1. Introduction
The elderly population is particularly vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe coronavirus disease 2019 (COVID-19)[
1]. Specifically, residents of long-term care facilities (LTCFs) are at a higher risk of SARS-CoV-2 transmission and infection [
2,
3]. LTCF population density may account for this increased risk of transmission. Described risk factors for infection and disease severity are sex, age, ethnicity, obesity, and cardiovascular and respiratory diseases. Current vaccines have successfully reduced both SARS-CoV-2 transmission and COVID-19 severity [
4,
5]. However, the development of new tools to predict virus transmission and disease burden may improve current and future treatments, as well as disease prevention strategies. Several large-scale genetic association studies have consistently identified human host single nucleotide polymorphisms (SNPs) that are implicated in the biology and epidemiology of COVID-19 [
6,
7,
8,
9,
10,
11]. These studies suggest different mechanisms to explain why some individuals are more prone to infection by SARS-CoV-2 or being more severely affected by the disease upon infection[
12,
13]. Host genetics can inform us about disease etiology and provide new means for patient management. Population-specific risk variants have also been reported [
8,
14,
15]. Due to the extreme importance of age as a risk factor for severe COVID-19, age should be considered in genetic analyses [
12]. In this study, we aimed to identify host genetic factors influencing susceptibility to SARS-CoV-2 infection in LTCF residents. Our study included individuals infected and uninfected with SARS-CoV-2 early in the pandemic before initiation of vaccination.
2. Materials and Methods
2.1. Study Participants
A total of 97 residents of three LTCFs located in the area of Barcelona, Spain, were randomly included in this prospective observational study (CoronAVI@S study). Plasma samples were collected before vaccination through September-November 2020[
12]. SARS-CoV-2 serology was performed to sub-classify participants into infected and uninfected individuals depending on their PCR and serology status.
2.2. Ethics
The CoronAVI@S study was approved by the Ethics Board of the Institut Universitari d’Investigació en Atenció Primària Jordi Gol (20-116P). All participants provided written informed consent before inclusion.
2.3. Single Nucleotide Polymorphism Genotyping
Genomic DNA was extracted from peripheral blood mononuclear cells using Quick Extract DNA Extraction 1.0 solution (Epicentre/Lucigen) as described previously [
16]. Two microliters of extracted DNA were used for SNP genotyping (Applied Biosystems). The reference numbers of the TaqMan assays used for genotyping on a Quantstudio 5.0 from Applied Biosystems were ADAR1/rs1127313, C_8724398_10; IFIH1/rs1990760, C_2780299_30; MMS19/rs2236575, C_1797584_30; OAS-1/rs4767027, C_28015278_10; OAS-1/ rs10774671, C_2567433_10; OAS-2/rs1293767, C_8920276_10; OAS-3/rs10735079, C_31831768_10; IFNL3/rs12979860, C_7820464_10; IFNAR2/rs2236757, C_11354003_30; and PNPLA3/rs738409, C_7241_10. The SNPs IFNL3/rs4803217 and TNFAIP8L1/rs1060555 were determined using IDT DNA assay Hs.GT.rs4803217.A.1 and Hs.GT.rs1060555.G.1, respectively, and also assayed by Quantstudio 5.0 (Applied Biosystems).
2.4. Statistical Analysis
Haplotype estimation and association with patient susceptibility to SARS-CoV-2 infection were achieved using Haploview 4.2 (Broad Institute of Harvard and MIT, Cambridge, Massachusetts, USA) and SNPSTATS [
17]. To enhance the robustness of our findings, we performed a bootstrap resampling with 100 interactions, and calculated empirical p-values as the proportion of times the observed p-value was greater than the p-value derived from the bootstrap resampling. Risk factors were included in the genetic analysis to adjust the association of each SNP to infection susceptibility. Hardy-Weinberg equilibrium, minor allele frequency, and SNP linkage disequilibrium (LD) were also determined by Haploview 4.2. Mann Whitney t and chi-squared tests to compare infected and uninfected individuals were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, California, USA).
