Submitted:
03 July 2024
Posted:
05 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
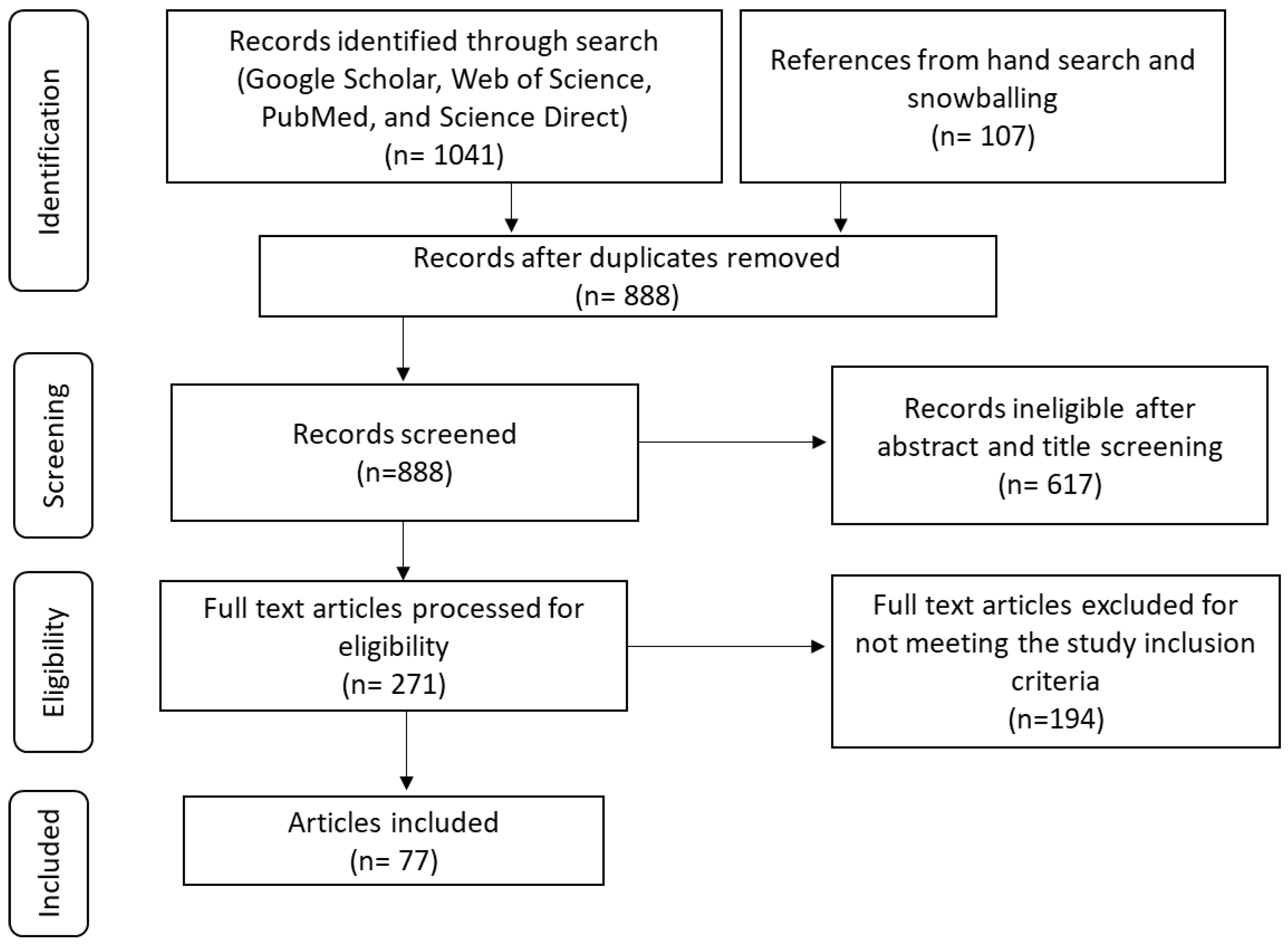
2.3. Data Extraction
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2020, 8, e191-e203. [CrossRef]
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol Lett 2020, 20, 2058-2074. [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J Obstet Gynaecol 2020, 40, 602-608. [CrossRef]
- Song, D.; Li, H.; Li, H.; Dai, J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol Lett 2015, 10, 600-606. [CrossRef]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Research 2019, 7, 176-179. [CrossRef]
- Gao, G.; Smith, D.I. Human Papillomavirus and the Development of Different Cancers. Cytogenetic and Genome Research 2017, 150, 185-193. [CrossRef]
- Sharma, R.; Aashima; Nanda, M.; Fronterre, C.; Sewagudde, P.; Ssentongo, A.E.; Yenney, K.; Arhin, N.D.; Oh, J.; Amponsah-Manu, F.; et al. Mapping Cancer in Africa: A Comprehensive and Comparable Characterization of 34 Cancer Types Using Estimates From GLOBOCAN 2020. Frontiers in Public Health 2022, 10. [CrossRef]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front Public Health 2020, 8, 552028. [CrossRef]
- Zayats, R.; Murooka, T.T.; McKinnon, L.R. HPV and the Risk of HIV Acquisition in Women. Front Cell Infect Microbiol 2022, 12, 814948. [CrossRef]
- Evans, A.M.; Salnikov, M.; Tessier, T.M.; Mymryk, J.S. Reduced MHC Class I and II Expression in HPV−Negative vs. HPV−Positive Cervical Cancers. Cells 2022, 11, 3911.
- Paaso, A.; Jaakola, A.; Syrjänen, S.; Louvanto, K. From HPV Infection to Lesion Progression: The Role of HLA Alleles and Host Immunity. Acta Cytol 2019, 63, 148-158. [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int J Cancer 2006, 119, 2105-2112. [CrossRef]
