Introduction
Pesticide, the combination of the English word “pest” and Latin word “cide” means “killer” (Hoy et al., 2023). Pesticides are mixture substances uses to control pests in agricultural lands, gardening, and also uses to control the vectors of diseases in normal human life (FAO 2002). Depending on the nature of target organisms, they are classified into insecticides, herbicides, fungicides, and etc. On the basis of chemical nature there are also classified in to inorganic pesticides which carries the arsenic, fluoride substances. And organic pesticides which are organophosphates, carbamates, pyrethroid, etc. (Bjorling-Poulsen et al. 2008). Additionally, pesticides are utilised in the health sector to eliminate disease-carrying insects like mosquitoes, flies, and cockroaches (Belluco et al., 2023). During the Green Revolution, pesticides were used more than necessary (
Table 1). As a result, biodiversity, soil health, and crop quality have been affected (Baweja et al., 2020). Pesticides are always spread through agriculture.
Nature and effects of pesticides on aquatic organisms
It has been discovered that the frequent application of pesticides including nitrogen, phosphate, and potassium increases the soil’s concentration of several elements, such as Zn, Pb, Ni, and Cr, which were previously only sometimes detected in soluble forms (Song et al., 2019). Synthetic pesticides have somewhat more solubility into lipids and organic substances but remain largely solvable due to being lipophilic in nature. Because of their endurance and toxicity to aquatic life, synthetic pesticides have been the focus of substantial studies to ascertain their toxicity for a certain range of species. Synthetic pesticides such as pyrethroid and organophosphate insecticides act as a toxicant for aquatic organisms (Kadiru et al., 2022). The major disadvantages of organophosphate insecticides are that their toxicity to mammals is considerable, and pyrethroids are very harmful to aquatic organisms (Goh et al., 2022). Pesticides are known to cause disruptions in the photosynthesis, respiration, and biosynthesis processes of microorganisms together with cell development, separation as well as molecular makeup.
Pesticides have become a threat to biodiversity
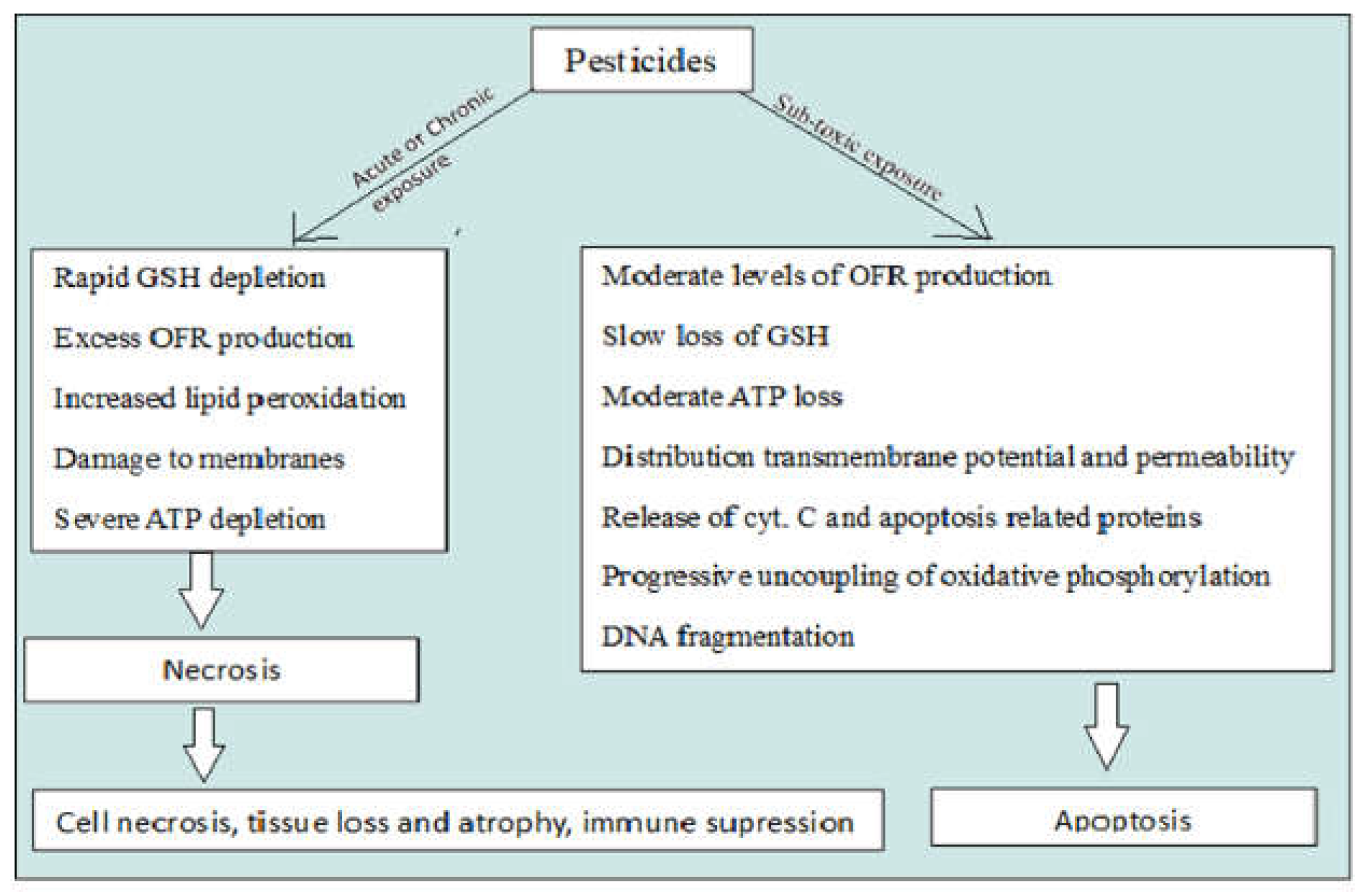
The harmful action of pesticides can play a vital role in biodiversity loss (AYDINALP et al., 2004). Pesticides are toxic substances, which means they can cause temporary, transient, or permanent impairment of one or more functions, impairments that can progress to total annihilation or death after entering the body at a relatively high dose once or long term process (Chaudhary et al., 2023) (
Figure 1). Day by day these pesticides become part of the ecosystem which means that the effects of pesticides in mammals is a serious matter to discuss as well as the effects on invertebrates (Bernardes et al. 2015). On one hand, Organophosphates and other pesticides are toxic in mammals on the other hand various insecticides are toxic to aquatic organisms (Mathivanan et al., 2023). The detrimental impact on soil denitrification and denitrifying microorganisms was noted (Alho et al., 2008). Algae and other aquatic vegetative creatures develop more quickly when the phosphorus level of the water is increased.
