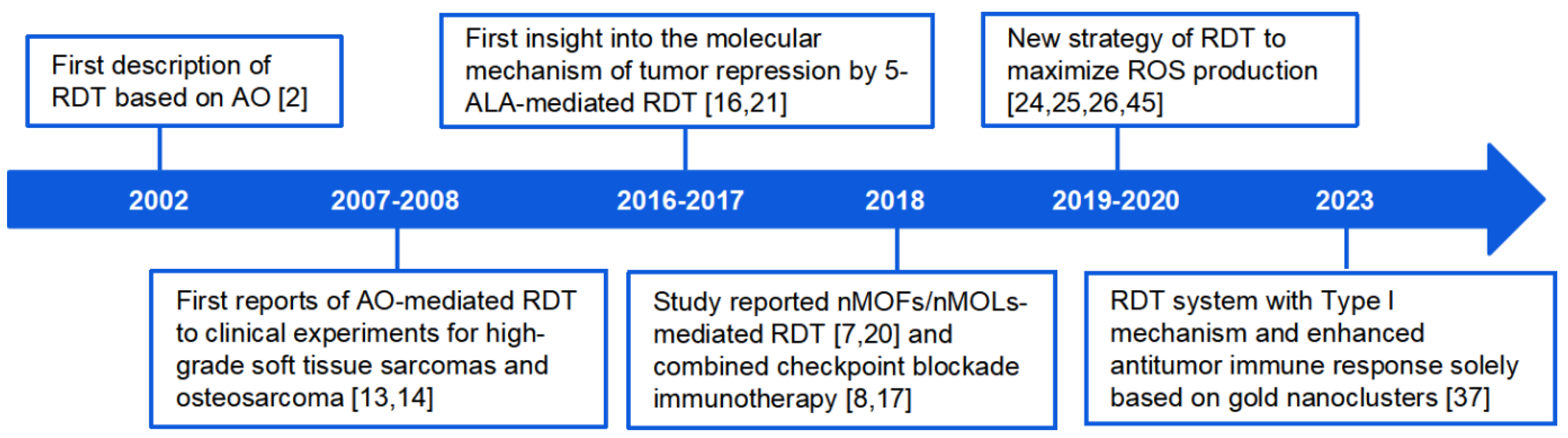
1. Introduction
Cancer has posed a significant threat to human health for decades, prompting the development of various treatment modalities. However, the three conventional methods, surgery, radiotherapy, and chemotherapy, each has its own limitations. Surgeries carry risks of metastasis and recurrence due to large incisions. Additionally, radiotherapy and chemotherapy result in local side effects on surrounding normal tissues under high-dose radiation and systemic side effects with the emergence of drug resistance. The shortcomings of these approaches underscore the urgent need for innovative cancer treatments that can overcome these limitations.
Over a century ago, the term “photodynamic” was introduced, marking the beginning of studies on photodynamic therapy (PDT) [
1]. PDT, a non-invasive technique, involves the co-localization of light, oxygen, and photosensitizers (PS) to produce highly cytotoxic reactive oxygen species (ROS). Compared to traditional tumor treatments such as surgery or chemotherapy, PDT is more advantageous because of the small wound it causes and its high selectivity and low side-effects. While PDT has shown success in local treatment for superficial cancers, its application to deep-seated or solid tumors is technically challenging due to limitations in light penetration and local irradiation.
To address the restricted penetration depth of visible and near-infrared lights, high-energy rays, such as X-rays and γ-rays, have emerged as promising sources for activating photosensitizers in deep-seated tumors. This approach, known as radio-dynamic therapy (RDT), combines low-dose high-energy radiation with photosensitizers to generate highly toxic ROS for dynamic therapy. RDT presents several advantages, including greater penetration depth, higher excitation efficiency than PDT, fewer side effects than traditional radiotherapy, non-drug resistance, and a non-invasive approach to prevent cancer metastasis and recurrence.
The inception of RDT dates back to 2002 when Shin Hashiguchi and colleagues proposed the use of X-rays to activate the photosensitizer acridine orange for deep-seated musculoskeletal sarcoma [
2]. Subsequent studies revealed the potential of utilizing 5-ALA-induced protoporphyrin IX (PpIX) to enhance RDT effects [
3]. However, challenges persisted, including the discrepancy between the energy of rays and the excitation energy of photosensitizers, leading to decreased energy utilization efficiency.
In recent years, nanomaterials containing high-Z atomic number elements have emerged as a solution to enhance the deposition of radiation energy at tumor sites during RDT. Notable developments include scintillating nanoparticles [
4], nanoscale metal-organic frameworks (nMOFs) [
5,
6,
7,
8] and gold nanoparticles (AuNPs) [
9], demonstrating enhanced energy transfer from X-rays to photosensitizers, increased cell destruction efficiency, and notable DNA damage.
This review aims to summarize the progress made in RDT over the past two decades (
Figure 1), focusing on therapeutic mechanisms, strategies for improving RDT efficacy through photosensitizer design, drug delivery, and microenvironmental control. We also highlight advances in combining RDT with other cancer treatments to enhance specificity and lethality to tumor cells. The discussion also encompasses existing challenges and potential directions for further exploration in the field of radio-dynamic therapy.
2. Radio-Dynamic Therapy (RDT) and Its Problems
Radio-dynamic Therapy (RDT) has emerged as a novel cancer treatment method, integrating low-dose high-energy radiation, such as X-rays, with photosensitizers [
10,
11,
12]. This innovative approach aims to exploit the cytotoxic reactive oxygen species (ROS) generated by the interaction of radiation and photosensitizers to induce cancer cell death. The fundamental components of the RDT system include scintillators and photosensitizers. For example, Hashiguchi et al. reported that the acridine orange (AO) could be excited by low-dose X-rays to generate singlet oxygen (
1O
2), and AO-mediated RDT was effective in eliminating mouse osteosarcoma cells in vitro and in vivo [
2]. Kusuzaki and Nakamura et al. then successively applied AO-mediated RDT to clinical studies treating patients with high-grade soft tissue sarcomas and osteosarcoma [
13,
14,
15]. Though clinical cases reported positive results such as lower local recurrence rate and increased specificity due to fluorescence, AO-mediated RDT displayed significant limitations in treating deep-seated or large tumors. Takahashi et al. investigated 5-aminolevulinic acid (5-ALA)-mediated RDT on radiation-resistant cancer melanoma and revealed corresponding molecular mechanism of tumor repression [
16]. Lu et al. reported two nMOFs constructed from Hf cluster scintillators and porphyrin-based photosensitizer ligands, 5,15-di(
p-benzoato)porphyrin-Hf (DBP-Hf) and 5,10,15,20-tetra(
p-benzoato) porphyrin-Hf (TBP-Hf), were structurally optimal for RDT and obtained positive clinical feedbacks by combining with checkpoint blockade immunotherapy [
17]. Subsequently, Lin group developed a series of Hf-based nanoscale metal-organic frameworks (nMOFs) and nanoscale metal-organic layers (nMOLs). These materials demonstrated tunable chemical compositions, multifunctionality, extremely high porosity, great biocompatibility, short-term stability, and long-term biodegradability, have been applied to RDT [
7,
8,
17,
18,
19,
20]. In principle, RDT can increase DNA double-strand break (DSB), halt cells at G2/M phase, enhance ROS generation, and induce immune responses that collectively result in the cell cycle disruption [
17,
21,
22,
23]. Nevertheless, the low photosensitivity, selectivity, and ROS quantum yield makes conventional PS still fall short of the expected therapeutic efficacy [
14,
15,
16].
The scintillator-mediated dual-component strategy utilizes scintillators to absorb and convert X-rays into visible light, which can activate nearby photosensitizers to produce cytotoxic ROS. However, this approach faces significant challenges, including insufficient energy transfer efficiency, low loading capacity, diffusion of photosensitizers, and limited ROS generation rate [
10,
24,
25]. These issues hinder the overall efficacy of the RDT system. Thus, developing photosensitizers that can be directly excited by low-dose X-ray independent of fluorescence resonance energy transfer (FRET) is necessary for high efficacy [
25].
3. Innovative Single-Component RDT Systems
To overcome the drawbacks of the dual-component strategy, recent developments have focused on one-component RDT systems based on nanomaterials containing high-Z metal elements. This innovative approach has demonstrated great potential in effectively treating deep-seated tumors, thereby addressing the limitations of loading capacity and energy transfer efficiency.
