1. Introduction
Addressing climate change mitigation necessitates a comprehensive understanding of natural processes and the impacts of human development on these processes. Within this context, the framework of social-ecological systems (SES) becomes pertinent. SES represent complex adaptive systems where human societies are intricately integrated with nature [
1,
2].
In the SES framework, the social component encompasses all human activities, while the ecological component pertains to the biosphere and its associated natural processes. [
3]. The two parts are interrelated and the boundaries between them depend on the perspective of the analysis. The interplay between these components is dynamic, with their boundaries contingent upon the analytical perspective adopted. Ecosystem services, defined as the benefits that society derives from ecosystems, illustrate this interaction [
3]. Analyzing the relationships within a defined social-ecological system facilitates the pursuit of integrated solutions to issues arising from human activities in specific ecosystems. This integrated approach is essential for developing strategies that not only mitigate the negative impacts of human development on natural processes but also enhance the resilience and sustainability of both human and ecological systems.
Mangroves are SES characterised by intricate social and ecological relationships, [
2,
4,
5,
6,
7], including the provision of ecosystem services [
3]. These services encompass supporting, provisioning, regulating, and cultural or spiritual services [
8,
9]. For instance, coastal ecosystems contribute to climate regulation through carbon sequestration. Mangroves, along with saltmarshes and seagrasses, are categorised as blue carbon ecosystems due to their significant capacity for atmospheric carbon sequestration. [
10,
11,
12].
Mangroves can absorb and sequester substantial amounts of carbon through both aboveground and belowground biomass [
13]. Additionally, the environmental conditions within these ecosystems favour the long-term accumulation of soil organic matter [
14]. Mangroves provide critical regulating services to coastal communities by mitigating the impacts of waves, wind, and floods [
15,
16]. The ecosystem service of climate regulation not only addresses the causes of climate change but also mitigates the impacts of climate events. Moreover, mangroves offer supporting, provisioning, and cultural services, [
9,
17,
18] characterised by high productivity, biodiversity, and ecological connectivity. Moreover, they contribute to the livelihoods of coastal communities by serving as a food resource and supporting the local economy [
19,
20,
21,
22].
Despite their importance, mangroves are threatened by human activities, primarily those that transform the spaces in which these ecosystems develop [
17,
23,
24]. Anthropogenic activities that degrade mangrove forests include the overexploitation of resources, land-use changes, agricultural development, damming of freshwater sources, and pollution. The reduction or loss of mangroves consequently leads to the reduction or loss of the ecosystem services they provide, thereby diminishing benefits to local communities [
16,
25,
26]. Recent research priorities in mangrove studies have been identified in the areas of ecosystem functionality, extreme events and interacting stressors, and social-ecological systems [
28].
In response, the international scientific community is striving to integrate the value of ecosystem services into human development decision-making processes [
8,
28]. Initiatives such as the Global Mangrove Alliance and the International Blue Carbon Initiative recognise mangroves as high-priority ecosystems for climate change mitigation through climate regulation [
17,
20].
How coastal communities perceive mangrove ecosystem services influences the success of conservation efforts [
27,
29,
30]. Therefore, the priorities and preferences of these communities should be integral to the decision-making process [
28,
31,
32]. It is essential to work with both the mangrove ecosystem and the surrounding social system to analyse how society benefits from nature [
33,
34]. Identifying the motives to protect ecosystem services helps to determine which services are relevant to stakeholders and informs the options to be considered for management decisions [
18].
In Cuba, mangroves are recognised as vulnerable and significant coastal wetlands [
24]. They encompass 5.1% of the national territory and constitute 27% of the total forested area. Approximately 35% of mangroves are legally safeguarded under the National System of Protected Areas (SNAP), managed through various conservation measures. [
35]. Globally, Cuba ranks 10th in terms of mangrove area [
36].
There is substantial evidence indicating that coastal communities significantly influence the ecological health of mangroves, as well as the quality of ecosystem services [
37,
38] and their governance structures [
39,
40,
41]. However, inadequate participation hinders the establishment of governance standards within the existing policy framework, thereby impacting mangrove management [
41].
Within Cuba’s National System of Protected Areas (SNAP), environmental education initiatives such as training sessions, workshops, and festivals are conducted exclusively in communities surrounding protected mangrove areas. These activities aim to foster community involvement in mangrove conservation [
42].
Despite limited research on the analysis of perceptions within coastal communities and governance for improved mangrove management in Cuba, existing evidence underscores the local recognition of the importance of coastal ecosystems, particularly mangroves, for sustaining livelihood activities such as fishing, tourism, and agriculture [
24,
43]. While mangroves are primarily valued for their role in shielding coastal communities and infrastructure from climate change impacts like coastal flooding and strong winds [
24], efforts to quantify the ecosystem services they provide in terms of provisioning, regulating, and supporting functions are gaining momentum [
44].
To date, comprehensive studies exploring the intricate social, ecological, and governance dynamics within mangrove habitats are lacking. Therefore, this study aims to elucidate SES and governance interactions within southeastern Cuba’s mangroves, focusing on community perceptions of this invaluable ecosystem, essential for its integrated and sustainable management.
2. Materials and Methods
2.1. Study Design
2.1.1. Design of the Social-Ecological Study
In this study, we employed a qualitative research approach [
45] with exploratory data summary, utilising a structured questionnaire technique [
27,
32,
46,
47,
48,
49]. The objective was to gather the perceptions of coastal communities regarding the following variables: 1) ecosystem services provided by mangroves, 2) utilisation of mangrove resources, 3) current management practices in place, 4) potential threats posing risks to these ecosystems, and 5) social-ecological relationships existing within the localities. Indeed, for Cuba, and particularly for this region, data are scarce and there is a need to obtain descriptive data to understand the mangrove social-ecological systems. In a later phase, hypotheses can be put forward as to the causes of similarities and differences within Cuba, between localities and in comparison, to insights elsewhere.
The questionnaire (Supplementary material, Appendix S1) was structured into three parts, as follows:
The following uses were considered: 1) the use of marine species (fish, molluscs, crustaceans) for eating or selling, 2) the use of mangroves bark or wood as as pigment resource A three-point Likert scale was used to determine the frequency of use for the following population groups: 1 (never used), 2 (sometimes) and 3 (always).
The perception of ecosystem services was assessed by evaluating the importance of various services to both individual and community life. These included: i) Supporting Services, such as providing shelter for the juvenile stages of many species, supporting the feeding and production of a diverse range of marine and terrestrial organisms, and offering habitat for numerous marine and terrestrial fauna. ii) Provisioning Services, acting as a source of food for consumption and sale and providing natural medicines for various diseases. iii) Regulatory Services, such as protecting coastal areas from storms, cyclones, and waves; maintaining seawater quality by trapping sediments; and mitigating climate change effects through carbon sequestration. iv) Cultural Services, serving as recreational and educational sites and representing symbols of local culture and heritage.
Participants rated the importance of these services using the following scale: 1 (not important), 2 (important), and 3 (very important), following the classification proposed by MEA (Millennial Ecosystem Assessment) [
8].
Awareness of mangrove management activities was assessed using a scale ranging from 1 to 3: 1 (unaware), 2 (somewhat aware), and 3 (fully aware). This evaluation focused on several aspects: i) The existence of a specific management plan or program for mangroves. ii) Participation in seminars or training sessions related to mangrove management. iii) Awareness of the presence of legal instruments governing mangrove conservation and iv) Knowledge about the existence of government officials responsible for monitoring and evaluating mangrove resources.
The perception of mangrove managers was gauged by identifying the institutions or agencies responsible for mangrove management within their locality. Additionally, perceived threats to mangroves were elicited through an open-ended question, allowing respondents to freely list events or factors they believed were impacting the ecosystem in their area. This structured approach aimed to capture a comprehensive understanding of community awareness, management perceptions, and perceived threats to mangroves in the study area.
2.1.2. Design of the Governance Study
To identify the type of governance present, interviews were conducted involving specialists from each of the three protected areas: MCOA, SAM, and HAT. These specialists play active roles in the development and management planning of their respective areas. An unstructured instrument with open-ended questions was used in the interview sessions. Key topics explored included: i) The degree of consultation of communities during the preparation of management plans; ii) The extent of community involvement in identifying issues, conflicts, and solutions. iii) The role of communities solely as recipients of management plan actions or as actively involved. iv) Features of management, distinguishing top-down, bottom-up, or co-management systems, with diverse perspectives analyzed regarding the specific governance system applied in each study area. The focus group surveys were aimed to gather insights into the governance structures and processes within each protected area, highlighting perspectives from various stakeholders involved in mangrove management.
In addition, through the interviews we examined six variables crucial for understanding mangrove management and decision-making in the study region: i) Transparency: Assessing the accessibility and clarity of information related to decision-making processes; ii) Knowledge of the Legal Framework: Understanding of the legal provisions concerning mangrove protection and their implementation; iii) Accountability: Inquiry into stakeholders’ awareness of their responsibility to demonstrate actions related to mangrove management and their ability to assess the consequences of these actions; iv) Citizen Participation: Evaluating the extent to which citizens can engage in mangrove decision-making processes and influence public policies; v) Equity: Analysis of the distribution of resources and opportunities related to mangrove management; vi) Inclusion: Examination of the participation of all social groups in mangrove decision-making processes.
To gauge the stakeholders’ level of influence and interest, we utilized an influence-interest matrix, specifically the Mendelow Stakeholder Matrix [
50]. This matrix helps categorize stakeholders based on their level of influence over mangrove management decisions and their level of interest in the outcomes. The interviews and the matrix analysis are intended to provide insights into the dynamics of mangrove governance, stakeholder engagement, and decision-making processes within the study area.
2.1.3. Integrated Matrix Analysis
Finally, to qualitatively assess the impacts of social-ecological and governance interactions, we constructed a matrix incorporating anthropic activities reported by respondents, mangrove ecosystem services, technical reports, public information, and reports from protected area management plans. Each component was evaluated to determine its influence on the overall state of SES. This qualitative weighting or evaluation process involves assessing how the development of specific activities, uses, or ecosystem services affect the SES comprehensively.

2.2. Sampling.
The study baseline was established through the review of the 2021-2025 management plans of the following protected areas: Monte Cabaniguán/Ojo de Agua Wildlife Refuge (MCOA), San Miguel de Parada Wildlife Refuge (SAM), and the Hatibonico Ecological Reserve (HAT). Guamá is considered a multiple-use area, not subject to any management regime [
51]. In addition, a total of 6 protected area specialists were interviewed to contrast the information obtained from the above paper review.
The sample size was determined based on the population size of coastal communities adjacent to the mangrove sites. A confidence level of 95% was chosen with a margin of error of 10% (see
Table 1 for details), corresponding to the final number of questionnaires administered per mangrove site. Individuals within these communities were approached selectly only ensuring that participants were over 18 years old. Prior to participation, all individuals were provided with information about the study’s objectives and requested to provide consent for their involvement.
2.2.1. Social-Demographic Profile of Respondents
Respondents across all sites ranged in age from 18 to 80 years, with a median age of 43 years. The gender balance was nearly equal, with 53.8% male and 46.2% female respondents overall (
Table 2).
Regarding occupational backgrounds as reported, the largest share of participants were employed in the state sector: 20.5% in MCOA, 41.3% in GUM, 51.4% in SAM, and 60.9% in HAT. These roles included forest rangers, specialists from the Flora and Fauna Company, teachers, and tourism sector workers. Approximately 14.1% of respondents were engaged in the private sector, and an equal percentage (14.1%) were farmers conducting agricultural activities in coastal areas. About 20% identified as housewives, and I 8.4% were retirees (
Table 2).
2.3. Statistical Analysis
The creation of the database and analysis of the frequency tables were conducted using SPSS (version 27). This facilitated the identification of patterns and highlighted the notable characteristics of the variables assessed in the communities for each mangrove site.
