1. Introduction
Burn wound infections are generally associated with poly-microbial, multi-drug resistant bacterial pathogens. Predominant bacterial isolates of burn wound infections were
Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and
Escherichia coli. Co-infection of these multi-drug resistant bacteria leads to the worse patient outcome. A high level of drug resistance and ability of in vivo biofilm formation, bacterial virulence, and immune evasion contributes to high mortality rates. Biofilms are densely packed communities of microorganisms growing on biotic and abiotic surfaces or surrounding themselves by secreting extracellular polymers [
1]. Within a biofilm, the bacteria communicate with each other by producing chemotactic factors or pheromones. This phenomenon is called quorum sensing [
2]. Bacteria move towards surfactants by chemotaxis; surface adhesions and the presence of surfactants are responsible for forming biofilms, which is one of the critical survival strategies of pathogens [
3,
4]. The formation of biofilms will begin when the bacteria sense unfavorable environmental conditions that trigger the transition to live on those surfaces. The structural and physiological complexity of biofilms has led to an idea enforced on coordinated and cooperated groups and analogs to multicellular organisms [
5,
6]. In humans, biofilms are responsible for developing many diseases, most of which are associated with medical devices. Significant problems of biofilms are their inherent tolerance to defense mechanisms and antibiotic therapy. Therefore, there is an urgent need to manifest alternative ways to prevent or control biofilm-associated infections [
7,
8].
Microorganisms in a biofilm are intrinsically more resistant to antimicrobial agents than planktonic cells [
9,
10]. A high dose of antimicrobial agents is required to inactivate the biofilm growth. According to the national institute of health (NIH) report, more than 80% of infections associated with biofilms are dental plaques, urogenital tract infections, peritonitis, and urogenital infections [
3,
11]. Both gram-positive and gram negative can form biofilms, including
Staphylococcus aureus, Streptococcus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and
proteus species. Pathogenic bacterial biofilms have been associated as a source of infection, and often it's challenging to treat with available antibiotics because they develop resistance to present antimicrobial treatment mechanisms and often act as a source of a high number of bacterial communities. The bacteria encased in the biofilm showed an elevated drug resistance nature and were even tricky for the host immune system to clear [
12,
13].
Several strategies are currently used to eradicate biofilm formation and minimize the microbial load on the infectious sites. One of the widely employed methods for biofilm treatment is bacteriophage therapy [
14]. Bacteriophages were used to treat bacterial diseases in plants, animals, and humans [
15]. The use of bacteriophages to control biofilms is one of the best methods because of its merits. Phages can replicate at the site of infection, thereby increasing the number of progenies, where the bacterial load is predominant, and biofilm is formed.
Moreover, a single virion will produce hundreds of progeny phages, and most the bacteriophages can produce degrading enzymes for the extracellular polysaccharides of bacteria. Hence, Bacteriophage possesses unique properties and shows considerable promise in controlling biofilms. However, such applications are still evolving in large-scale production or under development [
16,
17,
18]. Thus, identification is the most effective approach or required and speculative nature to reach the best practices for appropriate use. Biofilms are formed by associating bacterial communities on solid surfaces; bacterial cells are attached to the surface because of their extracellular polymeric matrix. Biofilms are generally multi-species in nature. Biofilms can adhere to a wide variety of biotic or abiotic surfaces such as human tissues, medical devices, and plastic apparatus, causing infections and economic burden. Current antibiotics and disinfectants have limited efficacy on biofilms; in this situation, phage or phage cocktails are proposed promising alternatives for biofilm eradication.
In this present study, we are mainly attentive to showing the significant comparative lytic effect of phage and phage cocktail on biofilm of single-species biofilm of P. aeruginosa, S. aureus, and interspecies competition of dual-species (P. aeruginosa + S. aureus) combination by employing scanning electron and confocal laser scanning electron microscopic studies, isolated from burn wound infections.
2. Methods and Materials
2.1. Bacterial Strains, Bacteriophages, and Growth Conditions
MDR-bacterial isolates were isolated from patients with burn wound infections and were previously reported to be used for this study [
19]. The experiments performed in this article were approved by the
Institutional Review Board (IEC). P. aeruginosa, S. aureus, were selected and grown on Luria agar (Himedia, Mumbai) at 37
°C. Bacteriophages vB_PAnP_PADP4 for (
P. aeruginosa) and vB_SAnS_SADP1 for (
S. aureus) were isolated as described previously [
20]. The bacteriophages were stored in salts of magnesium (SM) buffer (5.8 g /L NaCl, 2 g/L MgSO
4.7H
2O, 50 ml /L 1M-Tris-HCl pH 7.5) at 4
°C. The biofilm staining was performed using FilmTracer™ LIVE/DEAD
® Biofilm viability kit (Molecular Probes, Life Technologies Ltd.) according to the instructions provided by the manufacturer.