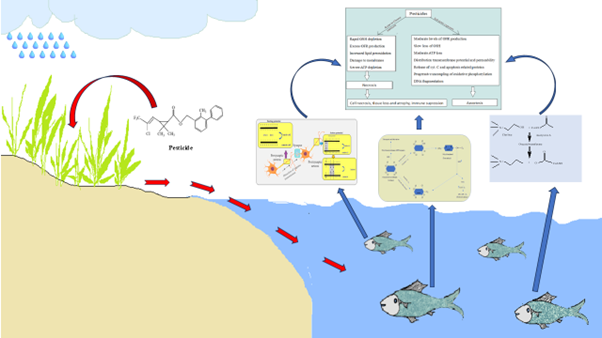
Route of pesticides enter into the water bodies and accumulate by aquatic organisms
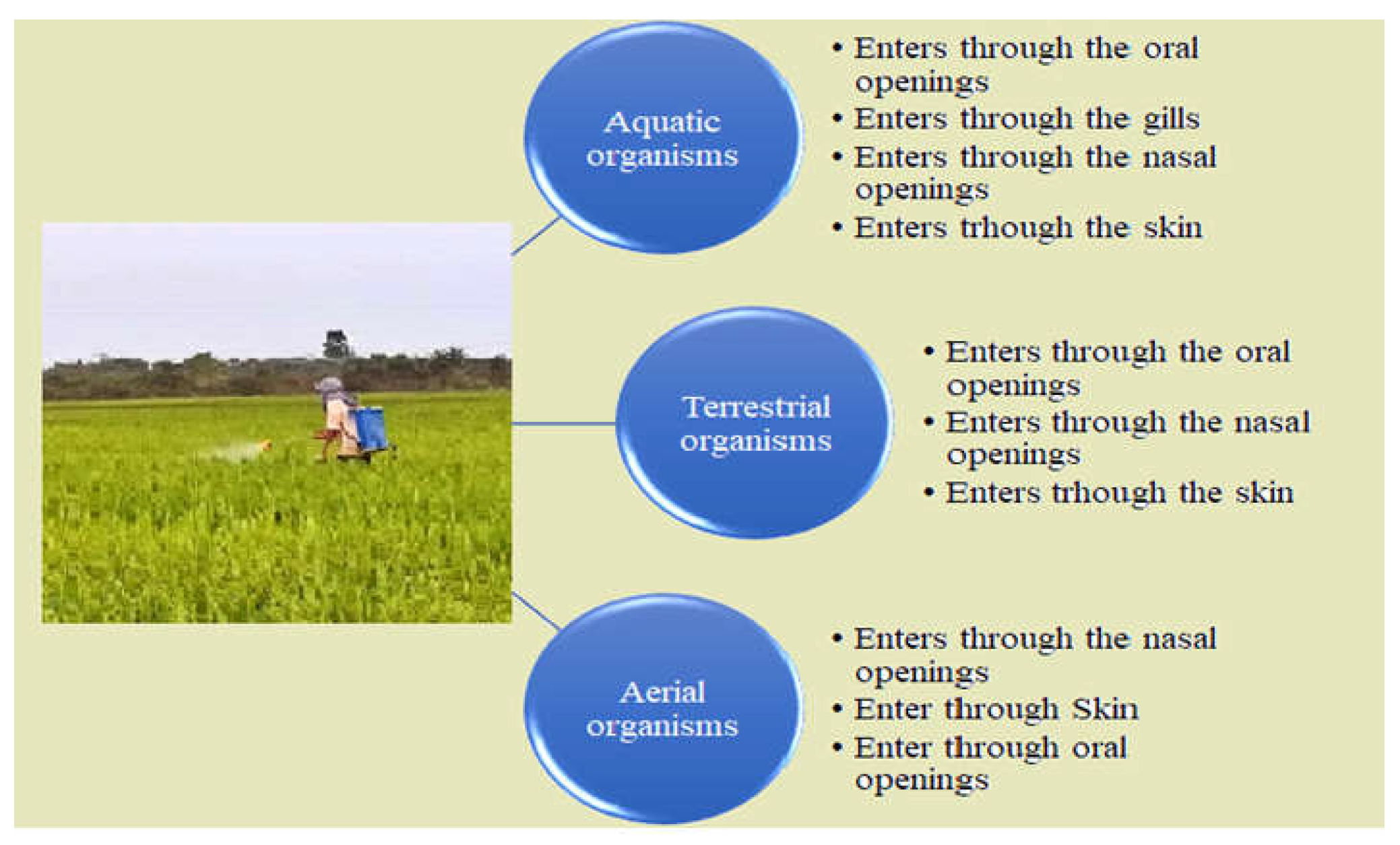
These pesticides may contaminate the drainage and irrigation system during agricultural activities. It can easily enter into the body through oral opening, nasal openings, skin contact and in fact through the gills in aquatic organisms (Ravula et al. 2021) (
Figure 2). It also spread through the ground water (Wanner et al., 2023). Pesticides are most commonly used by farmers as a shield against harmful organisms but a number of non-target organisms, such as freshwater organisms, are also adversely affected (Rajak et al., 2023). Aquatic organisms can counteract the effects of pesticide stress through their own toxic resistance mechanism (Regoli et al., 2014). Pesticides are nondegradable or less degradable ability in nature so if a limited quantity of pesticide use is may be tolerable but excessive use of pesticide cannot degrade in soil (Nahar et al., 2023) and when it comes contact with aquatic organisms it shows the harmful nature by reduce their growth rate, their reproductive rate and also their sensory organ’s activity which is very dangerous for aquatic organisms. Because aquatic organisms play vital roles in the ecological food chain. By their normal activity in nature helps the ecosystem clean. So the harmful activity of pesticides in aquatic organisms also creates a serious topic to think about.