2.4. Study Area
In marine and coastal biogeography, Cuba is part of the Northwest Atlantic Province [
53] and experiences significant influence from seasonal tropical cyclones, which bring heavy rainfall that reduces salinity and increases nutrient loads in watersheds draining into mangrove ecosystems [
54,
55]. Cuban mangroves typically form narrow strips [
54], due to the relatively small tidal range of about 25 to 80 cm [
55]. The extent and structure of mangroves in Cuba vary based on geomorphology, characteristics of rivers and tributaries, and local climatic conditions [
55]. Mangrove development is more extensive in the southern central part of the island, while the northern slope generally shows mangroves of a lower canopy height. Mangrove coverage is notably limited in eastern Cuba. Our study focused on the Southeastern Region of Cuba, defined as a natural and political entity extending along the eastern most part of the island, south of an imaginary line from the center of Guacanayabo Bay to the northern edge of Banes Bay [
56]. This region is bordered to the north by the Central Region, to the northeast by the Atlantic Ocean, to the east by the Paso de los Vientos, to the south by the Caribbean Sea, and to the west by the Gulf of Guacanayabo [
56]. The climate in this region is classified as tropical savannah (type AW according to the Köppen classification [
53,
57], with temperatures ranging from 25.6 C
0 in Las Tunas to 26.8 C
0 in Guantánamo. Annual rainfall varies from 792 mm in Santiago de Cuba to 1130 mm in Las Tunas. Coastal geomorphological and hydrodynamic characteristics create diverse environments, some of which are conducive to mangrove development.
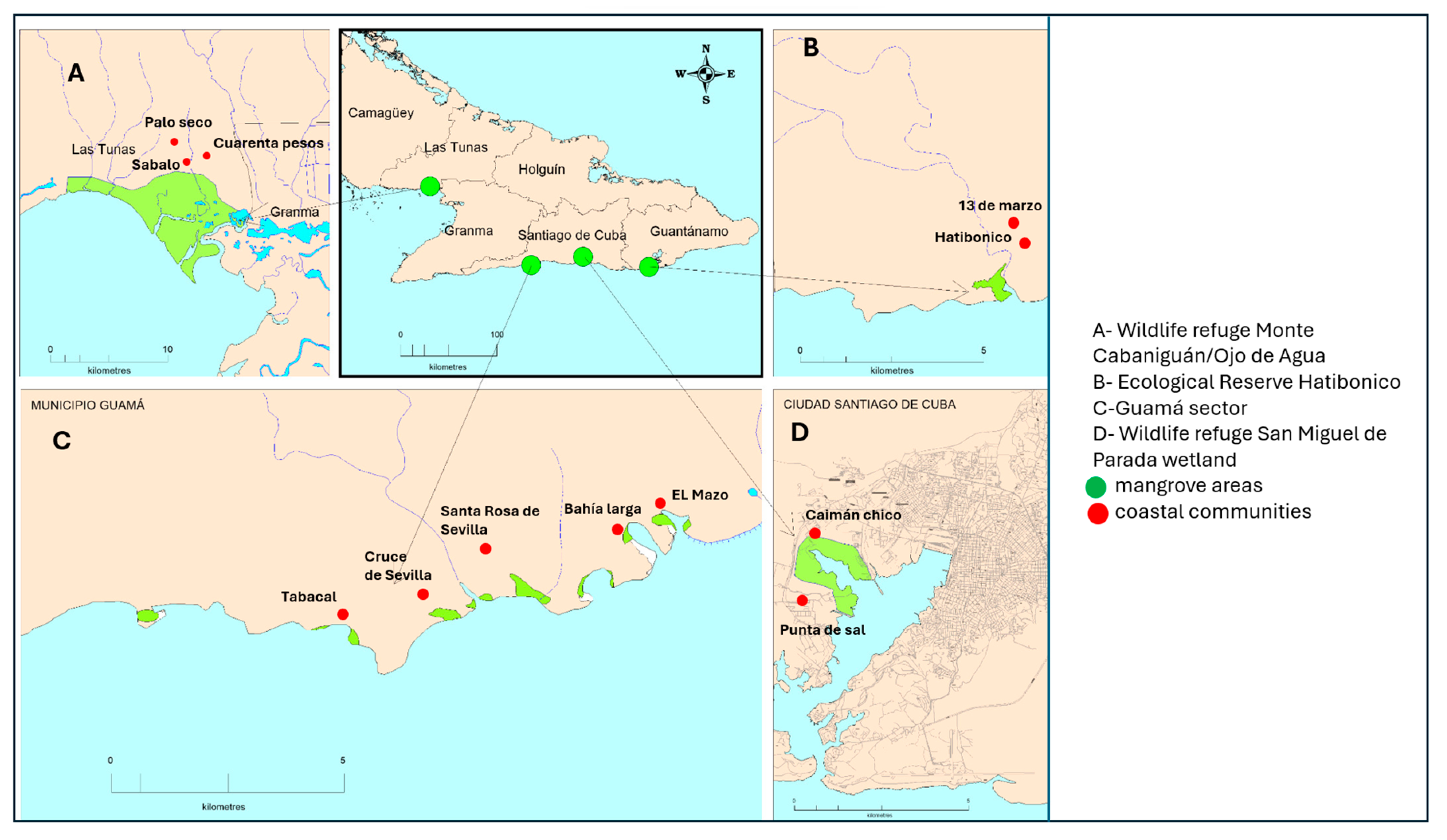
The social-ecological questionnaire was designed and administered from March to October 2023 across the four selected study sites in southeastern Cuba: Monte Cabaniguán/Ojo de Agua (MCOA) in Las Tunas province, Guamá (GUAM) and San Miguel de Parada (SAM) in Santiago de Cuba province, and Hatibonico (HAT) in Guantánamo province (
Figure 1). These sites were chosen based on criteria including proximity of communities to mangroves, community accessibility, and existing management practices at each location.
Features of the mangrove SESs studied are:
- 1)
Ojo de Agua-Monte Cabaniguán (MCOA) (See
Table 1)
This mangrove site is a fragment of the Delta del Cauto vegetation [
58], under the administration of the Ojo de Agua-Monte Cabaniguán Fauna Refuge of the National System of Protected Areas of Cuba. It is located between the municipalities of Jobabo and Colombia in the extreme southwest of the province of Las Tunas next to the Gulf of Guacanayabo (
Figure 1A). This sector was selected to represent the Cauto Delta Wetland ecosystem, which contains the largest extent of mangroves in the study area and the second largest in Cuba and the Caribbean Archipelago [
58]. Located in a coastal deltaic-alluvial plain, these mangroves, together with the marshes, form an extensive vegetation formation formed by tidal activity and contributions from the Cauto River delta. (
Figure 2) [
55]. The area covers 3929.18 ha and represents 57% of the Delta del Cauto Faunal Refuge [
59].
This area is predominantly rural. Economic activities include extensive livestock farming, commercial fishing, and charcoal production made of Dichrostachys cinerea Wight et Arn. Fishing is considered the most impactful activity, including the capture and illegal trade of key mangrove-associated species: Crocodylus acutus Cuvier, the American crocodile and Cyclura nubila Gray the Cuban iguana.
as, and hutias (
Capromys pilorides Say) [
59,
60]. Livestock production also has an impact, through uncontrolled animal entry into the reserve. It contributes to the spread of exotic species like marabou
Dichrostachys cinerea and soil compaction. Despite the measures taken to manage the reserve, these problems continue [
59,
60]. Finally, the supply of water and sediment to the mangroves has been significantly reduced by the construction of upstream reservoirs.
- 2)
This sector is located in Guamá municipality to the south of the Sierra Maestra in the province of Santiago de Cuba (
Figure 1C) [
61,
62,
63]. Mangroves in Guamá municipality are not under any type of official management system [
20]. They are located along the coastline and are subject to marine influence. However, they often receive local contributions from terrestrial runoff. The mangroves in the sector from Mazo Bay to Seville were selected because of the concentration of patches, the number and proximity of coastal settlements and the variety of activities that occur. The total mangrove area here is 162 ha. There are five coastal communities in the vicinity. Water inputs come from land runoff, the Los Lirios stream, and the Sevilla River. All four mangrove species reported in Cuba are present there [
20], with the highest abundance of
Avicennia germinans (L.) Stearn. and
Rhizophora mangle L. (
Figure 3). The estimated mangrove area of 162 ha and they generally occur as patches [
20].
The main activities are agriculture, livestock farming, and forestry. Agricultural impacts include changing land use, such as clearing mangroves to grow crops, which is mainly done by farmers. Livestock farming is considered one of the most impactful activities, mainly due to soil compaction and erosion. It requires the cutting of mangroves or other coastal vegetation to plant grasses and fodder. The forestry activities of the “Guamá” Forestal Integral Company currently include the production, collection, and marketing of a range of agricultural products. The company also promotes and manages forests and fruit trees. Mangrove bark production and marketing in local currency and wholesale are also included. Another activity in this area is aquaculture in the Bay of Mazo, where PESCASAN has its El Mazo oyster farm, which covers 30% of the bay. It is dedicated exclusively to the cultivation of oysters (Crassostrea sp.) for the local market.
- 3)
San Miguel de Parada Wildlife Refuge (SAM) (See
Table 1)
The San Miguel de Parada Wildlife Reserve is situated in the Bay of Santiago de Cuba, surrounded by the bay’s industry zone (
Figure 1D) [
64,
65]. Due to environmental changes caused by industries, settlements and the city of Santiago de Cuba (approx. 500,000 inhabitants) itself at 2 km across the bay, this area is currently considered disturbed. There is no industrial activity within the protected area itself, but there are several industrial and social service infrastructures in the vicinity that have an impact on the functioning of the ecosystem. However, the dumping and leaking of polluting products in the area, such as oil and its derivatives, is one of the main problems associated with the presence of these facilities. This wetland area covers an area of 55 hectares of mangroves [
58] (
Figure 4)
- 4)
Hatibonico Ecological Reserve (HAT) (See
Table 1)
The Hatibonico Ecological Reserve lies southwest of Guantánamo Province, between Niceto Pérez and Caimanera (
Figure 1B) [
66,
67]. According to the management plan [
66], the main function of the local mangrove is ‘coastal protection’, although it is considered important as a forest reserve. Subsistence fishing is allowed, supervised by the Forest Ranger Corps and the Alejandro de Humboldt Environmental Services Unit. Crab fishing is a traditional activity that is still allowed in the area. There, 64 ha of mangrove forest borders the mouth of the Hatibonico River (
Figure 5).
3. Results
3.1. Community Perceptions of the Use of Mangrove Ecosystem Resources
Most respondents (over 50%) considered mangrove products very important as a food source, with 65.2% of GUAM respondents always eating mangrove fish, shellfish (48.9%) and crustaceans (47.8%). Respondents in the MCOA (49.4%) said that they sometimes eat fish, but never shellfish (69.9%) nor crustaceans (63.9%). In SAMSAM, 45.8% of those interviewed said they never eat fish, shellfish (45.8%) nor crustaceans (51.4%). In HAT, 43.7% always ate crustaceans, 40.3% sometimes fish and 45.8% never crustaceans (supplementary material,
Figure S1).
Mangrove wood was never reported as a source of energy or for construction by most respondents (between 72.2% and 92.2%) (
Figure 7). Between 7.6% and 19.4% report to use mangroves for construction, mainly fencing. Between 7.6% and 25% sometimes used mangrove wood for cooking.
Most respondents (over 50%) reported not using mangrove resources for colouring or decoration communities in SAM (62.5%) and HAT (50.6%) rarely use mangroves for recreative bathing, while communities in GUAM (62%) and MCOA (56.6%) sometimes do. Respondents from MCOA (50.6%) and SAM (61.1%) reported that they do not use mangroves for traditional medicine, but in GUAM and HAT, 59.8% and 41.4% respectively reported using mangrove parts as a medicinal resource.
