1. Introduction
Muscle synergy analysis is becoming increasingly used to study gait patterns [
1,
2]. In accordance with Safavynia et al muscle synergies represent a library of motor subtasks, that the central nervous system can flexibly combine to produce complex and natural movements [
3]. In fine, it is a group of muscles contracting together as part of a functional unit [
4]. This method involves extracting muscle synergies from electromyography (EMG) signals and takes into account the muscle activity of the whole limb that underlies gait patterns and simplifies the analysis. Muscle synergies have been shown to be modified by different treatments (e.g. rehabilitation interventions, botulinum toxin type-A injections and selective dorsal rhizotomy) in a range of pathologies (e.g. cerebral palsy, spinal cord injury, Parkinson’s disease) [
4,
5,
6,
7]. Nonetheless, some studies found mixed results. Indeed, Routson et al. found that 12 weeks of treadmill training with body weight support modified both the composition and timing of lower limbs muscles synergies [
2]. In contrast, Ambrosini et al. noted only minor changes in muscle synergies after 3 weeks of cycle training with functional electrical stimulation for the stroke participants [
8].
The analysis of muscle synergy flexibility has been more rarely studied in stroke patients. Moreover the impact of an intervention that may modify muscles synergies immediately after it, remains largely unexplored. Gait disorders are a common sequel of stroke [
9,
10]. Although various gait deviations may occur, one gait pattern commonly observed after stroke is Stiff Knee Gait (SKG) which is defined by a reduction of peak knee flexion during swing phase. This reduction of knee flexion has some consequences among them decreased toe clearance which compromises the stability of gait and increases the risk of falling [
11]. Several mechanisms have been proposed as causes of SKG. For example, an increase in forces generated by the vastii [
12], a decrease of hip flexion moment [
13], a decrease in ankle plantar flexion moment or an over-activity of the rectus femoris muscle during the swing phase of gait [
14,
15]. Although SKG is often multifactorial, over-activity of the rectus femoris is frequently the predominant mechanism in stroke patients [
16]
. To help in the diagnostic of main cause of stiff knee gait, anaesthetic motor nerve block of the rectus femoris nerve, to temporarily stop nerve conduction and thus muscle activity, is commonly used to determine the role of this muscle in peak knee flexion alteration. The nerve block involves injecting an anesthetic around the rectus femoris nerve, reducing both sensory and motor activity for 30 to 60 minutes [
17]. It has been shown that the anesthesic motor nerve block of the nerve branch of the rectus femoris improved peak knee flexion during swing phase of the gait cycle when patients exhibited rectus femoris over-activity during swing phase of the gait cycle [
18]. However, the adaptability of the gait pattern of the other muscles involved during walking after such nerve block is poorly studied. Indeed, the extent to which the muscles synergies involved in gait pattern of the paretic limb are modified by the decrease of rectus femoris muscle overactivity has to our knowledge never been studied.
The main aim of this study was to investigate whether an intervention consisting of a sudden decrease in muscle overactivity of a paretic lower limb in a stroke patient induces flexibility of muscle synergy immediately thereafter. In another words, we wished to determine whether motor nerve block of rectus femoris in stroke patient who exhibited a SKG due to an overactivity of the rectus femoris during swing phase of the gait cycle changes the robustness of muscle synergies for the paretic lower limb in stroke patients. We define robust synergy as representative of motor organization, and therefore linked to extraction parameters such as imposed or unconstrained Variance Account For (VAF). We will define these aspects of signal processing in the methodology section. The second objective was to explore the possible variability of muscle synergies in the paretic leg and their modification induced by nerve block. To this end, muscle synergies were extracted before and after nerve block and compared. We hypothesize that the robustness of muscle synergies decreases after nerve block in parallel with changes in muscle gait pattern.
Participants
We recruited a convenience sample of eight adult patients with stroke who were attending a routine clinical consultation at the Raymond Poincaré University Hospital gait laboratory. Inclusion criteria were: a diagnosis of stroke more than 12 months previously (ischaemic or haemorrhagic), the ability to walk 10m barefoot with no walking aids, quadriceps spasticity of ≥ 1 on the modified Ashworth scale [
19], and a stiff knee gait, defined as a peak of knee flexion during swing of less than 45 ° [
20]. Here we analyze the results of the gait analyses enabling the inclusion of participants in the study of gait disorders due to post-stroke muscle overactivity (NCT01973023).
