschke, H.; Marhofer, P. Does regional anaesthesia really improve outcome?, Br. J. Anaesth. 107 Suppl 1 (2011) i90-95. [CrossRef]
- Salicath, J.H.; Yeoh, E.C.; Bennett, M.H. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults, Cochrane Database Syst. Rev. 8 (2018) CD010434. [CrossRef]
- Hutton, M.; Brull, R.; Macfarlane, A.J.R. Regional anaesthesia and outcomes, BJA Educ. 18 (2018) 52–56. [CrossRef]
- Goff, J.; Hina, M.; Malik, N.; McLardy, H.; Reilly, F.; Robertson, M.; Ruddy, L.; Willox, F.; Forget, P. Can Opioid-Free Anaesthesia Be Personalised? A Narrative Review, J. Pers. Med. 13 (2023) 500. [CrossRef]
- Guay, J.; Choi, P.; Suresh, S.; Albert, N.; Kopp, S.; Pace, N.L. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews, Cochrane Database Syst. Rev. 2014 (2014) CD010108. [CrossRef]
- Kapala, M.; Meterissian, S.; Schricker, T. Neuraxial anesthesia and intraoperative bilevel positive airway pressure in a patient with severe chronic obstructive pulmonary disease and obstructive sleep apnea undergoing elective sigmoid resection, Reg. Anesth. Pain Med. 34 (2009) 69–71. [CrossRef]
- Major, A.L.; Jumaniyazov, K.; Yusupova, S.; Jabbarov, R.; Saidmamatov, O.; Mayboroda-Major, I. Removal of a Giant Cyst of the Left Ovary from a Pregnant Woman in the First Trimester by Laparoscopic Surgery under Spinal Anesthesia during the COVID-19 Pandemic, Med. Sci. Basel Switz. 9 (2021) 70. [CrossRef]
- Major, A.L.; Jumaniyazov, K.; Yusupova, S.; Jabbarov, R.; Saidmamatov, O.; Mayboroda-Major, I. Laparoscopy in Gynecologic and Abdominal Surgery in Regional (Spinal, Peridural) Anesthesia, the Utility of the Technique during COVID-19 Pandemic, Med. Basel Switz. 8 (2021) 60. [CrossRef]
- Vincenzi, P.; Starnari, R.; Faloia, L.; Grifoni, R.; Bucchianeri, R.; Chiodi, L.; Venezia, A.; Stronati, M.; Giampieri, M.; Montalti, R.; Gaudenzi, D.; De Pietri, L.; Boccoli, G. Continuous thoracic spinal anesthesia with local anesthetic plus midazolam and ketamine is superior to local anesthetic plus fentanyl in major abdominal surgery, Surg. Open Sci. 2 (2020) 5–11. [CrossRef]
- Spannella, F.; Giulietti, F.; Damiani, E.; Faloia, L.; Stronati, M.; Venezia, A.; Vincenzi, P.; Castellani, D.; Boccoli, G.; Dellabella, M.; Giampieri, M.; Sarzani, R.; Starnari, R. Thoracic continuous spinal anesthesia for high-risk comorbid older patients undergoing major abdominal surgery: one-year experience of an Italian geriatric hospital, Minerva Anestesiol. 86 (2020) 261–269. [CrossRef]
- Macfarlane, A.J.R.; Harrop-Griffiths, W.; Pawa, A. Regional anaesthesia and COVID-19: first choice at last?, Br. J. Anaesth. 125 (2020) 243–247. [CrossRef]
- Ander, M.; Mugve, N.; Crouch, C.; Kassel, C.; Fukazawa, K.; Isaak, R.; Deshpande, R.; McLendon, C.; Huang, J. Regional anesthesia for transplantation surgery - A White Paper Part 2: Abdominal transplantation surgery, Clin. Transplant. 38 (2024) e15227. [CrossRef]
- Delvecchio, A.; Pavone, G.; Conticchio, M.; Piacente, C.; Varvara, M.; Ferraro, V.; Stasi, M.; Casella, A.; Filippo, R.; Tedeschi, M.; Pullano, C.; Inchingolo, R.; Delmonte, V.; Memeo, R. Awake robotic liver surgery: A case report, World J. Gastrointest. Surg. 15 (2023) 2954–2961. [CrossRef]
- Ilyas, C.; Jones, J.; Fortey, S. Management of the patient presenting for emergency laparotomy, BJA Educ. 19 (2019) 113–118. [CrossRef]
- Rodríguez, M.A.P.; Mencía, T.P.; Alvarez, F.V.; Báez, Y.L.; Pérez, G.M.S.; García, A.L. Low-dose spinal anesthesia for urgent laparotomy in severe myasthenia gravis, Saudi J. Anaesth. 7 (2013) 90–92. [CrossRef]
- Wais Farda, Ahmad Bashir Nawazish, EMERGENCY LAPAROTOMIES UNDER SPINAL ANESTHESIA: A RETROSPECTIVE, FACILITY BASED OBSERVATIONAL STUDY, IN KABUL, AFGHANISTAN, Int. J. Adv. Res. (n.d.). https://www.journalijar.com/article/ (accessed May 7, 2024).