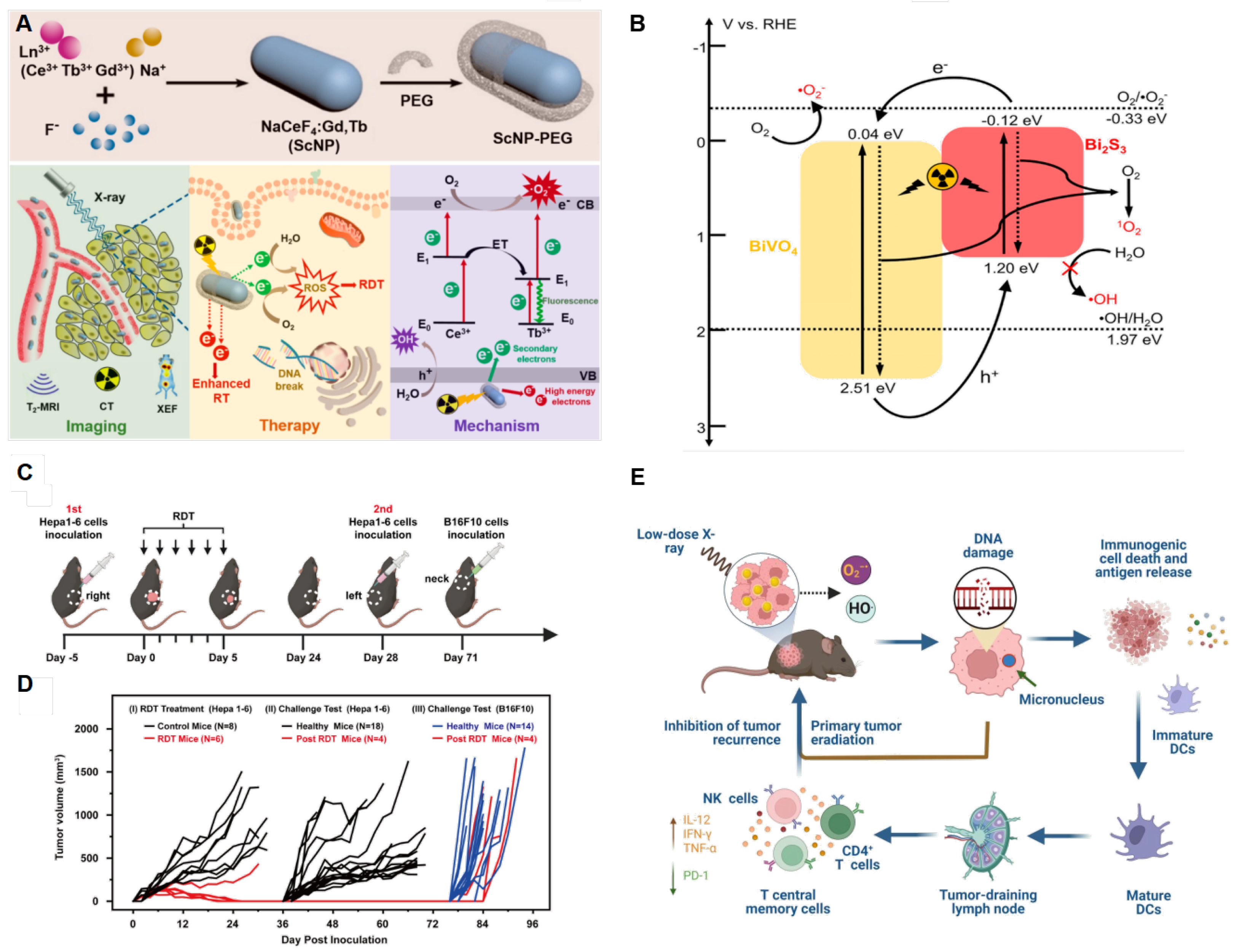
Zhong et al. firstly designed the Ce-doped NaCeF
4:Gd,Tb scintillator nanoparticles (ScNPs) and proved its X-ray responsive property [
24]. Their proposed mechanism suggests that ROS generation can be enhanced by inhibiting the recombination of electrons and holes of Ce
3+ ions and Tb
3+ ions. Herein, electrons in ground state of Ce
3+ ions and Tb
3+ ions can absorb the energy of one secondary electron to an excited state or move toward the conduction band and further reacts with O
2 in
Figure 2A [
24]. Meanwhile, in vivo NaCeF
4:Gd, Tb ScNPs-PEG demonstrated antitumor efficacy up to 63.67% with the least body weight loss. To maximize ROS production, researchers have focused on improving the reduction reaction of oxygen. BiVO
4@Bi
2S
3 heterojunction nanorods (HNRs) reported enhanced RDT therapeutic efficacy due to the long-lasting separation of electron-pairs that can excited directly by ionizing irritation [
26]. Under both X-ray and NIR irradiation, BiVO
4@Bi
2S
3 HNRs demonstrated extraordinary, complete inhibition of tumor growth with a relatively low body weight loss. The heterojunction structure enables electron migration between conduction band and valence band of BiVO
4@Bi
2S
3. Combined with oxygen vacancies on the surface of photosensitizers, the electron-pair lifetimes are prolonged, thereby increasing the possibility for it to reduce O
2 to form ROS in
Figure 2B [
26].
More recently, gold nanomaterials have been investigated for applications in PDT as photosensitizers [
27,
28,
29,
30] and radiotherapy as radio-sensitizers [
31,
32,
33,
34,
35,
36], owing to their stronger X-ray excited luminescence as gold being high-Z elements. Gold nanoparticle (AuNP) aggregates have shown size-dependent enhanced surface plasmonic resonance (SPR), enabling the higher singlet oxygen species formation than AuNP under same irradiation [
27,
28,
29,
30]. Han et al. proved that dihydrolipoic acid coated gold nanoclusters (AuNC@DHLA) obtain two-photon excitation property and that enhance type I energy-transfer which produces free radicals of ROS that are more responsive to PDT [
27]. After irradiation, cells demonstrate apoptotic morphological changes. Meanwhile, the excess ROS releases lysosomal proteases and damages mitochondria. Gold nanoclusters utilizing type I photochemical mechanism has proven to be the most efficient in reducing tumor sizes among conventional and type II photosensitizers, while also exhibiting the least toxicity. Therefore, they can be ideal substitutes for photosensitizers in RDT in the future [
27,
28,
29,
30]. Recently, AuNCs@DHLA RDT, without additional scintillator or photosensitizer assisted, has been developed [
37]. It has been proven to be highly efficient in in vivo treatment of solid tumors via single dose of drug administration and low-dose X-ray radiation. Interestingly, enhanced antitumor immune response have also been observed, which could be effective against tumor recurrence or metastasis (2C-E).