Intervention
Nerve block was performed according to the technique described by Sung et al. [
17]. The effectiveness of the nerve block was tested by comparing rectus femoris EMG signals recorded before and after the intervention during maximal voluntary isometric hip flexion. A reduction of at least 50% of activity calculated using the root mean square was considered effective [
18]. For both gait analyses, participants were asked to walk at spontaneous gait speed.
Motion analysis
3D gait analysis was performed in two conditions: before (PRE) and after (POST) nerve block, using a Motion Analysis system with 8 cameras (100 Hz, Motion Analysis Corporation, Santa Rosa, CA, USA). The trajectories of 24 reflective markers placed on anatomical landmarks [
21] were recorded. Surface electromyographic (EMG) signals (1000Hz) (MA311, Motion Lab Systems, LA, USA) were recorded from five muscles on the paretic leg: rectus femoris, vastus lateralis, semitendinosus soleus and tibialis anterior. Participants were asked to walk at their own comfortable pace, barefoot, along a 10m walkway. 8 trials were recorded before and after rectus femoris motor nerve block. The following gait cycle phases were manually identified by a single operator: initial double contact, single support phase, final double contact and swing phase.
Kinematic variables
The marker displacement was filtered at 6 Hz using a 3rd order Butterworth filter [
22] and following kinematic variables were calculated using Orthotrak 6.2.8 (Motion Analysis Corporation, USA): maximum peak knee flexion in swing and gait speed. The mean of all the trials was calculated for each variable and used in the analysis.
EMG variables
All EMG signals were analysed using custom written Matlab routine (version R2012b, The MathWorks, Inc., MA, USA). Before processing, EMG signals were visually checked to detect artifacts. EMG signals were band-pass filtered from 5 Hz to 500 Hz, centred, full wave rectified, and low-pass filtered at 10 Hz. The amplitude of the EMG signals was normalised to the maximum activation recorded for each participant by condition [
23,
