1. Introduction
Aortic stenosis (AS) is one of the most common cardiovascular diseases in the Western world [
1,
2]. It can be treated by cardiac surgery or catheter interventions. Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure to replace the aortic valve. TAVI has been established as standard treatment for patients with inoperable or high-risk AS [
3]. The first procedure was in 2002 in France by Cribier, and since then more than 500,000 TAVIs have been performed in more than 70 countries [
4,
5]. In Germany, more than 100,000 TAVIs have been performed since 2008, with more than 12,000 procedures annually, and in the U.S., 54,782 TAVIs were registered in 2016 in the Transcatheter Valve Therapy Registry.
Among the potential complications, local vascular events are the most common after cardiac catheterizations such as TAVI. Hemorrhages and hematomas typically occur within 12 hours after the procedure, whereas pseudoaneurysms may develop over days or weeks [
6] . The risk of iatrogenic aortic dissection (IAD) during TAVI ranges from 0.6% to 1.9%, often caused by manipulations of the guidance/delivery system or by valve repositioning, retrieval, or retraction. Dissections occur mainly in the aortic root, although they can also affect the ascending and descending aorta due to catheter or guidewire injuries [
7,
8].
2. Detailed Case Description
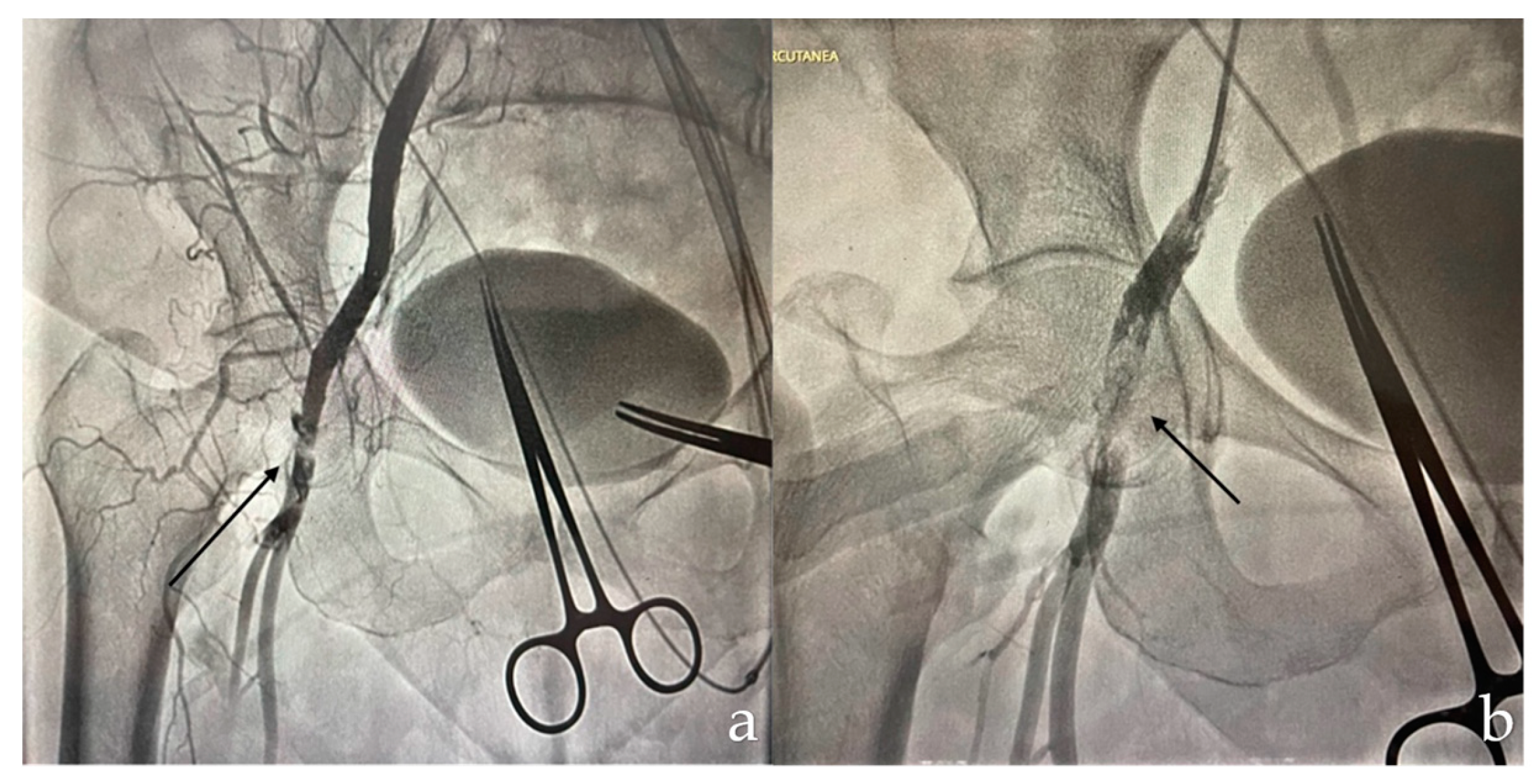
A 68-year-old patient with a history of open surgery for aortic and mitral valve replacement, in addition to coronary revascularization with bypass, presented severe aortic valve stenosis requiring TAVI. During the procedure, the patient experienced acute limb ischemia due to occlusion of the right common femoral artery after use of the ProGlide™ device (Abbott), requiring femoral stent placement (
Figure 1).
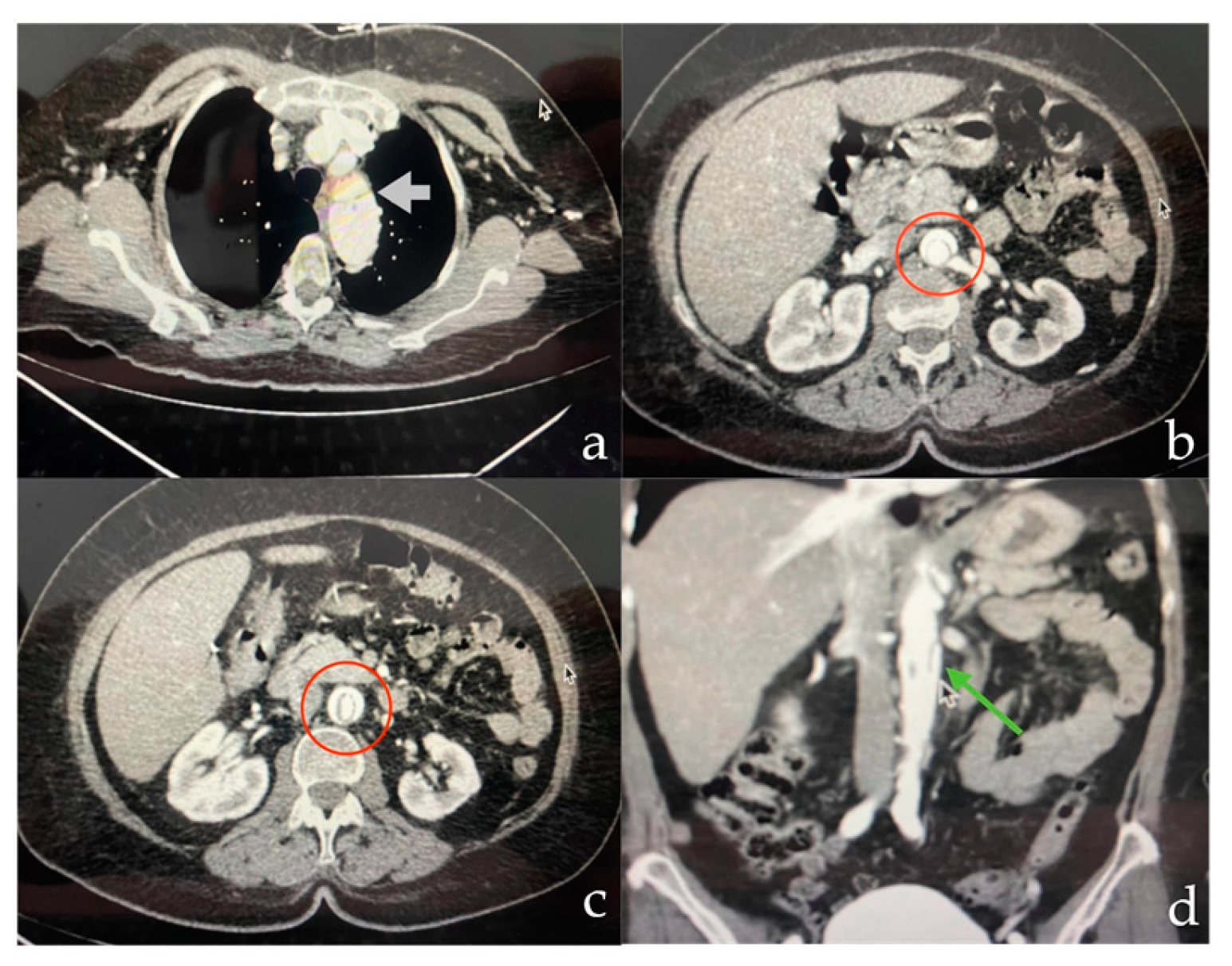
On postoperative day 8, the patient reported pain in the right lower extremity, prompting computed tomography angiography (CTA). This revealed a Stanford B-type aortic dissection extending into the abdominal aorta and right external iliac artery (
Figure 2).
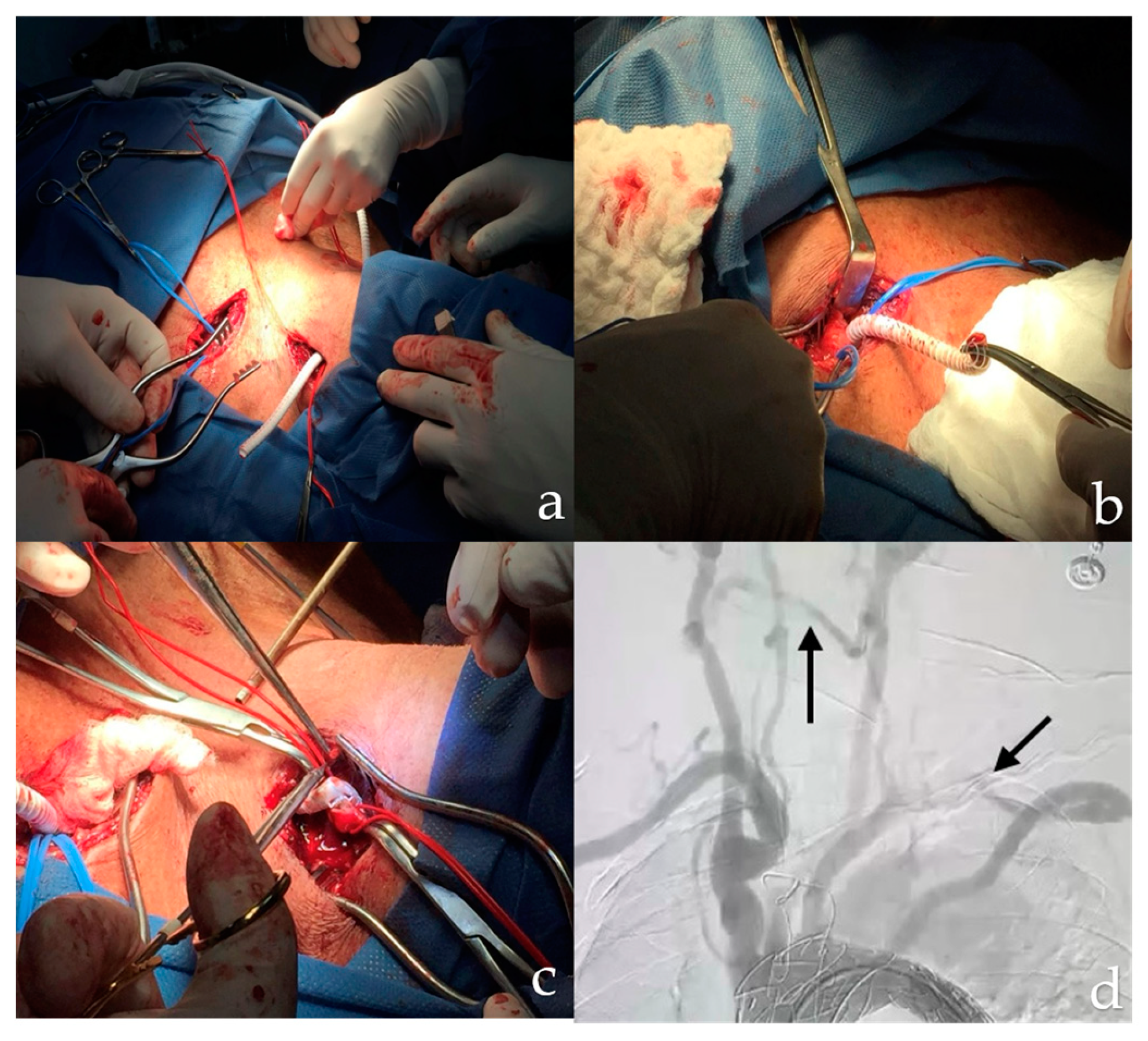