2.2. Determination of Biofilm Biomass of Single or Dual Species
To determine the bacteriophage inhibitory effect on the single or dual-species biofilms, 100 μL of bacterial culture (
S. aureus/
P. aeruginosa) and 100 μL of respective bacteriophages (10
9 PFU) were added to 24 well culture plate for single-species biofilm assay. For the dual-species study, the following combinations of bacterial cultures and bacteriophages were used as mentioned, 100 μL of
S. aureus + 100 μL of
P. aeruginosa (100 μL of SM buffer, 100 μL of phage SADP1 + 100 μL of Phage PADP4) and SM buffer were used as a control instead of bacteriophages. The above sets of cultures were incubated for 24 h at 37°C, and other groups were incubated with respective phage and phage cocktails for four h to determine the phage effect on biofilm biomass of single or dual species. After incubation, the planktonic bacteria were removed by washing twice with PBS buffer. A crystal violet assay measured the biomass attached to each well in 24 well tissue culture plates. The wells were washed four times with PBS-(pH 7.4), then biofilms were fixed with 200 µL of methanol for 15 min. Methanol was removed, and to each well was added 200 µL of crystal violet (1% v/v, Qualigens, Mumbai) and incubated for 15 min. The wells were then washed with water and dried for two h at room temperature, and 300 µL of ethanol (95%) was added to dissolve the stain. The absorbance of eluted stain was measured at 570 nm with a benchmark plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA), and triplicates were maintained [
21,
22].
Biofilms were grown on borosilicate glass coverslips earlier placed into the wells of a 24-well microtiter plate. Single and dual-species biofilm formed on the coverslips were incubated with 100 µL of respective bacteriophages (10
9 PFU) for four h. After treatment, the coverslips were washed twice with PBS and dried in an incubator for 20 h at 37°C. The biofilms coated on glass slides were fixed with glutaraldehyde (2.5%) and dehydrated through a series of graded ethanol (30-100%) for five minutes. Further, the glass slides were sputtered with gold after critical point drying, and the aggregated biofilms were examined using Scanning electron microscopy (FEI, Tecnai G-2S Twin) [
23,
24,
25].
- 2.
