1. Introduction
Medicinal plants used in traditional medicine, pose a new alternative for therapeutics of vascular diseases.
Larrea tridentata, also known as creosote bush, is an endemic plant of the northern arid Mexican plateau and south of the United States of America. This bush belongs to the Zygophillaceae family and has been widely used in traditional medicine to treat hypertension, renal stones and infection among others [
1,
2].
Several bioactive compounds have been isolated from this plant such as lignans, terpenes and flavonoids, showing a myriad of antioxidant, antimicrobial, antineoplasic and anti-inflammatory effects [
3,
4,
5,
6,
7]. Of these compounds, a particular flavone: 5-Hydroxy-2-(4-hydroxy-3-methoxyphenyl)-6,7,8-trimethoxy-4H-1- benzopyran-4-one, also known as 8-methoxycirsilineol (8-M) became of our interest due to its vasorelaxant effects.
8-M shares a similar structure with quercetin, which is well known for its vasorelaxant and anti-pressor effects. 8-M has been isolated from several plant species such as
Sideritis genus,
Thymus species,
Cleome droserifolia and
Rabdosia rubescent, among others [
8,
9,
10]. In this regard, Fushiya et al. [
11] have shown that two flavone analogs: 3’-Methoxycalycopterin and 8-M from
Cleome droserifolia suppress nitric oxide production in macrophages and reduce histamine and ß hexosaminidase release, which suggest anti- inflammatory properties observed in the traditional use of
L. tridentata [
11]. On the other hand, a calycopterin isolated from
Dracocephalum kotschyi increased the levels of reactive oxygen species and nitric oxide against the human hepatoblastoma cancer cell (HepG2) line [
12] and a similar compound isolated from
D. kotschyi Boiss induced apoptosis in 2 prostate cancer cell lines [
13] Nevertheless, the biological activity and the mechanisms of action underlying the vasorelaxant and vaso-protective effects of 3’-Methoxycalycopterin or 8-M isolated from any plant are poorly investigated and require further insight.
Vascular reactivity to hormones such as catecholamines, results from a localized increase in intracellular calcium which activates the contractile apparatus of smooth muscle cells. This phenomenon includes cell membrane depolarization, and thus depends on the activity of several ionic channels including potassium channels activated by voltage (KV), which counterbalance depolarization by allowing potassium efflux from the cell [
14].
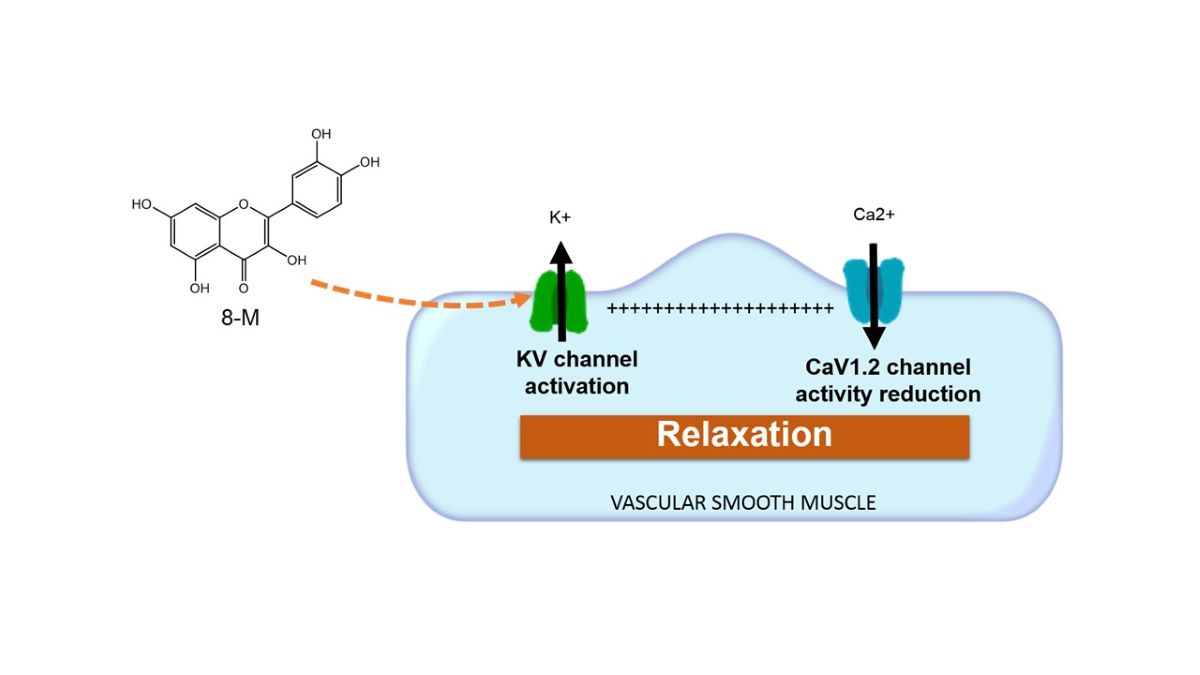
In the present work we observed a full vasorelaxant effect of 8-M isolated from L. tridentata on guinea pig mesenteric arteries stimulated by phenylephrine which depended on KV channel activity. Besides, our results suggest that 8-M activates KV channels and shifts the membrane potential to a hyperpolarized state. This resulting in a system less feasible to contract. These findings pose an interesting alternative for further study of compounds used in traditional medicine to treat cardiovascular pathologies.
2. Materials and Methods
2.1. Extraction and Purification of 8-M
Collection.
Larrea tridentata was collected during flowering time, at one Km from the road to the municipality of Guadalcázar, San Luis Potosí State, México, in several seasons from 1995 to 2011.
L. tridentata was identified by José García-Pérez with voucher specimen SLPM050796 in the Isidro Palacios Herbarium of Instituto de Zonas Desérticas of Universidad Autónoma of San Luis Potosí.
Larrea tridentata has been catalogued in México Voucher 50315052 [
15] and in the International Plant Names Index by the Life Sciences Identifier number: 135649-2 [
16].
