Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background of the Study
1.2. Statement of the Problems
1.3. Objective of the Study
1.3.1. General Objective
1.3.2. Specific Objectives
- ✓
- To estimate carbon stock in above, below, litter and soil pools
- ✓
- To examine the correlation of AGB/AGC with sentinel 2A derived vegetation indices
- ✓
- To illustrate the distribution of carbon stock with surface data
2. Materials and Methods
2.1. Location
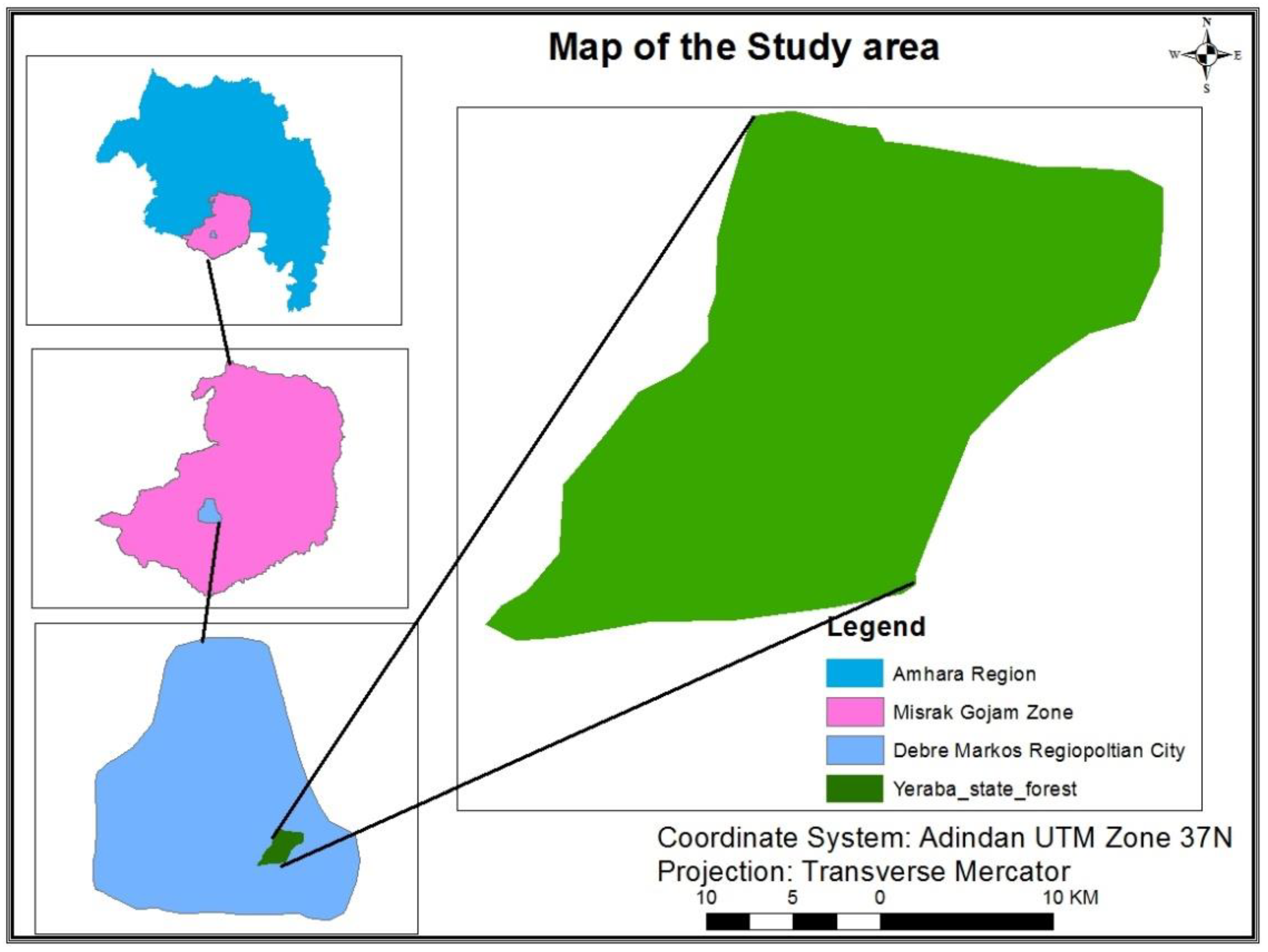