4. RDT Mechanisms
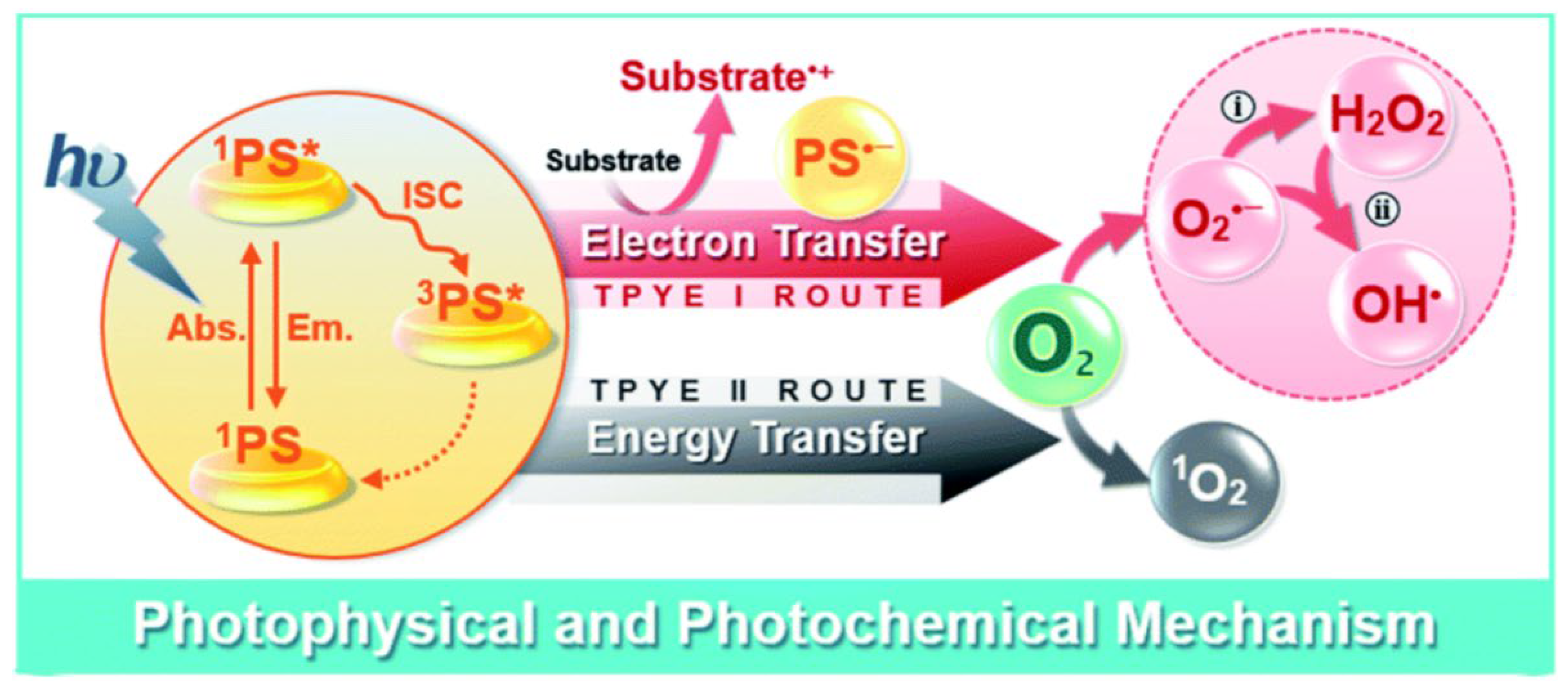
4.1. ROS Generation in RDT
RDT primarily relies on the generation of cytotoxic ROS to induce damage to tumor cells. Singlet oxygen (
1O
2), superoxide anion radical (O
2˙−), hydroxyl radical (HO˙), and hydrogen peroxide (H
2O
2) are the ROS species generally involved in RDT [
38,
39,
40,
41,
42,
43]. Based on the mechanisms of ROS generation, RDT can be classified into two types: type I and type II. Type II RDT generates highly reactive
1O
2 through energy transfer from activated PSs to the triplet ground molecular oxygen (
3O
2) [
28]. In contrast, type I RDT produces ROS via electron (or hole) transfer from PSs to O
2 (
Figure 3). Currently, RDT systems predominantly rely on type II photochemical reactions, which is heavily dependent on and consume large amounts of O
2 during treatment. However, the hypoxic microenvironment prevalent in deep-seated tumors poses a significant challenge. This issue has led to the exploration of type I RDT, where the photosensitizer (PS) and substrate molecules undergo direct electron transfer, typically forming free radicals. Importantly, this photodynamic pathway operates without requiring significant oxygen consumption.
4.2. The Biomedical Mechanism behind RDT
The precise biomedical mechanism of RDT is still not fully understood, yet the synergistic effect of PDT and hypofractionated radiotherapy is commonly associated with RDT [
44]. The followings five aspects are known for the biomedical mechanism behind RDT.
4.2.1. The Cytotoxic Effect of ROS
In the study of AO-mediated RDT on mouse osteosarcoma, Hashiguchi et al. reported that the inhibitor of
1O
2, L-histidine could promote survival rate of mouse osteosarcoma cells [
2]. Thus, they primarily proposed that the generation of
1O
2 might explain the cytocidal effect. Fan and his group worked on the synthesis of hollow mesoporous organosilica nanoparticles (HMONs), a radiosensitizer that hybridizes the organic/inorganic framework, to co-deliver tert-butyl hydroperoxide (TBHP) and iron pentacarbonyl (Fe(CO)
5) [
45]. They found that X-ray can break the O-OH bond of HMOP-TBHP/Fe(CO)
5 and form highly cytotoxic •OH that brings oxidative damage to DNA and other biological macromolecules in RDT. Studies on nanoscale metal organic frameworks (nMOFs) and nanoscale metal-organic layers (nMOLs) showed that the interaction between these materials and X-ray irradiation generate intracellular •OH,
1O
2, and O
2•− during radio therapy-radio dynamic therapy (RT-RDT) [
7,
17,
20]. Takahashi, Hasegawa and their colleagues proved that 5-ALA/protoporphyrin IX-mediated RDT promote the production of free oxygen radicals [
3,
16,
21,
22,
23]. Meanwhile, the increase of ROS in cells enhances expression of caspase-3 and caspase-9 (enzymes initiating proteolytic cleavage) in downstream apoptotic pathways, resulting in dysfunction of mitochondria and apoptosis [
7]. Though the enhancement of ROS production in RDT has been proved, evaluating the specific contribution of each type of ROS in killing tumors can be challenging due to the characteristics of ROS including short lifetime, high reactivity, and diffusion and interconversion [
20,
38,
43,
46]. Comprehensively, ROS generation destructs the DNA double strands, proteins, panniculus adiposus of organelles (i.e., mitochondria) of tumor cells and other active biological macromolecules to suppress cell proliferation and prevent cell reproduction [
3,
20].
4.2.2. The Disturbance of Cell Cycle
Apart from ROS generation, the inhibition of cell mitosis and disturbance of cell cycle also plays an important role in RDT. Kusuzaki and Takahashi et al. reported that aberrant nuclear morphologies and swollen cells were observed after RDT [
2,
13,
19]. DNA ploidy analysis showed that many of the surviving cancer cells were arrested at the G2 phase and had a polyploid DNA content more than octoploid [
2,
13,
19]. Additionally, taking in ROS scavenger, vitamin C, can prevent B16-BL6 melanoma cells from being arrested at G2/M phase [
16]. Those vitamin C-treated tumor cells can proceed the normal cell cycle of growth and cell division, indicating that ROS may disrupt the cell cycle [
16]. Many research also revealed that the oxidative damage of RDT resulted in DNA double-strand break (DSB, known as the most deleterious type of DNA damage) of tumor cell [
7,
17,
20,
24,
35,
45,
47]. The abnormality of DNA prevents cells from passing G2 checkpoint to enter M phase, proving that RDT is capable of inhibiting mitosis.
The cell cycle is precisely regulated by the interactions between cyclins (Ccn), cyclin-dependent kinase (CDKs), and cyclin-dependent kinase inhibitors in eukaryotic cells [
48]. Takahashi et al. reported that RDT could decrease the gene expression of Ccna1, Ccna2, Ccnb1, Ccnb2, Cdk1, and Cdk4, while up-regulate that of Gadd45a and Cdkn1a (p21) [
16]. CDKs are primarily regulated by binding to its cyclin partners and CDK inhibitors [
49]. Since cyclin B activates CDK1 to initiate mitosis [
51], decrease of cyclins and Cdk1 indicates cell arrest at G2 phase. GADD45 is a protein induced by DNA damage or captured by cell cycle, while p21 is an inhibitor of cell cycle dependent kinase [
16]. Thus, the up regulation of Gadd45a and Cdkn1a (p21) prevents the cells from progressing in the cell cycle at the G2/M checkpoint.
Malignant tumors are always considered life-threatening because of their uncontrolled growth of abnormal cells. Once damaged cells enter the cell cycle, they can proliferate uncontrollably due to the failure of checkpoints. RDT that can induce up-/down- expression of genes regulating cell cycle is prospective as a cancer therapy for compensating the unfunctional checkpoints. Meanwhile, the abnormality of DNA renders those cells incapable of passing the functional checkpoint, thereby reducing the aggregation of damaged cells.
