Submitted:
01 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Parameters | [28] | [23] | [8] | [20] | [29] | [30] |
| pH | 3.67 | 5.80 | 5.18 | 3.90 | 4.50 | 4.71 |
| DBO (g/l) | NA | NA | NA | 29.2 | NA | 6.3 |
| DCO (g/l) | 58.790 | 37.700 | 21.690 | 101.380 | 69.830 | 10.500 |
| Total solids (g/l) | NA | NA | 16.89 | 92.9 | 57.92 | NA |
| Volatile solids (g/l) | NA | 4. 332 | 16,480 | 73,400 | 42,270 | NA |
| Azote (%) | 0.710* | 0.028* | 0.030 | 2,140 | NA | 0.525* |
| Cyanure (mg/l) | NA | NA | NA | NA | 13.2 | 2.3 |
2. Materials and Methods
2.1. Materials
2.1. Methods
2.2.1. Substrate and Inoculum Preparation
2.2.2. Immediate Substrate Characterization
2.2.1.1. Dryness
2.2.1.2. Organic Matter Content
2.2.1.3. Determination of COD
2.2.1.4. Determination of Total Kjeldahl Nitrogen (N-NTK)
2.2.1.5. Determination of Total Carbon
2.2.2. Methanization
2.2.2.1. Digester Composition
2.2.2.2. Analysis of Biogas Composition
2.2.2.3. Conversion Efficiency
3. Results
3.1. Substrate Characteristics
| Reactor | TS (%) before digestion | TS (%) post-digestion | MOV (%)Before digestion | MOV (%)post digestion |
| Reactor 1 (Chicken droppings) | 4.13 | 2.18 | 78.65 | 62.15 |
| Reactor 2 (Cassava effluent) | 4.22 | 2.59 | 91.73 | 75.01 |
| Reactor 3 (25%Fp+ 75% EUM) | 4.32 | 1.83 | 84.81 | 59.45 |
| Reactor 4 (50%Fp+ 50%EUM) | 4.42 | 1.97 | 87.89 | 61.04 |