- Hocking, G.; Wildsmith, J.A.W. Intrathecal drug spread, Br. J. Anaesth. 93 (2004) 568–578. [CrossRef]
- Shen, Q.-H.; Li, H.-F.; Zhou, X.-Y.; Yuan, X.-Z.; Lu, Y.-P. Dexmedetomidine as an adjuvant for single spinal anesthesia in patients undergoing cesarean section: a system review and meta-analysis, J. Int. Med. Res. 48 (2020) 300060520913423. [CrossRef]
- Usta, B.; Gozdemir, M.; Demircioglu, R.I.; Muslu, B.; Sert, H.; Yaldiz, A. Dexmedetomidine for the prevention of shivering during spinal anesthesia, Clin. Sao Paulo Braz. 66 (2011) 1187–1191. [CrossRef]
- Pereira, A.P.M.; Teixeira, F.J.M.; Pereira, E.N.; Sampaio, J.C.P.; Gonçalves, D.C.R.P. Emergency exploratory laparotomy under continuous spinal anaesthesia in a patient with severe pulmonary comorbidities: A case-report, Anesth. Analg. (2021) 1730–1730.
- Niraj, G.; Basar, S.H.M.A.; Warusawitharana, C.; Sebastian, S.; Camacho, E.; Internationals, O. Continuous Spinal Anaesthesia (CSA) for Emergency Laparotomy in High-Risk Elderly patients: Technique and Outcomes of a Prospective Service Evaluation, J. Anesth. Surg. 4 (2017) 0–0.
- Le Roux, J.J.; Wakabayashi, K.; Jooma, Z. Emergency Awake Abdominal Surgery Under Thoracic Epidural Anaesthesia in a High-Risk Patient Within a Resource-Limited Setting, Cureus 15 (2023) e34856. [CrossRef]
- Del Buono, R.; Pascarella, G.; Costa, F.; Terranova, G.; Leoni, M.L.; Barbara, E.; Carassiti, M.; Agrò, F.E. Predicting difficult spinal anesthesia: development of a neuraxial block assessment score, Minerva Anestesiol. 87 (2021) 648–654. [CrossRef]
- Prytherch, D.R.; Whiteley, M.S.; Higgins, B.; Weaver, P.C.; Prout, W.G.; Powell, S.J. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity, Br. J. Surg. 85 (1998) 1217–1220. [CrossRef]
- McNelis, J.; Castaldi, M. “The National Surgery Quality Improvement Project” (NSQIP): a new tool to increase patient safety and cost efficiency in a surgical intensive care unit, Patient Saf. Surg. 8 (2014) 19. [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J. Chronic Dis. 40 (1987) 373–383. [CrossRef]
Figure 1.
Postoperative pain intensity (NRS) at 12, 24, 48 and 72 hours after surgery. Effective pain control was achieved postoperatively from 12 to 72 hours after surgery. Although there was a gradual and statistically significant increase in NRS over this period (p=0.014), the median NRS values remained below the threshold that would require additional analgesic intervention.