Most of the respondents (over 50%) reported that they never do bird watching activities nor use mangroves for research and education. A similar response was recorded when asked about religious or cultural use of mangroves. Few respondents use the mangrove for religious purposes, particularly in GUAM (1.1% always, 1.1% sometimes).
3.2. Community Perception of Mangrove Ecosystem Services
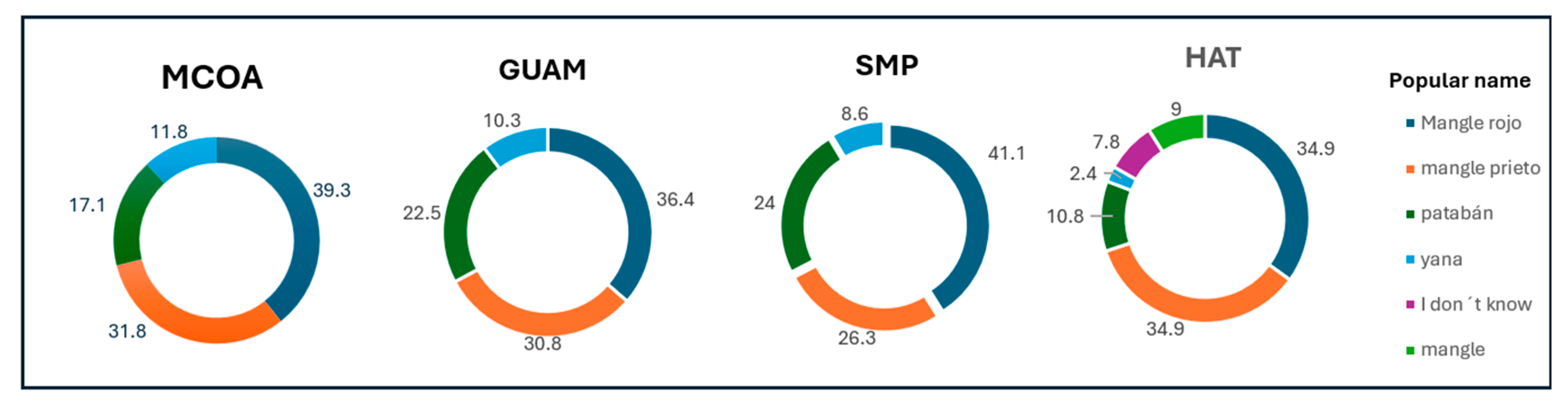
Respondents recognised the presence of red mangrove (
Rhizophora mangle, locally called mangle rojo) and black mangrove (
Avicennia germinans, locally called mangle prieto) in their area, followed by white mangrove (
Laguncularia racemosa (L.) C.F. Gaertn, locally called patabán). The least identified (
Figure 6) is the button mangrove (
Conocarpus erectus L., locally called yana). In HAT, between 2.4% and 7.8% of respondents did not know any of the mangrove species.
The local people were asked about the importance of the ecosystem services provided by mangroves (
Table 3) and the frequency of the use of resources (supplementary material,
Figure S1). Overall, the perception of ecosystem services was very high in all mangrove communities. Between 43.7% and 100% of respondents agreed that mangroves provide important support for fauna and fauna juveniles (nursery function). In addition, MCOA (88.0%) and GUAM (82.6%) communities identified sources of food and traditional medicines as very important provisioning services. Less frequently, the use of mangrove resources as a food source was reported by communities in SAM (27.8%) and HAT (35.6%).
There were three different levels of importance for mangrove regulating services defined. Coastal protection is considered very important, with between 65.2% and 95.7% of respondents indicating that mangroves help to protect against high waves and strong winds associated with extreme hydrometeorological events. In GUAM, 97.7% of respondents said that this service was very important. In HAT, there is less recognition of this service (i.e., 28.7% said it was important and 34.5% said it was very important). In terms of water purification, mangroves were perceived to be important and very important (PERCENTAGES see
Table 3). The role of mangroves in climate change mitigation through carbon sequestration was considered largely unimportant, i.e., 45.8% in MCOA, 40.2% in Guamá, 41% in SAM and 43.7% in HAT. The role of water quality maintenance is very important in all communities, i.e., 84.3% in MCOA, 70.7% in GUAM, 59.7% in SAM and 39.1% in HAT.
Most respondents from MCO, GUAM and SAM rated the role of mangroves for recreation and education as very important (
Table 3) (65.2%, 65.2% and 41.7% respectively). However, this service does not seem to be important for the members of the HAT community. In GUAM, local people consider the landscape value of mangroves as local heritage (64.1%).
3.3. Community Perceptions of Mangrove Management Framework
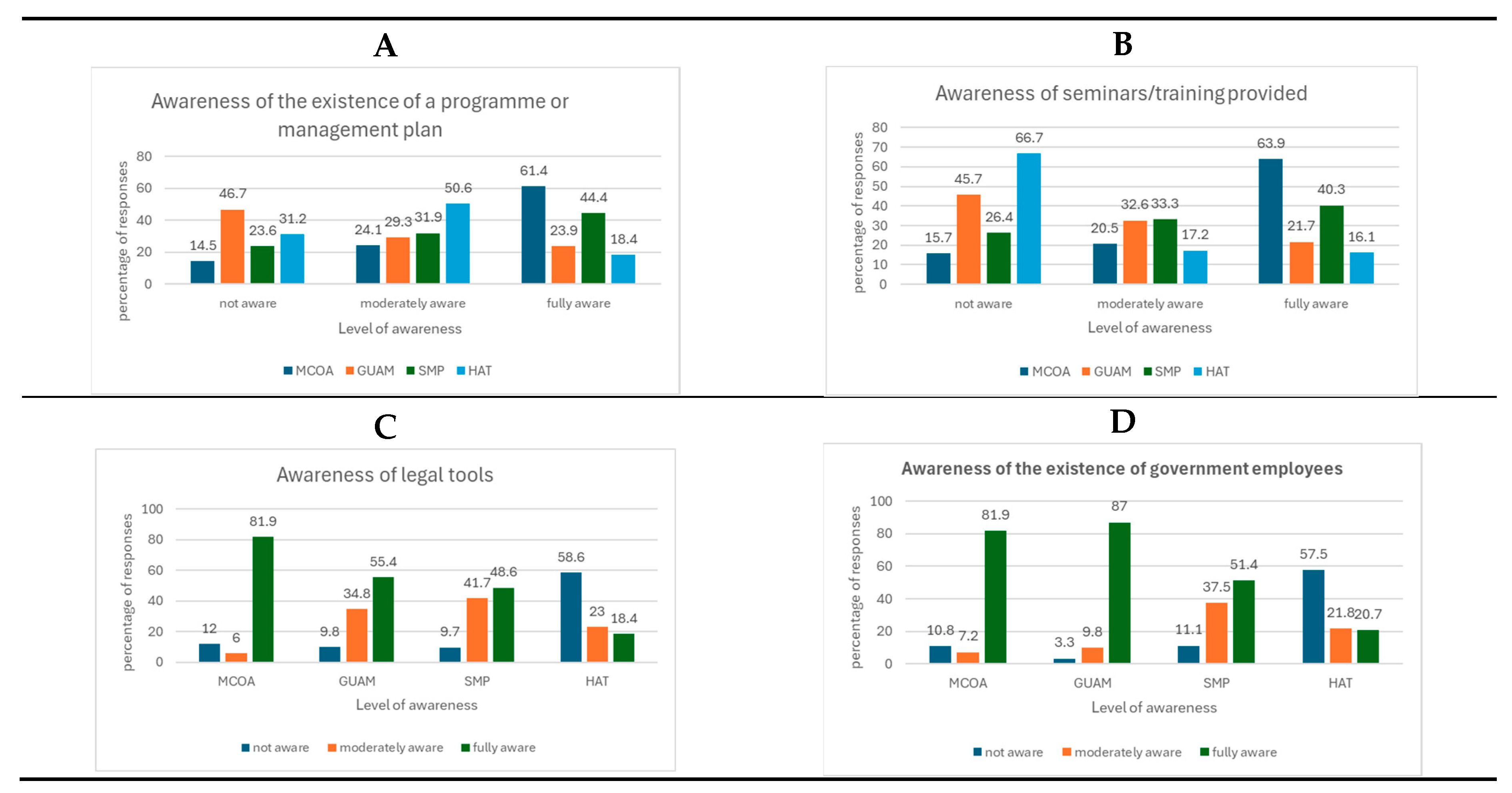
We asked the local people per site if they were aware of the existence of any management activity (
Figure 7A). Respondents were fully aware of the existence of the protected area and management activities in MCOA (61.4%) and SAM (44.4%). In GUAM, between 23.9% and 29.3% were aware and fully aware that there was no management plan. However, they referred to the activities of the rangers and the government to protect the mangrove forest (
Figure 7D). In HAT, 50.6% of villagers are moderately aware of the existence of management activities.
Figure 7.
A- Awareness of mangrove management in the study sites. Perception of management plan existence, B- awareness of management training activities, C- awareness of the existence of legal tools regarding mangrove management, and D- awareness of the existence of government employees for mangrove protection.
Figure 7.
A- Awareness of mangrove management in the study sites. Perception of management plan existence, B- awareness of management training activities, C- awareness of the existence of legal tools regarding mangrove management, and D- awareness of the existence of government employees for mangrove protection.
In the study sites, a significant percentage of respondents in MCOA (63.9%) and SAM (40.3%) indicated being very aware of ongoing management training activities such as seminars, workshops, and training sessions (
Figure 7B). In GUAM, where mangroves are not designated as a protected area, perceptions of management activities were linked to the implementation of the COSTASURESTE project, which was concluded in 2012 and no longer operational. Conversely, in HAT, local awareness of mangrove management activities was reported as moderate.
Across all sites, the communities displayed moderate to high levels of awareness regarding the existence of laws regulating mangrove use, penalties for non-compliance, and the governmental authorities responsible for enforcement (
Figure 7C,D).
3.4. Community Perception of Potential Threats to Mangroves
During the survey, villagers identified and ranked 15 natural and anthropogenic threats affecting their localities (
Table 4). Climate change and drought emerged as natural threats raising most concern across all areas. Tropical cyclones were highlighted by villagers in GUAM (16.1%) and SAM (9.0%) as significant threats. Salinisation was also noted as a relevant concern in MCOA (4.2%), GUAM (12.5%), and SAM (4.2%).
Locally, forest fires were identified as a threat by respondents in MCOA (14.4%) and HAT (24.5%). Among anthropogenic threats, logging was prominently mentioned in MCOA (24.2%), GUAM (24.7%), and SAM (12.7%). Industries were specifically noted in SAM (18.0%) as contributing to environmental risks.
Road construction was reported as impacting mangroves in MCOA (4.8%) and GUAM (4.8%). Damming of rivers was cited in MCOA (3.0%) and SAM (4.2%). Agricultural expansion in GUAM (5.1%) was noted for replacing mangrove areas with crops, while 4.5% reported conversion to pasture.
Local communities also expressed concern over the extraction of mangrove bark and roots for various purposes, posing threats to mangroves. The presence and spread of exotic species were identified as hazards in MCOA (5.3%), GUAM (4.1%), and HAT (4.3%), but were not mentioned in SAM.
