Submitted:
03 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methodology
Outbreaks Genesis
Risk Factors for Outbreak
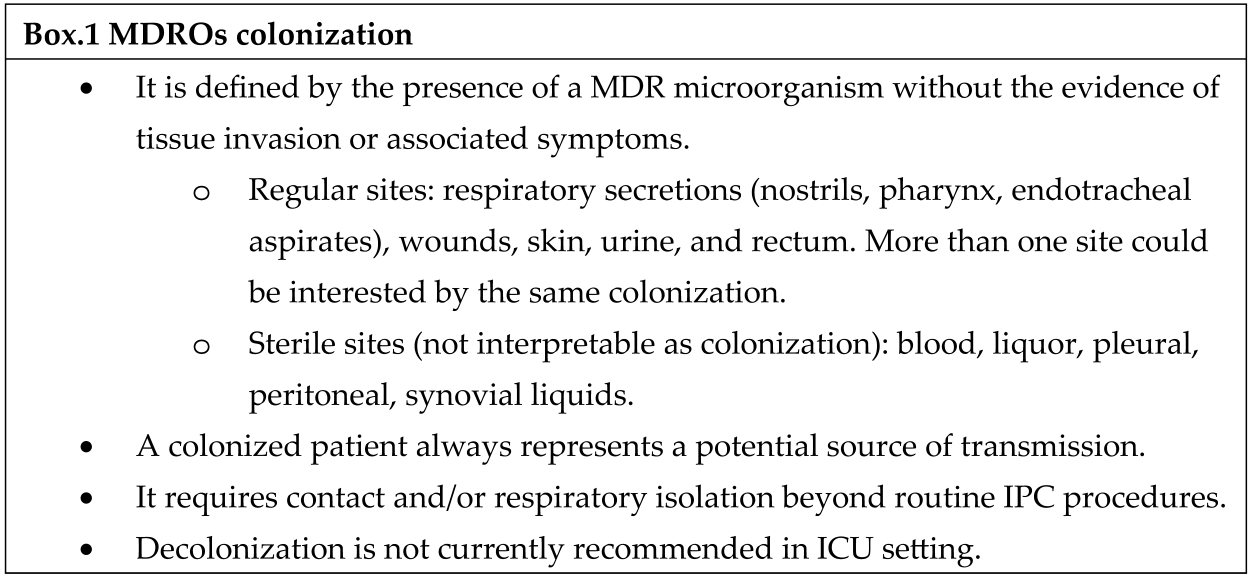
Strategies
- NURSE-TO-PATIENT RATIO
- 2.
- PHYSICIAN/PATIENT RATIO
- 3.
- EDUCATION
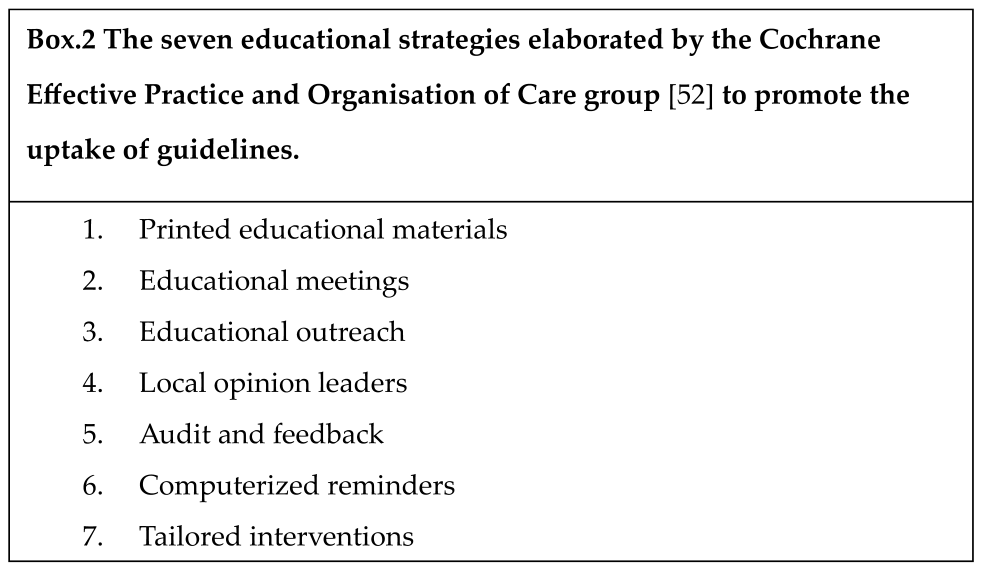

- 4.
- ISOLATION
Respiratory Isolation
- A negative-pressure room for patients who resulted to be colonized or infected by potential air-spreading pathogens;
- A positive-pressure for patients who are likely more susceptible of acquiring an infection, such as solid-organ transplanted (SOT) recipients, hematopoietic stem cell transplanted (HSCT) patients, presence of hematological disorders, chronic use of corticosteroids, calcineurin inhibitors, anti-metabolites and other immunosuppressants.
Contact Isolation
- 5.
- MDROs DECOLONIZATION
- 6.
- HAND HYGIENE
- 7.
- SHOE HYGIENE (SH)
- 8.
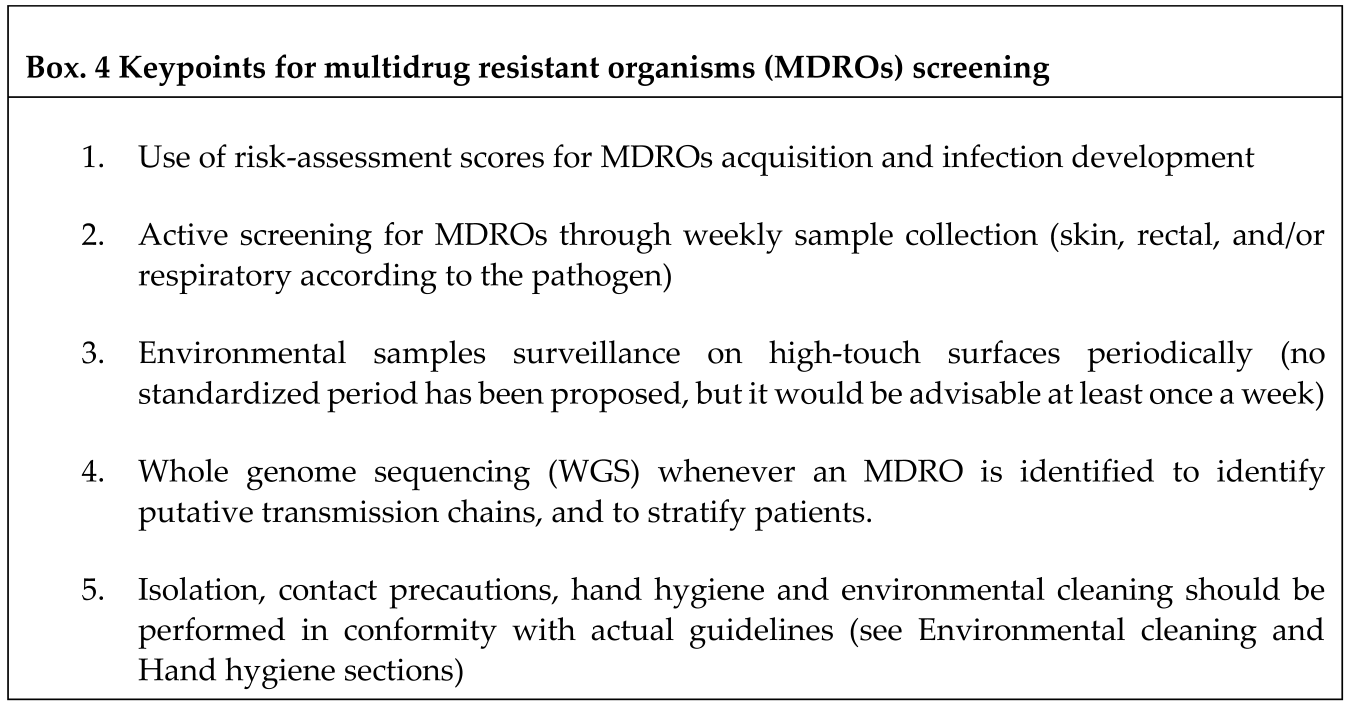
- SCREENING
- Risk-assessment scores
- b.