Extraction. 967g dried and pulverized leaves from Larrea tridentata were poured into a glass column. The secondary metabolites were extracted by percolated/maceration method. First, non-polar metabolites were extracted with hexane and next, the mixture of flavones was extracted with dichloromethane. All these were precipitated and washed with ethyl acetate, obtaining 1.3 g of yellow solid. The 8-M was obtained from the yellow solid by flash column stationary phase chromatography using gel silica and a mixture of chloroform and ethyl alcohol as mobile phase at a 99:1 ratio, obtaining 123 mg (0.01 % w/w) of yellow solid. Finally, the 8-M was re-crystallized with dimethylsulfoxide and washed with ethyl alcohol.
General considerations. The melting point was determined using a Sybron- Thermolyne melting point equipment and is uncorrected. The infrared spectra were collected on a Nicolet 205 FT-IR spectrophotometer with a KBr tablet by the transmission method. The NMR-1H spectra was recorded on Bruker equipment to 200.1 MHz, using DMSO-d6 as a solvent and internal TMS standard (0 ppm). Mass spectra was detected by electronic impact and recorded using Hewlett-Packard equipment to 70 eV by direct.
Purity Analysis. The purity of 8-M was determined by HPLC in a Water equipment composed of a 2996 photodiode array detector, a 600 controller and a 600 pump equipped with a YMC Pack Pro C-18 (250 mm x 4.6 I. D.) HPLC column, methyl alcohol/dimethylformamide 93:7 as mobile phase at 1 mL/min flow.
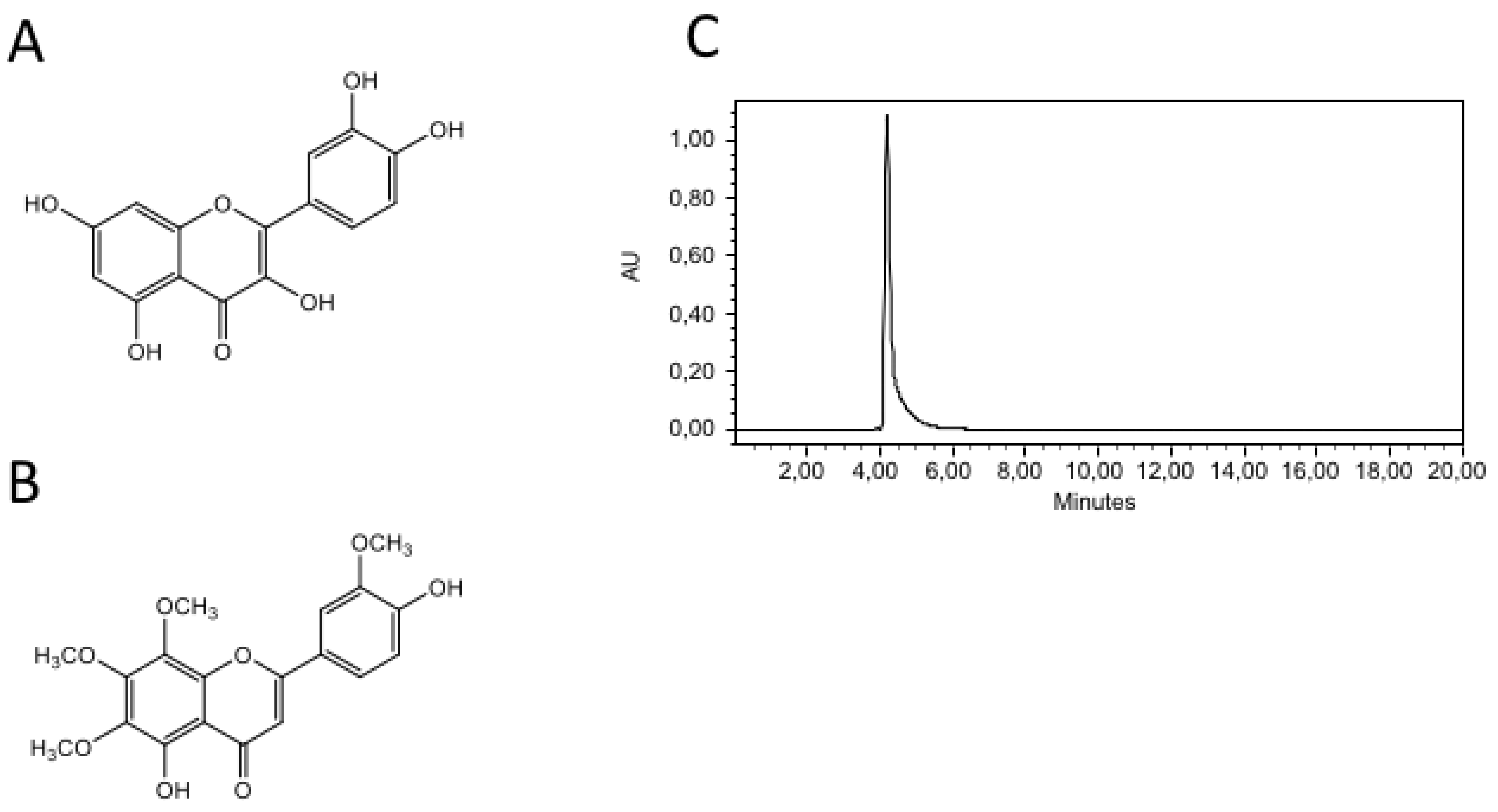
8-M: m. p.= 216-218 oC, HPLC purity = 98.3 %, r. t. = 4.1 min. , , UV (λ max, nm): 258.2 y 361.9, IR (cm-1): 3358, 1649, 1603, 1511, 1359, 1210. NMR-1H (δ, ppm): 3.83 (6H, s), 3.86 (3H, s), 3.92 (3H, s), 6.58 (1H, s), 7.04 (1H, d, Jo= 8.33 Hz), 7.64 (1H, d), 7.68 (1H, d), 10.1 (1H), 12.48 (1H, s). MS (m/z, %): 374 [M]+, 58%; 360, 21%; 359, 100%; 226, 0.4%; 151, 6%.