| Reactor 5 (75% Fp+25%EUM) | 4.51 | 3.34 | 90.97 | 46.14 |
| Reactor 6 (Inoculum) | 6.66 | 3.00 | 81.95 | 59.23 |
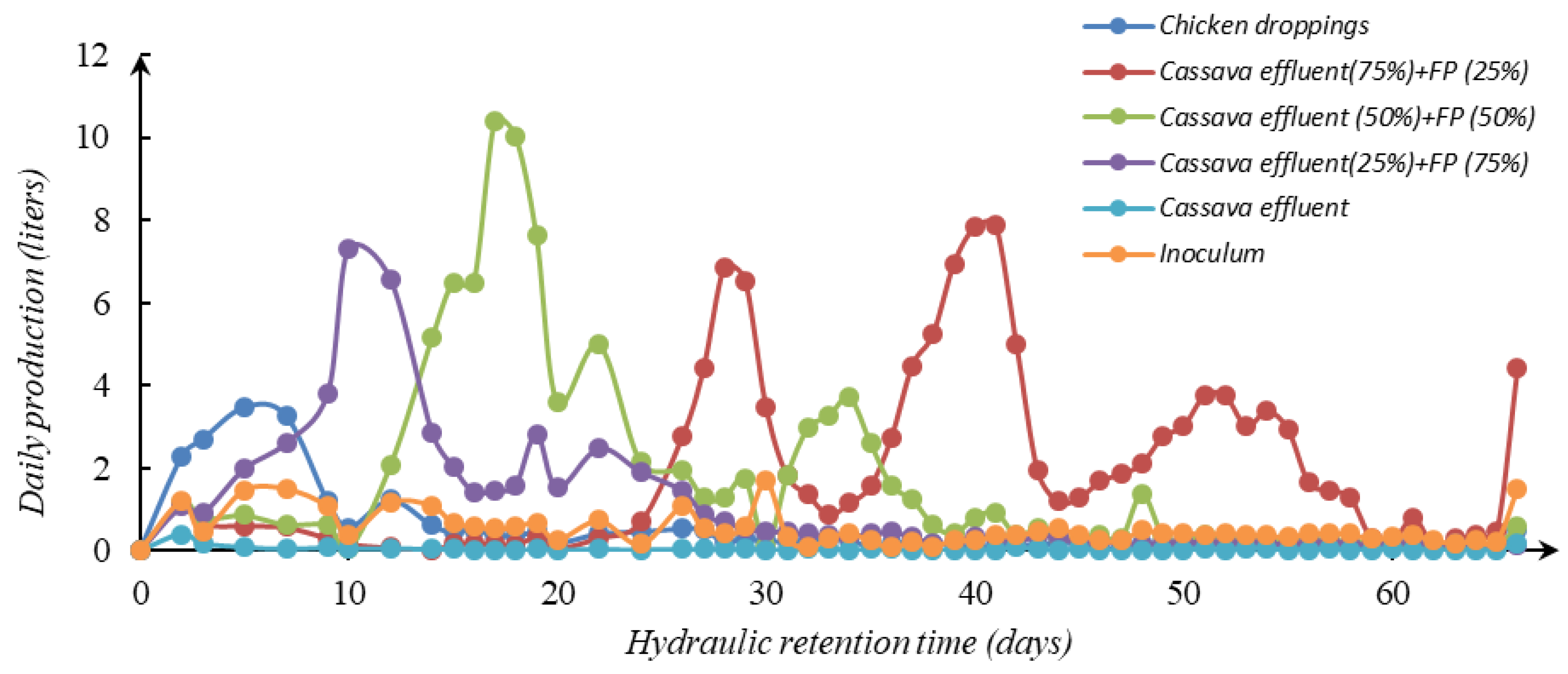
3.2. Daily Production
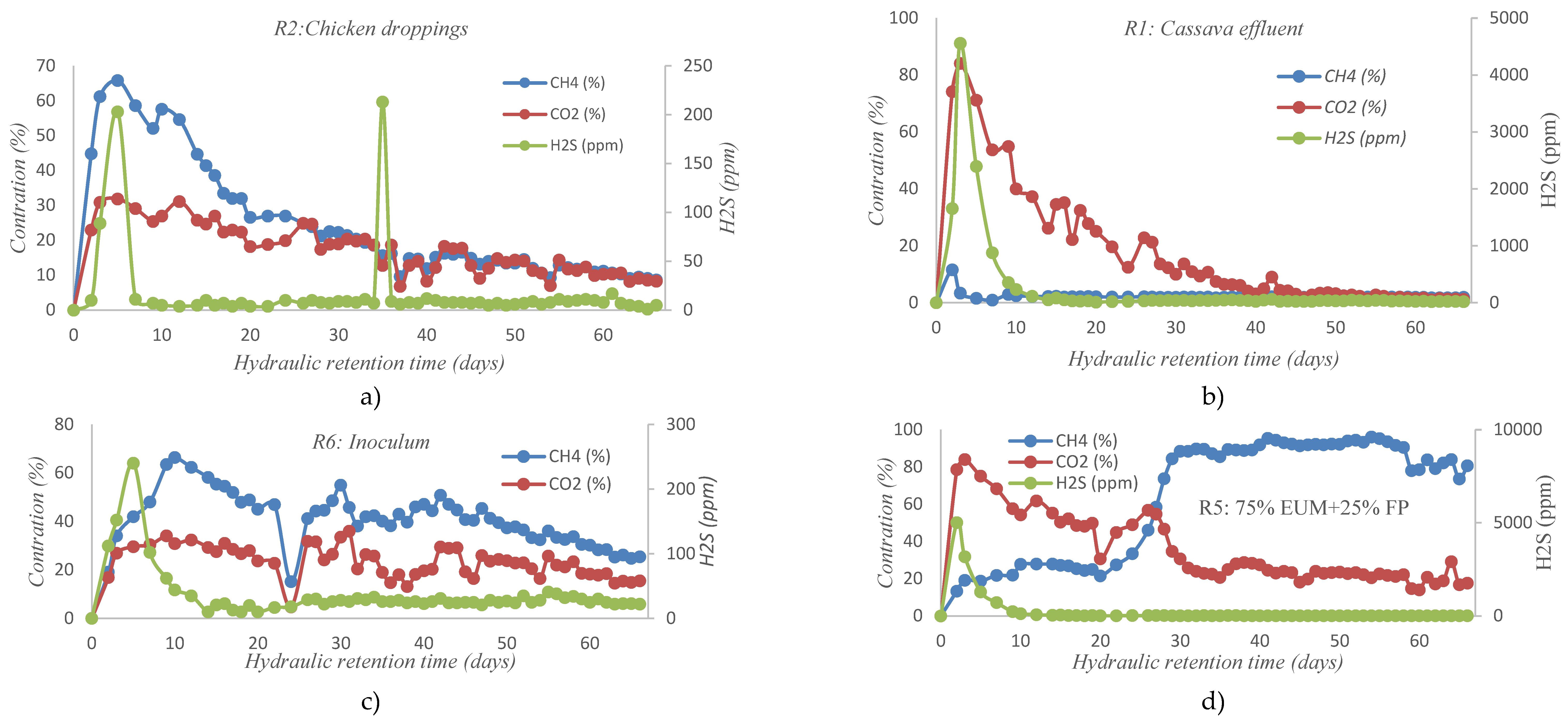
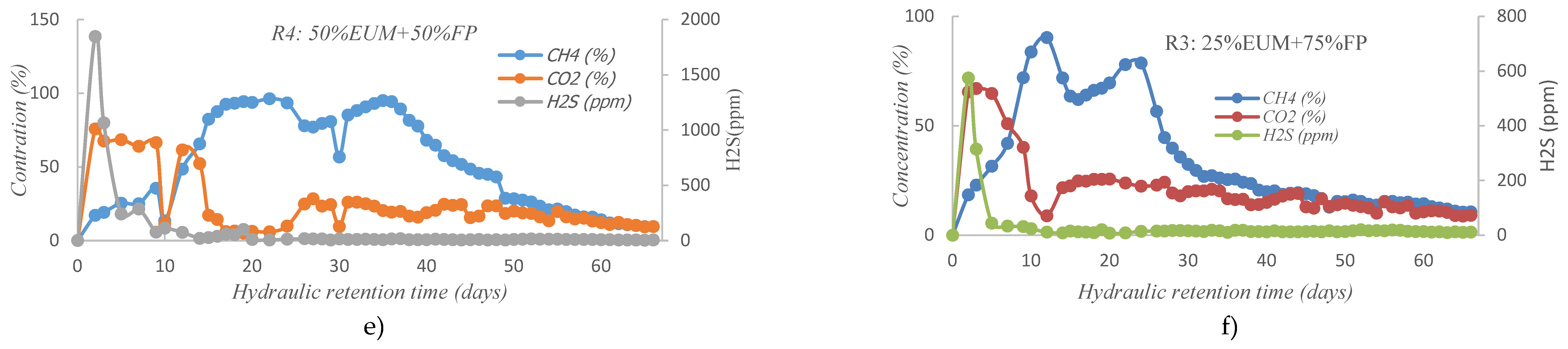
2.1. Evolution of Biogas Composition
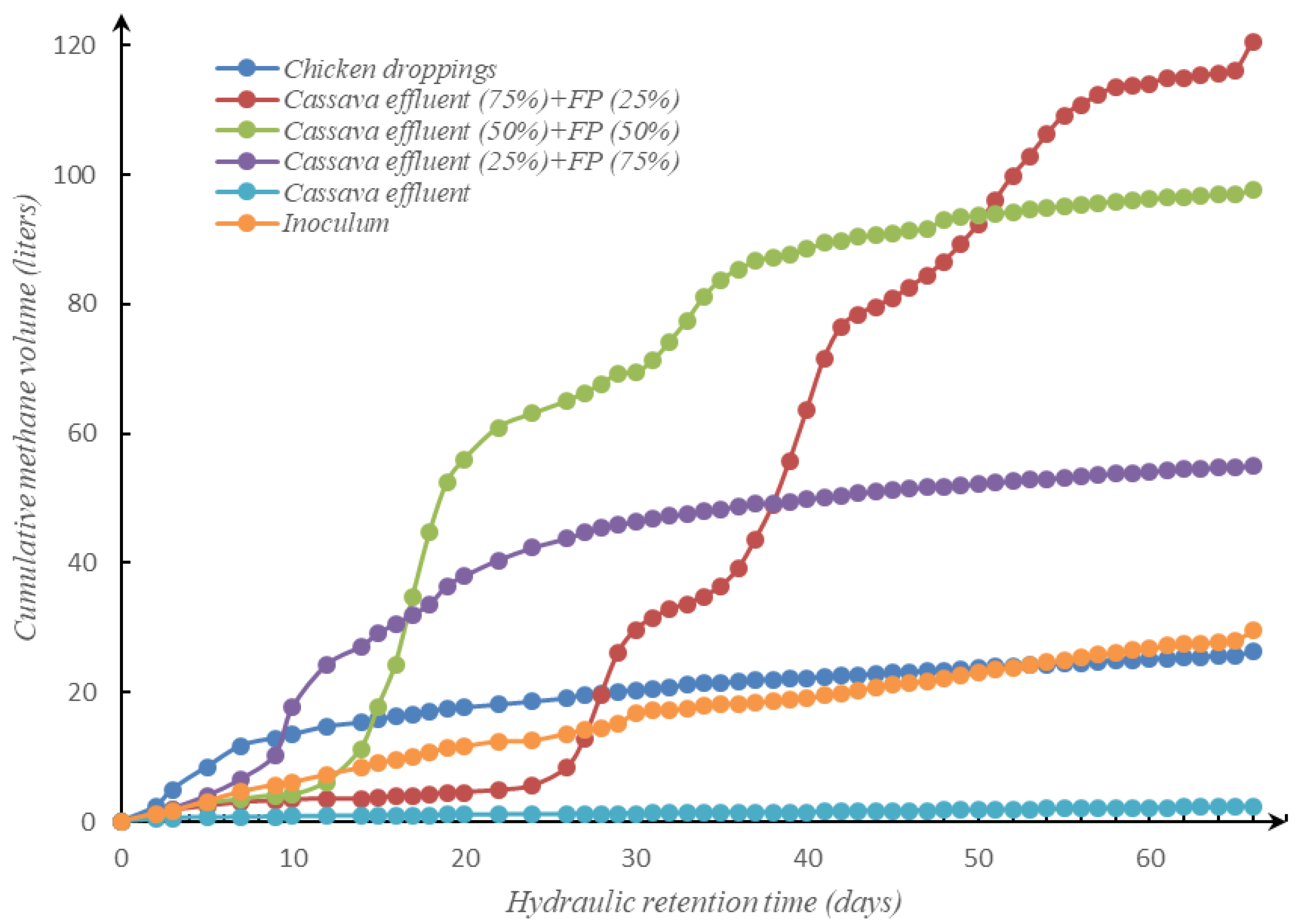
3.3. Cumulative Methane Production
3.4. Effect of Organic Nitrogen Addition on Digestion
3.5. Conversion Efficiency
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Y. Ma and Y. Liu, “Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach,” Biotechnol. Adv., vol. 37, no. 7, 2019. [CrossRef]
- S. K. Pramanik, F. B. Suja, S. M. Zain, and B. K. Pramanik, “The anaerobic digestion process of biogas production from food waste: Prospects and constraints,” Bioresour. Technol. Reports, vol. 8, p. 100310, 2019. [CrossRef]
- P. C. Slorach, H. K. Jeswani, R. Cuéllar-Franca, and A. Azapagic, “Environmental sustainability of anaerobic digestion of household food waste,” J. Environ. Manage., vol. 236, no. January, pp. 798–814, 2019. [CrossRef]
- Y. Sun, J. Zhang, Y. Pan, Y. Wang, T. Liao, and T. Song, “Short Communication A tm spheric P ollution during the implementation of pollution reduction measures,” vol. 5, pp. 789–795, 2014,. [CrossRef]
- FAO, “Conference Regionale de La FAO pour L ’Afrique,” Rabbat, 2024.