Pesticides can hamper molecular mechanisms in aquatic organisms
Due to their lipophilic nature, pesticides can hamper lipid metabolism in aquatic organisms. Due lipophilic nature pesticides can easily penetrate the lipid layer of cells in aquatic organisms which cause oxidative stress (Bashir et al., 2015). Oxidative stress leads to produce excessive reactive oxygen species (ROS) which cause molecular damages in aquatic organisms. Pesticide stress may induce the heat shock protein in aquatic organisms. So we need to find out the mode of action of pesticides in aquatic organisms. Pyrethroid insecticides are one of the synthetic insecticide which can triggres the lipid peroxidation in aquatic organism by reaction with the polyunsaturated fatty acids. Free radicals production indicates the cellular defence mechanisms fails which leads to the cellular death. Free radicals production cause the depletion of GSH in tissue. In aquatic organisms free radical production is normal process due to water pollution so their body defence mechanism against the production of free radicals is very high but when the pesticide particles mixed in to the water they can induces the lipid peroxidation by chain reaction process then their normal body defence mechanisms fails (Valavanidis et al., 2006). Many pesticides possess the capacity to induce apoptosis by altering redox homeostasis, which is brought on by a reduction in defences against ROS production in aquatic organisms (Lushchak et al., 2011). An important indicator of electrophilic (and oxidised) substances, such as xenobiotic metabolites, is the activation of transcription-dependent p53 signalling cascades and SAPKs, or stress-activated protein kinases, like N-terminal kinases of c-Jun (JNK). SAPK activation also causes apoptotic cell death. To address and mitigate the deleterious impacts of elevated intracellular reactive species levels, which therefore initiate apoptotic pathways, Moreover, pesticides trigger the activation of defence mechanisms such DNA repair Signalling cascades involving phosphatidylinositol 3-kinase/mitogen-activated protein kinase (MAPK/PI3K) as well as the activation of antioxidant defenses. Apoptotic and survival signalling pathways are typically activated in the moment of pesticide disclosure (Franco et al., 2009). Within aquatic insect larvae pesticides act as enzymatically as cytochrome P450 and as well as hydrolyzed carboxylesterases as main compound conjugates endogenously (Katagi et al., 2010). In previous works researchers see the enzymatic activity after pesticide exposure, immunological studies also doing for study the pesticides exposure level, various gene expression study also done to see the effects of pesticides in aquatic invertebrates have all been studied, but the work has mostly focused on annelida, arthropods, and mollusca. The goal of this essay is to review the previous scientific works on The way that insecticides work in watery organisms additionally identify any immediate areas that may require additional research.
Figure 3.
Pesticides can hamper molecular mechanisms in aquatic organisms.
Figure 3.
Pesticides can hamper molecular mechanisms in aquatic organisms.
Mechanism of Action of Pesticides
1. Organophosphate (OP)
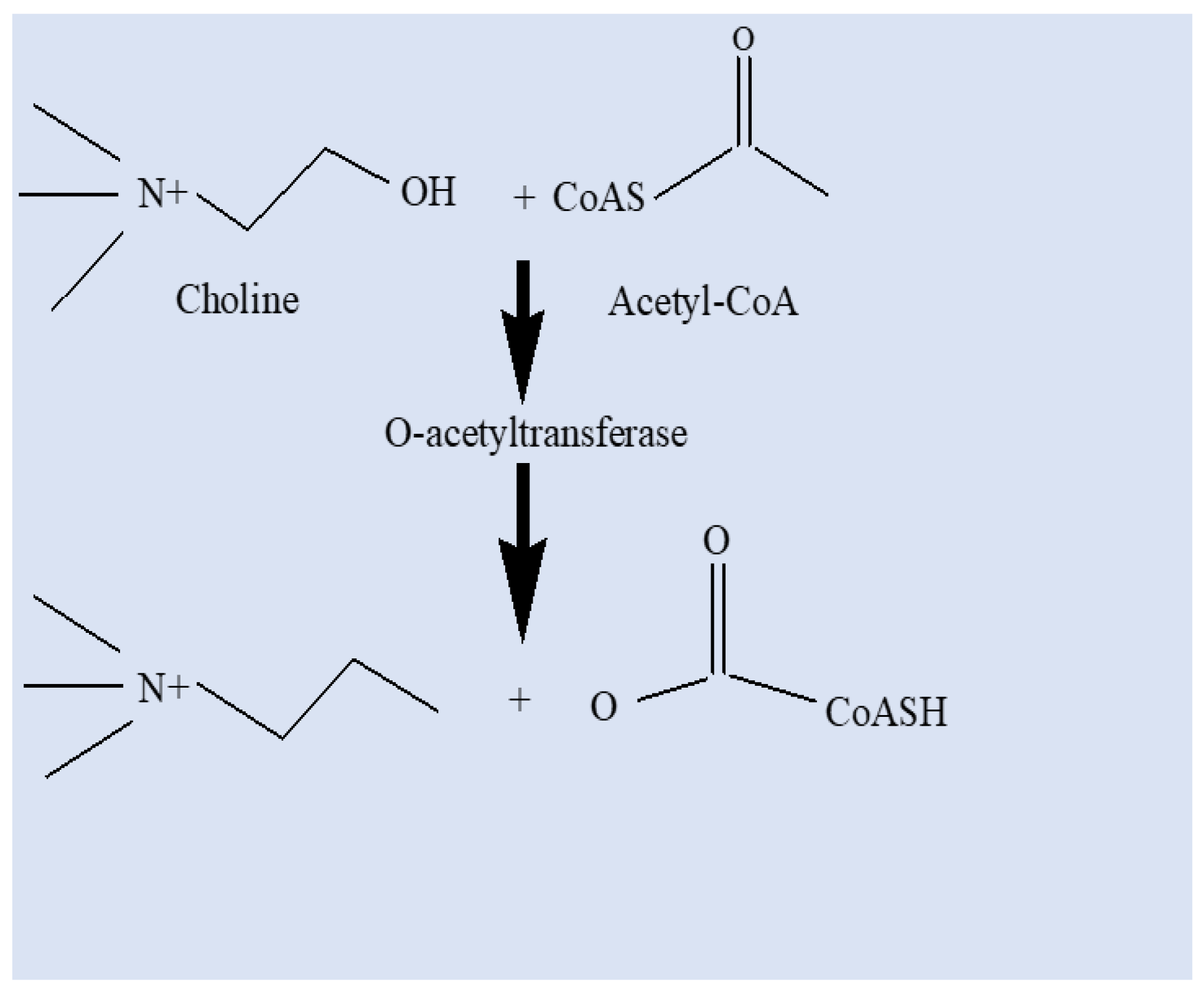
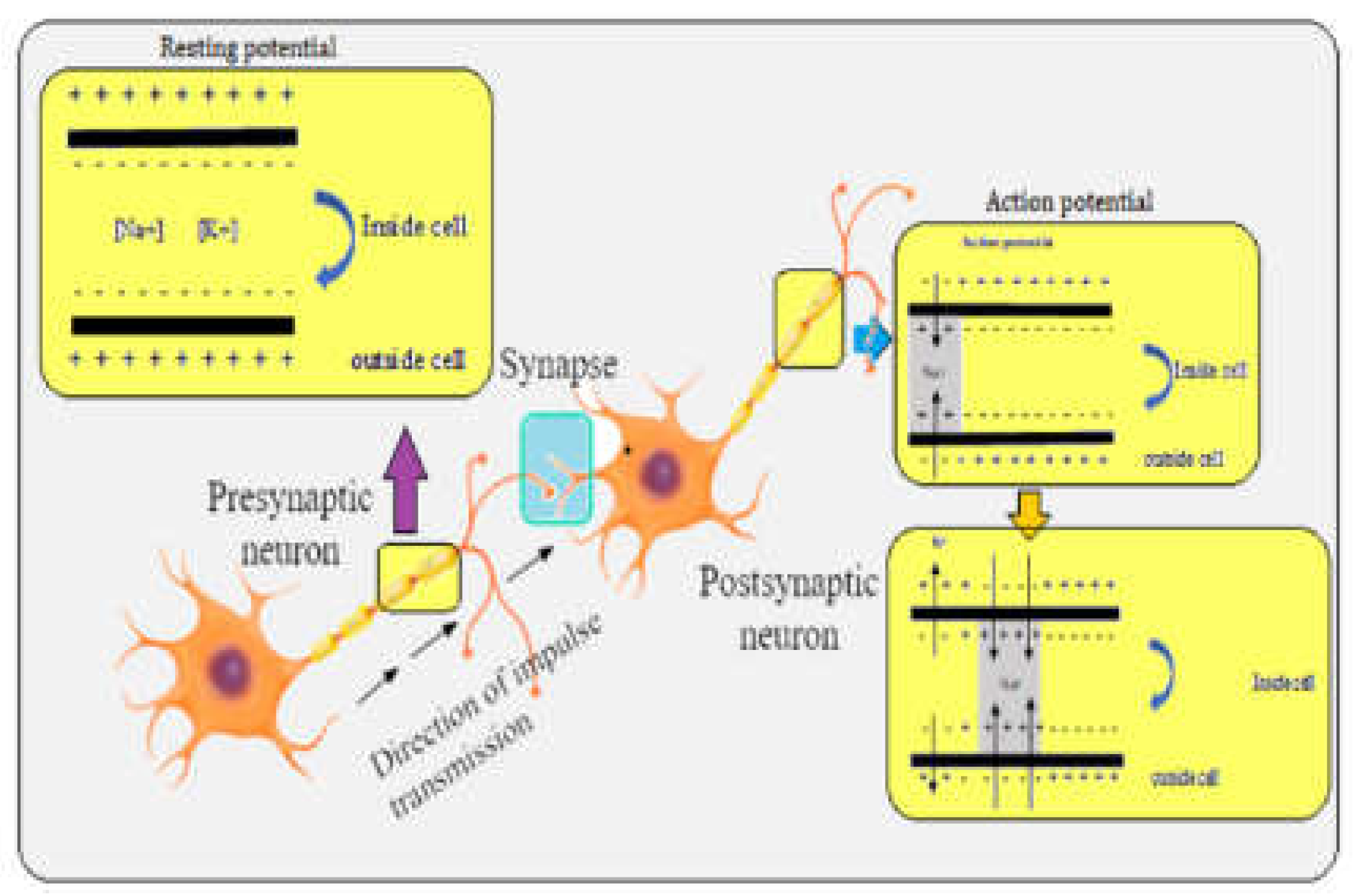
Phosphorus-containing insecticides should known as Organophosphates(OP). Some terms that were in vogue a while back are cousins of nerve fumes, esters of phosphoric acid, and phosphorus insecticides. Since they are generated from one of the phosphorus acids, organophosphates are the most dangerous class of insecticides to vertebrates overall. The “nerve gases” and OP chemical structures are similar, and as a result, so are their modes of action. At first, the finding was discovered in an effort to find alternatives to nicotine, which was in short supply in Germany yet was widely utilised as an insecticide. At least five different structural diversity types are present in organophosphate (OP) insecticides: (i)phosphates, (ii)thiophosphates,(iii)dithiophosphates, (iv)phosphnoates, and (v)phosphroamides (Costa et al.,2008). Alkoxy, substituted, and