The chemical structure for quercetine and 8-M are shown in
Figure 1 A and 1B, respectively. The purity and migration pattern of 8-M are shown on the HPLC chromatogram in
Figure 1C.
2.2. Tissue Preparation and Organ Bath Experiments.
Our protocols were approved by the Animal Care Committee of the Universidad Autónoma de San Luis Potosí. Under deep anesthesia induced by an overdose of sodium pentobarbital (130 mg/kg i.p.) mesenteric arteries from guinea pigs (400-600 g weight) were obtained and cleaned of connective and adipose tissue. Rings of 2-3 mm wide (3-4 from each mesenteric artery) were cut and mounted in a 4 ml temperature controlled (37°C) organ bath using stainless steel hooks inserted into de lumen. One hook was connected to the bottom of the bath and the other side to a FT- 03 force transducer (Grass Instruments Division Astro-Med, Inc., West Warwick, RI, USA). In some experiments the endothelium was removed by gently rubbing the lumen of the vascular ring with a cotton swab. Tissues were bathed with a physiological solution of the following composition (in mM): 135 NaCl, 4.7 KCl, 1.17 MgSO4, 1.15 KH2PO4, 1.1 CaCl2, 20 HEPES and 10 dextrose and maintained at 37°C (pH 7.4). Rings were gently stretched to impose a preload tension of 1 g, this in order to allow the maximal response and give them a 30 min equilibration period before experimental manipulation. Isometric changes in tension were amplified in a 79 D Grass Polygraph and stored in a hard disk for posterior offline analyses. At the beginning of every experiment rings were challenged with a solution containing 80mM KCl in order to evaluate tissue viability. Only those rings that developed a 1 g contraction were considered for further experimentation. Rings were then contracted with the alfa-1 adrenergic agonist phenylephrine at a concentration of 2 μM in all experiment. The absence of the endothelium was verified by adding L-NAME1 mM. If a contraction was developed after L-NAME was added suggested the endothelium was present. The lack of response to L-NAME indicated the absence of the endothelium. Also, abscense of endothelium was verified by addition of Charbachol 10 μM at the peak contraction to phenylephrine 2 μM.
Cumulative dose-response curves were built for 8-M (10-8 – 10-4 M) and quercetin (10- 8 – 10-4 M) upon maximal contraction to 2 µM phenylephrine. Other drugs used were also added directly to the organ bath. Half maximal effective concentration (EC50) values were calculated for each curve using statistical software (GraphPad Prism, Ver. 8.0), and considering this value to reflect the agonist concentration capable of eliciting the half-maximal response.
2.3. Mesenteric Artery Cell Isolation for Patch-Clamp Recording
Guinea pig mesenteric arteries were obtained as described above and dissected free of adipose and connective tissues. Endothelium was removed by rubbing the lumen with a silk thread. The tissue was then placed in a dissociation saline solution containing (in mM): 55 NaCl, 6 KCl, 80 Glutamic acid, 80 KOH, 5 MgCl2, 10 dextrose, 5 Dithiothreitol, 10 HEPES, 2 mg/ml BSA and 10 EDTA as well as 1.5 mg/ml papain. Digestion was carried out in a shaking bath at 120 rpm and at 32ºC for 30 minutes. Afterwards, 0.7mg/ml of F-type collagenase and 0.3 mg/ml of H-type collagenase were added to the saline solution and the tissue was incubated at 32ºC for another 6 minutes. The tissue was then placed in an enzyme-free dissociation saline solution and cells were dissociated with the help of a 1 ml micropipette tip.
2.4. Electrophysiological Recordings
Guinea pig mesenteric smooth muscle cells were placed directly onto the glass bottom of the experimental chamber and visualized using an inverted microscope (Zeiss Axio Vert.A1). Cells were left to stick onto the bottom for 10 – 15 min before an experiment was started. Pipettes were pulled from borosilicate glass capillary tubes (World Precision Instruments, Sarasota, FL, USA) on a programmable puller (Sutter Instruments, Novato, CA, USA). When pipettes were filled with the internal solution, tip resistance ranged from 2 – 3 mega Ω. Currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Data acquisition and generation of voltage-clamp pulse protocols were carried out with a Digidata 1440A interface (Molecular Devices) controlled by the pCLAMP 10 software (Molecular Devices). Current traces were filtered at 1 kHz with a low-pass 4-pole Bessel filter in the clamp amplifier, subjected to P/4 leak subtraction, digitized and stored for subsequent analysis.
In order to isolate calcium channel currents, the pipette was filled with a high Cs+ solution of the following composition (in mM): 132.5 CsCl, 3 ATP-Na2, 0.1 GTP, 3 MgCl2, 10 EGTA and 10 HEPES, pH 7.2 (titrated with CsOH). Ba2+ ion was used as a carrier of calcium channel currents. The Ba2+-containing solution consisted of (in mM): 10 BaCl2, 150 NaCl, 5.4 glucose and 5 HEPES, pH 7.4 (titrated with NaOH).
In order to record potassium currents, the cells were bathed with an external solution composed of (in mM): 140 NaCl, 5.4 KCl, 1.2 MgCl2, 10 HEPES, 1 EGTA, and 10 glucose, pH 7.4 (titrated with NaOH). The pipette solution contained (in mM): 140 KCl, 1 MgCl2, 10 EGTA, 10 HEPES, and 2 Na2ATP, pH 7.2 (titrated with KOH). All experiments were conducted at room temperature (22 – 24 °C).
2.5. Statistical Analysis
Data residuals were tested for variance homogeneity with Brown-Forsythe test and for normal distribution using Shapiro-Wilk test. A p value < 0.05 was considered statistically significant. Unpaired t test was used to determine differences and these results are presented as means standard deviations (SD). When needed, ANOVA was used for multiple comparisons with a post-hoc Tukey Honest Significant Difference test.