24]. To avoid time shifts induced by different gait speeds, time normalisation was performed with respect to gait phase duration [
25]: initial double contact = 20 time points, single support phase= 80 time points, final double contact = 20 time points, and swing phase = 80 time points. Thus each gait cycle was time-normalised on 200 points. The number of points per phase was chosen in accordance with the literature and the relative duration of each cycle [
26]. We applied these percentages in accordance with what is commonly described in the spatiotemporal parameters of the gait cycle: 10% of the duration of a gait cycle corresponded to the initial double contact, 40% to the single support phase, 10% to the final double contact and 40% to the oscillation phase. In the continuation of the manuscript we will call treated EMG the EMGs having undergone the whole of the stages described above
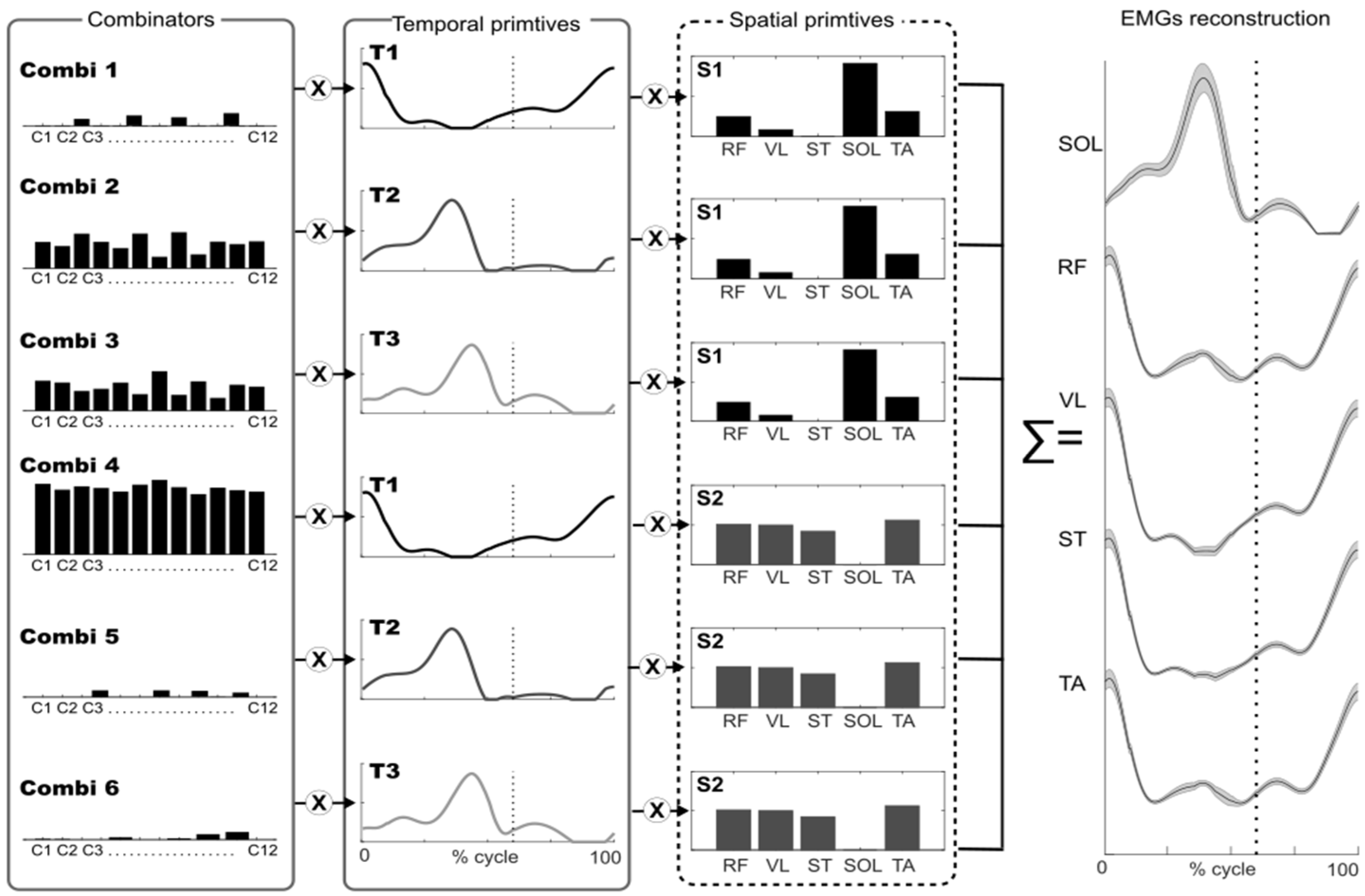
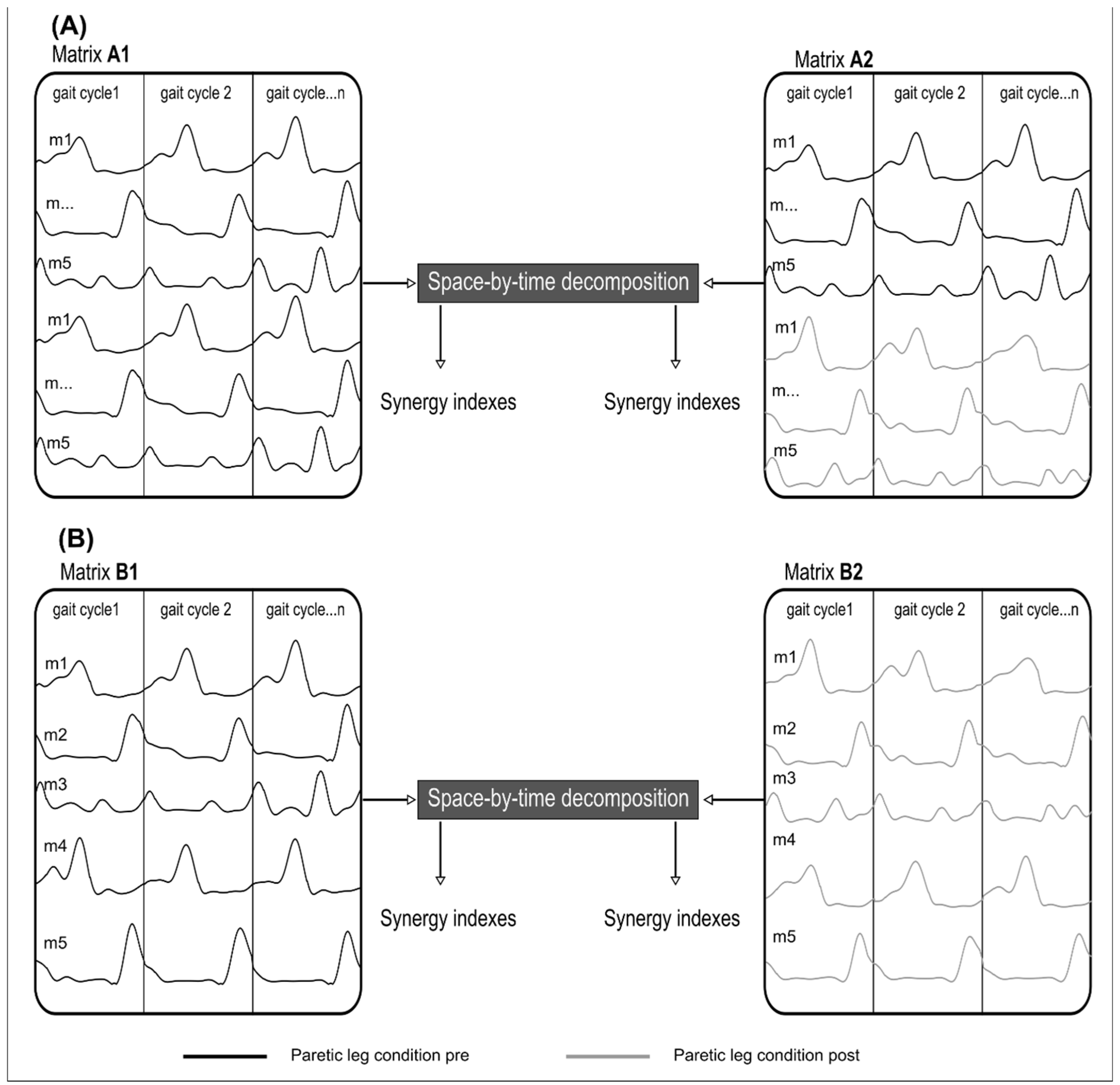
Muscle Synergy Indexes
The main outcome was the VAF from the first muscle synergy extraction, that provided an estimation of muscle synergy robustness in PRE and POST [
7].
The secondary outcome was an Index of Recruitment Selectivity (IRS) which reflects the degree of multiplexing of muscle synergies [
31]
, and an Index of Recruitment Consistency (IRC) which reflects muscle synergy variability. The IRS and IRC were calculated from two separate muscle synergy extractions.
The IRS index evaluates the actual (in-use) dimensionality of muscle synergy space for the task under investigation. Basically, it evaluates how many pairs of spatial/temporal primitives are really used for the task with respect to the total number of available pairs in the decomposition. The formula is based on the sparseness measure proposed by Hoyer [
32], applied to the combinatory values concatenated in a single vector of dimension N×P:
A large IRS value (i.e. large sparseness) would mean that only a few muscle synergies (much less than all N×P activable muscle synergies) are recruited to reconstruct the original muscle patterns.