3. Results
Study participants were tested by PCR during SARS-CoV-2 outbreaks at their respective LTCFs between September and November 2020. During this period, 81 of the participants tested positive for SARS-CoV-2 (80% female, mean age 87 years) and 16 tested negative (
Table 1). The number of females was significantly higher in the infected group than the uninfected group (p = 0.0104). Only three infected individuals required hospitalization after acute infection. Interestingly, the 16 uninfected individuals remained seronegative for antibodies against the virus nucleoprotein until vaccination (January and February 2021)[
18].
Table 1 shows the clinical and biochemical characteristics of the study patients. Compared with SARS-CoV-2-infected individuals, uninfected individuals had significantly higher glucose levels (
Table 1). The higher glucose level in the uninfected group may be due to the higher number of diabetic individuals in this group (n=8/16) compared with the infected group (n=17/81; p = 0.0153, chi-squared). No other differences were found between infected and uninfected individuals.
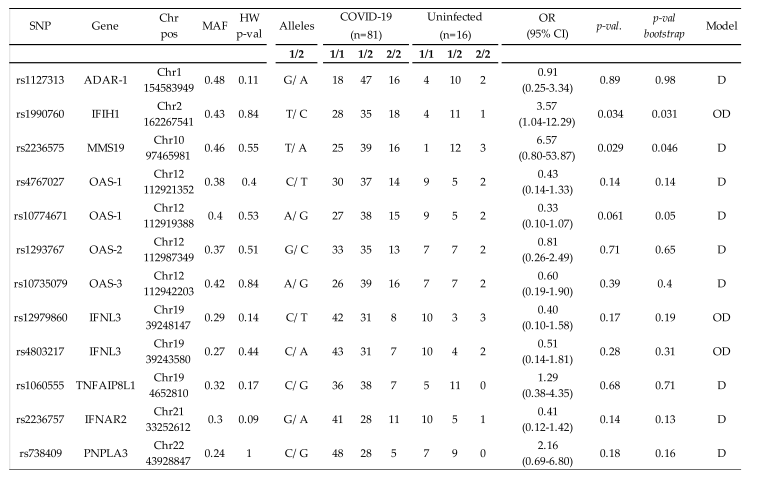
For the entire cohort of 97 individuals, we analyzed 12 SNPs from 7 host genes previously associated with the innate immune response [
9,
10,
11,
16,
19] or essential host cofactors involved in SARS-CoV-2 replication[
20,
21]. The observed allele SNP frequencies were characteristic of southern European populations. All of the variants had a minor allele frequency >0.01 and were in Hardy–Weinberg equilibrium (
Table 2). The four SNPs in the OAS gene were in high LD (R2 > 0.81). The SNPs in the IFNL3 gene were also in high LD (R2 = 0.94). No other significant LD was observed among the other study SNPs. For the association analyses for each SNP, we adjusted for sex and age, identifying genetic associations between susceptibility to SARS-CoV-2 infection and two SNPs: MMS19/rs2236575 in the dominant model (odds ratio [OR] 6.57, 95% CI 0.80-53.87, p = 0.029) and over dominant model (OR 3.60, 95% CI 0.97-13.30, p = 0.041), and IFIH1/rs1990760 in the over dominant model (OR 3.57, 95% CI 1.04-12.29, p = 0.034;
Table 2). After adjusting for multiple comparisons, by means of bootstrap techniques, MMS19/rs2236575 and IFIH1/rs1990760 remained significantly associated with susceptibility to SARS-CoV-2 infection (
Table 2). In the unadjusted analysis, MMS19/rs2236575 was the only variant related to susceptibility to SARS-CoV-2 infection in the dominant (p = 0.022) and over dominant (p = 0.049) models.
