Submitted:
04 July 2024
Posted:
05 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Screening and Informed Consent
2.2. Study Procedure and Data Collection
| Psychosomatic assessment |
Insomnia Severity Index (ISI) for measuring insomnia and sleep difficulties [19] |
| Fatigue Severity Scale (FSS) for measuring fatigue [20] | |
| Patient Health Questionnaire (PHQ-SADS) for measuring depression (PHQ-9), anxiety (GAD-7), and somatic symptoms (PHQ-15) [21] | |
| Hospital Anxiety and Depression Scale (HADS) for measuring depression and anxiety [22] | |
| Impact of Event Scale-Revised (IES-R) for measuring posttraumatic stress [23] | |
| 5-Level EQ-5D version (EQ-5D-5L) for measuring quality of life [24] | |
| Respiratory assessment | Current and persisting COVID-19 symptoms |
| Pulmonary function (spirometry, plethysmography, DLCO) | |
| Exercise tolerance (6-minute walk test; 6MWT) | |
| Sleep architecture (home overnight polygraphy with WatchPATTM) [18] | |
| Neurocognitive assessment | Montreal Cognitive Assessment (MoCA) for measuring cognitive dysfunction [25] |
| Bayer Activities of Daily Living Scale (B-ADL) for measuring deficits in the performance of everyday activitie [26] | |
| Digit Symbol Substitution Test (DSST) for measuring global cognitive function, especially attention, processing speed, and executive function [27] | |
| Clinical neurological examination for detection of a cerebral or neurodegenerative disease (yes/no). Assessment of overall cognitive performance: by neurologists (normal/borderline/impaired), subjective (same/worse), and by relatives (same/worse pre-post COVID-19) |
2.3. Statistical Analyses
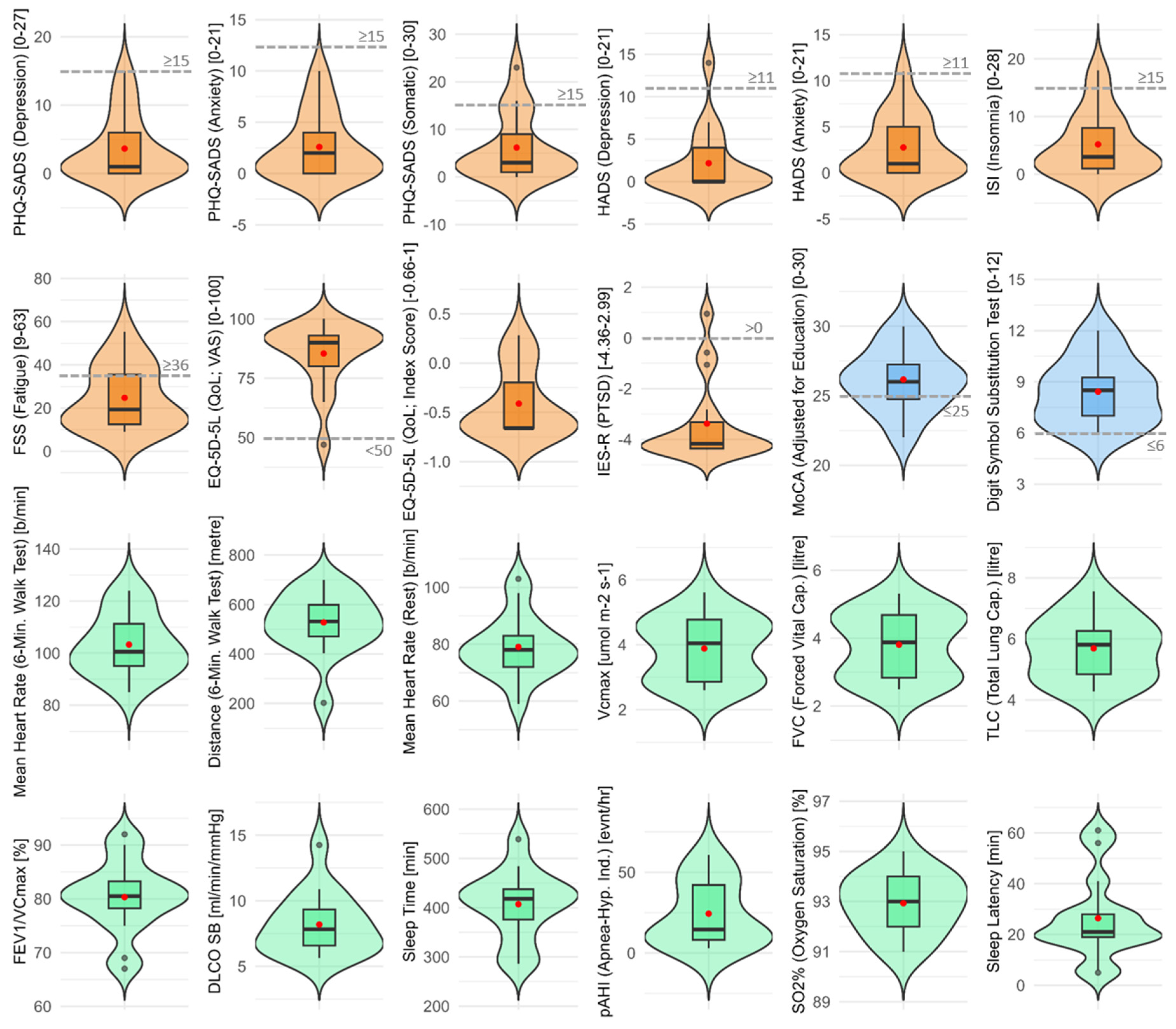
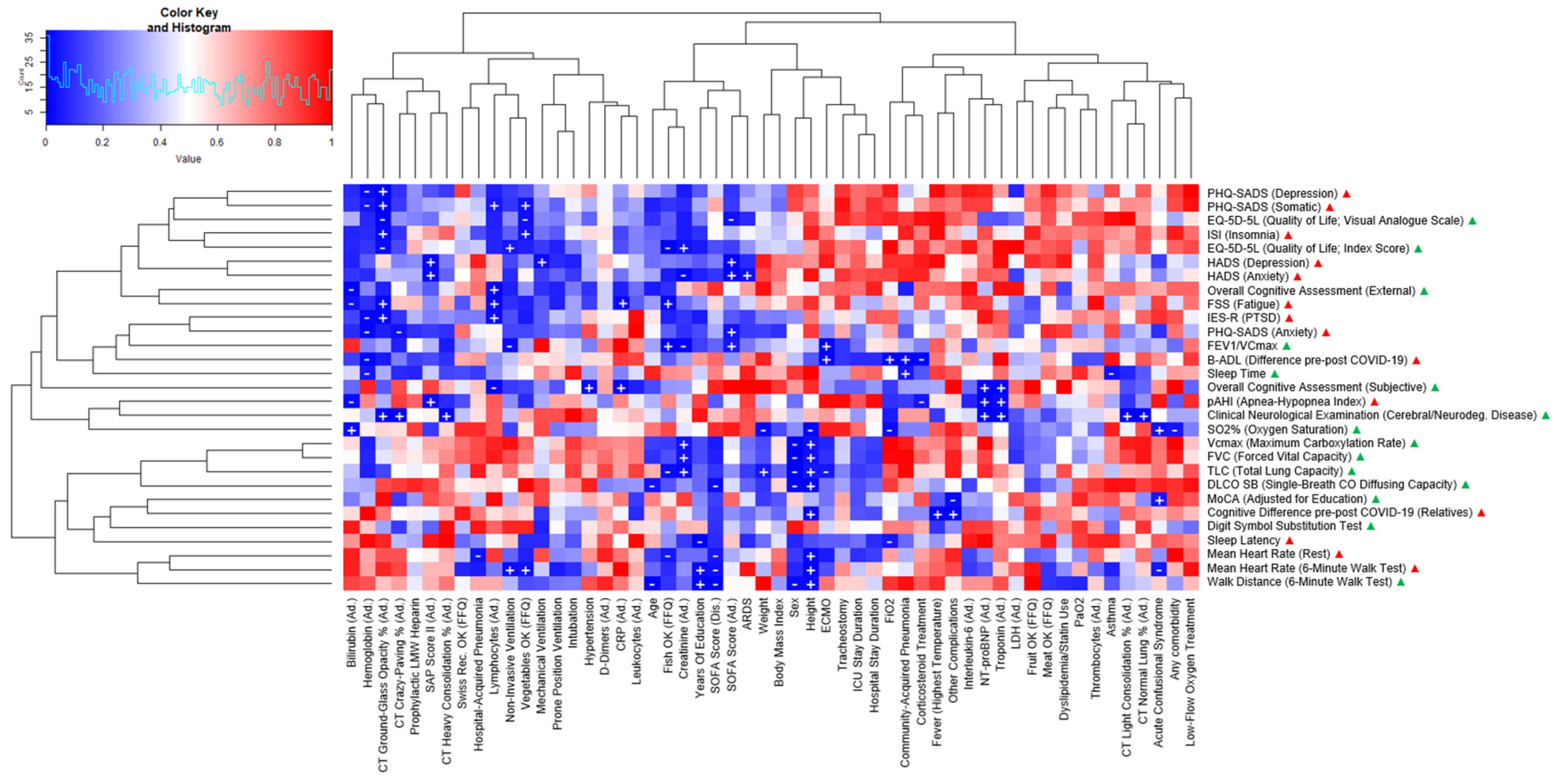
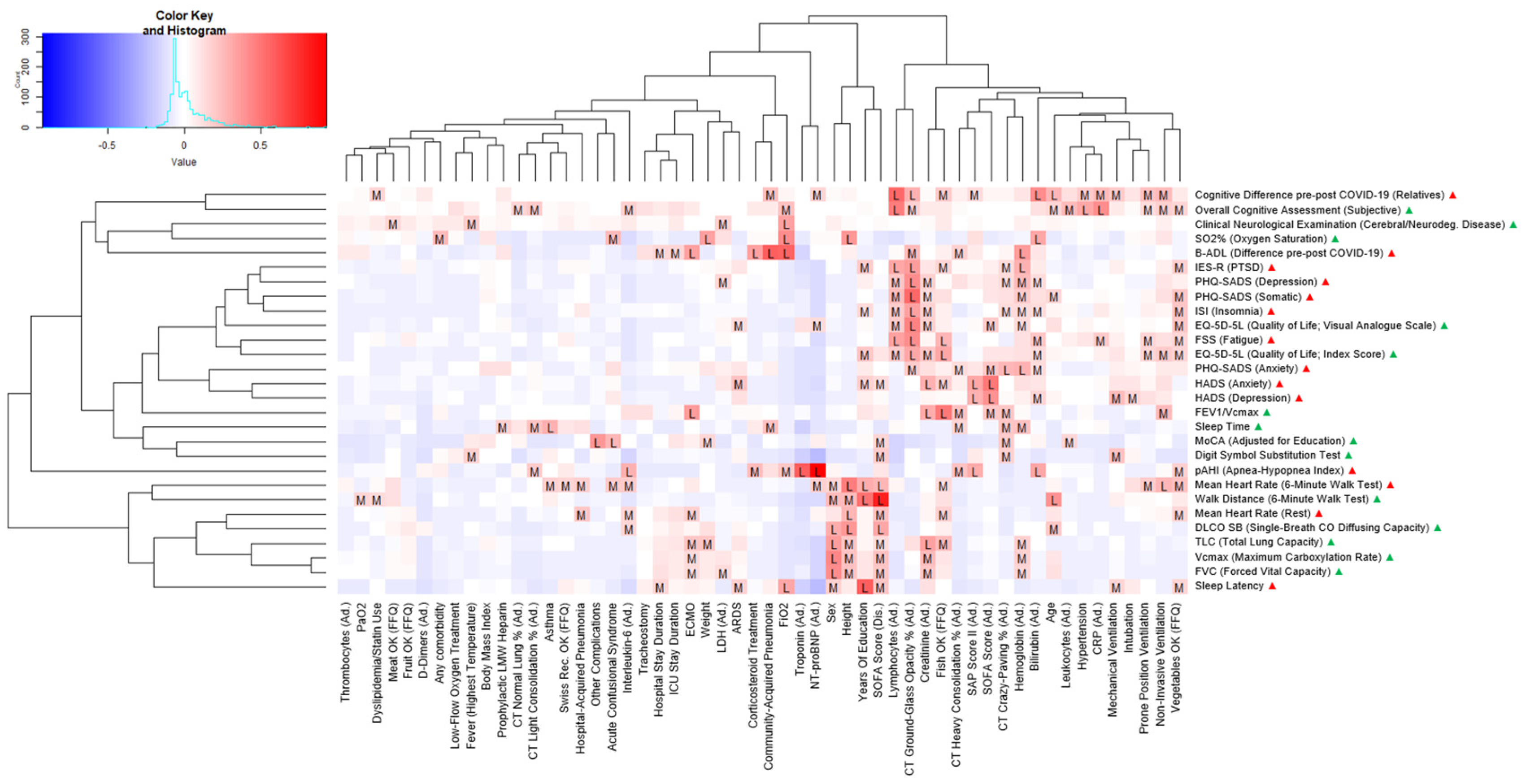
3. Results
| PATIENT CHARACTERISTICS | CASES (N = 17) |
|---|---|
|
Demographics Male sex, n (%) |
13 (76.5) |
| Age, years, mean (SD) | 60 (11.4) |
| 18-30 years, n (%) 31-45 years, n (%) 46-60 years, n (%) 61-75 years, n (%) >75 years, n (%) |
0 (0) 2 (11.8) 7 (41.2) 8 (47) 0 (0) |
| Weight, kg, mean (SD) Height, cm, mean (SD) Body mass index (BMI), kg/m2, mean (SD) Years of primary and secondary education, mean (SD) |
90.8 (18) 172.2 (7.3) 30.5 (5.2) 11.4 (3.3) |
|