The IRC quantifies the variability of the combinators for each muscle synergy from one gait cycle to another. This can be compared between the PRE and POST conditions. The IRC is defined as the sum of the variances of the activation coefficients across gait cycles, normalized by the total number of activation coefficients:
A low IRC value would mean that the combinators are relatively invariant and consistent across gait cycles.
Statistical analyses
All statistical tests were performed with Matlab (version R2012b, The MathWorks, Inc., MA, USA). All data are reported as means ± standard deviations. A non parametric Wilcoxon signed rank test was used to compare kinematic variables (the values of maximum peak knee flexion in swing and gait speed), and muscle synergy indexes (VAF, IRC, IRS) PRE and POST nerve block.
3. Results
The mean age of the 8 participants was 39 ± 10 years, mean height 174 ± 10 cm, mean weight 74 ± 06 kg. Seven were men, and the average time since stroke was at least 12 months. Spasticity of the rectus femoris ranged from 1 to 3 on the modified Ashworth scale in PRE and decreased of at least one point on the modified Ashworth scale in POST.
Gait Variables
The results of the gait variables are presented in
Table 1. Maximum peak knee flexion increased significantly from PRE to POST (p=0.007) for all participants, ranging from 26.68 ± 5.16° in PRE to 35.60 ± 7.07° in POST. This result confirmed the effect of the nerve block. There was no significant change in gait speed between conditions.
Muscle Synergy Indexes
Since the primitives can slightly differ between PRE and POST, we conducted the next analyses with extraction II where the primitives are allowed to vary in PRE and POST. We then focuses our analysis on the variability and selectivity of synergy recruitement between PRE and POST. The IRS quantifies selectivity (Table 2). Mean IRS values decreased significantly from 0.48 ± 0.03 in PRE to 0.43 ± 0.07 in POST (p=0.007), indicating an increase in interactions between spatial and temporal primitives and demonstrating that the degree of multiplexing of muscle synergies was increased by the nerve block (less sparseness). The IRC quantifies variability in the recruitment of primitives across gait cycles.
