Submitted:
02 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
Keywords:
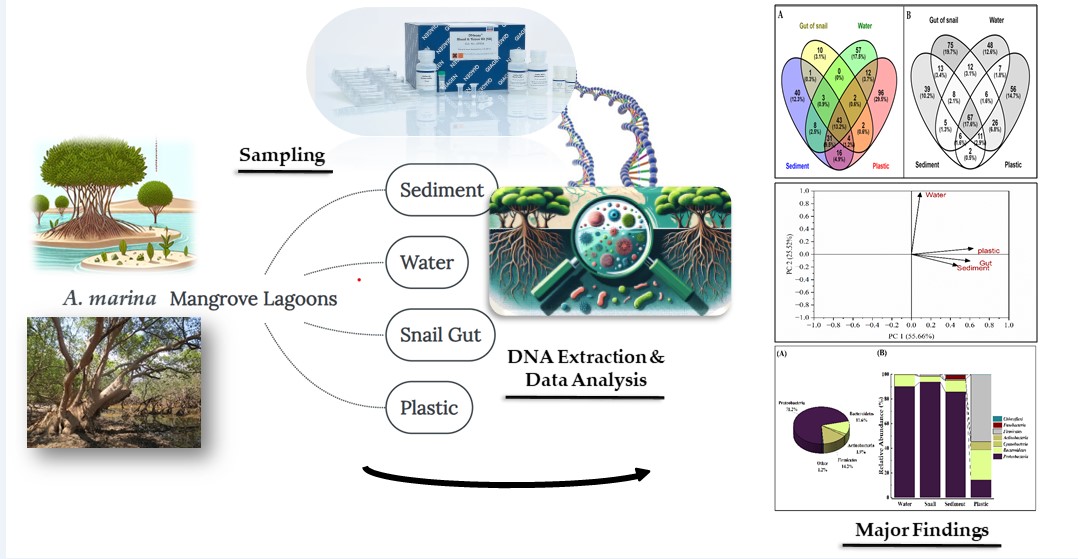
1. Introduction
2. Materials and Methods
2.1. Locations and Site Characteristics
2.2. Sediments Collection
2.3. Water Collection
2.4. Plastics Collection and Characterization
2.5. Snail Collection
2.6. Isolation of DNA
2.7. DNA sequencing and Identification of Bacteria
2.8. Data Analysis and Statistical Methods