4.2.3. The Effect of Directly Killing Tumor Cells
Apoptosis, necrosis, autophagy, and other cell death can serve as regulatory mechanisms of radio-dynamic therapy. Apoptosis is an autonomous cell death which is strictly regulated by multiple genes that are highly conservative among species, such as Bcl-2 family, caspase family, and p53 [
51,
52,
53,
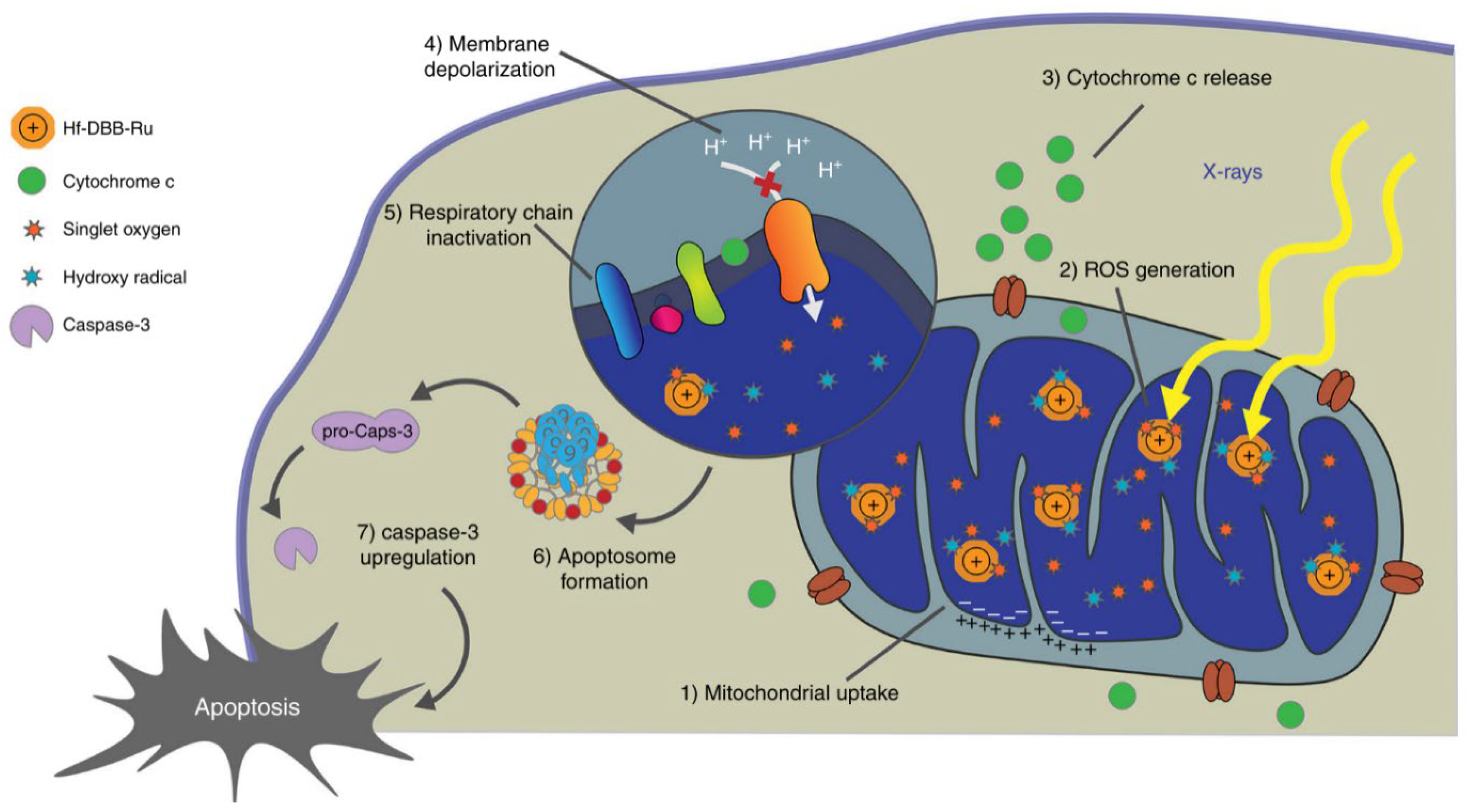
54]. Following RDT’s application, depolarization of mitochondrial membrane potential and release of cytochrome c from mitochondria was observed (
Figure 4) [
7]. Meanwhile, the expression of apoptosis related proteins, caspase 3, caspase 9, and p53 were distinctly up regulated [
7,
45,
47,
55]. Apoptotic cells initially show nucleus and cytoplasm condensation, DNA fragmentation, and then follow by blebbing of the plasma membrane to form apoptotic bodies which can rapidly be identified by neighboring phagocytes without induction of inflammation [
52,
53,
56].
Comparing to the immune-silent cell apoptosis [
57], necrosis is an uncontrolled type of cell death. It begins with cellular swelling, membrane integrity rupture, and spillage of cell contents into surrounding tissues. Eventually, it leads to activation of the immune system and extensive inflammation [
57,
58]. As early as 2002, Hashiguchi reported that the cellular morphological changes such as cytoplasmic swelling, nuclear swelling, and cell membrane rupture were observed after receiving acridine orange-mediated RDT [
2]. This suggests the high likelihood that RDT was able to induce necrosis in tumor cells.
Autophagy involves the engulfment of cellular components such as macromolecules or entire organelles by double-membrane vesicles (autophagosomes). Subsequently, these autophagosomes fuse with lysosome to degrade the enclosed substances [
57]. As expected, autophagy also contributed to the killing effect of RDT, as demonstrated by the cell death of B16G4F cells induced by 5-ALA-mediated RDT [
19].
Apoptosis and necrosis are merely induced under X-irradiation, while the effects vary across different structures and irradiation doses. Nanoscale metal organic frameworks (MOFs)-mediated RDT can enhance RT and induce RDT to increase the rate of apoptosis and necrosis of tumor cells. Under 2 Gy X-ray irradiation, DBP-Hf induces 31.5% apoptosis and 1.73% necrosis [
17]. Hf12-Ir can induce apoptosis up to 38.6% and necrosis up to 15.1% [
25], while Hf-DBB-Ru leads to 62.8% apoptosis and 3.68% necrosis [
7]. HMOP-TBHP/Fe(CO)5 caused 42.18% apoptosis and 0.65% necrosis under 6 Gy irradiation [
45].
Apoptosis and necrosis have been the primary types of tumor cell death that has been reported in RDT [
4,
7,
17,
20,
45], while other forms of cell death such as pyroptosis [
59], necroptosis [
57,
58], and ferroptosis [
60,
61] has received limited attention. Accordingly, further research is needed to investigate different cell death mechanisms in the future scientific research.
4.2.4. Activation of the Antitumor Immune Response
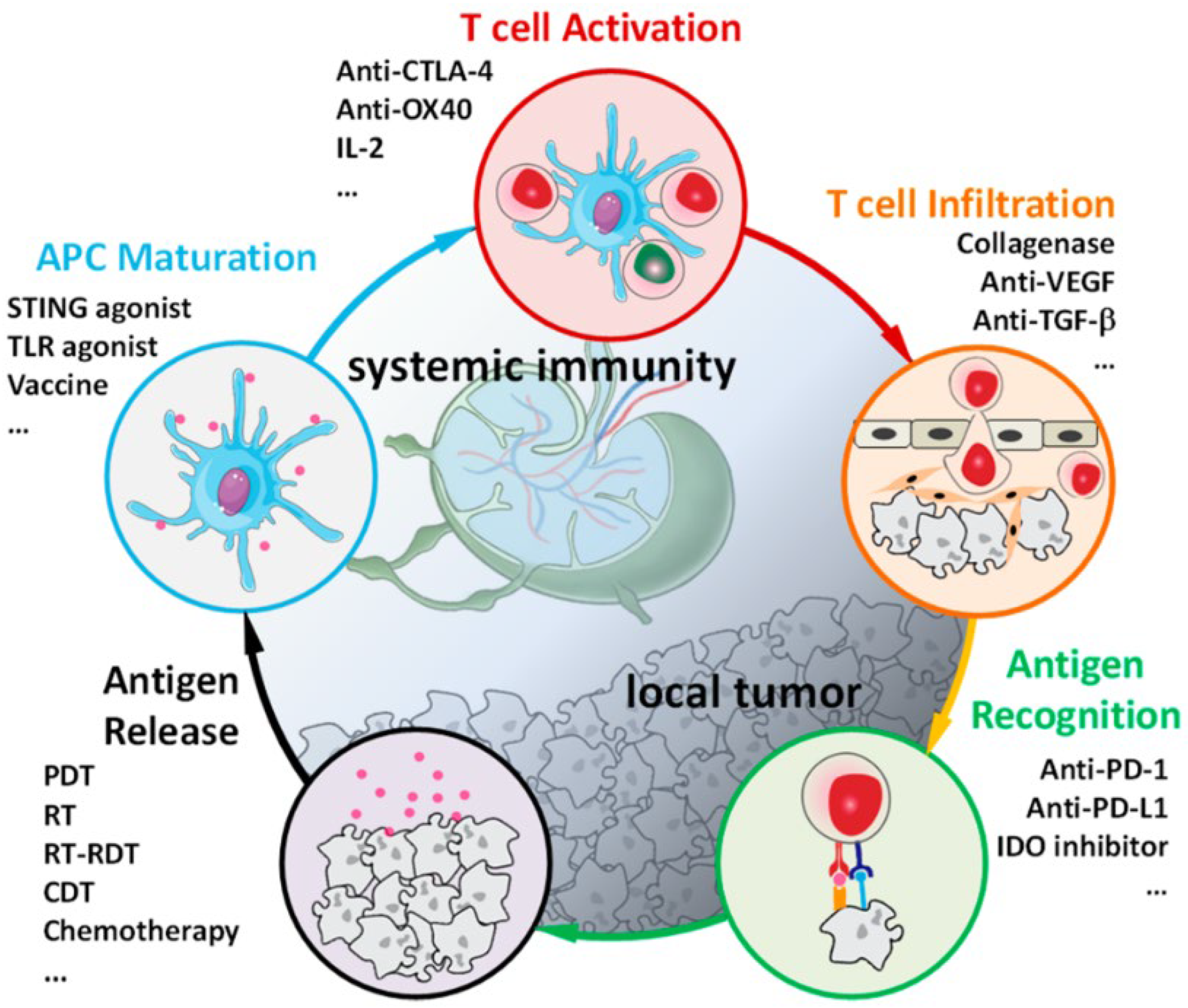
X-ray irradiation can activate the immune system and provoke both innate and systematic immune responses. As shown in
Figure 5, Ni reported immuno-oncology cycle and the use of immunotherapeutics to enhance essential steps of immune responses [
6]. Immunogenicity Calreticulin (CRT) is a chaperone protein, and High mobility group box-1 (HMGB-1) is a nucleosome-associated chromatin binding protein. Both are markers of the immunogenic cell death (ICD) as they elicit the immune responses from anti-tumor macrophages and dendritic cells. Lin group discovered that DBP-Hf mediated RDT can result in the ICD of CT26 cells by the eversion of CRT on plasma membrane [
17]. They also found that ICD can be induced by Hf-MOL (nanoscale metal-organic layer) + X-ray treatment, as evidenced by the detection of cell-surface exposure of CRT, HMGB-1 release, and adenosine triphosphate (ATP) excretion [
62]. Takahashi et al. found that RDT led to the up-regulation of representative genes of immune response, MHC class I, and death receptors such as
Fas,
Icam, interleukin
IL-6, interleukin
IL15, Toll-like receptor
Tlr6, and Toll-like receptor
Tlr7 [
16]. The RDT-mediated tumor cell death not only targets cells within the irradiation field but also induces systematic immune responses from metastatic tumors [
37]. The DBP-Hf binding to an interference molecule that can reverse immunosuppression were proven to effectively reduce the distant metastatic tumor size [
17]. Additionally, Formenti and his colleagues reported that irradiating normal tissues of mouse’s leg did not cause abscopal effect. Irradiation on normal tissues could not induce inhibitory regulation on metastasis in non-radiation site, indicating that anti-tumor systematic immune response can only be evoked after tumor cell death or other stress responses [
63].