Figure 1.
Postoperative pain intensity (NRS) at 12, 24, 48 and 72 hours after surgery. Effective pain control was achieved postoperatively from 12 to 72 hours after surgery. Although there was a gradual and statistically significant increase in NRS over this period (p=0.014), the median NRS values remained below the threshold that would require additional analgesic intervention.
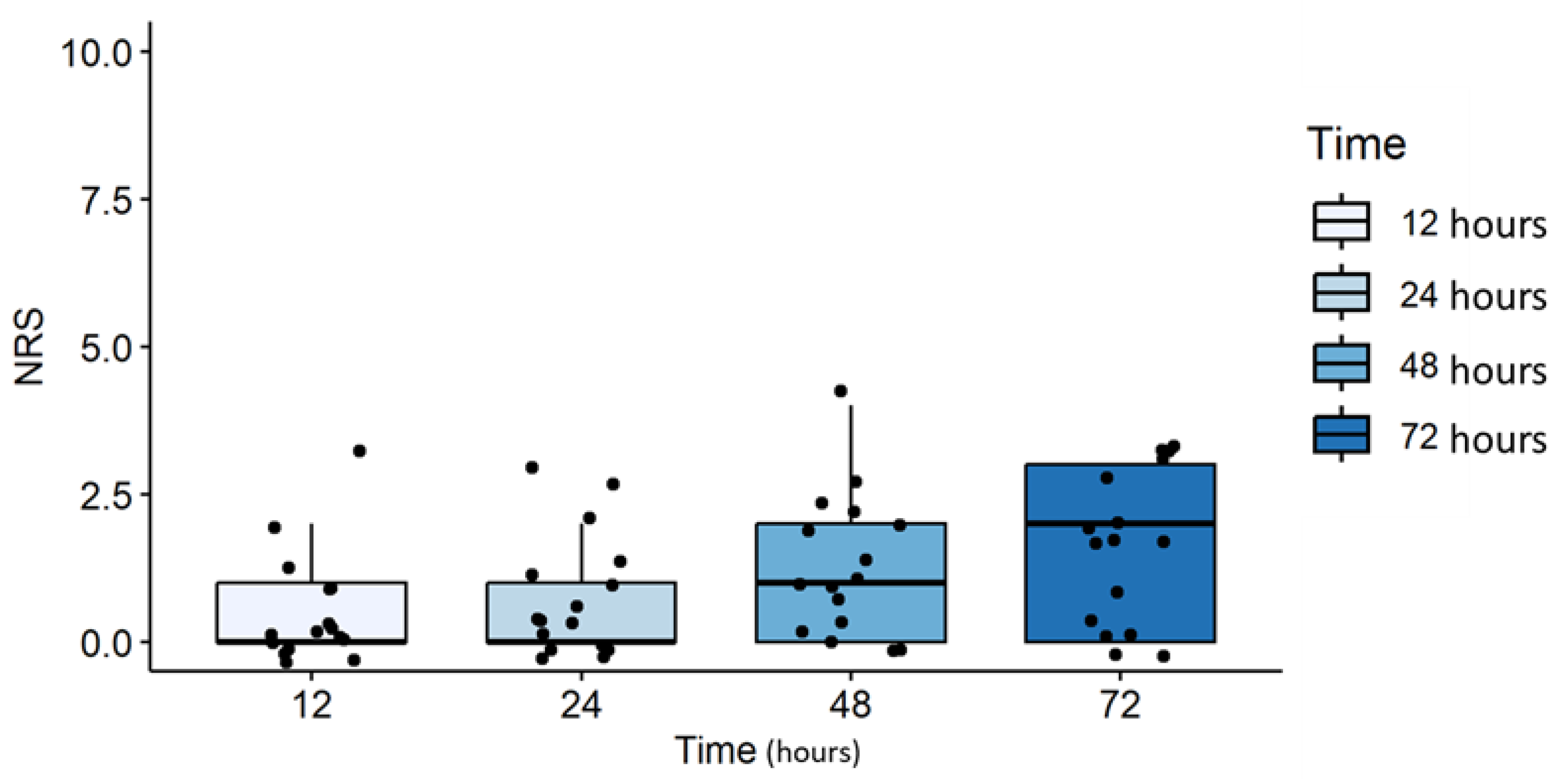
Table 1.
