Submitted:
03 July 2024
Posted:
04 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
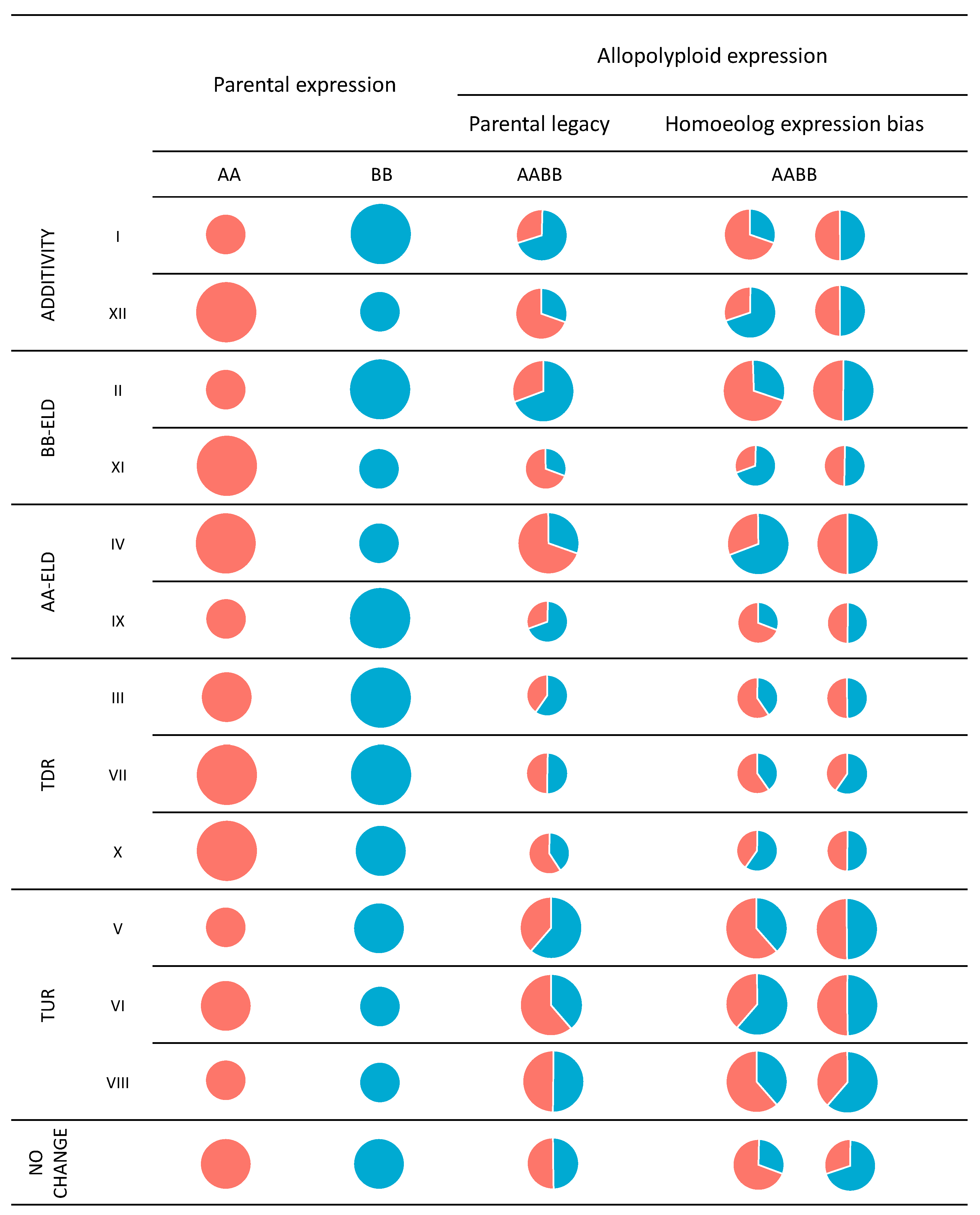
2. Terminology in Allopolyploid Gene Expression
3. How Allopolyploidy Affects Bioinformatics Transcriptome Analyses?
4. Sub-Genome Phasing
5. Homoeology Inference
6. RNA-Sequencing
7. Mapping Reads
- Similarity-based mapping: These pipelines compare allopolyploid reads to loci in the genomes of the parental species to identify homoeolog sequences.
- Genotype-aware mapping: These pipelines aim to detect genotypic differences and classify allopolyploids reads to the corresponding sub-genomes.