Personal medical history, n (%) Any comorbidity Hypertension Cardiovascular disease Chronic lung disease Asthma Dyslipidemia/statin use |
13 (76.5) 9 (52.9) 1 (5.9) 2 (11.8) 2 (11.8) 3 (17.6) |
|
Symptoms at COVID-19 onset, n (%) Fever Cough Headache Night sweats Chills Shivering Myalgia Joint pain Dyspnea Inspiratory chest pain Retrosternal chest pain Loss of appetite Weight loss |
10 (58.8) 13 (76.5) 11 (64.7) 9 (52.9) 10 (58.8) 10 (58.8) 8 (47.1) 8 (47.1) 9 (52.9) 10 (58.8) 9 (52.9) 10 (58.8) 8 (47.1) |
|
Findings on lung CT scans at ICU admission, mean (SD) Ground-glass opacity in % of normal lung Crazy-paving in % of normal lung Light consolidation in % of normal lung Heavy consolidation in % of normal lung |
39.0 (4.3) 26.9 (5.8) 8.0 (4.1) 0.4 (0.2) |
| Laboratory data at ICU admission, mean (SD) | |
| Hemoglobin in g/l Thrombocytes in G/l1 Troponin in ng/l2 NT-proBNP in ng/l Creatinine in umol/l3 Bilirubin in umol/l CRP in mg/l Leukocytes in G/l Interleukin-6 in pg/ml4 Lymphocytes in G/l D-dimers in ng/ml LDH in IU/l |
128.4 (18.2) 271.1 (117.2) 30.5 (49.7) 758.6 (615.7) 73.2 (24.4) 9.3 (6.1) 208.1 (93.6) 10.2 (3.6) 103.9 (119.3) 0.9 (0.5) 2045 (1384.5) 602.6 (224.8) |
| Clinical scores, syndromes and complications during ICU stay | |
| Admission SOFA5 score (SD) Discharge SOFA score (SD) Admission SAPS6 II (SD) Hospital-acquired pneumonia (HAP) more than 48h after hospital admission, n (%) Community-acquired pneumonia (CAP) at hospital admission or within 48h (other than COVID-19), n (%) Acute confusional syndrome, n (%) Acute respiratory distress syndrome (ARDS), n (%) Other complications (acute kidney/hepatic injury, septic shock), n (%) Partial arterial oxygen pressure (PaO2) in mmHg (mean, SD) Fraction of inspired oxygen (FiO2) in % (mean, SD) Highest body temperature in °C (mean, SD) ICU stay duration in days (mean, SD) Hospital stay duration in days (mean, SD) Intubation duration in mechanically ventilated patients in days (mean, SD) |
5.8 (3.4) 2.5 (0.9) 33.6 (12.7) 3 (17.6) 2 (11.8) 5 (29.4) 10 (58.8) 3 (17.6) 59.3 (8.0) 53.1 (18.4) 38.2 (0.8) 13 (9.5) 23 (12.6) 10 (4.1) |
|
ICU treatment, n (%) Corticosteroid treatment Noradrenalin treatment Prophylactic LMW heparin Low-flow oxygen treatment Non-invasive ventilation (NIV) Mechanical ventilation Extracorporeal membrane oxygenation (ECMO) Intubation Prone position ventilation Tracheostomy Hemofiltration/hemodialysis |