The table presents demographic and clinical data for 16 patients who underwent emergency laparotomy under neuraxial anaesthesia (NA).
Table 1.
The table presents demographic and clinical data for 16 patients who underwent emergency laparotomy under neuraxial anaesthesia (NA).
| Variable | Age | Gender | ASA | Comorbidities | Previous abdominal surgery |
Diagnosis | Surgery performed |
Anaesthesia |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 41 | M | 2 | None | No | Perforated Diverticulitis | Sigmoid colectomy + protective ileostomy | NA + EA |
| Patient 2 | 33 | F | 2 | None | Yes | Biliary Peritonitis | Ileal resection + ileo-ileal anastomosis | NA |
| Patient 3 | 71 | M | 2 | None | No | Tumour bowel perforation | Subtotal colectomy with ileosigmoid anastomosis | NA |
| Patient 4 | 73 | F | 3 | Hypertension, Hypothyroidism | Yes | Intraperitoneal sepsis due to anastomotic leakage | Left colectomy + protective ileostomy + cholecystectomy | NA |
| Patient 5 | 72 | M | 3 | Stroke with left hemiparesis, Hypertension, Prostatic Hypertrophy, COPD | Yes | Anastomotic leakage | Ileocolic resection + Ileo ascending colon anastomosis | NA |
| Patient 6 | 85 | M | 3 | Hypertension | Yes | Abdominal adhesions with intestinal obstruction | Lysis of adhesions | NA |
| Patient 7 | 77 | M | 3 | Obesity, Diabetes type II, Chronic liver disease, COPD | No | Volvulus | Sigmoid colectomy + protective ileostomy | NA |
| Patient 8 | 60 | F | 2 | None | No | Malignant bowel obstruction | Right hemicolectomy | NA |
| Patient 9 | 85 | F | 4 | COPD, rheumatoid arthritis, hypertension | No | Perforated Diverticulitis | Hartmann procedure | NA |
| Patient 10 | 89 | F | 4 | Dilatative cardiomyopathy with reduced ejection fraction (20%), fibrotic interstitial disease, hypothyroidism, diverticulosis, Parkinson disease | Yes | Volvulus | Ileal resection + ileo-ileal anastomosis | NA + EA |
| Patient 11 | 84 | F | 3 | Hypertension | No | Colon cancer with perforation + vaginal fistula | Hartmann procedure | NA + EA |
| Patient 12 | 77 | M | 3 | Obesity, Diabetes type II, Depression, Chronic liver disease, COPD | Yes | Bowel obstruction due to abdominal adhesions | Lysis of adhesions + ileostomy | NA |
| Patient 13 | 84 | F | 3 | COPD, Hypertension | No | Colon cancer with perforation | Right colectomy with ileotransverse astomosis + sigmoid colectomy with colostomy | NA |
| Patient 14 | 90 | F | 4 | Obesity, Diabetes type II, Hypertension, COPD | Yes | Bowel occlusion in incarcerated incisional Hernia | Lysis of adhesions + hernia repair | NA |
| Patient 15 | 48 | F | 3 | Tracheomalacia, renal tubulopathy, diabetes type 2, hypertension, asthma, myocardial fibrosis (preserved ejection fraction), sarcoidosis, Bechet vasculitis | Yes | Acute diverticulitis with sigma perforation | Left colectomy + protective ileostomy | NA + EA |
| Patient 16 | 70 | F | 2 | Hypertension, Chronic Diverticulitis | No | Volvulus | Ileal resection for bowel ischemia | NA |
* Abbreviation: F, female; M, male; ASA, American Society of Anaesthesiologists (ASA) score; COPD chronic obstructive pulmonary disease; EA, epidural analgesia.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