7. Normalization in Differential Expression Analysis
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flagel, L.E.; Wendel, J.F.; Udall, J.A. Duplicate Gene Evolution, Homoeologous Recombination, and Transcriptome Characterization in Allopolyploid Cotton. BMC Genomics 2012, 13, 302. [Google Scholar] [CrossRef]
- Roulin, A.; Auer, P.L.; Libault, M.; Schlueter, J.; Farmer, A.; May, G.; Stacey, G.; Doerge, R.W.; Jackson, S.A. The Fate of Duplicated Genes in a Polyploid Plant Genome. Plant J 2013, 73, 143–153. [Google Scholar] [CrossRef]
- Conant, G.C.; Birchler, J.A.; Pires, J.C. Dosage, Duplication, and Diploidization: Clarifying the Interplay of Multiple Models for Duplicate Gene Evolution over Time. Curr Opin Plant Biol 2014, 19, 91–98. [Google Scholar] [CrossRef]
- Mutti, J.S.; Bhullar, R.K.; Gill, K.S. Evolution of Gene Expression Balance Among Homeologs of Natural Polyploids. G3 (Bethesda) 2017, 7, 1225–1237. [Google Scholar] [CrossRef]
- Griffiths, A.G.; Moraga, R.; Tausen, M.; Gupta, V.; Bilton, T.P.; Campbell, M.A.; Ashby, R.; Nagy, I.; Khan, A.; Larking, A.; et al. Breaking Free: The Genomics of Allopolyploidy-Facilitated Niche Expansion in White Clover. The Plant Cell 2019, 31, 1466–1487. [Google Scholar] [CrossRef] [PubMed]
- Van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An Evolutionary and Ecological Force in Stressful Times. The Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Mo, R.; Li, H.; Ni, Z.; Sun, Q.; Liu, Z. The Transcriptional and Splicing Changes Caused by Hybridization Can Be Globally Recovered by Genome Doubling during Allopolyploidization. Molecular Biology and Evolution 2021, 38, 2513–2519. [Google Scholar] [CrossRef]
- Li, M.; Hu, M.; Xiao, Y.; Wu, X.; Wang, J. The Activation of Gene Expression and Alternative Splicing in the Formation and Evolution of Allopolyploid Brassica Napus. Horticulture Research 2022, 9, uhab075. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Lv, P.; Wang, W.; Wang, J.; He, Y.; Song, Z.; Cai, D. Full-Length Transcriptome Reconstruction Reveals Genetic Differences in Hybrids of Oryza Sativa and Oryza Punctata with Different Ploidy and Genome Compositions. BMC Plant Biology 2022, 22, 131. [Google Scholar] [CrossRef]
- Glover, N.M.; Redestig, H.; Dessimoz, C. Homoeologs: What Are They and How Do We Infer Them? Trends Plant Sci 2016, 21, 609–621. [Google Scholar] [CrossRef]
- Gruenheit, N.; Deusch, O.; Esser, C.; Becker, M.; Voelckel, C.; Lockhart, P. Cutoffs and K-Mers: Implications from a Transcriptome Study in Allopolyploid Plants. BMC Genomics 2012, 13, 92. [Google Scholar] [CrossRef]
- Krasileva, K.V.; Buffalo, V.; Bailey, P.; Pearce, S.; Ayling, S.; Tabbita, F.; Soria, M.; Wang, S.; IWGS Consortium; Akhunov, E. ; et al. Separating Homeologs by Phasing in the Tetraploid Wheat Transcriptome. Genome Biol 2013, 14, R66. [Google Scholar] [CrossRef]
- Boatwright, J.L.; McIntyre, L.M.; Morse, A.M.; Chen, S.; Yoo, M.-J.; Koh, J.; Soltis, P.S.; Soltis, D.E.; Barbazuk, W.B. A Robust Methodology for Assessing Differential Homeolog Contributions to the Transcriptomes of Allopolyploids. Genetics 2018, 210, 883–894. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Morales-Briones, D.F.; Passow, C.N.; Yang, Y. Performance of Gene Expression Analyses Using de Novo Assembled Transcripts in Polyploid Species. Bioinformatics 2019, 35, 4314–4320. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.C.Y.; Hatakeyama, M.; Tameshige, T.; Shimizu, K.K.; Sese, J. Homeolog Expression Quantification Methods for Allopolyploids. Briefings in Bioinformatics 2020, 21, 395–407. [Google Scholar] [CrossRef]
- Voshall, A.; Moriyama, E.N. Next-Generation Transcriptome Assembly and Analysis: Impact of Ploidy. Methods 2020, 176, 14–24. [Google Scholar] [CrossRef]
- Hu, G.; Grover, C.E.; Arick, M.A., II; Liu, M.; Peterson, D.G.; Wendel, J.F. Homoeologous Gene Expression and Co-Expression Network Analyses and Evolutionary Inference in Allopolyploids. Briefings in Bioinformatics 2021, 22, 1819–1835. [Google Scholar] [CrossRef]
