1. Introduction
Sparkling wines market is a growing worldwide industry which at 2023 reached the US
$ 39.3 Billion [
1,
2]. This so large market volume can be explained through the changes in the social perception of the sparkling wines and the increase on the diversity offered inside the sparkling wines category [
3]. While the market is still lead by Champagne and Prosecco, ancestral sparkling wines - also known by its French name
Pét-nat, are one of these “new” products that are gaining popularity between consumers who are looking for lower alcoholic content wines and for the wine insiders who like to try particular products directly from grapegrowers [
4,
5].
The effervescence of high quality natural sparkling wines must be obtained through an alcoholic fermentation in sealed vessels (bottle or tank fermentation) [
6,
7] called
prise de mousse. Different methodologies exist depending on the way in which the vessel-sealed fermentation is performed. Traditional method, used for Champagne, Cava or Franciacorta, and
Charmat method, used for Prosecco, are the most well-known [
2,
8]. Both methodologies follow a second fermentation over a low alcoholic base wine with an added mixture, called
liqueur de tirage, of sugar (20-24 g of sucrose/L), preadapted yeasts (1-2 million viable cells/mL) and a riddling agent to favour the sedimentation and compact the lees at the neck of the bottle (only in the case of traditional method). The main difference between these two methodologies is that in traditional method, the second fermentation is performed in closed individual bottles while in
Charmat method, the second fermentation is carried out in isobaric fermentation tanks, called autoclaves, which retain the generated pressure [
9].
By contrast, ancestral method, which seems to be historically the oldest procedure to elaborate sparkling wines, consists only in one fermentation [
10]. The alcoholic fermentation starts in a fermentation tank until the fermenting must’s sugars level is low enough (around 18 g/L). The fermenting must is then bottled to allow the fermentation to finish on sealed bottles [
11] adding or not a riddling agent. Some winemakers apply a cold settling and even filter the fermenting must to reduce the yeast population and slow down the fermentation rate. In some French winegrowing regions is still preserved as part of its heritage like in AOC Blanquette de Limoux or AOC Clairette de Die.
Lately, due to its increasing popularity, some wine awards have recognized the ancestral sparkling wines production by creating its own special categories, like “Effervescent du monde ®” since 2010, “Barcelona Rosé” awards since its creation at 2022, or 50 Great Sparkling wines contest since 2022 [
12,
13,
14]. Other contests have not incorporated an specific category for this kind of wines but it can be found that between 2015-2018 some important prizes have started to be given to ancestral sparkling wines [
15,
16,
17].
However, the procedure of elaboration of ancestral sparkling wines is not so well defined as traditional method and probably for that reason ancestral sparkling wines that can be found in the market present great heterogeneity on its parameters such as cloudiness, fizziness, aroma or colour [
18]. Even though theoretically ancestrals should have a clear appearance and without fermentation faults [
19], it is common to find faulty products because of the difficulties and the precision that the process requires. The reason for that is related with the difficulty of stopping or at least slowing down fermentation kinetics before bottling at the adequate moment, and in the control of the yeast population introduced inside the bottle. The lack of control of these aspects could cause excess or underpressure in the bottles and also an excess of yeast population. An excessive population of yeast in the bottle could difficult the riddling process and consequently could cause excess turbidity in the bottle and even some times the appearance off-flavours (reduction taint) [
20,
21]. In fact, these difficulties were the main reason that explains the progressive abandon of the method centuries ago in favour of the traditional method [
22,
23].
In Spain, Ancestral sparkling wines, are mainly produced in zones with old sparkling wine culture, like Catalan Cava region and its surroundings. This increasing phenomenon has lead some Protected Designation of Origin (PDO) such as PDO Penedès or PDO Tarragona to include and regulate the ancestral sparkling wines production under their normatives [
24,
25].
Ancestral sparkling wines are not just an uprising elaboration trend but are also a way to obtain sparkling wines under warm climate conditions like it happens in the Mediterranean basin because they do not require the addition of sugar for a second fermentation. For that reason, the harvest of grapes for ancestral sparkling wines can be done later than in the case of grapes for traditional method. This later harvest allows to obtain a higher aromatic development of the berries and avoids the occasional appearance of herbaceous characters [
26]. Furthermore, ancestral sparkling wines are usually conceived to be short aged which means that acidity levels from berries can be lower at harvest time. In addition, the needs of sulphite addition to protect against oxidation or microbiological spoilage are much lower in ancestral sparkling wines since they do not require a stabilization time between the two fermentations. This is without any doubt a great advantage since sulphites that have been shown to have adverse effects for human health [
27] and for environment [
28].
Because of its recent popularity, the scientific literature on ancestral sparkling wines is very scarce and only few scientific articles can be found [
29,
30,
31]. By contrast, traditional sparkling wines [
32,
33,
34,
35] and other natural sparkling wine methods [
9,
36,
37] have been widely studied. Given this lack of information, the aim of this work was to compare the physicochemical characteristics and sensory properties of a representative amount of commercial ancestral sparkling wines elaborated in the winegrowing zone of Catalonia with young aged sparkling wines elaborated by the traditional method.
2. Materials and Methods
2.1. Wine Samples
A total of 20 white sparkling wines, 9 elaborated by traditional method and 11 by ancestral method were collected from different wine-specialized shops. The Ancestral sparkling wines were all without any PDO label. The Traditional sparkling wines were mainly from the classic blend of the PDO Cava (Xarel·lo, Macabeu and Parellada cultivars). Ancestral sparkling wines were presented with cork or with crown cap and some of them were presented with transparent bottle while Traditional sparkling samples were all presented with cork cap (mandatory) and green opaque bottle. All Traditional sparkling wines were from the 2022 vintage while Ancestrals were from different vintages. All the analyses were carried out by triplicate using three bottles of each sample. The data from all the wines collected can be found at
Table 1.
2.2. Sample Preparation
All wine samples were centrifuged at 13,000xg (Biofuge Primo centrifuge, Heraeus, Hanau, Germany) for 15 min at 4oC to obtain clear samples and to remove carbon dioxide.
2.3. Analysis of General Wine Parameters
The internal CO
2 pressure of the bottles was measured using a non-invasive Laser Sensor (L.Sensor CO2, FTSystem, Alseno, Italy). The ethanol content was determined by ebulliometry (GAB Analysis Systems, Moja-Olerdola, Barcelona, Spain). Turbidity was measured with a 2100N IS TURBIDIMETER (HACH, Loveland,CO, USA). Titratable acidity and pH were determined following the OIV recommended methods [
38]. The total sulphur dioxide content was determined using a commercial kit (GAB Analysis Systems, Moja-Olerdola, Barcelona, Spain). The concentrations of residual fermentable sugars (D-glucose and D-fructose), glycerol, gluconic acid, L-(+)-tartaric acid, L-Malic acid, L and D Lactic acid, citric acid and acetic acid were determined using Y15 Autoanalyser (Biosystems, Barcelona, Spain).
2.4. Colour Parameters
The CIEL*a*b* coordinates were determined following the method described by Ayala et al. [
39] using a Helios Alpha UV VIS spectrophotometer (Thermo Fisher Scientific Inc., Waltman, MA, USA). Data were processed using MSCV® software. Total Polyphenol index (TPI) was determined by the dilution 1:10 of each sample and the measurement at 280 nm via spectrophotometer. The colour of each one of the samples was reproduced in power point software using the RGB signals after transforming the CIEL*a*b* coordinates (ColorMine.org).
2.5. Quantification of Proteins by HRSEC-DAD
The samples were processed and analysed using the methodology described by Canals et al. [
40]. Fifteen mL of each sample were concentrated in triplicate following a two steps dialysis in tubes with a molecular weight cutoff of 3.5 kDa (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA). The first step lasted 48h with 0.3 M ammonium acetate ≥ 98.0 % (Sigma–Aldrich, Madrid, Spain) solution with a rate of 1:10 (sample:solution) and constant agitation. The second step was carried out with water for another 48h. The dialyzed samples were subsequently lyophilised and preserved at −20 °C. The soluble fractions were analysed by high-resolution size-exclusion chromatography (HRSEC) in order to determine the molecular distribution and quantify the proteins obtained from the samples. The lyophilized samples were resuspended in 0.6 μL of ammonium acetate solution (300 mM) and centrifuged (12,000 g for 5 min). The supernatant was filtered through 0.22 μm acetate cellulose filters (Merck Millipore, Darmstadt, Germany) and then 100 μL of supernatant was injected into the chromatographic system. The analyses were carried out in HPLC Agilent 1200 Series system (Agilent Technologies Inc., Santa Clara, USA) equipped with a G1311A quaternary pump, a G1316A column oven, a G1329A autosampler (Agilent Technologies, Santa Clara, CA, USA) and with a diode array detector (G1315D - DAD) to monitor output at 230 and 320 nm. Separation was carried out at 20 °C using an S 165 Shodex gel permeation HPLC column 210 (OHpak 166 SB-803 HQ, 300 mm × 8 mm i.d.; Showa Denko, Tokyo, Japan). The mobile phase consisted of an aqueous solution of 300 mmol/L ammonium acetate applied at a constant flow of 0.6 mL/min for 70 min.
2.6. Polysaccharide Extraction and Determination by HRSEC-RID
The samples were processed using a variation of the methodology described by Ayestarán et al. [
41]. Briefly, 1 mL of sample was frozen to -20
oC and then freeze-dried using a lyophilizer (Telstar LyoQuest HT40, Barcelona, Spain). The pellet was resuspended with 0.2 mL of ultra-pure water and 1 mL of cold acidified ethanol (hydrochloric acid 0.3 M in absolute ethanol) and kept for 24 h at 4°C to allow soluble polysaccharides precipitation. Samples were centrifugated (14,000 RPM) 10 minutes, the supernatant was discarded and the pellet was dried using heating block (70
oC). The pellet was resuspended in mL of 50 mM ammonium formate ≥ 99.0 % (Sigma–Aldrich, Madrid, Spain) and filtered through 0.22 μm acetate cellulose filters (Merck Millipore, Darmstadt, Germany). Then 100 μL were injected into the chromatographic system. The analyses were carried out in an HPLC Agilent 1200 Series system (Agilent Technologies Inc., Santa Clara, USA) equipped with a G1311A quaternary pump, a G1316A column oven, a G1329A autosampler (Agilent Technologies, Santa Clara, CA, USA) and with a refractive index detector (G1362A - RID). Separation was carried out at 20 °C using two Shodex gel permeation HPLC columns (OHpak SB-186 803 HQ and SB-804 HQ, 300 mm × 8 mm I.D.; Showa Denko, Japan). The mobile phase consisted of an aqueous solution of 50 mM ammonium formate applied with a constant flow of 0.6 mL/min for 60 min, and the cell RID temperature was 35 °C.