15 (88.2) 5 (29.4) 12 (70.6) 3 (17.6) 5 (29.4) 7 (41.2) 2 (11.8) 9 (52.9) 8 (47.1) 3 (17.6) 0 (0) |
|
Nutritional habits at COVID-19 onset Fruit OK (≥2/day) Vegetables OK (≥3/day) Meat OK (≤5/week) Fish OK (≥1/week) Swiss recommendations OK (≥3 of the 4 recommendations above) |
9 (52.9%) 6 (35.3%) 9 (52.9%) 8 (47.1%) 6 (35.3%) |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | 109/l (giga/l) |
| 2 | 109 gram/l (nanogram/l) |
| 3 | 106 mol/l (micromole/l) |
| 4 | 1012 gram/ml (picogram/ml) |
| 5 | Sequential organ failure assessment score |
| 6 | Simplified acute physiology score |
References
- Eythorsson, E.; Helgason, D.; Ingvarsson, R. F.; Bjornsson, H. K.; Olafsdottir, L. B.; Bjarnadottir, V.; Runolfsdottir, H. L.; Bjarnadottir, S.; Agustsson, A. S.; Oskarsdottir, K.; Thorvaldsson, H. H.; Kristjansdottir, G.; Armannsdottir, B.; Bjarnason, A.; Johannsson, B.; Gudlaugsson, O.; Gottfredsson, M.; Sigurdsson, M. I.; Indridason, O. S.; Palsson, R. Clinical Spectrum of Coronavirus Disease 2019 in Iceland: Population Based Cohort Study. BMJ 2020, 371, m4529. [Google Scholar] [CrossRef]
- Anderegg, N.; Panczak, R.; Egger, M.; Low, N.; Riou, J. Survival among People Hospitalized with COVID-19 in Switzerland: A Nationwide Population-Based Analysis. BMC Med 2022, 20, 164. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; Cheng, Z.; Yu, T.; Xia, J.; Wei, Y.; Wu, W.; Xie, X.; Yin, W.; Li, H.; Liu, M.; Xiao, Y.; Gao, H.; Guo, L.; Xie, J.; Wang, G.; Jiang, R.; Gao, Z.; Jin, Q.; Wang, J.; Cao, B. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Carola, V.; Vincenzo, C.; Morale, C.; Pelli, M.; Rocco, M.; Nicolais, G. Psychological Health in COVID-19 Patients after Discharge from an Intensive Care Unit. Front Public Health 2022, 10, 951136. [Google Scholar] [CrossRef] [PubMed]
- Aguila, E. J. T.; Lontok, M. A. D.; Francisco, C. P. D. Follow Your Gut: Challenges in Nutritional Therapy During the COVID-19 Pandemic. Clin Gastroenterol Hepatol 2020, 18, 2638–2639. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S. C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P.; endorsed by the ESPEN Council. ESPEN Expert Statements and Practical Guidance for Nutritional Management of Individuals with SARS-CoV-2 Infection. Clin Nutr 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- PHOSP-COVID Collaborative Group. Clinical Characteristics with Inflammation Profiling of Long COVID and Association with 1-Year Recovery Following Hospitalisation in the UK: A Prospective Observational Study. Lancet Respir Med 2022, 10, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zang, C.; Xu, Z.; Zhang, Y.; Xu, J.; Bian, J.; Morozyuk, D.; Khullar, D.; Zhang, Y.; Nordvig, A. S.; Schenck, E. J.; Shenkman, E. A.; Rothman, R. L.; Block, J. P.; Lyman, K.; Weiner, M. G.; Carton, T. W.; Wang, F.; Kaushal, R. Data-Driven Identification of Post-Acute SARS-CoV-2 Infection Subphenotypes. Nat Med 2023, 29, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sahanic, S.; Tymoszuk, P.; Ausserhofer, D.; Rass, V.; Pizzini, A.; Nordmeyer, G.; Hüfner, K.; Kurz, K.; Weber, P. M.; Sonnweber, T.; Boehm, A.; Aichner, M.; Cima, K.; Boeckle, B.; Holzner, B.; Rumpold, G.; Puelacher, C.; Kiechl, S.; Huber, A.; Wiedermann, C. J.; Sperner-Unterweger, B.; Tancevski, I.; Bellmann-Weiler, R.; Bachler, H.; Piccoliori, G.; Helbok, R.; Weiss, G.; Loeffler-Ragg, J. Phenotyping of Acute and Persistent Coronavirus Disease 2019 Features in the Outpatient Setting: Exploratory Analysis of an International Cross-Sectional Online Survey. Clinical Infectious Diseases 2022, 75, e418–e431. [Google Scholar] [CrossRef] [PubMed]
- CDC. Post-COVID Conditions. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 12 May 2024).
- Soriano, J. B.; Murthy, S.; Marshall, J. C.; Relan, P.; Diaz, J. V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect Dis 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus disease (COVID-19): Post COVID-19 condition. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 12 May 2024).
- Pfaff, E. R.; Girvin, A. T.; Bennett, T. D.; Bhatia, A.; Brooks, I. M.; Deer, R. R.; Dekermanjian, J. P.; Jolley, S. E.; Kahn, M. G.; Kostka, K.; McMurry, J. A.; Moffitt, R.; Walden, A.; Chute, C. G.; Haendel, M. A.; Consortium, T. N. Who Has Long-COVID? A Big Data Approach [Preprint]. medRxiv 2021. [Google Scholar] [CrossRef]