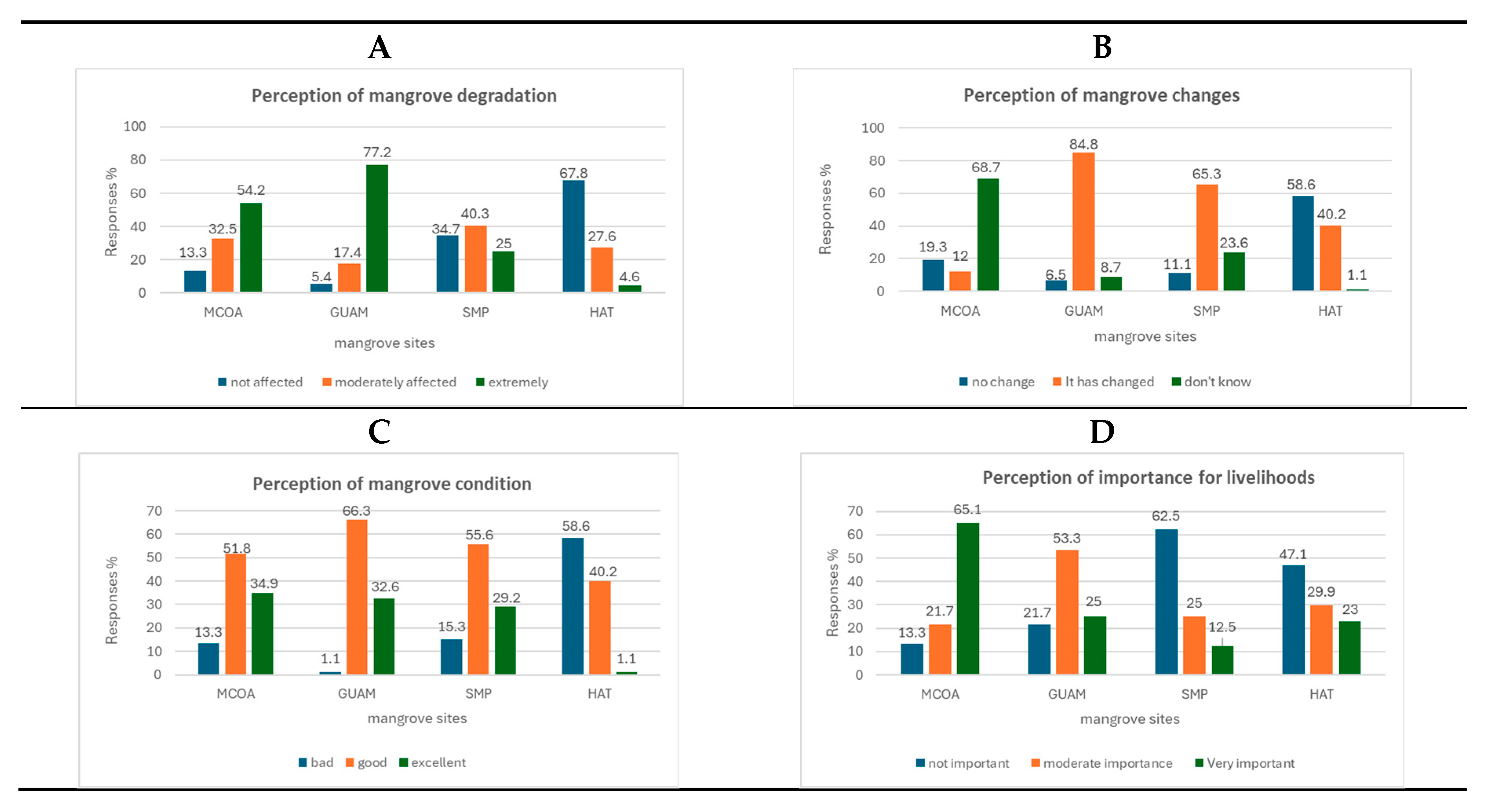
We queried local residents regarding their perceptions of changes and the current state of mangroves near their communities (
Figure 8). We surveyed local communities to assess their awareness and perception of the impact of mangrove degradation on their communities (
Figure 8A). In MCOA, 54.2% of respondents acknowledged the severe impact of mangrove degradation, while in GUAM, this figure was notably higher at 77.1%. Conversely, in SAM, 34.7% of respondents perceived mangrove degradation as having minimal or moderate negative consequences. In HAT, a majority of respondents (67.8%) believed that mangrove degradation would either be harmless or would only have a moderate negative impact on their communities.
In MCOA, 68.7% of respondents perceived no significant changes in the mangrove landscape, while 51.8% felt that mangroves were in good condition (
Figure 8B). Additionally, 65.1% of respondents emphasised the importance of mangroves to their livelihoods (
Figure 8D). In GUAM, a notable 84.8% of respondents perceived changes in local mangroves, with 66.3% believing these ecosystems were in good condition (
Figure 8B). About 53.3% of respondents indicated that mangroves were moderately important for their livelihoods, primarily as a food source (
Figure 8D). In SAM, 55.6% of respondents observed an increase in mangrove area within the wetland, and an equal percentage considered mangroves to be in good condition. Surprisingly, 62.5% of respondents stated that mangroves were not significant for their livelihoods (
Figure 8D). In HAT, 58.6% of respondents reported no noticeable changes in mangroves (
Figure 8B), and 40.2% assessed the mangroves as being in good condition (
Figure 8C). Moreover, a majority (64.4%) of respondents expressed that mangroves were not crucial for their livelihoods (
Figure 8D).
3.5. Results of the Governance Analysis
Based on insights gathered from interviews and management plans, distinct approaches to mangrove management and community involvement were observed across different sites.
In MCOA, mangrove management plans are formulated exclusively by area specialists without direct community participation or voting rights. The community’s role is limited to providing information during stakeholder workshops, where only one community representative is invited.
In SAM, workshops serve as platforms to reconcile diverse interests, gathering inputs from various experts within the protected area. However, community participation is here also restricted to providing information rather than influencing decision-making directly.
HAT employs a more inclusive approach where workshops precede plan development or updates. These sessions involve community participation in defining tasks and reporting on previous plans. Decision-makers and community representatives are integral to these workshops, held locally with support from government entities such as the Ministry of the Revolutionary Armed Forces (MINFAR) and civil defense. Economic challenges sometimes hinder plan implementation, especially for continuity plans, which often rely solely on data updates.
In GUAM, mangrove management falls under the responsibility of the Integral Forestal Guamá Enterprise and the State Forestry Service of the Ministry of Agriculture (MINAGRI), with involvement from the municipal government and the Ministry of Science Technology and Environment (CITMA) in decision-making. However, there is a recognised need to expand involvement to include other local institutions and community members.
The analysis of stakeholder relationships identified key entities such as the Cuban State, CITMA, MINAGRI, provincial governments, and municipal councils as having significant influence and interest in mangrove management (Supplementary material,
Figure S2). In contrast, entities like the National Hydraulic Institute (INRH) and the Ministry of Food Industry (MINAL) exhibit lower current influence and interest, necessitating minimal involvement in the management framework.
Communities and the Ministry of Tourism (MINTUR) are identified as stakeholders with high levels of interest but low influence in shaping outcomes within the mangrove social-ecological system. This suggests that they are significantly affected by the results of actions impacting mangroves, yet their ability to influence these outcomes through management decisions is limited. Conversely, stakeholders such as MINFAR and the Ministry of the Interior (MININT) possess high influence but exhibit low interest in mangrove-related activities.
3.6. Description of the Observed Social-Ecological Relationships
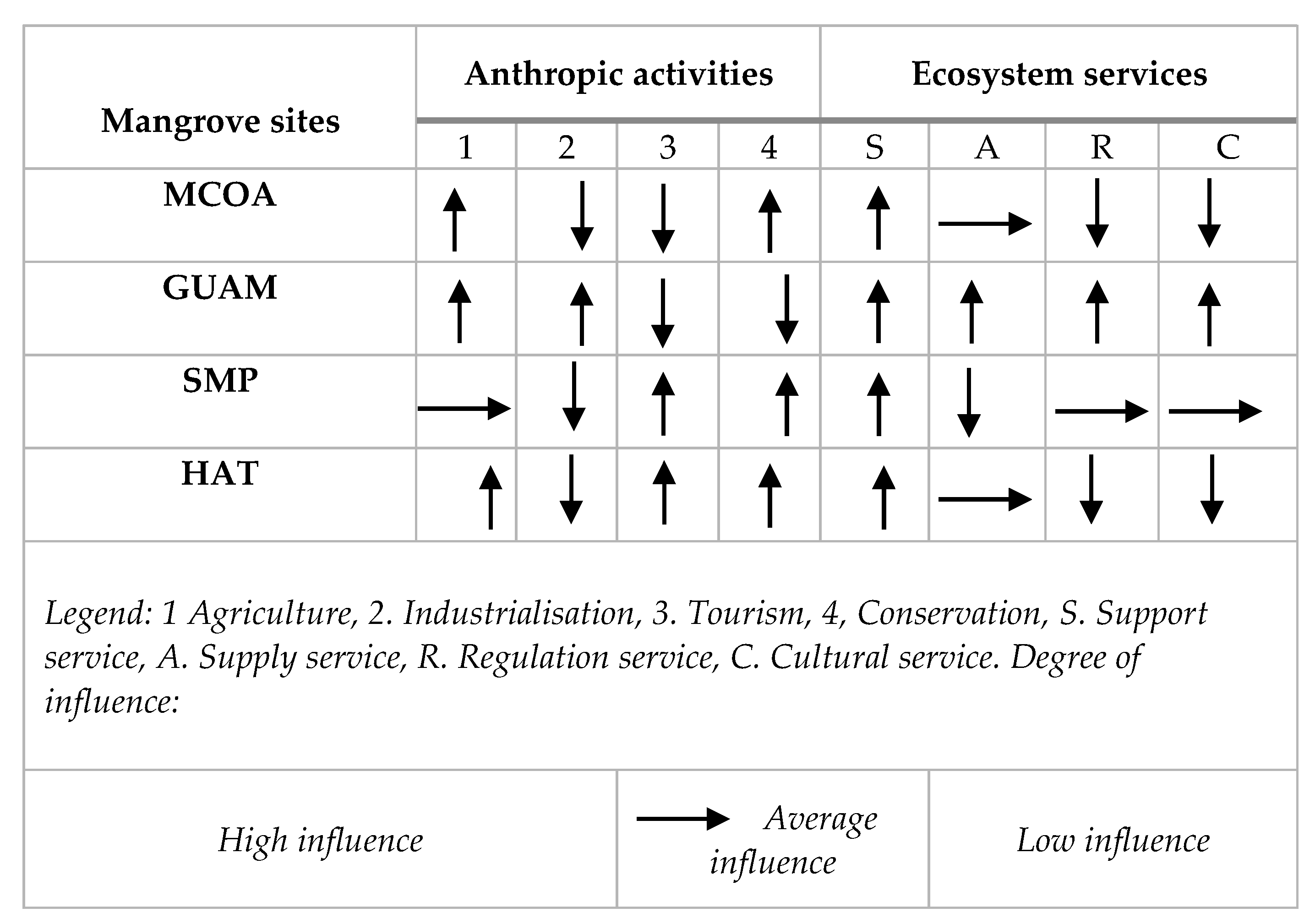
Our analysis emphasizes how human activities (social, economic, and cultural) affect the mangrove ecosystem in each locality analyzed. The matrix (Figure 11) synthesises the social-ecological relationships observed, revealing three distinct interaction types: urban/industrial, rural/agricultural, and rural/agricultural/tourist.
In MCOA, significant interaction occurs with the agricultural sector and expanding livestock farming. These activities have minimal impact on mangroves, likely due to the expansive nature of the local ecosystem. The mangroves primarily provide supporting services, crucial for justifying conservation efforts, with provisioning services playing a secondary role, according to respondents.
GUAM exhibits a high level of interaction between tourism and agriculture activities. Agricultural expansion poses the greatest threat to mangrove areas through land use changes. Tourism, while not directly impacting mangroves, focuses on exploiting the scenic and cultural values of the local forest, fostering strong social relations based on goods supply and cultural services.
SAM is characterised by intense urban-industrial interaction, situated within the industrial zone of Santiago de Cuba’s bay. Here, mangroves face significant challenges from pollution and land use changes driven by industrial growth, compromising ecosystem services. Supporting services, particularly the mangrove’s role in sheltering migratory birds, dominate despite these pressures.
