Submitted:
04 July 2024
Posted:
05 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
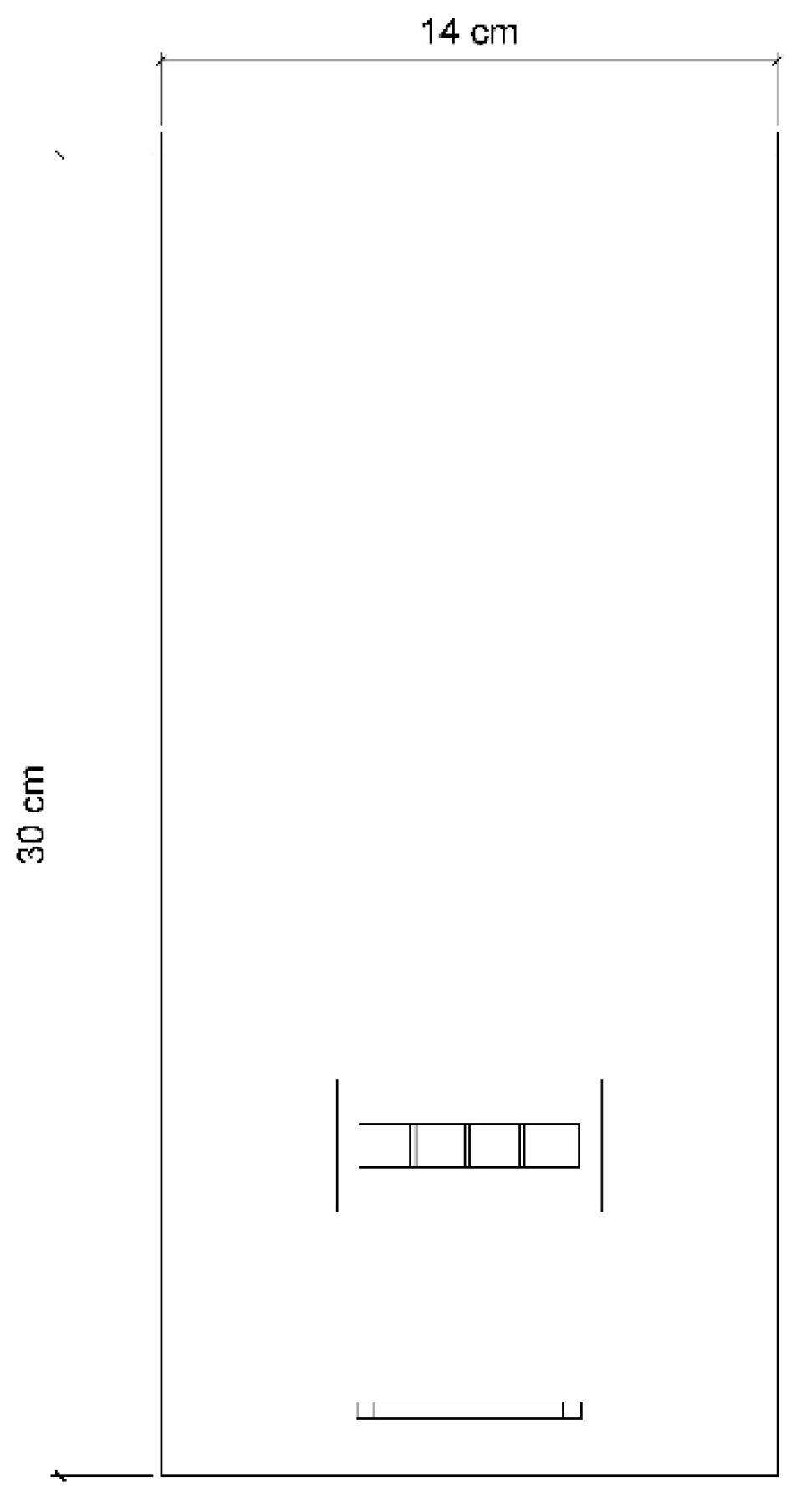
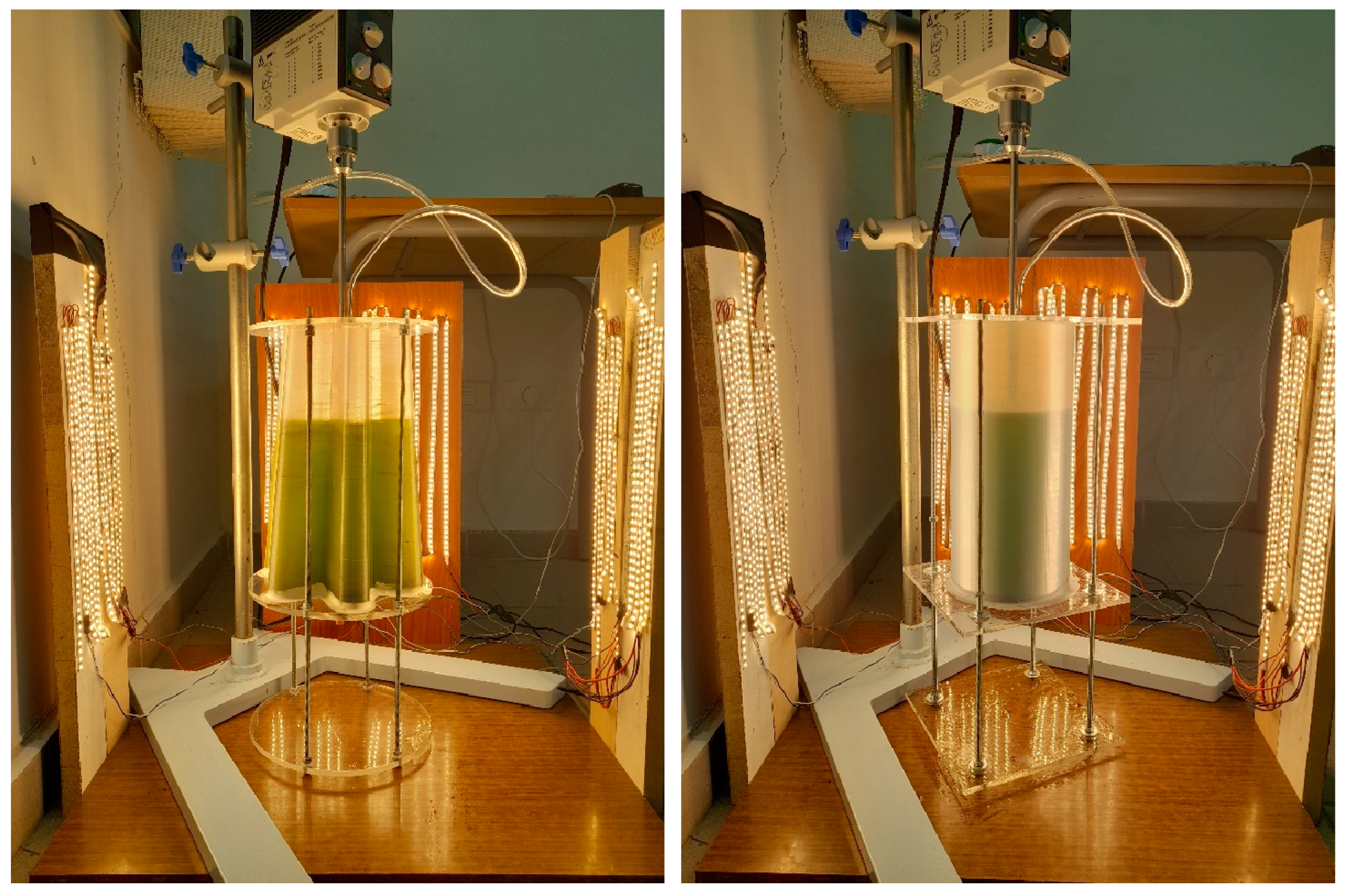
2.1. PBR Geometry Description

2.2. Cultivation Experiment
2.3. Light Intensity Measurement
2.4. Biomass Measurement
2.4.1. Specific Growth Rate
2.4.2. Productivity
2.4.3. Energy Efficiency
2.5. Statistical Analysis
3. Results and Discussion
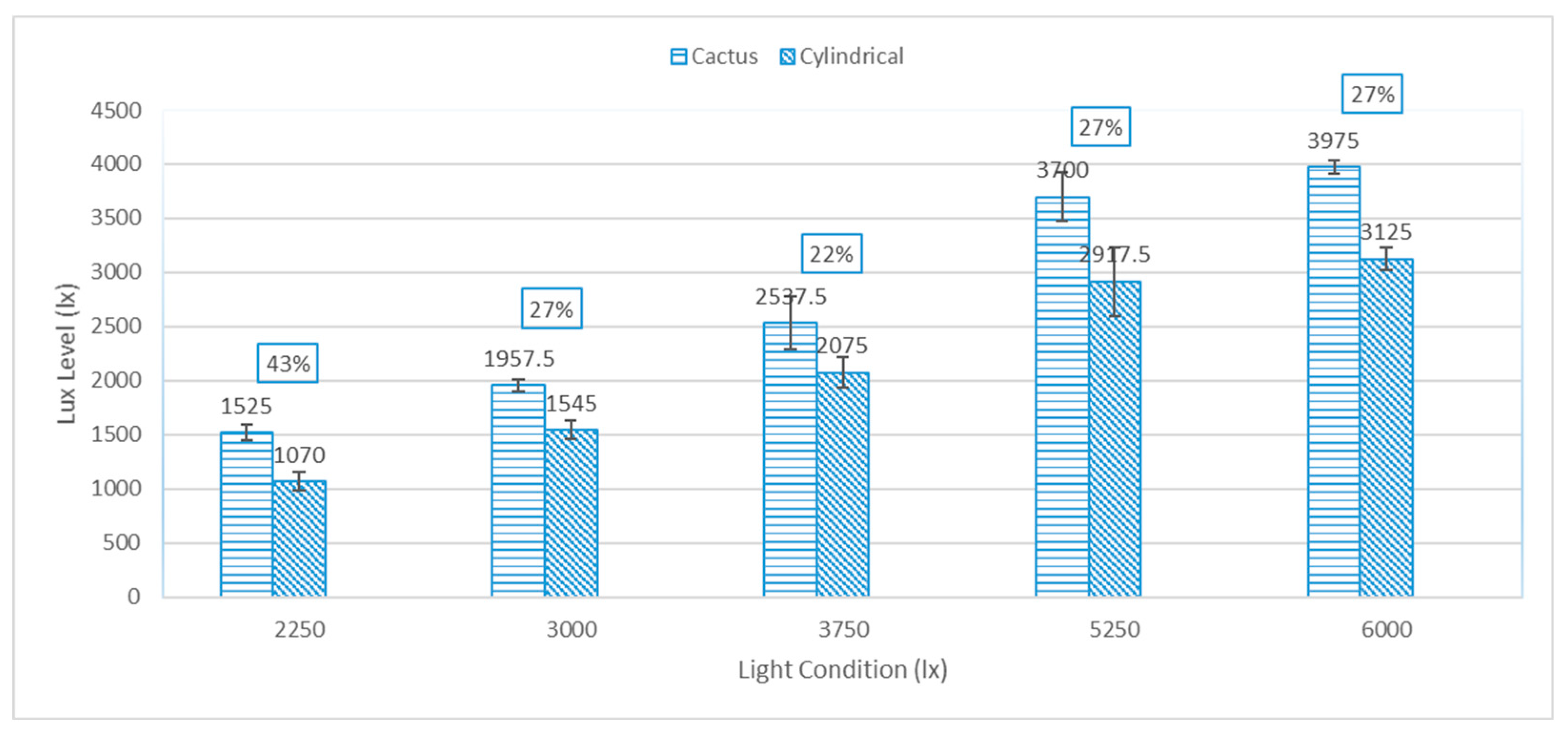
3.1. Irradiance
3.2. Biomass Production
3.2.1. Specific Growth Rate
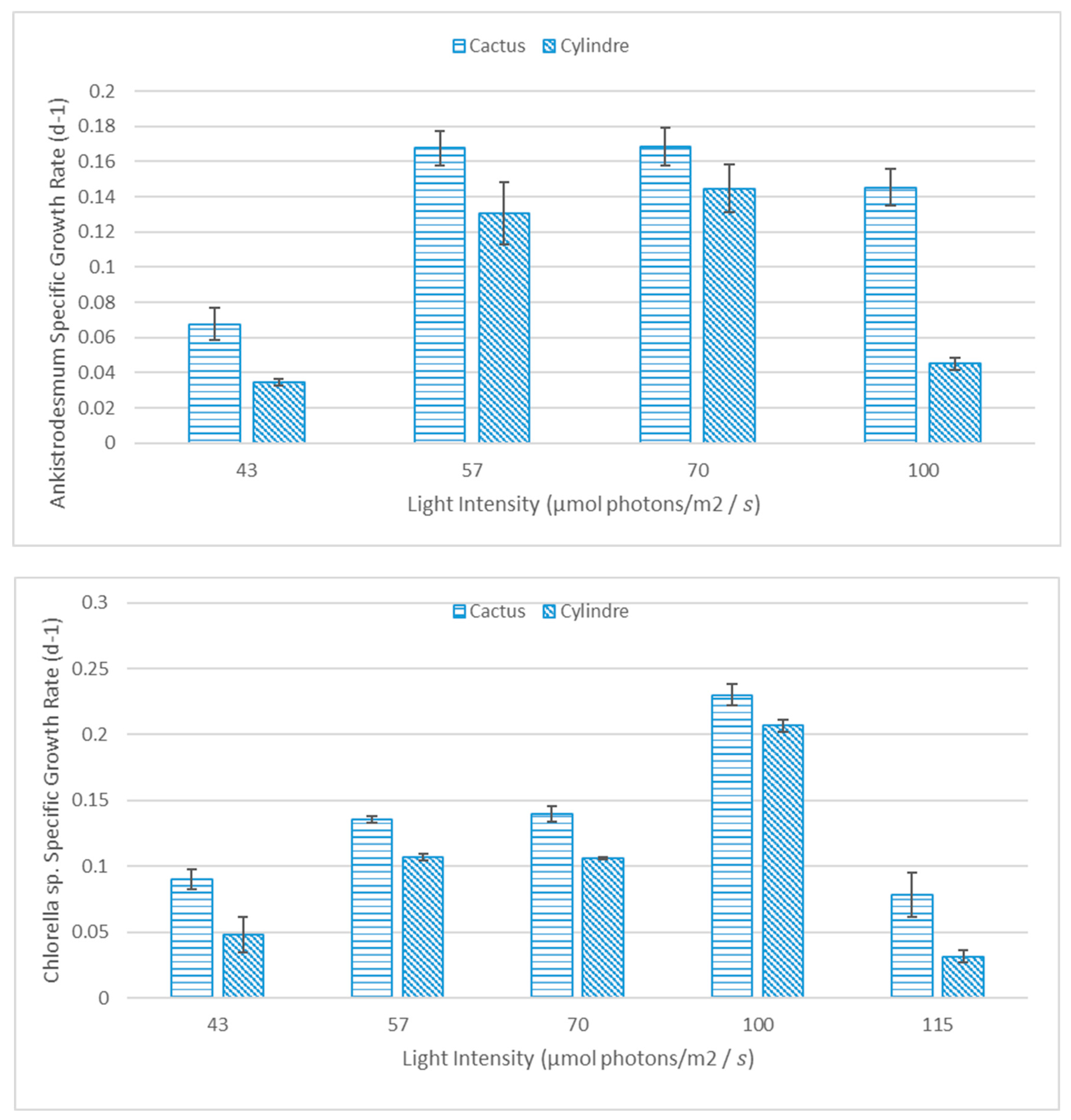
3.2.2. Microalgae Productivity