- Albrich, W. C.; Ghosh, T. S.; Ahearn-Ford, S.; Mikaeloff, F.; Lunjani, N.; Forde, B.; Suh, N.; Kleger, G.-R.; Pietsch, U.; Frischknecht, M.; Garzoni, C.; Forlenza, R.; Horgan, M.; Sadlier, C.; Negro, T. R.; Pugin, J.; Wozniak, H.; Cerny, A.; Neogi, U.; O’Toole, P. W.; O’Mahony, L. A High-Risk Gut Microbiota Configuration Associates with Fatal Hyperinflammatory Immune and Metabolic Responses to SARS-CoV-2. Gut Microbes 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Baz, Y. E.; Scanferla, G.; Graf, N.; Waldeck, F.; Kleger, G.-R.; Frauenfelder, T.; Bremerich, J.; Kobbe, S. S.; Pagani, J.-L.; Schindera, S.; Conen, A.; Wildermuth, S.; Leschka, S.; Strahm, C.; Waelti, S.; Dietrich, T. J.; Albrich, W. C. Comparison of Temporal Evolution of Computed Tomography Imaging Features in COVID-19 and Influenza Infections in a Multicenter Cohort Study. European Journal of Radiology Open 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Chung, M.; Bernheim, A.; Keir, G.; Mei, X.; Huang, M.; Li, S.; Kanne, J. P. Computed Tomography Features of Coronavirus Disease 2019 (COVID-19): A Review for Radiologists. J Thorac Imaging 2020, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, D.; Guessous, I.; Vaucher, J.; Preisig, M.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Low Compliance with Dietary Recommendations for Food Intake among Adults. Clin Nutr 2013, 32, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Pang, K. P.; Gourin, C. G.; Terris, D. J. A Comparison of Polysomnography and the WatchPAT in the Diagnosis of Obstructive Sleep Apnea. Otolaryngol Head Neck Surg 2007, 137, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Morin, C. M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Valko, P. O.; Bassetti, C. L.; Bloch, K. E.; Held, U.; Baumann, C. R. Validation of the Fatigue Severity Scale in a Swiss Cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R. L.; Williams, J. B. W.; Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A Systematic Review. Gen Hosp Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A. S., & Snaith. The Hospital Anxiety and Depression Scale, 1983. [CrossRef]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A Measure of Subjective Stress. Psychosom Med 1979, 41, 209–218. [Google Scholar] [CrossRef] [PubMed]
- EQ-5D-5L – EQ-5D. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed 2023-12-04).