- Sun, J.; Okada, M.; Tameshige, T.; Shimizu-Inatsugi, R.; Akiyama, R.; Nagano, A.J.; Sese, J.; Shimizu, K.K. A Low-Coverage 3′ RNA-Seq to Detect Homeolog Expression in Polyploid Wheat. NAR Genomics and Bioinformatics 2023, 5, lqad067. [Google Scholar] [CrossRef]
- Rapp, R.A.; Udall, J.A.; Wendel, J.F. Genomic Expression Dominance in Allopolyploids. BMC Biology 2009, 7, 18. [Google Scholar] [CrossRef]
- Flagel, L.E.; Wendel, J.F. Evolutionary Rate Variation, Genomic Dominance and Duplicate Gene Expression Evolution during Allotetraploid Cotton Speciation. New Phytologist 2010, 186, 184–193. [Google Scholar] [CrossRef]
- Bardil, A.; de Almeida, J.D.; Combes, M.C.; Lashermes, P.; Bertrand, B. Genomic Expression Dominance in the Natural Allopolyploid Coffea Arabica Is Massively Affected by Growth Temperature. New Phytologist 2011, 192, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Chelaifa, H.; Monnier, A.; Ainouche, M. Transcriptomic Changes Following Recent Natural Hybridization and Allopolyploidy in the Salt Marsh Species Spartina × Townsendii and Spartina Anglica (Poaceae). New Phytologist 2010, 186, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Carvalho, J.; Boutte, J.; Bourdaud, P.; Chelaifa, H.; Ainouche, K.; Salmon, A.; Ainouche, M. Gene Expression Variation in Natural Populations of Hexaploid and Allododecaploid Spartina Species (Poaceae). Plant Syst Evol 2017, 303, 1061–1079. [Google Scholar] [CrossRef]
- Giraud, D.; Lima, O.; Rousseau-Gueutin, M.; Salmon, A.; Aïnouche, M. Gene and Transposable Element Expression Evolution Following Recent and Past Polyploidy Events in Spartina (Poaceae). Frontiers in Genetics 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Schnable, J.C.; Springer, N.M.; Freeling, M. Differentiation of the Maize Subgenomes by Genome Dominance and Both Ancient and Ongoing Gene Loss. Proceedings of the National Academy of Sciences 2011, 108, 4069–4074. [Google Scholar] [CrossRef]
- Schnable, J.; Wang, X.; Pires, J.; Freeling, M. Escape from Preferential Retention Following Repeated Whole Genome Duplications in Plants. Frontiers in Plant Science 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Edger, P.P.; Poorten, T.J.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.D.L.; Wai, C.M.; et al. Origin and Evolution of the Octoploid Strawberry Genome. Nat Genet 2019, 51, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bird, K.A.; Niederhuth, C.E.; Ou, S.; Gehan, M.; Pires, J.C.; Xiong, Z.; VanBuren, R.; Edger, P.P. Replaying the Evolutionary Tape to Investigate Subgenome Dominance in Allopolyploid Brassica Napus. New Phytologist 2021, 230, 354–371. [Google Scholar] [CrossRef]
- Glombik, M.; Copetti, D.; Bartos, J.; Stoces, S.; Zwierzykowski, Z.; Ruttink, T.; Wendel, J.F.; Duchoslav, M.; Dolezel, J.; Studer, B.; et al. Reciprocal Allopolyploid Grasses (Festuca × Lolium) Display Stable Patterns of Genome Dominance. The Plant Journal 2021, 107, 1166–1182. [Google Scholar] [CrossRef]
- Kopecký, D.; Scholten, O.; Majka, J.; Burger-Meijer, K.; Duchoslav, M.; Bartoš, J. Genome Dominance in Allium Hybrids (A. Cepa × A. Roylei). Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef]
- Grover, C.E.; Gallagher, J.P.; Szadkowski, E.P.; Yoo, M.J.; Flagel, L.E.; Wendel, J.F. Homoeolog Expression Bias and Expression Level Dominance in Allopolyploids. New Phytologist 2012, 196, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants. Chromosomal evolution in higher plants. 1971. [Google Scholar]
- Buggs, R.J.A.; Wendel, J.F.; Doyle, J.J.; Soltis, D.E.; Soltis, P.S.; Coate, J.E. The Legacy of Diploid Progenitors in Allopolyploid Gene Expression Patterns. Philos Trans R Soc Lond B Biol Sci 2014, 369, 20130354. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.-J.; Szadkowski, E.; Wendel, J.F. Homoeolog Expression Bias and Expression Level Dominance in Allopolyploid Cotton. Heredity 2013, 110, 171–180. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Xu, M.; Chen, P.; Liu, D.; Sun, Q.; Ran, L.; Wang, Y. Homoeolog Expression Bias and Expression Level Dominance in Resynthesized Allopolyploid Brassica Napus. BMC Genomics 2018, 19, 586. [Google Scholar] [CrossRef]