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624-2642.
- McBride, A.A. Oncogenic human papillomaviruses. Philos Trans R Soc Lond B Biol Sci 2017, 372. [CrossRef]
- Graham, Sheila V. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clinical Science 2017, 131, 2201-2221. [CrossRef]
- Ashique, S.; Hussain, A.; Fatima, N.; Altamimi, M.A. HPV pathogenesis, various types of vaccines, safety concern, prophylactic and therapeutic applications to control cervical cancer, and future perspective. Virusdisease 2023, 34, 1-19. [CrossRef]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 Suppl 5, F55-70. [CrossRef]
- Stanley, M.A.; Pett, M.R.; Coleman, N. HPV: from infection to cancer. Biochem Soc Trans 2007, 35, 1456-1460. [CrossRef]
- Burk, R.D.; Chen, Z.; Van Doorslaer, K. Human papillomaviruses: genetic basis of carcinogenicity. Public Health Genomics 2009, 12, 281-290. [CrossRef]
- Stanley, M.A. Immunobiology of papillomavirus infections11Paper presented by invitation at the Second International Conference on Experimental and Clinical Reproductive Immunobiology, Amsterdam, The Netherlands, November 2000. Journal of Reproductive Immunology 2001, 52, 45-59. [CrossRef]
- Fusconi, M.; Grasso, M.; Greco, A.; Gallo, A.; Campo, F.; Remacle, M.; Turchetta, R.; Pagliuca, G.; M, D.E.V. Recurrent respiratory papillomatosis by HPV: review of the literature and update on the use of cidofovir. Acta Otorhinolaryngol Ital 2014, 34, 375-381.
- Ball, S.L.; Winder, D.M.; Vaughan, K.; Hanna, N.; Levy, J.; Sterling, J.C.; Stanley, M.A.; Goon, P.K. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol 2011, 83, 1345-1350. [CrossRef]
- Williamson, A.-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440.