- Nasreddine, Z. S.; Phillips, N. A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J. L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J Am Geriatr Soc 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, I.; Lehfeld, H.; de Jongh, P.; Erzigkeit, H. The Bayer Activities of Daily Living Scale (B-ADL). Dement Geriatr Cogn Disord 1998, 9 Suppl 2, 20–26. [Google Scholar] [CrossRef]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J Clin Psychopharmacol 2018, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A General Power Analysis Program. Behavior Research Methods, Instruments, & Computers 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Buuren, S. V.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Soft. 2011, 45. [Google Scholar] [CrossRef]
- Von Hippel, P. T. 4. Regression with Missing Ys: An Improved Strategy for Analyzing Multiply Imputed Data. Sociological Methodology 2007, 37, 83–117. [Google Scholar] [CrossRef]
- Wickham, H. (2016). Ggplot2: Elegant Graphics for Data Analysis. https:\ggplot2.tidyverse.o.
- Warnes, Maintainer Gregory R., et al. “Package ‘Gplots’.” Various R Programming Tools for Plotting Data.
- Team, R. R: A Language and Environment for Statistical Computing. MSOR connections 2014. [Google Scholar]
- Armstrong, R. A. When to Use the Bonferroni Correction. Ophthalmic and Physiological Optics 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M. R.; Olmstead, R.; Carroll, J. E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.; Vgontzas, A. N.; Fernandez-Mendoza, J.; He, F.; Calhoun, S. L.; Liao, D.; Bixler, E. O. Increased Inflammation from Childhood to Adolescence Predicts Sleep Apnea in Boys: A Preliminary Study. Brain Behav Immun 2017, 64, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Jokela, M.; Virtanen, M.; Batty, G. D.; Kivimäki, M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatry 2016, 73, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Orre, I. J.; Reinertsen, K. V.; Aukrust, P.; Dahl, A. A.; Fosså, S. D.; Ueland, T.; Murison, R. Higher Levels of Fatigue Are Associated with Higher CRP Levels in Disease-Free Breast Cancer Survivors. J Psychosom Res 2011, 71, 136–141. [Google Scholar] [CrossRef]
- Cho, H. J.; Seeman, T. E.; Bower, J. E.; Kiefe, C. I.; Irwin, M. R. Prospective Association between C-Reactive Protein and Fatigue in the Coronary Artery Risk Development in Young Adults Study. Biol Psychiatry 2009, 66, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front Immunol 2017, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H. K.; Al-Rubaye, H. T.; Al-Hadrawi, D. S.; Almulla, A. F.; Maes, M. Long-COVID Post-Viral Chronic Fatigue and Affective Symptoms Are Associated with Oxidative Damage, Lowered Antioxidant Defenses and Inflammation: A Proof of Concept and Mechanism Study. Molecular Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K. G.; Heuschmann, P. U.; Hildesheim, H.; Hinz, A.; Hopff, S.; Horn, A.; Krawczak, M.; Krist, L.; Kudelka, J.; Lieb, W.; Maetzler, C.; Mehnert-Theuerkauf, A.; Montellano, F. A.; Morbach, C.; Schmidt, S.; Schreiber, S.; Steigerwald, F.; Störk, S.; Maetzler, W.; Finke, C. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. EClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef] [PubMed]
- Swanink, C. M.; Vercoulen, J. H.; Galama, J. M.; Roos, M. T.; Meyaard, L.; van der Ven-Jongekrijg, J.; de Nijs, R.; Bleijenberg, G.; Fennis, J. F.; Miedema, F.; van der Meer, J. W. Lymphocyte Subsets, Apoptosis, and Cytokines in Patients with Chronic Fatigue Syndrome. J Infect Dis 1996, 173, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol Immunol 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Naudé, P. J. W.; Roest, A. M.; Stein, D. J.; de Jonge, P.; Doornbos, B. Anxiety Disorders and CRP in a Population Cohort Study with 54,326 Participants: The LifeLines Study. World J Biol Psychiatry 2018, 19, 461–470. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Cardoso, T.; Silva, R. H.; Fernandes, J. L.; Arent, C. O.; Amboni, G.; Borba, L. A.; Padilha, A. P. Z.; Botelho, M. E. M.; Maciel, A. L.; Barichello, T.; Morales, R.; Soares, S. J. B.; Bagatini, M. D.; Dallagnol, C.; Brighenti, M. E.; Ignácio, Z. M.; Quevedo, J.; Ceretta, L. B.; Réus, G. Z. Stress Levels, Psychological Symptoms, and C-Reactive Protein Levels in COVID-19: A Cross-Sectional Study. J Affect Disord 2023, 330, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, O. J.; Friedman, L. A.; Colantuoni, E.; Dinglas, V. D.; Sepulveda, K. A.; Mendez-Tellez, P.; Shanholz, C.; Pronovost, P. J.; Needham, D. M. Psychiatric Symptoms after Acute Respiratory Distress Syndrome: A 5-Year Longitudinal Study. Intensive Care Med 2018, 44, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J. A.; Krall, J. T. W.; Belfield, L. T.; Files, D. C. Long-Term Outcomes in Acute Respiratory Distress Syndrome: Epidemiology, Mechanisms, and Patient Evaluation. Crit Care Clin 2021, 37, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Tomlinson, G.; Chu, L.; Robles, P.; Matte, A.; Burns, S.; Thomas, C.; Lamontagne, F.; Adhikari, N. K. J.; Ferguson, N.; Friedrich, J. O.; Rudkowski, J. C.; Skrobik, Y.; Meggison, H.; Cameron, J.; Herridge, M.; Herridge, M. S.; Chu, L. M.; Matte, A.; Tomlinson, G.; Chan, L.; Thomas, C.; Friedrich, J. O.; Mehta, S.; Lamontagne, F.; Levasseur, M.; Ferguson, N. D.; Adhikari, N. K. J.; Rudkowski, J. C.; Meggison, H.; Skrobik, Y.; Flannery, J.; Bayley, M.; Batt, J.; Santos, C. dos; Abbey, S. E.; Tan, A.; Lo, V.; Mathur, S.; Parotto, M.; Morris, D.; Flockhart, L.; Fan, E.; Lee, C. M.; Wilcox, M. E.; Ayas, N.; Choong, K.; Fowler, R.; Scales, D. C.; Sinuff, T.; Cuthbertson, B. H.; Rose, L.; Robles, P.; Burns, S.; Cypel, M.; Singer, L.; Chaparro, C.; Chow, C.-W.; Keshavjee, S.; Brochard, L.; Hebert, P.; Slutsky, A. S.; Marshall, J. C.; Cook, D.; Cameron, J. I. Determinants of Depressive Symptoms at 1 Year Following ICU Discharge in Survivors of ≥ 7 Days of Mechanical Ventilation: Results From the RECOVER Program, a Secondary Analysis of a Prospective Multicenter Cohort Study. Chest 2019, 156, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Lever-van Milligen, B. A.; Vogelzangs, N.; Smit, J. H.; Penninx, B. W. J. H. Hemoglobin Levels in Persons with Depressive and/or Anxiety Disorders. J Psychosom Res 2014, 76, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Yeh, H.-L.; Tsai, S.-J. Association of Lower Hemoglobin Levels with Depression, Though Not with Cognitive Performance, in Healthy Elderly Men. Psychiatry Clin Neurosci 2012, 66, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, M.; Kumari, M.; Steptoe, A. Sleep and Biomarkers in the English Longitudinal Study of Ageing: Associations with C-Reactive Protein, Fibrinogen, Dehydroepiandrosterone Sulfate and Hemoglobin. Psychoneuroendocrinology 2013, 38, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; El Salamony, O. Depression, Quality of Life and Malnutrition-Inflammation Scores in Hemodialysis Patients. Am J Nephrol 2008, 28, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Simic Ogrizovic, S.; Jovanovic, D.; Dopsaj, V.; Radovic, M.; Sumarac, Z.; Bogavac, S. N.; Stosovic, M.; Stanojevic, M.; Nesic, V. Could Depression Be a New Branch of MIA Syndrome? Clin Nephrol 2009, 71, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Ciciarelli, C.; Di Stasio, E.; Conte, G. L.; Vulpio, C.; Luciani, G.; Tazza, L. Correlates of Symptoms of Depression and Anxiety in Chronic Hemodialysis Patients. Gen Hosp Psychiatry 2010, 32, 125–131. [Google Scholar] [CrossRef]
- Ringdal, M.; Plos, K.; Lundberg, D.; Johansson, L.; Bergbom, I. Outcome after Injury: Memories, Health-Related Quality of Life, Anxiety, and Symptoms of Depression after Intensive Care. J Trauma 2009, 66, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Mazeraud, A.; Polito, A.; Sivanandamoorthy, S.; Porcher, R.; Heming, N.; Stoclin, A.; Hissem, T.; Antona, M.; Blot, F.; Gaillard, R.; Chrétien, F.; Annane, D.; Bozza, F. A. B.; Siami, S.; Sharshar, T.; Groupe d’Explorations Neurologiques en Réanimation (GENER). Association Between Anxiety and New Organ Failure, Independently of Critical Illness Severity and Respiratory Status: A Prospective Multicentric Cohort Study. Crit Care Med 2020, 48, 1471–1479. [Google Scholar] [CrossRef]
- Sasaki, N.; Yamamoto, H.; Ozono, R.; Maeda, R.; Kihara, Y. Sleeping Difficulty and Subjective Short Sleep Duration Are Associated with Serum N-Terminal Pro-Brain Natriuretic Peptide Levels in the Elderly Population. Intern Med 2020, 59, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F. P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M. A. Sleep Duration Predicts Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. Eur Heart J 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, L. E.; Vatten, L. J.; Platou, C.; Janszky, I. Insomnia and the Risk of Acute Myocardial Infarction.