acid derivative amino groups are varied chemical chains linked to the phosphorus base, and most organophosphorus insecticides follow this molecular pattern. This comprises the aminohalogen phosphates (Dimefox), orthophosphates (dichlorovos), thionphosphates (EPN), dithiophosphates (malathion), orthothionphosphates (diazinon, parathion), and pyrophosphates (TEPP). Although there are wide Variations in this group’s toxicity, environmental Often, persistence is not a property of the parent substance. The problem of organophosphates’ toxicological impression on aquatic life remains extremely challenging when combined with acaricides, nematicides, and rodenticides (Parra-Arroyo et al., 2022). Aquatic species’ peripheral and central nervous systems include the acetylcholinesterase (AChE) enzyme, which organophosphate (OP) pesticides aim to inhibit (Fulton et al., 2001). Ops’ principal target is AChE, a B-esterase with a physiological function that is to hydrolyze the essential neurotransmitter acetylcholine. Acetylcholine (ACh) could not be broken down by the affected organism into acetic acid and choline. Non-decomposed AChs build in synapses and cause constant neuronal stimulation that eventually kills aquatic life (Jeon et al., 2023). The action of the enzyme on the physiological substrate is hindered by OPs containing a P moiety with O, which A hydroxyl group is phosphorylated on the serine part at the enzyme’s active site. Water hydrolyzes phosphorylated AChE extremely slowly. Conversely, however, Because of their high neurological system resemblance to insects, organic pollutants (OPs) may be extremely hazardous to non-target creatures, such as terrestrial and aquatic vertebrates. Phosphorylated orthophosphates (OPs) have in the active region, a P moiety with O phosphorylates a hydroxyl bond on serine an enzyme to stop it from functioning on the physiological substrate. The esteratic site of the enzyme and the phosphorus atom form a significantly more stable connection than the acetate’s carbonyl carbon (in acetylcholine). The enzyme-carbon bond can be broken in a matter of microseconds, However, depending on the OP’s chemical makeup, the phosphorus-enzyme relationship may take a while to break (Costa et al., 2008). Acetylcholine builds up at cholinergic synapses when AChE is inhibited, overstimulating nicotinic and muscarinic receptors and resulting in neurological problems (Colovic et al., 2013). Indeed, even brief exposures to OPs can have negative neurological repercussions (Fulton et al., 2001). Acetylcholine and other neurotransmitters have unique trophic functions in the development of the central and peripheral nervous systems of freshwater species (Slotkin et al., 2004). Acetylcholine buildup as a result of OPs inhibiting AChE may therefore potentially impede this development (Colovic et al., 2013). The majority of investigations have discovered a link between increased neurologic symptom prevalence in freshwater organisms and OP exposure (Kamel et al., 2004). Exposure to chlorpyrifos during pregnancy modifies the programming of synapse formation. Additionally, fish introduced to the sublethal dosage of dichlorvos showed less inhibition of enzyme activity and higher oxidation and reduction in glutathione levels when subjected to the lethal quantity of organophosphate insecticide (Peña-Llopis et al., 2003). In both adult zebrafish, triphenyl phosphate (TPP) upsets the balance of sex hormones. Fish require thyroid stimulating hormone, also known as TSH, to control the growth and development of the thyroid gland, which produces and secretes T4 and, to a lesser extent, T3. Because these hormones are critical for an organism’s growth, development, and energy consumption, fluctuations in thyroid hormone control have significant toxicological ramifications. In order to control thyroid hormones, the intricate web that makes up the hypothalamus-pituitary-thyroid (HPT) circuit is essential. Prior research has demonstrated that organophosphates can alter the expression of the trα and trβ genes, allowing triphenyl phosphate (TPP) to communicate with thyroid hormone transporters in fish pituitary glands (Freitas et al., 2011).