3. Results
3.1. Chemical Composition of Plastics
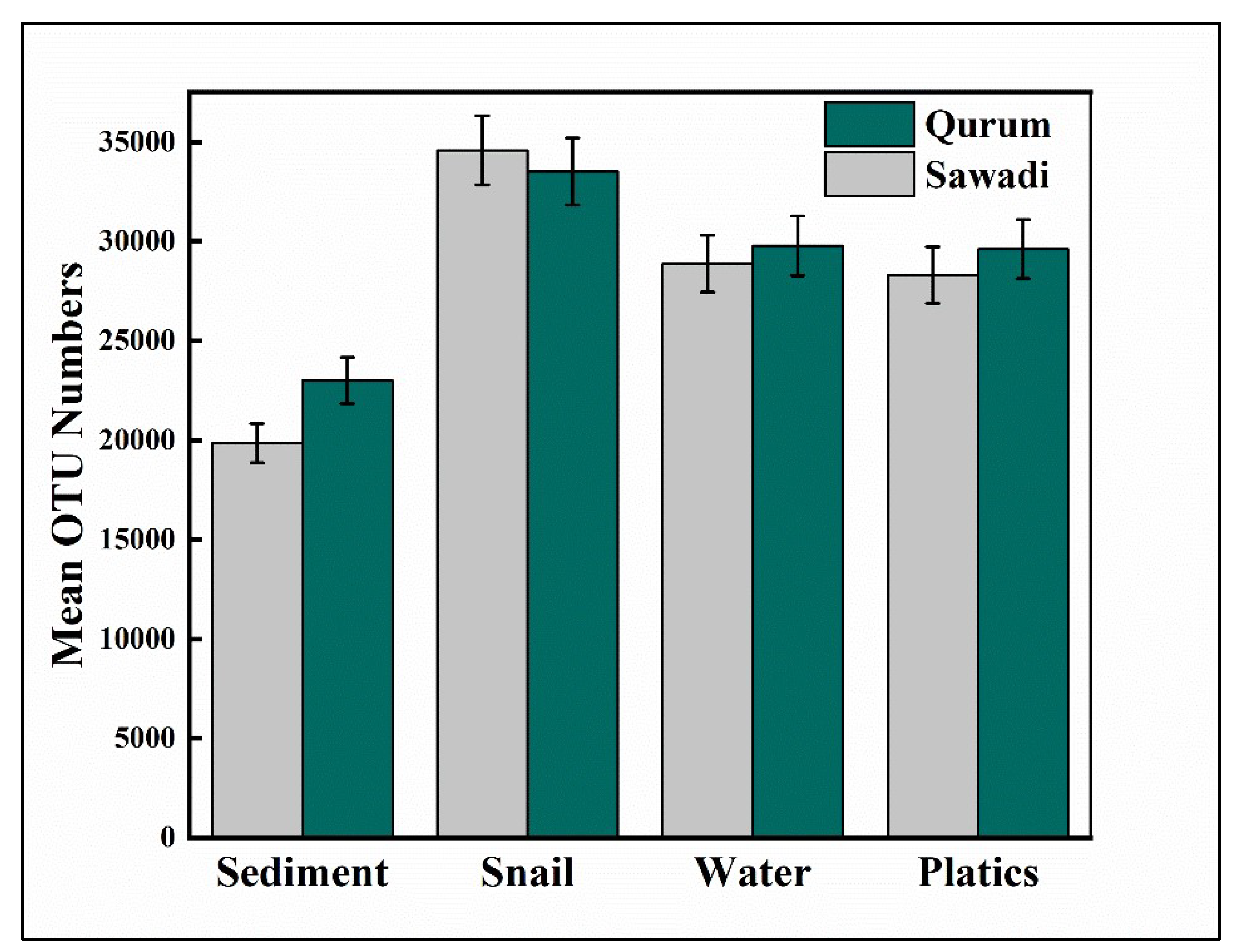
3.2. Diversity of the Bacterial Communities
3.3. Abundance of Bacterial Communities
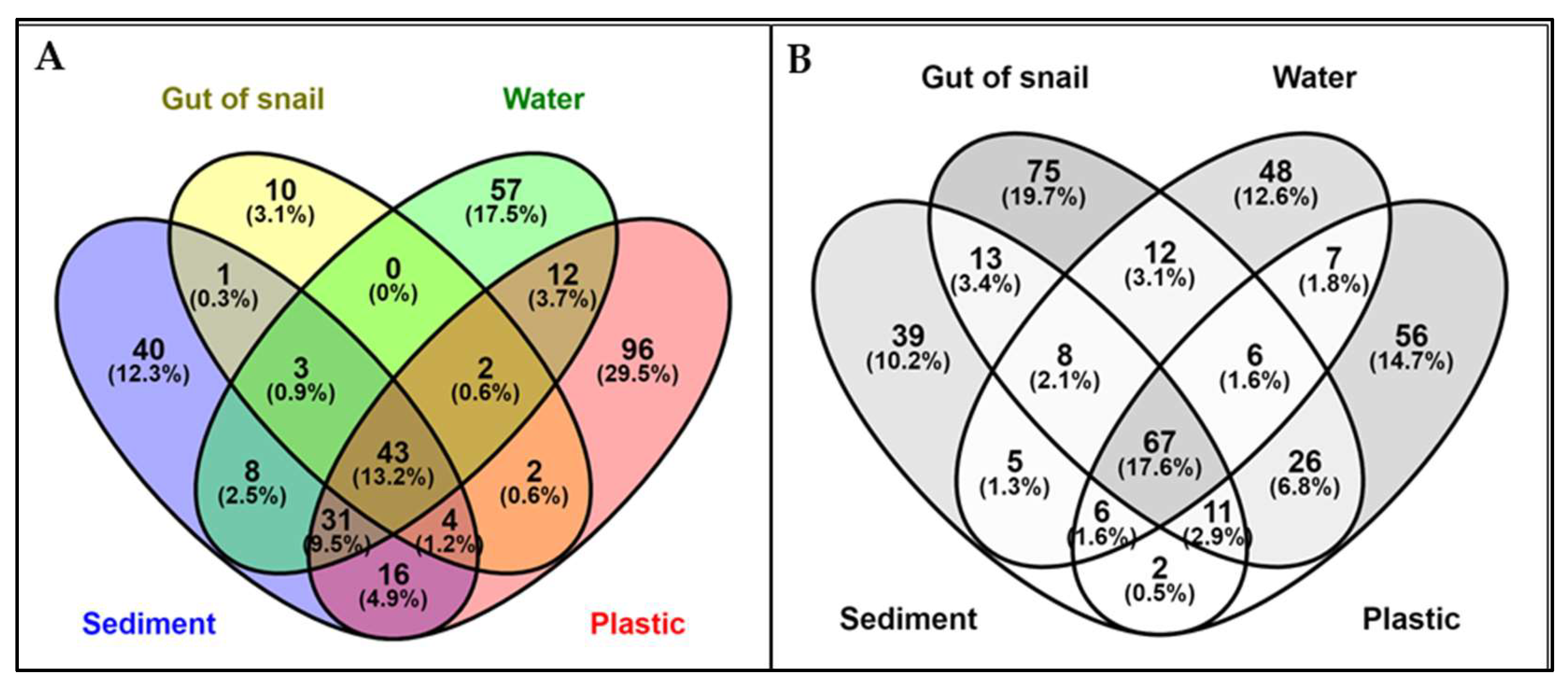
3.3.1. OTUs From all Substrates in Two Lagoons
3.3.2. OTUs from Different Substrates in Two Lagoons
3.4. Taxonomic Analysis of Bacterial Communities
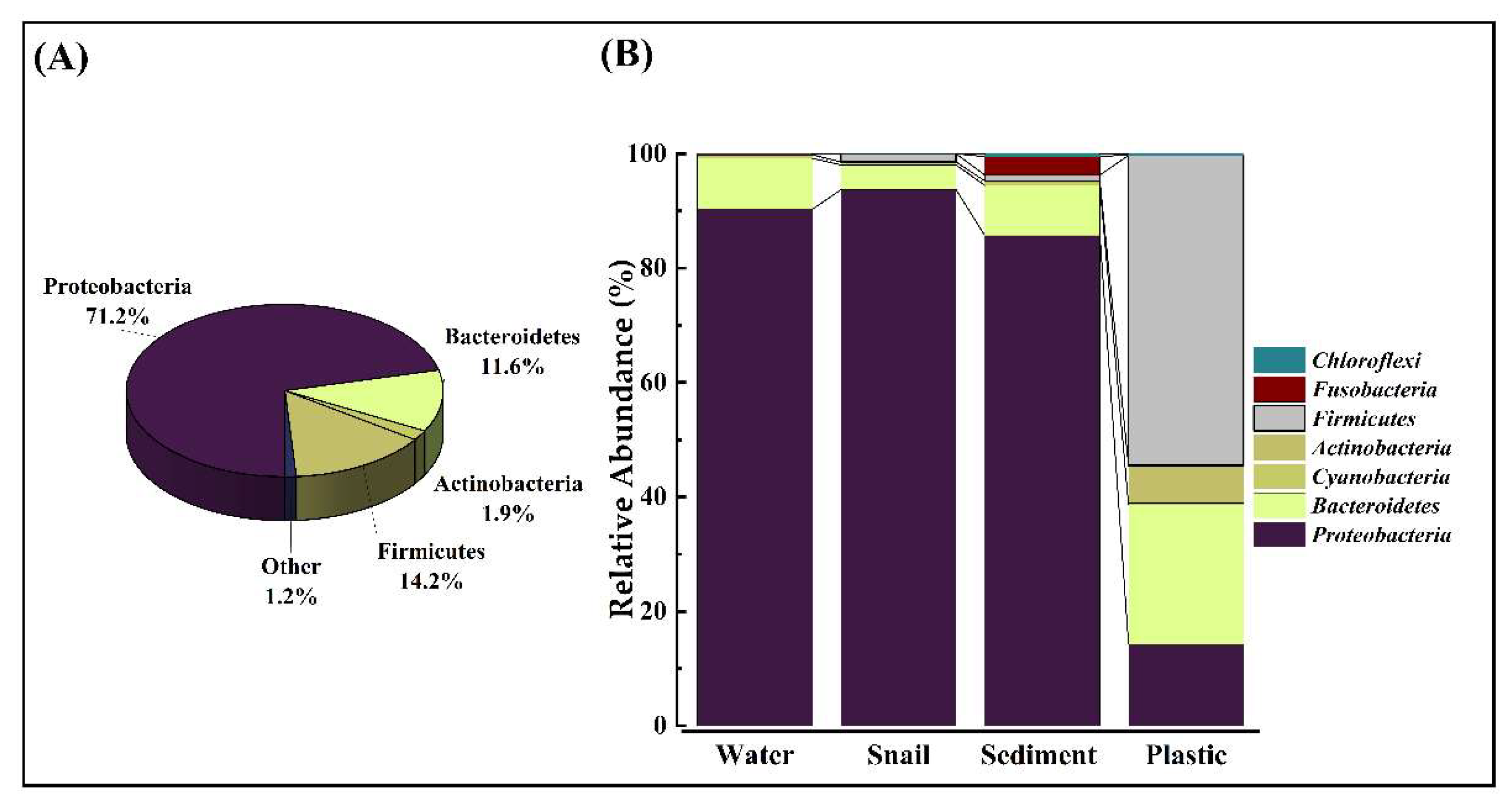