4. Discussion
In this study, we tested the association between host genetic polymorphisms and susceptibility to SARS-CoV-2 infection. After adjusting for sex and age, we found that residents of LTCFs carrying genetic variants in MMS19/rs2236575 and IFIH1/rs1990760 were more refractory to SARS-CoV-2 infection.
The IFIH1/rs1990760 TT variant was previously reported to confer resistance to SARS-CoV-2 infection and explain the epidemiological features of the pandemic in different countries [
22]. Importantly, COVID-19 patients with the IFIH1/rs1990760 TT variant have an attenuated inflammatory response and better outcomes[
23]. The human gene IFIH1 encodes a helicase, melanoma differentiation-associated gene-5, which acts as a cytoplasmic virus receptor and triggers the transcription of type-1 interferon genes and the systemic inflammatory response. IFIH1/rs1990760 has been previously linked to bowel disease, type 1 diabetes, vitiligo, and liver disease progression in HIV-1 patients [
16], [
24].
In contrast to the IFIH1/rs1990760 variant, we are the first to describe a possible implication of MMS19/rs2236575 variants in susceptibility to SARS-CoV-2 infection. MMS19 is a component of the cytosolic iron-sulfur protein assembly machinery that transfers Fe/S clusters to various DNA metabolism-associated Fe/S proteins [
25]. Whether MMS19/rs2236575 associates with loss-of-function (LOF) of MMS19 remains to be clarified. This LOF would then likely reduce ability of the virus to replicate because of its dependence on MMS19 for acquisition of its required iron sulfur cofactors. MMS19 is implicated in DNA replication and repair, and its deregulation has been linked to numerous human diseases, including cancer[
26]. MMS19 degradation and its downregulation is a common feature in cancer and genomic stability and is evolutionarily controlled [
27]. MMS19/rs2236575 has been explored as a possible marker of advanced epithelial ovarian cancer[
28]. Finally, the MMS19 protein has been described as a potential cofactor of the SARS-CoV-2 RNA-dependent RNA polymerase[
20]. The virus helicase (nonstructural protein 3) has a Fe/S cluster that modulates its RNA-binding and unwinding activities[
21].
Isoforms in the Neanderthal haplotype on chromosome 12 in the gene encoding oligoadenylate synthetase (OAS) play a protective role for individuals of European ancestry against COVID-19 susceptibility and severity [
9,
10,
11]. An additional finding of our study is that we did not find support for a protective role of OAS after interaction with SARS-CoV-2.
Although our findings require validation in larger cohorts, we identified two host genetic variants associated with susceptibility to SARS-CoV-2 infection in an elderly population. Current SARS-CoV-2 variants tend to cause mild symptoms, with few hospitalizations and deaths [
13,
29,
30]. Nevertheless, our findings can lead to better understanding of SARS-CoV-2 infections.
Author Contributions
Conceptualization, S.F. and MA.M.; methodology, S.F. and MA.M.; validation S.F. and MA.M.; formal analysis, S.F., M.T., L.M., M.M. and MA.M.; investigation, S.F. and MA.M.; resources, D.P., JM.B-S, M.MI, N.M., L.M., N.P. and M.M.; data curation, D.P., JM.B-S, MM.I., N.M., L.M., N.P., M.M. ; writing—original draft preparation, MA.M.; writing—review and editing, S.F., M.T., D.P., JM.B-S, MM.I., N.M., L.M., N.P., M.M. and MA.M.; supervision, D.P, JM.B-S, M.MI and N.M.; funding acquisition, M.M. and MA.M.
Funding
This work was supported by the Ministerio de Ciencia e Innovación (PID2019-103955RB-100), Gloria Soler Foundation and the crowdfunding initiative
https://www.yomecorono.com. MT was supported by a doctoral fellowship from the Departament de Salut from Generalitat de Catalunya (SLT017/20/000095). MM was granted with RYC2020-028934-I/AEI/10.13039/501100011033 from the Ministerio de Ciencia e Innovación and State Research Agency, and the European Social Fund “investing in your future”.