Mean IRC values increased from 3.93 ± 1.20 in PRE to 4.03 ± 2.00 in POST, however this difference was not significant (p=0.843) suggesting that the combinators were relatively invariant and consistent across gait cycles in both PRE and POST. In other words, once a strategy was found, it was quite consistent across gait cycles.
4. Discussion
The results of this study confirmed our hypothesis that the characteristics of the paretic limb’s muscle synergies during gait were modified after rectus femoris nerve block. Spatial and temporal primitives are slightly different in PRE vs POST, and their activation is more intertwined in POST compared to PRE.
Gait Variables
After the nerve block, our results showed that the maximum peak knee flexion in swing phase was increased. In addition, there was no statistical change in speed. These finding confirm the efficiency of the nerve block. Moreover, they are in accordance with previous studies which have not observed any changes in spatio-temporal parameters (spontaneous gait speed) after the nerve block [
17,
18].
Muscle Synergy Indexes
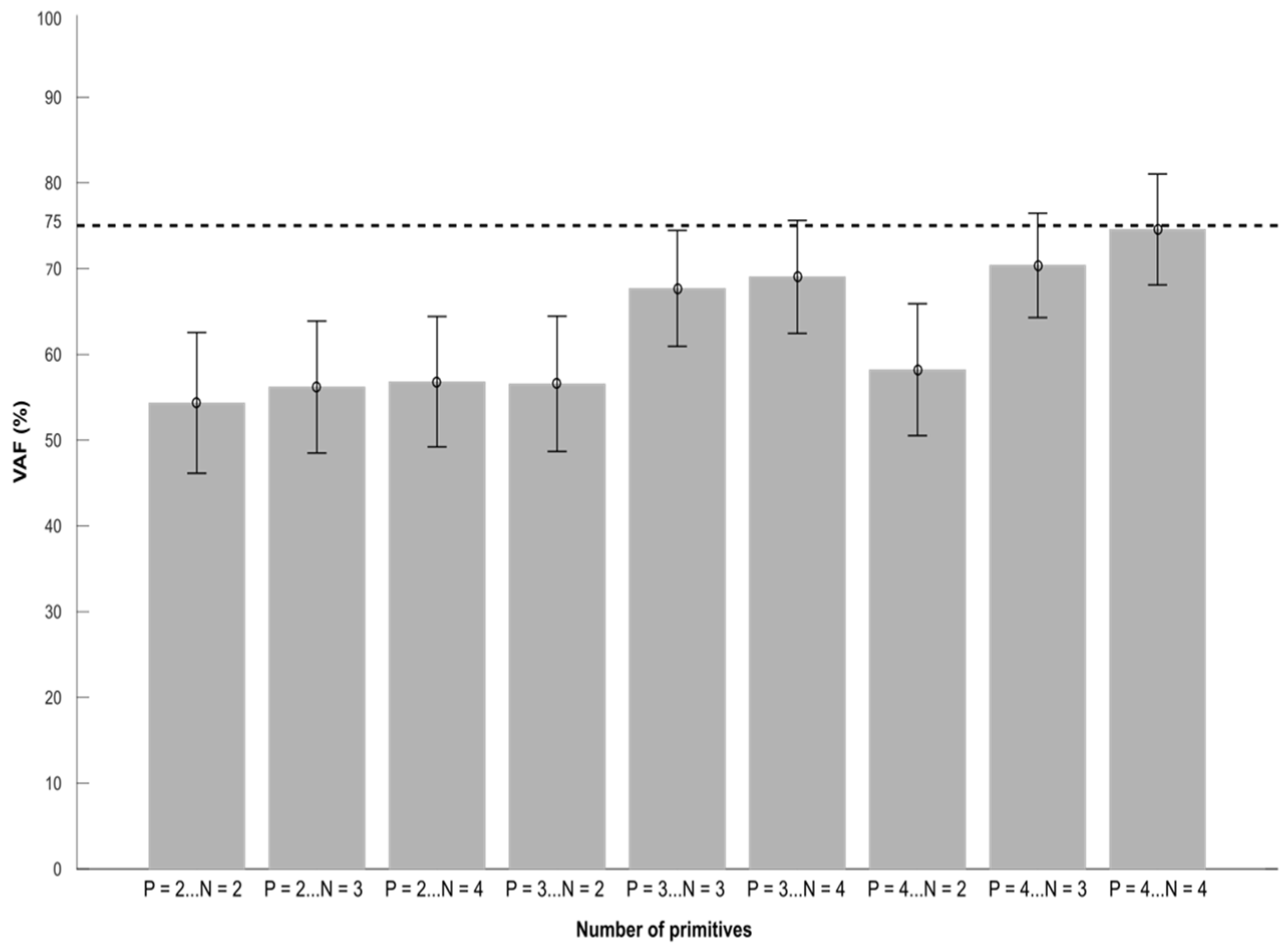
Four spatial and four temporal primitives were needed to obtain VAF values that were close to 75% in PRE. This is consistent with a recent systematic review that found that 4 primitives are common in stroke patients [
4]. Furthermore, after stroke the number of primitives in the paretic limb is the same as healthy subjects or reduced during gait [
4]. These four primitives have been associated with the biomechanical constraints of walking: body support, forward propulsion and swing initiation [
2]. These results suggest that the neural structures required for the activation of synergies were still intact [
4]. Nevertheless, it is common for studies to specify a minimum VAF cut-off of 90% to identify the number of synergies. These cut-offs differences can be explained by differences in the choice of the method used to extract muscle synergies. Most published studies extracted muscle synergies from averaged EMG signals [
32,
33] which was not the case here. If we used averaged EMG signals, the IRC and IRS could not have been calculated but the VAF would be similar to other reports. As a result, we had to use a more general method that allows to account for inter-cycle variability but hardly allows us to reach 90% of VAF.
After the nerve block, the VAF value decreased from 74.50% (6.90) to 70.30% (8.00) (p=0.015), suggesting a modification of the muscle synergies in order to adapt to the new biomechanical constraints. Kargo and Nitz showed that during a new motor task, the composition of muscle synergies was modulated until a stable state emerged with a new temporal profile [
34]. So the higher the VAF, the more the identified synergies explain the motor organization. Therefore, the formation of new muscle synergies is an adaptive process that is related to the experiences of each subject [
38], thus repeated movement practice could lead to the development of new muscle synergies or change the composition of existing ones [