2.2. Research Methods
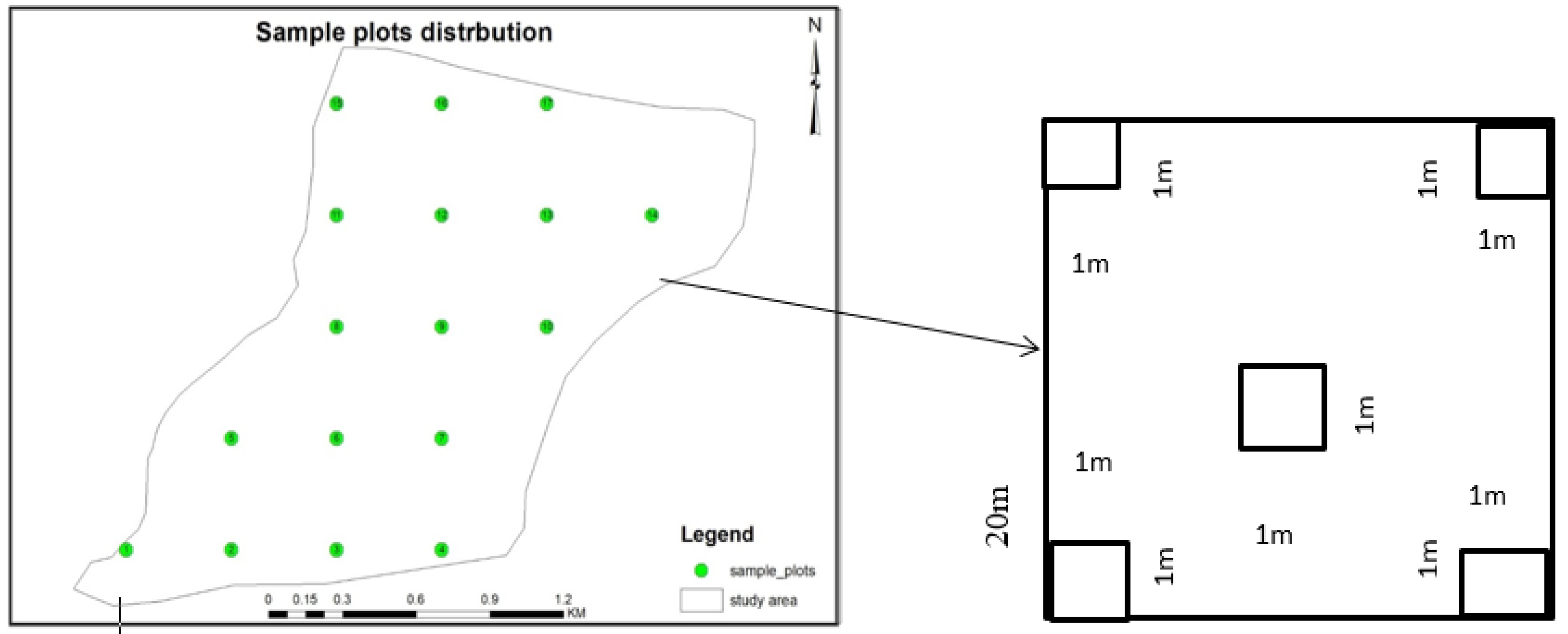
2.2.1. Sampling Design
2.2.2. Sampling Technique and Sample Size
2.2.3. Data Sources and Methods of Data Collection
2.2.4. Materials and Software
| Tools and software’s with their function | |
|---|---|
| Tools | Function |
| Garmin 72 GPS | Navigation and to indicate plot central coordinate |
| Clinometer | Tree height measurement |
| Diameter tape | To outline the plot |
| Caliper | DBH measurement |
| Soil ogre | To take soil sample |