- J. C. de Carvalho, I. A. Borghetti, L. C. Cartas, A. L. Woiciechowski, V. T. Soccol, and C. R. Soccol, “Biorefinery integration of microalgae production into cassava processing industry: Potential and perspectives,” Bioresour. Technol., vol. 247, pp. 1165–1172, 2018. [CrossRef]
- N. Glanpracha and A. P. Annachhatre, “Anaerobic co-digestion of cyanide containing cassava pulp with pig manure,” Bioresour. Technol., vol. 214, pp. 112–121, 2016. [CrossRef]
- P. Wadjeam, A. Reungsang, T. Imai, and P. Plangklang, “Co-digestion of cassava starch wastewater with buffalo dung for bio-hydrogen production,” Int. J. Hydrogen Energy, vol. 44, no. 29, pp. 14694–14706, 2019. [CrossRef]
- A. Cruz et al., “Valorization of cassava residues for biogas production in Brazil based on the circular economy: An updated and comprehensive review,” Clean. Eng. Technol., vol. 4, no. December 2020, p. 100196, 2021. [CrossRef]
- S. Sánchez, Y. L. Silva, R. A. Kalid, E. Cohim, and E. A. Torres, “Waste bio-refineries for the cassava starch industry: New trends and review of alternatives,” Renew. Sustain. Energy Rev., vol. 73, no. February, pp. 1265–1275, 2017. [CrossRef]
- Y. Shapovalov, S. Zhadan, G. Bochmann, A. Salyuk, and V. Nykyforov, “Dry anaerobic digestion of chicken manure: A review,” Appl. Sci., vol. 10, no. 21, pp. 1–24, 2020. [CrossRef]
- W. CZEKAŁA, J. DACH, A. LEWICKI, K. GAJEWSKA, and Ż. STASZAK, “Utilization of Digestate Obtained From Methane Fermentation of Chicken Manure,” in Farm Machinery and Processes Management in Sustainable Agriculture, 2017, pp. 92–96. [CrossRef]
- F. Abouelenien et al., “Optimization of biomethane production via fermentation of chicken manure using marine sediment: A modeling approach using response surface methodology,” Int. J. Environ. Res. Public Health, vol. 18, no. 22, 2021. [CrossRef]
- Konkol, L. Świerczek, and A. Cenian, “Chicken Manure Pretreatment for Enhancing Biogas and Methane Production,” Energies, vol. 16, no. 14, 2023. [CrossRef]