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Frontiers in Public Health 2021, 8. [CrossRef]
- Stanley, M.A.; Sterling, J.C. Host responses to infection with human papillomavirus. Curr Probl Dermatol 2014, 45, 58-74. [CrossRef]
- Ashique, S.; Hussain, A.; Fatima, N.; Altamimi, M.A. HPV pathogenesis, various types of vaccines, safety concern, prophylactic and therapeutic applications to control cervical cancer, and future perspective. VirusDisease 2023, 34, 172-190. [CrossRef]
- Ebrahimi, N.; Yousefi, Z.; Khosravi, G.; Malayeri, F.E.; Golabi, M.; Askarzadeh, M.; Shams, M.H.; Ghezelbash, B.; Eskandari, N. Human papillomavirus vaccination in low- and middle-income countries: progression, barriers, and future prospective. Front Immunol 2023, 14, 1150238. [CrossRef]
- Bardají, A.; Mindu, C.; Augusto, O.J.; Casellas, A.; Cambaco, O.; Simbine, E.; Matsinhe, G.; Macete, E.; Menéndez, C.; Sevene, E.; et al. Awareness of cervical cancer and willingness to be vaccinated against human papillomavirus in Mozambican adolescent girls. Papillomavirus Res 2018, 5, 156-162. [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Preventive Medicine 2021, 144, 106399. [CrossRef]
- Asempah, E. HPV vaccine and cervical cancer policy and policymaking research interest in sub-Saharan Africa: A scoping review. Journal of Cancer Policy 2020, 26, 100258. [CrossRef]
- El-Zein, M.; Richardson, L.; Franco, E.L. Cervical cancer screening of HPV vaccinated populations: Cytology, molecular testing, both or none. J Clin Virol 2016, 76 Suppl 1, S62-s68. [CrossRef]
- Conageski, C. Human Papillomavirus Vaccines. Clin Obstet Gynecol 2023, 66, 433-447. [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines (Basel) 2020, 8. [CrossRef]
- Sasagawa, T.; Takagi, H.; Makinoda, S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother 2012, 18, 807-815. [CrossRef]
- Bedoya, A.M.; Jaramillo, R.; Baena, A.; Castaño, J.; Olaya, N.; Zea, A.H.; Herrero, R.; Sanchez, G.I. Location and density of immune cells in precursor lesions and cervical cancer. Cancer Microenvironment 2013, 6, 69-77.
- Hewavisenti, R.V.; Arena, J.; Ahlenstiel, C.L.; Sasson, S.C. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Frontiers in Immunology 2023, 14. [CrossRef]
- Muntinga, C.L.P.; de Vos van Steenwijk, P.J.; Bekkers, R.L.M.; van Esch, E.M.G. Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy. J Clin Med 2022, 11. [CrossRef]
- Sheu, B.-C.; Chang, W.-C.; Lin, H.-H.; Chow, S.-N.; Huang, S.-C. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. Journal of Obstetrics and Gynaecology Research 2007, 33, 103-113. [CrossRef]
- Mosaad, Y.M. Clinical Role of Human Leukocyte Antigen in Health and Disease. Scandinavian Journal of Immunology 2015, 82, 283-306. [CrossRef]
- Janeway Jr, C.A. Immunobiology the immune system in health and disease. Artes Medicas 1997.
- Bhaskaran, M.; Murali, S.V.; Rajaram, B.; Krishnasamy, S.; Devasena, C.S.; Pathak, A.; Ravi, V.; Swaminathan, K.; Ayyappa, A.; Vedhantham, S.; et al. Association of HLA-A, -B, DRB, and DQB Alleles with Persistent HPV-16 Infection in Women from Tamil Nadu, India. Viral Immunol 2019, 32, 430-441. [CrossRef]
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Frontiers in Immunology 2017, 8. [CrossRef]
- Holoshitz, J. The quest for better understanding of HLA-disease association: scenes from a road less travelled by. Discovery medicine 2013, 16, 93.
- Djaoud, Z.; Parham, P. HLAs, TCRs, and KIRs, a Triumvirate of Human Cell-Mediated Immunity. Annual Review of Biochemistry 2020, 89, 717-739. [CrossRef]
- Jiang, N.; Yu, Y.; Wu, D.; Wang, S.; Fang, Y.; Miao, H.; Ma, P.; Huang, H.; Zhang, M.; Zhang, Y.; et al. HLA and tumour immunology: immune escape, immunotherapy and immune-related adverse events. Journal of Cancer Research and Clinical Oncology 2023, 149, 737-747. [CrossRef]
- Espinoza, H.; Ha, K.T.; Pham, T.T.; Espinoza, J.L. Genetic Predisposition to Persistent Human Papillomavirus-Infection and Virus-Induced Cancers. Microorganisms 2021, 9, 2092.