| Condition 1 | Condition 2 | Condition 3 | Condition 4 | Condition 5 | |||
|---|---|---|---|---|---|---|---|
| Ankistrodesmum | Productivity (g/L/d) | Cactus | 0.5885 ± 0.0624 | 0.6888 ± 0.0452 | 0.9660 ± 0.0814 | 0.6566 ± 0.0630 | - |
| Cylindrical | 0.4122 ± 0.0192 | 0.4162 ± 0.0603 | 0.4858 ± 0.0040 | 0.2273 ± 0.0052 | - | ||
| Specific growth rate (d-1) | Cactus | 0.06766 ± 0.0090 | 0.1677 ±0.0097 | 0.1684 ± 0.0106 | 0.1452± 0.0102 | - | |
| Cylindrical | 0.0346± 0.0021 | 0.1305 ± 0.01758 | 0.1446 ± 0.0135 | 0.0450 ± 0.0037 | - | ||
| Energy efficiency (g/d/kWh) | Cactus | 0.3139 ±0.0333 | 0.1837 ±0.0120 | 0.1288 ±0.0108 | 0.0437 ±0.0042 | - | |
| Cylindrical | 0.2199 ±0.0102 | 0.1110 ±0.0160 | 0.0649 ±0.0035 | 0.0151 ±0.0003 | - | ||
| Chlorella | Productivity (g/L/d) | Cactus | 0.4285 ± 0.0070 | 0.4898 ± 0.0182 | 0.5160 ± 0.0080 | 0.9994 ± 0.0322 | 0.2398 ± 0.0028 |
| Cylindrical | 0.3219 ± 0.0072 | 0.2910 ± 0.0078 | 0.2777 ± 0.0031 | 0.7422 ± 0.0435 | 0.1879 ± 0.0125 | ||
| Specific growth rate (d-1) | Cactus | 0.0901 ± 0.0072 | 0.1353 ± 0.0024 | 0.1396 ± 0.0059 | 0.2300 ± 0.0082 | 0.0782 ±0.0169 | |