Confocal Laser Scanning Microscopy
24 h old biofilms of MDR-bacterial isolates of burn wounds and their respective bacteriophage treated (4 h) slides were stained with Syto ®9 stain and propidium iodide nucleic acid dyes. Briefly, a working solution of fluorescent stains was prepared by adding 3 µL of Syto ®9 stains and 3 µL of propidium iodide (PI) stain to 1 mL of filter-sterilized water. 200 µL of staining solution was deposited on a glass coverslip surface coated with biofilms and treated with respective phages. After 15 min incubation at room temperature in the dark, samples were washed with sterile saline to remove the excess dye and rinsed with water from the base of the support material.
MDR-bacterial biofilms and respective phage-treated coverslips were subjected to CLSM to detect the effect of bacteriophages on the MDR-bacterial biofilms. The staining with FilmTracer™ LIVE/DEAD
® Biofilm viability kit (Molecular Probes, Life Technologies Ltd.) was performed according to the instructions provided by the manufacturer [
26].
3. Results
Lytic phages can lyse the bacteria, release progeny, and gradually spread around them, inhibiting bacterial growth and cell number. Indirectly phage lytic action on bacteria decreases the biomass of biofilms. Since biofilms incubated for four h with phage and phage cocktail showed decreased biofilm biomass, we used SEM and CLSM to gather more evidence on the morphological changes during this process.
3.1. Phage or Phage Cocktail Action on Biofilm Biomass
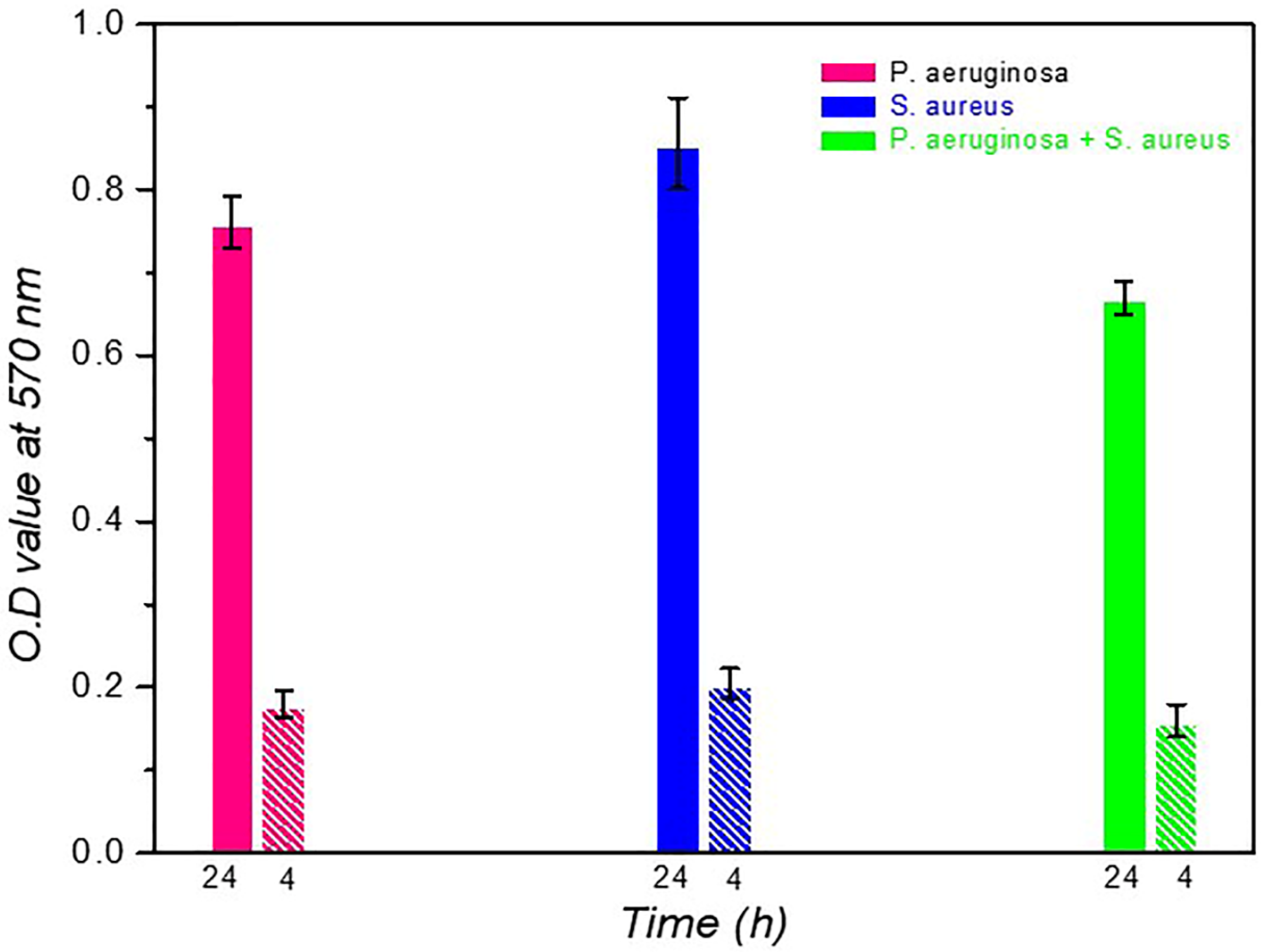
S. aureus produced a higher amount of biomass (0.856) than
P. aeruginosa (0.761). In contrast, in dual-species, combinations of
S. aureus +
P. aeruginosa (0.67) produced low concentrations of biomass than the individual states. Interspecies competition between these gram-positive and gram-negative bacteria may be responsible for decreased OD values at 37
oC at 24 h of incubation. The phage lytic action was measured on single and dual species at 37
oC for four h of incubation. The phage cocktail showed efficient bacteriolytic activity; its OD value (0.16 ±0.02) is much less than in the individual state documented in
Table 1. The reduced biomass was noticed in the presence of a phage cocktail, represented in