HAT experiences minimal interaction, primarily centred on agricultural activities and non-productive forestry endeavors. Social interactions thrive without adverse effects on mangroves. This area exhibits low ecological impact, with supporting services being most pronounced and provisioning services playing a moderate role.
Figure 9.
Evaluation matrix of social-ecological interactions in the study sites.
Figure 9.
Evaluation matrix of social-ecological interactions in the study sites.
4. Discussion
Our study underscores that knowledge and utilisation of mangroves are deeply intertwined with the daily lives and cultural practices of southeastern Cuban communities, consistent with findings by authors such as [
7,
18,
32]. This increased awareness among locals about ecosystem services and threats like climate change likely stems from environmental education initiatives integrated into Cuba’s Environmental Strategy implementation [
70].
Cuba in the Atlantic East Pacific mangrove region exhibits a relatively low mangrove species richness as compared to the Indo-West Pacific and against the background of the global total of approximately 70 species. Mangroves in Cuba comprise primarily
Rhizophora mangle (red mangrove),
Avicennia germinans (black mangrove), and further
Laguncularia racemosa (white mangrove) and
Conocarpus erectus (button mangrove) [
62]. These species, particularly red and black mangroves, also dominate the mangrove forests across the study sites in southeastern Cuba [
20,
58,
64]. Red mangroves, in particular, are well-recognised by coastal communities [
20,
58].
We intended to provide descriptive data for the mangrove social-ecological systems of southeastern Cuba in support of a better understanding of the issues at stake for good management and conservation. Despite not performing an in-depth quantitative analysis, we propose several inferences towards management and conservation from our study.
4.1. Use of Mangrove Resources by Communities
Fishing and forest product exploitation, whether commercial or for subsistence, have historically provided primary livelihoods for coastal communities near mangroves [
4,
6]. However, our study indicates a shift in southeastern Cuba where local livelihoods are increasingly diversifying towards inland activities [
65,
66], employment in industries [
67], and emerging agricultural and livestock sectors [
55,
68]. Despite these changes, the use of mangrove resources for food remains prominent, alongside medicinal and recreational uses like bathing areas. Notably, while mangrove wood is valued elsewhere for fuel and construction [
4,
48], in this study respondents viewed its use as illegal, reflecting Cuba’s stringent regulations against mangrove logging [
69].
Studies on mangroves in Mexico, Brazil, Bangladesh, and Madagascar highlight uses such as recreation, tourism, and cultural preservation tied to mangroves [
7,
71,
72,
73]. In southeastern Cuba, local communities also utilise red mangrove for traditional medicine, such as in the treatment of kidney infections and skin diseases. Although there is potential for nature tourism and ecotourism, given the high scenic value attributed to mangroves, particularly for wildlife observation within protected areas, this is not very common in the area. Sustainable practices for these activities remain underdeveloped.
4.2. Perception of Ecosystem Services in Communities
Communities near mangroves in southeastern Cuba demonstrate a robust understanding of ecosystem goods and services, consistent with what is globally known for mangroves, and also reported elsewhere, as in Mauritius [
6]. Habitat support for fauna, especially juveniles, and provisioning services such as food and medicine are widely recognised [
7,
22,
74]. In GUAM and MCOA, mangroves play a crucial role in supporting local fisheries, highlighting their significant economic importance [
59]. This dependency is less linked to demographic factors and more influenced by community livelihood strategies, if compared to other studies [
7,
74,
75].
The perception of the regulatory function of climate mitigation services depends on the level of awareness of the population regarding the protective role of mangroves against the effects of extreme hydro-meteorological phenomena. Knowledge on this topic extends beyond the experience of the respondents with national environmental policies [
24], where the emphasis is put on this ecosystem service [
4,
32].
While awareness about mangroves’ regulatory role in climate mitigation is evident, the perception of their carbon sequestration capacity remains limited, possibly due to the abstract nature of this service in local contexts [
76,
77]. This suggests a need for enhanced environmental education and capacity building to leverage local understanding for future blue carbon initiatives and to exploit the potential they offer, Cuba ranking 10th worldwide in mangrove area.
4.3. Perception of Mangrove Ecosystem Threats and Management Framework
Coastal residents are usually well aware of ongoing mangrove conservation efforts and the regulatory frameworks governing their use in Cuba. However, clarity on responsible management entities remains a concern despite established legal structures [
24]. Apparently involving local governments is essential for successful management programmes [
32,
48]. Management plans often prioritise charismatic fauna over mangrove ecosystems, reflecting funding biases. In GUAM, initiatives like the COSTASURESTE project [
78] have bolstered local environmental awareness, albeit without specific protected area designations or integrated coastal zone management (ICZM) programmes. Though concluded in 2012, the awareness lingers on.
Governance types around the world could be divided roughly into bottom-up, top-down and co-management [
79], amongst others. In Cuba, the top-down management system is still prevailing [
41,
80]. Moving forward, embracing bottom-up or co-management models could enhance local participation and governance efficacy, aligning with international best practices and ensuring sustainable mangrove management [
79].
4.3.2. Potential Threats to Mangroves
Mangroves face varied threats, categorised as natural (e.g., drought, cyclones) and anthropogenic (e.g., logging, agricultural expansion, pollution) hazards, each posing different levels of risk [
16,
81]. Natural forces have caused significant mangrove loss [
23]. Climate change has natural and anthropogenic roots, but locals identified it as a natural threat. Climate change and drought are perceived as the most significant natural threats in southeastern Cuba, exacerbated by local climatic conditions and regional forecasts [
82]. Cyclones are a major threat, causing area loss and damage, though mangroves can recover [
23,
83]. Salinisation and forest fires are other concerns expressed, while sea level rise is less perceived due to the elevated eastern coast.
Anthropogenic threats such as logging and agricultural expansion are significant concerns, driven by economic activities that alter land use and hydrology, impacting mangrove ecosystems [
16,
84,
85,
86]. Effective governance and community involvement are crucial for mitigating these threats, aligning policies with local realities and sustainable practices [
16,
79,
80].
4.3.3. New Perspective for Governance and Management of Mangroves in Eastern of Cuba
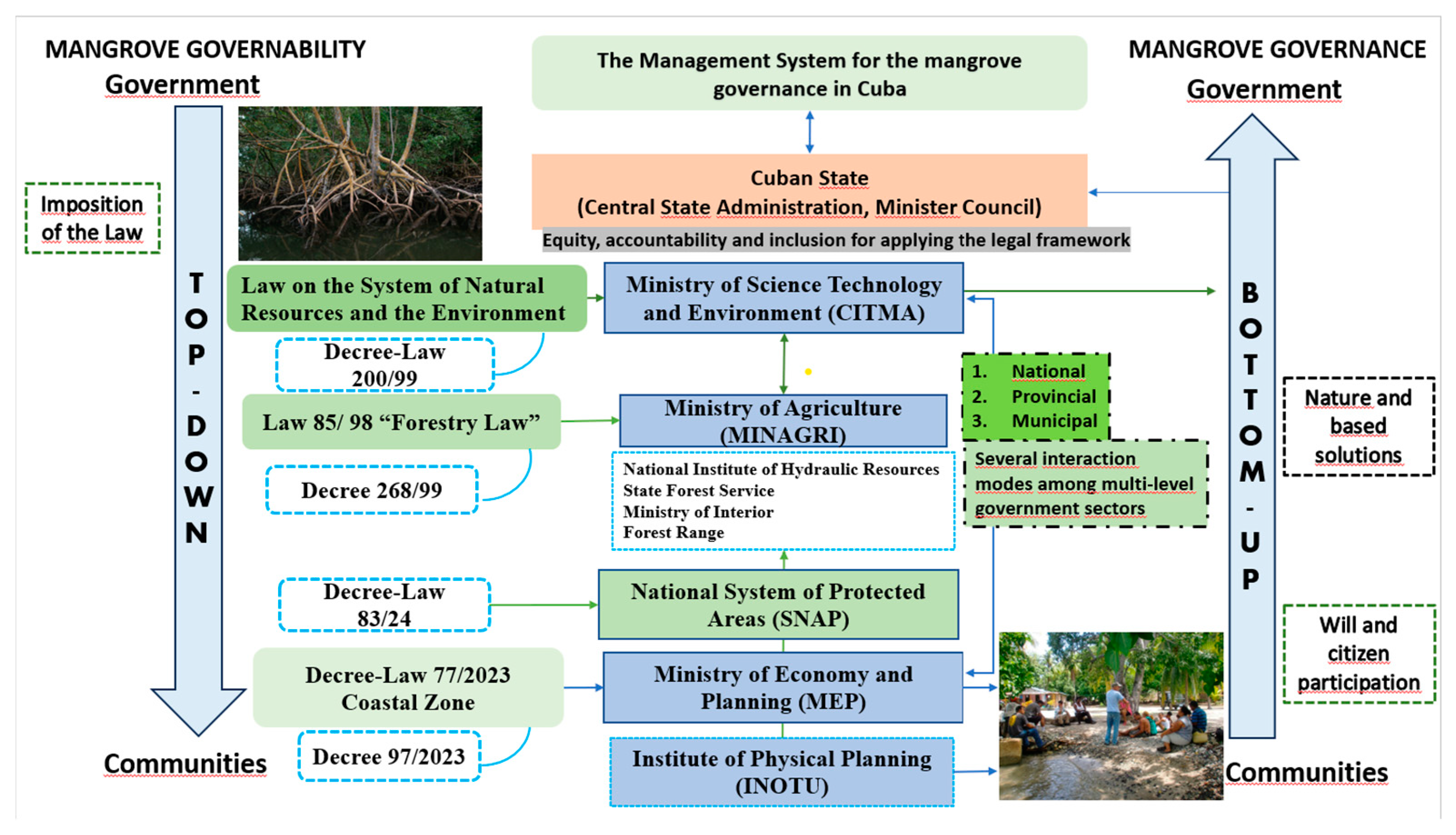
While Cuba has established institutional mechanisms for environmental governance, their effectiveness in incorporating community voices and ensuring equitable, accountable management of mangroves remains a challenge. Enhancing stakeholder engagement and fostering inclusive governance models could bridge existing gaps, facilitating more sustainable mangrove conservation practices [
40,
41]. The proposed model (
Figure 10) outlines actionable steps for integrating local knowledge and fostering collaborative management approaches, building on Cuba’s existing legal frameworks [
24].
4.3.4. Description of the Observed Social-Ecological Relationships
A preliminary analysis (qualitative, descriptive) was performed by integrating the results of the survey and structuring the documentary review. For future studies, we recommend introducing indicators and measurable variables to analyse these social-environmental interactions. Our research suggests that protected area management programmes should align with the principles of integrated coastal zone management. This alignment will facilitate the emergence of social-ecological system approaches and increase governance efficacy.
We found gaps in local knowledge on the role of mangrove ecosystems in carbon sequestration. There is a need for training and identifying opportunities for implementing local development projects. Emphasising capacity building could address market issues for ecosystem services, including carbon sequestration, and raise awareness of this intangible service. Understanding the complex international carbon market assessments and realising the ecosystem’s potential for this service in Cuba is crucial.
Our study concludes that communities have different social-environmental relationships based on the rural, urban, and coastal characteristics of these settlements. There are also different manifestations of anthropic activities depending on the dominant sector of economic development and its impact on mangrove functioning.
5. Conclusions
This study examined the knowledge, use patterns, interdependencies, and social-cultural constructs surrounding the social-ecological dynamics between four southeastern Cuban communities and coastal mangrove ecosystems. Understanding these relationships provides already insights for decision-makers to promote conservation and highlights shortcomings in existing management programmes, including training programmes tailored to each community, local public, and private stakeholders.