| Hard plastic box | To hold the individual soil sample |
| Software’s | Function |
| SNAP | Preprocessing sentinel data |
| ARCGIS | Regression and correlation analysis, mapping the result |
| Excel | Field data organization |
2.2.5. Method of Data Analysis
2.2.5.1. Aboveground Biomass Carbon Stock Estimation
| Species name | Specific wood density (P) | References |
| Cupressus lusitanica | 0.414 | (ICRAF Database - Wood Density, n.d.) |
| Acacia dicurusne | 0.557 | (ICRAF Database - Wood Density, n.d.) |
| eucallyptus globuse | 0.7093 | (ICRAF Database - Wood Density, n.d.) |
| Acacia melanoxylon | 0.538 | (ICRAF Database - Wood Density, n.d.) |
| Grevillea roubsta | 0.536 | (ICRAF Database - Wood Density, n.d.) |
| pinus patula | 0.45 | (ICRAF Database - Wood Density, n.d.) |
2.2.5.2. Belowground Biomass Carbon Stock Estimation
2.2.5.3. Estimation of Carbon in the Litter Biomass
2.2.5.4. Estimation of Soil Organic Carbon
2.2.5.5. Total Carbon Stock Density (TCSD)
2.2.5.6. Scaling up AGB via Remote Sensing