Consequently, the patient was transferred to the operating room for staged hybrid management. It was decided to perform Zone 1 coverage, starting with a cervical debridement procedure that included a carotid-carotid bypass. Access was gained through a retropharyngeal route and space was created for the tunnel by blunt dissection medial to the common carotid artery and retroesophageal. After completing the carotid-carotid bypass, the anterior scalene muscle was divided to access the subclavian artery. A lateral arteriotomy was performed on the left common carotid artery and an end-to-end anastomosis was performed using a 6 mm polytetrafluoroethylene (PTFE) ringed graft (Gore-Tex) with 5/0 prolene sutures (
Figure 3). Hemostasis was ensured and revascularization of the left subclavian artery was achieved before removal of the vascular clamp. After the procedure, the patient was transferred to the Intensive Care Unit (ICU).
For endovascular treatment, a left femoral and right brachial approach was used. A hydrophilic guidewire was introduced through a pigtail catheter, navigating the area of abdominal and thoracic aortic dissection. The wire was positioned in the ascending aorta and replaced with a high-support LUNDERQUIST wire through the left approach. Through this guidewire, a Medtronic Valiant VAMF3834C150TE aortic stent graft was placed and released in zone 1. After securing the thoracic aortic stent graft, the pigtail catheter was removed and aortography was performed to verify adequate flow and patency of the brachiocephalic trunk.
A pigtail catheter was then introduced into the abdominal aorta for further aortography, identifying the visceral vessels and the area of dissection. A guide wire was passed and an ABRE AB9-20x100 aortic endograft was deployed below the ostium of the superior mesenteric artery. Once the endograft was in place, another aortogram was performed confirming resolution of the aortic dissection and patency of all aortic branches. A coda balloon was then used to secure the position of the endograft, removing the devices and closing the arteriotomy with 7/0 prolene sutures.
The patient tolerated the procedure without complications and was transferred to the Intensive Care Unit (ICU), from where he was subsequently discharged.