Figure 4.
Mode of action of organophosphates.
Figure 4.
Mode of action of organophosphates.
2. Carbamates
Carbamate insecticides are made from carbamic acid, same like OPs are from phosphoric acid. Like the OPs, they also work by blocking the crucial cholinesterase (ChE) enzyme in freshwater organisms. Carbaryl (Sevin®). In 1956 the researchers saw the introduction of the first efficient carbamate pesticide. Its usage is more than that for each of the other carbamates combined. When it comes to suppressing cholinesterase (ChE), carbamates and OPs function nearly the same in biological systems, with two notable deviations. Occasionally, carbamates’ selectivity against the ChE of different species can be stronger, and some of them are potent aliesterase inhibitors (a class of aliphatic esterases whose specific activities are unknown). Secondly, the inhibition of ChE by carbamates is reversible. When a carbamate blocks CHE, it is referred to as being carbamylated, much like an OP induces an enzyme to become phosphorylated. Some carbamates lose their stability in boiling water and emit an isocyanate odour as a result of the quick breakdown of quaternary ammonium groups. Due to the lack of a cholinergic neural-muscular junction in insects and the corresponding impermeability within the insect nerve membrane to ionised compounds, this type of smell breaks down and releases (Casida et al., 1963). According to Thompson et al., 1999, The interaction among the OP and the serine at the enzyme’s active site inhibits B esterases, the category to which ChE belongs. The phosphorylated enzyme takes a very long time to reactivate after this very low inhibition concentration. Carbamate molecules have the ability to carbamylate the serine, which is a moiety in the enzyme’s active region of B esterase, however, the connection is weaker, and carbamylated esterase can spontaneously reactivate over time in response to dilution or substrate competition. According to a study, the fish Sarotherodon mossambic’s acetylcholinesterase activity is inhibited by the carbamate insecticide Sevin. According to Brzezinski and Ludwicki,1973, an increase in acetylcholine levels occurs when the action of acetylcholinesterase is inhibited. Increased catecholamine release from this state may increase the level of cyclic AMP, which could therefore have an impact on the function of the enzymes responsible for glycogenolysis and synthesis. Consequently, changes happen to the blood plasma levels of glucose and insulin. As a result, elevated catecholamine levels could result in hyperglycemia. Increased blood lactate levels, decreased muscle glycogen stores, and elevated LDH activity in the muscles all suggested that fish exposed to sevin had faster rates of glycolysis. The reduction in PDH and SDH metabolism in the muscles and liver indicated that pesticide exposure negatively affected fish aerobic oxidation via the Krebs cycle. Unmistakable decreases in PDH and SDH, as well as an increase in LDH, show that fish under sevin stress prefer anaerobic metabolism According to Koundinya and Ramamurthi, sumithion and sevin reduced Tilapia mossambica’s breathing and consumption of oxygen by the muscles, gills, and other tissues because of a condition known as “coagulation film anoxia,” in which the fish’s capacity to take in oxygen from its surroundings was negatively impacted by mucus leakage from the gills. According to their research, sevin may trigger metabolic depression (Koundinya et al., 1979). Channa punctatus exposed to a lower sevin dose (1.05 mg/l) altered the amounts of LDH, PDH, and SDH in various organs as well as the chemical makeup of the liver, blood, and tissues. An increased rate of glycogenolysis is indicated by declining liver glycogen levels and increasing blood sugar levels. Another possibility for the decline in the quantity of glycogen in the muscles and liver is a reduction in either gluconeogenesis or glycogenesis rate (Sastry et al., 1982). Carbaryl is another well-liked carbamate pesticide. Numerous investigations revealed that interlocking clusters of cilia covering the gill filaments and hypoplasia of epithelial cells were among the additional injuries sustained by fish following carbaryl exposure. Certain changes in the connective tissue’s core have led to vacuolization, capillary enlargement, and normal structural collapse. A fish given 1 mg/L carbaryl, on the other hand, had entirely damaged gill structures and damaged interlamellar connections. The gill filaments had been extensively injured, and necrosis had changed their morphologies, although little tufts of cilia were visible on the top and lateral sides.