- CRAB screening
- c.
- Rectal screening for carbapenem resistant Gram negative bacteria (CR- GNB)
- d.
- Skin screening for MRSA
- e.
- Environmental samples surveillance
- f.
- Whole genome sequencing (WGS)
- 9.
- ENVIRONMENTAL CLEANING
Air Cleaning
Surfaces Cleaning
- 10.
- ANTIMICROBIAL STEWARDSHIP PROGRAM
- 11.
- OUTBREAK REPORTING
- 12.
- CRE PREVENTION AMONG SPECIAL POPULATIONS
- 13.
- COST-EFFECTIVENESS AND MDROs REPRODUCTIVE NUMBER (R0)
- 14.
- NEW EXPERIMENTED STRATEGIES

- 15.
- FUTURE PERSPECTIVES OF IPC
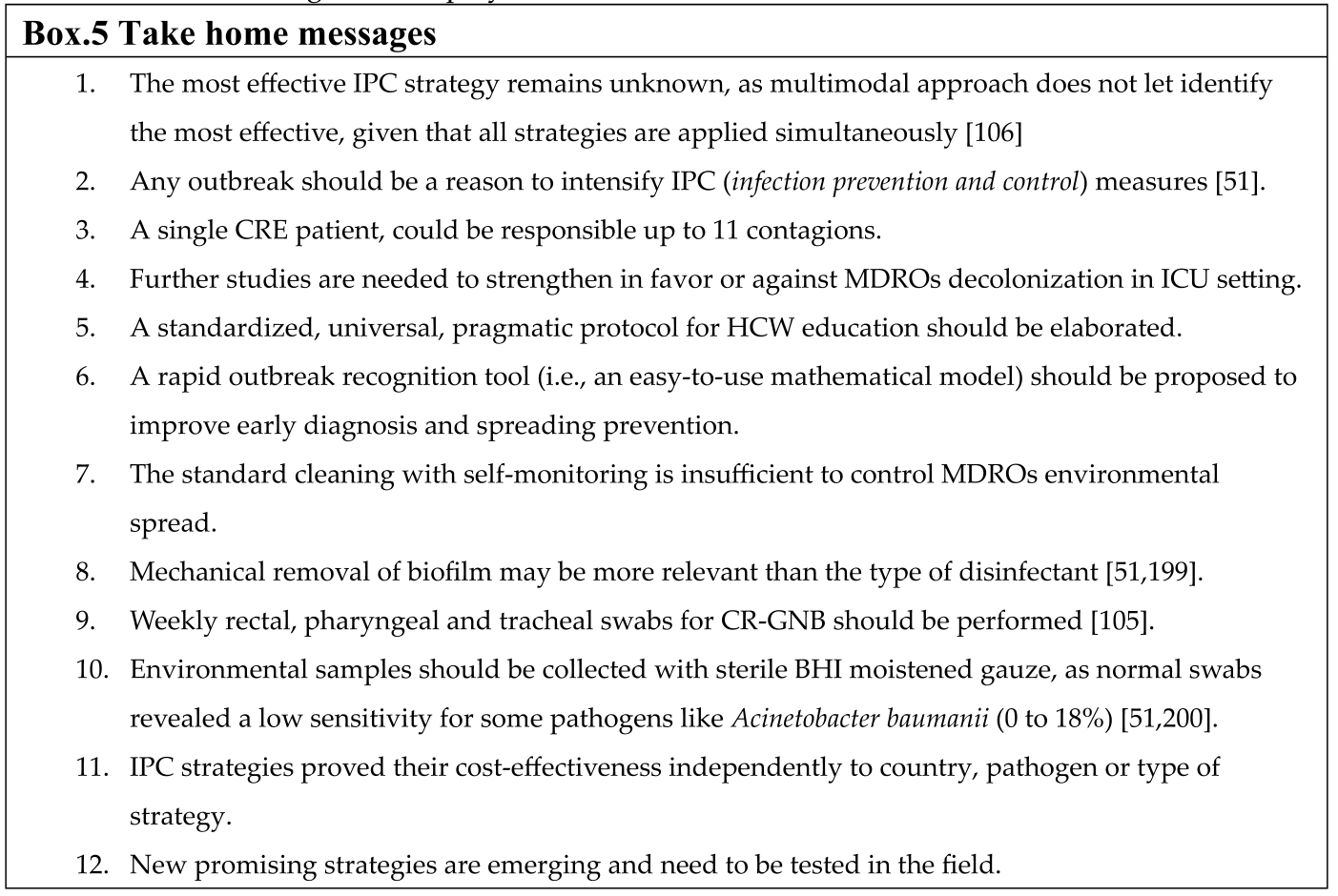
Limitations
Conclusions
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Blot, S.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit Care Nurs 2022, 70, 103227–2022. [Google Scholar] [CrossRef] [PubMed]
- WHO launches first ever global report on infection prevention and control. https://www.who.int/news/item/06-05-2022-who-launches-first-ever-global-report-on-infection-prevention-and-control.
- Markwart, R.; et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med 2020, 46, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Zahar - 2018 - Dilemmas in infection control in the intensive car.pdf.
- Bixler, D. Outbreak Investigation. ( 2014.
- World Health Organization. World Health Organization outbreak communication planning guide. Guide de l’OMS sur la planification de la communication lors des flambées de maladies.
- Al-Dorzi, H. M. & Arabi, Y. M. Outbreaks in the adult ICUs. Current Opinion in Infectious Diseases 2017, 30, 432–439. [Google Scholar] [PubMed]
- Jackson, K. C. , Short, C. T., Toman, K. R., Mietchen, M. S. & Lofgren, E. Transient dynamics of infection transmission in a simulated intensive care unit. PLoS One 2022, 17, e0260580. [Google Scholar]
- Tacconelli, E.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clinical Microbiology and Infection 2014, 20, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Langford, D. & Williams, V. What does ESBL mean, and why does my patient require contact isolation? Crit Care Nurs Q 2011, 34, 46–51. [Google Scholar]
- Kovacic, A.; et al. Transmission and survival of carbapenem-resistant Acinetobacter baumannii outside hospital setting. International Microbiology. Official journal of the Spanish Society for Microbiology. [CrossRef]
- Popovich, K. J.; et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol 2022, 44, 1039–1067. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S. J. Hospital cleaning in the 21st century. Eur J Clin Microbiol Infect Dis 2011, 30, 1473–1481. [Google Scholar] [CrossRef]
- Giovagnorio, F.; et al. Successful control measures to treat the transmission of Candida auris in Northern Italian Hospital. New Microbiol 2024, 46, 395–399. [Google Scholar]
- Luplertlop, N. Pseudallescheria/Scedosporium complex species: From saprobic to pathogenic fungus. Journal de Mycologie Médicale 2018, 28, 249–256. [Google Scholar] [CrossRef]
- Beam, E. L.; et al. Ebola Virus Disease: Clinical Challenges, Recognition, and Management. Nurs Clin North Am 2019, 54, 169–180. [Google Scholar] [CrossRef]
- Kanamori, H. Healthcare-Associated Outbreaks Associated with a Water Reservoir and Infection Prevention Strategies.
- Hoenigl, M.; et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. The Lancet Infectious Diseases 2021, 21, e246–e257. [Google Scholar] [PubMed]
- Tacconelli, *!!! REPLACE !!!*; et al. - 2014 - ESCMID guidelines for the management of the infect.pdf.