3. Discussion
Aortic dissection (AD) is a serious but rare vascular complication [
8]. In most cases, it occurs in the ascending aorta and is usually related to trauma caused by medical instruments such as catheters, guidewires, and vascular sheaths used during procedures such as TAVI [
9]. In contrast, TAVI-related descending aortic dissection is even rarer. In these cases, the main mechanisms involved are usually associated with improper handling of the instruments during valve repositioning or during retraction of the devices used in the procedure [
10]. Given the high mortality associated with complications such as aortic dissection in procedures such as TAVI, it is crucial to implement meticulous evaluation and preventive measures at all stages of treatment [
11,
12]. Preoperatively, it is essential to perform a thorough diagnostic imaging not only at the access point, but throughout the aorta, to detect possible anomalies that may predispose to vascular complications. During the procedure, strict protocols should be followed to handle the instruments with care and precision. It is essential to avoid injury to the vascular system when positioning and manipulating the catheter, maintain sheath stability and correctly position the guidewire under fluoroscopic control to ensure safe and accurate navigation and postoperatively, follow-up transesophageal echocardiography (TEE) is recommended to detect any early signs of major vascular complications such as aortic dissection [
13]. This allows rapid and effective intervention if any complication is suspected, thus improving the patient’s prognosis.
At present, there are no specific guidelines for the treatment of iatrogenic descending aortic dissection, probably due to its low incidence [
14]. However, conservative treatment may be appropriate for limited and uncomplicated dissections and is usually the most recommended treatment. In complicated cases, such as impending rupture, uncontrollable pain, poor perfusion, early aortic expansion, or unstable blood pressure, TEVAR should be considered to reduce mortality, morbidity, and risk of paraplegia compared with conventional open repair. In this specific case, early expansion of the descending aortic dissection led us to opt for TEVAR instead of a conservative approach [
15].
Traditionally, TEVAR has been the main treatment for complex Stanford B dissections, although in specific cases hybrid repair is required. Ideally, at least 2 cm of healthy aorta should be secured above the entry point of the tear to ensure an adequate proximal sealing zone and prevent retrograde propagation into the ascending aorta. However, achieving this 2-cm landing zone may not always be feasible without partially or completely covering one or more branches of the aortic arch. Therefore, hybrid repair presents itself as a suitable option in these circumstances [
16].
Iliac artery dissections can occur during the passage of wires or devices. It is crucial to identify and adequately treat these dissections, even if they do not cause significant clinical problems during the procedure. We recommend a final angiography of the iliac arteries. The usual treatment consists of implanting bare nitinol stents, or covered stents if thrombus formation is suspected. On the other hand, common femoral artery occlusions often occur following the use of vascular closure devices, especially when needle-based systems such as Perclose ProGlide™ (Abbott) are used in combination with collagen-based systems such as AngioSeal (Terumo, Japan). In addition, advancement of large lumen devices can dislodge plaques and lead to occlusion of the vessel lumen. This occlusion presents as acute or subacute ischemia, which should be identified before the patient leaves the catheterization laboratory. Clinical monitoring of the foot of the punctured leg and Doppler ultrasound the following day are crucial.
4. Conclusions
AD is a serious but uncommon complication associated with procedures such as TAVI. It most commonly occurs in the ascending aorta, but can also occur in the descending aorta, often due to errors during valve manipulation. This case underscores the importance of preventive measures, meticulous preoperative planning, implementation of precise procedural protocols, and rigorous postoperative follow-up to detect complications early. Treatment options range from conservative approaches for simple cases to TEVAR for complex dissections, highlighting the need for careful patient selection and procedural expertise to minimize risks and improve clinical outcomes.
Author Contributions
Conceptualization, HT, AT, IM, AT, NP, MJ and WD ; methodology, HT, AT, IM, AT, NP, MJ and WD ; formal analysis and investigation, HT, AT, IM, AT, NP, MJ and WD ; writing—original draft preparation, HT, AT, IM, AT, NP, MJ and WD; writing—review and editing, HT, AT, IM, AT, NP, MJ and WD ; visualization, HT, AT, IM, AT, NP, MJ and WD ; supervision, AT and WD. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of the study and the minimal risk to participants.