3. Pyrethroid synthetic insecticides
Pyrethrins are one type of insecticide which are made from Chrysanthemum plants. It is highly dangerous for aquatic organisms as compared to mammals. It is naturally collected and then modified into pesticide manufacturer companies to provide its photo-stability and high insecticide ability (Chakraborty et al., 2023). Because it is expensive and unstable in sunlight, natural pyrethrum hasn’t been utilized much in agriculture. Many synthetic materials that resemble pyrethrin have recently become available. When these insecticides are synthetically manufactured in factories they are known as synthetic insecticides. This insecticides are highly active for agricultural insects, it means it’s lower dose can be effective for pests. But when it mixed into the water bodies day by day, it is not soluble in water. So due to this insolubility they remain keep their structure remain intact. When the aquatic organisms intake these substances they are highly affective by this chemical particles (Sanyal et al., 2024). With the property of neuronal damages in insects, pyrethroids are also damages the neuronal function of aquatic organisms. It acts on neuronal gated channels which are maintained by sodium and potassium. It blocks or reduced the activity of this channels which cause serious damages in signalling activity in bodies. This pesticides can cause a reduction in neuronal signalling transfer in aquatic organisms. With the other hand this substances can be the reason for serious damages in cell which is caused by the free oxidative radicals. This particles easily moved in to the membranes which are made up of lipids. That induces the permeability rates in lipid membranes in aquatic organisms. Even these excessive oxidative radicals can form reactive oxygen species (ROS) in aquatic organisms. These ROS can act as molecular cutter, that means it can damages the DNA, proteins. Aquatic organisms as mollucs has the ability to reduce the action of ROS. But when these particles produce more ROS in the body, the antioxidants fails or can not control the normal cellular functions in aquatic organisms. This insecticides have several structural modifications which is contain with different moieties such as acid, alcohol and also the esters. Several isomers presence in this insectides which is stereospecific that means these insectides has different binding sites which are specific (Clark et al., 2012). Pyrethroids are generally divided into two classes (Tan et al., 2023). Type I chemicals are found in freshwater species. T syndrome is a syndrome characterised by high behavioural arousal, stopping, eating less, staying apart, exquisite body tremor that progresses to swaying of the entire body, and bending (Ono et al., 1975). On the other hand Type II substances cause cholinic seizures (CS syndrome), excessive salivation, and coarse tremor that progresses to choreoatetosis (Costa et al., 2008). Those groups are different from each other by among the alpha groups, a cyano group (Takasaki et al., 2013; de Brum Vieira et al., 2023). In addition to depolarizing the membrane and persistently keeping the sodium channels within the changed open state, the chemical also blocks the action potential without causing recurrent firing (Alcivar-Warren et al., 2023). The alpha cyano group-free pesticides do not affect the resting potential; instead, they cause significant depolarizing afterpotentials, recurrent firing, and brief maintenance of sodium channels in a changed open state in aquatic organisms. National Pesticide Information Centre (NPIC) states that because biphenthrin one of the commonly used insecticides is scarcely soluble in water, the majority of it would remain in the sediments while being extremely detrimental to aquatic life. Bifenthrin has an impact on fish and other aquatic species even at low doses. Fish have high sensitivity in part because of their slow metabolism. The fish’s body will retain bifenthrin for a longer time. The effects of bifenthrin as an ATPase inhibitor are another factor contributing to fish’s high sensitivity. To regulate the osmotic equilibrium of oxygen, the gills require ATP. The fish will perish if it is unable to absorb oxygen since ATP can no longer be utilised. Due to the osmoregulatory disruption and ionic balance dysfunction these substances are highly dangerous for aquatic organisms (Antwi et al., 2015). The lengthening of the kinetics of sodium channels controlled by voltage, which are in charge of producing the forward sodium current that excites excitable cells’ action potential, is the primary mechanism via which pyrethroids cause acute toxicity (Von Stein et al., 2011). The interaction of the pyrethroid-sodium channel causes disturbance by slows down the channel’s activation and inactivation characteristics, creating a hyperexcitable state (Deuis et al., 2017). Excitable cells have such an abundance of sodium channels that even while activation slows down at one channel level, there are always enough unaltered channels to guarantee that the action potential’s activation phase is not delayed (Contetet al., 2016). Nonetheless, even a small percentage of altered channels can provide enough more current during the action potential’s falling phase to postpone inactivation (Rajak et al., 2023). This delay results in protracted depolarization, which can set off if the current is, another action potential will be high enough then persists long enough aimed at nearby unaffected channels to become excitable again (Renigunta et al., 2015). These variations in the timing of sodium channel opening mostly cause variations among the T and CS syndromes (Costa et al., 2008). Type II pyrethroids may additionally inhibit GABAA-gated chloride channels, but only at higher doses than those that are sufficient to affect sodium channels. At low concentrations, Type II pyrethroids additionally inhibit voltage-dependent chloride channels (Rajak et al., 2023). These two outcomes are thought to be mediators of the CS syndrome’s choreoatetosis, salivation, and seizures (Costa et al., 2008). Pyrethroid can cause the induction of degradative metabolic systems in freshwater organisms (Lavaríaset al., 2023). In aquatic animals, exposure to pyrethroids can cause an increase in the overall quantity of cytochrome P-450 and related proteins, especially in the liver, within a few hours or days (Wilkinson et al., 1983). In order for freshwater species to detoxify pesticide compounds, oxidative degradation mechanisms must include cytochrome P-450 proteins (Alonso et al., 2023). They are the ones who bind and oxidise pesticide molecules directly. The inducer phenomenon is not limited to cytochrome P-450s; it also affects a wide range of other systems, including those linked to mixed-function oxidases, glutathione-S-transferases, and glucuronyl transferases (Lobodaet al., 2016). Furthermore, the increased concentration of antioxidant enzymes in hepatopancreatic tissue, such as glutathione (GSH) and superoxide dismutase (SOD) and catalase, which break down H2O2, is likely an early adaptive response to elevated oxidative stress in freshwater creatures intoxicated with pyrethroids (Sanchez-Hernandez et al., 2023). Redox (reduction/oxidation) cycle features, mitochondrial respiration, as well as reactive oxygen species (ROS), considered to be byproducts of detoxifying metabolism, are among the established mechanisms by which pesticides cause oxidative stress. In the redox cycle, the parent chemical is frequently enzymatically reduced first to form the pesticide radical by a reducer that depends on NADPH, it comprises cytochrome P450 reductase NADPH. The radical (.O2-) and parent chemical are created by giving up the unshared electron of the O2 radical. The latter is susceptible to further cycles. Consequently, at each cycle turn, two potentially dangerous events have occurred: the creation of an oxyradical and the oxidation of a reductant. Previous studies have shown that more freshwater fish are sensitive to cypermethrin. Fishes are exceedingly susceptible to growth impacts, as shown by sublethal and chronic tests of pyrethroids, and reduced survivorship has been recorded in various species, particularly impacting the early life stages.
Figure 5.
mode of action of pyrethroid insecticides.
Figure 5.
mode of action of pyrethroid insecticides.