- Xu, H.-H.; Yan, W.-H.; Lin, A. The Role of HLA-G in Human Papillomavirus Infections and Cervical Carcinogenesis. Frontiers in Immunology 2020, 11. [CrossRef]
- Gameiro, S.F.; Zhang, A.; Ghasemi, F.; Barrett, J.W.; Nichols, A.C.; Mymryk, J.S. Analysis of Class I Major Histocompatibility Complex Gene Transcription in Human Tumors Caused by Human Papillomavirus Infection. Viruses 2017, 9. [CrossRef]
- Ekanayake Weeramange, C.; Shu, D.; Tang, K.D.; Batra, J.; Ladwa, R.; Kenny, L.; Vasani, S.; Frazer, I.H.; Dolcetti, R.; Ellis, J.J.; et al. Analysis of human leukocyte antigen associations in human papillomavirus–positive and –negative head and neck cancer: Comparison with cervical cancer. Cancer 2022, 128, 1937-1947. [CrossRef]
- Gomez, F.; Hirbo, J.; Tishkoff, S.A. Genetic variation and adaptation in Africa: implications for human evolution and disease. Cold Spring Harb Perspect Biol 2014, 6, a008524. [CrossRef]
- Fan, S.; Kelly, D.E.; Beltrame, M.H.; Hansen, M.E.B.; Mallick, S.; Ranciaro, A.; Hirbo, J.; Thompson, S.; Beggs, W.; Nyambo, T.; et al. African evolutionary history inferred from whole genome sequence data of 44 indigenous African populations. Genome Biology 2019, 20, 82. [CrossRef]
- Pfennig, A.; Petersen, L.N.; Kachambwa, P.; Lachance, J. Evolutionary Genetics and Admixture in African Populations. Genome Biology and Evolution 2023, 15. [CrossRef]
- Adolf, I.C.; Almars, A.; Dharsee, N.; Mselle, T.; Akan, G.; Nguma, I.J.; Nateri, A.S.; Atalar, F. HLA-G and single nucleotide polymorphism (SNP) associations with cancer in African populations: Implications in personal medicine. Genes & Diseases 2022, 9, 1220-1233.
- Mellet, J.; Tshabalala, M.; Agbedare, O.; Meyer, P.W.A.; Gray, C.; Pepper, M. Human leukocyte antigen (HLA) diversity and clinical applications in South Africa. South African Medical Journal 2019, 109, 29. [CrossRef]
- Adebamowo, S.N.; Adeyemo, A.; Adebayo, A.; Achara, P.; Alabi, B.; Bakare, R.A.; Famooto, A.O.; Obende, K.; Offiong, R.; Olaniyan, O.; et al. Genome, HLA and polygenic risk score analyses for prevalent and persistent cervical human papillomavirus (HPV) infections. European Journal of Human Genetics 2024. [CrossRef]
- Stanley, M.A. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev 2012, 25, 215-222. [CrossRef]
- Stanley, M. Immunology of HPV Infection. Current Obstetrics and Gynecology Reports 2015, 4, 195-200. [CrossRef]
- Sapp, M.; Bienkowska-Haba, M. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. Febs j 2009, 276, 7206-7216. [CrossRef]
- Raff, A.B.; Woodham, A.W.; Raff, L.M.; Skeate, J.G.; Yan, L.; Da Silva, D.M.; Schelhaas, M.; Kast, W.M. The evolving field of human papillomavirus receptor research: a review of binding and entry. Journal of virology 2013, 87, 6062-6072.