Figure 1. 4 h of phage incubation with single or dual-species biofilms were effectively removed, a 2-fold reduction of biofilm biomass.
3.2. Determination of Phage or Phage Cocktail Lytic Action on Single or Dual-Species Biofilm by Using SEM and CLSM
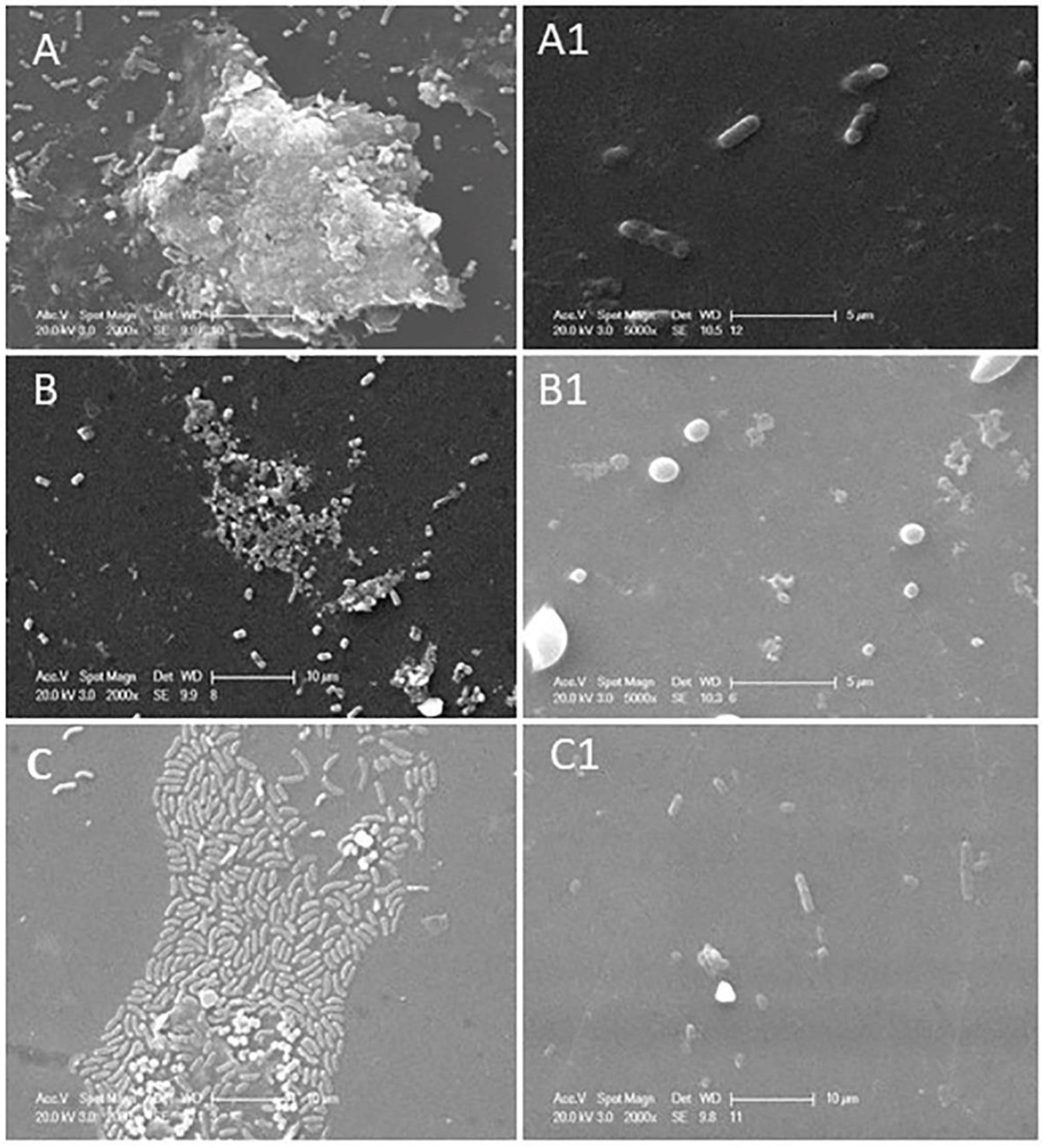
Structural architecture of single or dual-species biofilms of
P. aeruginosa and S. aureus were determined by employing scanning electron and confocal laser scanning electron microscopic images. Scanning electron microscopic images of single or dual-species biofilms were represented in
Figure 2.
P. aeruginosa (
Figure 2A) forms multi-layered complexed biofilm after 24 h of incubation, whereas
S. aureus (
Figure 2B) forms less complexed biofilm than the
P. aeruginosa. In the case of dual-species biofilm (
Figure 2C), i.e., a combinational biofilm of
P. aeruginosa and
S. aureus formed a single-layered structure even after 24 h of incubation at 37
oC. Dual species biofilm is initiated with the same concentration (10
9 CFU/mL) of these bacteria, even though a more significant number of
P. aeruginosa were found than
S. aureus when observed with SEM; this study proved that
P. aeruginosa inhibits
S. aureus proliferation in dual-species biofilms. Exoproducts of
P. aeruginosa cause toxic effects on
S. aureus leading to a lower population density. These bacteria' polysaccharide matrixes help prevent antibiotic regime action in biological conditions. Incubation with phage and phage cocktail for four h showed reduced biomass and biofilm of bacteria in both single and dual-species biofilm combinations. Biofilm inhibitory action of phage or phage cocktail was shown in
Figure 2. where
Figure 2A1, phage (
vB_PAnP_PADP4) treatment with four h of incubation, showed a trace amount of biomass and deformed rod-shaped bacteria; Phage vB_SAnS_SADP1 destructed the biofilm integrity, and single coccus was noticed with limited biofilm matrix (Figure 2B1). Dual species biofilm is treated with a phage cocktail consisting of phages vB_SAnS_SADP1 + vB_PAnP_PADP4 showed excellent lytic action against their host bacteria were represented in Figure 2C1. Interspecies competition between this gram-positive and gram-negative bacterium leads to forming a single-layered biofilm. The phage cocktail effectively destroyed the formed biofilm within four h of incubation at 37
oC.