3.4.1. Distribution and Relative Abundance of Bacterial Communities Across Locations and Substrates: Phylum-Level
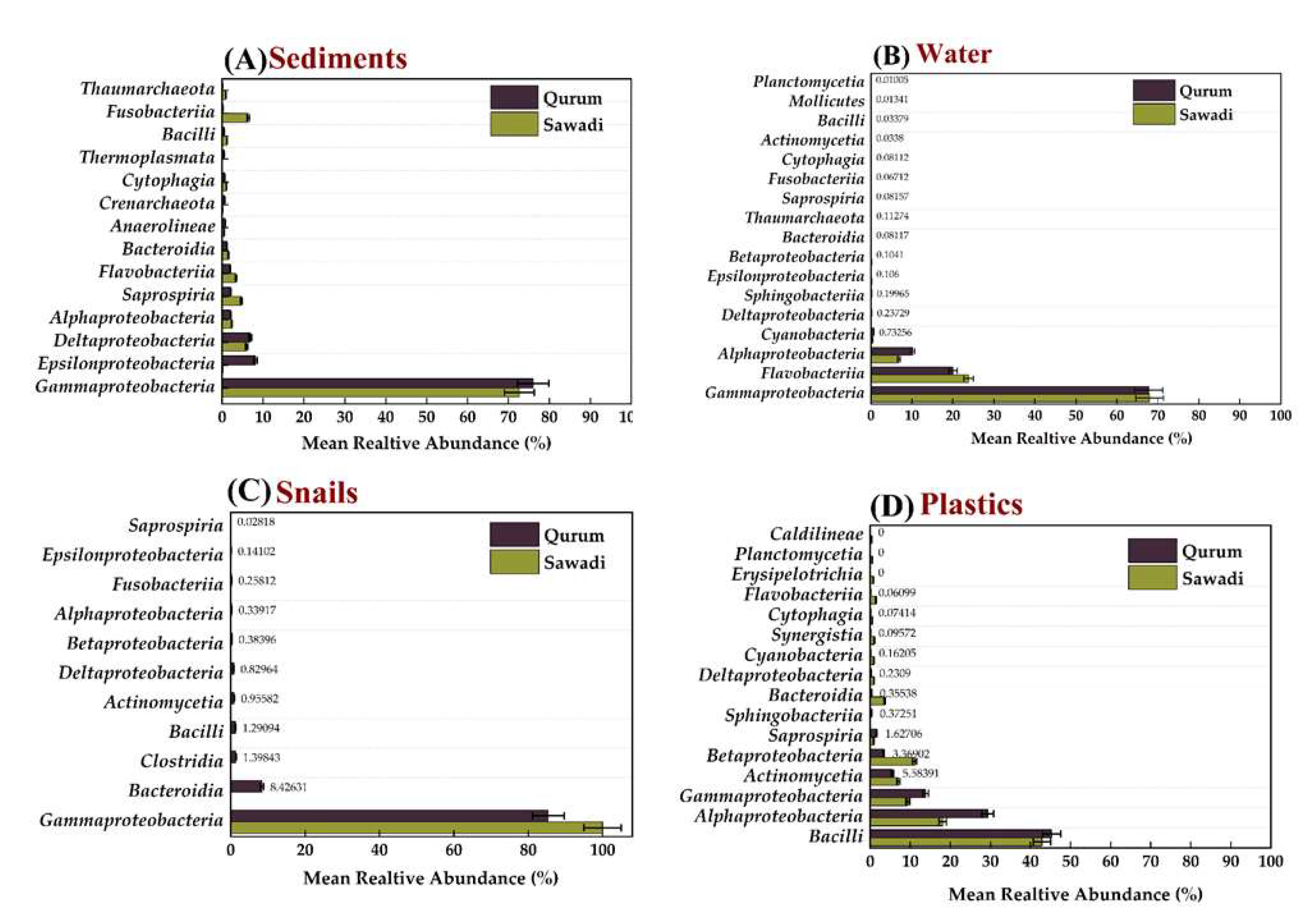
3.4.2. Distribution and Relative Abundance of Bacterial Communities Across Locations and Substrates: Class-Level
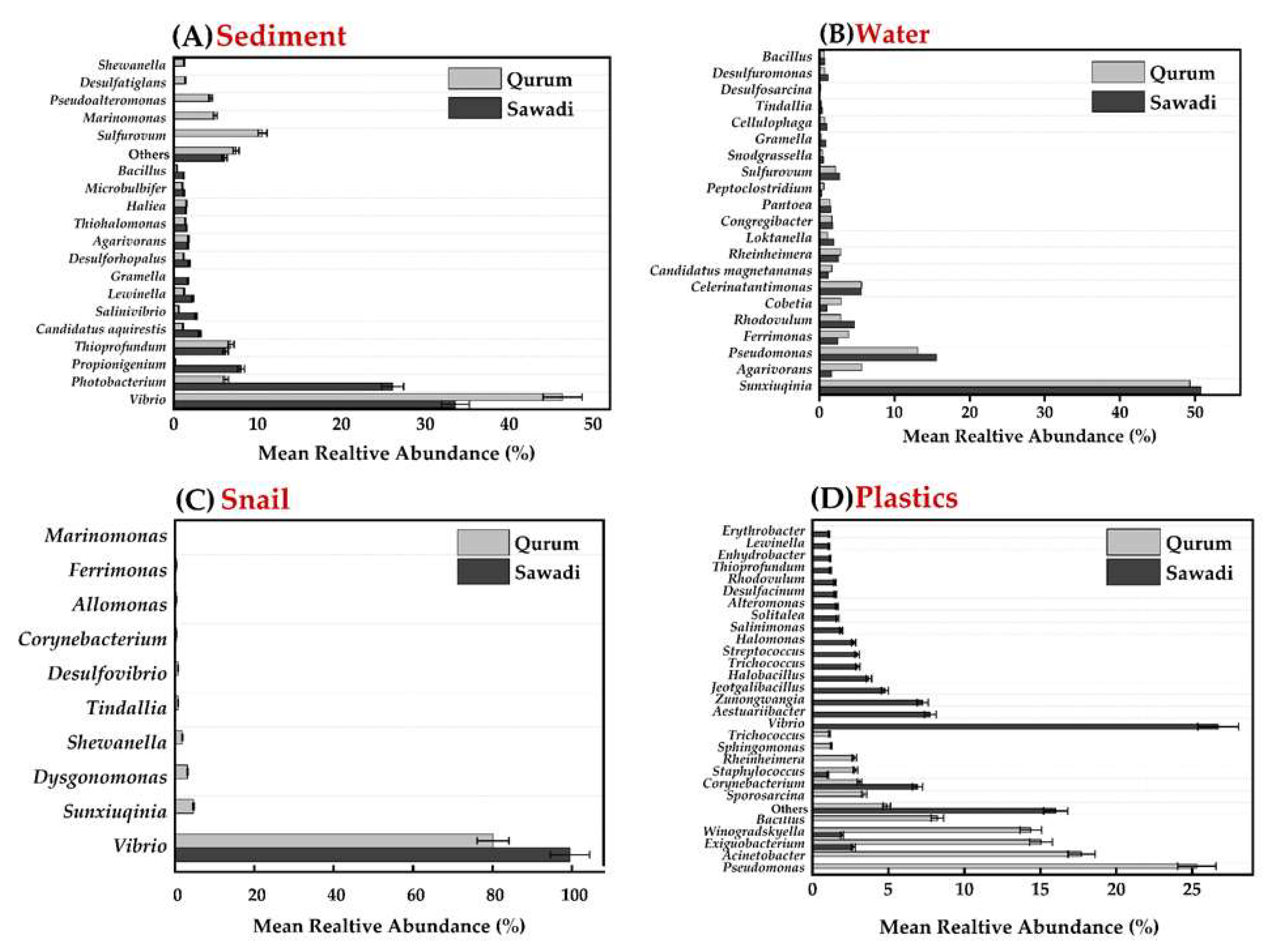
3.4.3. Distribution and Relative Abundance of Bacterial Communities Across Locations and Substrates: Genus-Level
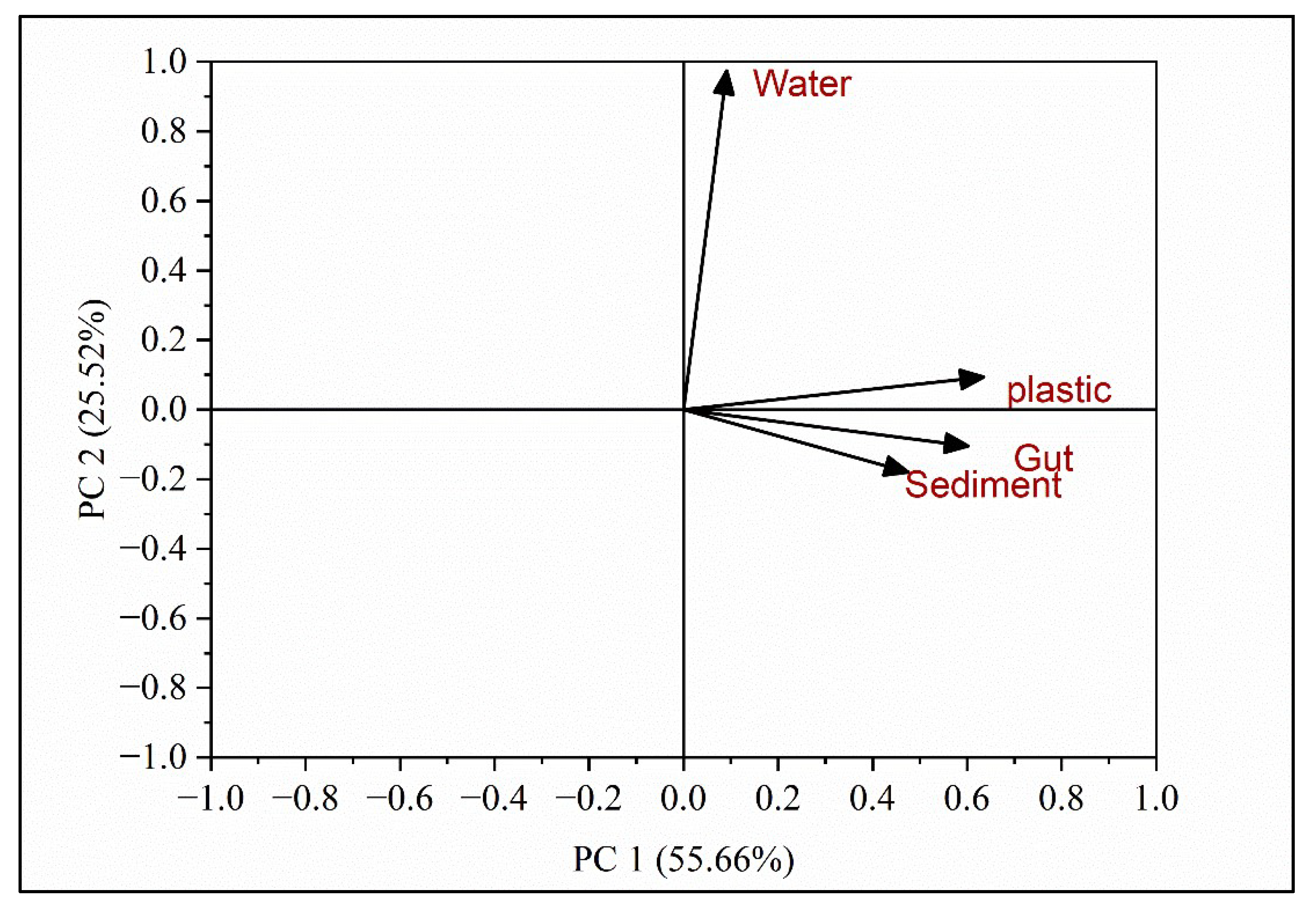
3.5. Structure of Bacterial Communities
4. Discussion
4.1. Diversity of Microbes between Two Lagoons and Across Different Substrates in the Study Area
4.2. Dominant Groups of Bacteria
4.3. Bacterial Communities on Different Substrates
4.3. Bacterial Communities in Different Lagoons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- R. L. Marinelli and G. G. Waldbusser, “Sediments ’ Closing the Ecological Loop,” 2005.