| Cylindrical | 0.0477 ± 0.0136 | 0.1069 ± 0.0025 | 0.1060 ± 0.0011 | 0.2068 ± 0.0044 | 0.0314 ± 0.0043 | ||
| Energy efficiency (g/d/kWh) | Cactus | 0.2286 ±0.0037 | 0.1306 ±0.0048 | 0.0688 ±0.0010 | 0.0666 ±0.0021 | 0.0107 ±0.0001 | |
| Cylindrical | 0.1717 ±0.0038 | 0.0776 ±0.0020 | 0.0370 ±0.0004 | 0.0494 ±0.0029 | 0.0083 ±0.0005 |
3.2.3. Energy Efficiency
4. Conclusions
Author Contributions
Funding
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.F.; Rafa, S.J.; Mehjabin, A.; Tasannum, N.; Ahmed, S.; Mofijur, M.; Lichtfouse, E.; Almomani, F.; Badruddin, I.A.; Kamangar, S. Bio-oil from microalgae: Materials, production, technique, and future. Energy Reports 2023, 10, 3297–3314. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technologies and Sustainability 2023, 2, 100060. [Google Scholar] [CrossRef]
- Murthy, G.S. Overview and assessment of algal biofuels production technologies. In Biofuels; Academic Press, 2011; pp. 415–437. [Google Scholar] [CrossRef]
- Hijazi, R.; Mounsef, J.R.; Kanaan, H.Y. Design considerations for photo-bioreactors: a review. In Proceedings of the 2020 5th International Conference on Renewable Energies for Developing Countries (REDEC); IEEE; pp. 1–7.