3.3. Phage or Phage Cocktail Lytic Action on Single or Dual-Species Biofilm by Using CLSM
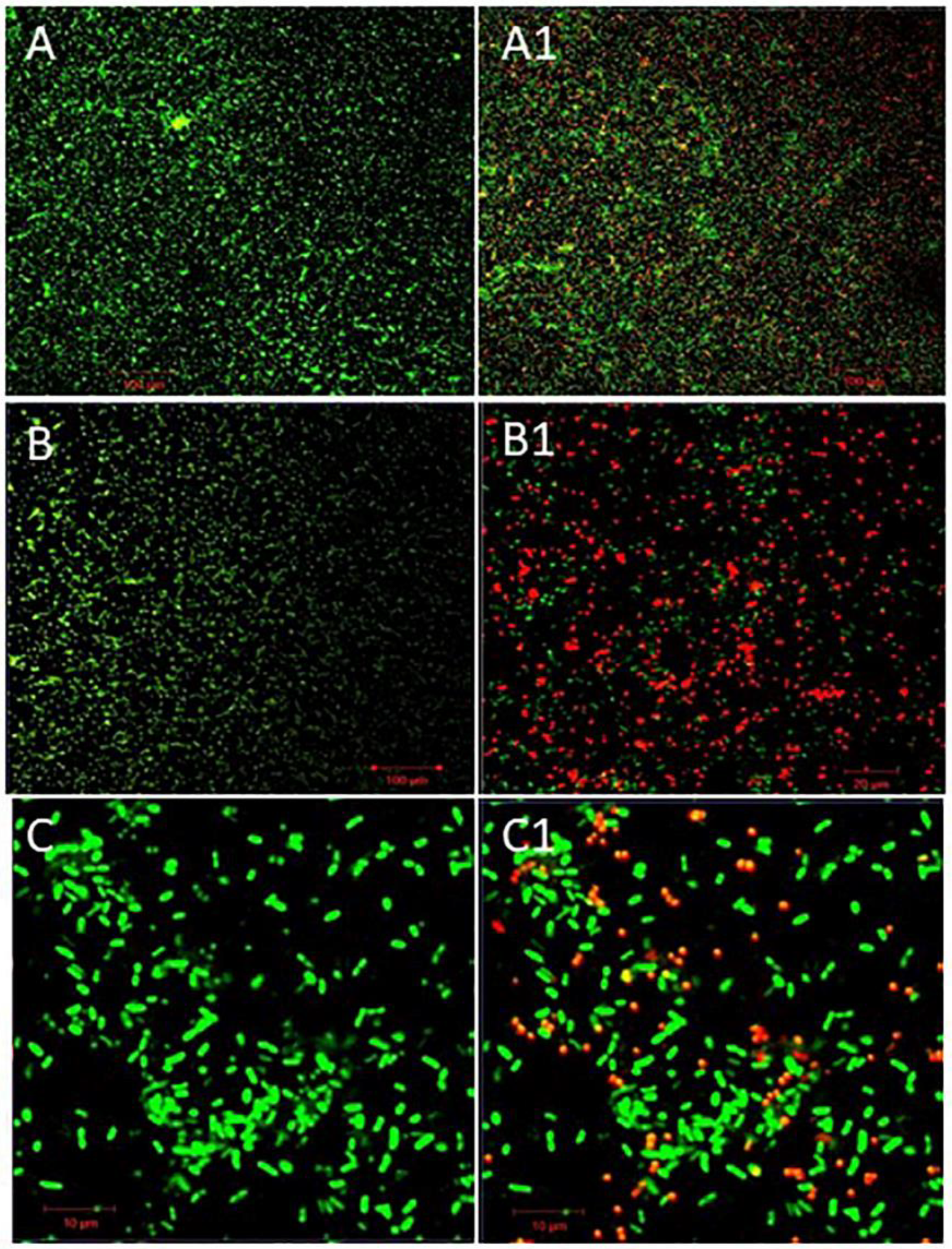
Confocal laser scanning electron microscopic images of phage or phage cocktail on single or dual-species biofilms were represented in
Figure 3. where
Figure 3A, B, and C represented 24 h old native biofilm formed (Control) on a coverslip were appeared in green color (stained with Syto
®9) by
P. aeruginosa,
S. aureus and Combinational biofilm of these two bacteria respectively. The appearance of green color in controls, due to nucleic acid dye, i.e., Syto
®9, its stain only live bacterial cells. Single species formed densely packed biofilms than the dual-species biofilm. After treatment with respective phage or phage cocktails against single or dual biofilms were represented in
Figure 3A1, B1, C1 (Test). Propidium iodide (PI) stains nucleic acid of dead cells. Red-colored spots illustrated in
Figure 3A1, B1, and C1 are because by dead bacterial cells. This is because of the bacteriolytic action of the phage and phage cocktail.
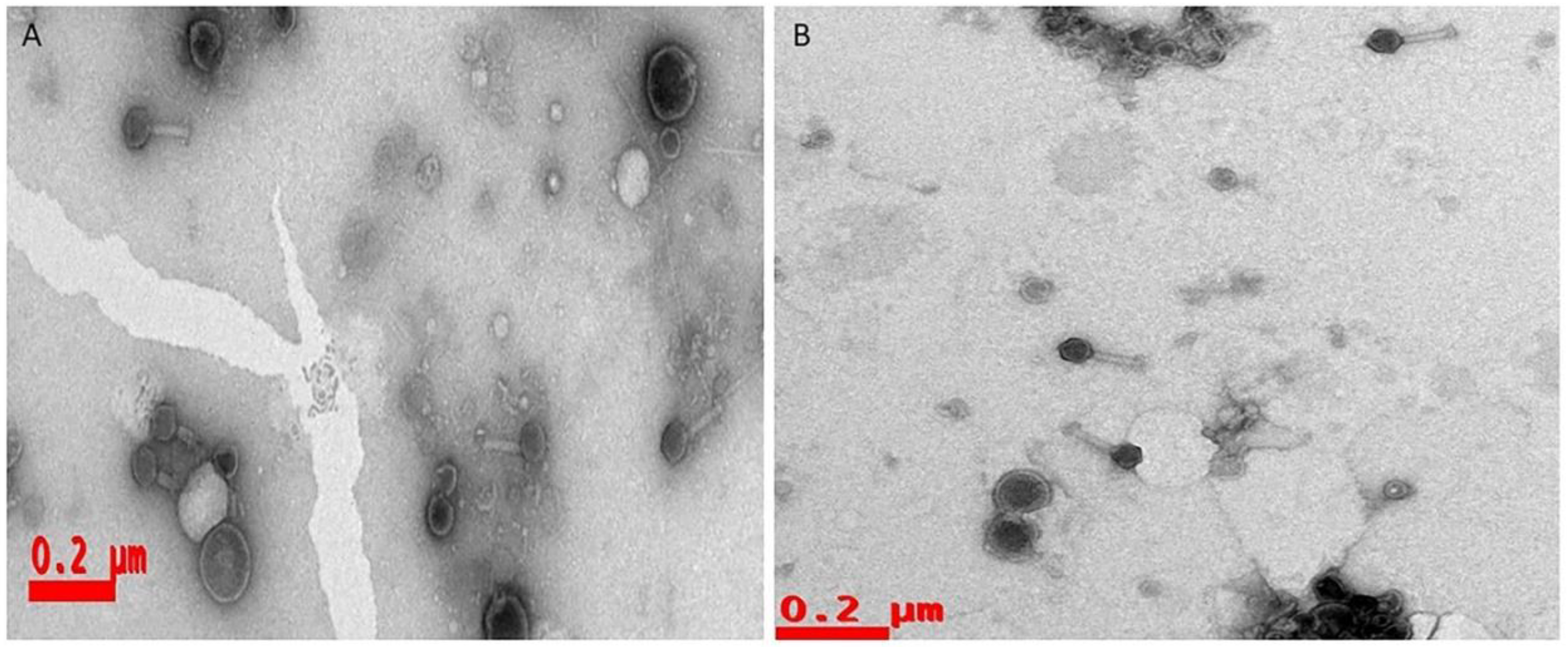
Our observational studies of SEM and CLSM disclosed that single-species bacteria could form densely packed biofilms, whereas dual-species biofilms were less dense because of their interspecies competition. Phage or phage cocktails effectively lyse the biofilms and provide a path for therapeutic applications of phages as antibiofilm agents. Generally, antibiotics cannot penetrate the biofilms because of the polysaccharide matrix, but phage progeny disturbs the matrix and lyse the bacteria. This is one of the premier advantages of phages and renewed interest as alternatives to antibiotics soon. The phages which are isolated and employed against to both single and dual species is belonging to Myoviridae family (Contractile tails were observed under TEM studies (
Figure 4).
4. Discussion
Biofilms are formed by the aggregation of prokaryotic or eukaryotic cells, surrounded by a matrix of extracellular polymeric substances (EPS), consisting of long polysaccharide chains, DNA, and biological macromolecules. Biofilm formation is one of the essential characteristic features of pathogenic bacteria and a dangerous threat to human healthcare. The bacteria encased in a polysaccharide matrix form complex multicellular structure and are more resistant to antimicrobial agents than planktonic cells. It’s complicated to destroy multi-drug resistant bacteria if it forms biofilms or is encased in biofilms. So, therefore an urgent need to find alternative strategies to combat biofilm-forming bacteria. In this scenario, phage-based antimicrobials are becoming a promising alternative to treat biofilms of pathogenic bacterial infections. Phages can lyse the bacterial biofilm by producing lytic enzymes. A single dose of phage administration is efficient to lyse entire bacterial communities.