- Adams, C. E. & Dancer, S. J. Dynamic Transmission of Staphylococcus Aureus in the Intensive Care Unit. Int J Environ Res Public Health 2020, 17, 2109. [Google Scholar] [PubMed]
- Demuyser, T. , De Cock, E. & Sermijn, E. Airborne Aspergillus fumigatus contamination in an intensive care unit: Detection, management and control. J Infect Public Health 2019, 12, 904–906. [Google Scholar] [PubMed]
- Fiore, V.; et al. Mood Reactive Disorders among COVID-19 Inpatients: Experience from a Monocentric Cohort. Med Princ Pract 2021, 30, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P. C. , Moschopoulos, C. D., Karofylakis, E., Kelesidis, T. & Tsiodras, S. Update in Viral Infections in the Intensive Care Unit. Front Med (Lausanne) 2021, 8, 575580–2021. [Google Scholar]
- Shariff, M. , Aditi, A. & Beri, K. Corynebacterium striatum: an emerging respiratory pathogen. J Infect Dev Ctries 2018, 12, 581–586. [Google Scholar]
- Wang, X.; et al. Whole-Genome Sequencing Reveals a Prolonged and Persistent Intrahospital Transmission of Corynebacterium striatum, an Emerging Multidrug-Resistant Pathogen. J Clin Microbiol 2019, 57, e00683–19. [Google Scholar] [CrossRef]
- Zahar, J.-R. Dilemmas in infection control in the intensive care unit. ( 2018. [CrossRef]
- Elliott, T. M.; et al. Cost-effectiveness analysis of whole-genome sequencing during an outbreak of carbapenem-resistant Acinetobacter baumannii. Antimicrob Steward Healthc Epidemiol 2021, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; et al. Predicting the occurrence of multidrug-resistant organism colonization or infection in ICU patients: development and validation of a novel multivariate prediction model. Antimicrob Resist Infect Control 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; et al. ESCMID/EUCIC clinical practice guidelines on perioperative antibiotic prophylaxis in patients colonized by multidrug-resistant Gram-negative bacteria before surgery. Clinical Microbiology and Infection 2023, 29, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Gordin, F. M.; et al. A cluster of hemodialysis-related bacteremia linked to artificial fingernails. Infect Control Hosp Epidemiol 2007, 28, 743–744. [Google Scholar] [CrossRef]
- Moolenaar, R. L.; et al. A prolonged outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit: did staff fingernails play a role in disease transmission? Infect Control Hosp Epidemiol 2000, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Price, A. M. , Sarween, N., Gupta, I. & Baharani, J. Risk factors and short-term outcomes for methicillin-resistant Staphylococcus aureus and methicillin-sensitive Staphylococcus aureus colonization among hemodialysis patients. Saudi J Kidney Dis Transpl 2019, 30, 1351–1363. [Google Scholar] [PubMed]
- Olsen, K.; et al. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 2013, 8, e63716. [Google Scholar] [CrossRef]
- Dossett, L. A.; et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009, 10, 137–142. [Google Scholar] [CrossRef]
- Bray, K.; et al. Standards for nurse staffing in critical care units determined by: The British Association of Critical Care Nurses, The Critical Care Networks National Nurse Leads, Royal College of Nursing Critical Care and In-flight Forum. Nurs Crit Care 2010, 15, 109–111. [Google Scholar] [CrossRef]
- Sharma, S. K. & Rani, R. Nurse-to-patient ratio and nurse staffing norms for hospitals in India: A critical analysis of national benchmarks. J Family Med Prim Care 2020, 9, 2631–2637. [Google Scholar]
- Falk, A.-C. Nurse staffing levels in critical care: The impact of patient characteristics. Nurs Crit Care 2023, 28, 281–287. [Google Scholar] [CrossRef] [PubMed]
- West, E.; et al. Nurse staffing, medical staffing and mortality in Intensive Care: An observational study. Int J Nurs Stud 2014, 51, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J. M. , Yabes, J. G., Bukowski, L. A. & Davis, B. S. Intensivist physician-to-patient ratios and mortality in the intensive care unit. Intensive Care Med 2023, 49, 545–553. [Google Scholar] [PubMed]
- Neuraz, A.; et al. Patient Mortality Is Associated With Staff Resources and Workload in the ICU: A Multicenter Observational Study*. Critical Care Medicine 2015, 43, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Gershengorn, H. B.; et al. Association of Intensive Care Unit Patient-to-Intensivist Ratios With Hospital Mortality. JAMA Intern Med 2017, 177, 388. [Google Scholar] [CrossRef] [PubMed]
- Gershengorn, H. B.; et al. Association of patient-to-intensivist ratio with hospital mortality in Australia and New Zealand. Intensive Care Med 2022, 48, 179–189. [Google Scholar] [CrossRef]
- Kerlin, M. P. & Caruso, P. Towards evidence-based staffing: the promise and pitfalls of patient-to-intensivist ratios. Intensive Care Med 2022, 48, 225–226. [Google Scholar] [PubMed]
- Dara, S. I. & Afessa, B. Intensivist-to-Bed Ratio. Chest 2005, 128, 567–572. [Google Scholar] [PubMed]
- Agarwal, A.; et al. SWEAT ICU—An Observational Study of Physician Workload and the Association of Physician Outcomes in Academic ICUs. Crit Care Explor 2022, 4, e0774. [Google Scholar] [CrossRef]
- Kahn, J. M. , Yabes, J. G., Bukowski, L. A. & Davis, B. S. Intensivist physician-to-patient ratios and mortality in the intensive care unit. Intensive Care Med 2023, 49, 545–553. [Google Scholar]
- Estenssoro, E.; et al. Organizational Issues, Structure, and Processes of Care in 257 ICUs in Latin America: A Study From the Latin America Intensive Care Network. Critical Care Medicine 2017, 45, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on core components of infection prevent.pdf.
- Menegueti, M. G.; et al. Long-term prevention of catheter-associated urinary tract infections among critically ill patients through the implementation of an educational program and a daily checklist for maintenance of indwelling urinary catheters: A quasi-experimental study. Medicine (Baltimore) 2019, 98, e14417. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. https://www.who.int/publications-detail-redirect/9789241549929.
- Meschiari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob Resist Infect Control 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Flodgren, G.; et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database Syst Rev 2016, 2016, CD010669. [Google Scholar] [CrossRef] [PubMed]
- Moghnieh, R.; et al. Mapping of infection prevention and control education and training in some countries of the World Health Organization’s Eastern Mediterranean Region: current situation and future needs. Antimicrobial Resistance & Infection Control 2023, 12, 90. [Google Scholar]
- McNett, M.; et al. A Scoping Review of Implementation Science in Adult Critical Care Settings. Crit Care Explor 2020, 2, e0301. [Google Scholar] [CrossRef] [PubMed]
- Barbash, I. J. , Pike, F., Gunn, S. R., Seymour, C. W. & Kahn, J. M. Effects of Physician-targeted Pay for Performance on Use of Spontaneous Breathing Trials in Mechanically Ventilated Patients. Am J Respir Crit Care Med 2017, 196, 56–63. [Google Scholar] [PubMed]
- Mogyoródi, B.; et al. Effect of an educational intervention on compliance with care bundle items to prevent ventilator-associated pneumonia. Intensive and Critical Care Nursing 2023, 75, 103342. [Google Scholar] [CrossRef] [PubMed]
- Phan, H. T.; et al. An educational intervention to improve hand hygiene compliance in Vietnam. BMC Infect Dis 2018, 18, 116. [Google Scholar] [CrossRef]
- your-5-moments-for-hand-hygiene-poster.pdf.
- World Health Organization & WHO Patient Safety. WHO guidelines on hand hygiene in health care. 262 ( 2009.