2.7. Volatile Compound Analysis by Gas Chromatography
Volatile compounds were extracted using a modification of the methodology described by Ortega et al. [
42]. the volatile compounds were liquid/liquid extracted with 400 μL of dichloromethane in presence of 2.5 g (NH
4)
2SO
4 using 4-methyl-2-pentanol (0.8 g/L), heptanoic acid (0.7 g/L) and heptadecanoic acid (0.7 g/L) as internal standards.
The organic phase was extracted and 2 μL was injected in split mode (10:1, 30 mL/min) into a gas chromatograph (Agilent Technologies Inc., Santa Clara, USA) with a FFAP column of 30 m × 0.25 mm × 0.25 μm. All aromatic volatile compounds were identified and quantified by comparison with standards. They included fatty acids ethyl esters (ethyl butyrate, ethyl hexanoate, ethyl octanoate, ethyl decanoate and ethyl dodecanoate), fusel alcohols (cis-3-hexen-1-ol, 2-phenylethanol and 1-hexanol), acetate esters ( isoamyl acetate, hexyl acetate, 2-phenyletanol acetate), short-chain fatty acids (propionic, isobutyric, butyric, 3-methyl butanoic and valeric acids), medium-chain fatty acids (hexanoic, octanoic, decanoic and dodecanoic acids) and long-chain fatty acids (myristic acid, palmitic acid, stearic acid and oleic acid).
2.8. Measurement of Foaming Properties
The foam properties were measured using the Mosalux method (Station Oenotechnique de Champagne, Epernay, France) according to the procedure described by Maujean et al [
43]. Two parameters were measured: HM, the maximum foam height, and HS, the stable foam height. HM represents foamability while HS represents foam stability.
2.9. Sensory Analysis
All the samples were tasted by a trained panel of 15 tasters, nine males and six females aged between 24 and 60. For each sample, the tasters were required to evaluate the intensity of eight sensory attributes (Colour, Bubble size and stability, reduction/oxidation balance, ageing impact, gas aggressivity, body, and acidity) and 4 aromatic attributes ( tropical fruit, aniseed, white fruits, yeast/bread) on a scale of 1 to 10 ( 1 = “slight intensity”, 10 = “maximum intensity”. In the case of Reduction/oxidation balance, the scale goes from evident reduction notes (1) to high oxidation notes (10). The value of each descriptor was expressed as the average of all tasters. The sensory analysis was performed by triplicate in order to avoid random results.
2.10. Statistical Analysis
The data shown are the arithmetic means of triplicates with the standard deviation for each parameter. One-way ANOVA and Tukey comparison tests were carried out using the XLSTAT software version 2022.5.1 (Addinsoft, Paris, France). The sensorial analysis results were analysed also with the PanelCheck V1.4.2 software (Nofima Mat, Technical University of Denmark & University of Copenhagen).
3. Results and Discussion
3.1. General Parameters
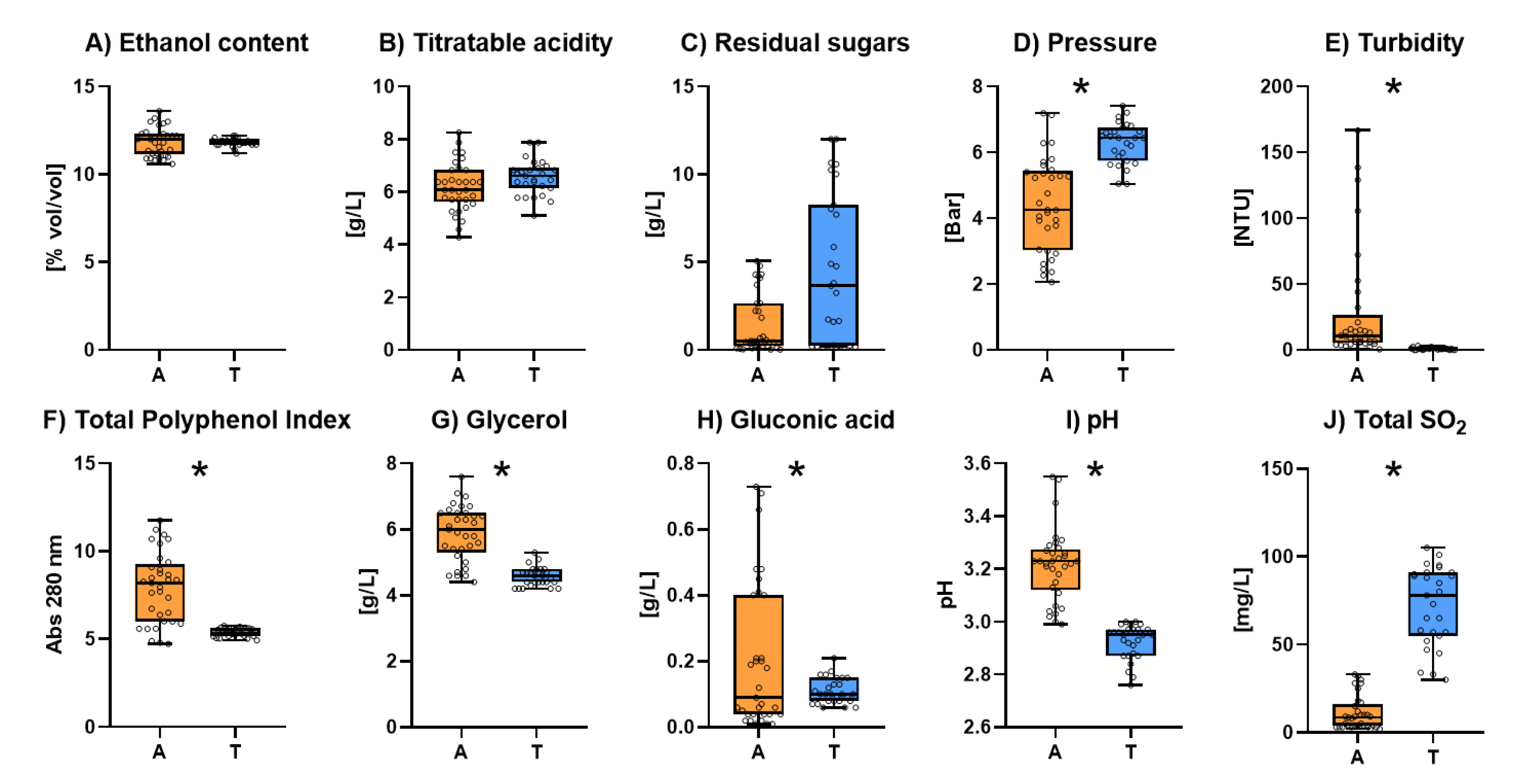
All figures include only the average ± standard deviation of the different parameters of both types of sparkling wines. The results for all the studied sparkling wines are shown as .
The results corresponding to the general parameters are shown in
Figure 1. The individual results for general parameters of all the studied sparkling wines are shown in
Table S1. No significant differences were found between ancestral and traditional sparkling wines for ethanol content (
Figure 1.A) and total titratable acidity (
Figure 1.B). Neither significant difference was found for residual sugars (
Figure 1.C) even though some of the traditional sparkling wine samples were classified as “Brut” and therefore contained some added sugar.
The average internal pressure (
Figure 1.D) of Traditional sparkling wines was significantly higher than that of ancestral sparkling wines. In addition, all the Traditional sparkling wines accomplished with the normative [
6] having a homogeneous internal pressure above 3.00 bar whereas Ancestral sparkling wines showed greater heterogeneity being some of the samples below the minimal value.
Figure 1.E shows the turbidity of the different samples. Turbidity of Traditional sparkling wines was always below 5 NTU which should be considered as a clear appearance for the consumers. In contrast, the turbidity of Ancestral sparkling wines was more heterogenous and the average was significantly higher than that of Traditional sparkling wines. Some of the Ancestral sparkling wines were also below 5 NTU but others had turbidity levels over 100 NTU which could lead to unstable and faulty wines. It should be highlighted that although turbidity is not regulated by OIV, a high turbidity is considered a fault [
44].
The total polyphenol index (TPI) (
Figure 1.F) was also significantly higher in Ancestral sparkling wines than in Traditional ones. This higher value can be related to the higher turbidity of these sparkling wines probably because of the lack of cold settling before fermentation or because the lower use of fining agents according with the minimal intervention philosophy of most of Ancestral sparkling wine producers. A high TPI in white wines is also related to a loss of sensorial attributes in wines and higher colour oxidability [
45].
Glycerol (
Figure 1.G) content was found to be significantly higher on Ancestral sparkling wines than in Traditional ones. Glycerol is mainly produced by yeast throughout glyceropyruvic pathway [
46]. It has been described that glycerol is in part produced by yeast to compensate the osmotic pressure of the fermenting matrix [
47]. A possible explanation of the higher levels of this metabolite may be therefore related with the fact that Ancestral sparkling wines have got only one alcoholic fermentation step with an initial total sugar concentration higher than Traditional sparkling wines. Glycerol can also be produced when the grapes are infected by
Botrytis cinerea [
48]. Consequently, another possible explanation may be related with the fact that grapes for Ancestral sparkling wine production are usually harvested later than those for traditional sparkling wines increasing the risk of appearance of this filamentous fungus. This last data is confirmed by the significantly higher levels of gluconic acid (
Figure 1.H) in Ancestral sparkling wines since this acid is considered as a marker of the presence of
B. cinerea [
49].
Ancestral sparkling wines had also significant higher pH levels (
Figure 1.I). This data could be easily explained because of the harvest date of the grapes for ancestral sparkling wines is generally later than for traditional sparkling wines. Total Sulphur dioxide content (
Figure 1.J) was found to be significantly lower in Ancestral sparkling wines, always below 30 mg/L. These lower levels of sulphur dioxide are mainly because Ancestral sparkling wines are directly bottled in the adequate moment of the fermentation and therefore, they do not need the addition of this additive to protect them during the stabilization period [
50].