- M. Fusi et al., “Bioturbation Intensity Modifies the Sediment Microbiome and Biochemistry and Supports Plant Growth in an Arid Mangrove System,” Microbiology Spectrum, vol. 10, no. 3, 2022. [CrossRef]
- S. Ghosh, J. K. Sinha, S. Ghosh, K. Vashisth, S. Han, and R. Bhaskar, “Microplastics as an Emerging Threat to the Global Environment and Human Health,” Sustainability (Switzerland), vol. 15, no. 14, 2023. [CrossRef]
- K. R. Arrigo, “Marine microorganisms and global nutrient cycles,” Nature, vol. 437, no. 7057, pp. 349–355, 2005. [CrossRef]
- W. Zhuang et al., “Diversity, function and assembly of mangrove root-associated microbial communities at a continuous fine-scale,” npj Biofilms and Microbiomes, vol. 6, no. 1, pp. 1–10, 2020. [CrossRef]
- M. Ghose, A. S. Parab, C. S. Manohar, D. Mohanan, and A. Toraskar, “Unraveling the role of bacterial communities in mangrove habitats under the urban influence, using a next-generation sequencing approach,” Journal of Sea Research, vol. 198, no. January, p. 102469, 2024 . [CrossRef]
- C. O. De Santana, P. Spealman, V. M. M. I. Melo, D. Gresham, T. B. De Jesus, and F. A. Chinalia, “Effects of tidal influence on the structure and function of prokaryotic communities in the sediments of a pristine Brazilian mangrove,” Biogeosciences, vol. 18, no. 7, pp. 2259–2273, 2021 . [CrossRef]
- B. C. Crump and J. L. Bowen, “The Microbial Ecology of Estuarine Ecosystems,” Annual Review of Marine Science, vol. 16, pp. 335–360, 2024. [CrossRef]
- A. Gupta, R. Gupta, and R. L. Singh, “Microbes and Environment BT - Principles and Applications of Environmental Biotechnology for a Sustainable Future,” R. L. Singh, Ed., Singapore: Springer Singapore, 2017, pp. 43–84. [CrossRef]
- N. Narula, E. Kothe, and R. K. Behl, “Role of root exudates in plant-microbe interactions,” Journal of Applied Botany and Food Quality, vol. 82, no. 2, pp. 122–130, 2009.
- J. R. Gaiero, C. A. McCall, K. A. Thompson, N. J. Day, A. S. Best, and K. E. Dunfield, “Inside the root microbiome: Bacterial root endophytes and plant growth promotion,” American Journal of Botany, vol. 100, no. 9, pp. 1738–1750, 2013 . [CrossRef]
- A. Mukherjee and D. Chattopadhyay, “Exploring environmental systems and processes through next-generation sequencing technologies: insights into microbial response to petroleum contamination in key environments,” The Nucleus, vol. 60, no. 2, pp. 175–186, 2017. [CrossRef]
- C. Zhao et al., “Mangrove species mapping in coastal China using synthesized Sentinel-2 high-separability images,” Remote Sensing of Environment, vol. 307, p. 114151, 2024.
- T. Tong, R. Li, S. Wu, and S. Xie, “The distribution of sediment bacterial community in mangroves across China was governed by geographic location and eutrophication,” Marine Pollution Bulletin, vol. 140, no. December 2018, pp. 198–203, 2019. [CrossRef]
- A. C. C. Pires et al., “Denaturing gradient gel electrophoresis and barcoded pyrosequencing reveal unprecedented archaeal diversity in mangrove sediment and rhizosphere samples,” Applied and Environmental Microbiology, vol. 78, no. 16, pp. 5520–5528, 2012 . [CrossRef]
- L. W. Mendes and S. M. Tsai, “Variations of bacterial community structure and composition in mangrove sediment at different depths in Southeastern Brazil,” Diversity, vol. 6, no. 4, pp. 827–843, 2014. [CrossRef]
- S. Dobretsov et al., “Living on the edge: biofilms developing in oscillating environmental conditions,” Biofouling, vol. 34, no. 9, pp. 1064–1077, Oct. 2018. [CrossRef]
- I. E. Napper and R. C. Thompson, “Plastic Debris in the Marine Environment: History and Future Challenges,” Global Challenges, vol. 4, no. 6, 2020. [CrossRef]
- L. Miao et al., “Distinct microbial metabolic activities of biofilms colonizing microplastics in three freshwater ecosystems,” Journal of Hazardous Materials, vol. 403, no. April 2020, p. 123577, 2021. [CrossRef]
- M. C. Rillig, S. W. Kim, and Y.-G. Zhu, “The soil plastisphere,” Nature Reviews Microbiology, vol. 22, no. 2, pp. 64–74, 2024. [CrossRef]
- A. M. Osborn and S. Stojkovic, “Marine microbes in the Plastic Age,” Microbiology Australia, vol. 35, no. 4, p. 207, 2014. [CrossRef]
- J. A. Bryant et al., “Diversity and Activity of Communities Inhabiting Plastic Debris in the North Pacific Gyre,” mSystems, vol. 1, no. 3, 2016. [CrossRef]
- E. R. Zettler, T. J. Mincer, and L. A. Amaral-Zettler, “Life in the ‘plastisphere’: Microbial communities on plastic marine debris,” Environmental Science and Technology, vol. 47, no. 13, pp. 7137–7146, 2013 . [CrossRef]
- L. Miao et al., “Spatio-temporal succession of microbial communities in plastisphere and their potentials for plastic degradation in freshwater ecosystems,” Water Research, vol. 229, no. November 2022, p. 119406, 2023. [CrossRef]
- N. Wu et al., “Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary,” Science of the Total Environment, vol. 708, p. 134876, 2020 . [CrossRef]
- H. F. Dos Santos et al., “Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: Bacterial proxies for oil pollution,” PLoS ONE, vol. 6, no. 3, pp. 1–8, 2011. [CrossRef]
- M. V. R. Conceição et al., “Amazonia Seasons Have an Influence in the Composition of Bacterial Gut Microbiota of Mangrove Oysters (Crassostrea gasar),” Frontiers in Genetics, vol. 11, no. February, pp. 1–7, 2021. [CrossRef]
- Pacific Consultants International, “the Master Plan Study on Restoration, Conservation and Management of Mangrove in the Sultanate of Oman,” vol. 1, no. July, pp. 1–146, 2004.