- Horvath, C.A.; Boulet, G.A.; Renoux, V.M.; Delvenne, P.O.; Bogers, J.P. Mechanisms of cell entry by human papillomaviruses: an overview. Virol J 2010, 7, 11. [CrossRef]
- Day, P.M.; Lowy, D.R.; Schiller, J.T. Heparan sulfate-independent cell binding and infection with furin-precleaved papillomavirus capsids. J Virol 2008, 82, 12565-12568. [CrossRef]
- Moody, C. Mechanisms by which HPV Induces a Replication Competent Environment in Differentiating Keratinocytes. Viruses 2017, 9. [CrossRef]
- Sakakibara, N.; Mitra, R.; McBride, A.A. The papillomavirus E1 helicase activates a cellular DNA damage response in viral replication foci. J Virol 2011, 85, 8981-8995. [CrossRef]
- Stanley, M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010, 117, S5-10. [CrossRef]
- Paaso, A.; Koskimaa, H.M.; Welters, M.J.P.; Grenman, S.; Syrjanen, K.; van der Burg, S.H.; Syrjanen, S. Cell mediated immunity against HPV16 E2, E6 and E7 peptides in women with incident CIN and in constantly HPV-negative women followed-up for 10-years. Journal of Translational Medicine 2015, 13. [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Frontiers in Microbiology 2020, 10. [CrossRef]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 2011, 6, 45-57. [CrossRef]
- Bodily, J.; Laimins, L.A. Persistence of human papillomavirus infection: keys to malignant progression. Trends Microbiol 2011, 19, 33-39. [CrossRef]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int J Mol Sci 2018, 19. [CrossRef]
- Basukala, O.; Banks, L. The Not-So-Good, the Bad and the Ugly: HPV E5, E6 and E7 Oncoproteins in the Orchestration of Carcinogenesis. Viruses 2021, 13, 1892.
- Zhou, C.; Tuong, Z.K.; Frazer, I.H. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front Oncol 2019, 9, 682. [CrossRef]
- de Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R., Jr.; Venuti, A. hrHPV E5 oncoprotein: immune evasion and related immunotherapies. J Exp Clin Cancer Res 2017, 36, 71. [CrossRef]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137-142. [CrossRef]
- Senba, M.; Mori, N. Mechanisms of virus immune evasion lead to development from chronic inflammation to cancer formation associated with human papillomavirus infection. Oncol Rev 2012, 6, e17. [CrossRef]
- Georgopoulos, N.T.; Proffitt, J.L.; Blair, G.E. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene 2000, 19, 4930-4935. [CrossRef]
- Einstein, M.H.; Leanza, S.; Chiu, L.G.; Schlecht, N.F.; Goldberg, G.L.; Steinberg, B.M.; Burk, R.D. Genetic variants in TAP are associated with high-grade cervical neoplasia. Clinical Cancer Research 2009, 15, 1019-1023.
- Wilting, S.M.; Steenbergen, R.D.M. Molecular events leading to HPV-induced high grade neoplasia. Papillomavirus Research 2016, 2, 85-88. [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Reviews in Medical Virology 2015, 25, 2-23. [CrossRef]
- Pešut, E.; Đukić, A.; Lulić, L.; Skelin, J.; Šimić, I.; Milutin Gašperov, N.; Tomaić, V.; Sabol, I.; Grce, M. Human Papillomaviruses-Associated Cancers: An Update of Current Knowledge. Viruses 2021, 13. [CrossRef]
- McBride, A.A.; Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog 2017, 13, e1006211. [CrossRef]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of host immune defenses by human papillomavirus. Virus Research 2017, 231, 21-33. [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. British Journal of Cancer 2021, 124, 359-367. [CrossRef]
- Reusser, N.M.; Downing, C.; Guidry, J.; Tyring, S.K. HPV Carcinomas in Immunocompromised Patients. J Clin Med 2015, 4, 260-281. [CrossRef]
- Wang, C.; Xiong, C.; Hsu, Y.-C.; Wang, X.; Chen, L. Human leukocyte antigen (HLA) and cancer immunotherapy: HLA-dependent and-independent adoptive immunotherapies. Ann. Blood 2020, 5, 10.21037.
- Cassioli, C.; Baldari, C.T. The Expanding Arsenal of Cytotoxic T Cells. Frontiers in Immunology 2022, 13. [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. Journal of Cellular Physiology 2019, 234, 8509-8521. [CrossRef]
- Martina, J.A.; Wu, X.S.; Catalfamo, M.; Sakamoto, T.; Yi, C.; Hammer, J.A., 3rd. Imaging of lytic granule exocytosis in CD8+ cytotoxic T lymphocytes reveals a modified form of full fusion. Cell Immunol 2011, 271, 267-279. [CrossRef]
- Li, K.; Qiu, J.; Pan, J.; Pan, J.P. Pyroptosis and Its Role in Cervical Cancer. Cancers (Basel) 2022, 14. [CrossRef]
- Wang, J.L.; Hua, S.N.; Bao, H.J.; Yuan, J.; Zhao, Y.; Chen, S. Pyroptosis and inflammasomes in cancer and inflammation. MedComm 2023, 4, e374.