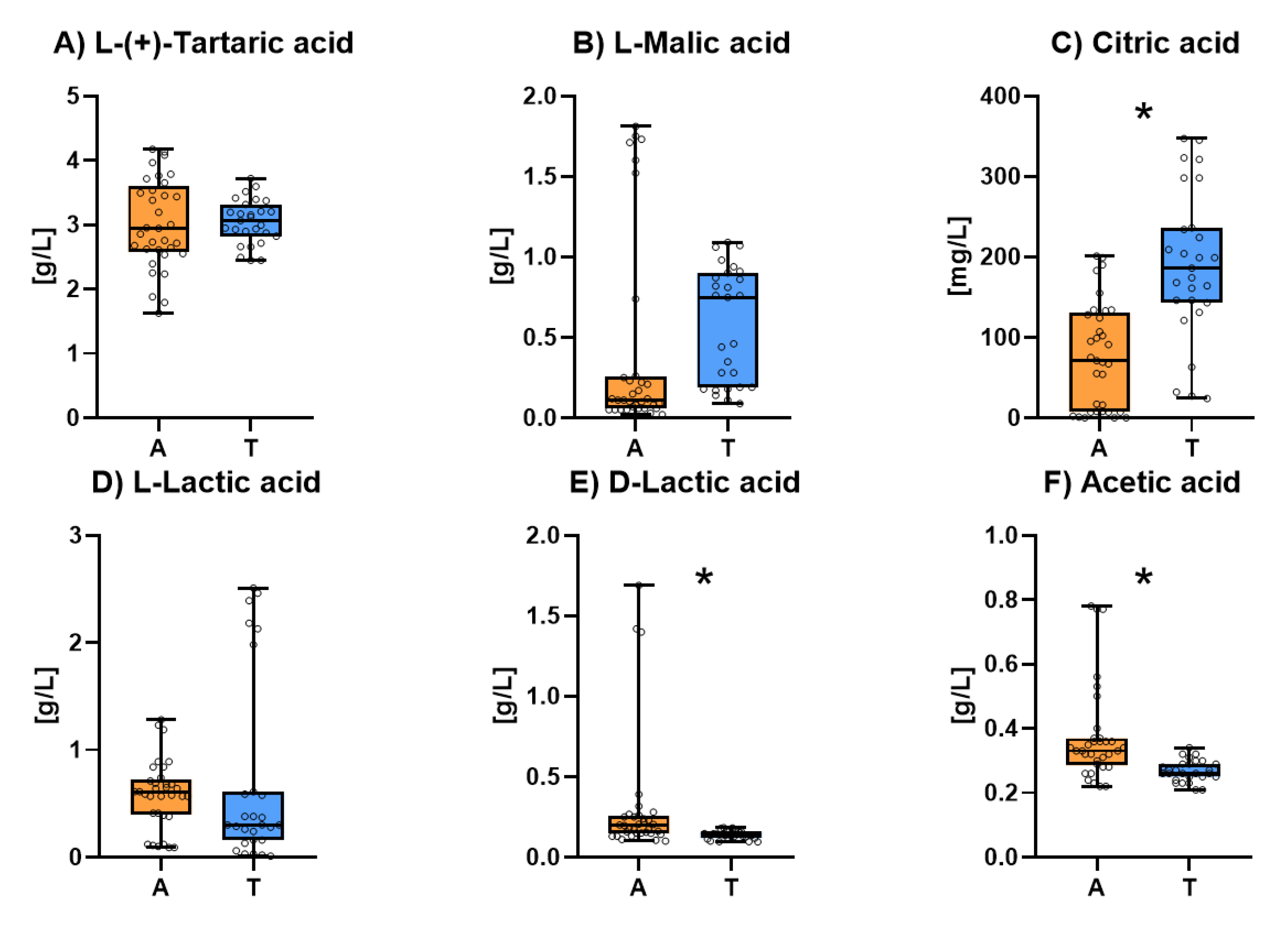
3.2. Acidic characterization of sparkling wines
The concentration of the major acids of the different sparkling wines is showed in
Figure 2. The individual results for acidic composition of all the studied sparkling wines are shown in
Table S2. The levels of L-(+)-tartaric acid (
Figure 2.A), L-Malic acid (
Figure 2.B) and L-lactic acid (
Figure 2.C) were similar in both types of sparkling wines whereas the concentration of citric acid (
Figure 2.D) was significantly higher in Traditional sparkling wines. It should be highlighted that in both groups there were samples in which malolactic fermentation was carried out and other samples in which it was not.
The average content of D-lactic acid (
Figure 2.E) and acetic acid (
Figure 2.F) were significantly higher in Ancestral sparkling wines. Nevertheless, this difference was mainly due to one of the Ancestral samples in which lactic taint seems to have been produced (D-lactic acid levels over 1g/L and acetic acid levels around 0.8 g/L). Excessive levels of D-lactic acid are related to the metabolism of D-Fructose produced by an uncontrolled growth of lactic acid bacteria in the presence of sugars. Acetic acid is also generated when heterofermentative lactic acid bacteria metabolize D-fructose [
51,
52].
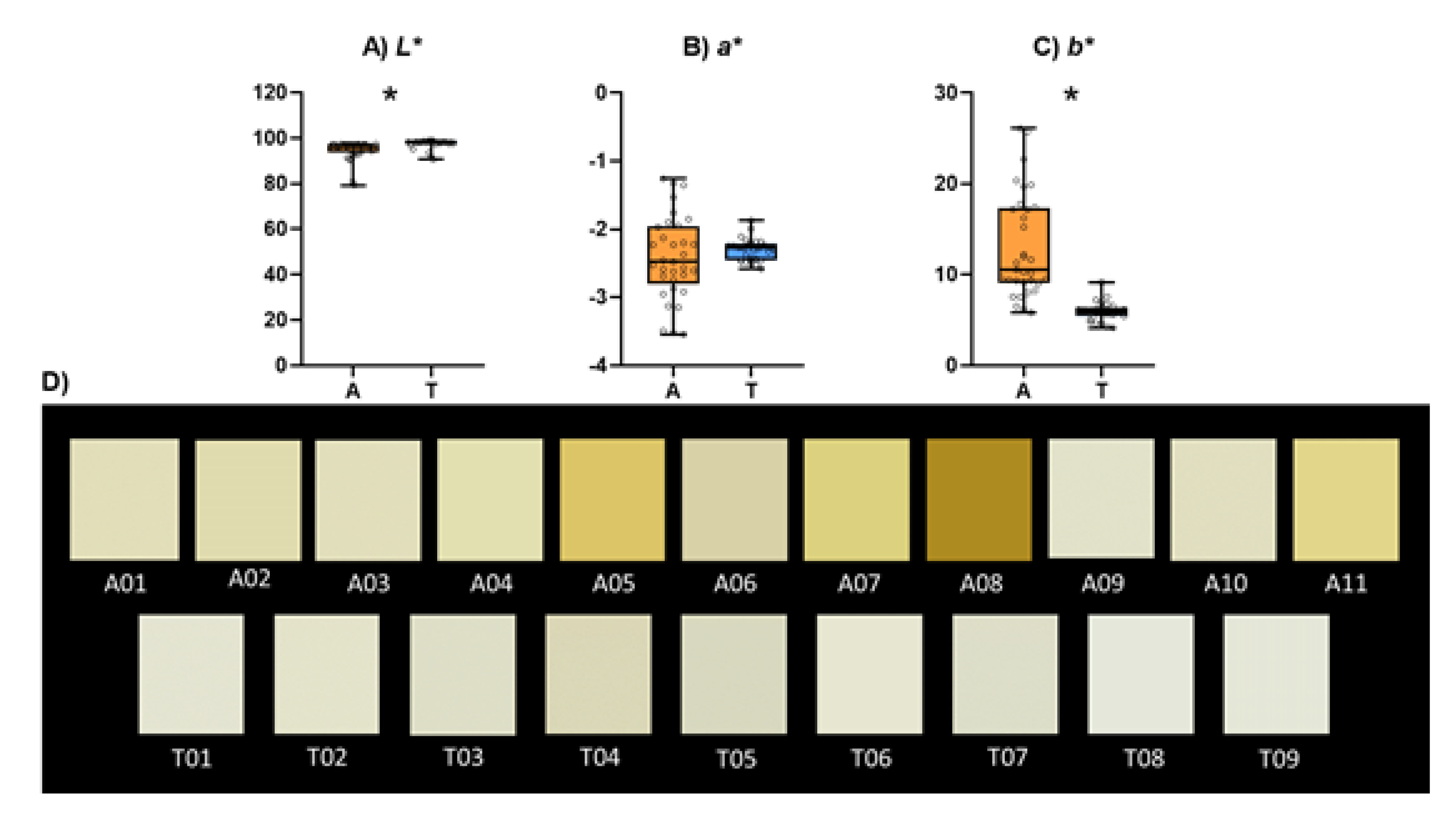
3.3. Colour
Figure 3 shows the Cie
L*a*b* coordinates values and the colour appearance of the different sparkling wines. The individual results for colour parameters of all the studied sparkling wines are shown in
Table S3. Lightness (
L*) (
Figure 3.A) of Ancestral sparkling wines was significantly lower and the blue-yellow component of the colour (
b*) (
Figure 3.C) significantly higher than in Traditional sparkling wines whereas no significant differences were found in the green-red component (
a*) (
Figure 3.B). These data indicates that the colours of Ancestral sparkling wines were in general less clear and more yellowish than in Traditional sparkling wines as it can be seen in the picture (
Figure 3.D). This larger palette of colours observed in Ancestral sparkling wines reflects more brownish tonalities in some of these wines which could be related to the lower sulphur dioxide usage that could entail a higher oxidation [
53].