Biofilms are thought to underlie much of the resistance reported to antibiotics. As an outline of the life cycles of bacterial biofilms, it is exemplified that
P. aeruginosa is a motile bacterium that can produce more complex biofilms than the non-motile except
S. aureus, which forms extensive biofilms. Bacterial communities in the extracellular matrix showed special features that deviated from the planktonic bacterial cells, such as a) Intercellular signals between the community (Quorum sensing) usually regulate the maturation and detachment of the biofilms to objects. b) Activation of secondary messengers, which plays a role in forming biofilms, flagellar movements, and production of extracellular polysaccharides. c) bap protein 12 plays a role in the matrix formation with the help of matrix scaffold proteins and creates a suitable environment for the bacteria to live in the biofilm [
9,
27,
28]. The formation of biofilms depends on the many internal and external factors such as moist surfaces, energy sources on the site of the wound, type of bacterial association, availability of receptors for the bacterial attachment, temperature, and pH (J. Liu et al. 2020; Peng et al. 2020).
Most of the studies reported that predominant bacterial isolates of septic wound infections are Pseudomonas aeruginosa, staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli, Streptococcus pyogenes, Acinetobacter spp, Citrobacter, Proteus, and Enterobacter which are almost multi-drug resistant and biofilm-forming bacterial isolates [
31,
32,
33]; consistent with our study. Pseudomonas aeruginosa and Staphylococcus aureus are opportunistic pathogens commonly associated with polymicrobial diseases. These bacteria form the biofilms and contribute to increased tolerance to antibiotics. Alarming levels of drug resistance and biofilm-forming capabilities lead to the search for alternative strategies. Phage and phage cocktails have shown promising antibiofilm activity due to their mode of action. In this study, 24 h old mono and dual-species biofilms were treated with phages (
vB_SAnS_SADP1, vB_PAnP_PADP4), for four h alone and in combinations. Within four h of incubation, both single and dual-species biofilms were eradicated; this study is consistent with a study reported by Ergun Akturk et al., 2019, where single or dual-species biofilms were reduced by employing both phages and various antibiotic combinations at six h of incubation [
34].