- S. M. Ashekuzzaman and T. G. Poulsen, “Optimizing feed composition for improved methane yield during anaerobic digestion of cow manure based waste mixtures,” Bioresour. Technol., vol. 102, no. 3, pp. 2213–2218, 2011. [CrossRef]
- M. N. I. Siddique and Z. A. Wahid, “Achievements and perspectives of anaerobic co-digestion: A review,” J. Clean. Prod., vol. 194, pp. 359–371, 2018. [CrossRef]
- Beniche, “Optimisation Du Rapport Carbone / Azote Des Résidus Agricoles Et Des Déchets Alimentaires Par Co-Diges,” UNIVERSITE IBN TOFAIL, 2021. [Online]. Available: https://helvia.uco.es/xmlui/bitstream/handle/10396/21433/2021000002272.pdf?sequence=1&isAllowed=y.
- H. Jiang, Y. Qin, S. I. Gadow, A. Ohnishi, N. Fujimoto, and Y. Y. Li, “Bio-hythane production from cassava residue by two-stage fermentative process with recirculation,” Bioresour. Technol., vol. 247, pp. 769–775, 2018. [CrossRef]
- Y. Njankouo Ndam et al., “Influence of cultivars and processing methods on the cyanide contents of cassava (Manihot esculenta Crantz) and its traditional food products,” Sci. African, vol. 5, 2019,. [CrossRef]
- S. Peres, M. R. Monteiro, M. L. Ferreira, A. F. do Nascimento Junior, and M. de Los Angeles Perez Fernandez Palha, “Anaerobic Digestion Process for the Production of Biogas from Cassava and Sewage Treatment Plant Sludge in Brazil,” Bioenergy Res., vol. 12, no. 1, pp. 150–157, 2019,. [CrossRef]
- J. G. Ferreira Madeira et al., “Optimum Co-Digestion Ratio of Cattle Manure and Manipueira in a Single-Stage Anaerobic Digester for Biogas Production,” Clean - Soil, Air, Water, vol. 48, no. 12, 2020. [CrossRef]
- M. C. C. De Amorim, P. T. De Souza Silva, P. S. Barbosa, and N. E. Montefusco, “Anaerobic biodegradation of cassava wastewater under different temperatures and inoculums,” Comun. Sci., vol. 10, no. 1, pp. 65–76, 2019. [CrossRef]
- L. M. Villa, A. C. A. Orrico, L. A. Akamine, J. de L. Junior, and N. da S. Sunada, “Anaerobic co-digestion of swine manure with sweet potato or cassava in different c/n ratios,” Cienc. Rural, vol. 50, no. 10, pp. 1–9, 2020. [CrossRef]