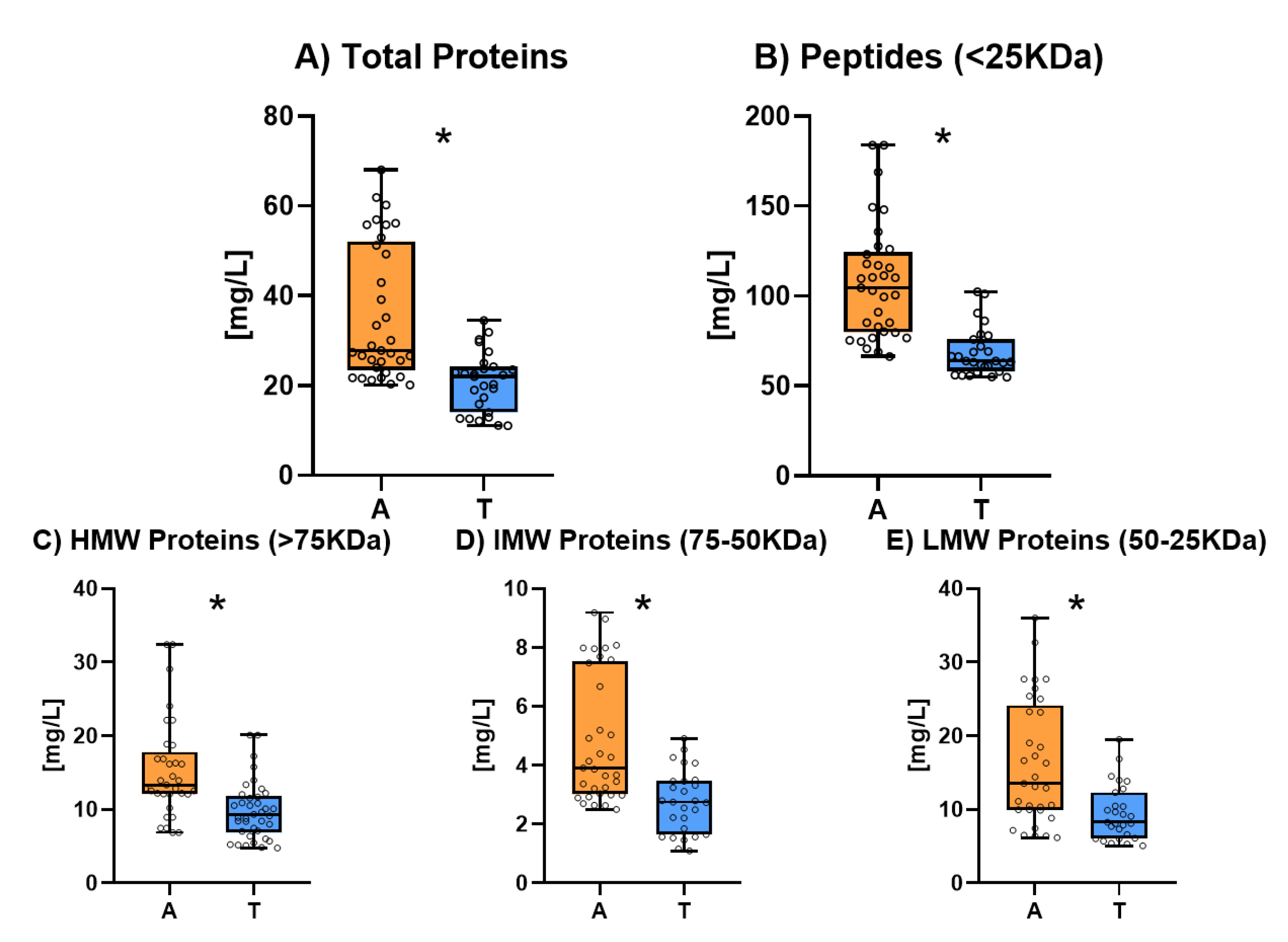
3.4. Proteins
Protein and peptide content determined via HPLC are showed in
Figure 4. The individual results for protein and peptide composition of all the studied sparkling wines are shown in
Table S4. These results show that Ancestral sparkling wines had significantly higher protein content than Traditional sparkling wines. Total proteins (
Figure 4.A) were around an average of 30 mg/L in Ancestral sparkling wines while in Traditional wines the average content was around 20 mg/L. All molecular weight protein fractions (
Figure 4.C, 4.D, 4.E) showed a similar behaviour, being high (HMW) and the low molecular weight (LMW) fractions the most abundant. Peptide content (
Figure 4.B) was also found to be significantly higher in Ancestral sparkling wines
This higher concentration of proteins and peptides may be due to two different reasons. In one hand, the base wines of Traditional sparkling wines are usually treated with bentonite for fining and riddling which eliminates a substantial amount of natural wine occurring proteins [
54,
55]. By contrast, Ancestral sparkling wines are not usually bentonite fined and some elaborators neither add bentonite to favour lees sedimentation during riddling. On the other hand, it is quite probable that some of the Ancestral sparkling wines were bottled with higher yeasts populations than Traditional sparkling wines which should favour the release of proteins and peptides from yeast autolysis[
56,
57,
58].
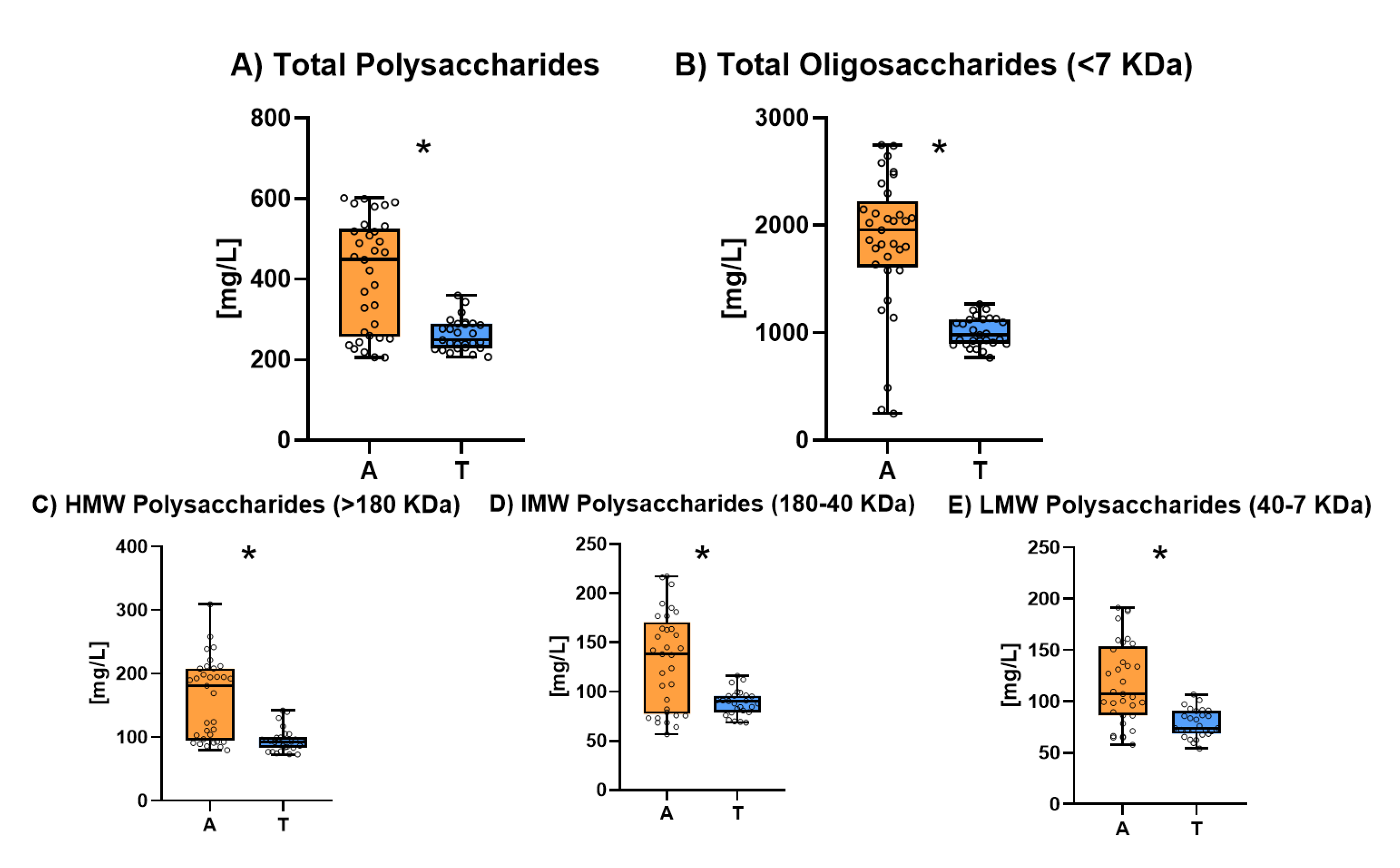
3.5 Polysaccharides
Figure 5 shows the polysaccharide and oligosaccharide content. The individual results for polysaccharide and oligosaccharide composition of all the studied sparkling wines are shown in
Table S5. Ancestral sparkling wines had significantly higher concentrations of polysaccharides (
Figure 5.A) (from 200 to 600 mg/L) than Traditional method sparkling wines (all around 200 mg/L). Oligosaccharide content (
Figure 5.B) was also significantly higher for Ancestral Sparkling wines. The reasons why ancestral wine has significantly higher levels of these molecules would be the same as those that have been put forward for proteins.