Our findings emphasise the need for a comprehensive approach to mangrove management and conservation, with implications extending beyond Cuba. Policymakers should recognise NbS as an opportunity for inclusive solutions and establish partnerships for mangrove management in southeastern Cuba. Reducing the gap between NbS and formal governance, addressing diverse opinions, and providing continuous support for community participation is essential. Developing NbS capacities based on regional contexts is crucial for effective management.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Questionnaire; Table S2. Variables used in statistical analysis and types of data, Figure S1: Frequency of mangrove resource use in the sites studied, Figure S2. Level of specific stakeholders in the management system for the Mangrove governance in Cuba (Mendelow’s Stakeholder Matrix).
Author Contributions
Conceptualisation, YC, NK, CM, FDG and OP; methodology, YC, NK, CM, FDG and OP software, YC; validation, YC, CM and OP; formal analysis, YC, NK, NB, OJR, CM and OP; investigation, YC; writing—original draft preparation, YC; writing—review and editing, YC, NK, NB, OJR, CM, FDG and OP; visualisation, YC and CM; supervision, NK, NB, OJR and OP; funding acquisition, YC, NK, NB, OJR and OP. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Flemish Development Cooperation through the Flemish Interuniversity Council-University Cooperation for Development (VLIR-UOS) as part of an Institutional University Cooperation program with Universidad de Oriente in Santiago de Cuba, Cuba. YC received a grant from Global Minds, Hasselt University, Belgium. Moreover, we received support from Science, Technology, and Innovation research project: Adaptive governance to Climate Change in coastal municipalities of Cuba, PN211LH012-018, Associated with the National Local Development Program in Cuba.
Acknowledgments
The authors thank MSc. Agustin Garzón for valuable discussions and insights, also to MSc. Yusneida Alarcón Jorge”, MSc. Yairén Alonso Jiménez, Ing. Yuri Drebros Trutié, Msc. Hayler M. Pérez Trejo for assistance with field trips and questionnaire implementation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chapin, F.S.; Kofinas, G.P.; Folke, C. Principles of Ecosystem Stewardship Resilience-Based Natural Resource Management in a Changing World; Springer Science+Business Media: New York, NY, USA, 2009; p. 402. [Google Scholar]
- Dahdouh-Guebas, F.; Hug, J.; Abuchahla, G.M.O.; Cannicci, S.; Jayatissa, L.P.; Kairo, J.G.; Kodikara, S.; Koedam, N.; Mafaziya, T.W.G.F.; Mukherjee, N.; et al. Estuarine, Coastal and Shelf Science Reconciling nature, people and policy in the mangrove social-ecological system through the adaptive cycle heuristic. Estuarine 2021, 248, 1–29. [Google Scholar]
- SARAS Institute. Key Concepts: Socio-ecological systems. South American Institute for Resilience and Sustainability Studies 2019, p. 2. https://saras-institute.org/social-ecological-systems/.
- Walters, B.B.; Rönnbäckb, P.; Kovacsc, J.M.; Cronab, B.; Hussain, S.A.; Badola, R.; Primavera, J.H.; Barbier, E.B.; Dahdouh-Guebas, F. Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat. Bot. 2008, 89, 220–236. [Google Scholar] [CrossRef]
- Nagendra, H. Reforestation and regrowth in the human-dominated landscapes of South Asia. Reforesting Landscapes, Springer 2009, 1 49–1 74.
- Abib, S.; Appadoo, C. Local people and mangroves: Ecosystem perception and valuation on the southwest coast of Mauritius. West. Indian Ocean. J. Mar. Sci. 2021, 20, 11–19. [Google Scholar] [CrossRef]
- Merven, R.; Appadoo, C.; Florens, V.; Iranah, P. Dependency on mangrove ecosystem services is modulated by socioeconomic drivers and socio-ecological changes–insights from an insular biodiversity hotspot. Res. Sq. 2023, 1–18. [Google Scholar]
- MEA (Millennium Ecosystem Assessment). Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Mitra, A.; Mitra, A. Ecosystem services of mangroves: An overview. Mangrove Forests in India: Exploring Ecosystem Services 2020, 1-32.
- Nellemann, C.; Corcoran, E. , Duarte, C.M.; Valdés, L.; De Young, C.; Fonseca, L.; Grimsditch, G. Blue carbon: A rapid response assessment. Blue Carbon 2009, vol. 80. Arendal, Norway: United Nations Environment Programme, GRID-Arendal).
- Kauffman, J.B.; Adame, M.F.; Arifanti, V.B.; Schile-Beers, L.M.; Bernardino, A.F.; Bhomia, R.K.; Donato, D.C.; Feller, I.C.; Ferreira, T.O.; Jesus Garcia, M.C.; et al. Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol. Monogr. 2020, 90, 1–18. [Google Scholar] [CrossRef]
- P. I. Macreadie, A. Anton, J.A. Raven, N. Beaumont, R.M. Connolly, D.A. Friess, J.J. Kelleway, H. Kennedy, T. Kuwae, Lavery, P.S.; et al. The future of Blue Carbon science. Nat. Commun. 2019, 10. [Google Scholar]
- Cummings, A.R.; Shah, M. Mangroves in the global climate and environmental mix. Geogr. Compass 2017, 1–17. [Google Scholar] [CrossRef]
- Bouillon, E. Carbon cycle: Storage beneath mangroves. Nature Geoscience 2011, 4, 1–3. [Google Scholar] [CrossRef]
- Das, S.C.; Das, S.; Tah, J. Mangrove Forests and People’s Livelihoods. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Pullaiah, T., Ashton, E.C., Eds.; Springer Nature: Singapore, 2022; pp. 153–173. [Google Scholar]
- H. Akram, S. Hussain, P. Mazumdar, K.O. Chua, T.E. Butt, JA. Harikrishna. Mangrove Health: A Review of Functions, Threats, and Challenges Associated with Mangrove Management Practices. Forests 2023, 14, 1698. [CrossRef]
- D.A. Friess, E.S. Yando, J.B. Alemu, L.W. Wong, S.D. Soto N. Bhatia. Ecosystem services and disservices of mangrove forests and salt marshes. Oceanography and marine biology 2020.
- J. Come, N. Peer, J.L. Nhamussua, N.A. Miranda, C.C. Macamo, A.S. Cabral, H. Madivadua, D. Zacarias, J. Narciso, and B. Snow, “A socio-ecological survey in Inhambane Bay mangrove ecosystems: Biodiversity, livelihoods, and conservation. Ocean & Coastal Management, 2023; 244. [CrossRef]
- S.O. Bandeira, C.F. Macamo, J.G. Kairo, F. Amade, N. Jiddawi, & J. Paula. Evaluation of mangrove structure and condition in two transboundary areas in the Western Indian Ocean. Aquatic Conservation: Marine and Freshwater Ecosystems 2009, 19(S1), S46-S55. [CrossRef]
- Portorreal, Y.C.; Montero, O.P. Evaluación de impactos a la salud del manglar en el municipio Guamá, Santiago de Cuba, Cuba. Madera Y Bosques 2017, 23, 27–41. [Google Scholar] [CrossRef]
- V.M. Alati, J. Olunga, M. Olendo, L. Nduku Daudi, K. Osuka, C. Odoli, P. Tuda, Nordlund, L.M. Mollusc shell fisheries in coastal Kenya: Local ecological knowledge reveals overfishing. Ocean and Coastal Management, 2020; 17. [CrossRef]
- S.E. zu Ermgassen, N. Mukherjee, T.A. Worthington, A. Acosta, A.R. da Rocha Araujo, C.M. Beitl, G.A. Castellanos-Galindo, M. Cunha-lignon, F. Dahdouh-Guebas, Diele, K.; et al. Fishers who rely on mangroves: Modelling and mapping the global intensity of mangrove-associated fisheries. Estuar. Coast. Shelf Sci. 2021, 248.
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Change Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef] [PubMed]
- Y.C. Portorreal, O.J.R. Dominguez, B. Cuker, C.B. Milanes, and O.P. Montero, “Environmental policy and regulatory framework for managing mangroves as a carbon sink in Cuba. Water 2022, 14, 1–24.
- R.T. Munang, I. Thiaw and M. Rivington Ecosystem management: tomorrow’s approach to enhancing food security under a changing climate. Sustainability 2011. 3:937–954. [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species : Mangrove Extinction Risk and Geographic Areas of Global Concern. PloS ONE 2010, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- B. Satyanarayana, P. Bhanderi, M. Debry, D. Maniatis, F. Foré, D. Badgie, K. Jammeh, T. Vanwing, C. Farcy, N. Koedam, and F. Dahdouh-Guebas, “A Socio-Ecological Assessment Aiming at Improved Forest Resource Management and Sustainable Ecotourism Development in the Mangroves of Tanbi Wetland National Park, The Gambia, West Africa. Sciences-New York 2012, 41, 513–526.
- F. Dahdouh-Guebas, D.A. Friess, C.E. Lovelock, R.M. Connolly, I.C. Feller, K. Rogers, and S. Cannicci, “Cross-cutting research themes for future mangrove forest research. Nature Plants 2022, 8, 1131–1135. [CrossRef]
- F. Dahdouh-Guebas, G.N. Ajonina, A.A. Amir, D.A. Andradi-Brown, I. Aziz, T. Balke, E.B. Barbier, S. Cannicci, S.M. Cragg, M. Cunha-Lignon, D.J. Curnick, C.M. Duarte, N.C. Duke, C. Endsor, S. Fratini, I.C. Feller, F. Fromard, J. Hugé, M. Huxham, J.G. Kairo, T. Kajita, K. Kathiresan, N. Koedam, S.Y. Lee, H.-J. Lin, J.R. Mackenzie, M.M. Mangora, C. Marchand, T. Meziane, T.E. Minchinton, N. Pettorelli, J. Polanía, G. Polgar, M. Poti, J. Primavera, A. Quarto, S.M. Rog, B. Satyanarayana, Y. Schaeffer-Novelli, M. Spalding, T. Van Der Stocken, D. Wodehouse, J.W.H. Yong, M. Zimmer, and A.D.A. Friess, “Public Perceptions of Mangrove Forests Matter for Their Conservation. Frontiers in Marine Science 2020, 7, 1–5. [CrossRef]
- H. Nguyen, R.J. Harper, and B. Dell, “Examining local community understanding of mangrove carbon mitigation: A case study from Ca Mau province, Mekong River Delta, Vietnam. Marine Policy 2023, 148, 10.
- B.P. Nyangoko, H. Berg, M.M. Mangora, M. Gullström, and M.S. Shalli, “Community Perceptions of Mangrove Ecosystem Services and Their Determinants in the Rufiji Delta, Tanzania. Sustainability 2021, 13, 63. [CrossRef]
- J.M.D. Quevedo, Y. Uchiyama, and R. Kohsaka, “Perceptions of local communities on mangrove forests, their services and management: implications for Eco-DRR and blue carbon management for Eastern Samar, Philippines. Journal of Forest Research 2020, 25, 12.
- B. Martín-López, I. Iniesta-Arandia, M. García-Llorente, I. Palomo, I. Casado-Arzuaga, Amo, et al. (Uncovering Ecosystem Service Bundles through Social Preferences. PLoS ONE 2012, 7, e38970. [CrossRef]
- K. Bimrah, R. Dasgupta, S. Hashimoto, I. Saizen, and S. Dhyani, “Ecosystem Services of Mangroves: A Systematic Review and Synthesis of Contemporary Scientific Literature. Sustainability 2022, 14, 2051. [CrossRef]
- S.P. Valderrama, A.H. Ávila, J.G. Méndez, O.M. Martínez, D.C. Rojas, H.F. Azcona, E.M. Hernández, H.C. Aragón, P.M. Alcolado, F. Pina-Amargós, Z. Hernández González, L.E. Pantoja, and L.F.R. Farrat, Marine protected areas in Cuba. Bulletin of Marine Science, 2018; 94, 423–442.