35]. In our case, the 4 primitives explain part of the motor organization, but not all of it. The duration of the effect of the nerve block was likely too short for new muscles synergies to be created, thus the changes that occurred were more likely due to the modification of existing muscles synergies. In addition, previous studies have highlighted that during learning new motor task subjects exhibited variability across muscle synergies [
35]. This variability could be a ‘fine-tuning’ produce by central nervous system in an attempt to balance the opposing demands of achievement of the motor goal and energy efficiency. This fine-tuning is determined by ‘feed forward’ based on knowledge of prior performance and ‘feedback’. Finally, the increase in variability could be increased by the local anaesthetic nerve block which induces disturbances on motor nerves [
37]. We hypothesize that in the spastic patient the use of the nerve block almost induces a new locomotor stain due to its disturbance [VAF from 74.50% to 70.30%].
The nerve block did not affect the IRC values showing a invariance and consistency of the combinators across gait cycles, despite the decrease in rectus femoris over-activity. After the nerve block, the central nervous system must adapt the muscle synergies. We hypothesis that it is the same slightly modified muscle synergies which are at the origin of the modification of the paretic gait pattern and not the development of a new muscle synergy. Consequently muscle synergies are less robust which may also explain the decrease in VAF. In fact, if stroke patients had created a new muscle synergies gait pattern then the recruitment variability should have increased. Indeed learning new coordination is characterised by the continued experimentation enabling the development of new muscle synergy strategies, which will introduce some variability in the movement but might favour the optimisation and the transferred of this motor behaviour across other contexts [
38]. In another words IRC seems to reflect more a long term adaptation of a subject after an intervention than a short one
Analysis of the IRS values showed a higher level of muscle synergy selectivity after the nerve block (PRE =0.48 ±0.03 POST =0.43 ± 0.07, p=0.007), suggesting that the central nervous system recruited additional combinations of primitives to cope with the new biomechanical constraints. This likely suggests that the IRS reflects mainly short term adaptations. this hypothesis is in accordance with other results. For example, Santuz et al. showed in healthy subjects that during gait on an uneven-surface treadmill, the central nervous system produced a temporal rearrangement of the shape of the motor primitives, but their structure was unaltered [
39]. Another study in healthy subjects found that the same muscle synergies used for unperturbed gait were via supraspinal activity in challenging gait conditions [
40]. Comparison of synergy behaviour between healthy individuals and those with stroke showed a reduction in gait adaptability after stroke, with simplified movement strategies [
41,
42]. Muscle synergy in stroke patients were less ‘complex [
1,
8]. This might indicate a reduction in cortical adaptability and flexible synergy recruitment. We therefore concluded that the central nervous system flexibility controls the modification and activation of muscle synergies in case of peripheral disturbance.