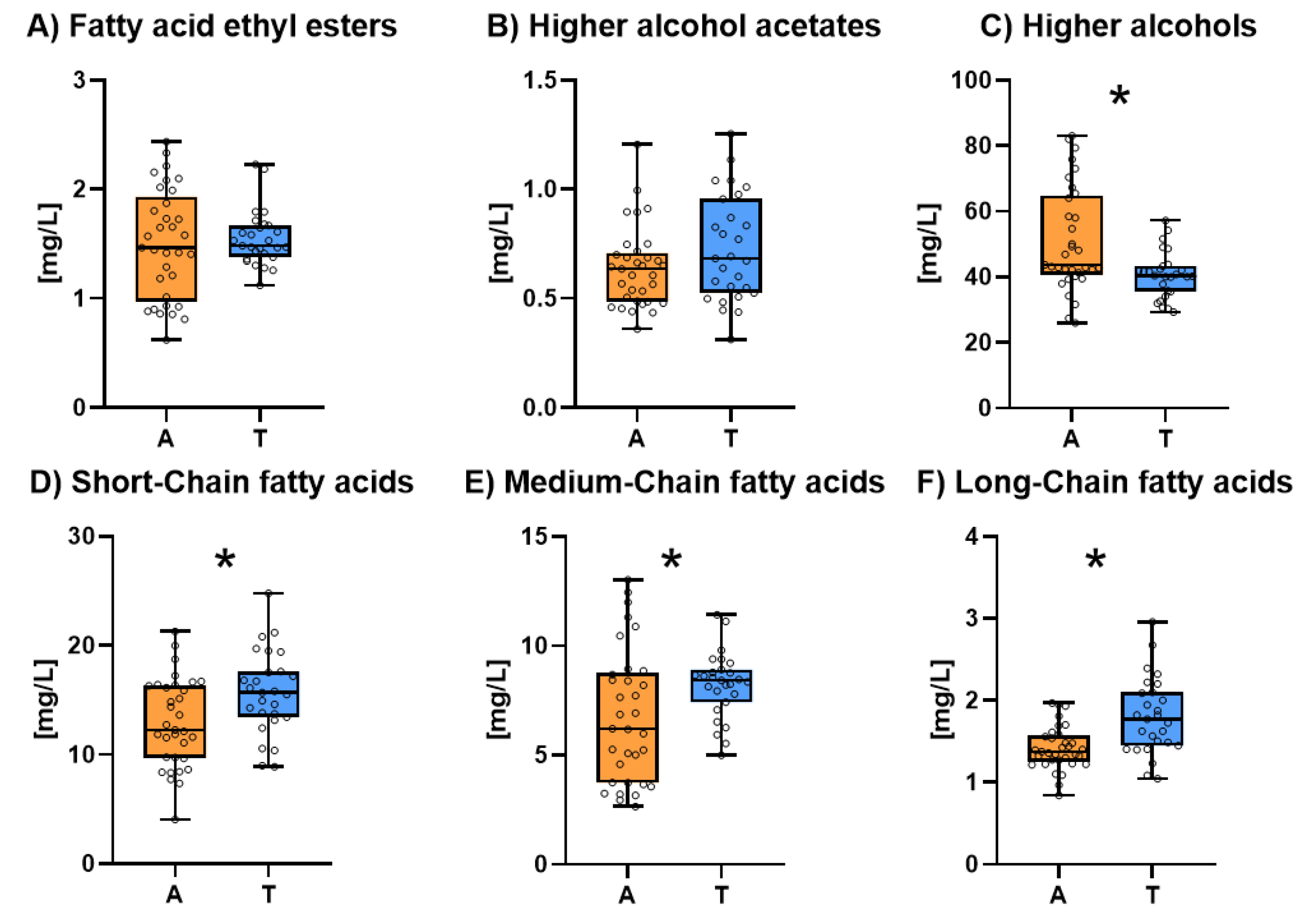
3.6. Volatile Substances
Figure 6 shows the volatile substances composition of both types of sparkling wines. The individual results for each one of the six families of all the studied sparkling wines are shown in
Table S6. The volatile substances were grouped in this figure in six families. No significant differences were found in the concentration of fatty acids ethyl esters (
Figure 6.A) and total higher alcohol acetates (
Figure 6.B). However, Ancestral sparkling wines showed higher levels of higher alcohols (
Figure 6.C) whereas Traditional sparkling wines showed significantly higher levels of short (
Figure 6.D), medium (
Figure 6.E) and long-chain fatty acids (
Figure 6.F).
Higher alcohols are the result of amino-acidic metabolism of yeasts when the nitrogen is scarce during fermentation [
59]. They are considered as not pleasant aromas once they achieve concentrations above their threshold (300 mg/L) and some authors described them as foam antagonists in sparkling wines [
43]. Nevertheless, their concentration in Ancestral sparkling wines was always below 100 mg/L, which means it should not affect negatively the aromatic profile of the wines but their presence could affect its foamability. It should be also highlighted that there is a close relationship between turbidity during alcoholic fermentation and higher alcohols concentration [
60]. In fact, non-settled grape musts give rise to wines with a higher concentration of higher alcohols [
61]. This data suggest that the grape must of some of the ancestral sparkling wines was not well settled.
Fatty acids generally are mainly produced by yeast during alcoholic fermentation but can also be released from grape skin [
62]. Short (SCFA) and medium (MCFA) chain fatty acids have been described as toxic for yeast being sometimes responsible of stuck and sluggish fermentations [
63,
64]. By contrast, long-chain fatty acids (LCFA) are principal constituents of the yeast cell membrane and its presence increases with aereation during fermentation [
65]. A possible explanation of the higher levels of fatty acids in Traditional sparkling wines could be that these wines have been elaborated by a refermentation of a base wines which implies a higher stress whereas Ancestral sparkling wines have been obtained with only one fermentation. Another possibility is that ancestral sparkling wines, which have a greater population of yeasts than Traditional wines, have absorbed more fatty acids [
64,
66].
3.7. Foaming Properties
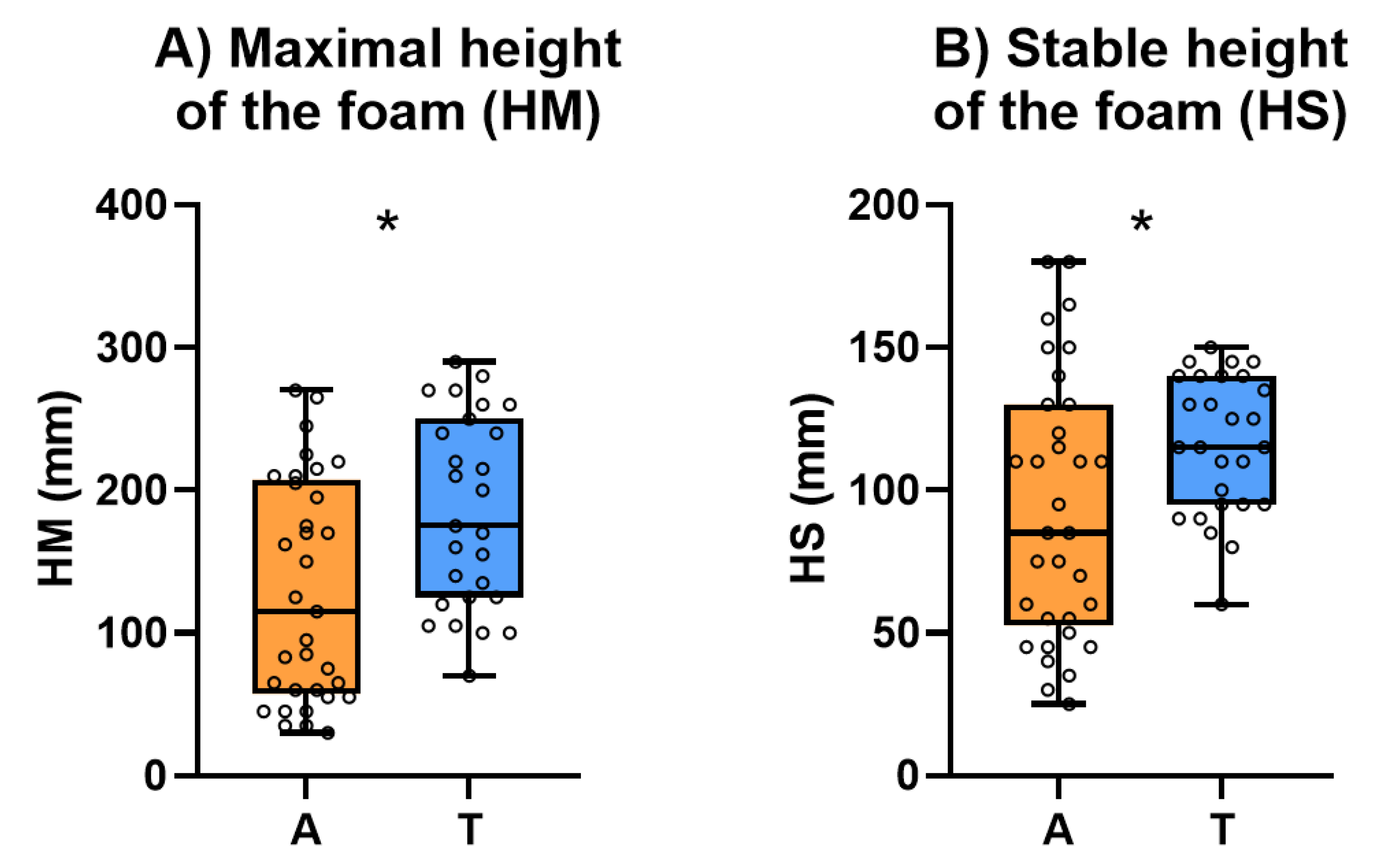
The maximal height of the foam (HM) (
Figure 7.A) that represents the wine foamability and stable height of the foam (HS) (
Figure 7.B) that represents the foam stability of both types of sparkling wines are shown in
Figure 7. The individual results for HM and HS of all the studied sparkling wines are shown in
Table S7.
In general, Traditional sparkling wines had significantly higher values of HM and HS than Ancestral sparkling wines although there was a great heterogeneity in both groups. It has been described that peptides, proteins and mannoproteins favour the integration of carbon dioxide improving foamability [
67,
68]. Consequently, the higher HM and HS of Traditional sparkling wines is an unexpected result because their peptide, protein and polysaccharide concentration were significantly higher than in Ancestral sparkling wines. However, it must be highlighted that Ancestral sparkling wines have significant higher levels of gluconic acid than Traditional sparkling wines. Gluconic acid is an indicator of the development of
B. cinerea in the grapes and it is well known that their presence affects negatively the foamability of sparkling wines [
49,
69]. In addition, Ancestral sparkling wines also have significantly higher concentration of higher alcohols which have been reported as negative for foamability [
43].