Institutional Review Board Statement
The CoronAVI@S study was approved by the Ethics Board of the Institut Universitari d’Investigació en Atenció Primària Jordi Gol (20-116P).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy of the patient.
Acknowledgments
We are deeply grateful to all participants and their families, to the LTCF who participated in this study. We also thank the technical staff of Direcció d’Atenció Primària de la Metropolitana Nord for sample collection in LTCF (S. Reyes Carrión, N. Salarich Solà, A. Vidal, R. Alvarez Viñallonga, J. Tornero, E. Vilamala, C. Suarez, T. Gonzalo, L. Perez, D. Sans, A. Blancas Loras, A. Garcia Archer, J. Borràs, S. Cervelló) and staff of IrsiCaixa for sample processing (L. Ruiz, R. Ayen, L. Gomez, C. Ramirez, M. Martinez, T Puig). We thank CERCA Programme/Generalitat de Catalunya for institutional support and Foundation Dormeur for financial support for the acquisition of Quantstudio 5.0 real time.
Conflicts of Interest
The authors declare no conflicts of interest
References
- T. Levin, W. P. Hanage, N. Owusu-Boaitey, K. B. Cochran, S. P. Walsh, and G. Meyerowitz-Katz, “Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications,” European Journal of Epidemiology, vol. 35, no. 12. 2020. [CrossRef]
- U. Meis-Pinheiro et al., “Clinical characteristics of COVID-19 in older adults. A retrospective study in long-term nursing homes in Catalonia,” PLoS ONE, vol. 16, no. 7 July. 2021. [CrossRef]
- D. Prieto-Alhambra et al., “Filling the gaps in the characterization of the clinical management of COVID-19: 30-day hospital admission and fatality rates in a cohort of 118 150 cases diagnosed in outpatient settings in Spain,” Int J Epidemiol, vol. 49, no. 6, 2020. [CrossRef]
- Y. M. Bar-On et al., “Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel,” New England Journal of Medicine, vol. 385, no. 15, 2021. [CrossRef]
- F. P. Polack et al., “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine,” New England Journal of Medicine, vol. 383, no. 27, 2020. [CrossRef]
- J. E. Horowitz et al., “Genome-wide analysis provides genetic evidence that ACE2 influences COVID-19 risk and yields risk scores associated with severe disease,” Nat Genet, vol. 54, no. 4, 2022. [CrossRef]
- J. Hu, C. Li, S. Wang, T. Li, and H. Zhang, “Genetic variants are identified to increase risk of COVID-19 related mortality from UK Biobank data,” Hum Genomics, vol. 15, no. 1, 2021. [CrossRef]
- H. Namkoong et al., “DOCK2 is involved in the host genetics and biology of severe COVID-19,” Nature, vol. 609, no. 7928, 2022. [CrossRef]
- H. Zeberg and S. Pääbo, “A genomic region associated with protection against severe COVID-19 is inherited from Neandertals,” Proc Natl Acad Sci U S A, vol. 118, no. 9, 2021. [CrossRef]
- H. Zeberg and S. Pääbo, “The major genetic risk factor for severe COVID-19 is inherited from Neanderthals,” Nature, vol. 587, no. 7835, 2020. [CrossRef]
- S. Zhou et al., “A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity,” Nat Med, vol. 27, no. 4, 2021. [CrossRef]
- M. E. K. Niemi, M. J. Daly, and A. Ganna, “The human genetic epidemiology of COVID-19,” Nature Reviews Genetics, vol. 23, no. 9. 2022. [CrossRef]