From 1969 to 2014, 46 cases of abscopal response caused by radiotherapy has been reported [
64,
65]. The innate immune response non-specifically induced cell death to adjacent cells through destabilizing genome and mutating genes, while the abscopal effect on metastasis is anti- or pro-tumor is synergistically determined by the radiation dose and tissue type. The corresponding signal pathway of RDT induced systematic immune response has not been clearly investigated yet, but it is anticipated to not only eliminate primary tumors but also prevent their metastasis and recurrence after manipulation.
4.2.5. Activation of the Antitumor Immune Response
The tumor microenvironment (TME) is uniquely distinct from that in normal tissues. It is often characterized by the asymmetric distribution of nutrients, low level oxygen (hypoxia), acidic pH (acidosis), high level of reductant (glutathione), and elevated hydrogen peroxide concentration [
10,
66]. Substantial limitations on RDT efficacy are primarily posed by the tumor microenvironment, including hypoxia and immunosuppression. Recent breakthroughs aimed at overcoming these obstacles and enhancing RDT efficacy are discussed.
The oxygen deficiency in tumor reduces radiation-induced DNA damages through reduction of DNA radicals and promotes radiation resistance by upregulating the expression of hypoxia inducible factor 1α (HIF-1α) [
67,
68]. HIF-1α that upregulates genes controlling angiogenesis, metabolism, and metastasis is an important indicator of poor prognosis after radiotherapy [
64,
69]. As the cellular oxygen level in a solid tumor determines the extent of cancer cell damage during radiation, it is necessary to develop effective strategies to alleviate tumor hypoxia to promote the radio-dynamic therapy. Tumor hypoxia is primarily caused by the disorder blood vessels that fail to transport O
2 to the cells. Therefore, the artificial oxygen carriers are designed in replace of red blood cells to address the insufficient oxygen delivery. Yu et al. reported that PFC (a carbon-fluorine compound) emulsions developed to combine with carbogen (95% O
2 + 5% CO
2) showed distinct radio-sensitization that enhances oxygen concentration in the tumor microenvironment [
67]. The sensitization was limited in the tumor hypoxic cells, minimizing cytotoxicity to the surrounding normal cells with appropriate oxygen level [
67]. Beyond increasing the amount of O
2 transported to the tumor site, drugs have been designed to actively generate oxygen. Ni et al. reported a biomimetic Hf-DBP-Fe nMOF that can decompose elevated concentration of hydrogen peroxide in hypoxic tumor to produce oxygen and hydroxyl radicals to optimize RDT effects [
25]. Apart from degrading H
2O
2 in situ to generate oxygen, Fan et al. developed a biodegradable thioether-hybridized HMONs to deliver tert-butyl hydroperoxide (TBHP), where the peroxy bond could be cleaved to generate •OH for RDT [
45]. This strategy is distinctly effective under hypoxia in TME since the generation of •OH is oxygen independent [
67,
68]. In addition, Sang et al. discovered that Hb@Hf-Ce
6 NPs might take a dual responsibility of increasing the absorbance of radiation energy and boost the generation of ROS from Hf-polyphenol coordination platform [
70].
The hypoxic microenvironment of deep-seated tumors poses challenges for type II RDT. Although the mechanisms of type I are still debated [
71], numerous studies have demonstrated that type I PDT remains effective under low O
2 levels. This suggests that type I PDT can be a basis for innovative solutions to overcome hypoxia limitations in type II PDT [
71,
72,
73,
74,
75,
76].
Recently, highly efficient RDT system with type I radiodynamic mechanism has been developed solely based on gold nanoclusters (AuNC@DHLA) [
37]. The mechanism behind the efficient type I process likely involves the complex energy level structure of AuNC and the ultra-long triplet state. Upon X-ray irradiation, the excited singlet state (
1AuNC@DHLA*) is generated, which then undergoes efficient intersystem crossing (ISC) to an excited triplet state (
3AuNC@DHLA*). In other words, the triplet formation originates from an effective excited-state relaxation, where the initially populated singlet (S1) forms triplet (T1) states via an intermediate triplet (T2) state. The low reduction potential and ultralong lifetime of the T1 state facilitate the efficient generation of O
2−• through inter-molecular charge transfer to molecular oxygen. Additionally, the energy gap of T1-S0 is smaller than that between
3O
2 and
1O
2, thereby precluding the generation of singlet oxygen by the type-II process [
77]. Meanwhile, previous studies have proved that thiol compounds could generate superoxide via autoxidation reaction [
78,
79]. Thus, excess thiols or free thiols liberated from the Au surface may also participate in oxidations catalyzed by thiol-protected AuNCs to generate ROS.
Tumor cells survive and thrive in highly acidic environment by controlling their intracellular pH. While the acidic extracellular pH facilitates the invasion of tumors and inhibits the immune surveillance, neutralization of acidity can reverse it to inhibit tumor cell growth [
80]. Prasad et al. discovered that MnO
2 Nano Particles (NPs) can react with endogenous H
2O
2 and H+ to produce significant amounts of oxygen (~6-fold increase) in situ under radiation, decreasing the intracellular pH and producing ROS [
81]. Similarly, MnO
2 conjugated with albumin was proven to decrease tumor hypoxia and pH for a prolonged time. This system downregulates HIF-1α and its downstream proteins involved in tumor cell metabolism, angiogenesis, and metastasis [
67,
68,
81].
Characterized by the aberrant oxygen concentration, nutrients distribution, and acidity, TME impedes normal cell division and regulation but boosts the tumor cell growth and evasion towards its surrounding areas. It would be important to disrupt the complex interactions between tumor cells and their microenvironment as for an effective cancer therapy.
5. Combination Therapy
The ideal cancer therapy is expected to both eradicate primary tumors and prevent their metastasis and recurrence. In recent years, radio-dynamic therapy has developed to combine with other cancer therapies, such as radiotherapy, checkpoint blockade immunotherapy, gas therapy, and photothermal therapy, for enhanced therapeutic effects.