3.8. Sensorial Analysis
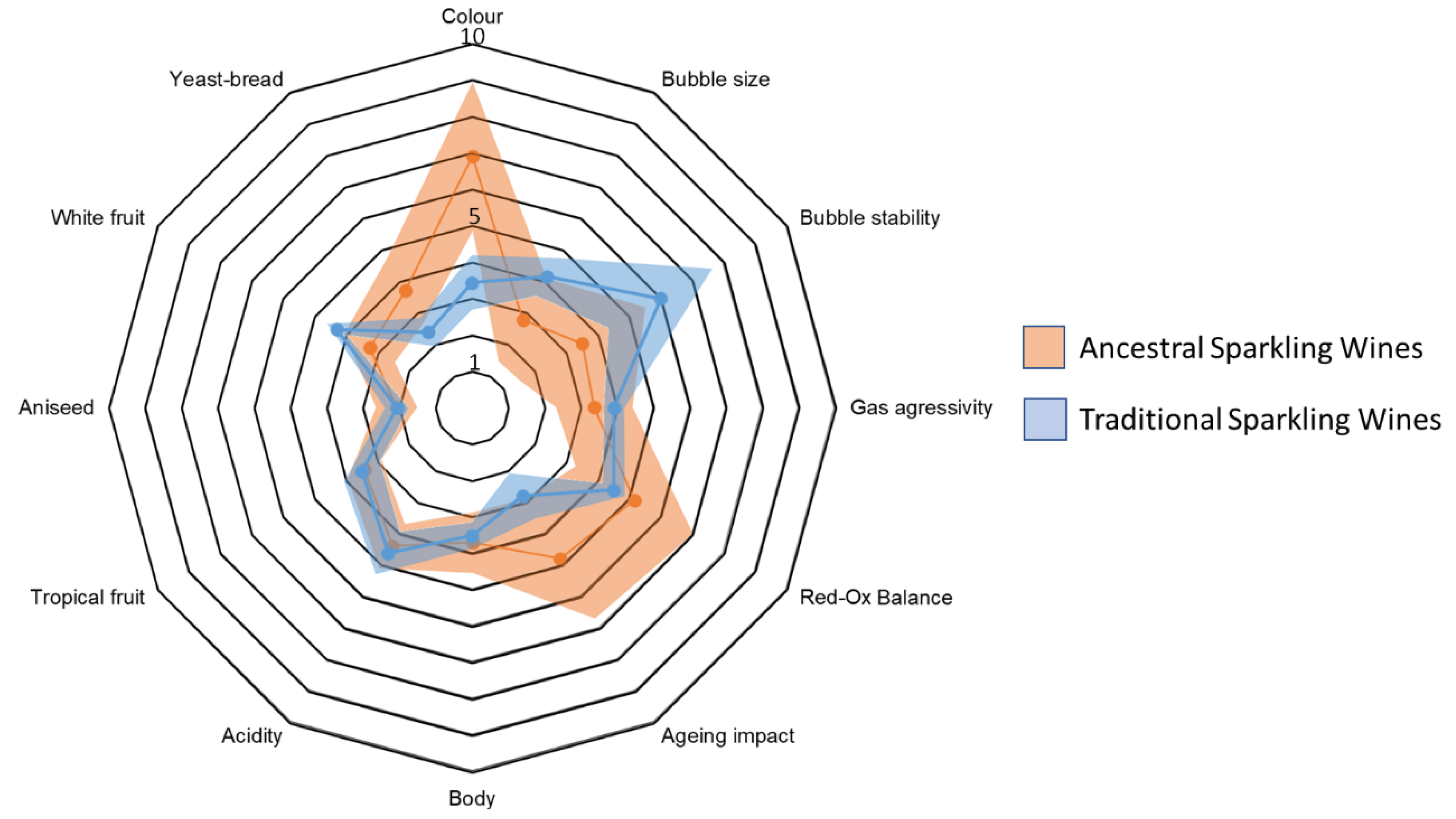
Sensorial analysis results are shown in the
Figure 8 in form of a spider web chart in which the average value for each one of the sensory attributes is indicated by means of a continuous line whereas the standard deviation is showed as a shadowed ring surrounding it. In general, Traditional sparkling wines showed greater homogeneity than Ancestral sparkling wines as it can be noticed by the narrower shadowed ring. The panel scored very similarly the following sensory attributes: gas aggressivity, reduction/oxidation balance, body, acidity, tropical fruit and aniseed notes. By contrast, the panellists considered that Ancestral sparkling wines showed more intense colour, ageing impact and yeast-bread aroma, and less intense notes of white fruit. They also considered that Ancestral sparkling wines have smaller bubble size and lower bubble stability.
3.9. Principal Component Analysis
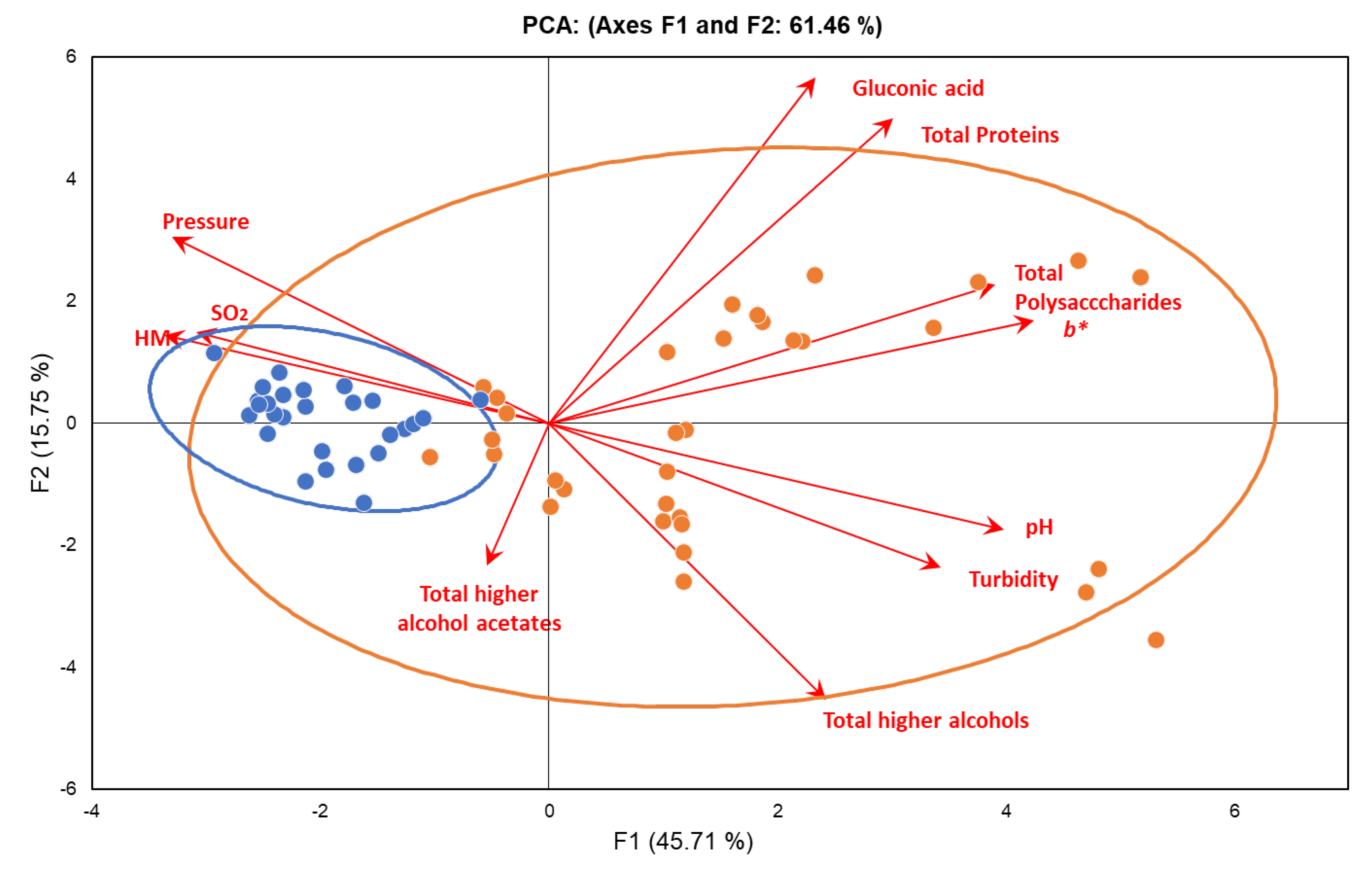
In order to better understand the differences between Ancestral and Traditional sparkling wines a principal component analysis was performed.
Figure 9 shows the plot of varimax-rotated corresponding to the principal components analysis. The first component (PC1) explains 45.71% of the variance, and the second (PC2) explains 15.75% (so the explained aggregate variance was 61.46% for the two components). The loadings are presented as arrows, the length and direction of which indicate the contribution made by both components. PC1 showed a significant correlation with all variables except total higher alcohol acetates. Specifically, PC1 was positively correlated with gluconic acid, total proteins, total polysaccharides, Cie
L*a*b*
b* parameter, pH, turbidity and total higher Alcohols. In turn, PC2 showed positive significant correlation with gluconic acid, total proteins and pressure and negative significant correlation with total higher alcohol acetates, total higher alcohols and turbidity. With regard to the different variables, total polysaccharides,
b*, pH, turbidity and total higher alcohols showed a strong negative correlation with HM, SO
2 and pressure. In addition, gluconic acid and total proteins had a negative correlation with total higher alcohol acetates.
All the samples corresponding to the Traditional sparkling wines group were placed in a relatively small confidence ellipse (95%) which indicates the existence of a great homogeneity among the samples. By contrast Ancestral sparkling wines were more ubiquitously placed throughout the entire diagram which confirms the much greater heterogeneity among the samples.
It must be highlighted that the arrows corresponding to pressure, SO2 and HM were directed towards the left coinciding with the location of the Traditional sparkling wines. This data indicates that Traditional sparkling wines have higher pressure and sulphur dioxide and better foamability than Ancestral sparkling wines. The arrow corresponding to total higher alcohol acetates was directed also towards the left, but downwards, indicating that Traditional Sparkling wines are richer in these volatile substances. By contrast, the arrows corresponding to gluconic acid, total proteins, total polysaccharides, b*, pH, turbidity and total higher alcohols were directed to the right, indicating that the samples placed there have higher levels of all these parameters. It must be highlighted that most of the Ancestral sparkling wines were located to a greater or lesser extent at the right side of the diagram. However, some of the Ancestral sparkling wines were placed on the left side, very close to the Traditional sparkling wines confidence ellipse. This data confirms that some of the Ancestral sparkling wines were very similar to Traditional sparkling wines whereas others were quite different.