3. Results
3.1. 8-Methoxycirsilineol (8-M) Has a Potent Relaxant Effect on Guinea Pig Mesenteric Artery Rings.
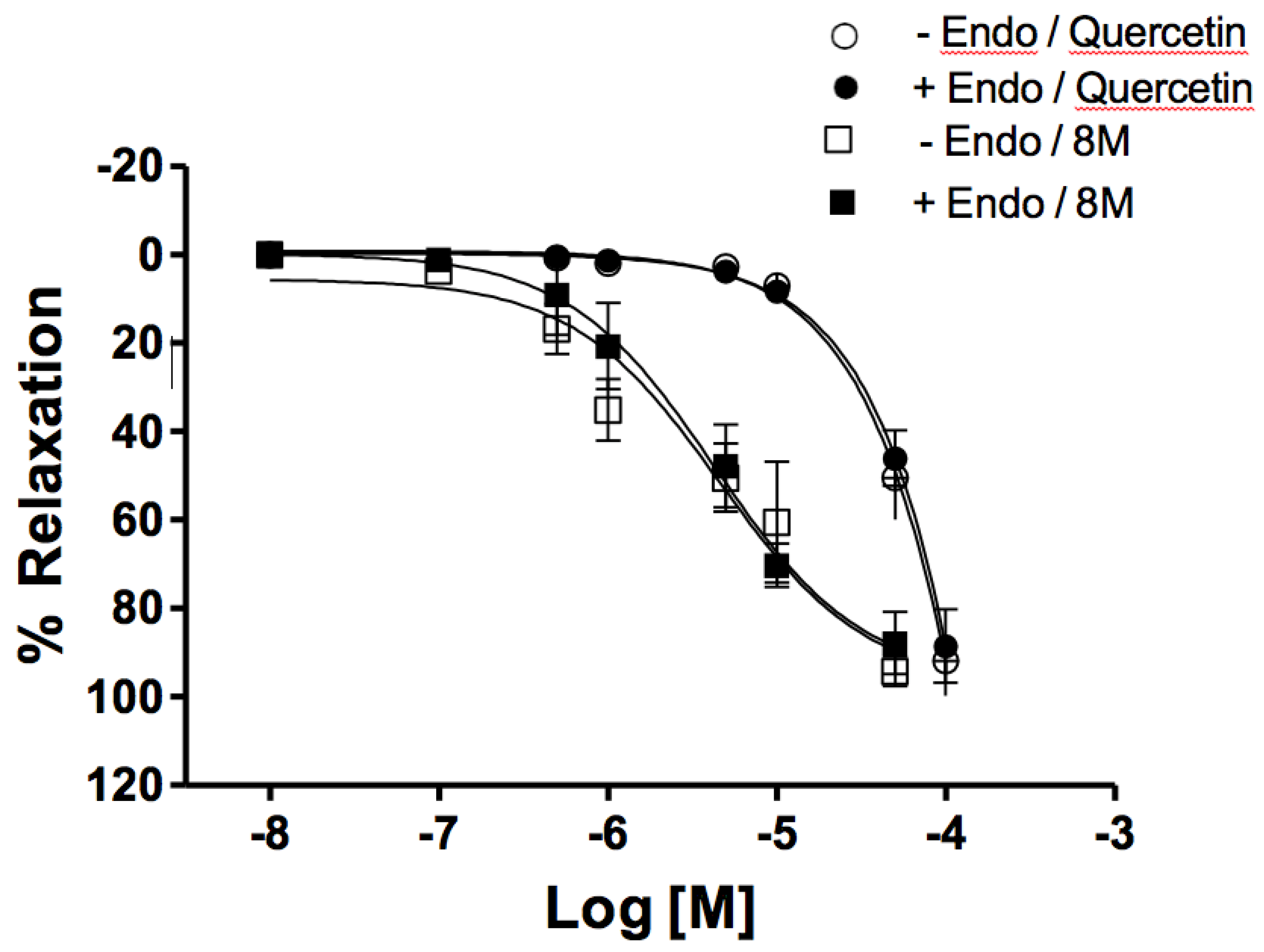
Guinea-pig mesenteric artery rings with and without endothelium were pre-contracted with 2 µM phenylephrine to maximal contraction. Once contraction was established, 8-M was added within a range of increasing concentrations until complete relaxation was observed (
Figure 2). 8-M caused a complete relaxation with a EC50 value of
4.26 ± 1.16 x 10-6 M and 4.56 ± 1.64 x 10-6 M; n=6 with or without endothelium, respectively. There was no difference between EC50 values between these conditions. Vehicle (DMSO) was added to one arterial ring in every experiment, at increasing concentrations reaching a maximal concentration of 0.8%, and no significant relaxation was observed (data not shown).
8-M relaxations were compared to relaxations observed with quercetin as a standard. Quercetin induced complete relaxations with higher EC50 values (5,36 ± 1.78 x10-5 M and 7.82 ± 2.15 10-5 M, n= 6, with or without endothelium, respectively).
3.2. 8-Methoxycirsilineol (8-M) Induced Relaxations Were Independent of CaV1.2 channel Inhibition.