- Jiraprasertwong, K. Maitriwong, and S. Chavadej, “Production of biogas from cassava wastewater using a three-stage upflow anaerobic sludge blanket (UASB) reactor,” Renew. Energy, vol. 130, pp. 191–205, 2019. [CrossRef]
- Mao, Y. Feng, X. Wang, and G. Ren, “Review on research achievements of biogas from anaerobic digestion,” Renew. Sustain. Energy Rev., vol. 45, pp. 540–555, 2015. [CrossRef]
- P. Thanwised, W. Wirojanagud, and A. Reungsang, “Effect of hydraulic retention time on hydrogen production and chemical oxygen demand removal from tapioca wastewater using anaerobic mixed cultures in anaerobic baffled reactor (ABR),” Int. J. Hydrogen Energy, vol. 37, no. 20, pp. 15503–15510, 2012. [CrossRef]
- S. D. M. L. Corbari, C. L. Andreani, D. G. B. Torres, F. Eng, and S. D. Gomes, “Strategies to improve the biohydrogen production from cassava wastewater in fixed-bed reactors,” Int. J. Hydrogen Energy, vol. 44, no. 32, pp. 17214–17223, 2019. [CrossRef]
- K.-K. Nazo Edith, K. K. Felix, K. Y. Francis, G. Theophile, and T. Kablan, “Characterization of Waste From Attiéké Factory: Case of Azito Village (Abidjan, Côte d’Ivoire),” Eur. Sci. Journal, ESJ, vol. 12, no. 35, p. 73, 2016. [CrossRef]
- L. R. S. Andrade et al., “Oyster shell-based alkalinization and photocatalytic removal of cyanide as low-cost stabilization approaches for enhanced biogas production from cassava starch wastewater,” Process Saf. Environ. Prot., vol. 139, pp. 47–59, 2020. [CrossRef]
- H. Sun, I. Angelidaki, S. Wu, R. Dong, and Y. Zhang, “The potential of bioelectrochemical sensor for monitoring of acetate during anaerobic digestion: Focusing on novel reactor design,” Front. Microbiol., vol. 10, no. JAN, pp. 1–10, 2019. [CrossRef]
- Prayitno and S. Rulianah, “Production of biogas using AnF2B reactor from cassava starch wastewater with consortium bacteria as biocatalyst,” IOP Conf. Ser. Earth Environ. Sci., vol. 969, no. 1, pp. 2–8, 2022. [CrossRef]
- K. Sharma, P. K. Sahoo, M. Mukherjee, and A. Patel, “Assessment of Sustainable Biogas Production from Co-Digestion of Jatropha De-Oiled Cake and Cattle Dung Using Floating Drum Type Digester under Psychrophilic and Mesophilic Conditions,” Clean Technol., vol. 4, no. 2, pp. 529–541, 2022. [CrossRef]