- Verderber, S. , Gray, S., Suresh-Kumar, S., Kercz, D. & Parshuram, C. Intensive Care Unit Built Environments: A Comprehensive Literature Review (2005–2020). HERD 2021, 14, 368–415. [Google Scholar]
- Mo, Y.; et al. Duration of Carbapenemase-Producing Enterobacteriaceae Carriage in Hospital Patients. Emerg Infect Dis 2020, 26, 2182–2185. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E. S. , Paras, M. L., Noubary, F., Walensky, R. P. & Hooper, D. C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis 2014, 14, 177. [Google Scholar]
- Scanvic, A.; et al. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis 2001, 32, 1393–1398. [Google Scholar] [CrossRef]
- Sanford, M. D. , Widmer, A. F., Bale, M. J., Jones, R. N. & Wenzel, R. P. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 1994, 19, 1123–1128. [Google Scholar] [PubMed]
- Larsson, A.-K.; et al. Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: a four-year experience from southern Sweden. Scand J Infect Dis 2011, 43, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Yiek, W.-K.; et al. Success rates of MRSA decolonization and factors associated with failure. Antimicrobial Resistance & Infection Control 2022, 11, 143. [Google Scholar]
- Tacconelli, E.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clinical Microbiology and Infection 2019, 25, 807–817. [Google Scholar] [CrossRef]
- Debby, B. D.; et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis 2012, 31, 1811–1817. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; et al. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis 2013, 77, 169–173. [Google Scholar] [CrossRef]
- Pisney, L. M. , Barron, M. A., Kassner, E., Havens, D. & Madinger, N. E. Carbapenem-resistant Enterobacteriaceae rectal screening during an outbreak of New Delhi metallo-β-lactamase-producing Klebsiella pneumoniae at an acute care hospital. Infect Control Hosp Epidemiol 2014, 35, 434–436. [Google Scholar] [PubMed]
- Dickstein, Y.; et al. Carbapenem-resistant Enterobacteriaceae colonization and infection in critically ill patients: a retrospective matched cohort comparison with non-carriers. J Hosp Infect 2016, 94, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Saidel-Odes, L.; et al. A randomized, double-blind, placebo-controlled trial of selective digestive decontamination using oral gentamicin and oral polymyxin E for eradication of carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control Hosp Epidemiol 2012, 33, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oren, I.; et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: A prospective controlled trial. Am J Infect Control 2013, 41, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. & Herwaldt, L. Nasal decolonization: What antimicrobials and antiseptics are most effective before surgery and in the ICU. Am J Infect Control 2023, 51, A64–A71. [Google Scholar] [PubMed]
- Ridenour, G.; et al. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. S.; et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013, 368, 2255–2265. [Google Scholar] [CrossRef]
- Kohler, P. , Bregenzer-Witteck, A., Rettenmund, G., Otterbech, S. & Schlegel, M. MRSA decolonization: success rate, risk factors for failure and optimal duration of follow-up. Infection 2013, 41, 33–40. [Google Scholar]
- Safdar, N. & Bradley, E. A. The Risk of Infection after Nasal Colonization with Staphylococcus Aureus. The American Journal of Medicine 2008, 121, 310–315. [Google Scholar]
- Samuel, P.; et al. Methicillin-Resistant Staphylococcus aureus Colonization in Intensive Care and Burn Units: A Narrative Review. Cureus 2023, 15, e47139. [Google Scholar]
- Olsen, K.; et al. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLoS One 2013, 8, e63716. [Google Scholar]
- Cheng, V. C.; et al. Decolonization of gastrointestinal carriage of vancomycin-resistant Enterococcus faecium: case series and review of literature. BMC Infect Dis 2014, 14, 514. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; et al. Comprehensive, multisystem, mechanical decolonization of Vancomycin-Resistant Enterococcus and Carbapenem-Resistant Enterobacteriacease without the use of antibiotics. Medicine (Baltimore) 2021, 100, e23686. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Professionals FAQ | Candida auris | Fungal Diseases | CDC. https://www.cdc.gov/fungal/candida-auris/c-auris-health-qa.html (2023).
- Key facts and figures. https://www.who.int/campaigns/world-hand-hygiene-day/2021/key-facts-and-figures.
- Lambe, K. A.; et al. Hand Hygiene Compliance in the ICU: A Systematic Review. Crit Care Med 2019, 47, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- DalBen, M. D. F.; et al. A Model-Based Strategy to Control the Spread of Carbapenem-Resistant Enterobacteriaceae: Simulate and Implement. Infect. Control Hosp. Epidemiol. 2016, 37, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T. , VonVille, H. M., Hasan, I. & Garey, K. W. Shoe soles as a potential vector for pathogen transmission: a systematic review. J Appl Microbiol 2016, 121, 1223–1231. [Google Scholar] [PubMed]
- Dee, S. , Deen, J. & Pijoan, C. Evaluation of 4 intervention strategies to prevent the mechanical transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res 2004, 68, 19–26. [Google Scholar] [PubMed]
- Funk, C. R.; et al. Passenger pathogens on physicians. American Journal of Infection Control 2023, 51, 807–811. [Google Scholar] [CrossRef]
- DalBen, M. F.; et al. Colonization pressure as a risk factor for colonization by multiresistant Acinetobacter spp and carbapenem-resistant Pseudomonas aeruginosa in an intensive care unit. Clinics (Sao Paulo) 2013, 68, 1128–1133. [Google Scholar] [CrossRef]
- Tacconelli, E.; et al. Prediction models to identify hospitalized patients at risk of being colonized or infected with multidrug-resistant Acinetobacter baumannii calcoaceticus complex. Journal of Antimicrobial Chemotherapy 2008, 62, 1130–1137. [Google Scholar] [CrossRef]
- Meschiari, M.; et al. Risk factors for nosocomial rectal colonization with carbapenem-resistant Acinetobacter baumannii in hospital: a matched case–control study. Antimicrob Resist Infect Control 2021, 10, 69. [Google Scholar] [CrossRef]
- Logan, C. , Martin-Loeches, I. & Bicanic, T. Invasive candidiasis in critical care: challenges and future directions. Intensive Care Med 2020, 46, 2001–2014. [Google Scholar]
- Pittet, D. , Monod, M., Suter, P. M., Frenk, E. & Auckenthaler, R. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg 1994, 220, 751–758. [Google Scholar] [PubMed]
- León, C.; et al. A bedside scoring system (‘Candida score’) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med 2006, 34, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit: Prediction rule for Candida in ICU. Mycoses 2011, 54, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; et al. Identifying Patients Harboring Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae on Hospital Admission: Derivation and Validation of a Scoring System ▿. Antimicrob Agents Chemother 2011, 55, 3485–3490. [Google Scholar] [CrossRef] [PubMed]
- Augustine, M. R.; et al. Clinical Risk Score for Prediction of Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae in Bloodstream Isolates. Infect. Control Hosp. Epidemiol. 2017, 38, 266–272. [Google Scholar] [CrossRef]
- Papafotiou, C.; et al. Predictive score for patients with carbapenemase-producing enterobacterales colonization upon admission in a tertiary care hospital in an endemic area. Journal of Antimicrobial Chemotherapy 2022, 77, 3331–3339. [Google Scholar] [CrossRef]
- Cogliati Dezza, F.; et al. Risk factors for carbapenem-resistant Acinetobacter baumannii (CRAB) bloodstream infections and related mortality in critically ill patients with CRAB colonization. JAC Antimicrob Resist 2023, 5, dlad096. [Google Scholar] [CrossRef]
- Cosentino, F. , Viale, P. & Giannella, M. MDR/XDR/PDR or DTR? Which definition best fits the resistance profile of Pseudomonas aeruginosa? Curr Opin Infect Dis 2023, 36, 564–571. [Google Scholar]
- Torres, K. & Sampathkumar, P. Predictors of methicillin-resistant Staphylococcus aureus colonization at hospital admission. American Journal of Infection Control 2013, 41, 1043–1047. [Google Scholar] [PubMed]
- Boeing, C.; et al. Development and Validation of a Tool for the Prediction of Vancomycin-Resistant Enterococci Colonization Persistence—the PREVENT Score. Microbiol Spectr.
- Hur, E. Y. , Jin, Y. J., Jin, T. X. & Lee, S. M. Development and evaluation of the automated risk assessment system for multidrug-resistant organisms (autoRAS-MDRO). Journal of Hospital Infection 2018, 98, 202–211. [Google Scholar] [PubMed]
- Garnacho-Montero, J.; et al. Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med 2015, 41, 2057–2075. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Martín, R.; et al. A multimodal intervention program to control a long-term Acinetobacter baumannii endemic in a tertiary care hospital. Antimicrob Resist Infect Control 2019, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A. , Lerner, A., Schwartz, D. & Carmeli, Y. Evaluation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect 2016, 22, 949–e5. [Google Scholar]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities. (World Health Organization, Geneva, 2017).