- P. Bunting, A. Rosenqvist, L. Hilarides, R.M. Lucas, N. Thomas, T. Tadono, T.A. Worthington, M. Spalding, N.J. Murray, and L.-M. Rebelo, “Global Mangrove Extent Change 1996–2020: Global Mangrove Watch Version 3.0. Remote Sensing, 2022; 14, 1–32.
- Turschwell, M.P.; Tulloch, V.J.; Sievers, M.; Pearson, R.M.; Andradi-Brown, D.A.; Ahmadia, G.N.; Connolly, R.M.; Bryan-Brown, D.; Lopez-Marcano, S.; Adame, M.F.; et al. Multi-scale estimation of the effects of pressures and drivers on mangrove forest loss globally. Biol. Conserv. 2020, 247, 11. [Google Scholar] [CrossRef]
- Rodríguez-Crespo, G.C.; Domínguez-Junco, O. Servicios ecosistémicos en manglares: beneficios a: resiliencia del ecosistema ante cambios climáticos, la comunidad y su desarrollo local. Rev. Transdiscipl. De Estud. Soc. Y Tecnológicos 2022, 2, 5–10. [Google Scholar] [CrossRef]
- Mahardika, S.H.; Yulianda, F.; Adrianto, L. Sulistiono; Interactive Governance for Mangrove Social-Ecological System in Tangerang Regency: A DPSIR Approach. Int. J. Adv. Sci. Eng. Inf. Technol. 2023, 13, 1249–1257. [Google Scholar] [CrossRef]
- Gayo, L. Local community perception on the State Governance of mangroves in Western Indian coast of Kinondoni and Bagamoyo, Tanzania. Global Ecology and Conservation 2022, 39, 11. [Google Scholar] [CrossRef]
- Ahmed, J.; Kathambi, B.; Kibugi, R. Policy perspective on governance standards setting using community participation for sustainable mangrove management in Lamu Kenya. International journal Of conservation science 2023, 14, 315–326. [Google Scholar]
- S. Valderrama, A. Hernández, H. Ferro-Azcona, D. Cobián-Rojas, J. González-Méndez, H. Caballero-Argón, E.D.L. Guardia, R.-P. A., Z.H. -Gonzales, L. Espinosa-Pantoja, and Lara, A. Increasing marine ecosystems conservation linking marine protected areas and integrated coastal management in southern Cuba. Ocean & Coastal Management 2020, 196, 105300. [CrossRef]
- Ferro-Azcona, H.; Espinoza-Tenorio, A.; Calderón-Contreras, R.; Ramenzoni, V.C.; País, M.d.L.M.G.; Mesa-Jurado, M.A. Adaptive capacity and social-ecological resilience of coastal areas: A systematic review. Ocean and Coastal Management 2019, 173, 36–51. [Google Scholar] [CrossRef]
- M.A.V. García and B.A. González, “MANGLAR VIVO EN CUBA: COSTOS Y BENEFICIOS DE LAS ACCIONES BASADAS EN ECOSISTEMAS. Análisis económico-ecológico en las provincias Sur Artemisa y Mayabeque. Revista Iberoamericana de Economía Ecológica 2021, 34, 86–110.
- Hernández Sampieri, R.; Fernández Collado, C.; Baptista Lucio, M.d.P. Metodología de la Investigación, Mexico: McGRAW-HILL, 2014. Sexta Edición. Impreso en México, p. 634. ISBN 978-1-4562-2396-0.
- Dahdouh-Guebas, F.; Mathenge, C.; Kairo, J.G.; Koedam, N. Exploitation of mangrove wood products from a subsistence perspective: a case study in Mida Creek, Kenya. Economic Botany 2000, 54, 513–527. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Collin, S.; Seen, D.L.; Rönnbäck, P.; Depommier, D.; Ravishankar, T.; Koedam, N. Analysing ethnobotanical and fishery-related importance of mangroves of the East-Godavari Delta (Andhra Pradesh, India) for conservation and management purposes. J. Ethnobiol. Ethnomedicine 2006, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Gallup, L.; Sonnenfeld, D.A.; Dahdouh-Guebas, F. Mangrove use and management within the Sine-Saloum Delta, Senegal. Ocean and Coastal Management 2020, 185, 105001. [Google Scholar] [CrossRef]
- Asante, F.; Hugé, J.; Asare, N.K.; Dahdouh-Guebas, F. Does mangrove vegetation structure reflect human utilisation of ecosystem goods and services? iScience 2023, 26, 106858. [Google Scholar] [CrossRef] [PubMed]
- Krkač, K. “Stakeholder Mapping”. In: S. Idowu, R. Schmidpeter, N. Capaldi, L. Zu, M. Del Baldo, R. Abreu (eds) “Encyclopedia of Sustainable Management”. Springer, Cham 2022. [CrossRef]
- Oficina Nacional de Estadísticas e Información (ONEI), “Anuario Estadístico de Cuba. Capítulo 1”: Territorio. Oficina Nacional de Estadísticas e información República de Cuba, la Habana, Cuba, 2021, p. 15.
- Worthington, P.S.E. Ermgassen, D.A. Friess, K.W. Krauss, C.E. Lovelock, J. Thorley, R. Tingey, C.D. Woodroffe, P. Bunting, N. Cormier, D. Lagomasino, R. Lucas, N.J. Murray, W.J. Sutherland, Spalding, M. A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Denis, D.; Cruz-Flores, D.D.; Testé, E. Biodiversity in Cuba. In Global Biodiversity; Apple Academic Press: 2018; pp. 139–176.
- Menéndez, L. The mangrove ecosystem in the Cuban archipelago: bases for its management.” Thesis in option for the scientific degree of Doctor in Forest Sciences, University of Alicante, Spain 2013.
- Martínez Quesada, E. Relación entre morfología foliar de antófitos y factores abióticos en las principales pluvisilvas de la Región Oriental cubana”. Rev. Biol. Trop. 2009, 57, 235–256. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification” updated, 2006.
- Reyes, O.J.; Acosta-Cantillo, F. Principales fitocenosis en el humedal del delta del río Cauto, Cuba Oriental. I. Vegetación lacustre y herbazal de humedal. For. Veracruzana 2007, 9, 15–22. [Google Scholar]
- M. Alonso-Tabet, M. López-Salcedo, R. Olano-Labrada, Y. Alarcón Jorge, A. Gil Rivero, Y. Alonso Jiménez, D. Egard Tamayo, O. Rodríguez González, Y. Borrero Nieves. “Plan de Manejo 2021-2025 Refugio de Fauna Ojo de Agua-Monte Cabaniguán. 2021. (Unpublished work).
- J. P. Cabrera-Díaz y L. A. Naranjo-Peña, “Estudio de vulnerabilidad de paisajes para la gestión ambiental del área protegida Reserva Ecológica Monte Cabaniguán-Ojo del Agua del municipio Jobabo, Las Tunas” pp. 172-181.
- W.V. Ferrera Bergues, O. Pérez Montero, O. Soler Nariño, “Población y vulnerabilidad social ante los efectos del cambio climático en el municipio costero de Guamá”. Novedades en Población 2020, 16, 190–217.
- O. Pérez, M.A. Carbonero, I. Poveda, M. Gómez, M.A. Oliver, “Cuando la mujer migra. Una mirada a las migraciones internas, desde la perspectiva del desarrollo sostenible, en el municipio costero de Guamá, Santiago de Cuba” Novedades en Población 2018, (28), 1-9. RNPS: 2106 ISSN: 1817-4078 No.28 julio-diciembre de 2018.
- Ferrera, A.; Pérez, O.; Soler, O. Población, cambio climático y percepción del riesgo en la región sur oriental” Santiago 2018, p 237-252 No. Especial https://santiago.uo.edu.cu/index.php/stgo/article/view/4618.
- L. Fernández-Rodríguez, S. L. Hechavarria, K. Mestril Cosme, J.A. Bouza Alonso; Y. Debros Trutié, “Plan de Manejo Refugio de Fauna San Miguel de Parada 2021-2025. 2021. (Unpublished work).
- Milanés, B.C.; Pérez, M.O. Ordenamiento y manejo integrado de la zona costera frente a los riesgos del cambio climático en la región Suroriental de Cuba. Revista Anales de la Academia de Ciencias de Cuba 2016. Vol 6 No3 2016 ISSN: 2304-0106 http://www.revistaccuba.cu/index.php/revacc/article/view/572.
- D. González-Rivera, I. Manet-Bombú, M. Aroche-Rodríguez, A. Frómeta-Barrera, I. Rodriguez-Munivel, A. Leiva Cueto. “Management Plan 2021-2025 Hatibonico Ecological Reserve. 2021. (Unpublished work).
- González Rivera, D. Conservación de Leptocereus nudiflorus en la Reserva Ecológica Hatibonico, Guantánamo. Bissea 2023, 15, 1. [Google Scholar]
- Cabrera Díaz, J.P.; Naranjo Peña, L.A. Estudio de vulnerabilidad de paisajes para la gestión ambiental del área protegida Reserva Ecológica Monte Cabaniguán-Ojo del Agua del municipio Jobabo. Las Tunas pp. 172–181 Estudios del Desarrollo Social: Cuba y América Latina RPNS 2346 ISSN 2308-0132 Vol. 7, No. Extraordinario, 2019.
- Ley 85 “Ley Forestal”. 1998. Gaceta Oficial de la República de Cuba. Edición Ordinaria Número No. 46: 773.
- Soto, H. Priorización de los ODS en Cuba: articulación de la Agenda 2030 con el Plan Nacional de Desarrollo e Identificación de prioridades de Desarrollo sostenible. Sede Subregional de la CEPAL en México (Estudios e Investigaciones) 49071, Naciones Unidas Comisión Económica para América Latina y el Caribe (CEPAL) 2023, p. 164.
- M.S. Uddin, M.A.R. Shah, S. Khanom, & M.K. Nesha, “Climate change impacts on the Sundarbans mangrove ecosystem services and dependent livelihoods in Bangladesh”. Asian J. Conserv. Biol. 2013, 2, 152–156.
- Queiroz, L.d.S.; Rossi, S.; Calvet-Mir, L.; Ruiz-Mallén, I.; García-Betorz, S.; Salvà-Prat, J.; Meireles, A.J.d.A. Neglected ecosystem services: Highlighting the socio-cultural perception of mangroves in decision-making processes. Ecosyst. Serv. 2017, 26, 137–145. [Google Scholar] [CrossRef]
- Reyes-Arroyo, N.; Camacho-Valdez, V.; Saenz-Arroyo, A.; Infante-Mata, D. Socio-cultural analysis of ecosystem services provided by mangroves in La Encrucijada Biosphere Reserve, southeastern Mexico. Local Environ. 2021, 26, 86–109. [Google Scholar] [CrossRef]
- Afonso, F.; Félix, P.M.; Chainho, P.; Heumüller, J.A.; de Lima, R.F.; Ribeiro, F.; Brito, A.C. Community perceptions about mangrove ecosystem services and threats. Reg. Stud. Mar. Sci. 2022, 49, 102114. [Google Scholar] [CrossRef]
- Mallick, B.; Priodarshini, R.; Kimengsi, J.N.; Biswas, B.; Hausmann, A.E.; Islam, S.; Huq, S.; Vogt, J. Livelihoods dependence on mangrove ecosystems: Empirical evidence from the Sundarbans. Curr. Res. Environ. Sustain. 2021, 3, 100077. [Google Scholar] [CrossRef]
- Warren-Rhodes, K.; Schwarz, A.-M.; Boyle, L.N.; Albert, J.; Agalo, S.S.; Warren, R.; Bana, A.; Paul, C.; Kodosiku, R.; Bosma, W. Mangrove ecosystem services and the potential for carbon revenue programs in the Solomon Islands. Environ. Conserv. 2011, 38, 485–496. [Google Scholar] [CrossRef]
- Song, A.M.; Dressler, W.H.; Satizábal, P.; Fabinyi, M. From conversion to conservation to carbon: The changing policy discourse on mangrove governance and use in the Philippines. J. Rural. Stud. 2021, 82, 184–195. [Google Scholar] [CrossRef]
- Chircop, A. Local Integrated Coastal Zone Management in Cuba (COSTASURESTE Project)”. Report to the Department of Foreign Affairs 2015, S06499. [Google Scholar]
- Golebie, E.J.; Aczel, M.; Bukoski, J.J.; Chau, S.; Ramirez-Bullon, N.; Gong, M.; Teller, N. A qualitative systematic review of governance principles for mangrove conservation. Conserv. Biol. 2021, 1–15. [Google Scholar] [CrossRef]
- Walker, J.E.; Ankersen, T.; Barchiesi, S.; Meyer, C.K.; Altieri, A.H.; Osborne, T.Z.; Angelini, C. Governance and the mangrove commons: Advancing the cross-scale, nested framework for the global conservation and wise use of mangroves. J. Environ. Manag. 2022, 312, 114823. [Google Scholar] [CrossRef]
- Alongi, D.M. Responses of mangrove ecosystems to climate change in the Anthropocene. In Mangroves: Ecology, Biodiversity and Management; Rastogi, R.P., Phulwaria, M., Gupta, D.K., Eds.; 2021, pp. 201–224.