- M. Mirabi, M. Karrabi, and B. Shahnavaz, “Anaerobic co-digestion of lignocellulosic/lipidic wastes with cattle manure: Investigating biogas production and methane yield,” Fuel, vol. 366, no. June, p. 131286, 2024. [CrossRef]
- N. E. Kpata-Konan, K. M. Kouamé, Y. F. Kouamé, S. A. Yaovi, K. F. Konan, and T. Gnagne, “Anaerobic Digestion of Liquid Waste from an Attiéké Factory: From the Experimental Scale to the Semi-Industrial Scale,” J. Environ. Prot. (Irvine,. Calif)., vol. 11, no. 07, pp. 531–539, 2020. [CrossRef]
- Moukazis, F. M. Pellera, and E. Gidarakos, “Slaughterhouse by-products treatment using anaerobic digestion,” Waste Manag., vol. 71, pp. 652–662, 2018. [CrossRef]
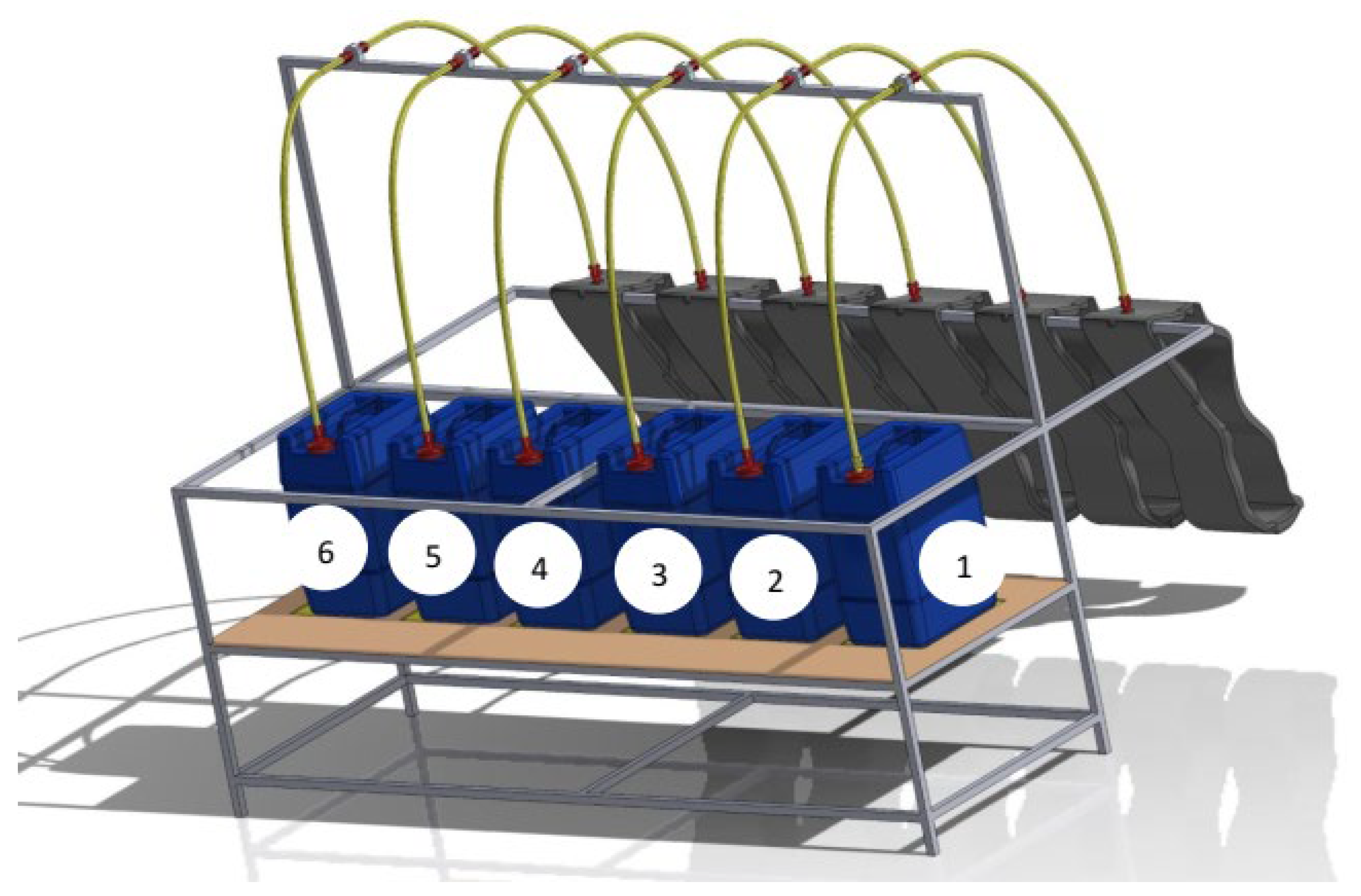
| Reactor | Ratio | Substrate volume (FP: EUM) (liters) | Inoculum volume (liters) |
| 1 | 100:00 | 10.5:0 | 4.5 |
| 2 | 00:100 | 0:10.5 | |
| 3 | 25:75 | 2,625:7,875 | |
| 4 | 50:50 | 5.25:5.25 | |
| 5 | 75:25 | 7,875:2,625 | |
| 6 | Inoculum | - | 15 |
| Substrat | Dry matter (DM (%)) | Organic matter (MOV (%)) | N (%) | C/N | DCO (mgO2/l) |
| Cassava effluent | 3.60 | 94.83 | 0.028 | 761.75 | 208,350 |
| Chicken droppings | 97.96 | 77.24 | 1.71 | 20.48 | NA |
| Inoculum | 6.66 | 81.95 | NA | NA | NA |
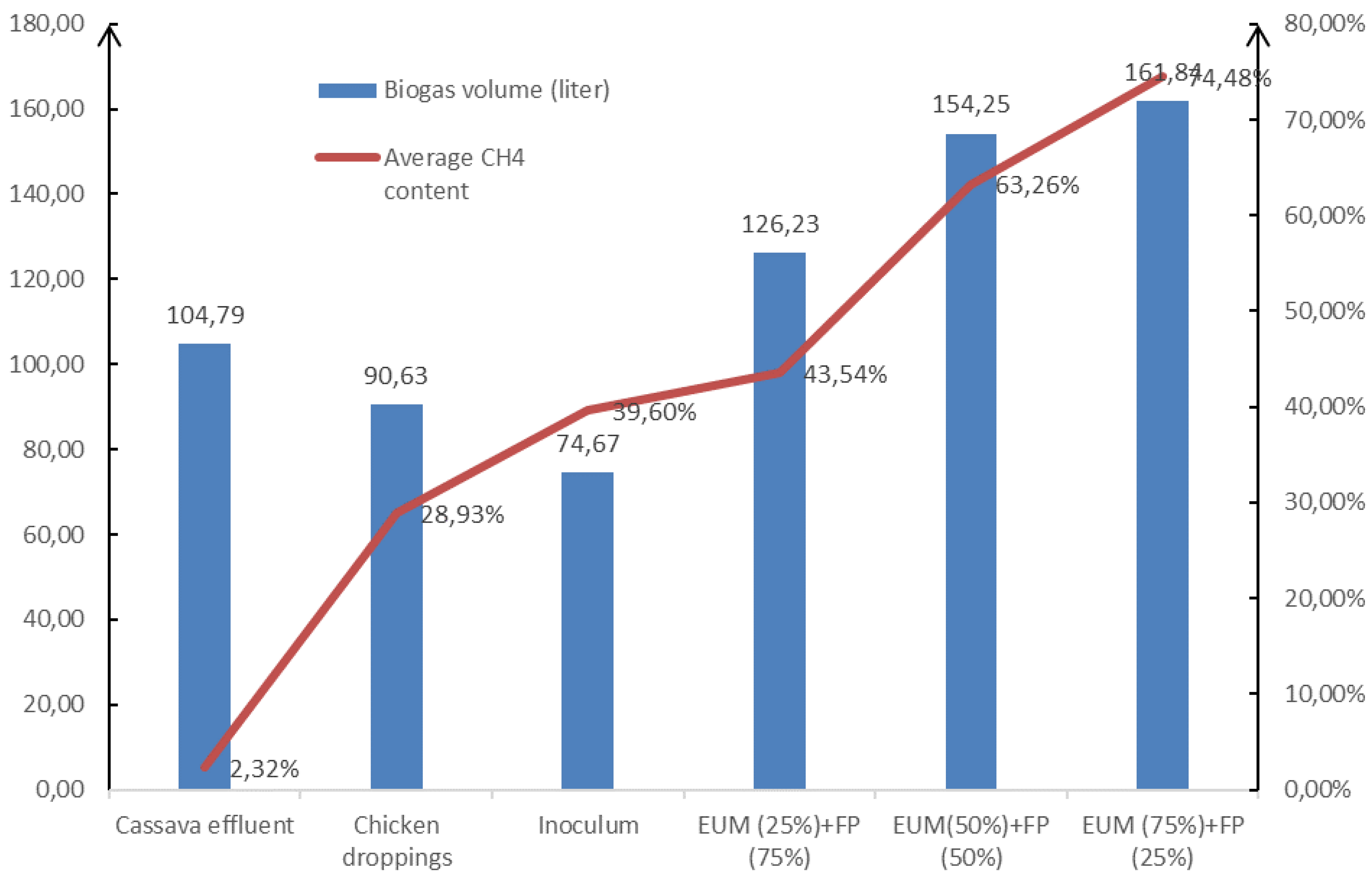
| Substrat | R1 (Cassava effluent) | R2 (Chicken droppings) | R3 (EUM (25%)+FP (75%)) | R4 (EUM (50%)+FP (50%)) | R5 (EUM (75%)+FP (25%)) | R6 (Inoculum) |
| Biogas volume (liter) | 104.79 | 90.63 | 126.23 | 154.25 | 161.84 | 74.67 |
| Methane volume (liter) | 2.43 | 26.22 | 54.96 | 97.57 | 120.54 | 29.57 |
| BMP (mL/g MOV) | 45.47 | 585.27 | 527.39 | 1184. 60 | 1075.27 | 290.52 |
| Reactors | Conversion efficiency (%) |
| R1 (Cassava effluent) | 18.23 |
| R2 (Chicken droppings) | 20.46 |
| R3 (EUM (25%)+FP (75%)) | 30.55 |
| R4 (EUM (50%)+FP (50%)) | 49.28 |
| R5 (EUM (75%)+FP (25%)) | 27.72 |
| R6 (Inoculum) | 29.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