- Hijazi, R.; Mounsef, J.R.; Kanaan, H.Y. Design and Computational Fluid Dynamics Simulation of a Novel Stirred Photo-Bioreactor. In Proceedings of the IECON 2021–47th Annual Conference of the IEEE Industrial Electronics Society; IEEE; pp. 1–6. [CrossRef]
- Chen, P.; Min, M.; Chen, Y.; Wang, L.; Li, Y.; Chen, Q.; Wang, C.; Wan, Y.; Wang, X.; Cheng, Y.; et al. Review of the biological and engineering aspects of algae to fuels approach. International Journal of Agricultural and Biological Engineering 2009, 2, 1–30. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renewable and Sustainable Energy Reviews 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Guler, B.A.; Deniz, I.; Demirel, Z.; Oncel, S.S.; Imamoglu, E. Computational fluid dynamics modelling of stirred tank photobioreactor for Haematococcus pluvialis production: Hydrodynamics and mixing conditions. Algal Research 2020, 47, 101854. [Google Scholar] [CrossRef]
- Sergejevová, M.; Malapascua, J.R.; Kopecký, J.; Masojídek, J. Photobioreactors with internal illumination. Algal Biorefineries: Volume 2: Products and Refinery Design 2015, 213–236. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Soejima, T.; Tanaka, H. An integrated solar and artificial light system for internal illumination of photobioreactors. J Biotechnol 1999, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Sato, T. A photobioreactor for microalgae cultivation with internal illumination considering flashing light effect and optimized light-source arrangement. Energy Conversion and Management 2017, 133, 558–565. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. A mini review: photobioreactors for large scale algal cultivation. World Journal of Microbiology and Biotechnology 2015, 31, 1409–1417. [Google Scholar] [CrossRef]
- Marconi, P.L.; Trentini, A.; Zawoznik, M.; Nadra, C.; Mercadé, J.M.; Sánchez Novoa, J.G.; Orozco, D.; Groppa, M. Development and testing of a 3D-printable polylactic acid device to optimize a water bioremediation process. AMB Express 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): research, development and industrialization. Biotechnology Journal 2010, 5, 1125–1136. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: a review. Frontiers of Chemistry in China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Kadic, E.; Heindel, T.J. An introduction to bioreactor hydrodynamics and gas-liquid mass transfer. John Wiley & Sons, 2014. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnology Advances 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Bitog, J.P.; Lee, I.B.; Lee, C.G.; Kim, K.S.; Hwang, H.S.; Hong, S.W.; Seo, I.H.; Kwon, K.S.; Mostafa, E. Application of computational fluid dynamics for modelling and designing photobioreactors for microalgae production: A review. Computers and Electronics in Agriculture 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological Reviews 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Mounsef, J.R.; Lteif, R. Pigment production by Scenedesmus dimorphus using different low-cost and alternative culture media. Journal of Chemical Technology & Biotechnology 2022, 97, 287–294. [Google Scholar]
- Waveform Lighting, Waveform Lighting, LLC. 2024. https://www.waveformlighting.com/horticulture/convert-lux-to-ppfd-online-calculator.
- Pozza, C.; Schmuck, S.; Mietzel, T. A novel photobioreactor with internal illumination using Plexiglas rods to spread the light and LED as a source of light for wastewater treatment using microalgae. 2013. [Google Scholar]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. Journal of Applied Phycology 2012, 24, 35–43. [Google Scholar] [CrossRef]
- Calvo, F.; Bula, A.; Di Mare, L.; Garcia, S. CFD simulation of multiphase (liquid–solid–gas) flow in an airlift column photobioreactor. Acta Mechanica 2017, 228, 2413–2427. [Google Scholar] [CrossRef]
- Lee, E.; Jalalizadeh, M.; Zhang, Q. Growth kinetic models for microalgae cultivation: A review. Algal Research 2015, 12, 497–512. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. The effects of light and temperature on microalgal growth and nutrient removal: an experimental and mathematical approach. RSC Advances 2016, 6, 22896–22907. [Google Scholar] [CrossRef]
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined effects of light intensity, light-path and culture density on output rate of Spirulina platensis (Cyanobacteria). European Journal of Phycology 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Wang, C.Y.; Fu, C.C.; Liu, Y.C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochemical Engineering Journal 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Sankar, V.; Daniel, D.K.; Krastanov, A. Carbon dioxide fixation by Chlorella minutissima batch cultures in a stirred tank bioreactor. Biotechnology & Biotechnological Equipment 2011, 25, 2468–2476. [Google Scholar] [CrossRef]