Radiotherapy utilizes high energy photons to ionize radiosensitizers, inducing Compton scattering and photoelectric emission, then releases excess energy as the Auger electrons (short-range secondary electrons) [
10]. The Compton and emissive electrons directly damage double-strand DNA, while the Auger electrons transfer the absorbed energy to surrounding water and oxygen molecules [
3,
17], generating ROS to damage biomacromolecules. By employing high-energy radiation rays to excite photosensitizers, RDT enhances both PDT and radiotherapy as those photosensitizers can absorb radiation energies as radiosensitizers. For example, NaCeF
4:Gd, Tb ScNPs, and POM nanoclusters mitigate the excitation energy gap by internal energy transfer, while their intrinsic absorption of X-ray enables them to produce auger electrons as well [
24,
82]. The Hf-based nMOFs and nMOLs are also proven to amplify the RT effect by producing hydroxyl radicals. In this way, the proper photosensitizers can take advantage of RT-RDT upon X-ray irradiation [
7,
20].
Immune checkpoint blockade (ICB) inhibits immune checkpoint pathways cytotoxic T-lymphocyte protein 4 (CTLA4) and programmed cell death protein 1 (PD-1), which negatively regulate T cells, to reinvigorate the antitumor immunity [
64]. However, the low exposure to the antigen of patients leads to limited immunotherapy responses [
6,
83]. In recent years, Lin laboratory has reported that nMOFs-mediated RDT synergizes with checkpoint blockade immunotherapy and can eradicate metastatic and recurrent tumors [
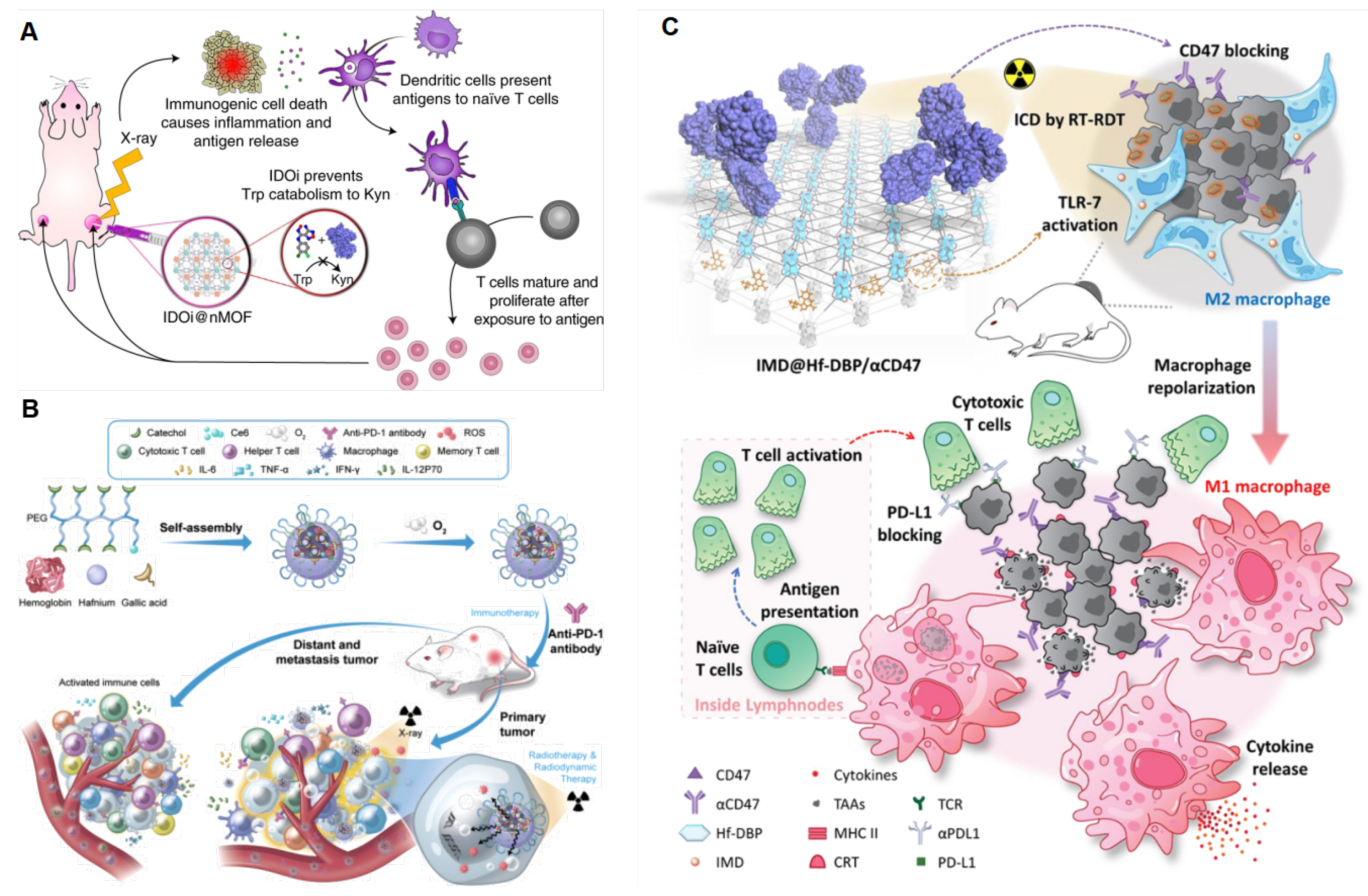
6]. In 2018, they firstly combined Hf-DBP-mediated RDT with immune checkpoint molecule indoleamine 2,3-dioxygenase (IDO) [
17]. As shown in
Figure 6A, IDO inhibitors reject the IDO-catalyzed oxidation of tryptophan and boost the production of kynurenine to reactivate T cells in the tumor microenvironment. Hf secondary building units absorbed X-ray irradiation energy and transferred energy to the anthracene ligand DBP to generate
1O
2 in vitro and in cells by the RDT mechanism. Intratumorally injected IDOi @nMOFs treated with low doses of X-ray irradiation were proven to eradicate primary tumors and distal tumors in mouse models of breast and colorectal cancer. Soon afterward, Ni et al. reported that nMOFs-mediated RDT combined with anti-PD-L1 antibody significantly diminished tumors in situ and metastatic tumors [
62]. PD-L1 blockade reversed T cell exhaustion to promote the increase in the ratio of CD8
+ T cells to immunosuppressive Treg cells and T cell oligoclonales to overcome immunosuppression [
84]. In addition to targeting adaptive immune checkpoints for multiple metastatic and treatment-refractory cancers, recent studies have shown that innate immune checkpoint CD47 is critical for cancer cells to escape from immune surveillance such as macrophages attack and phagocytes [
85,
86]. Sang reported that an oxygen-enriched X-ray nanoprocessor Hb@Hf-Ce6 nanoparticle is developed for improving the therapeutic effect of RT-RDT, enhancing modulation of hypoxia tumor microenvironment (TME) and promoting anti-tumor immune response in combination with PD-1 immune checkpoint blockade in
Figure 6B [
79]. Lin group reported that surface modification of Hf-DBP reactivates innate immunity by co-delivering a toll-like receptor 7 (TLC-7) agonist, imiquimod, and an anti-CD47 antibody (αCD47) in
Figure 6C. TLC-7 agonists initiate the repolarization immunosuppressive M2 phenotype and transform it into an immunostimulatory M1 phenotype to promote tumor cell phagocytosis. Under X-ray irradiation, IMD@Hf-DBP/αCD47 effectively activated innate immunity and IMD@Hf-DBP/αCD47(+) + αPDL1 orchestrated adaptive immunity that completely eradicate both primary and distant tumors on a bilateral colorectal tumor model [
18].
Other emerging combination strategies for improved radio-dynamic therapy are gas therapy and photothermal therapy. Gas therapy releases CO molecules to cause mitochondria and induce cell apoptosis [
45]. The synergistic radio-dynamic/gas therapy is bridged by RDT-produced hydroxyl radical (•OH) that cleaves the coordination bond and releases CO. Fan et al. designed HMONs to be loaded with TBHP, a radiosensitizer, and Fe(CO)
5, a CO releasing molecule. As the irradiation dose increases, CO molecules released from HMOP-TBHP/Fe(CO)
5 increase independent of oxygen concentration. Correspondingly, radiotherapy up-regulates caspase 3 and p53 and the treatment of HMOP-TBHP/Fe(CO)
5 leads to more than 95% apoptosis and a higher rate of necrosis. TME can not only bring resistance to cancer treatments but also initiate metastasis. Photothermal therapy (PTT) can alleviate tumor hypoxia by generating regional hyperthermia to ablate tumor cells and boost intratumoral blood flow that brings in oxygen [
26]. Wang et al. reported that BiVO
4@Bi
2S
3 HNRs can be converted into a photothermal agent under NIR irradiation. Depending on laser power density, they can demonstrate excellent thermal stability up to ~40°C. The HNRs+NIR+X-ray treatment which employs both RDT and PTT leads to the lowest tumor cell survival rate without damaging surrounding tissues.