- Magiorakos, A. P.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrobial Resistance & Infection Control 2017, 6, 113. [Google Scholar]
- Popovich, K. J.; et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol 2022, 44, 1039–1067. [Google Scholar] [CrossRef] [PubMed]
- Huslage, K. , Rutala, W. A., Sickbert-Bennett, E. & Weber, D. J. A quantitative approach to defining ‘high-touch’ surfaces in hospitals. Infect Control Hosp Epidemiol 2010, 31, 850–853. [Google Scholar] [PubMed]
- Enfield, K. B.; et al. Control of simultaneous outbreaks of carbapenemase-producing enterobacteriaceae and extensively drug-resistant Acinetobacter baumannii infection in an intensive care unit using interventions promoted in the Centers for Disease Control and Prevention 2012 carbapenemase-resistant Enterobacteriaceae Toolkit. Infect Control Hosp Epidemiol 2014, 35, 810–817. [Google Scholar]
- Mangioni, D.; et al. Genomic Characterization of Carbapenem-Resistant Acinetobacter baumannii (CRAB) in Mechanically Ventilated COVID-19 Patients and Impact of Infection Control Measures on Reducing CRAB Circulation during the Second Wave of the SARS-CoV-2 Pandemic in Milan, Italy. Microbiol Spectr 2023, 11, e0020923. [Google Scholar]
- Bogaty, C.; et al. Investigation of a Carbapenemase-producing Acinetobacter baumannii outbreak using whole genome sequencing versus a standard epidemiologic investigation. Antimicrob Resist Infect Control 2018, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S. , Gikas, A., Astrinaki, E. & Kritsotakis, E. I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J Hosp Infect 2020, 106, 447–453. [Google Scholar] [PubMed]
- Dickstein, Y.; et al. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin Infect Dis 2019, 69, 769–776. [Google Scholar] [CrossRef]
- Jones, C. L.; et al. Fatal outbreak of an emerging clone of extensively drug-resistant Acinetobacter baumannii with enhanced virulence. Clin Infect Dis 2015, 61, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Influence of High-Efficiency Particulate Air Filtr.pdf.
- Arıkan, I.; et al. Effectiveness of air purifiers in intensive care units: an intervention study. J Hosp Infect 2022, 120, 14–22. [Google Scholar] [CrossRef]
- Rapti, V. , Iliopoulou, K. & Poulakou, G. The Gordian Knot of C. auris: If You Cannot Cut It, Prevent It. Pathogens 2023, 12, 1444. [Google Scholar] [PubMed]
- Cadnum, J. L.; et al. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect Control Hosp Epidemiol 2017, 38, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A. O. , Abu-Hanna, J., Carmeli, Y. & Schechner, V. Environmental contamination by carbapenem-resistant Acinetobacter baumannii: The effects of room type and cleaning methods. Infect Control Hosp Epidemiol 2020, 41, 166–171. [Google Scholar] [PubMed]
- Nutman, A. , Lerner, A., Schwartz, D. & Carmeli, Y. Evaluation of carriage and environmental contamination by carbapenem-resistant Acinetobacter baumannii. Clinical Microbiology and Infection 2016, 22, 949–e5. [Google Scholar]
- Carling, P. C.; et al. Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infect Control Hosp Epidemiol 2008, 29, 1035–1041. [Google Scholar] [CrossRef]
- CDC Environmental Checklist for Monitoring Terminal Cleaning.
- Gray, A. P.; et al. Management of a hospital outbreak of extensively drug-resistant Acinetobacter baumannii using a multimodal intervention including daily chlorhexidine baths. J Hosp Infect 2016, 93, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Molter, G.; et al. Outbreak of carbapenem-resistant Acinetobacter baumannii in the intensive care unit: a multi-level strategic management approach. J Hosp Infect 2016, 92, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Cornistein, W.; et al. Synergy between infection control and antimicrobial stewardship programs to control carbapenem-resistant Enterobacterales. Antimicrob Steward Healthc Epidemiol 2023, 3, e162. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J. P. , Johansson, A. C., Schonberg, M. A. & Howell, M. D. Elements of a High-Quality Inpatient Consultation in the Intensive Care Unit. A Qualitative Study. Annals ATS 2013, 10, 220–227. [Google Scholar]
- Vazquez Guillamet, M. C.; et al. Antimicrobial stewardship for sepsis in the intensive care unit: Survey of critical care and infectious diseases physicians. Infect Control Hosp Epidemiol, 1374. [Google Scholar]
- Bonaconsa, C.; et al. Optimizing infection control and antimicrobial stewardship bedside discussion: a scoping review of existing evidence on effective healthcare communication in hospitals. Clin Microbiol Infect 2024, 30, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Zwerwer, L. R.; et al. Identifying the need for infection-related consultations in intensive care patients using machine learning models. Sci Rep 2024, 14, 2317. [Google Scholar] [CrossRef] [PubMed]
- Kampmeier, S. , Correa-Martinez, C. L., Peters, G., Mellmann, A. & Kahl, B. C. Personal microbiological consultations improve the therapeutic management of Staphylococcus aureus bacteremia. J Infect 2018, 77, 349–356. [Google Scholar]
- Lazure, P.; et al. Gaps and barriers in the implementation and functioning of antimicrobial stewardship programmes: results from an educational and behavioural mixed methods needs assessment in France, the United States, Mexico and India. JAC Antimicrob Resist 2022, 4, dlac094. [Google Scholar] [CrossRef]
- Raineri, E.; et al. Role of the infectious diseases specialist consultant on the appropriateness of antimicrobial therapy prescription in an intensive care unit. Am J Infect Control 2008, 36, 283–290. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med 2009, 169, 1290–1298. [Google Scholar] [CrossRef]
- Sellier, E.; et al. Factors and outcomes associated with physicians’ adherence to recommendations of infectious disease consultations for inpatients. J Antimicrob Chemother 2010, 65, 156–162. [Google Scholar] [CrossRef]
- Forsblom, E. , Ruotsalainen, E., Ollgren, J. & Järvinen, A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis 2013, 56, 527–535. [Google Scholar]
- WHO AWaRe antibiotic book.pdf.
- Seah, V. X. F.; et al. Impact of a Carbapenem Antimicrobial Stewardship Program on Patient Outcomes. Antimicrob Agents Chemother 2017, 61, e00736–17. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; et al. Antimicrobial Stewardship with and without Infectious Diseases Specialist Services to Improve Quality-of-Care in Secondary and Tertiary Care Hospitals in Germany: Study Protocol of the ID ROLL OUT Study. Infect Dis Ther 2022, 11, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Wieland, K. , Chhatwal, P. & Vonberg, R.-P. Outbreak reporting a decade after ORION: where do we stand? The Lancet Infectious Diseases 2017, 17, 476. [Google Scholar] [PubMed]
- Stone, S. P.; et al. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect Dis 2007, 7, 282–288. [Google Scholar] [CrossRef]
- Wieland, K. , Chhatwal, P. & Vonberg, R.-P. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: Results of a systematic review. American Journal of Infection Control 2018, 46, 643–648. [Google Scholar] [PubMed]
- Chen, X. , Wen, X., Jiang, Z. & Yan, Q. Prevalence and factors associated with carbapenem-resistant Enterobacterales (CRE) infection among hematological malignancies patients with CRE intestinal colonization. Ann Clin Microbiol Antimicrob 2023, 22, 3. [Google Scholar]
- Ferreira, A. M.; et al. Epidemiology, risk factors and outcomes of multi-drug-resistant bloodstream infections in haematopoietic stem cell transplant recipients: importance of previous gut colonization. J Hosp Infect 2018, 100, 83–91. [Google Scholar] [CrossRef]
- Zhang, L.; et al. Carbapenem-resistant Enterobacteriaceae in hematological patients: Outcome of patients with Carbapenem-resistant Enterobacteriaceae infection and risk factors for progression to infection after rectal colonization. Int J Antimicrob Agents 2019, 54, 527–529. [Google Scholar] [CrossRef]
- Price, A. M. , Sarween, N., Gupta, I. & Baharani, J. Risk factors and short-term outcomes for methicillin-resistant Staphylococcus aureus and methicillin-sensitive Staphylococcus aureus colonization among hemodialysis patients. Saudi J Kidney Dis Transpl 2019, 30, 1351–1363. [Google Scholar] [PubMed]
- WHO launches first ever global report on infection.pdf.