- Li, M.; Wang, R.; Wu, X.; Wang, J. Homoeolog Expression Bias and Expression Level Dominance (ELD) in Four Tissues of Natural Allotetraploid Brassica Napus. BMC Genomics 2020, 21, 330. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, H.; Sun, G.; Pan, Z.; Wang, X.; Geng, X.; He, S.; Du, X. Expression Patterns and Functional Divergence of Homologous Genes Accompanied by Polyploidization in Cotton (Gossypium Hirsutum L.). Sci China Life Sci 2020, 63, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Pootakham, W.; Sonthirod, C.; Naktang, C.; Yundaeng, C.; Yoocha, T.; Kongkachana, W.; Sangsrakru, D.; Somta, P.; Tangphatsornruang, S. Genome Assemblies of Vigna Reflexo-Pilosa (Créole Bean) and Its Progenitors, Vigna Hirtella and Vigna Trinervia, Revealed Homoeolog Expression Bias and Expression-Level Dominance in the Allotetraploid. Gigascience 2023, 12, giad050. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.-J.; Liu, X.; Pires, J.C.; Soltis, P.S.; Soltis, D.E. Nonadditive Gene Expression in Polyploids. Annual Review of Genetics 2014, 48, 485–517. [Google Scholar] [CrossRef]
- Bird, K.A.; VanBuren, R.; Puzey, J.R.; Edger, P.P. The Causes and Consequences of Subgenome Dominance in Hybrids and Recent Polyploids. New Phytologist 2018, 220, 87–93. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, Z.; Liu, B. The Effects of Hybridization and Genome Doubling in Plant Evolution via Allopolyploidy. Mol Biol Rep 2020, 47, 5549–5558. [Google Scholar] [CrossRef]
- Shimizu, K.K. Robustness and the Generalist Niche of Polyploid Species: Genome Shock or Gradual Evolution? Current Opinion in Plant Biology 2022, 69, 102292. [Google Scholar] [CrossRef] [PubMed]
- Nomaguchi, T.; Maeda, Y.; Yoshino, T.; Asahi, T.; Tirichine, L.; Bowler, C.; Tanaka, T. Homoeolog Expression Bias in Allopolyploid Oleaginous Marine Diatom Fistulifera Solaris. BMC Genomics 2018, 19, 330. [Google Scholar] [CrossRef] [PubMed]
- Buggs, R.J.A. Unravelling Gene Expression of Complex Crop Genomes. Heredity 2013, 110, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A. A Criticism of the Value of Midparent in Polyploidization. Journal of Experimental Botany 2013, 64, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Pumphrey, M.; Bai, J.; Laudencia-Chingcuanco, D.; Anderson, O.; Gill, B.S. Nonadditive Expression of Homoeologous Genes Is Established Upon Polyploidization in Hexaploid Wheat. Genetics 2009, 181, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Chagué, V.; Just, J.; Mestiri, I.; Balzergue, S.; Tanguy, A.-M.; Huneau, C.; Huteau, V.; Belcram, H.; Coriton, O.; Jahier, J.; et al. Genome-Wide Gene Expression Changes in Genetically Stable Synthetic and Natural Wheat Allohexaploids. New Phytologist 2010, 187, 1181–1194. [Google Scholar] [CrossRef]
- Chelaifa, H.; Chagué, V.; Chalabi, S.; Mestiri, I.; Arnaud, D.; Deffains, D.; Lu, Y.; Belcram, H.; Huteau, V.; Chiquet, J.; et al. Prevalence of Gene Expression Additivity in Genetically Stable Wheat Allohexaploids. New Phytologist 2013, 197, 730–736. [Google Scholar] [CrossRef]
- Song, K.; Lu, P.; Tang, K.; Osborn, T.C. Rapid Genome Change in Synthetic Polyploids of Brassica and Its Implications for Polyploid Evolution. Proceedings of the National Academy of Sciences 1995, 92, 7719–7723. [Google Scholar] [CrossRef]
- Salmon, A.; Ainouche, M.L.; Wendel, J.F. Genetic and Epigenetic Consequences of Recent Hybridization and Polyploidy in Spartina (Poaceae). Molecular Ecology 2005, 14, 1163–1175. [Google Scholar] [CrossRef]
- Hu, G.; Koh, J.; Yoo, M.-J.; Chen, S.; Wendel, J.F. Gene-Expression Novelty in Allopolyploid Cotton: A Proteomic Perspective. Genetics 2015, 200, 91–104. [Google Scholar] [CrossRef]
- Adams, K.L.; Cronn, R.; Percifield, R.; Wendel, J.F. Genes Duplicated by Polyploidy Show Unequal Contributions to the Transcriptome and Organ-Specific Reciprocal Silencing. Proceedings of the National Academy of Sciences 2003, 100, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S.; et al. One Thousand Plant Transcriptomes and the Phylogenomics of Green Plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef]