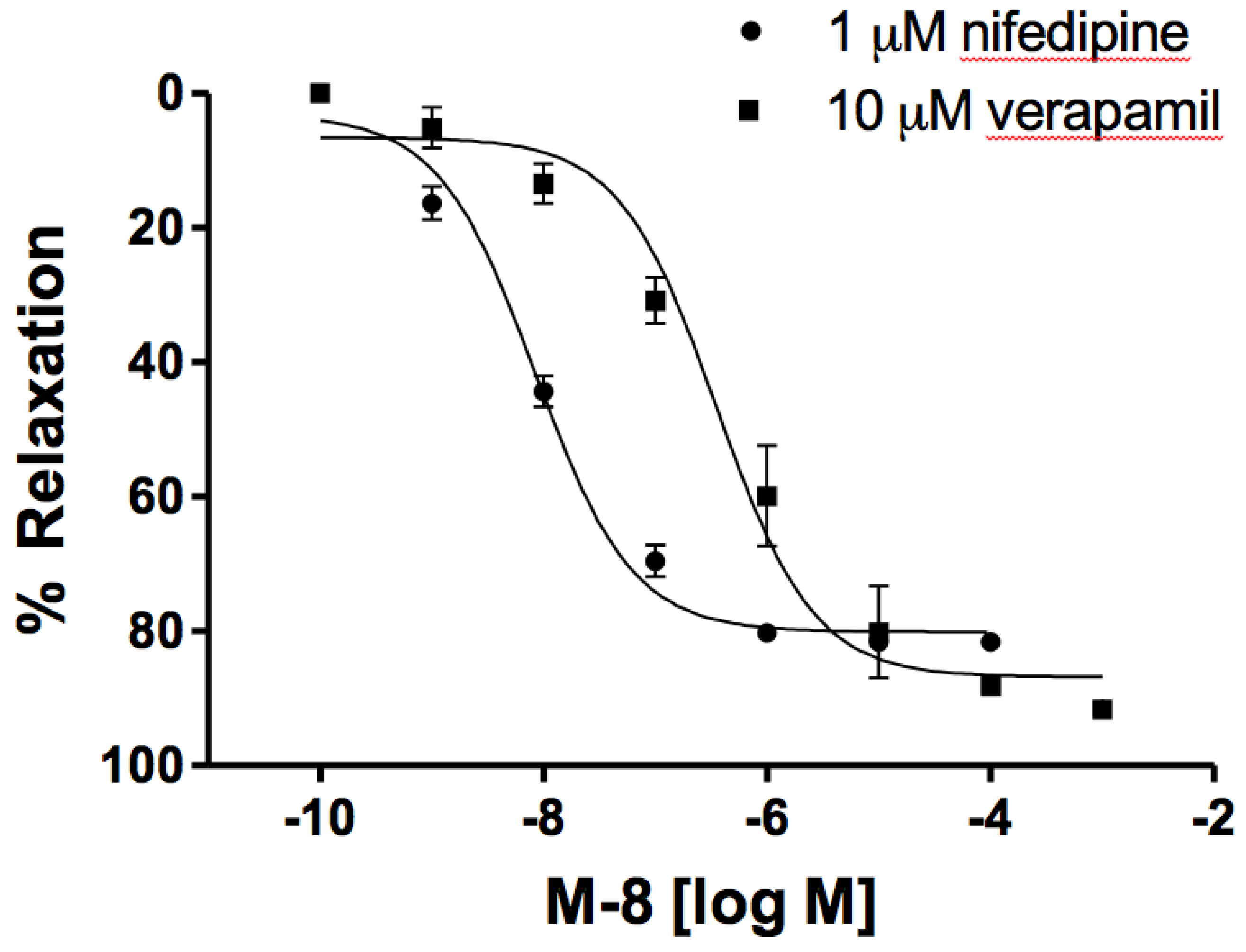
In order to test the role of CaV1.2 channels on the relaxant effect of 8-M, verapamil (10 µM) or nifedipine (1 µM) was added upon full contraction with 2 µM phenylephrine was obtained and incubated for 10 min or until maximal relaxation to these inhibitors was reached. Afterwards, increasing concentrations of 8-M (1 nM – 1mM) were added to the bath solution. Such concentrations of verapamil and nifedipine have been shown to completely inhibit the contractile response derived from Ca
V1.2 calcium entry [
17], and thus, residual tension after such treatment implies other mechanisms. We observed that 8-M in these conditions caused a complete relaxation (8.4 ± 1.28 10-9 M for nifedipine, and 3.53 ± 1.43 10-7 M for verapamil, n = 8 for both.
Figure 3). Pre-incubation with 10 µM nifedipine 15 min prior to phenylephrine stimulation was also tested, showing that the residual contraction to phenylephrine was completely relaxed by 8-M (10 µM) after 40 min. These results suggest that 8-M relaxant effect is not exclusively due to Ca
V1.2 channel activity modulation.
3.3. 8-M Induced Relaxations Depend on KV Channel Activity and Were Independent of BKCa Channel Activity
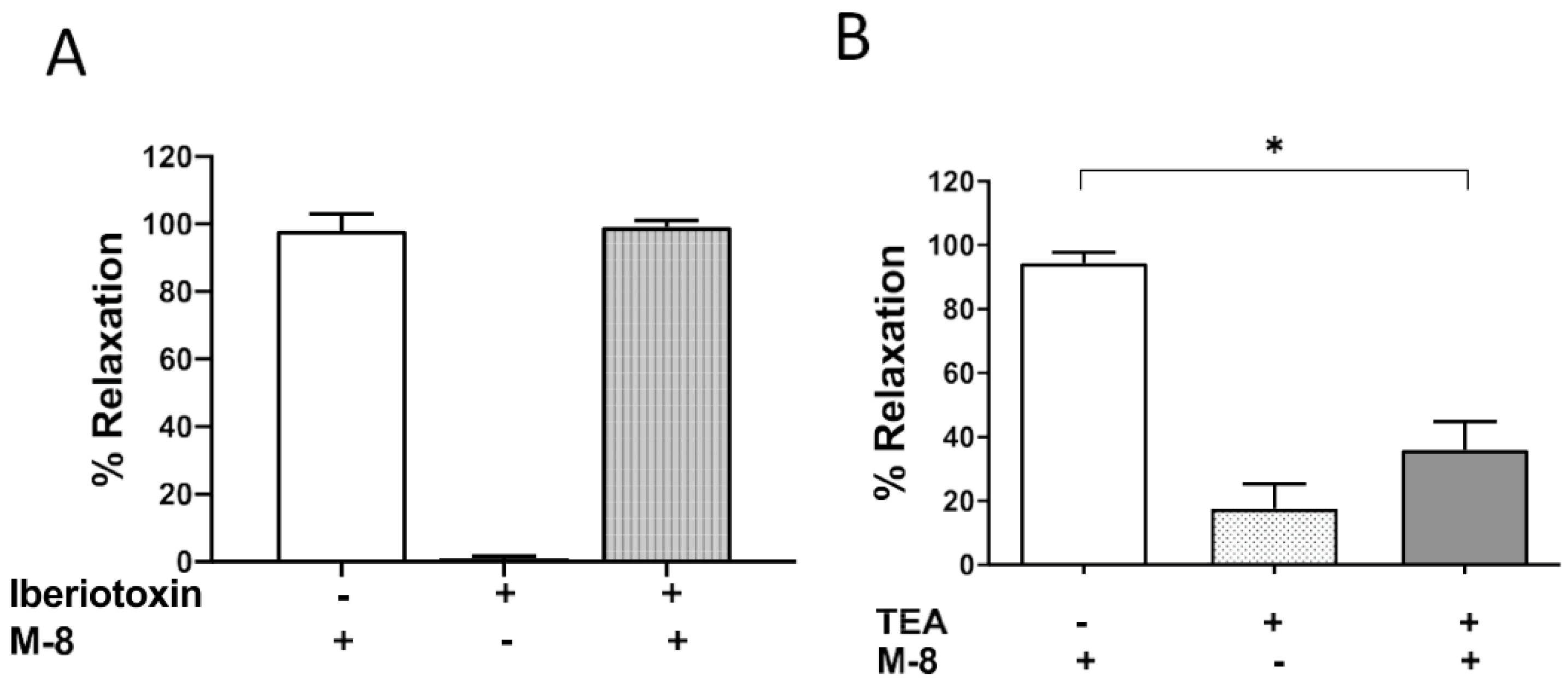
It has been described that vascular smooth muscle resting membrane potential and contraction depends on potassium channel activity. In order to test if potassium channel activation underlied the relaxant effect of 8-M, we used inhibitors directed towards BKCa channels (iberiotoxin, 10 nM) and KV (TEA, 10 mM) on phenylephrine contracted guinea pig mesenteric arteries. After a 15 min incubation with these