Dual species biofilms of
Pseudomonas aeruginosa and
Staphylococcus aureus are less densely arranged than single-species biofilms; this loose arrangement of biofilm is because of an inhibitory effect of these two species due to their inter-species competition. Our Scanning electron microscopic graphs clearly show the arrangement of bacterial biofilms in single and dual-species biofilms. Our observations were consistent with other reports [
35,
36]. Mixed species biofilms are easily treated using phage or cocktails [
37]. Phage cocktail effectively lysed the dual-species biofilm of
Pseudomonas aeruginosa and
Staphylococcus aureus and was shown in
Figure 2C and C1 after four h of incubation. Our study proved that only phage or phage cocktails are sufficient to remove single or dual-species biofilms; our study is consistent with other research by Tkhilaishvili et al., 2020 reported that dual-species biofilms of
Pseudomonas aeruginosa and MRSA-
Staphylococcus aureus were killed by phage or phage cocktail.
In contrast, Ciprofloxacin is active against only the planktonic stage, but biofilms were eradicated at high concentrations ranging from 256 to 512 mg/L [
38]. Ana Catarina Duarte et al. 2021 reported that 24 h old biofilms were treated with protein CHAPSH3b and phage phiLPLA-RODI alone and in combination; after incubation, the biofilm thickness was reduced in combinational treatment, and results were visualized by employing confocal microscopy. The biofilm showed a higher number of dead or compromised cells, which appeared red in colour due to staining with propidium iodide, and live cells appeared in green color, consistent with our reports. This study proved that combinational therapy plays a vital role in eradicating
S. aureus biofilms [
10].
5. Conclusions
Alarming level of multidrug resistance and biofilm formation in bacteria has becoming the sweltering problem for the human health care systems. The use of bacteriophages or phage cocktails in treating against various bacterial pathogens is increasing because of their potential action against bacteria irrespective of their multi-drug resistance. The isolated phages and phage cocktails showed the excellent lytic activity towards single or dual species biofilms. Inter-species competition promotes the phage activity against biofilms.
Author Contributions
RRP and VRPD conceived and designed the experiments. RRP performed the experiments, data collection and analysis. RRP drafted the manuscript. RRP, GDR, VRPD, VLD, JC analysis, interpretation of findings. RRP, GDR, VLD, VRPD, JC read and revised the manuscript. All authors were involved in reviewing the manuscript and approval for publication.
Data Availability Statement
Data will be available on request to the corresponding and first author.
Acknowledgments
Authors highly acknowledgeable to central facilities of Yogi Vemana University and Department of Nanotechnology, University of Hyderabad for CLSM, SEM and TEM service. Dr. PRR gratefully acknowledges the University Grants Commission for financial support in the form of JRF and SRF fellowships. This work is supported by the National Foundation of Korea (NRF) grant (NRF-2021R1A2C1007887).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage – A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, ume 14, 205–217. [Google Scholar] [CrossRef]
- Sagar, S.S.; Kumar, R.; Kaistha, S.D. Efficacy of Phage and Ciprofloxacin Co-therapy on the Formation and Eradication of Pseudomonas aeruginosa Biofilms. Arab. J. Sci. Eng. 2016, 42, 95–103. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. 2002, 99, 3129–3134. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Fernández, L.; Rodríguez, A.; García, P. Are Phage Lytic Proteins the Secret Weapon To Kill Staphylococcus aureus? mBio 2018, 9, e01923–e17. [Google Scholar] [CrossRef]
- Fernández, L.; González, S.; Campelo, A.B.; Martínez, B.; Rodríguez, A.; García, P. Downregulation of Autolysin-Encoding Genes by Phage-Derived Lytic Proteins Inhibits Biofilm Formation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e02724–16. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance - development, composition and regulation - therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Duarte AC, Fernández L, De Maesschalck V, et al. Synergistic action of phage phiIPLA-RODI and lytic protein CHAPSH3b: a combination strategy to target Staphylococcus aureus biofilms. NPJ Biofilms Microbiomes. 2021;7(1):1–10.
- Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses 2020, 12, 235. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as Weapons Against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Chen, L.; Fan, J.; Yan, T.; Liu, Q.; Yuan, S.; Zhang, H.; Yang, J.; Deng, D.; Huang, S.; Ma, Y. Isolation and Characterization of Specific Phages to Prepare a Cocktail Preventing Vibrio sp. Va-F3 Infections in Shrimp (Litopenaeus vannamei). Front. Microbiol. 2019, 10, 2337. [Google Scholar] [CrossRef]
- Motlagh, A.M.; Bhattacharjee, A.S.; Goel, R. Biofilm control with natural and genetically-modified phages. World J. Microbiol. Biotechnol. 2016, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Razem F, Al-Aloul H, Ishnaiwer M,, et. al. Isolation and partial characterization of Salmonella Gallinarum bacteriophage. Saudi J Biol Sci. 2022, 29, 3308–12.
- Bragg RR, Boucher CE, van der Westhuizen WA, et al. The Potential Use of Bacteriophage Therapy as a Treatment Option in a Post-Antibiotic Era. In: Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches. 2016, 15, 309–328.