- M. Al-Tarshi, S. Dobretsov, and W. Gallardo, “Marine litter and microplastic pollution in mangrove sediments in the Sea of Oman,” Marine Pollution Bulletin, vol. 201, no. February, p. 116132, 2024. [CrossRef]
- N. Wu et al., “Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary,” Science of the Total Environment, vol. 708, p. 134876, 2020. [CrossRef]
- D. R. Chaudhary, M. Kumar, and V. Kalla, “Sediment microbial community structure, enzymatic activities and functional gene abundance in the coastal hypersaline habitats,” Archives of Microbiology, vol. 205, no. 2, pp. 1–20, 2023. [CrossRef]
- C. L. Ettinger, S. E. Voerman, J. M. Lang, J. J. Stachowicz, and J. A. Eisen, “Microbial communities in sediment from Zostera marina patches, but not the Z. marina leaf or root microbiomes, vary in relation to distance from patch edge,” PeerJ, vol. 2017, no. 4, pp. 1–25, 2017. [CrossRef]
- A. M. Noguez, H. T. Arita, A. E. Escalante, L. J. Forney, F. García-Oliva, and V. Souza, “Microbial macroecology: Highly structured prokaryotic soil assemblages in a tropical deciduous forest,” Global Ecology and Biogeography, vol. 14, no. 3, pp. 241–248, 2005. [CrossRef]
- P. Li et al., “Comparison of extraction methods of total microbial DNA from freshwater,” Genetics and Molecular Research, vol. 14, no. 1, pp. 730–738, 2015. [CrossRef]
- D. M. Alongi, “Present state and future of the world’s mangrove forests,” Environmental Conservation, vol. 29, no. 3, pp. 331–349, 2002. [CrossRef]
- S.-L. Jia, Z. Chi, G.-L. Liu, Z. Hu, and Z.-M. Chi, “Fungi in mangrove ecosystems and their potential applications,” Critical Reviews in Biotechnology, vol. 40, no. 6, pp. 852–864, Aug. 2020. [CrossRef]
- S. M. Allard et al., “Introducing the Mangrove Microbiome Initiative: Identifying Microbial Research Priorities and Approaches To Better Understand, Protect, and Rehabilitate Mangrove Ecosystems.,” mSystems, vol. 5, no. 5, Oct. 2020. [CrossRef]
- H. Thatoi, B. C. Behera, R. R. Mishra, and S. K. Dutta, “Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review,” Annals of Microbiology, vol. 63, no. 1, pp. 1–19, 2013. [CrossRef]
- S. R. Cotta, L. L. Cadete, J. D. van Elsas, F. D. Andreote, and A. C. F. Dias, “Exploring bacterial functionality in mangrove sediments and its capability to overcome anthropogenic activity,” Marine Pollution Bulletin, vol. 141, pp. 586–594, 2019. [CrossRef]
- J. Shentu et al., “Disturbance and restoration of soil microbial communities after in-situ thermal desorption in a chlorinated hydrocarbon contaminated site,” Journal of Hazardous Materials, vol. 448, p. 130870, 2023.
- A. Oren, “Microbial life at high salt concentrations: phylogenetic and metabolic diversity,”Saline systems, vol. 4, pp. 1–13, 2008.
- G. Stotzky, “Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses,” Interactions of soil minerals with natural organics and microbes, vol. 17, pp. 305–428, 1986.
- J. P. Chen, S. L. Kim, and Y. P. Ting, “Optimization of membrane physical and chemical cleaning by a statistically designed approach,” Journal of Membrane Science, vol. 219, no. 1–2, pp. 27–45, Jul. 2003. [CrossRef]
- N. Annegret et al., “Inter-Population Differences and Seasonal Dynamic of the Bacterial Gut Community in the Endangered Land Snail Helix pomatia (Gastropoda: Helicidae),” 2015.
- M. JICA, “The master plan study on restoration, conservation and management of mangrove in the Sultanate of Oman,” Japan International Cooperation Agency & Ministry of Regional Municipalities, Environment and Water Resources (MRMEWR), The Sultanate of Oman, Muscat, 2004.
- M. M. Uddin, M. M. Hossain, A. A. Aziz, and C. E. Lovelock, “Ecological development of mangrove plantations in the Bangladesh Delta,” Forest Ecology and Management, vol. 517, p. 120269, 2022.
- D. M. Alongi, “Mangrove–microbe–soil relations,” Interactions between macro-and microorganisms in marine sediments, vol. 60, pp. 85–103, 2005.
- C. J. Robinson, B. J. Bohannan, and V. B. Young, “From structure to function: the ecology of host-associated microbial communities,” Microbiology and Molecular Biology Reviews, vol. 74, no. 3, pp. 453–476, 2010.
- M. Al-Tarshi, S. Dobretsov, and W. Gallardo, “Marine litter and microplastic pollution in mangrove sediments in the Sea of Oman,” Marine Pollution Bulletin, vol. 201, p. 116132, 2024.
- J. Wang, C. Peng, H. Li, P. Zhang, and X. Liu, “The impact of microplastic-microbe interactions on animal health and biogeochemical cycles: a mini-review,” Science of the Total Environment, vol. 773, p. 145697, 2021.
- A. M. Ibekwe, J. Ma, and S. E. Murinda, “Bacterial community composition and structure in an Urban River impacted by different pollutant sources,” Science of the Total Environment, vol. 566, pp. 1176–1185, 2016.
- T. Liu et al., “Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River,” Microbiome, vol. 6, pp. 1–14, 2018.
- C. Kivistik, “The impact of environmental disturbances on the gastrointestinal bacterial community and the viability of aquatic gastropods,” 2022.