inhibitors, 8-M was added at a concentration of 10 µM in order to observe maximum relaxation. We observed that inhibition KV channels with TEA significantly reduced the relaxant effect of 8-M (95 ± 3 % relaxation for vehicle and 8-M, vs 39.3 ± 8.7 % relaxation for TEA and 8-M, n=9,11 respectively; p<0.0001). On the other hand, inhibition of BKCa channels with Iberiotoxin did not alter the effect of 8-M (98 ± 5.0 % relaxation for vehicle and 8-M, vs 99.0 ± 1.4 % relaxation for iberiotoxin and 8-M, n=6 respectively; p=0.85,
Figure 4)
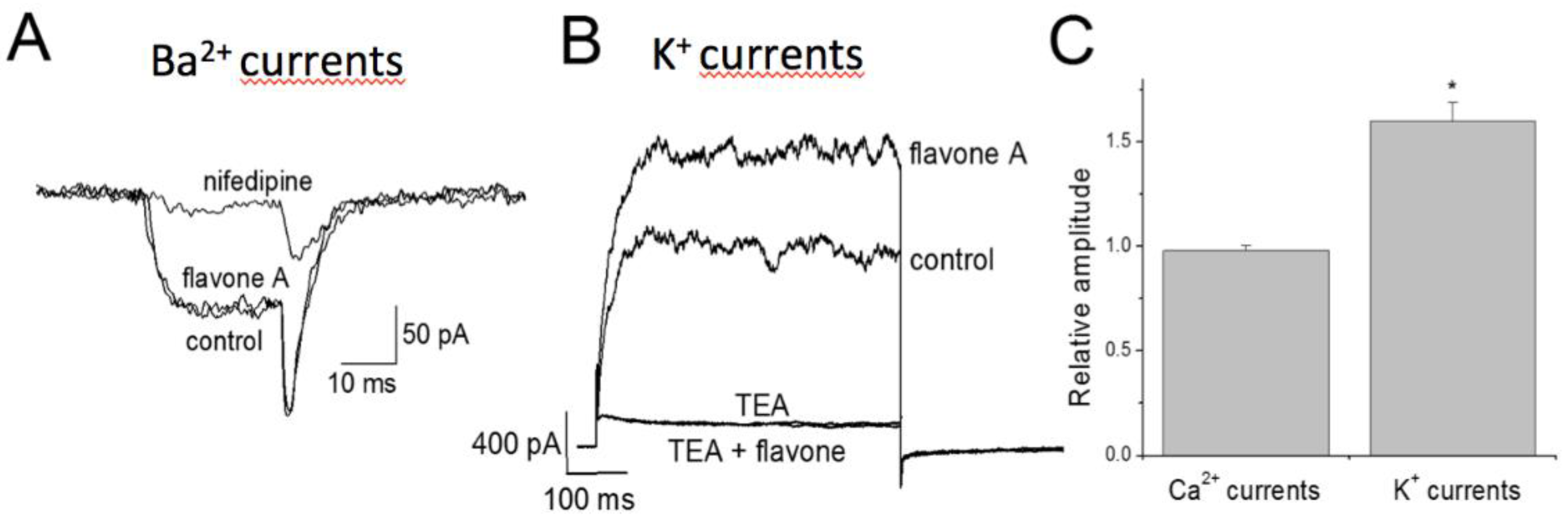
3.4. 8-M Increases KV Channel Activity on Freshly Isolated Vascular Smooth Muscle Cells but Has No Effect on CaV1.2 Channels
In order to understand the isolated effect of the 8-M on each ionic channel population, barium (through voltage-dependent calcium channels, Cav) and potassium currents were recorded in freshly isolated guinea pig mesenteric artery myocytes. CaV channel currents, sensitive to 1 μM nifedipine, were not altered by incubation with 8-M, expressed as the relative amplitude resulting from normalizing the amplitude after incubation with 8-M and that before incubation (0.98 ± 0.02, n = 6). On the other hand, potassium currents sensitive to TEA, were increased after incubation with 8-M leading to a relative amplitude of 1.6 ± 0.09 (n= 7), which represents roughly a 60% increase in macroscopic current. Simultaneous incubation with TEA + M-8 resulted in a complete ablation of current, similar to addition of TEA alone (
Figure 5). These results indicate that 8-M had no direct effect on Cav channels, but had a significant activating effect on KV channels.