- Halabi, K.; Shafir, A.; Mayrose, I. PloiDB: The Plant Ploidy Database. New Phytologist 2023, 240, 918–927. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, M.; Li, W.; Xu, M.; Shao, L.; Liu, Y.; Zhao, G.; Liu, Z.; Xu, Z.; You, J.; et al. Single-Cell RNA-Seq Reveals Fate Determination Control of an Individual Fibre Cell Initiation in Cotton (Gossypium Hirsutum). Plant Biotechnology Journal 2022, 20, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.J.; Uhl, M.A.; Miller, M.G.; Johnson, A.D. Identification and Characterization of a Candida Albicans Mating Pheromone. Mol Cell Biol 2003, 23, 8189–8201. [Google Scholar] [CrossRef]
- Langham, R.J.; Walsh, J.; Dunn, M.; Ko, C.; Goff, S.A.; Freeling, M. Genomic Duplication, Fractionation and the Origin of Regulatory Novelty. Genetics 2004, 166, 935–945. [Google Scholar] [CrossRef]
- Tate, J.A.; Joshi, P.; Soltis, K.A.; Soltis, P.S.; Soltis, D.E. On the Road to Diploidization? Homoeolog Loss in Independently Formed Populations of the Allopolyploid Tragopogon Miscellus (Asteraceae). BMC Plant Biol 2009, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Cai, X.; Liang, J.; Freeling, M.; Wang, X. Gene Retention, Fractionation and Subgenome Differences in Polyploid Plants. Nature Plants 2018, 4, 258–268. [Google Scholar] [CrossRef]
- Thomas, B.C.; Pedersen, B.; Freeling, M. Following Tetraploidy in an Arabidopsis Ancestor, Genes Were Removed Preferentially from One Homeolog Leaving Clusters Enriched in Dose-Sensitive Genes. Genome Res. 2006, 16, 934–946. [Google Scholar] [CrossRef]
- Gaeta, R.T.; Chris Pires, J. Homoeologous Recombination in Allopolyploids: The Polyploid Ratchet. New Phytologist 2010, 186, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.S.; Wendel, J.F. Homoeologous Exchanges, Segmental Allopolyploidy, and Polyploid Genome Evolution. Frontiers in Genetics 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.K.; Edger, P.P.; Pires, J.C.; McKain, M.R. Patterns, Mechanisms, and Consequences of Homoeologous Exchange in Allopolyploid Angiosperms: A Genomic and Epigenomic Perspective. New Phytologist 2023, 238, 2284–2304. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.; Combes, M.-C.; Cenci, A.; Lashermes, P.; Dereeper, A. SNiPloid: A Utility to Exploit High-Throughput SNP Data Derived from RNA-Seq in Allopolyploid Species. International Journal of Plant Genomics 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Freyman, W.A.; Johnson, M.G.; Rothfels, C.J. Homologizer: Phylogenetic Phasing of Gene Copies into Polyploid Subgenomes. Methods in Ecology and Evolution 2023, 14, 1230–1244. [Google Scholar] [CrossRef]
- Schiavinato, M.; Bodrug-Schepers, A.; Dohm, J.C.; Himmelbauer, H. Subgenome Evolution in Allotetraploid Plants. The Plant Journal 2021, 106, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Page, J.T.; Gingle, A.R.; Udall, J.A. PolyCat: A Resource for Genome Categorization of Sequencing Reads From Allopolyploid Organisms. G3 Genes|Genomes|Genetics 2013, 3, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Belfield, E.J.; Harberd, N.P.; Mithani, A. HANDS2: Accurate Assignment of Homoeallelic Base-Identity in Allopolyploids despite Missing Data. Sci Rep 2016, 6, 29234. [Google Scholar] [CrossRef] [PubMed]
- Page, J.T.; Udall, J.A. Methods for Mapping and Categorization of DNA Sequence Reads from Allopolyploid Organisms. BMC Genetics 2015, 16, S4. [Google Scholar] [CrossRef]
- Sun, P.; Jiao, B.; Yang, Y.; Shan, L.; Li, T.; Li, X.; Xi, Z.; Wang, X.; Liu, J. WGDI: A User-Friendly Toolkit for Evolutionary Analyses of Whole-Genome Duplications and Ancestral Karyotypes. Molecular Plant 2022, 15, 1841–1851. [Google Scholar] [CrossRef]
- Schiavinato, M.; Marcet-Houben, M.; Dohm, J.C.; Gabaldón, T.; Himmelbauer, H. Parental Origin of the Allotetraploid Tobacco Nicotiana Benthamiana. The Plant Journal 2020, 102, 541–554. [Google Scholar] [CrossRef]