- Lin, G.; et al. Cost-effectiveness of carbapenem-resistant Enterobacteriaceae (CRE) surveillance in Maryland. Infect Control Hosp Epidemiol 2022, 43, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- DalBen, M. de F. et al. A Model-Based Strategy to Control the Spread of Carbapenem-Resistant Enterobacteriaceae: Simulate and Implement. Infect Control Hosp Epidemiol 2016, 37, 1315–1322. [Google Scholar]
- Giacobbe, D. R.; et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: a changing landscape. J Antimicrob Chemother 2023, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Mac, S. , Fitzpatrick, T., Johnstone, J. & Sander, B. Vancomycin-resistant enterococci (VRE) screening and isolation in the general medicine ward: a cost-effectiveness analysis. Antimicrob Resist Infect Control 2019, 8, 168. [Google Scholar]
- Lloyd-Smith, P.; et al. Economic analysis of vancomycin-resistant enterococci at a Canadian hospital: assessing attributable cost and length of stay. J Hosp Infect 2013, 85, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Vancomycin-Resistant Enterococci Isolation and Screening Strategies: Clinical Evidence and Cost-Effectiveness.
- Satilmis, L. , Vanhems, P. & Bénet, T. Outbreaks of Vancomycin-Resistant Enterococci in Hospital Settings: A Systematic Review and Calculation of the Basic Reproductive Number. Infection Control & Hospital Epidemiology 2016, 37, 289–294. [Google Scholar]
- Chaix, C. , Durand-Zaleski, I., Alberti, C. & Brun-Buisson, C. Control of endemic methicillin-resistant Staphylococcus aureus: a cost-benefit analysis in an intensive care unit. JAMA 1999, 282, 1745–1751. [Google Scholar] [PubMed]
- Haddadin, A. S. , Fappiano, S. A. & Lipsett, P. A. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgraduate Medical Journal 2002, 78, 385–392. [Google Scholar] [PubMed]
- Samuel, P.; et al. Methicillin-Resistant Staphylococcus aureus Colonization in Intensive Care and Burn Units: A Narrative Review. Cureus.
- Steinig, E.; et al. Phylodynamic signatures in the emergence of community-associated MRSA. Proc Natl Acad Sci U S A 2022, 119, e2204993119. [Google Scholar] [CrossRef]
- Coyle, J. R.; et al. Effectiveness and cost of implementing an active surveillance screening policy for Acinetobacter baumannii: A Monte Carlo simulation model. American Journal of Infection Control 2014, 42, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Young, L. S. , Sabel, A. L. & Price, C. S. Epidemiologic, Clinical, and Economic Evaluation of an Outbreak of Clonal Multidrug-Resistant Acinetobacter baumannii Infection in a Surgical Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2007, 28, 1247–1254. [Google Scholar] [PubMed]
- Taori, S. K.; et al. Candida auris outbreak: Mortality, interventions and cost of sustaining control. Journal of Infection 2019, 79, 601–611. [Google Scholar] [CrossRef]
- Otter, J. A.; et al. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae: an economic evaluation from a hospital perspective. Clin Microbiol Infect 2017, 23, 188–196. [Google Scholar] [CrossRef] [PubMed]
- van Beurden, Y. H.; et al. Cost analysis of an outbreak of Clostridium difficile infection ribotype 027 in a Dutch tertiary care centre. J Hosp Infect 2017, 95, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; et al. Impact of In-house Candida auris Polymerase Chain Reaction Screening on Admission on the Incidence Rates of Surveillance and Blood Cultures With C. auris and Associated Cost Savings. Open Forum Infect Dis 2023, 10, ofad567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; et al. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis 2016, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Otete, E. H.; et al. Parameters for the Mathematical Modelling of Clostridium difficile Acquisition and Transmission: A Systematic Review. PLoS One 2013, 8, e84224. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; et al. Cost-effectiveness of bezlotoxumab and fidaxomicin for initial Clostridioides difficile infection. Clin Microbiol Infect 2021, 27, 1448–1454. [Google Scholar] [CrossRef]
- Elsaid, M. , Nasef, M. A. & Huy, N. T. R0 of COVID-19 and its impact on vaccination coverage: compared with previous outbreaks. Hum Vaccin Immunother 2021, 17, 3850–3854. [Google Scholar]
- Merler, S. & Ajelli, M. Deciphering the relative weights of demographic transition and vaccination in the decrease of measles incidence in Italy. Proc Biol Sci 2014, 281, 20132676. [Google Scholar]
- Majumder, M. S. , Rivers, C., Lofgren, E. & Fisman, D. Estimation of MERS-Coronavirus Reproductive Number and Case Fatality Rate for the Spring 2014 Saudi Arabia Outbreak: Insights from Publicly Available Data. PLoS Curr, 3382. [Google Scholar]
- Liu, W. , Tang, S. & Xiao, Y. Model Selection and Evaluation Based on Emerging Infectious Disease Data Sets including A/H1N1 and Ebola. Comput Math Methods Med 2015, 2015, 207105. [Google Scholar] [PubMed]
- Menardo, F. Understanding drivers of phylogenetic clustering and terminal branch lengths distribution in epidemics of Mycobacterium tuberculosis. eLife.
- Shrestha, S.; et al. Model-based Analysis of Tuberculosis Genotype Clusters in the United States Reveals High Degree of Heterogeneity in Transmission and State-level Differences Across California, Florida, New York, and Texas. Clin Infect Dis 2022, 75, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A. D.; et al. Maintenance of Measles Elimination Status in the United States for 20 Years Despite Increasing Challenges. Clinical Infectious Diseases 2022, 75, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F. M.; et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis 2017, 17, e420–e428. [Google Scholar] [CrossRef] [PubMed]
- Villela, D. a. M.; et al. Zika in Rio de Janeiro: Assessment of basic reproduction number and comparison with dengue outbreaks. Epidemiol Infect 2017, 145, 1649–1657. [Google Scholar] [CrossRef]
- Chowell, G.; et al. The basic reproduction number R0 and effectiveness of reactive interventions during dengue epidemics: the 2002 dengue outbreak in Easter Island, Chile. Math Biosci Eng 2013, 10, 1455–1474. [Google Scholar] [PubMed]
- Khan, M. A. & Fatmawati. Dengue infection modeling and its optimal control analysis in East Java, Indonesia. Heliyon 2021, 7, e06023. [Google Scholar]
- Navarro Valencia, V. A. , Díaz, Y., Pascale, J. M., Boni, M. F. & Sanchez-Galan, J. E. Using compartmental models and Particle Swarm Optimization to assess Dengue basic reproduction number R 0 for the Republic of Panama in the 1999-2022 period. Heliyon 2023, 9, e15424. [Google Scholar]
- Muzembo, B. A.; et al. The basic reproduction number (R0) of ebola virus disease: A systematic review and meta-analysis. Travel Med Infect Dis 2024, 57, 102685. [Google Scholar] [CrossRef]
- Nsubuga, R. N. , White, R. G., Mayanja, B. N. & Shafer, L. A. Estimation of the HIV basic reproduction number in rural south west Uganda: 1991-2008. PLoS One 2014, 9, e83778. [Google Scholar]
- DalBen, M. de F. et al. S: Strategy to Control the Spread of Carbapenem- Resistant Enterobacteriaceae.