5. Conclusions
The aim of this study was to explore the muscle synergies flexibility before and after a motor nerve block. Results showed that an efficient lower limb motor nerve block in stroke patients induces a decrease of VAF suggesting a decrease of the robustness of the gait pattern. our results also indicate that the motor nerve block did not involve the creation, of new muscle synergies gait pattern. They also suggest that there is a flexibility of muscles synergies after muscle nerve block in stroke patients which seems to be more variable than in healthy subject probably due to the brain lesion.
Author Contributions
conceptualization, A.S., D.P. N.R., and B.B.; methodology, A.S., D.P., N.R. and B.B.; software, A.S. and B.B.; formal analysis, A.S. and D.P.; investigation, A.S. N.R.; resources, A.S., D.P. and B.B.; data curation, A.S. and B.B.; writing—original draft preparation, A.S.; writing—review and editing, A.S., B.B. and D.P.; visualization, A.S. and B.B.; supervision, D.P. N.R. and B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. NCT01973023
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully thank all the participants involved in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hashiguchi, Y.; Ohata, K.; Kitatani, R.; Yamakami, N.; Sakuma, K.; Osako, S.; … Yamada, S. Merging and Fractionation of Muscle Synergy Indicate the Recovery Process in Patients with Hemiplegia: The First Study of Patients after Subacute Stroke. Neural Plasticity 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Routson, R.L.; Clark, D.J.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait & Posture 2013, 38, 511–517. [Google Scholar] [CrossRef]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Topics in Spinal Cord Injury Rehabilitation 2011, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Vermeulen, J.; Wagemans, K.; Schröder, J.; Embrechts, E.; Truijen, S.; Hallemans, A.; Saeys, W. Lower limb muscle synergies during walking after stroke: a systematic review. Disabil Rehabil 2020, 42, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.T.C.; van der Krogt, M.M.; Harlaar, J.; Dominici, N.; Buizer, A.I. Muscle synergies in response to biofeedback-driven gait adaptations in children with cerebral palsy. Front Physiol 2019, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Mileti, I.; Zampogna, A.; Santuz, A.; Asci, F.; Del Prete, Z.; Arampatzis, A.; Palermo, E.; Suppa, A. Muscle synergies in parkinson's disease. Sensors 2020, 20, 3209. [Google Scholar] [CrossRef] [PubMed]
- Shuman, B.R.; Schwartz, M.H.; Steele, K.M. Electromyography data processing impacts muscle synergies during gait for unimpaired children and children with cerebral palsy. Front Comput Neurosci 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; Parati, M.; Peri, E.; De Marchis, C.; Nava, C.; Pedrocchi, A.; Ferriero, G.; Ferrante, S. Changes in leg cycling muscle synergies after training augmented by functional electrical stimulation in subacute stroke survivors: a pilot study. J Neuroeng Rehabil 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.; Eng, J.; Garland, S. Clinical measurement of walking balance in people post stroke: a systematic review. Clin Rehabil 2011, 25, 693–708. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: a systematic review. The Lancet Neurology 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Weerdesteyn, V.; de Niet, M.; van Duijnhoven, H.J.; Geurts, A.C. Falls in individuals with stroke. J Rehabil Res Dev 2008, 45, 1195–213. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Anderson, F.C.; Pandy, M.G.; Delp, S.L. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. J Biomech 2004, 37, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, D.C.; Bang, M.S.; Burke, D.T. An algorithm to assess stiff-legged gait in traumatic brain injury. J Head Trauma Rehabil 1999, 14, 136–45. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.J.; Delp, S.L. The influence of muscles on knee flexion during the swing phase of gait. J Biomech 1996, 29, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.O.; Kerrigan, D.C. Torque action of two-joint muscles in the swing period of stiff-legged gait: a forward dynamic model analysis. J Biomech 1998, 31, 835–40. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Bonnyaud, C.; Geiger, M.; Bussel, B.; Bensmail, D. Relationship between hip flexion and ankle dorsiflexion during swing phase in chronic stroke patients. Clin Biomech 2015, 30, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Sung Duk, H.; Bang, H.J. Motor branch block of the rectus femoris: Its effectiveness in stiff-legged gait in spastic paresis. Arch Phys Med Rehabil 2000, 81, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.V.G.; Pradon, D.; Bensmail, D.; Fermanian, C.; Bussel, B.; Roche, N. Relevance of botulinum toxin injection and nerve block of rectus femoris to kinematic and functional parameters of stiff knee gait in hemiplegic adults. Gait Posture 2009, 29, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987, 67, 206–7. [Google Scholar] [CrossRef]