- Li, D.; Cong, W.; Cai, Z.; Shi, D.; Ouyang, F. Effect of Iron Stress, Light Stress, and Nitrogen Source on Physiological Aspects of Marine Red Tide Alga. J. Plant Nutr. 2004, 27, 29–41. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquaculture International 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Choochote, W.; Paiboonsin, K.; Ruangpan, S.; Pharuang, A. Effects of Urea and Light Intensity on the Growth of Chlorella sp. In Proceedings of the 8th International Symposium on Biocontrol and Biotechnology; 2010; pp. 127–134. [Google Scholar]
- Yeh, K.L.; Chang, J.S.; Chen, W.M. Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Engineering in Life Sciences 2010, 10, 201–208. [Google Scholar] [CrossRef]
- Lee, C.G.; Palsson, B.Ø. High-density algal photobioreactors using light-emitting diodes. Biotechnology and Bioengineering 1994, 44, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. Journal of Water Process Engineering 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Kamla, Y.; Bouzit, M.; Ameur, H.; Arab, M.I.; Hadjeb, A. Effect of the inclination of baffles on the power consumption and fluid flows in a vessel stirred by a Rushton turbine. Chinese Journal of Mechanical Engineering 2017, 30, 1008–1016. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, S.; Yan, C.; Wu, X.; Wen, S.; Cong, W. Installation of flow deflectors and wing baffles to reduce dead zone and enhance flashing light effect in an open raceway pond. Bioresource Technology 2015, 198, 150–156. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, W.; Song, Y.; Kumar, S.; Ali, K.A.; Zhou, J. Enhancing vorticity magnitude of turbulent flow to promote photochemical efficiency and trichome helix pitch of Arthrospira platensis in a raceway pond with conic baffles. Bioresource Technology 2018, 269, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.; Ahammad, S.Z.; Sallis, P.J.; Mota, C.R. Energy-efficient stirred-tank photobioreactors for simultaneous carbon capture and municipal wastewater treatment. Water Science and Technology 2014, 69, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.; Ahammad, Z.S.; Sallis, P.J.; Mota, C.R. Optimisation of red light-emitting diodes irradiance for illuminating mixed microalgal culture to treat municipal wastewater. WIT Transactions on Ecology and the Environment 2013, 178, 263–270. [Google Scholar]
- Cerón-García, M.C.; Macías-Sánchez, M.D.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. A Process for Biodiesel Production Involving the Heterotrophic Fermentation of Chlorella Protothecoides with Glycerol as the Carbon Source. Applied Energy 2013, 103, 341–349. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renewable and Sustainable Energy Reviews 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Senge, M.; Senger, H. Response of the photosynthetic apparatus during adaptation of Chlorella and Ankistrodesmus to irradiance changes. Journal of Plant Physiology 1990, 136, 675–679. [Google Scholar] [CrossRef]
- Aleya, L.; Dauta, A.; Reynolds, C.S. Endogenous regulation of the growth-rate responses of a spring-dwelling strain of the freshwater alga, Chlorella minutissima, to light and temperature. European Journal of Protistology 2011, 47, 239–244. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Yada, H.; Masui, H.; Tanaka, H. A novel internally illuminated stirred tank photobioreactor for large-scale cultivation of photosynthetic cells. Journal of Fermentation and Bioengineering 1996, 82, 61–67. [Google Scholar] [CrossRef]
- Sánchez-Contreras, M.I.; Morales-Arrieta, S.; Okoye, P.U.; Guillén-Garcés, R.A.; Sebastian, P.J.; Arias, D.M. Recycling industrial wastewater for improved carbohydrate-rich biomass production in a semi-continuous photobioreactor: effect of hydraulic retention time. Journal of Environmental Management 2021, 284, 112065. [Google Scholar] [CrossRef]
- Deniz, I. Scaling-up of Haematococcus pluvialis production in stirred tank photobioreactor. Bioresource Technology 2020, 310, 123434. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Maximizing biomass and lipid production in heterotrophic culture of Chlorella vulgaris: techno-economic assessment. Recent Patents on Food, Nutrition & Agriculture 2019, 10, 115–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