4. Fungicides
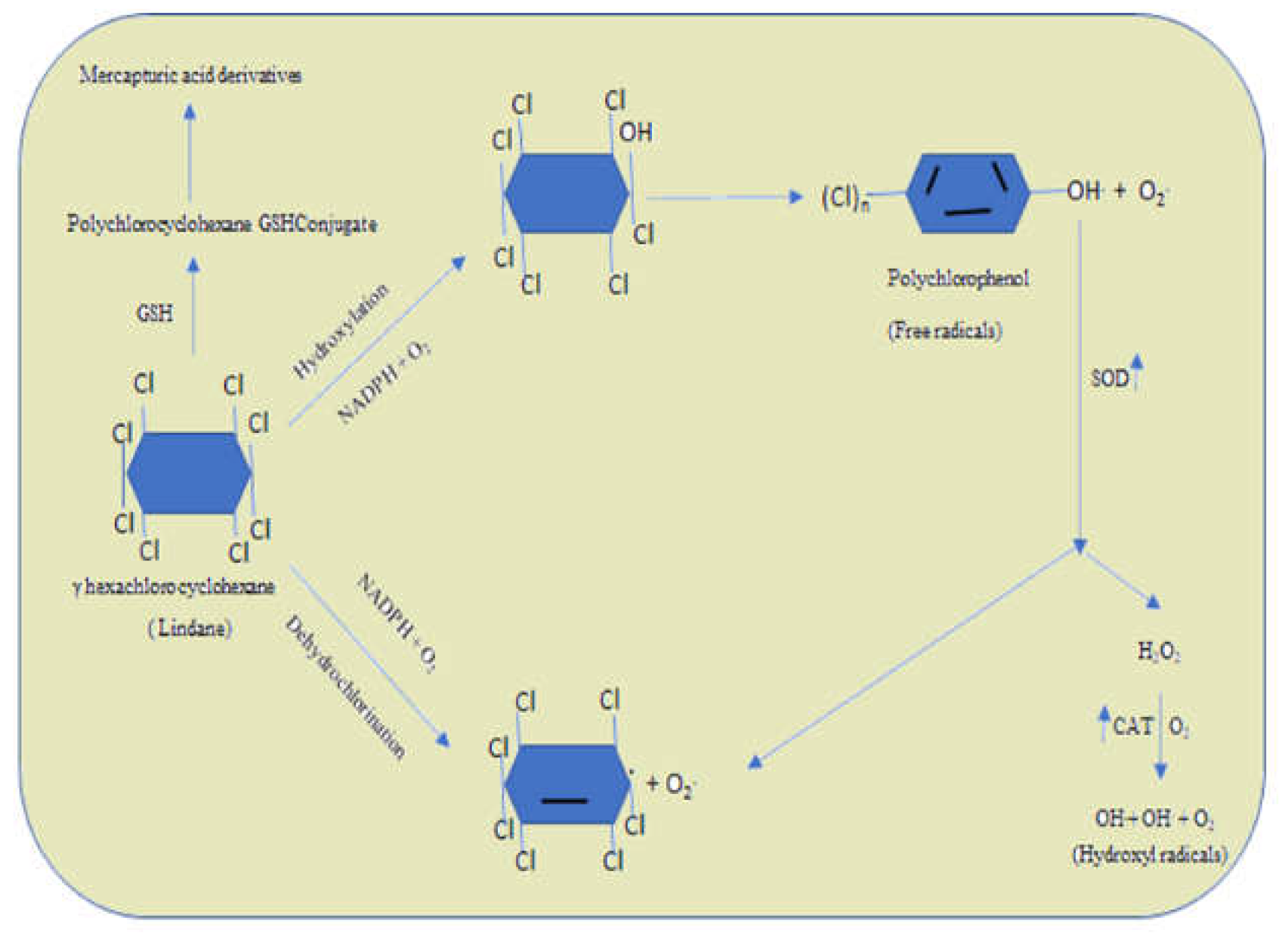
Substances classified as organochlorines (OCP) include a minimum of one chlorine atom bonded by a covalent bond. Chemical properties and structural changes pertaining to organochlorines are diverse. These compounds are discovered to be thicker than water due to the high atomic weight of chlorine. The element carbon, chlorine as well, and hydrogen are components of insecticides known as organochlorines. They go by the names chlorinated hydrocarbons, chlorinated synthetics, and chlorinated insecticides as well. Microorganisms are important inhabitants of aquatic environments, where they play vital roles in primary production, nutrient cycling, and decomposition. There is little information known about the harmful effects that agricultural fungicides in to aquatic microorganisms, according to a study by DeLorenzo et al., 2001. There are around 50,000 distinct species of microorganisms in the three kingdoms of bacteria, algae, fungi, and protozoa. Pesticides have been demonstrated to affect cellular growth, division, and molecular structure in microorganisms, as well as respiration, photosynthesis, and biosynthetic activities (DeLorenzo et al., 2001). In an aquatic environment, pesticides may absorb or remove on suspended particles. They can then settle deeper in bottom sediments, where they can absorb fishes and other aquatic life. Organochlorines may enter a living thing’s body by the skin, lungs, or intestinal wall. While dicofol, toxaphene, DDT, mirex, and methoxychlor are less absorbed via the skin than cyclodienes, hexachlorocyclohexane, endosulfan, and lindane (Costa et al., 2008). Due to lipophilicity in nature, organochlorines are stored in tissues, and repeated small exposure may result in accumulation and eventual toxicity (Krithiga et al., 2022). Their impact on the brain’s nerve cells results in hyperexcitable states in the brain, convulsions, hyperreflexia, ataxia, tremor, and tremor. Organic molecules decompose to produce the energy required for organisms to exist. A component of this catabolic process occurs in the mitochondria of fungi and other eukaryotes, where it produces the high-energy intermediate ATP. A number of fungicide groups disrupt the fungi’s energy source, and all such substances are potent spore germination inhibitors. Younis et al. (2002) discovered the DDT-targeted proteins in insects recently. The target protein was found to be an element of ATPase, which is necessary for healthy nerve function and plays a critical role in neural repolarization. DDT also has an impact on humans via at least three more routes. Compared to DDT and methoxychlor, cyclodienes, lindane, and mirex have the potential to have more negative consequences. DDT has been thoroughly tested for any possible negative effects using a range of animal models (de Perre et al., 2014). DDT boosted photorespiration and prevented the C4-dicarboxylic acid metabolism from incorporating 14C from 14CO2, according to research on the toxicity of DDT on algae. Most likely by disruption of cyclic photophosphorylation, DDT caused the metabolism to switch from an efficient to an inefficient pathway. Endosulfan’s toxicity to amphibians was assessed in a number of research. Rana pipiens tadpoles that were exposed to endosulfan at environmentally appropriate doses for seven weeks fared worse than controls in terms of survival (Shenoy et al., 2009). According to Shelley, 2009 the treatment with pentachlorophenol for 28 days act as boosting agent for phagocytic leukocytes in rainbow trout. According to a 2009 study by Hassold, Daphnia magna was chronically toxic to five fungicides that block demethylase. According to Ochoa-Acuna, 2009, Pseudokirchneriella subcapitata and Daphnia magna were poisoned by fungicides commonly used on soybeans, including strobilurin and conazole. According to Akoto, 2016, the organophosphates contaminate the water bodies which are transferred to the higher organisms by fishes through the food chain. By creating GSH conjugates, Enzymes reliant on glutathione have been linked to detoxification of toxic chemicals. An enzyme that depends on glutathione and is likely a glutathione-S-transferase detoxifies the insecticide DDT in resistant houseflies. Organochlorines has the potential to raise reactive oxygen species (ROS). in living things in water and a reduction in antioxidant defences, which can affect redox homeostasis and cause apoptosis (Valavanidis et al., 2006). Increased lipid peroxidation, changed GSH, and oxygen-free free radical-scavenging enzymatic levels in the gastrointestinal tract are reviewed in the context of oxidative stress (Vijayavel et al., 2004).
Figure 6.
Pathway of organochlorine metabolism and free radicle generation.
Figure 6.
Pathway of organochlorine metabolism and free radicle generation.