- P. Zhang, W. Li, H. Qiu, M. Liu, Y. Li, and E. He, “Metal resistant gut microbiota facilitates snails feeding on metal hyperaccumulator plant Sedum alfredii in the phytoremediation field,” Ecotoxicology and Environmental Safety, vol. 236, p. 113514, 2022.
- A. Bouchez et al., “Mangrove microbial diversity and the impact of trophic contamination,” Marine pollution bulletin, vol. 66, no. 1–2, pp. 39–46, 2013.
- A. Rosato et al., “Bacterial colonization dynamics of different microplastic types in an anoxic salt marsh sediment and impact of adsorbed polychlorinated biphenyls on the plastisphere,” Environmental Pollution, vol. 315, p. 120411, 2022.
- E. M. Eckert, S. Amalfitano, A. Di Cesare, C. Manzari, G. Corno, and D. Fontaneto, “Different substrates within a lake harbour connected but specialised microbial communities,” Hydrobiologia, vol. 847, pp. 1689–1704, 2020.
- K. H. Nealson and W. Berelson, “Sediment Habitats, Including Watery,” in Encyclopedia of Microbiology (Third Edition), M. Schaechter, Ed., Oxford: Academic Press, 2009, pp. 350–360. [CrossRef]
- L. H. Zeglin, “Stream microbial diversity in response to environmental changes: review and synthesis of existing research,” Front. Microbiol., vol. 6, May 2015. [CrossRef]
- Z. Hu et al., “Compositional and predicted functional analysis of the gut microbiota of Radix auricularia (Linnaeus) via high throughput Illumina sequencing,” 2018. [CrossRef]
- K. S. Stenger, O. G. Wikmark, C. C. Bezuidenhout, and L. G. Molale-Tom, “Microplastics pollution in the ocean: Potential carrier of resistant bacteria and resistance genes,” Environmental Pollution, vol. 291, p. 118130, Dec. 2021. [CrossRef]
- N. L. Fahrenfeld et al., “Study of marine debris around a tourist city in East China: Implication for waste management,” Science of the Total Environment, vol. 161, no. 1, pp. 834–840, 2019.
- L. Frère et al., “Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size,” Environmental Pollution, vol. 242, pp. [CrossRef]
- P. Behera, M. Mohapatra, J. Y. Kim, T. K. Adhya, A. K. Pattnaik, and G. Rastogi, “Spatial and temporal heterogeneity in the structure and function of sediment bacterial communities of a tropical mangrove forest,” Environmental Science and Pollution Research, vol. 26, no. 4, pp. 3893–3908, 2019. [CrossRef]
- X. X. Ma et al., “Effect of mangrove restoration on sediment properties and bacterial community,” Ecotoxicology, vol. 30, no. 8, pp. 1672–1679, 2021 . [CrossRef]
- N. C. Marcial Gomes, L. R. Borges, R. Paranhos, F. N. Pinto, L. C. S. Mendonça-Hagler, and K. Smalla, “Exploring the diversity of bacterial communities in sediments of urban mangrove forests,” FEMS Microbiology Ecology, vol. 66, no. 1, pp. 96–109, 2008. [CrossRef]
- A. Ghosh, R. Saha, and P. Bhadury, “Metagenomic insights into surface water microbial communities of a South Asian mangrove ecosystem,” PeerJ, vol. 10, p. e13169, 2022. [CrossRef]
- Z. Zhou, H. Wu, D. Li, W. Zeng, J. Huang, and Z. Wu, “Comparison of gut microbiome in the Chinese mud snail (Cipangopaludina chinensis) and the invasive golden apple snail (Pomacea canaliculata),” PeerJ, vol. 10, pp. 1–19, 2022. [CrossRef]
- C. Combustion and P. Engineering, “Pr ep rin t n ot pe er re v iew Pr ep rin t n ot pe er ed,” pp. 2018–2019, 1913.
- R. M. Puthusseri, H. P. Nair, T. K. Johny, and S. G. Bhat, “Insights into the response of mangrove sediment microbiomes to heavy metal pollution: Ecological risk assessment and metagenomics perspectives,” Journal of Environmental Management, vol. 298, no. April, p. 113492, 2021. [CrossRef]
- L. L. Rocha, G. B. Colares, V. L. R. Nogueira, F. A. Paes, and V. M. M. Melo, “Distinct Habitats Select Particular Bacterial Communities in Mangrove Sediments,” International Journal of Microbiology, vol. 2016, 2016. [CrossRef]
- S. Rampadarath, K. Bandhoa, D. Puchooa, R. Jeewon, and S. Bal, “Metatranscriptomics analysis of mangroves habitats around Mauritius,” World Journal of Microbiology and Biotechnology, vol. 34, no. 4, p. 59, 2018. [CrossRef]
- C.-J. Zhang, Y.-L. Chen, Y.-H. Sun, J. Pan, M.-W. Cai, and M. Li, “Diversity, metabolism and cultivation of archaea in mangrove ecosystems,” Marine Life Science & Technology, vol. 3, no. 2, pp. 252–262, 2021. [CrossRef]
- B. Fernández-Gómez et al., “Ecology of marine bacteroidetes: A comparative genomics approach,” ISME Journal, vol. 7, no. 5, pp. 1026–1037, 2013. [CrossRef]
- F. L. Thompson, T. Iida, and J. Swings, “Biodiversity of Vibrios,” Microbiology and Molecular Biology Reviews, vol. 68, no. 3, pp. 403–431, 2004. [CrossRef]
- J. L. Romalde, A. L. Diéguez, A. Lasa, and S. Balboa, “New Vibrio species associated to molluscan microbiota: A review,” Frontiers in Microbiology, vol. 4, no. JAN, pp. 1–11, 2014. [CrossRef]
- K. Kesy, M. Labrenz, B. S. Scales, B. Kreikemeyer, and S. Oberbeckmann, “Vibrio colonization is highly dynamic in early microplastic-associated biofilms as well as on field-collected microplastics,” Microorganisms, vol. 9, no. 1, pp. 1–13, 2021. [CrossRef]
- R. Metcalf, D. M. Oliver, V. Moresco, and R. S. Quilliam, “Quantifying the importance of plastic pollution for the dissemination of human pathogens: The challenges of choosing an appropriate ‘control’material,” Science of the Total Environment, vol. 810, p. 152292, 2022.