4. Conclusions
The physicochemical and sensory analyses of different sparkling wines elaborated by Traditional or Ancestral methods reveal the existence of some differences between them. Traditional sparkling wines are much more homogeneous than Ancestral sparkling wines having in general higher values of pressure, sulphur dioxide and better foamability. By contrast, Ancestral sparkling wines were much more heterogenous showing in general higher values of gluconic acid, total proteins and polysaccharides, more intense yellow colour, pH and higher turbidity. However, these differences are mainly since Ancestral method is not so well defined as Traditional method making that sometimes the quality of the product can be affected seriously. More specifically, the control of the sugar concentration and yeast population at bottling, are critical points that must be better defined in the elaboration protocols of Ancestral wines because they conditionate the final internal pressure and probably the turbidity of the product. This lack of definition in the Ancestral sparkling wine elaboration procedure may be responsible of the greater heterogeneity of these wines and that even in some cases they present some taints. However, Ancestral method allows working with riper grapes which represents a great advantage in the face of climate change. An additional advantage is that Ancestral methods allows working with lower doses of sulphur dioxide. Today these advantages make the Ancestral method an interesting alternative to the Traditional method to produce sparkling wines.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
A.J.-B.: methodology, investigation, writing—original draft, and data curation; M.A.-H.: methodology and research; A.G.-R.: methodology and research; M.B.: methodology and research; J.G.: methodology and research; P.C.: supervision; N.R.: supervision; J.M.C.: supervision; F.Z.: supervision, methodology, research, writing—original draft, and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant 2020PMF-PIPF-32 awarded by the University Rovira i Virgili.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no competing financial interests or personal relationships that could influence the work reported in this paper.
References
- Imarc, “Sparkling Wine Market Report by Type (Red, Rose, White), Product (Cava, Champagne, Cremant, Prosecco, and Others), Price Point (Economy, Mid-range, Luxury), Sales Channel (Supermarket and Hypermarket, Specialty Stores, On Trade, and Others), and Region 20,” 2023.
- S. Viciana, “Vinos Espumosos Buscando la premiumización ante el reto inflacionista,” Alimarket, 2023.
- P. Vachon, “The State of the Sparkling Wine Market,” SevenFiftyDaily, 2023.
- Concours Mondial de Bruxelles, “Tendencias que definen el mercado mundial de los vinos espumosos,” 2022. [Online]. Available: https://concoursmondial.com/es/tendencias-que-definen-el-mercado-mundial-de-los-vinos-espumosos/. [Accessed: 01-Mar-2024].
- K. T. Studeman, “Champagne’s Cooler Cousin: 5 Pét-Nat Sparkling Wines to Try Now,” VOGUE, 2015. [Online]. Available: https://www.vogue.com/article/pet-net-sparkling-wine-champagne-alternative. [Accessed: 01-Mar-2024].
- European council, “Council Regulation (EC) No 491/2009,” 2009.
- OIV, International Code of Oenological Practices, 20tH G.A. Ensenada, Mexico, 2023.
- F. Bassi, F. Pennoni, and L. Rossetto, “Market Segmentation and Dynamic Analysis of Sparkling Wine Purchases in Italy,” J. Wine Econ., vol. 16, no. 3, pp. 283–304, 2021.
- V. Caliari, C. P. Panceri, J. P. Rosier, and M. T. Bordignon-Luiz, “Effect of the traditional, charmat and asti method production on the volatile composition of moscato giallo sparkling wines,” Lwt, vol. 61, no. 2, pp. 393–400, 2015.
- Rose, Fizz! Champagne and sparkling wines of the world. Cassington, United Kingdom: Infinite Ideas Limited, 2021.
- Robinson and, J. Harding, The Oxford Companion to Wine, 4th ed., no. July. New York, USA: Oxford University Press, 2015.
- Forum Œnologie Association, “Effervescents du Monde,” 2023. [Online]. Available: https://www.effervescents-du-monde.com/pages/20_result/index.en.html.
- R. Roset, “Els ‘Barcelona Rosé’ accepten els escumosos ancestrals,” VAdeVI, 2024. [Online]. Available: https://vadevi.elmon.cat/premis-vinari/barcelona-rose-abracen-escumosos-ancestrals-119416/. [Accessed: 02-Mar-2024].
- Wine Pleasures, “50 Great Sparkling Wines of the World Awards & Scores 2022,” 2022. [Online]. Available: https://www.winepleasures.com/contest/50-great-sparkling-wines-of-the-world-awards-scores-2022/. [Accessed, 2024.
- Concours Mondial de Bruxelles, “Resultados 2023 CMB,” 2023. [Online]. Available: https://results.concoursmondial.com/es/resultados/2023?_token=B8OdtEIuzj3u7qu1a97siYfrMABvX9HVJSxhVMRC&search%5Bsession%5D=concours-mondial-de-bruxelles-sparkling-wine-session&search%5Bname%5D=&search%5Breward%5D=all&search%5Bcountry%5D=all&search%5Bregio. [Accessed: 01-Mar-2024].
- Decanter, “Decanter World Wine Awards,” 2024. [Online]. Available: https://awards.decanter.com/DWWA/2016/search/wines?competitionType=DWWA&style=Sparkling. [Accessed: 01-Mar-2024].
- International Wine Challange, “International Wine challange,” 2024. [Online]. Available: https://www.internationalwinechallenge.com/. [Accessed: 02-Mar-2024].
- E. Asimov, “Bubbles, With Joy: Pétillant Naturel’s Triumphant Return,” The New York Times, New York, USA, 2018. [Online]. Available: https://www.nytimes.com/2018/03/08/dining/drinks/wine-review-petillant-naturel.html. [Accessed: 02-Mar-2024].
- F. Wallace, “Natural wine 101: what’s pét-nat?,” VOGUE Australia, 2020. [Online]. Available: https://www.vogue.com.au/vogue-living/entertaining/natural-wine-101-whats-ptnat/image-gallery/4baa3e661f47c328197b519745c7854b. [Accessed: 02-Mar-2024].
- P. Ribéreau-Gayon, D. Dubourdieu, B. Donèche, and A. Lonvaud, Handbook of Enology: Volume 1, The Microbiology of Wine and Vinifications, vol. 1. 2006.
- C. Dubois, C. Flanzy, and J. M. Sablayrolles, “Blanquette methode ancestrale,” in Oenologie: Principes scientifiques et technologiques, C. Flanzy, Ed. Tec & Doc Lavoisier, 1998, p. 833.
- P. Jeandet, Y. Vasserot, G. Liger-Belair, and R. Marchal, “Sparkling wine production,” in Handbook of Enology: Principles, Practices and Recent Innovations, 1st ed., vol. III, no. 9, V. K. Joshi, Ed. New Delhi, India: Asiatech Publishers, 2011, pp. 2–52.
- P. S. Panesar, V. K. Joshi, V. Bali, and R. Panesar, “Technology for Production of Fortified and Sparkling Fruit Wines,” in Science and Technology of Fruit Wine Production, M. R. Kosseva, V. K. Joshi, and P. S. Panesar, Eds. Amsterdam, Netherlands: Elsevier Inc., 2017, pp. 487–530.
- DO Penedès, “Pliego de condiciones de la Denominación de Origen Protegida Penedès,” vol. C, pp. 1–23, 2020.
- DO Tarragona, “Pliego de condiciones DOP Tarragona,” 2021.
- F. Zamora, “Adapting red winemaking to climate change conditions,” J. Int. des Sci. la Vigne duVin, vol. Spécial Laccave, pp. 71–76, 2014.
- H. Vally and N. L. A. Misso, “Adverse reactions to the sulphite additives,” Gastroenterol. Hepatol. from Bed to Bench, vol. 5, no. 1, pp. 16–23, 2012.
- C.. Stockley, “Wine and health: sulfur dioxide and the wine consumer,” Aust. New Zeal. Grapegrow. Winemak., vol. 501, pp. 73–76, 2005.
- B. Dachery, K. C. Hernandes, C. A. Zini, J. E. Welke, and V. Manfroi, “Volatile and sensory profile of sparkling wines produced by faster and alternative methods (Ancestral and Single Tank Fermentation) compared to the usual methods (Charmat and Traditional),” Eur. Food Res. Technol., vol. 249, no. 9, pp. 2363–2376, 2023.
- A. S. Makarov and I. P. Lutkov, “Yeast race effect on the quality of base and young sparkling wines,” Foods Raw Mater., vol. 9, no. 2, pp. 290–301, 2021.
- J. S. Rossier, V. Maury, L. Gaillard, and E. Pfammatter, “Use of isotope ratio determination (13C/12C) to assess the production method of sparkling wine,” Chimia (Aarau)., vol. 70, no. 5, pp. 338–344, 2016.
- C. Berbegal et al., “Influence of the Dry Yeast Preparation Method on Final Sparkling Wine Characteristics,” Fermentation, vol. 8, no. 7, pp. 1–16, 2022.
- C. Cilindre et al., “Does the temperature of the prise de mousse affect the effervescence and the foam of sparkling wines?,” Molecules, vol. 26, no. 15, 2021.
- G. Liger-Belair, “The physics and chemistry behind the bubbling properties of champagne and sparkling wines: A state-of-the-art review,” J. Agric. Food Chem., vol. 53, no. 8, pp. 2788–2802, 2005.
- R. Martínez-García, T. R. Martínez-García, T. García-Martínez, A. Puig-Pujol, J. C. Mauricio, and J. Moreno, “Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle,” Food Chem., vol. 237, pp. 1030–1040, 2017.
- B. Cisilotto, F. J. Scariot, L. V. Schwarz, R. K. M. Rocha, A. P. L. Delamare, and S. Echeverrigaray, “Are the characteristics of sparkling wines obtained by the Traditional or Charmat methods quite different from each other?,” Oeno One, vol. 57, no. 1, pp. 321–331, 2023.
- J. A. Culbert et al., “Influence of Production Method on the Chemical Composition, Foaming Properties, and Quality of Australian Carbonated and Sparkling White Wines,” J. Agric. Food Chem., vol. 65, no. 7, pp. 1378–1386, 2017.
- OIV, Compendium of International Methods of Wine and Must Analysis. Dijon, France: OIV, 2023.
- F. Ayala, J. F. Echávarri, and A. I. Negueruela, “A new simplified method for measuring the color of wines. II. White wines and brandies,” Am. J. Enol. Vitic., vol. 48, no. 3, pp. 364–369, 1997.
- J. M. Canals, F. Zamora, and L. Arola, “Protein fraction analysis of white wine by FPLC,” Am. J. Enol. Vitic., vol. 49, no. 4, pp. 383–388, 1998.
- B. Ayestarán, Z. Guadalupe, and D. León, “Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process,” Anal. Chim. Acta, vol. 513, no. 1, pp. 29–39, 2004.
- C. Ortega, R. Lopez, J. Cacho, and V. Ferreira, “Fast analysis of important wine volatile compounds Development and validation of a new method based on gas chromatographic – flame ionisation detection analysis of dichloromethane microextracts,” vol. 923, pp. 205–214, 2001.