The synergistic antitumor treatment combining RDT with other therapies enhances RDT effects, ROS generation, and cytotoxicity, mitigates the suppressive tumor microenvironment towards therapeutics, and targets the metastatic and recurrent tumor cells in distant. Along with the development of nanoscale drugs, future RDT administrations are expected to combine with various therapies for stability and biocompatibility.
6. Conclusions
This comprehensive review summarizes the recent progress in radio-dynamic cancer therapy over the past two decades. Evolving from photodynamic therapy, RDT distinguishes itself by leveraging X-rays and high-energy irradiation rays. The flexibility and loading capacity of nanomaterials have led to the development of responsive nanoscale photosensitizers, addressing challenges such as limited tissue penetration, low energy transfer efficiency, and hypoxic tumor microenvironments. These photosensitizers serve as a crucial link between RDT and other cancer therapies, including radiotherapy, gas therapy, and photothermal therapy, amplifying their therapeutic effects. RDT mechanisms involve the production of reactive oxygen species (ROS) upon low-dose irradiation, direct excitation of high-efficiency photosensitizers by low-dose X-rays, and the use of nanoparticles for targeted drug delivery to modulate the tumor microenvironment. Notably, ROS production, particularly singlet oxygen (1O2), hydroxyl radical (•OH), superoxide anion (O2•−), and hydrogen peroxide (H2O2), proves highly cytotoxic to tumor cells, inducing damage to biomacromolecules and triggering cell cycle disruptions. Strategies employing composite nanomaterials, scintillators, and photosensitizers for treating deep-seated tissues have shown promising potentials, though further improvements in energy transfer efficiency and loading capacity are expected. Designing photosensitizers that matches the excitation energy of low-dose X-rays, exploring nanoclusters as efficient and less toxic PSs, and developing biodegradable photosensitizers are essential considerations for advancing RDT. Emerging combined strategies, including photothermal therapy, gas therapy, and checkpoint blockade immunotherapy, present innovative approaches to enhance RDT therapeutic outcomes. The ideal cancer therapy necessitates eradicating primary tumors, preventing metastasis, and overcoming the challenges posed by the tumor microenvironment. Multifunctional drugs responding to irradiation as photosensitizers and inducing other cancer therapies hold promise as a developing strategy.
Treating deep-seated tumors with RDT, especially in combination with other modalities, showcases significant progress and promising perspectives. The ability to enhance radiotherapy, modulate immune responses, and synergize with gas and photothermal therapies positions RDT as a cornerstone in the evolving landscape of cancer treatment. Continued research and clinical exploration will unveil further breakthroughs, bringing us closer to more effective and targeted cancer therapies.
Author Contributions
Shengcang Zhu: Methodology, investigation, validation, writing-original draft preparation, writing-reviewing and editing. Siyue Lin: Investigation, validation, writing-reviewing and editing. Rongcheng Han: Conceptualization, writing-reviewing and editing, supervision, project administration.
Funding Information
This work was jointly supported by the Beijing National Laboratory for Molecular Sciences (BNLMS-CXXM-202001) and the National Natural Science Foundation of China (grant 91961103).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nature Reviews Cancer. 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, S.; Katsuyuki, K.; Murata, H.; et al. Acridine Orange Excited by Low-Dose Radiation Has a Strong Cytocidal Effect on Mouse Osteosarcoma. Oncology. 2002, 62, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takahashi, J.; Nagasawa, S.; Doi, M.; Moriyama, A.; Iwahashi, H. DNA Strand Break Properties of Protoporphyrin IX by X-ray Irradiation against Melanoma. International Journal of Molecular Sciences. 2020, 21, 2302. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano. 2016, 10, 3918–3935. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Lin, W. Nanoscale Metal–Organic Frameworks Generate Reactive Oxygen Species for Cancer Therapy. ACS central science. 2020, 6, 861–868. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Nash, G.T.; Lin, W. Nanoscale Metal–Organic Frameworks for Cancer Immunotherapy. Accounts of Chemical Research. 2020, 53, 1739–1748. [Google Scholar] [CrossRef]
- Ni, K.; Lan, G.; Veroneau, S.S.; Duan, X.; Song, Y.; Lin, W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nature Communications. 2018, 9, 4321. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Chan, C.; et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nature Communications. 2018, 9, 2351. [Google Scholar] [CrossRef]
- Sun, W.; Luo, L.; Feng, Y.; et al. Aggregation-Induced Emission Gold Clustoluminogens for Enhanced Low-Dose X-ray-Induced Photodynamic Therapy. Angewandte Chemie International Edition. 2020, 59, 9914–9921. [Google Scholar] [CrossRef]
- Liu, T.; Yang, K.; Liu, Z. Recent advances in functional nanomaterials for X-ray triggered cancer therapy. Progress in Natural Science: Materials International. 2020, 30, 567–576. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; et al. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics. 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, A.L.; Harmon, A.C.; et al. 177Lu-Labeled Eu-Doped Mesoporous SiO2 Nanoparticles as a Theranostic Radiopharmaceutical for Colorectal Cancer. ACS applied nano materials. 2020, 3, 8691–8701. [Google Scholar] [CrossRef]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; et al. Acridine orange could be an innovative anticancer agent under photon energy. PubMed. 2007, 21, 205–214. [Google Scholar]
- Kusuzaki, K.; Murata, H.; Matsubara, T.; et al. Review. Acridine orange could be an innovative anticancer agent under photon energy. PubMed. 2007, 21, 205–214. [Google Scholar]
- Nakamura, T.; Kusuzaki, K.; Matsubara, T.; Matsumine, A.; Murata, H.; Uchida, A. A new limb salvage surgery in cases of high-grade soft tissue sarcoma using photodynamic surgery, followed by photo- and radiodynamic therapy with acridine orange. Journal of Surgical Oncology. 2008, 97, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kusuzaki, K.; Matsubara, T.; et al. Long-term clinical outcome in patients with high-grade soft tissue sarcoma who were treated with surgical adjuvant therapy using acridine orange after intra-lesional or marginal resection. Photodiagnosis and Photodynamic Therapy. 2018, 23, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Combined treatment with X-ray irradiation and 5-aminolevulinic acid elicits better transcriptomic response of cell cycle-related factors than X-ray irradiation alone. International journal of radiation biology. 2016, 92, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Guo, N.; et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nature Biomedical Engineering. 2018, 2, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Culbert, A.; Kaufmann, M.; Jiang, X.; Lin, W. Nanoscale Metal–Organic Framework Co-delivers TLR-7 Agonists and Anti-CD47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. Journal of the American Chemical Society. 2020, 142, 12579–12584. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, R.; et al. Nanoscale Metal-Organic Layers for Deeply Penetrating X-ray-Induced Photodynamic Therapy. Angewandte Chemie. 2017, 129, 12270–12274. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal–Organic Layers for Radiotherapy–Radiodynamic Therapy. Journal of the American Chemical Society. 2018, 140, 16971–16975. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Iwahashi, H. Introduction to 5-Aminolevulinic Acid-Protoporphyrin IXMediated Radiodynamic Therapy (RDT). Clinics in oncology. 2017, 2, 1330. [Google Scholar]
- Takahashi, J.; Murakami, M.; Mori, T.; Iwahashi, H. Verification of radiodynamic therapy by medical linear accelerator using a mouse melanoma tumor model. Scientific Reports. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Nagasawa, S.; Ikemoto, M.J.; Sato, C.; Sato, M.; Iwahashi, H. Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. International Journal of Molecular Sciences. 2019, 20, 5155. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Zhan, G.; et al. NaCeF4:Gd,Tb Scintillator as an X-ray Responsive Photosensitizer for Multimodal Imaging-Guided Synchronous Radio/Radiodynamic Therapy. Nano Letters. 2019, 19, 8234–8244. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Song, Y.; Hao, Z.; Lin, W. Biomimetic nanoscale metal–organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chemical science. 2020, 11, 7641–7653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, S.; Wang, L.; et al. BiVO4@Bi2S3 Heterojunction Nanorods with Enhanced Charge Separation Efficiency for Multimodal Imaging and Synergy Therapy of Tumor. ACS applied bio materials. 2020, 3, 5080–5092. [Google Scholar] [CrossRef]
- Han, R.; Zhao, M.; Wang, Z.; et al. Super-efficient in Vivo Two-Photon Photodynamic Therapy with a Gold Nanocluster as a Type I Photosensitizer. ACS nano. 2019, 14, 9532–9544. [Google Scholar] [CrossRef]