- E. Pairo-Castineira et al., “GWAS and meta-analysis identifies 49 genetic variants underlying critical COVID-19,” Nature, vol. 617, no. 7962, 2023. [CrossRef]
- R. Mathur et al., “Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform,” The Lancet, vol. 397, no. 10286, 2021. [CrossRef]
- P. Wu et al., “Trans-ethnic genome-wide association study of severe COVID-19,” Commun Biol, vol. 4, no. 1, 2021. [CrossRef]
- S. Franco et al., “Single nucleotide polymorphisms in PNPLA3, ADAR-1, and IFIH1 are associated with advanced liver fibrosis in patients co-infected with HIV-1/HCV.,” AIDS, Sep. 2021. [CrossRef]
- X. Solé, E. Guinó, J. Valls, R. Iniesta, and V. Moreno, “SNPStats: a web tool for the analysis of association studies.,” Bioinformatics, vol. 22, no. 15, pp. 1928–9, Aug. 2006. [CrossRef]
- M. Trigueros et al., “Reduced humoral response 3 months following BNT162b2 vaccination in SARS-CoV-2 uninfected residents of long-term care facilities,” Age Ageing, vol. 51, no. 5, 2022. [CrossRef]
- M. Pujantell et al., “ADAR1 affects HCV infection by modulating innate immune response,” Antiviral Res, vol. 156, 2018. [CrossRef]
- N. Maio et al., “Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets,” Science (1979), vol. 373, no. 6551, 2021. [CrossRef]
- N. Maio et al., “An iron–sulfur cluster in the zinc-binding domain of the SARS-CoV-2 helicase modulates its RNA-binding and -unwinding activities,” Proc Natl Acad Sci U S A, vol. 120, no. 33, 2023. [CrossRef]
- K. Maiti, “The African-American population with a low allele frequency of SNP rs1990760 (T allele) in IFIH1 predicts less IFN-beta expression and potential vulnerability to COVID-19 infection,” Immunogenetics, vol. 72, no. 6–7, 2020. [CrossRef]
- L. Amado-Rodríguez et al., “Effects of IFIH1 rs1990760 variants on systemic inflammation and outcome in critically ill COVID-19 patients in an observational translational study,” Elife, vol. 11, 2022. [CrossRef]
- E. Della Mina, M. P. Rodero, and Y. J. Crow, “Polymorphisms in IFIH1: the good and the bad.,” Nat Immunol, vol. 18, no. 7, pp. 708–709, Jun. 2017. [CrossRef]
- Stehling et al., “MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity,” Science (1979), vol. 337, no. 6091, 2012. [CrossRef]
- S. V. Torti and F. M. Torti, “Iron and cancer: More ore to be mined,” Nature Reviews Cancer, vol. 13, no. 5. 2013. [CrossRef]
- J. L. Weon, S. W. Yang, and P. R. Potts, “Cytosolic Iron-Sulfur Assembly Is Evolutionarily Tuned by a Cancer-Amplified Ubiquitin Ligase,” Mol Cell, vol. 69, no. 1, 2018. [CrossRef]
- K. M. Moxley et al., “The role of single nucleotide polymorphisms of the ERCC1 and MMS19 genes in predicting platinum-sensitivity, progression-free and overall survival in advanced epithelial ovarian cancer,” Gynecol Oncol, vol. 130, no. 2, 2013. [CrossRef]
- R. P. Karyakarte et al., “Chasing SARS-CoV-2 XBB.1.16 Recombinant Lineage in India and the Clinical Profile of XBB.1.16 Cases in Maharashtra, India,” Cureus, 2023. [CrossRef]
- E. Callaway, “COVID’s future: mini-waves rather than seasonal surges,” Nature, vol. 617, no. 7960. 2023. [CrossRef]
Table 1.
Clinical and biochemical characteristics of participants.
Table 1.