- Gordon, S.P.; Levy, J.J.; Vogel, J.P. PolyCRACKER, a Robust Method for the Unsupervised Partitioning of Polyploid Subgenomes by Signatures of Repetitive DNA Evolution. BMC Genomics 2019, 20, 580. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.-H.; Wang, Z.-X.; Wang, L.; Li, G.-Y.; Zhang, W.; Wang, X.-L.; Xu, F.-J.; Jiao, S.-Q.; Zhou, S.-S.; Liu, H.; et al. SubPhaser: A Robust Allopolyploid Subgenome Phasing Method Based on Subgenome-Specific k-Mers. New Phytologist 2022, 235, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-G.; Shang, H.-Y.; Jia, K.-H.; Ma, Y.-P. Subgenome Phasing for Complex Allopolyploidy: Case-Based Benchmarking and Recommendations. Briefings in Bioinformatics 2024, 25, bbad513. [Google Scholar] [CrossRef]
- Session, A.M. Allopolyploid Subgenome Identification and Implications for Evolutionary Analysis. Trends in Genetics 2024, 0. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.M. Distinguishing Homologous from Analogous Proteins. Systematic Biology 1970, 19, 99–113. [Google Scholar] [CrossRef]
- Altenhoff, A.M.; Studer, R.A.; Robinson-Rechavi, M.; Dessimoz, C. Resolving the Ortholog Conjecture: Orthologs Tend to Be Weakly, but Significantly, More Similar in Function than Paralogs. PLOS Computational Biology 2012, 8, e1002514. [Google Scholar] [CrossRef]
- Nevers, Y.; Defosset, A.; Lecompte, O. Orthology: Promises and Challenges. In Evolutionary Biology—A Transdisciplinary Approach; Pontarotti, P., Ed.; Springer International Publishing: Cham, 2020; pp. 203–228. ISBN 978-3-030-57246-4. [Google Scholar]
- Sonnhammer, E.L.L.; Koonin, E.V. Orthology, Paralogy and Proposed Classification for Paralog Subtypes. Trends in Genetics 2002, 18, 619–620. [Google Scholar] [CrossRef]
- Ouzounis, C. Orthology: Another Terminology Muddle. Trends in Genetics 1999, 15, 445. [Google Scholar] [CrossRef]
- Kristensen, D.M.; Wolf, Y.I.; Mushegian, A.R.; Koonin, E.V. Computational Methods for Gene Orthology Inference. Brief Bioinform 2011, 12, 379–391. [Google Scholar] [CrossRef]
- Glover, N.M.; Altenhoff, A.; Dessimoz, C. Assigning Confidence Scores to Homoeologs Using Fuzzy Logic. PeerJ 2019, 6, e6231. [Google Scholar] [CrossRef]
- Koonin, E.V. Orthologs, Paralogs, and Evolutionary Genomics. Annual Review of Genetics 2005, 39, 309–338. [Google Scholar] [CrossRef]
- Altenhoff, A.M.; Glover, N.M.; Dessimoz, C. Inferring Orthology and Paralogy. Methods Mol Biol 2019, 1910, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Fonstein, M.; D’Souza, M.; Pusch, G.D.; Maltsev, N. The Use of Gene Clusters to Infer Functional Coupling. Proceedings of the National Academy of Sciences 1999, 96, 2896–2901. [Google Scholar] [CrossRef] [PubMed]
- Glover, N.; Sheppard, S.; Dessimoz, C. Homoeolog Inference Methods Requiring Bidirectional Best Hits or Synteny Miss Many Pairs. Genome Biology and Evolution 2021, 13, evab077. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. Journal of Molecular Biology 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dalquen, D.A.; Dessimoz, C. Bidirectional Best Hits Miss Many Orthologs in Duplication-Rich Clades Such as Plants and Animals. Genome Biology and Evolution 2013, 5, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- McCouch, S.R. Genomics and Synteny. Plant Physiology 2001, 125, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.N. Positional Orthology: Putting Genomic Evolutionary Relationships into Context. Briefings in Bioinformatics 2011, 12, 401–412. [Google Scholar] [CrossRef]
- Train, C.-M.; Glover, N.M.; Gonnet, G.H.; Altenhoff, A.M.; Dessimoz, C. Orthologous Matrix (OMA) Algorithm 2.0: More Robust to Asymmetric Evolutionary Rates and More Scalable Hierarchical Orthologous Group Inference. Bioinformatics 2017, 33, i75–i82. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic Orthology Inference for Comparative Genomics. Genome Biology 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, R.; Wang, B.; Xun, H.; Liu, B.; Xu, C. Effects of Allopolyploidization and Homoeologous Chromosomal Segment Exchange on Homoeolog Expression in a Synthetic Allotetraploid Wheat under Variable Environmental Conditions. Plants (Basel) 2023, 12, 3111. [Google Scholar] [CrossRef]
- Vilella, A.J.; Severin, J.; Ureta-Vidal, A.; Heng, L.; Durbin, R.; Birney, E. EnsemblCompara GeneTrees: Complete, Duplication-Aware Phylogenetic Trees in Vertebrates. Genome Res. 2009, 19, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Coghlan, A.; Ruan, J.; Coin, L.J.; Hériché, J.-K.; Osmotherly, L.; Li, R.; Liu, T.; Zhang, Z.; Bolund, L.; et al. TreeFam: A Curated Database of Phylogenetic Trees of Animal Gene Families. Nucleic Acids Res 2006, 34, D572–580. [Google Scholar] [CrossRef]