5. Herbicides
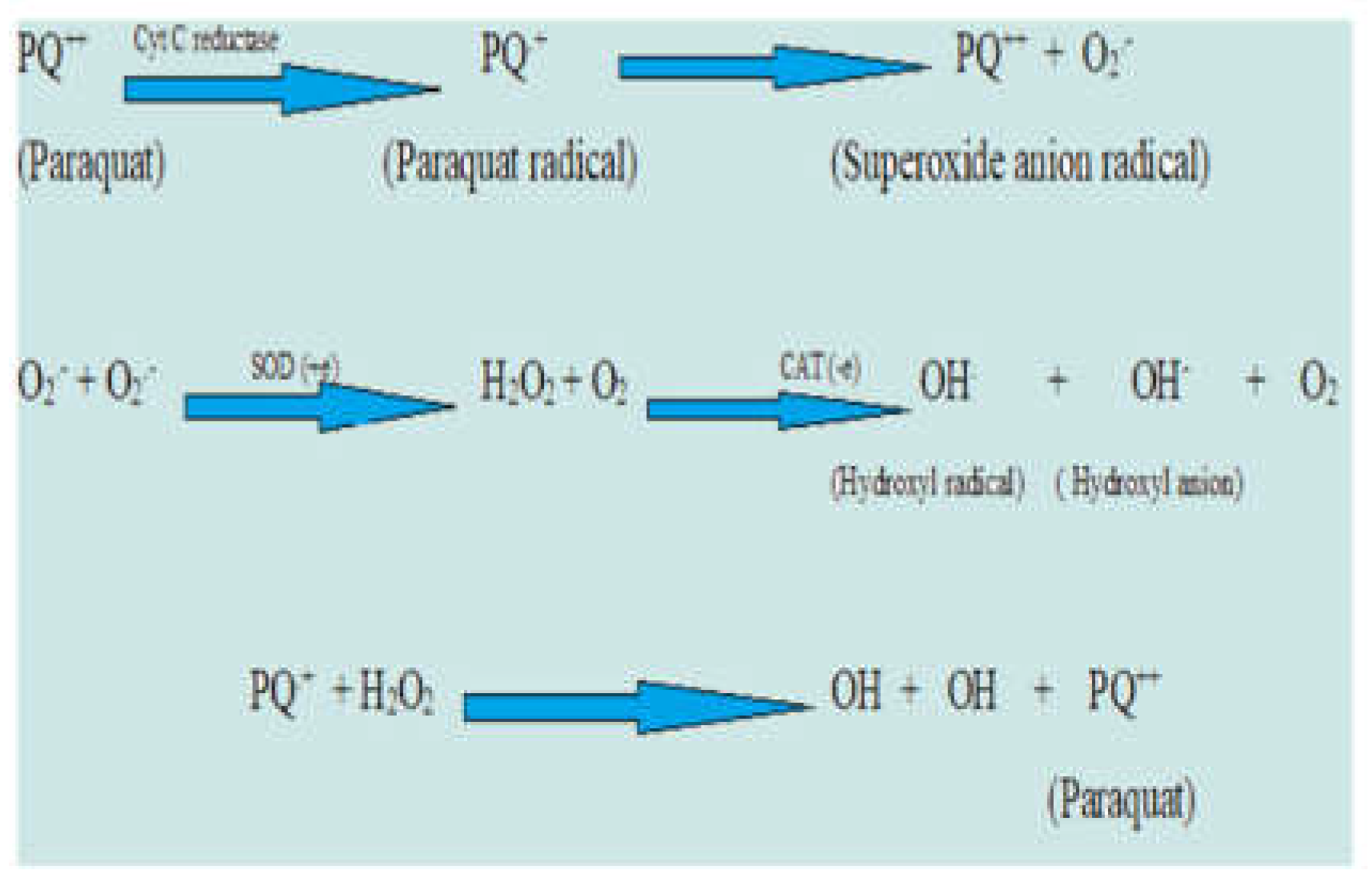
There are three different bipyridyl herbicides on the market: paraquat, diquat, and a combination of these two. Diquat and paraquat are extremely powerful systemic toxins. A structure is said to be bipyridyl if it has two pyridine rings on them, composed of aromatic rings joined by an ethylene molecule and in where a single carbon atom is replaced by a nitrogen atom. Usually, bromide and chlorine ions are used to generate the compounds diquat and paraquat, respectively. Herbicide spills have been observed to have an impact on a variety of freshwater creatures. Freshwater organisms’ tissues have been found to accumulate numerous herbicide families (Farcy et al., 2013). Reproductive harm in freshwater creatures exposed to atrazine has been documented in several investigations. One of the most significant sources of hazardous data for aquatic creatures is breeding under atrazine exposure. This data includes information on genetics, behaviour, development, and the area of biochemistry (Zheng et al., 2017). With an EC50 of 0.070 M, the highest algal lethality recorded by any herbicide, atrazine suppressed Chlorella fusca reproduction throughout the duration of one generation cycle. (Faust et al., 1993). Since atrazine inhibits vitellogenin synthesis, female crayfish have smaller oocytes in their ovaries and hepatopancreas (Silveyra et al., 2018). Long-term atrazine exposure causes reproductive failure in freshwater cladocerans (Phyu et al., 2013). On the other hand, atrazine decreases vitellogenic oocytes and increases the quantity of incorrectly hatched larvae in estuary crabs. Another research found that atrazine affects coastal crabs by reducing down their ovarian development (Silveyra et al., 2018). Tadpoles exposed to atrazine at low concentrations have shown altered gonadogenesis in several investigations (Hayes et al., 2003; Tavera-Mendoza et al., 2002). Figueiredo-Fernandes et al.,2006, report that oxidative indicators present in hepatocytes in Nile tilapia exposed to paraquat are influenced by temperature and gender. Males did better than females in their studies at both of the degrees (17 °C and 27 °C) in terms of SOD levels. Comparing the SOD activity in both genders of the control or reference groups, it was greater. However, at both temperatures, GR activity was higher in females, with no discernible variations between males. Paraquat can be converted by GR to its cation radical, which leads to the oxidation of GSH and an upsurge in GR activity (Richmond et al., 1982; Stephensen et al., 2000). Simazine is an algicide that may be used in ponds, pools, and aquariums. It is a specific chloro-S-triazine herbicide. The effects of subjecting the common carp to simazine, which were documented by Oropesa et al., 2009. Their field investigation revealed that their hepatopancreas had greater GSH levels and that carp from polluted lakes had higher tissue MDA levels. Due to the production of ROS in bluegill sunfish, atrazine exposure causes stress oxidative phenomena (Elia et al. 2002).
Figure 7.
Reactions leading to the generation of herbicide (paraquat) radicals.
Figure 7.
Reactions leading to the generation of herbicide (paraquat) radicals.