- Nakagawa, M.; Stites, D.P.; Farhat, S.; Sisler, J.R.; Moss, B.; Kong, F.; Moscicki, A.-B.; Palefsky, J.M. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. Journal of Infectious Diseases 1997, 175, 927-931.
- Bontkes, H.J.; De Gruijl, T.D.; van den Muysenberg, A.J.; Verheijen, R.H.; Stukart, M.J.; Meijer, C.J.; Scheper, R.J.; Stacey, S.N.; Duggan-Keen, M.F.; Stern, P.L. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. International journal of cancer 2000, 88, 92-98.
- Valdespino, V.; Gorodezky, C.; Ortiz, V.; Kaufmann, A.M.; Roman-Basaure, E.; Vazquez, A.; Berumen, J. HPV16-specific cytotoxic T lymphocyte responses are detected in all HPV16-positive cervical cancer patients. Gynecologic oncology 2005, 96, 92-102.
- Nakagawa, M.; Stites, D.P.; Patel, S.; Farhat, S.; Scott, M.; Hills, N.K.; Palefsky, J.M.; Moscicki, A.-B. Persistence of Human Papillomavirus Type 16 Infection Is Associated with Lack of Cytotoxic T Lymphocyte Response to the E6 Antigens. The Journal of Infectious Diseases 2000, 182, 595-598. [CrossRef]
- Woo, Y.L.; van den Hende, M.; Sterling, J.C.; Coleman, N.; Crawford, R.A.F.; Kwappenberg, K.M.C.; Stanley, M.A.; van der Burg, S.H. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. International Journal of Cancer 2010, 126, 133-141. [CrossRef]
- Adebamowo, S.N.; Famooto, A.; Dareng, E.O.; Olawande, O.; Olaniyan, O.; Offiong, R.; Adebamowo, C.A. Clearance of Type-Specific, Low-Risk, and High-Risk Cervical Human Papillomavirus Infections in HIV-Negative and HIV-Positive Women. J Glob Oncol 2018, 4, 1-12. [CrossRef]
- Kuguyo, O.; Dube Mandishora, R.S.; Thomford, N.E.; Makunike-Mutasa, R.; Nhachi, C.F.B.; Matimba, A.; Dandara, C. High-risk HPV genotypes in Zimbabwean women with cervical cancer: Comparative analyses between HIV-negative and HIV-positive women. PLoS One 2021, 16, e0257324. [CrossRef]
- Mbuya, W.; Held, K.; Mcharo, R.D.; Haule, A.; Mhizde, J.; Mnkai, J.; Mahenge, A.; Mwakatima, M.; Sembo, M.; Mwalongo, W.; et al. Depletion of Human Papilloma Virus E6- and E7-Oncoprotein-Specific T-Cell Responses in Women Living With HIV. Frontiers in Immunology 2021, 12. [CrossRef]
- Steele, J.C.; Mann, C.H.; Rookes, S.; Rollason, T.; Murphy, D.; Freeth, M.G.; Gallimore, P.H.; Roberts, S. T-cell responses to human papillomavirus type 16 among women with different grades of cervical neoplasia. British Journal of Cancer 2005, 93, 248-259. [CrossRef]
- Li, X.C.; Raghavan, M. Structure and function of major histocompatibility complex class I antigens. Curr Opin Organ Transplant 2010, 15, 499-504. [CrossRef]
- Hopkins, J.R.; MacLachlan, B.J.; Harper, S.; Sewell, A.K.; Cole, D.K. Unconventional modes of peptide–HLA-I presentation change the rules of TCR engagement. Discovery Immunology 2022, 1. [CrossRef]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012, 4, 155ra138. [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front Immunol 2018, 9, 14. [CrossRef]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst 2013, 105, 1172-1187. [CrossRef]
- Zhao, M.; Qiu, L.; Tao, N.; Zhang, L.; Wu, X.; She, Q.; Zeng, F.; Wang, Y.; Wei, S. HLA DRB allele polymorphisms and risk of cervical cancer associated with human papillomavirus infection: a population study in China. European journal of gynaecological oncology 2013, 34, 54-59.