4. Discussion
The main finding of this work was that 8-M compound extracted from Larrea tridentata showed a strong vasodilating effect in guinea pig mesenteric arteries which depends on KV channel activity. This vasorelaxation was independent of endothelium, hence we propose that 8-M is mainly affecting smooth muscle contractile mechanisms which involve membrane depolarization and calcium handling.
Vascular smooth muscle cell contraction in response to agonist stimulation is initiated by membrane depolarization leading to calcium entry through CaV1.2 channels, and activation of signaling pathways which promote calcium release from the sarcoplasmic reticulum and calcium sensitization of the contractile apparatus. Vascular resting membrane potential is approximately -50 mV [
18] and depolarizations due to agonist stimulation reach -20 mV depending on the agonist involved [
19]. At these values, a proportion of CaV1.2 channels are activated leading to calcium entry and contraction. Membrane potential in these cells is highly influenced by the activity of potasium channels which counterbalance inward cationic currents and is responsible for maintaining resting membrane potential at negative values [
20,
21]. In this work, we observed that the magnitude of KV currents were significantly increased by addition of 8-M on freshly isolated guinea pig mesenteric artery cells. We suggest that this current activation explains most of the vasodilatory effect of 8-M in mesenteric artery rings.
Similar compounds have been reported to cause potassium channel activation in vascular smooth muscle. For example, in rat coronary artery, quercetin reduced high extracellular potassium induced elevation of intracellular calcium and induced vasorelaxation. This effect was inhibited by 4AP (KV inhibitor) but not by other potassium channel inhibitors such as glybenclamide or iberiotoxin suggesting Kv channel activation by this 8-M [
22]. Baicalin, a flavone extracted from
Sutellaria baicalensis showed vasorelaxant effects in rat thoracic aortic rings stimulated with phenylephrine and angiotensin II. This complete vasorelaxation was sensitive to KATP inhibition by glybenclamide but not by other potassium channel inhibitors such as 4AP and BaCl2, neither by CaV1.2 inhibition with nifedipine [
23]. Han Jun et al., showed an indirect pathway for BK channel activation in rat cerebral basal artery in response to
Rhododendron simsii flavonoids, which involved transient receptor potential cation channel subfamily V member 4 (TRPV4) activation [
24]. These studies together with our results suggests that flavonoids have a common vasorelaxant pathway involving potassium channels and membrane hyperpolarization, yet the type of potassium channel involved probably depends on the flavone studied.
Regarding the role of calcium channel inhibition by 8-M, according to our results, inhibition is indirect via membrane hyperpolarization due to potassium channel activation. Direct inhibition of CaV1.2 channels by 8-M was discarded in accordance with the electrophysiological experiments performed on isolated cells. In contrast with our results, other flavonoids such as quercetin have a direct inhibitory effect con CaV 1.2 channels. Experiments performed in rat basilar artery, showed that quercetin induces vasodilation to endothelin and 5-hydroxytryptamine (serotonin) induced contractions by directly inhibiting calcium channels independently of potassium channels. This effect was partially dependent on endothelium in contrast to what we found, suggesting that in this vascular bed, quercetin requires endothelium mediated relaxation [
25]. On the other side, Hou et al. reported that 10 µM quercetin directly inhibits CaV1.2 channels in rat renal artery independent of endothelium [
26]. According to these results, flavonoids like quercetin have a clear vasorelaxant effect, yet the mechanisms involved depend on which vascular bed studied.
In our study, we observed that the vasodilatory effect of 8-M was not completely abolished by KV channel inhibition. This poses the question of other mechanisms involved such as calcium de-sensitization induced by de-phosphorylation and activation of non-selective cationic currents. Similar compounds have shown vasorelaxant effects produced by calcium desensitization mechanisms in smooth muscle. Jeon et al. reported that commercial flavone caused rat aortic vasorelaxation by inhibiting myosin light chain (MLC 20) phosphorylation in the classic Thr855 residue [
27]. Similar to these results, quercetin was found to cause rat aortic vasorelaxation to phenylephrine by activation of AMPK pathway leading to decreased expression of myosin light chain kinase (MLCK) [
28].
Results herein reported show and confirm that some compounds derived from endemic plants have potential medicinal effects. Likewise, the vasorelaxant effect produced by 8-M explain the medical use of L. tridentata.
Author Contributions
PAS Performed some tension experiments and analyzed the data and wrote up the manuscript MPNH Performed some tension experiments MMGC Perfomed 8-M purification and characterization XOR Collected leaves of L tridentata leaves and contributed during the chemical extraction procedure. Also, she performed some tension experiments. AARM Performed the electrophysiological recordings and analyzed those data IAAF Performed the electrophysiological recordings MDBO Isolated vascular smooth muscle cells JRVA Contributed to critical discussion on the experimental design, results, presentation, writing and submission RET Designed the protocol and discussion on the results and contributed to critical discussion on the experimental design, results, presentation, writing and submission.
Funding
This work was supported by the UASLP [ C14-FAI-04-34.34, 2014] to PAS. We would also like to thank LQ. Daniel Olivares Bucio for technical assistance.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Arteaga, S.; Andrade-Cetto, A.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sagaste, C. A., G. Montero, M. A. Coronado, J. R. Ayala, J. León, C. García, B. A. Rojano, S. Rosales and D. G. Montes, 2019. Creosote Bush. Molecules, 24(9), 1786.