- Li, Y.; Zhang, X.; Winkelman, J. W.; Redline, S.; Hu, F. B.; Stampfer, M.; Ma, J.; Gao, X. Association Between Insomnia Symptoms and Mortality: A Prospective Study of US Men. Circulation 2014, 129, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Laugsand, L. E.; Strand, L. B.; Platou, C.; Vatten, L. J.; Janszky, I. Insomnia and the Risk of Incident Heart Failure: A Population Study. Eur Heart J 2014, 35, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.-H.; El Mokhtari, N. E.; Freitag, S.; Rausche, T.; Göder, R.; Tiroke, A.; Lins, M.; Simon, R.; Bewig, B. NT-proBNP Is Not Elevated in Patients with Obstructive Sleep Apnoea. Respir Med 2008, 102, 134–142. [Google Scholar] [CrossRef]
- Tasci, S.; Manka, R.; Scholtyssek, S.; Lentini, S.; Troatz, C.; Stoffel-Wagner, B.; Lüderitz, B. NT-pro-BNP in Obstructive Sleep Apnea Syndrome Is Decreased by Nasal Continuous Positive Airway Pressure. Clin Res Cardiol 2006, 95, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Vartany, E.; Imevbore, M.; O’Malley, M.; Manfredi, C.; Pasquarella, C.; Scinto, L.; Fine, J. N-terminal Pro-brain Natriuretic Peptide for Detection of Cardiovascular Stress in Patients with Obstructive Sleep Apnea Syndrome. Journal of Sleep Research 2006, 15, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, A. I.; Hamer, M.; Gaze, D.; Collinson, P.; Rumley, A.; Lowe, G.; Steptoe, A. The Interaction between Systemic Inflammation and Psychosocial Stress in the Association with Cardiac Troponin Elevation: A New Approach to Risk Assessment and Disease Prevention. Preventive Medicine 2016, 93, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, A. I.; Hamer, M.; Gaze, D.; Collinson, P.; Steptoe, A. The Association Between Cortisol Response to Mental Stress and High-Sensitivity Cardiac Troponin T Plasma Concentration in Healthy Adults. Journal of the American College of Cardiology 2013, 62, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Solh, T. Takotsubo Cardiomyopathy: Review of Broken Heart Syndrome. JAAPA 2020, 33, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Gupta, G.; Narang, R.; Ray, A.; Pandey, R. M.; Malhotra, A.; Sinha, S. The Impact of Obstructive Sleep Apnoea Severity on Cardiac Structure and Injury. Sleep Medicine 2021, 77, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Einvik, G.; Røsjø, H.; Randby, A.; Namtvedt, S. K.; Hrubos-Strøm, H.; Brynildsen, J.; Somers, V. K.; Omland, T. Severity of Obstructive Sleep Apnea Is Associated with Cardiac Troponin I Concentrations in a Community-Based Sample: Data from the Akershus Sleep Apnea Project. Sleep 2014, 37, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; Zhang, X.; Qu, Y.; Fan, Y.; Li, X.; Li, C.; Yu, T.; Xia, J.; Wei, M.; Chen, L.; Li, Y.; Xiao, F.; Liu, D.; Wang, J.; Wang, X.; Cao, B. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H. K.; Al-Jassas, H. K.; Morris, G.; Maes, M. Increased ACE2, sRAGE, and Immune Activation, but Lowered Calcium and Magnesium in COVID-19. Recent Adv Inflamm Allergy Drug Discov 2022, 16, 32–43. [Google Scholar] [CrossRef] [PubMed]
- The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis - eClinicalMedicine. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00491-6/fulltext (accessed on 17 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