- Othmane, Y.B.; Ghazouani, E.; Mezlini, A.; Lagha, A.; Raïs, M.; Kochkar, R.; Zidi, S.; Afrit, M.; Mota-Vieira, L.; Loueslati, B.Y. HLA class II susceptibility to cervical cancer among Tunisian women. Bulletin du cancer 2012, 99, E81-E86.
- Steinbach, A.; Riemer, A.B. Immune evasion mechanisms of human papillomavirus: An update. International Journal of Cancer 2018, 142, 224-229. [CrossRef]
- Cicchini, L.; Blumhagen, R.Z.; Westrich, J.A.; Myers, M.E.; Warren, C.J.; Siska, C.; Raben, D.; Kechris, K.J.; Pyeon, D. High-Risk Human Papillomavirus E7 Alters Host DNA Methylome and Represses HLA-E Expression in Human Keratinocytes. Sci Rep 2017, 7, 3633. [CrossRef]
- Ferguson, R.; Ramanakumar, A.V.; Richardson, H.; Tellier, P.P.; Coutlée, F.; Franco, E.L.; Roger, M. Human leukocyte antigen (HLA)-E and HLA-G polymorphisms in human papillomavirus infection susceptibility and persistence. Hum Immunol 2011, 72, 337-341. [CrossRef]
- Mora, M.J.; de los Ángeles Bayas-Rea, R.; Mejía, L.; Cruz, C.; Guerra, S.; Calle, P.; Sandoval, D.M.; Galarza, J.M.; Zapata-Mena, S. Identification of human leukocyte antigen in precancerous and cancerous cervical lesions from Ecuadorian women. Infection, Genetics and Evolution 2022, 105, 105365. [CrossRef]
- Adebamowo, S.N.; Adeyemo, A.A. Classical HLA alleles are associated with prevalent and persistent cervical high-risk HPV infection in African women. Hum Immunol 2019, 80, 723-730. [CrossRef]
- Muchiri, L.W.; Sekadde-Kigondu, C.B.; Estambale, B.; Temmerman, M.T. Risk association between human leucocyte antigens (HLA) and cervical neoplasia in Kenyan women. African Journal of Pharmacology and Therapeutics 2012, 1.
- Dong, D.D.; Yang, H.; Li, K.; Xu, G.; Song, L.H.; Fan, X.L.; Jiang, X.L.; Yie, S.M. Human Leukocyte Antigen-G (HLA-G) Expression in Cervical Lesions: Association With Cancer Progression, HPV 16/18 Infection, and Host Immune Response. Reproductive Sciences 2010, 17, 718-723. [CrossRef]
- Da Silva, M.L.R.; De Albuquerque, B.; Allyrio, T.; De Almeida, V.D.; Cobucci, R.N.O.; Bezerra, F.L.; Andrade, V.S.; Lanza, D.C.F.; De Azevedo, J.C.V.; De Araújo, J.M.G.; et al. The role of HPV-induced epigenetic changes in cervical carcinogenesis (Review). Biomed Rep 2021, 15, 60. [CrossRef]
- Mbulawa, Z.Z.A.; Phohlo, K.; Garcia-Jardon, M.; Williamson, A.L.; Businge, C.B. High human papillomavirus (HPV)-35 prevalence among South African women with cervical intraepithelial neoplasia warrants attention. Plos One 2022, 17. [CrossRef]
- Mudini, W.; Palefsky, J.M.; Hale, M.J.; Chirenje, M.Z.; Makunike-Mutasa, R.; Mutisi, F.; Murahwa, A.; Mario, A. Human Papillomavirus Genotypes in Invasive Cervical Carcinoma in HIV-Seropositive and HIV-Seronegative Women in Zimbabwe. J Acquir Immune Defic Syndr 2018, 79, e1-e6. [CrossRef]
- Fitzpatrick, M.B.; Hahn, Z.; Mandishora, R.S.D.; Dao, J.; Weber, J.; Huang, C.; Sahoo, M.K.; Katzenstein, D.A.; Pinsky, B.A. Whole-Genome Analysis of Cervical Human Papillomavirus Type 35 from rural Zimbabwean Women. Scientific Reports 2020, 10, 7001. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