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How Phages Overcome the Challenges of Drug Resistant Bacteria in Clinical Infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Viertel, T.M.; Ritter, K.; Horz, H.-P. Viruses versus bacteria--novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Pallavali, R.R.; Degati, V.L.; Lomada, D.; Reddy, M.C.; Durbaka, V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLOS ONE 2017, 12, e0179245. [Google Scholar] [CrossRef] [PubMed]
- Pallavali RR, Degati VL, Narala VR, et. al. Lytic Bacteriophages Against Bacterial Biofilms Formed by Multidrug-Resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus Isolated from Burn Wounds. Phage. 2021, 2, 120–30.
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J.; Sullivan, M.B. Improving Phage-Biofilm In Vitro Experimentation. Viruses 2021, 13, 1175. [Google Scholar] [CrossRef]
- Wagner, E.M.; Fischel, K.; Rammer, N.; Beer, C.; Palmetzhofer, A.L.; Conrady, B.; Roch, F.-F.; Hanson, B.T.; Wagner, M.; Rychli, K. Bacteria of eleven different species isolated from biofilms in a meat processing environment have diverse biofilm forming abilities. Int. J. Food Microbiol. 2021, 349, 109232. [Google Scholar] [CrossRef] [PubMed]
- González S, Fernández L, Campelo AB, et al. The behavior of Staphylococcus aureus dual-species biofilms treated with bacteriophage phiIPLA-RODI depends on the accompanying microorganism. Appl Environ Microbiol. 2017, 83, e02821–16.
- Drago, L.; Agrappi, S.; Bortolin, M.; Toscano, M.; Romanò, C.L.; De Vecchi, E. How to Study Biofilms after Microbial Colonization of Materials Used in Orthopaedic Implants. Int. J. Mol. Sci. 2016, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Choi, J.; Ahn, Y.-H. Biofouling reduction in a MBR by the application of a lytic phage on a modified nanocomposite membrane. Environ. Sci. Water Res. Technol. 2018, 4, 1624–1638. [Google Scholar] [CrossRef]
- Baudin, M.; Cinquin, B.; Sclavi, B.; Pareau, D.; Lopes, F. Understanding the fundamental mechanisms of biofilms development and dispersal: BIAM (Biofilm Intensity and Architecture Measurement), a new tool for studying biofilms as a function of their architecture and fluorescence intensity. J. Microbiol. Methods 2017, 140, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- O’Neill E, Pozzi C, Houston P, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol. 2008, 190, 3835–50.
- Liu, J.; Gao, S.; Dong, Y.; Lu, C.; Liu, Y. Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol. 2020, 20, 1811. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Borg, R.E.; Dow, L.P.; Pruitt, B.L.; Chen, I.A. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc. Natl. Acad. Sci. USA 2020, 117, 1951–1961. [Google Scholar] [CrossRef]
- Vicar, E.K.; Acquah, S.E.K.; Williams, W.; Kuugbee, E.D.; Saba, C.K.S.; Mensah, G.I. Antibiotic Resistant Bacteria Infecting Wounds of Rural Community Dwellers in Northern Ghana. Eur. J. Med Heal. Sci. 2021, 3, 112–117. [Google Scholar] [CrossRef]
- Kabanangi, F.; Joachim, A.; Nkuwi, E.J.; Manyahi, J.; Moyo, S.; Majigo, M. High Level of Multidrug-Resistant Gram-Negative Pathogens Causing Burn Wound Infections in Hospitalized Children in Dar es Salaam, Tanzania. Int. J. Microbiol. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Islam, N.; Hawlader, M.D.H.; Ahmed, S.; Wahab, A.; Islam, M.; Uddin, K.R.; Hossain, A. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: A cross-sectional study. Int. J. Surg. Open 2021, 28, 56–62. [Google Scholar] [CrossRef]
- Akturk, E.; Oliveira, H.; Santos, S.B.; Costa, S.; Kuyumcu, S.; Melo, L.D.R.; Azeredo, J. Synergistic Action of Phage and Antibiotics: Parameters to Enhance the Killing Efficacy Against Mono and Dual-Species Biofilms. Antibiotics 2019, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Radlinski, L.; Rowe, S.E.; Kartchner, L.B.; Maile, R.; Cairns, B.A.; Vitko, N.P.; Gode, C.J.; Lachiewicz, A.M.; Wolfgang, M.C.; Conlon, B.P. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLOS Biol. 2017, 15, e2003981. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Rice, A.; Sutton, B.; Gabrilska, R.; Wessel, A.K.; Whiteley, M.; Rumbaugh, K.P. Albumin Inhibits Pseudomonas aeruginosa Quorum Sensing and Alters Polymicrobial Interactions. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- González S, Fernández L, Campelo AB, et al. The behavior of Staphylococcus aureus dual-species biofilms treated with bacteriophage phiIPLA-RODI depends on the accompanying microorganism. Appl Environ Microbiol. 2017, 83, 1–14.
- Tkhilaishvili, T.; Wang, L.; Perka, C.; Trampuz, A.; Moreno, M.G. Using Bacteriophages as a Trojan Horse to the Killing of Dual-Species Biofilm Formed by Pseudomonas aeruginosa and Methicillin Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 695. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).