- Zhuang, Z.; Dai, J.; Yu, M.; et al. Type I photosensitizers based on phosphindole oxide for photodynamic therapy: Apoptosis and autophagy induced by endoplasmic reticulum stress. Chemical Science. 2020, 11, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.; Du, H.; Wang, H. Intracellular gold nanoparticle aggregation and their potential applications in photodynamic therapy. Chemical Communications. 2014, 50, 7287. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small. 2015, 11, 5860–5887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Luo, Z.; Chen, J.; et al. Ultrasmall Au10−12(SG)10−12Nanomolecules for High Tumor Specificity and Cancer Radiotherapy. Advanced Materials. 2014, 26, 4565–4568. [Google Scholar] [CrossRef] [PubMed]
- Amirhosein Kefayat, Ghahremani, F.; Hasan Motaghi, Alireza Amouheidari. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomedicine 2019, 16, 173–184. [CrossRef] [PubMed]
- Jia, T.T.; Yang, G.; Mo, S.J.; et al. Atomically Precise Gold–Levonorgestrel Nanocluster as a Radiosensitizer for Enhanced Cancer Therapy. ACS Nano. 2019, 13, 8320–8328. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer. Small. 2019, 15, 1900968. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Jiang, Y.; et al. Glutathione-Depleting Gold Nanoclusters for Enhanced Cancer Radiotherapy through Synergistic External and Internal Regulations. ACS Applied Materials & Interfaces. 2018, 10, 10601–10606. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, J.; Luo, Z.; et al. Enhanced Tumor Accumulation of Sub-2 nm Gold Nanoclusters for Cancer Radiation Therapy. Advanced Healthcare Materials. 2013, 3, 133–141. [Google Scholar] [CrossRef]
- Zhu, S.; Yan, F.; Yang, L.; et al. Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response. Theranostics. 2023, 13, 1042–1058. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chemical Reviews. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Nakayama, M.; Sasaki, R.; Ogino, C.; et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiation Oncology. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring Singlet Oxygen and Hydroxyl Radical Formation with Fluorescent Probes During Photodynamic Therapy. Photochemistry and Photobiology. 2009, 85, 1177–1181. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Liu, B.; et al. Detection and analysis of reactive oxygen species (ROS) generated by nano-sized TiO2 powder under ultrasonic irradiation and application in sonocatalytic degradation of organic dyes. Ultrasonics Sonochemistry. 2011, 18, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine: Nanotechnology, Biology and Medicine. 2011, 7, 604–614. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, G.; Sun, Q.; et al. Investigation of the Mechanisms of Radio-Dynamic Therapy. Mathews Journal of Cancer Science. 2020, 5, 10024. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Shen, Z.; et al. Generic synthesis of small-sized hollow mesoporous organosilica nanoparticles for oxygen-independent X-ray-activated synergistic therapy. Nature Communications. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Tew, L.S.; Cai, M.T.; Lo, L.W.; Khung, Y.L.; Chen, N.T. Pollen-Structured Gold Nanoclusters for X-ray Induced Photodynamic Therapy. Materials. 2018, 11, 1170. [Google Scholar] [CrossRef]
- Ghelli Luserna di Rora’, A.; Iacobucci, I.; Martinelli, G. The cell cycle checkpoint inhibitors in the treatment of leukemias. Journal of Hematology & Oncology. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Duronio, R.J.; Xiong, Y. Signaling Pathways that Control Cell Proliferation. Cold Spring Harbor Perspectives in Biology. 2013, 5, a008904. [Google Scholar] [CrossRef]
- Rhind, N.; Russell, P. Signaling Pathways that Regulate Cell Division. Cold Spring Harbor Perspectives in Biology. 2012, 4, a005942. [Google Scholar] [CrossRef] [PubMed]
- Dickens, L.S.; Powley, I.R.; Hughes, M.A.; MacFarlane, M. The “complexities” of life and death: Death receptor signalling platforms. Experimental Cell Research. 2012, 318, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of Apoptosis in disease. Aging. 2012, 4, 330–349. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harbor perspectives in biology. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harbor Perspectives in Biology. 2013, 5, a008714. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.J.; Ranjan, A. Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: Recent progress and challenges in treatment and tracking response to immunotherapy. Pharmacology & Therapeutics 2020, 207, 107456. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Tomita, K.; Urushihara, Y.; Sato, T.; Kurimasa, A.; Fukumoto, M. Association between radiation-induced cell death and clinically relevant radioresistance. Histochemistry and cell biology. 2018, 150, 649–659. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biology International. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Martin, S.J.; Henry, C.M. Distinguishing between apoptosis, necrosis, necroptosis and other cell death modalities. Methods. 2013, 61, 87–89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; et al. Gasdermin E suppresses tumor growth by activating anti-tumor immunity. Nature. 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death & Differentiation. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Riegman, M.; Sagie, L.; Galed, C.; et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nature Cell Biology. 2020, 22, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Lan, G.; Chan, C.; et al. Ultrathin metal-organic layer-mediated radiotherapy-radiodynamic therapy enhances immunotherapy of metastatic cancers. PubMed. 2019, 1, 1331–1353. [Google Scholar]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. The Lancet Oncology. 2009, 10, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends in Immunology. 2018, 39, 644–655. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Current Problems in Cancer. 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Advanced Materials. 2017, 29, 1700996. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Dai, M.; Liu, Q.; Xiu, R. Oxygen carriers and cancer chemo- and radiotherapy sensitization: Bench to bedside and back. Cancer Treatment Reviews. 2007, 33, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nature Reviews Cancer. 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Sang, W.; Xie, L.; Wang, G.; et al. Oxygen-Enriched Metal-Phenolic X-Ray Nanoprocessor for Cancer Radio-Radiodynamic Therapy in Combination with Checkpoint Blockade Immunotherapy. Advanced Science. 2020, 8, 2003338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angewandte Chemie International Edition. 2018, 57, 11522–11531. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, H.; Dong, Y.; et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2011, 156, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Gilson, R.C.; Black, K.C.L.; Lane, D.D.; Achilefu, S. Hybrid TiO2 -Ruthenium Nano-photosensitizer Synergistically Produces Reactive Oxygen Species in both Hypoxic and Normoxic Conditions. Angewandte Chemie (International Ed in English). 2017, 56, 10717–10720. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, J.; Wan, Y.; et al. Dual Fenton Catalytic Nanoreactor for Integrative Type-I and Type-II Photodynamic Therapy Against Hypoxic Cancer Cells. ACS applied bio materials. 2019, 2, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, K.; Bu, W.; et al. Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angewandte Chemie. 2015, 54, 1770–1774. [Google Scholar] [CrossRef]
- Lv, Z.; Wei, H.; Li, Q.; et al. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(II) photosensitizer through type I photochemical process. Chemical Science. 2018, 9, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.; Chen, W.; Niu, L.; Fang, W.; Cui, G.; Yang, Q. BODIPY-Based Photodynamic Agents for Exclusively Generating Superoxide Radical over Singlet Oxygen. Angewandte Chemie. 2021, 60, 19912–19920. [Google Scholar] [CrossRef]
- Misra, H.P. Generation of Superoxide Free Radical during the Autoxidation of Thiols. Journal of Biological Chemistry. 1974, 249, 2151–2155. [Google Scholar] [CrossRef]
- Kaewmati, P.; Somsook, E.; Dhital, R.N.; Sakurai, H. Aerobic oxygenation of phenylboronic acid promoted by thiol derivatives under gold-free conditions: A warning against gold nanoparticle catalysis. Tetrahedron letters. 2012, 53, 6104–6106. [Google Scholar] [CrossRef]
- Ibrahim-Hashim, A.; Estrella, V. Acidosis and cancer: From mechanism to neutralization. Cancer and Metastasis Reviews 2019, 38, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; et al. Multifunctional Albumin–MnO2Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano. 2014, 8, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Zhong, J.; Zhang, Z.; et al. Polyoxomolybdate (POM) nanoclusters with radiosensitizing and scintillating properties for low dose X-ray inducible radiation-radiodynamic therapy. Nanoscale Horizons. 2020, 5, 109–118. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nature Reviews Cancer. 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jiang, W.; Kim, B.Y.; Zhang, C.; Fu, Y.X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nature reviews Cancer. 2019, 19, 568–586. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature. 2019, 574, 45–56. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).