- Xu, M.-R.-X.; Liao, Z.-Y.; Brock, J.R.; Du, K.; Li, G.-Y.; Chen, Z.-Q.; Wang, Y.-H.; Gao, Z.-N.; Agarwal, G.; Wei, K.H.-C.; et al. Maternal Dominance Contributes to Subgenome Differentiation in Allopolyploid Fishe. Nat Commun 2023, 14. [Google Scholar] [CrossRef]
- Pereira, C.; Denise, A.; Lespinet, O. A Meta-Approach for Improving the Prediction and the Functional Annotation of Ortholog Groups. BMC Genomics 2014, 15, S16. [Google Scholar] [CrossRef]
- Chorostecki, U.; Molina, M.; Pryszcz, L.P.; Gabaldón, T. MetaPhOrs 2.0: Integrative, Phylogeny-Based Inference of Orthology and Paralogy across the Tree of Life. Nucleic Acids Research 2020, 48, W553–W557. [Google Scholar] [CrossRef]
- Altenhoff, A.M.; Dessimoz, C. Phylogenetic and Functional Assessment of Orthologs Inference Projects and Methods. PLOS Computational Biology 2009, 5, e1000262. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of Age: Ten Years of next-Generation Sequencing Technologies. Nat Rev Genet 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Heather, J.M.; Chain, B. The Sequence of Sequencers: The History of Sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Gault, C.M.; Kremling, K.A.; Buckler, E.S. Tripsacum De Novo Transcriptome Assemblies Reveal Parallel Gene Evolution with Maize after Ancient Polyploidy. The Plant Genome 2018, 11, 180012. [Google Scholar] [CrossRef]
- Kamitani, M.; Kashima, M.; Tezuka, A.; Nagano, A.J. Lasy-Seq: A High-Throughput Library Preparation Method for RNA-Seq and Its Application in the Analysis of Plant Responses to Fluctuating Temperatures. Sci Rep 2019, 9, 7091. [Google Scholar] [CrossRef]
- Wang, M.; Wang, P.; Liang, F.; Ye, Z.; Li, J.; Shen, C.; Pei, L.; Wang, F.; Hu, J.; Tu, L.; et al. A Global Survey of Alternative Splicing in Allopolyploid Cotton: Landscape, Complexity and Regulation. New Phytol 2018, 217, 163–178. [Google Scholar] [CrossRef]
- Gonzalez-Garay, M.L. Introduction to Isoform Sequencing Using Pacific Biosciences Technology (Iso-Seq). In Transcriptomics and Gene Regulation; Wu, J., Ed.; Translational Bioinformatics; Springer Netherlands: Dordrecht, 2016; pp. 141–160. ISBN 978-94-017-7450-5. [Google Scholar]
- Pyatnitskiy, M.A.; Arzumanian, V.A.; Radko, S.P.; Ptitsyn, K.G.; Vakhrushev, I.V.; Poverennaya, E.V.; Ponomarenko, E.A. Oxford Nanopore MinION Direct RNA-Seq for Systems Biology. Biology 2021, 10, 1131. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Y.; Jia, E.; Pan, M.; Bai, Y.; Ge, Q. Bias in RNA-Seq Library Preparation: Current Challenges and Solutions. Biomed Res Int 2021, 2021, 6647597. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Chen, X.; Wang, S.; Wang, J.; Guo, L.; Yin, Z.; Chen, Q.; Ye, W. Temporal Salt Stress-Induced Transcriptome Alterations and Regulatory Mechanisms Revealed by PacBio Long-Reads RNA Sequencing in Gossypium Hirsutum. BMC Genomics 2020, 21, 838. [Google Scholar] [CrossRef]
- Yao, S.; Liang, F.; Gill, R.A.; Huang, J.; Cheng, X.; Liu, Y.; Tong, C.; Liu, S. A Global Survey of the Transcriptome of Allopolyploid Brassica Napus Based on Single-Molecule Long-Read Isoform Sequencing and Illumina-Based RNA Sequencing Data. The Plant Journal 2020, 103, 843–857. [Google Scholar] [CrossRef]
- Navajas-Pérez, R.; Paterson, A.H. Patterns of Tandem Repetition in Plant Whole Genome Assemblies. Mol Genet Genomics 2009, 281, 579–590. [Google Scholar] [CrossRef]
- Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Current Strategies of Polyploid Plant Genome Sequence Assembly. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Y.; Zhang, S.; Yu, J.; Zhang, X. Recent Advances in Assembly of Complex Plant Genomes. Genomics Proteomics Bioinformatics 2023, 21, 427–439. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Garvin, D.F. De Novo Transcriptome Assembly in Polyploid Species. Methods Mol Biol 2017, 1536, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat Biotechnol 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Akama, S.; Shimizu-Inatsugi, R.; Shimizu, K.K.; Sese, J. Genome-Wide Quantification of Homeolog Expression Ratio Revealed Nonstochastic Gene Regulation in Synthetic Allopolyploid Arabidopsis. Nucleic Acids Research 2014, 42, e46. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Reeder, J.; Lawrence, M.; Becker, G.; Brauer, M.J. GMAP and GSNAP for Genomic Sequence Alignment: Enhancements to Speed, Accuracy, and Functionality. In Statistical Genomics: Methods and Protocols; Mathé, E., Davis, S., Eds.; Springer: New York, NY, 2016; pp. 283–334. ISBN 978-1-4939-3578-9. [Google Scholar]
- Kuo, T.; Frith, M.C.; Sese, J.; Horton, P. EAGLE: Explicit Alternative Genome Likelihood Evaluator. BMC Medical Genomics 2018, 11, 28. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. ; 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Zyprych-Walczak, J.; Szabelska, A.; Handschuh, L.; Górczak, K.; Klamecka, K.; Figlerowicz, M.; Siatkowski, I. The Impact of Normalization Methods on RNA-Seq Data Analysis. BioMed Research International 2015, 2015, 621690. [Google Scholar] [CrossRef]
- Evans, C.; Hardin, J.; Stoebel, D.M. Selecting Between-Sample RNA-Seq Normalization Methods from the Perspective of Their Assumptions. Brief Bioinform 2017, 19, 776–792. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat Biotechnol 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef]
- Zhao, S.; Ye, Z.; Stanton, R. Misuse of RPKM or TPM Normalization When Comparing across Samples and Sequencing Protocols. RNA 2020, 26, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biol 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Nat Prec 2010, 1–1. [Google Scholar] [CrossRef]
- Aanes, H.; Winata, C.; Moen, L.F.; Østrup, O.; Mathavan, S.; Collas, P.; Rognes, T.; Aleström, P. Normalization of RNA-Sequencing Data from Samples with Varying mRNA Levels. PLOS ONE 2014, 9, e89158. [Google Scholar] [CrossRef]
- Coate, J.E.; Doyle, J.J. Variation in Transcriptome Size: Are We Getting the Message? Chromosoma 2015, 124, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Pirrello, J.; Deluche, C.; Frangne, N.; Gévaudant, F.; Maza, E.; Djari, A.; Bourge, M.; Renaudin, J.-P.; Brown, S.; Bowler, C.; et al. Transcriptome Profiling of Sorted Endoreduplicated Nuclei from Tomato Fruits: How the Global Shift in Expression Ascribed to DNA Ploidy Influences RNA-Seq Data Normalization and Interpretation. The Plant Journal 2018, 93, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Coate, J.E.; Doyle, J.J. Quantifying Whole Transcriptome Size, a Prerequisite for Understanding Transcriptome Evolution Across Species: An Example from a Plant Allopolyploid. Genome Biology and Evolution 2010, 2, 534–546. [Google Scholar] [CrossRef]
- Visger, C.J.; Wong, G.K.-S.; Zhang, Y.; Soltis, P.S.; Soltis, D.E. Divergent Gene Expression Levels between Diploid and Autotetraploid Tolmiea Relative to the Total Transcriptome, the Cell, and Biomass. American Journal of Botany 2019, 106, 280–291. [Google Scholar] [CrossRef]
- Fomina-Yadlin, D.; Du, Z.; McGrew, J.T. Gene Expression Measurements Normalized to Cell Number Reveal Large Scale Differences Due to Cell Size Changes, Transcriptional Amplification and Transcriptional Repression in CHO Cells. Journal of Biotechnology 2014, 189, 58–69. [Google Scholar] [CrossRef]
- Orr-Weaver, T.L. When Bigger Is Better: The Role of Polyploidy in Organogenesis. Trends Genet 2015, 31, 307–315. [Google Scholar] [CrossRef]
- Coate, J.E. Beyond Transcript Concentrations: Quantifying Polyploid Expression Responses per Biomass, per Genome, and per Cell with RNA-Seq. Methods Mol Biol 2023, 2545, 227–250. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Z.; Xia, Z.; Zhao, D.; Li, W.; Tyler, J.K. The Overlooked Fact: Fundamental Need for Spike-In Control for Virtually All Genome-Wide Analyses. Mol Cell Biol 2016, 36, 662–667. [Google Scholar] [CrossRef]
- Almeida-Silva, F.; Prost-Boxoen, L.; Van de Peer, Y. Hybridexpress: An R/Bioconductor Package for Comparative Transcriptomic Analyses of Hybrids and Their Progenitors. New Phytologist 2024, 243, 811–819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