- Iturralde, M.A.; Serrano Méndez, H. Peligros y Vulnerabilidades de la zona marino-costera de Cuba: estado actual y perspectivas ante el cambio climático hasta el 2100. Editorial Academia, La Habana, Cuba, 2015, p. 86. ISBN 978-959-270-338-4.
- Krauss, K.W.; Osland, M.J. Tropical cyclones and the organization of mangrove forests: a review. Ann. Bot. 2020, 125, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, G.; Herold, M.; De Sy, V. Drivers of deforestation and forest degradation: A synthesis report for EDD+ policymakers; Lexeme Consulting: Vancouver, BC, Canada, 2012. [Google Scholar]
- Madhav, S.; Nazneen, S.; Singh, P. Coastal Ecosystems Environmental importance, current challenges, and conservation measures”; Springer Nature Switzerland AG 2022; p. 392.
- Friess, D.A.; Adame, M.F.; Adams, J.B.; Lovelock, C.E. Mangrove Forests under Climate Change in a 2°C World. Wiley Interdiscip. Rev. Clim. Change 2022, 13, e792. [Google Scholar] [CrossRef]
Figure 1.
Map of eastern Cuba (inset: Cuba island) with mangrove localities under survey (Maps A, B, C and D). Sections with green shades show the mangrove areas while the red circles indicate the coastal villages that were surveyed.
Figure 1.
Map of eastern Cuba (inset: Cuba island) with mangrove localities under survey (Maps A, B, C and D). Sections with green shades show the mangrove areas while the red circles indicate the coastal villages that were surveyed.
Figure 2.
R. mangle and A.germinans mixed in Ojo de Agua-Monte Cabaniguán Fauna Refuge (Nico Koedam, 2020).
Figure 2.
R. mangle and A.germinans mixed in Ojo de Agua-Monte Cabaniguán Fauna Refuge (Nico Koedam, 2020).
Figure 3.
Rhizophora mangle stand in Guamá Sector (by Yanet Cruz Portorreal, 2023).
Figure 3.
Rhizophora mangle stand in Guamá Sector (by Yanet Cruz Portorreal, 2023).
Figure 4.
Stands with a low canopy of Avicennia germinans in San Miguel de Parada Wildlife Reserve (by Yanet Cruz Portorreal, 2023).
Figure 4.
Stands with a low canopy of Avicennia germinans in San Miguel de Parada Wildlife Reserve (by Yanet Cruz Portorreal, 2023).
Figure 5.
Low canopy stands of Rhizophora mangle in Hatibonico Ecological Reserve (by Hayler María Pérez Trejo, 2012).
Figure 5.
Low canopy stands of Rhizophora mangle in Hatibonico Ecological Reserve (by Hayler María Pérez Trejo, 2012).
Figure 6.
Percentage of responses related to the mangrove species identification per site.
Figure 6.
Percentage of responses related to the mangrove species identification per site.
Figure 8.
Perception of the studied communities regarding degradation, condition, changes in the mangroves and importance for their livelihood. A- Perception of mangrove degradation impact on the community, B- Perception of mangroves changes as a landscape, C- Perception of mangrove condition, D- Perception of the importance of the mangrove for the livelihood of the local people.
Figure 8.
Perception of the studied communities regarding degradation, condition, changes in the mangroves and importance for their livelihood. A- Perception of mangrove degradation impact on the community, B- Perception of mangroves changes as a landscape, C- Perception of mangrove condition, D- Perception of the importance of the mangrove for the livelihood of the local people.
Figure 10.
The Management System proposed for the mangrove governance in Cuba, particularly southeastern Cuba, studied in this work. Photos credit Nico Koedam (top left) and Yanet Cruz Portorreal (bottom right).
Figure 10.
The Management System proposed for the mangrove governance in Cuba, particularly southeastern Cuba, studied in this work. Photos credit Nico Koedam (top left) and Yanet Cruz Portorreal (bottom right).
Table 1.
Mangrove SES localities: status, extent, mangrove type, resp. communities, population size and general appraisal of pollution status.
Table 1.
Mangrove SES localities: status, extent, mangrove type, resp. communities, population size and general appraisal of pollution status.
| Mangrove site |
Management classification (SNAP) |
Administration |
Extension
(ha) |
Mangrove typology
[52] |
Community |
Population (number of respondents) |
| MCOA |
Faunal Refuge |
Enterprise for Flora and Faunal Protection Las Tunas |
3929.18 |
Deltaic |
Sabalo |
596
(83) |
| Palo Seco |
| Cuarenta pesos |
| GUAM |
Multiple use area |
No management regime |
162 |
Bay
Open coast
estuary |
Santa Rosa de Sevilla
Cruce de Sevilla
Bahía Larga
El Mazo
Tabacal |
1525
(92) |
| SAM |
Faunal Refuge |
Enterprise for Flora and Faunal Protection, Santiago de Cuba |
55 |
Bay
|
Punta de Sal |
267
(72) |
| Caimán Chico |
| HAT |
Ecological Reserve |
Ministry of Science Technology and Environment (CITMA) |
64 |
Open coast
|
Hatibonico
13 de Agosto |
1003
(87) |
| |
Number of questionnaires |
334 |
Table 2.
Distribution of the demographic profile of respondents (in percentage, n=334).
Table 2.
Distribution of the demographic profile of respondents (in percentage, n=334).
| Indicators |
Percentage |
| MCOA |
GUAM |
SAM |
HAT |
| Age groups |
17.7 |
20.7 |
30.6 |
14.9 |
| 18-30 |
| f31-40 |
28.9 |
19.6 |
16.7 |
25.3 |
| 41-50 |
24.1 |
18.5 |
16.7 |
25.3 |
| 51-60 |
16.9 |
23.9 |
16.7 |
20.7 |
| 61-70 |
7.2 |
10.9 |
15.3 |
6.9 |
| 71-80 |
7.2 |
6.5 |
4.2 |
6.9 |
| Gender |
|
|
|
|
| Male |
50.6 |
53.3 |
57.2 |
56.3 |
| Female |
49.4 |
46.7 |
45.8 |
43.7 |
| Occupation |
|
|
|
|
| State sector |
20.5 |
41.3 |
51.4 |
60.9 |
| Private sector |
13.3 |
14.2 |
15.3 |
14.9 |
| Farmers |
35.0 |
15.2 |
4.2 |
1.2 |
| Retired |
7.2 |
9.8 |
8.3 |
8.1 |
| Housewife |
24.0 |
19.5 |
20.8 |
14.9 |
Table 3.
Percentage distribution of respondents at each level of importance of mangrove ecosystem services.
Table 3.
Percentage distribution of respondents at each level of importance of mangrove ecosystem services.
| Ecosystem services by mangrove localities |
Importance level (%) |
| Not important |
Important |
Very important |
Monte
Cabaniguán/Ojo de agua
(MCOA)
|
1. Nursery |
3.6 |
7.2 |
89.2 |
| 2. Wildlife habitat |
|
|
100 |
| 3. Food source |
1.2 |
10.8 |
88.0 |
| 4. Coastal protection |
7.2 |
18.1 |
74.7 |
| 5. Water purification |
7.2 |
7.2 |
84.3 |
| 6. Carbon sequestration |
45.8 |
18.1 |
36.1 |
| 7. Recreation/education |
10.8 |
28.3 |
65.2 |
| 8. Natural medicine |
7.2 |
7.2 |
85.5 |
Guama
(GUAM)
|
1. Nursery |
- |
1.1 |
98.9 |
| 2. Wildlife habitat |
- |
- |
100 |
| 3. Food source |
9.8 |
7.6 |
82.6 |
| 4. Coastal protection |
2.2 |
2.2 |
95.7 |
| 5. Water purification |
6.5 |
22.8 |
70.7 |
| 6. Carbon sequestration |
40.2 |
23.9 |
35.9 |
| 7. Recreation/education |
6.5 |
28.3 |
65.2 |
| 8. Natural medicine |
2.2 |
12.0 |
85.9 |
San Miguel de Parada
(SAM)
|
1. Nursery |
6.9 |
23.6 |
69.4 |
| 2. Fauna habitat |
5.6 |
22.2 |
72.2 |
| 3. Food source |
27.8 |
47.2 |
25.0 |
| 4. Coastal protection |
6.9 |
27.8 |
65.3 |
| 5. Water purification |
12.5 |
27.8 |
59.7 |
| 6. Carbon sequestration |
41.7 |
31.9 |
26.4 |
| 7. Recreation/education |
8.3 |
50.0 |
41.7 |
| 8. Natural medicine |
6.9 |
55.6 |
37.5 |
Hatibonico
(HAT)
|
1. Nursery |
26.4 |
20.7 |
52.9 |
| 2. Fauna habitat |
17.2 |
32.2 |
50.6 |
| 3. Food source |
35.6 |
20.7 |
43.7 |
| 4. Coastal protection |
34.5 |
28.7 |
36.8 |
| 5. Water purification |
32.2 |
28.7 |
39.1 |
| 6. Carbon sequestration |
43.7 |
27.6 |
28.7 |
| 7. Recreation/education |
34.5 |
28.7 |
36.8 |
| 8. Natural medicine |
33.3 |
19.5 |
47.1 |
Table 4.
Threats to the mangrove perceived in the sites studied.
Table 4.
Threats to the mangrove perceived in the sites studied.
| |
Answers (%) |
| Mangrove threats/localities |
MCOA |
GUAM |
SAM |
HAT |
| Climate change |
24.6 |
18.5 |
19.6 |
31.9 |
| Tropical cyclones |
- |
16.1 |
9.0 |
- |
| Fires |
14.4 |
- |
- |
24.5 |
| Drought |
16.3 |
5.5 |
20.1 |
39.3 |
| Rising sea level |
- |
1.4 |
0.5 |
- |
| Salinization |
4.2 |
12.5 |
4.2 |
- |
| Logging |
24.2 |
24.7 |
12.7 |
- |
| Pollution/waste dumping |
6.4 |
9.9 |
11.6 |
- |
| River damming |
3.0 |
- |
4.2 |
- |
| Road construction |
4.8 |
4.8 |
- |
- |
| Agriculture |
- |
5.1 |
- |
- |
| Exotic species |
5.3 |
4.1 |
- |
4.3 |
| Industries |
- |
- |
18.0 |
- |
| Bark and roots removal |
- |
6.8 |
- |
- |
| Grazing |
- |
4.5 |
- |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).