- Stachel, A.; et al. Implementation and evaluation of an automated surveillance system to detect hospital outbreak. American Journal of Infection Control 2017, 45, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Piaggio, D.; et al. The use of smart environments and robots for infection prevention control: A systematic literature review. Am J Infect Control 2023, 51, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A. , Schwebke, I. & Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006, 6, 130. [Google Scholar]
- Corbella, X.; et al. Environmental sampling of Acinetobacter baumannii: moistened swabs versus moistened sterile gauze pads. Infect Control Hosp Epidemiol 1999, 20, 458–460. [Google Scholar] [CrossRef]
- Schwab, F. , Meyer, E., Geffers, C. & Gastmeier, P. Understaffing, overcrowding, inappropriate nurse:ventilated patient ratio and nosocomial infections: which parameter is the best reflection of deficits? J Hosp Infect 2012, 80, 133–139. [Google Scholar] [PubMed]
- Oliveira, A. C. de, Garcia, P. C. & Nogueira, L. de S. Nursing workload and occurrence of adverse events in intensive care: a systematic review. Rev Esc Enferm USP 2016, 50, 683–694. [Google Scholar]
- Bracco, D. , Dubois, M.-J., Bouali, R. & Eggimann, P. Single rooms may help to prevent nosocomial bloodstream infection and cross-transmission of methicillin-resistant Staphylococcus aureus in intensive care units. Intensive Care Med 2007, 33, 836–840. [Google Scholar] [PubMed]
- Hatfull, G. F. , Dedrick, R. M. & Schooley, R. T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu Rev Med 2022, 73, 197–211. [Google Scholar]
- Wang, J.; et al. Isolation and identification of a novel phage targeting clinical multidrug-resistant Corynebacterium striatum isolates. Front Cell Infect Microbiol 2024, 14, 1361045. [Google Scholar] [CrossRef]
- Skurnik, D.; et al. Extended-spectrum antibodies protective against carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2016, 71, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Kalfopoulou, E. & Huebner, J. Advances and Prospects in Vaccine Development against Enterococci. Cells 2020, 9, 2397. [Google Scholar] [PubMed]
- Miller, L. S. , Fowler, V. G., Shukla, S. K., Rose, W. E. & Proctor, R. A. Development of a vaccine against Staphylococcus aureus invasive infections: Evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev 2019, 44, 123–153. [Google Scholar]
- Bae, J. Y.; et al. Association between Pneumonia Development and Virulence Gene Expression in Carbapenem-Resistant Acinetobacter baumannii Isolated from Clinical Specimens. Can J Infect Dis Med Microbiol 2023, 2023, 8265683. [Google Scholar] [CrossRef]
- Wong, F. , de la Fuente-Nunez, C. & Collins, J. J. Leveraging artificial intelligence in the fight against infectious diseases. Science 2023, 381, 164–170. [Google Scholar] [PubMed]
- Ben-Chetrit, E.; et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: long-term impact for the ICU and hospital. Crit Care 2018, 22, 319. [Google Scholar] [CrossRef] [PubMed]
- Enfield, K. B.; et al. Control of simultaneous outbreaks of carbapenemase-producing enterobacteriaceae and extensively drug-resistant Acinetobacter baumannii infection in an intensive care unit using interventions promoted in the Centers for Disease Control and Prevention 2012 carbapenemase-resistant Enterobacteriaceae Toolkit. Infect Control Hosp Epidemiol 2014, 35, 810–817. [Google Scholar]
- Landelle, C. , Pagani, L. & Harbarth, S. Is patient isolation the single most important measure to prevent the spread of multidrug-resistant pathogens? Virulence 2013, 4, 163–171. [Google Scholar]
- DalBen, M. D. F.; et al. A Model-Based Strategy to Control the Spread of Carbapenem-Resistant Enterobacteriaceae: Simulate and Implement. Infect. Control Hosp. Epidemiol. 2016, 37, 1315–1322. [Google Scholar] [CrossRef]
| Routes of transmission in ICU |
|---|
| Direct or indirect contact Bacteria: MDR-GNB [9] (including CRE, ESBL carriers [10], MDR-Klebsiella spp, MDR-Acinetobacter baumanii [11], and MDR-Pseudomonas aeruginosa), MRSA [12], VRE [13], Clostridium difficile [13] Fungi: Candida auris [14], Scedosporium spp [15] Virus: Ebola virus [16] |
| Water contamination [17] Bacteria: Legionella spp., Pseudomonas spp, Acinetobacter spp, and Serratia Fungi: Aspergillus spp., Mucor spp., Trichosporon spp., Scedosporium spp [18] and Fusarium Virus: Norovirus |
| Air contamination Bacteria [19]: CRE, Acinetobacter baumanii, Pseudomonas aeruginosa, Corynebacterium striatum*, Legionella spp., MRSA [20] Fungi: Aspergillus spp [21], Fusarium spp [18], Scedosporium spp [15,18], Lomentospora spp. [18] Virus: human coronaviruses (including SARS-CoV-2 [22]), Ebola virus [16] |
| Droplet and airborne spread [23] Bacteria: Mycobacterium tuberculosis, Bordetella spp, Pertussis Virus: human coronaviruses (including SARS-CoV-2 [22]), Varicela-Zoster virus, Measles virus, influenza viruses (including H1N1, H2N3, H5N1), Parainfluenza viruses, Respiratory Syncytial Virus, Adenoviruses |
| Type of study | Study author and year of publishing | Country | Time period | Sample size | Suggested ICU PNR | Higher ratios are associated with higher mortality |
|---|---|---|---|---|---|---|
| Guidelines | Bray K et al. 2010 (the British Association of Critical Care Nurses, the Critical Care Networks National Nurse Leads) [35] | UK | - | - | 1: 1 | Yes |
| American Nurses Association (ANA) and California Legislation (Assembly Bill No. 394) | California, USA | - | - | 2 : 1 | Yes | |
| Narrative review | Suresh K. Sharma et Ritu Rani 2020 [36] | India | - | - | 1 : 1 | Yes |
| Retrospective observational study | Falk AC 2023 [37] | Sweden | 15 years | 2 ICUs (9,814 patients) | 1 : 1 | Yes |
| Cross-sectional, retrospective, risk adjusted observational study | West E et al. 2014 [38] | UK | 16 years | 65 ICUs (38,168 patients) | 0,5 : 1 | Yes* |
| Study author and year of publication | Country | Type of study | Time period | Sample size | Median PIR | Higher ratios resulted to be associated with higher mortality |
|---|---|---|---|---|---|---|
| Neuraz A et al. 2015 [40] | France | Multicenter observational study | 2013 |
5,718 patients (8 ICUs) |
5.6 | Yes |
| Gershengorn HB et al. 2017 [41] | UK | Retrospective cohort study | 2010-2013 |
49,686 patients (94 ICUs) |
8.5 | Yes |
| Dara SI et al. 2005 [44] | USA | Retrospective cohort study | 2001-2003 | 2,492 patients (1 ICU) |
8.4* |
No |
| Gershengorn HB et al. 2022 [42] | Australia and New Zealand | Retrospective cohort study | 2016-2019 | 27,380 patients (67 ICUs) in the “narrow cohort” and 91,206 patients (73 ICUs) in the “broad cohort” | 10.1 | No |
| Agarwal A et al. 2022 [45] | USA | Cross-sectional observational study | 2020-2021 | 1,322 patients (62 ICUs) |
12 | No |
| Kahn JM et al. 2023 [46] | USA | Retrospective cohort study | 2018-2020 | 51,656 patients (29 ICUs) |
11.8 | No |
| Estenssoro E et al. 2017 [47] | Latin America (51% from Brazil, 17% Chile, 13% Argentina, 6% Ecuador, 5% Uruguay, 3% Colombia, and 5% between Mexico, Peru, and Paraguay.) | Cross-sectional observational study | 2015-2016 | 257 ICUs | 1:1-1:3 (11%) 1:4 to 1:7 (46%) > 8 (41%) |
Not evaluated |
| Study author and year of publication | Country | Type of infection | Most relevant proposed solution |
|---|---|---|---|
| Menegueti MG et al. 2019 [49] | Brazil | CAUTI |
|
| McNett et al 2020 [54] | USA | VAP |
|
| Mogyodi et al. 2023 [56] | Hungary | VAP |
|
| Phan et al. 2018 [57] | Vietnam | All HCAI |
|