- Gnabre, J.; Bates, R.; Huang, R.C. Creosote bush lignans for human disease treatment and prevention: Perspectives on combination therapy. J. Tradit. Complement. Med. 2015, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchyo-Tenorio, G.; Rodríguez-Cruz, M.; Andrade-Cetto, A.; Cárdenas-Vázquez, R. Creosote Bush (Larrea tridentata) Improves Insulin Sensitivity and Reduces Plasma and Hepatic Lipids in Hamsters Fed a High Fat and Cholesterol Diet. Front. Pharmacol. 2016, 07, 194. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, B.; Li, L.; Bains, T.; Debnath, A.; LaBarbera, D.V. Larrea tridentata: A novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PLOS Neglected Trop. Dis. 2017, 11, e0005832–e0005832. [Google Scholar] [CrossRef]
- Skouta, R.; Morán-Santibañez, K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef] [PubMed]
- Morán-Santibañez, K.; Vasquez, A.H.; Varela-Ramirez, A.; Henderson, V.; Sweeney, J.; Odero-Marah, V.; Fenelon, K.; Skouta, R. Larrea tridentata Extract Mitigates Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. Antioxidants 2019, 8, 427. [Google Scholar] [CrossRef]
- Watanabe, J.; Shinmoto, H.; Tsushida, T. Coumarin and Flavone Derivatives from Estragon and Thyme as Inhibitors of Chemical Mediator Release from RBL-2H3 Cells. Biosci. Biotechnol. Biochem. 2005, 69, 1–6. [Google Scholar] [CrossRef]
- Brahmi, Z.; Niwa, H.; Yamasato, M.; Shigeto, S.; Kusakari, Y.; Sugaya, K.; Onose, J.-I.; Abe, N. Effective Cytochrome P450 (CYP) Inhibitor Isolated from Thyme (Thymus saturoides) Purchased from a Japanese Market. Biosci. Biotechnol. Biochem. 2011, 75, 2237–2239. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, S., N. Anzani, A. Orrù, C. Floris, P. Caboni, S. Alcaro, E. Maccioni, S. Distinto and F. Cottiglia, 2015. Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: in silico, in vitro, and in vivo studies. J Nat Prod, 78(1), 69-76.
- Fushiya, S.; Kishi, Y.; Hattori, K.; Batkhuu, J.; Takano, F.; Singab, A.N.B.; Okuyama, T. Flavonoids from Cleome droserifolia Suppress NO Production in Activated Macrophages in Vitro. Planta Medica 1999, 65, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Farimani, M.M.; Kiaei, M. Anticancer effect of calycopterin via PI3K/Akt and MAPK signaling pathways, ROS-mediated pathway and mitochondrial dysfunction in hepatoblastoma cancer (HepG2) cells. Mol. Cell. Biochem. 2014, 397, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lotfizadeh, R.; Sepehri, H.; Attari, F.; Delphi, L. Flavonoid Calycopterin Induces Apoptosis in Human Prostate Cancer Cells In-vitro. . 2020, 19, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Brozovich, F.; Nicholson, C.; Degen, C.; Gao, Y.Z.; Aggarwal, M.; Morgan, K. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- CONABIO, 2009. Catálogo taxonómico de especies de México, en Capital Natural de México. Vol. 1.
- Coville, F.V. 1893. A Report On The Botany Of The Expedition Sent Out In 1891 By The U. S. Department Of Agriculture To Make A Biological Survey Of The Region Of Death Valley California. Contr. U.S. Natl. Herb 4: 75.
- Algara-Suárez, P., C. Romero-Méndez, T. Chrones, S. Sánchez-Armass, U. Meza, S. M. Sims and R. Espinosa-Tanguma, 2007. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 293(1), L191-198.
- Worley, J. F., J. W. Deitmer and M. T. Nelson,1986. Single nisoldipine-sensitive calcium channels in smooth muscle cells isolated from rabbit mesenteric artery. Proc Natl Acad Sci U S A, 83(15), 5746-5750.
- Nelson, M.T.; Standen, N.B.; Brayden, J.E.; Worley, J.F. Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature 1988, 336, 382–385. [Google Scholar] [CrossRef]
- Tykocki, N. R., E. M. Boerman and W. F. Jackson, 2017. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr Physiol, 7(2), 485-581.
- Jackson, W.F. KV channels and the regulation of vascular smooth muscle tone. Microcirculation 2017, 25. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liu, Y.; Niu, L.; Cui, L.; Zhang, M. Enhancement of Voltage-Gated K+ Channels and Depression of Voltage-Gated Ca2+ Channels Are Involved in Quercetin-Induced Vasorelaxation in Rat Coronary Artery. Planta Medica 2014, 80, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Jia, C.; Zhang, Y.; Wang, W.; Zhu, W.; Chen, Y.; Zhang, T. Baicalin relaxes vascular smooth muscle and lowers blood pressure in spontaneously hypertensive rats. Biomed. Pharmacother. 2018, 111, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Han, J., H. H. Xu, X. L. Chen, H. R. Hu, K. M. Hu, Z. W. Chen and G. W. He, 2018.
- Total Flavone of Rhododendron Improves Cerebral Ischemia Injury by Activating Vascular TRPV4 to Induce Endothelium-Derived Hyperpolarizing Factor-Mediated Responses. Evid Based Complement Alternat Med, 2018: 8919867.
- Yuan, T.-Y.; Niu, Z.-R.; Chen, D.; Chen, Y.-C.; Zhang, H.-F.; Fang, L.-H.; Du, G.-H. Vasorelaxant effect of quercetin on cerebral basilar artery in vitro and the underlying mechanisms study. J. Asian Nat. Prod. Res. 2018, 20, 477–487. [Google Scholar] [CrossRef]
- Hou, X.-M.; Zhang, M.-S.; Qin, X.-J. [Vasodilation of quercetin on rat renal artery and the relationship with L-type voltage-gated Ca2+ channels and protein kinase C]. . 2017, 69, 775–780. [Google Scholar]
- Jeon, S.B.; Kim, G.; Kim, J.I.; Seok, Y.M.; Kim, S.; Suk, K.; Shin, H.; Lee, Y.; Kim, I.K. FLAVONE INHIBITS VASCULAR CONTRACTION BY DECREASING PHOSPHORYLATION OF THE MYOSIN PHOSPHATASE TARGET SUBUNIT. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1116–1120. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.-R.; Choi, H.C. Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction Through LKB1-AMPK Signaling Pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).