Clinical and biochemical characteristics of participants.
| |
SARS-CoV-2 Infected |
SARS-CoV-2 Uninfected |
p-value |
| N (%) |
81 (83.5) |
16 (16.5) |
|
| Age, years |
87 (81-90) |
80 (74-91) |
0.1668 |
| Female |
65 (80.2) |
8 (50) |
0.01042
|
| AGM level1
|
|
|
0.14602
|
| 1 |
0 |
0 |
|
| 2 |
4 |
3 |
|
| 3 |
38 |
6 |
|
| 4 |
39 |
7 |
|
| Albumin (g/L) |
37.55 (35.9-39.75) |
38.2 (36.93-39.5) |
0.2725 |
| Alanine aminotransferase (U/L) |
11 (8.5-14) |
12.5 (9.25-17.75) |
0.3694 |
| Aspartate aminotransferase (U/L) |
17 (14-20) |
18 (15.25-21.25) |
0.3431 |
| Creatine kinase (U/L) |
42.5 (33.25-71) |
47 (34.5-73.25) |
0.7907 |
|
High-density lipoprotein (mg/dL)
|
46.2 (40.73-56.53) |
42.4 (36-47.8) |
0.1135 |
| Total cholesterol (mg/dL) |
193 (162-228) |
198.5 (131.8-230.8) |
0.6147 |
| Creatinine mg/dL |
0.81 (0.675-1.035) |
0.82 (0.6925-1.25) |
0.8186 |
| Alkaline phosphatase (U/L) |
78 (66.25-104) |
89 (72.25-112.8) |
0.2704 |
| Ferritin (ng/mL) |
66.5 (34-144.3) |
108 (50-195.8) |
0.1604 |
| Fibrinogen (mg/L) |
484 (415-539.5) |
471.5 (386.3-535.5) |
0.8820 |
| Phosphate (mmol/L) |
3.4 (3.1-3.8) |
3.4 (3.225-3.6) |
0.9087 |
| Gamma-glutamyltransferase (U/L) |
17 (13-26) |
25.5 (15-32) |
0.1615 |
| Glucose (mg/dL) |
92 (83-106) |
111 (90-136) |
0.0329 |
| Hematocrit (%) |
38.2 (35.55-40.35) |
37.25 (32.88-40.3) |
0.5044 |
| Hemoglobin (g/dL) |
33.5 (33-34.1) |
33.95 (33.33-34.45) |
0.0891 |
| Lactate dehydrogenase (U/L) |
171 (143.8-187.8) |
172.5 (156.5-192.5) |
0.7683 |
| Leucocyte count (×109/L) |
6.2 (5-7) |
6.3 (4.97-7.8) |
0.8605 |
| Lymphocyte count (×109/L) |
1.7 (1.35-2.05) |
1.75 (1.225-2.35) |
0.8263 |
| Magnesium (mg/dL) |
2.06 (1.898-2.188) |
2.03 (1.893-2.115) |
0.2356 |
| Platelet (× 109/L) |
205 (168-249.5) |
180 (145.8-221.5) |
0.1236 |
| Potassium (mmol/L) |
4.32 (4.153-4.53) |
4.39 (4.073-4.588) |
0.6842 |
| Serum protein (g/L) |
65.95 (63.15-68.78) |
65.35 (61.18-68.98) |
0.7870 |
| Sodium (mmol/L) |
140.9 (139.1-142) |
140 (138.6-141.6) |
0.2790 |
|
Prothrombin time (s)
|
11.75 (11.2-12.4) |
11.8 (11.1-13.2) |
0.6207 |
|
Triglycerides (mg/dL)
|
115.5 (81.5-148.8) |
111 (88.25-165.5) |
0.8935 |
| Partial thromboplastin Time (s) |
30.05 (28.53-33.15) |
30.55 (28.8-35.68) |
0.3548 |
| Urea (mg/dL) |
40 (34-51) |
43.5 (32-53) |
0.7094 |
Table 2.
Association of 12 single nucleotide polymorphisms from 7 host genes with susceptibility to SARS-CoV-2 infection.
Table 2.
Association of 12 single nucleotide polymorphisms from 7 host genes with susceptibility to SARS-CoV-2 infection.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).