- G. Caruso et al., “Effects of microplastics on trophic parameters, abundance and metabolic activities of seawater and fish gut bacteria in mesocosm conditions,” Environmental Science and Pollution Research, vol. 25, no. 30, pp. 30067–30083, 2018. [CrossRef]
- K. Takai et al., “Sunxiuqinia faeciviva sp. nov., a facultatively anaerobic organoheterotroph of the Bacteroidetes isolated from deep subseafloor sediment,” International journal of systematic and evolutionary microbiology, vol. 63, no. Pt_5, pp. 1602–1609, 2013.
- Y. Ye et al., “Pseudomonas mangrovi sp. nov., isolated from mangrove soil,” International Journal of Systematic and Evolutionary Microbiology, vol. 69, no. 2, pp. 377–383, 2019.
- B. Yin, J.-D. Gu, and N. Wan, “Degradation of indole by enrichment culture and Pseudomonas aeruginosa Gs isolated from mangrove sediment,” International biodeterioration & biodegradation, vol. 56, no. 4, pp. 243–248, 2005.
- S. Dobretsov et al., “Living on the edge: biofilms developing in oscillating environmental conditions,” Biofouling, vol. 34, no. 9, pp. 1064–1077, 2018.
- E. North and R. L. Minton, “Diversity and predicted function of gut microbes from two species of viviparid snails,” Freshwater Mollusk Biology and Conservation, vol. 24, no. 2, pp. 104–113, 2021.
- Z. Hu et al., “Host species of freshwater snails within the same freshwater ecosystem shapes the intestinal microbiome,” Frontiers in Ecology and Evolution, vol. 12, p. 1341359, 2024.
- A. M. Cardoso et al., “Gut bacterial communities in the giant land snail Achatina fulica and their modification by sugarcane-based diet,” PloS one, vol. 7, no. 3, p. e33440, 2012.
- H. Doi, A. Chinen, H. Fukuda, and Y. Usuda, “Vibrio algivorus sp. nov., an alginate-and agarose-assimilating bacterium isolated from the gut flora of a turban shell marine snail,” International Journal of Systematic and Evolutionary Microbiology, vol. 66, no. 8, pp. 3164–3169, 2016.
- W. Guoliang, Z. Tianlun, L. Tongxia, W. Yinong, Y. Hong, and J. Shan, “Bacteriological analysis of the digestive tube of the mud snail (Bullacta exarata Philippi) and its rearing shoal,” Journal of Ocean University of Qingdao, vol. 1, pp. 161–164, 2002.
- Y. Song et al., “Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica,” Science of the Total Environment, vol. 746, p. 141289, 2020.
- M. A. Dar, K. D. Pawar, and R. S. Pandit, “Prospecting the gut fluid of giant African land snail, Achatina fulica for cellulose degrading bacteria,” International Biodeterioration & Biodegradation, vol. 126, pp. 103–111, 2018.
- C. Dussud et al., “Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters,” Environmental Pollution, vol. 236, pp. 807–816, 2018. [CrossRef]
- D. Didier, M. Anne, and T. H. Alexandra, “Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders,” Science of the Total Environment, vol. 599–600, pp. 1222–1232, 2017. [CrossRef]
- A. Alvarez et al., “Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals,” Chemosphere, vol. 166, pp. 41–62, 2017. [CrossRef]
- J. Liao and Q. Chen, “Biodegradable plastics in the air and soil environment: Low degradation rate and high microplastics formation,” Journal of Hazardous Materials, vol. 418, no. June, p. 126329, 2021. [CrossRef]
- Y. Gu et al., “Reconstruction of xylose utilization pathway and regulons in Firmicutes,” BMC Genomics, vol. 11, no. 1, pp. 1–14, 2010. [CrossRef]
- F. A. de Vogel et al., “Biodegradable plastics in Mediterranean coastal environments feature contrasting microbial succession,” Science of the Total Environment, vol. 928, p. 172288, 2024.
- G. Erni-Cassola, R. J. Wright, M. I. Gibson, and J. A. Christie-Oleza, “Early colonization of weathered polyethylene by distinct bacteria in marine coastal seawater,” Microbial ecology, vol. 79, pp. 517–526, 2020.
- A. Parthasarathy et al., “Polystyrene degradation by Exiguobacterium sp. RIT 594: preliminary evidence for a pathway containing an atypical oxygenase,” Microorganisms, vol. 10, no. 8, p. 1619, 2022.
- D. Chauhan, G. Agrawal, S. Deshmukh, S. S. Roy, and R. Priyadarshini, “Biofilm formation by Exiguobacterium sp. DR11 and DR14 alter polystyrene surface properties and initiate biodegradation,” RSC advances, vol. 8, no. 66, pp. 37590–37599, 2018.
- L. Maroof, I. Khan, H. Hassan, S. Azam, and W. Khan, “Microbial degradation of low density polyethylene by Exiguobacterium sp. strain LM-IK2 isolated from plastic dumped soil,” World Journal of Microbiology and Biotechnology, vol. 38, no. 11, p. 197, 2022.
- Y. Sun, Y. Zhang, X. Hao, X. Zhang, Y. Ma, and Z. Niu, “A novel marine bacterium Exiguobacterium marinum a-1 isolated from in situ plastisphere for degradation of additive-free polypropylene,” Environmental Pollution, vol. 336, p. 122390, 2023.
- H. Wang, J. A. Gilbert, Y. Zhu, and X. Yang, “Salinity is a key factor driving the nitrogen cycling in the mangrove sediment,” Science of the Total Environment, vol. 631, pp. 1342–1349, 2018.
- M. Ikenaga, R. Guevara, A. L. Dean, C. Pisani, and J. N. Boyer, “Changes in Community Structure of Sediment Bacteria Along the Florida Coastal Everglades Marsh–Mangrove–Seagrass Salinity Gradient,” Microb Ecol, vol. 59, no. 2, pp. 284–295, Feb. 2010. [CrossRef]
- S. P. Meera, M. Bhattacharyya, A. Nizam, and A. Kumar, “A review on microplastic pollution in the mangrove wetlands and microbial strategies for its remediation,” Environ Sci Pollut Res, vol. 29, no. 4, pp. 4865–4879, Jan. 2022. [CrossRef]
- Y. Li, L. Zheng, Y. Zhang, H. Liu, and H. Jing, “Comparative metagenomics study reveals pollution induced changes of microbial genes in mangrove sediments,” Sci Rep, vol. 9, no. 1, p. 5739, Apr. 2019. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