| Moghnieh et al. 2023 [53] | Eastern Mediterranean Region (Afghanistan. Barhain, Iraq, Kuwait, Jordan, Lebanon, Oman, Pakistan, Palestine, Qatar, Sudan, Syria, United Arab Emirates, Yemen) | All HCAI |
|
| Candida spp. [93] | Colonization | Candida Colonization Index [94] | Ratio of the number of (non-blood) sites colonized with Candida spp /total number of sites cultured Threshold = 0.5 |
PPV = 66% NPV = 100% |
| Infection | Candida score [95] | Candida Score = TPN (1 point), surgery (1 point), severe sepsis (2 points), Multifocal Candida colonization (1 point). Threshold = 2.5 |
Sensitivity = 81% Specificity = 74% PPV = 16% NPV = 98% |
|
| Ostrosky-Zeichner Clinical Prediction Rule [96] |
Mechanical ventilation ≥ 48hours AND Systemic antibiotic AND CVP (on any of day 1–3 of ICU admission) plus ≥1 of: any major surgery (days 7–0), pancreatitis (days 7–0), use of steroids/other immunosuppressive agents (days 7–0), use of TPN (days 1–3), or dialysis (days 1–3) | Sensitivity = 50% Specificity= 83% PPV = 10% NPV = 97% |
||
| ESBL-producing Enterobacteriacae | Colonization | Tumbarello et al. [97] | Recent (≤12 months before admission) hospitalization, transfer from another health care facility, Charlson comorbidity score ≥ 4, recent (≤3 months before admission) β-lactam and/or fluoroquinolone treatment, recent urinary catheterization, and age ≥ 70 years. | With cutoff score ≥3: Sensitivity = 94% Specificity = 41% PPV = 44% NPV = 93% |
| Infection (BSI) | ESBL Prediction Score (ESBL-PS) [98] | Outpatient procedures within 1 month, prior infections or colonization with ESBLE within 12 months, and number of prior courses of β-lactams and/or fluoroquinolones used within 3 months of BSI. | With cutoff score ≥1: Sensitivity = 88% Specificity = 77% PPV = 16% NPV = 99% With cutoff score ≥3: Sensitivity = 43% Specificity = 96% PPV = 33% NPV = 97% |
|
| CPE | Colonization | Papafotiou et al. [99] | Karnofsky score, previous hospitalization, stay in a Long-term care facilty, history of ≥2 different interventional procedures, previous CPE colonization or infection, renal replacement therapy, and diabetes with end-organ damage | With cutoff score ≥27: Sensitivity = 72% Specificity = 81% PPV = 15% NPV = 98% |
| CRAB | Infection | Cogliati Dezza et al. [100] | CRAB colonization, higher CCI, multisite colonization and the need for mechanical ventilation. | Unknown |
| XDR A. baumanii | Colonization | Moghnieh et al. [101] | Urinary catheter placement >6 days, ICU contact pressure for >4 days, presence of gastrostomy tube, and previous use of carbapenems or piperacillinetazobactam | Unknown |
| MRSA | Colonization | Torres et Sampathkumar [102] | Nursing home residence, diabetes, hospitalization in the past year, and chronic skin condition/infection | With cutoff score ≥8: Sensitivity = 54% Specificity = 80% |
| VRE | Colonization | The PREVENT score [103] | Age of ≥60 years, hemato-oncological disease, cumulative antibiotic treatment for >4 weeks, and a VRE infection | Sensitivity = 82% Specificity = 77% PPV = 57% NPV = 92% |
| MDROs | Colonization | AutoRAS- MDRO [104] | Electronic health records (EHRs) | Sensitivity = 81% Specificity= 79% PPV = 49% NPV = 94% |
| Type of pathogen | Estimated mean single-patient cost per hospital length of stay i | Estimated mean R₀ | Estimated mean outbreak cost | IPC implementation threshold (up to) |
|---|---|---|---|---|
| CRE | $ 639,48 | 11 | €1.1 millions | $572,000 |
| CRAB | $55,122-$ 60,000 | 1.5 | €1.0 millions | $75,000- $93,822/QALY |
| VRE | $17,949 | 1.32 | € 60.524 | $50.000/QALY |
| MRSA | $ 9.275 | 0.97-1.6 [160] | $30.225 | $9.275 |
| C. auris | € 35.818* | Unknown | € 1.2 millions | $3.730.480,26 |
| (HO-CDI) | $30,049 - $34,149 [165,167] | 0.55-7.0 [168] | €1.2 millions | $150 000/QALY [169] |
| Type of transmission | Type of pathogen | Estimated R₀ (mean) | Country |
|---|---|---|---|
| Airborne | SARS-CoV-2 | 1.4 to 6.7 [170] (4.1) | China, Italy, Korea, Peru |
| SARS virus | 1.7 to 1.9 [171] (1.8) | Hong Kong | |
| MERS virus | 2.0 to 6.7 [172] (4.4) | Saudi Arabia | |
| H1N1 | 1.9 [173] | China | |
| Mycobacterium tuberculosis (MTB) | 0.8 to 1.2 [174] 0.2 to 0.4 [175] (0.29) |
USA |
|
| Measles virus | 0.7 [176] to 25.3 [171] (13)* 12-18 (15) [177] |
USA, Italy, Japan Systemic review |
|
| Vectorborne | Zika virus | 2.3 [178] to 27.2 [179] (14.9) | Brazil, Chile |
| Dengue virus | 1.1 [180]-1.7 [178,181] (1.4) | Indonesia, Brazil | |
| Bloodborne/ Body fluids contact |
Ebola virus | 1.1 to 10 [182] (1.95) [182] | West Africa |
| HIV (viremic) | (36.8) [183] | Uganda |
| Study author and year of publication | Country | Study design | Pathogen | Experimental period | Name of the new strategies |
|---|---|---|---|---|---|
| De Freitas DalBen et al. 2016 [184] | Brazil | Prospective study | CRE | Baseline period: 10 monthsIntervention period: 24 weeks | Educational model based on:
|
| Stachel et al. 2017 [185] | USA | Prospective study | MDROs | 8 months | Automated surveillance system to detect hospital outbreak |
| Fitzpatrick et al. 2020 | Ireland | Narrative review | All pathogens | - | Artificial Intelligence in IPC: driven by “big data”, it could find correlations that may indicate medically relevant conditions or identify potential risk factors for outbreaks |
| Meschiari et al. 2021 [51] | Italy | Prospective study | CRAB | 6-years (2013-2019) | Cycling radical cleaning and disinfection |
| Piaggio et al. 2023 [186] | Italy | Systemic review | All pathogens | - |
|
| Zwerwer et al. 2024 [132] | Netherlands | Prospective study | All pathogens | 3-years (2014-2017) | Machine-learning model to predict the need for infection-related consultations in ICU |
| First Author and year of publication | Country | Target pathogen | Aim of the study | Suggested technique |
|---|---|---|---|---|
| Hatfull GF et al. 2022 [192] | USA | MDRB | Fighting antibiotic resistance | Phage therapy |
| Wang J et al. 2024 [193] | China | MDR-Corynebacterium striatum | Fighting antibiotic resistance | Phage therapy |
| Skurnik et al. 2016 [194] | USA | CPE | Vaccine against CPE (including NDM-producers E. coli, E. cloacae, K. pneumoniae, K. pneumoniae carbapenemase (KPC)-producing and PNAG-producing P. aeruginosa) | Vaccine targeting polysaccharide poly-(β-1,6)-N-acetyl glucosamine (PNAG) in CPE |
| Kalfopoulou et Huebner. 2020 [195] | Germany | VRE | Vaccine against Enterococci and VRE | Vaccine targeting capsular polysaccharides and surface-associated proteins in Enterococci |
| Miller et al. 2020 [196] | USA | MRSA | Vaccine against MRSA | Vaccine targeting superantigens and pore-forming toxins in MRSA |
| Meschiari et al. 2021 [51] | Italy | CRAB | IPC measures in CRAB’s outbreaks | Targeting inactivated adeN gene in CRAB |
| Ji Yun Bae et al. 2023 [197] | Korea | CRAB | Identifying virulents CRAB’s genes associated with higher mortality in VAP | Targeting hisF and uspA genes in CRAB |
| Choi et al. 2022 [82] | South Korea | VRE and CRE | New non-antibiotic decolonization strategy | 4-items bundle:
|
| Wong et al. 2023 [198] | USA | All pathogens | Use of artificial intelligence for new antinfective drugs discovery, pathogens’ pathophysiology and transmission understanding, and diagnostics | Artificial intelligence implementation |
| Zwerwer et al. 2024 [132] | Netherlands | All pathogens | Use a machine-learning model to predict the need for infection-related consultations in ICU | Machine-learning model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