- Merlo, A.; Campanini, I. Impact of instrumental analysis of stiff knee gait on treatment appropriateness and associated costs in stroke patients. Gait Posture 2019, 72, 195–201. [Google Scholar] [CrossRef]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J Orthop Res 1990, 8, 383–92. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Devarajan, K.; Cheung, V.C.K. On non-negative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput 2014, 26, 1128–1168. [Google Scholar] [CrossRef] [PubMed]
- Santuz, A.; Ekizos, A.; Janshen, L.; Baltzopoulos, V.; Arampatzis, A. The Influence of Footwear on the Modular Organization of Running. Front Physiol 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hebenstreit F, Leibold A, Krinner S, Welsch G, Lochmann, M.; Eskofier BM. Effect of walking speed on gait sub phase durations. Hum Mov Sci 2015, 43, 118–24. [CrossRef] [PubMed]
- Perry, J.; Burnfield, J.M. ; 1992. Gait analysis: normal and pathological function, 2nd ed. ed. SLACK, Thorofare, NJ.
- Delis, I.; Panzeri, S.; Pozzo, T.; Berret, B. A unifying model of concurrent spatial and temporal modularity in muscle activity. J. Neurophysiol 2014, 111, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.D.; Seung, H.S. Algorithms for non-negative matrix factorization. In Advances in Neural Information Processing Systems 13 - Proceedings of the 2000 Conference, NIPS 2000. Neural information processing systems foundation. 2001. [Google Scholar]
- Hayes, H.B.; Chvatal, S.A.; French, M.A.; Ting, L.H.; Trumbower, R.D. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin Neurophysiol 2014, 125, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Chvatal, S.A.; Ting, L.H. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 2012, 32, 12237–50. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, P.O. Non-negative matrix factorization with sparseness constraints. J Mach Learn Res 2004, 5, 1457–1469. [Google Scholar]
- Oliveira Anderson, S.; Gizzi, L.; Farina, D.; Kersting, U.G. Motor modules of human locomotion: influence of EMG averaging, concatenation, and number of step cycles. Front Hum Neurosci 2014, 8. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J Neurophysiol 2010, 103, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Kargo, W.J.; Nitz, D.A. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J Neurosci 2003, 23, 11255–11269. [Google Scholar] [CrossRef] [PubMed]
- Frère, J.; Hug, F. Between-subject variability of muscle synergies during a complex motor skill. Front Comput Neurosci 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Safavynia, S.; Torres-Oviedo, G.; Ting, L. Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top Spinal Cord Inj Rehabil 2011, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ririe, D.G.; Walker, F.P.O.; James, R.L.; Butterworth, J. Effect of alkalinization of lidocaine on median nerve block. Br J Anaesth. 2000, 84, 163–8. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Bastian, A.J. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophysiol 2012, 107, 346–56. [Google Scholar] [CrossRef]
- Santuz, A.; Ekizos, A.; Eckardt, N.; Kibele, A.; Arampatzis, A. Challenging human locomotion: stability and modular organisation in unsteady conditions. Scientific Reports 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, Gizzi, L.; Kersting, U.G.; Farina, D. Modular organization of balance control following perturbations during walking. J Neurophysiol 2012, 108, 1895–1906. [CrossRef] [PubMed]
- Den Otter, A.R.; Geurts, A.C.H.; de Haart, M.; Mulder, T.; Duysens, J. Step characteristics during obstacle avoidance in hemiplegic stroke. Exp Brain Res 2005, 161, 180–192. [Google Scholar] [CrossRef]
- van Swigchem, R.; van Duijnhoven, H.J.R.; den Boer, J.; Geurts, A.C.; Weerdesteyn, V. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabil and Neural Repair 2013, 27, 230–239. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).