Informed Consent Statement
Informed consent was obtained from the patient and healthcare proxy/decision maker for the publication of the case report and accompanying images.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient privacy and confidentiality
Conflicts of Interest
The authors declare no conflict of interest related to this manuscript.
References
- Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular Heart Disease: Diagnosis and Management. Mayo Clin Proc. 2010 May;85(5):483–500. [CrossRef]
- Ito S, Oh JK. Aortic Stenosis: New Insights in Diagnosis, Treatment, and Prevention. Korean Circ J. 2022;52(10):721. [CrossRef]
- Liu H, Liu S, Lu Y, Yang Y, Wang W, Zhu L, et al. Transapical transcatheter aortic valve implantation for predominant aortic regurgitation with a self-expandable valve. J Thorac Dis. 2020 Mar;12(3):538–49.
- Cribier A. Development of transcatheter aortic valve implantation (TAVI): A 20-year odyssey. Arch Cardiovasc Dis. 2012 Mar;105(3):146–52.
- Elbaz-Greener G, Rozen G, Kusniec F, Marai I, Carasso S, Ko DT, et al. Comparing Trajectory of Surgical Aortic Valve Replacement in the Early vs. Late Transcatheter Aortic Valve Replacement Era. Front Cardiovasc Med. 2021 Jun 22;8.
- Avvedimento M, Nuche J, Farjat-Pasos JI, Rodés-Cabau J. Bleeding Events After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2023 Feb;81(7):684–702.
- Geile J, Doberentz E, Madea B. Rapid development of an iatrogenic aortic dissection following transcatheter aortic valve implantation. Forensic Sci Med Pathol. 2020 Jun 14;16(2):335–9.
- Chaudhry MA, Sardar MR. Vascular complications of transcatheter aortic valve replacement: A concise literature review. World J Cardiol. 2017;9(7):574. [CrossRef]
- Rujirachun P, Junyavoraluk A, Jakrapanichakul D, Wongpraparut N, Chunhamaneewat N, Maneesai A, et al. Immediate aortic dissection after transcatheter aortic valve replacement: A case report and review of the literature. Clin Case Rep. 2021 Jul 6;9(7). [CrossRef]
- de Agustin JA, Jiménez-Quevedo P, Nombela-Franco L, Almeria C, Gomez de Diego JJ, Rodrigo JL, et al. Descending Aorta Rupture During Transcatheter Aortic Valve Replacement. Circulation. 2016 Jan 12;133(2). [CrossRef]
- Sardar MR, Goldsweig AM, Abbott JD, Sharaf BL, Gordon PC, Ehsan A, et al. Vascular complications associated with transcatheter aortic valve replacement. Vascular Medicine. 2017 Jun 11;22(3):234–44.
- Avvedimento M, Nuche J, Farjat-Pasos JI, Rodés-Cabau J. Bleeding Events After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2023 Feb;81(7):684–702.
- Angellotti D, Manzo R, Castiello DS, Immobile Molaro M, Mariani A, Iapicca C, et al. Echocardiographic Evaluation after Transcatheter Aortic Valve Implantation: A Comprehensive Review. Life. 2023 Apr 24;13(5):1079.
- Iatrogenic aortic dissection of the descending aorta after percutaneous coronary intervention. British Journal of Cardiology. 2022;
- Thieme M, Moebius-Winkler S, Franz M, Baez L, Schulze CP, Butter C, et al. Interventional Treatment of Access Site Complications During Transfemoral TAVI: A Single Center Experience. Front Cardiovasc Med. 2021 Nov 15;8. [CrossRef]
- Nasser MM, Nassif BS, Eldessoki MH, Elsamadoni A, Balboula AM. Supra-aortic debranching as a preliminary surgery prior to aortic endografting in patients with type B aortic dissection: immediate-term and short-term outcomes. The Egyptian Journal of Surgery. 2023 Jan;42(1):192–9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).