- A. Maujean, P. Poinsaut, H. Dantan, F. Brissonnet, and E. Cossiez, “Étude de la tenue et de la qualité de mousse des vins effervescents. II. Mise au point d’une technique de mesure de la moussabilité de la tenue et de la stabilité de la mousse des vins efervescents.,” Bull. l’OIV, pp. 711-712,405–426, 1990.
- R. S. Jackson, Wine Tasting. A professional Handboook, 2nd ed., vol. 1, no. 1. Academic Press, 2009.
- R. Gutiérrez-Escobar, M. J. Aliaño-González, and E. Cantos-Villar, “Wine polyphenol content and its influence on wine quality and properties: A review,” Molecules, vol. 26, no. 3, 2021.
- F. Zamora, “Biochemistry of Alcoholic Fermentation,” in Wine Chemistry and Biochemistry, M. V. Moreno-Arribas and M. C. Polo, Eds. New York, USA: Springer, 2009, pp. 3–26.
- F. Remize, J. L. Roustan, J. M. Sablayrolles, P. Barre, and S. Dequin, “Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase,” Appl. Environ. Microbiol., vol. 65, no. 1, pp. 143–149, 1999.
- H. H. Nieuwoudt, B. A. Prior, L. S. Pretorius, and F. F. Bauer, “Glycerol in South African Table Wines: An Assessment of its Relationship to Wine Quality,” South African J. Enol. Vitic., vol. 23, no. 1, pp. 22–30, 2017. [CrossRef]
- M. Esteruelas et al., “Influence of grape maturity on the foaming properties of base wines and sparkling wines (Cava),” J. Sci. Food Agric., vol. 95, no. 10, pp. 2071–2080, 2015.
- B. Cisilotto, F. J. Scariot, L. V. Schwarz, R. K. M. Rocha, A. P. L. Delamare, and S. Echeverrigaray, “Yeast stress and death caused by the synergistic effect of ethanol and so2 during the second fermentation of sparkling wines,” Oeno One, vol. 55, no. 4, pp. 49–69, 2021.
- E. J. Bartowsky, “Bacterial spoilage of wine and approaches to minimize it,” Lett. Appl. Microbiol., vol. 48, pp. 149–156, 2009.
- C. Delfini, R. Schellino, M. Minetto, A. Pagliara, and S. Ambro, “Biometric Study on the Ability of Wine Yeasts to Produce and / or Degrade DL -Lactic Acid during Alcoholic Fermentation,” J. Wine Res., vol. 13, no. 2, pp. 101–115, 2002.
- P. Giménez et al., “Development of a synthetic model to study browning caused by laccase activity from Botrytis cinerea,” Lwt, vol. 154, p. 112871, 2022.
- F. N. Salazar, F. Zamora, J. M. Canals, and F. Lopez, “Protein stabilization in sparkling base wine using zirconia and bentonite: influence on the foam parameters and protein fractions,” J. Int. des Sci. la Vigne du Vin, no. special issue Macrowine 2010, pp. 51–58, 2010.
- G. Vanrell, R. Canals, M. Esteruelas, F. Fort, J. M. Canals, and F. Zamora, “Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava),” Food Chem., vol. 104, 2006.
- C. Fornairon-Bonnefond, C. Camarasa, M. Moutounet, and J. M. Salmon, “New trends on yeast autolysis and wine ageing on lees: A bibliographic review,” J. Int. des Sci. la Vigne du Vin, vol. 36, no. 2, pp. 49–69, 2002.
- V. Moreno-Arribas, E. Pueyo, and M. C. Polo, “Peptides in musts and wines. Changes dur- ing the manufacture of Cavas (Sparkling Wines),” J. Agric. Food Chem., vol. 44, pp. 3783–3788, 1996.
- P. Pons-Mercadé et al., “Monitoring yeast autolysis in sparkling wines from nine consecutive vintages produced by the traditional method,” Aust. J. Grape Wine Res., vol. 28, no. 3, pp. 347–357, 2022.
- M. Ciani and F. Comitini, Yeasts in the Production of Wine. New York, USA: Springer, 2019.
- G. Nicolini, S. Moser, T. Román, E. Mazzi, and R. Larcher, “Effect of juice turbidity on fermentative volatile compounds in white wines,” Vitis - J. Grapevine Res., vol. 50, no. 3, pp. 131–135, 2011.
- Bertrand, “Influence du debourbage et de la temperature de fermentation sur les teneurs en substances volatiles des vins blancs,” Ann. Technol. Agric., vol. 27, pp. 231–233, 1978.
- M. Rid, A. Markheiser, C. Hoffmann, and J. Gross, “Waxy bloom on grape berry surface is one important factor for oviposition of European grapevine moths,” J. Pest Sci. (2004)., vol. 91, no. 4, pp. 1225–1239, 2018. [CrossRef]
- L. F. Bisson, “Stuck and sluggish fermentations,” Am. J. Enol. Vitic., vol. 50, no. 1, pp. 107–119, 1999.
- S. Lafon-Lafourcade, C. Geneix, and P. Ribereau-Gayon, “Inhibition of alcoholic fermentation of grape must by fatty acids produced by yeasts and their elimination by yeast ghosts,” Appl. Environ. Microbiol., vol. 47, no. 6, pp. 1246–1249, 1984.
- S. Restrepo, L. Espinoza, A. Ceballos, and A. Urtubia, “Production of Fatty Acids during Alcoholic Wine Fermentation under Selected Temperature and Aeration Conditions,” vol. 2, pp. 169–176, 2019.
- Martín-Garcia, C. Abarca-Rivas, M. Riu-Aumatell, and E. López-Tamames, “Comparison of volatile compounds during biological ageing and commercial storage of Cava (Spanish sparkling wine): The role of lees,” Heliyon, vol. 9, no. 8, 2023.
- Kemp, B. Condé, S. Jégou, K. Howell, Y. Vasserot, and R. Marchal, “Chemical compounds and mechanisms involved in the formation and stabilization of foam in sparkling wines,” Crit. Rev. Food Sci. Nutr., vol. 59, no. 13, pp. 2072–2094, 2019. [CrossRef]
- Martínez-Rodríguez, A. V. Carrascosa, J. M. Barcenilla, M. Angeles Pozo-Bayón, and M. Carmen Polo, “Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production,” Food Microbiol., vol. 18, no. 2, pp. 183–191, 2001.
- Cilindre, A. J. Castro, C. Clément, P. Jeandet, and R. Marchal, “Influence of Botrytis cinerea infection on Champagne wine proteins (characterized by two-dimensional electrophoresis/immunodetection) and wine foaming properties,” Food Chem., vol. 103, no. 1, pp. 139–149, 2007.
Figure 1.
Box-plots for standard parameters of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 1.
Box-plots for standard parameters of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 2.
Box-plots for organic acids of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 2.
Box-plots for organic acids of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 3.
Box-plots for colour coordinates (A-C) and the palette of colours in RGB signals (D) of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant difference.
Figure 3.
Box-plots for colour coordinates (A-C) and the palette of colours in RGB signals (D) of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant difference.
Figure 4.
Box-plots for proteins and peptides of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 4.
Box-plots for proteins and peptides of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 5.
Box-plots for polysaccharides and oligosaccharides of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 5.
Box-plots for polysaccharides and oligosaccharides of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 6.
Box-plots for volatile substances of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 6.
Box-plots for volatile substances of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 7.
Box-plots for foaming properties of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 7.
Box-plots for foaming properties of sparkling wines elaborated by ancestral (A, n = 11) or traditional (T, n = 9) methods. The presence of an asterisk indicates the existence of significant differences at p < 0.05.
Figure 8.
Spider web chart for the sensorial analysis results. The average value for each one of the sensory attributes is indicated by a continuous line whereas the standard deviation is showed as a shadowed ring surrounding it.
Figure 8.
Spider web chart for the sensorial analysis results. The average value for each one of the sensory attributes is indicated by a continuous line whereas the standard deviation is showed as a shadowed ring surrounding it.
Figure 9.
Plot of varimax-rotated principal component analysis for the different sparkling wines. Orange points: Ancestral sparkling wines. Blue points: Traditional sparkling wines.
Figure 9.
Plot of varimax-rotated principal component analysis for the different sparkling wines. Orange points: Ancestral sparkling wines. Blue points: Traditional sparkling wines.
Table 1.
Characteristics of studied wines.
Table 1.
Characteristics of studied wines.
| Method |
Code |
Variety |
Prize (€) |
Expedition cap |
Dosification |
Vintage |
| Ancestralsparkling wines |
A.01 |
Garnatxa blanca |
13 |
Crown cap |
Non dosed |
2022 |
| A.02 |
Macabeu |
10.95 |
Cork |
Non dosed |
2022 |
| A.03 |
Xarel·lo |
8.95 |
Crown cap |
Non dosed |
2022 |
| A.04 |
Parellada |
7.83 |
Crown cap |
Non dosed |
2021 |
| A.05 |
Macabeu |
8.96 |
Crown cap |
Non dosed |
2021 |
| A.06 |
Parellada |
18.15 |
Crown cap |
Non dosed |
2022 |
| A.07 |
Macabeu & Sauvignon Blanc |
4.5 |
Cork |
Non dosed |
2020 |
| A.08 |
Xarel·lo |
10.85 |
Cork |
Non dosed |
2020 |
| A.09 |
Parellada |
9.8 |
Cork |
Non dosed |
2022 |
| A.10 |
Macabeu & Xarel·lo |
10.1 |
Cork |
Non dosed |
2022 |
| A.11 |
Xarel·lo |
13.55 |
Crown cap |
Non dosed |
2022 |
| Traditional sparkling wines |
T.01 |
Blend 1 |
8.1 |
Cork |
Brut |
2022 |
| T.02 |
Blend 1 |
5.22 |
Cork |
Brut |
2022 |
| T.03 |
Blend 1 |
13.1 |
Cork |
Brut |
2022 |
| T.04 |
Blend 1 |
3.4 |
Cork |
Brut Nature |
2022 |
| T.05 |
Blend 1 |
5.5 |
Cork |
Brut Nature |
2022 |
| T.06 |
Blend 1 |
6.75 |
Cork |
Brut Nature |
2022 |
| T.07 |
Blend 2 |
6.7 |
Cork |
Brut |
2022 |
| T.08 |
Blend 1 |
6 |
Cork |
Brut |
2022 |
| T.09 |
Blend 1 |
4.95 |
Cork |
Brut Nature |
2022 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).