| No | Vegetation indices | Formula | Reference |
| 1 | NDII/Normalized difference VI) | (B8-B12)/(B8+B12) | (Hunt & Rock, 1989) |
| 2 | ARVI /Atmospheric resistant VI) | (B8-(B4-B2))/(B8+(B4) | Kaufman, 1992 |
| 3 | GNDVI /Green normalized difference VI) | (B8-B3)/(B8+B3) | (Gitelson et al., 1996) |
| 4 | VDVI (Visible band difference VI) | (2*green-red-blue)/(2*green + red + blue) | Wang et al., 2015 |
| 5 | NDVI (Normalized difference VI) | (B8-B4)/(B8+B4) | Rouse et al., 1974 |
2.2.5.7. Correlation and regression Statistical data analysis
2.2.5.8. Producing Carbon Sink and Stock Potential Map of the Forest
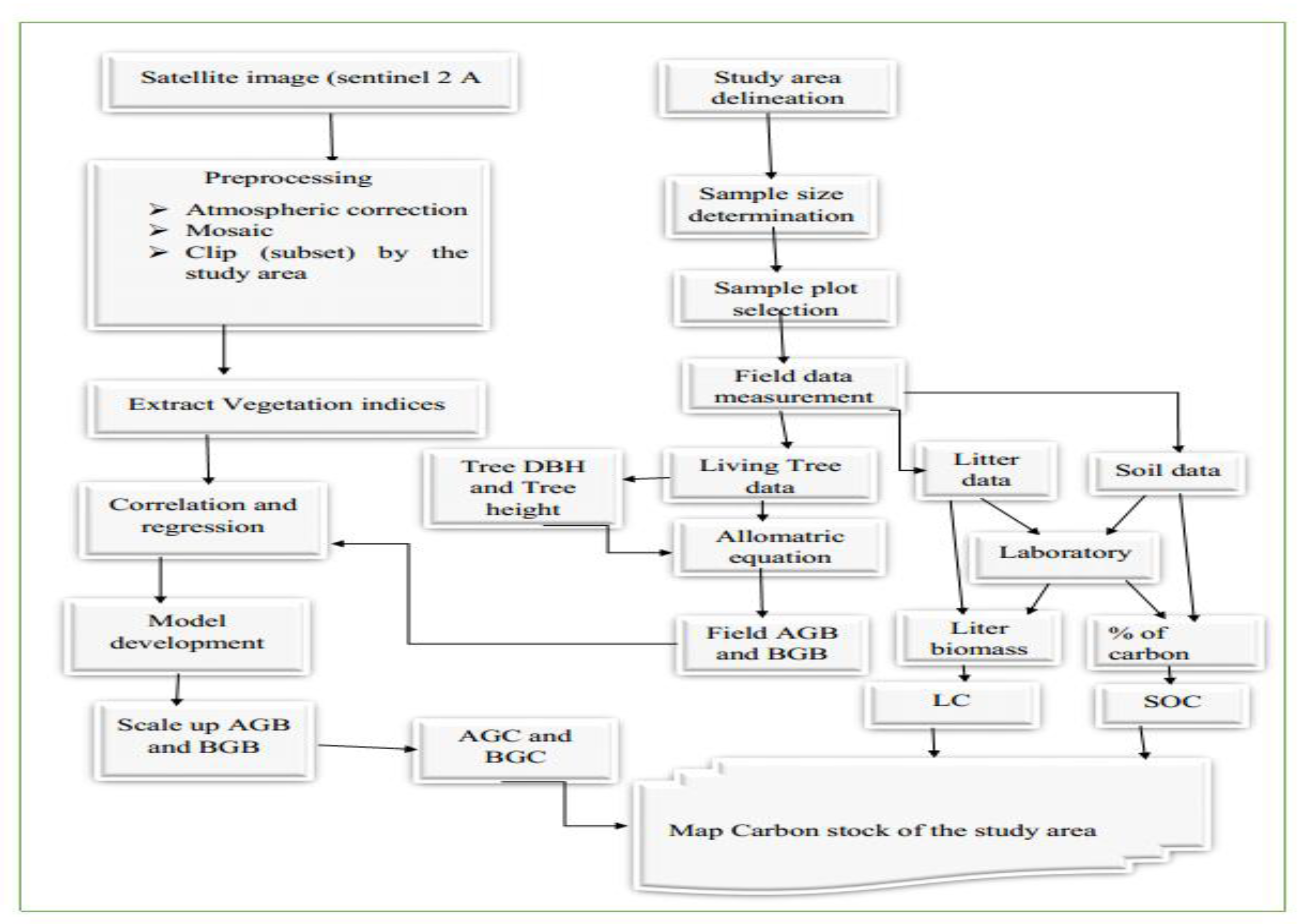
3. Result and Discussion
3.1. Results
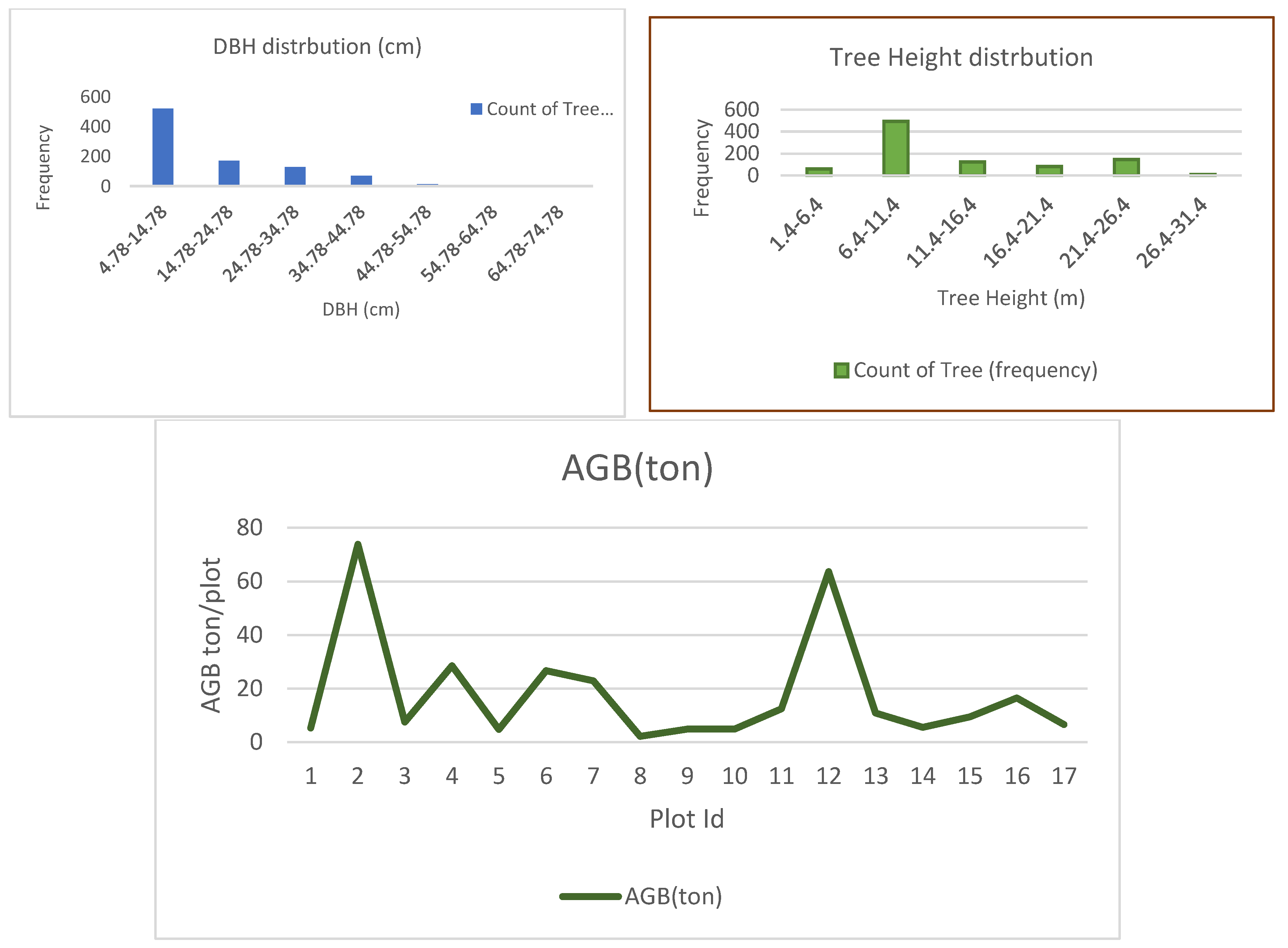
3.1.1. Field above Ground Biomass of the Study Area
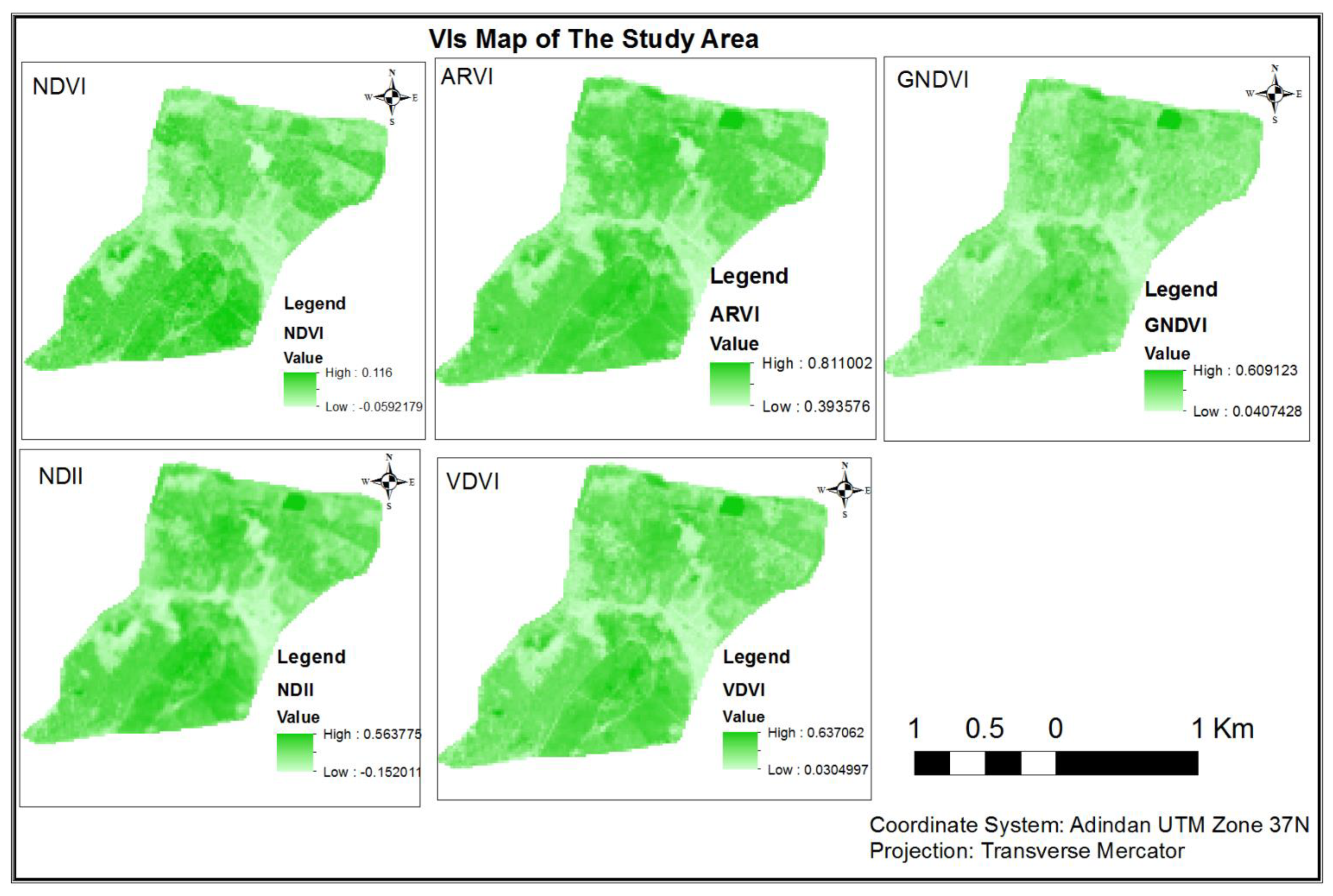
3.1.2. Comparison of Vegetation Indices for AGB/AGC Estimation
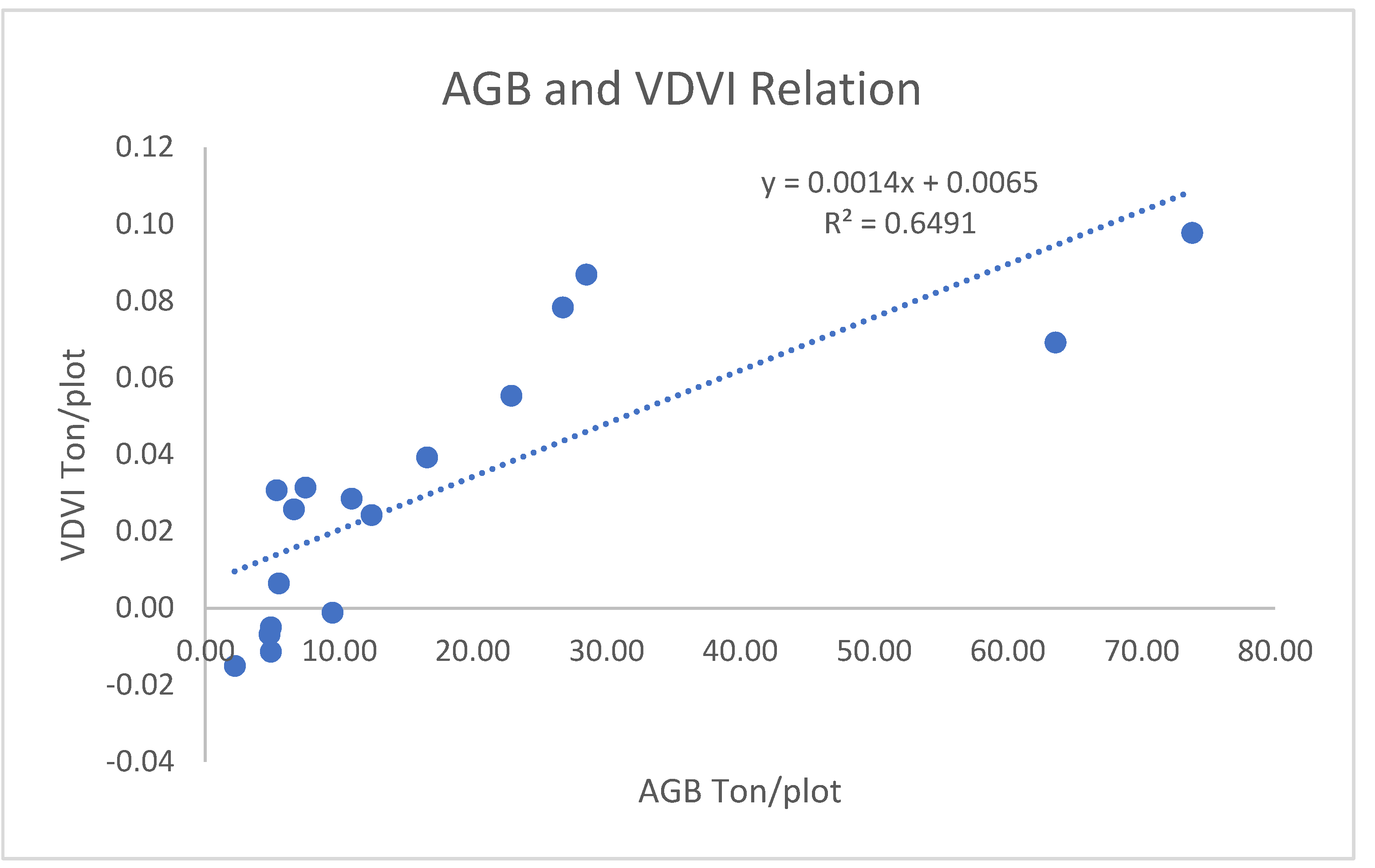
3.1.2.1. Correlation of AGB/AGC with VDVI
3.1.2.2. Correlation of AGB/AGC with NDVI
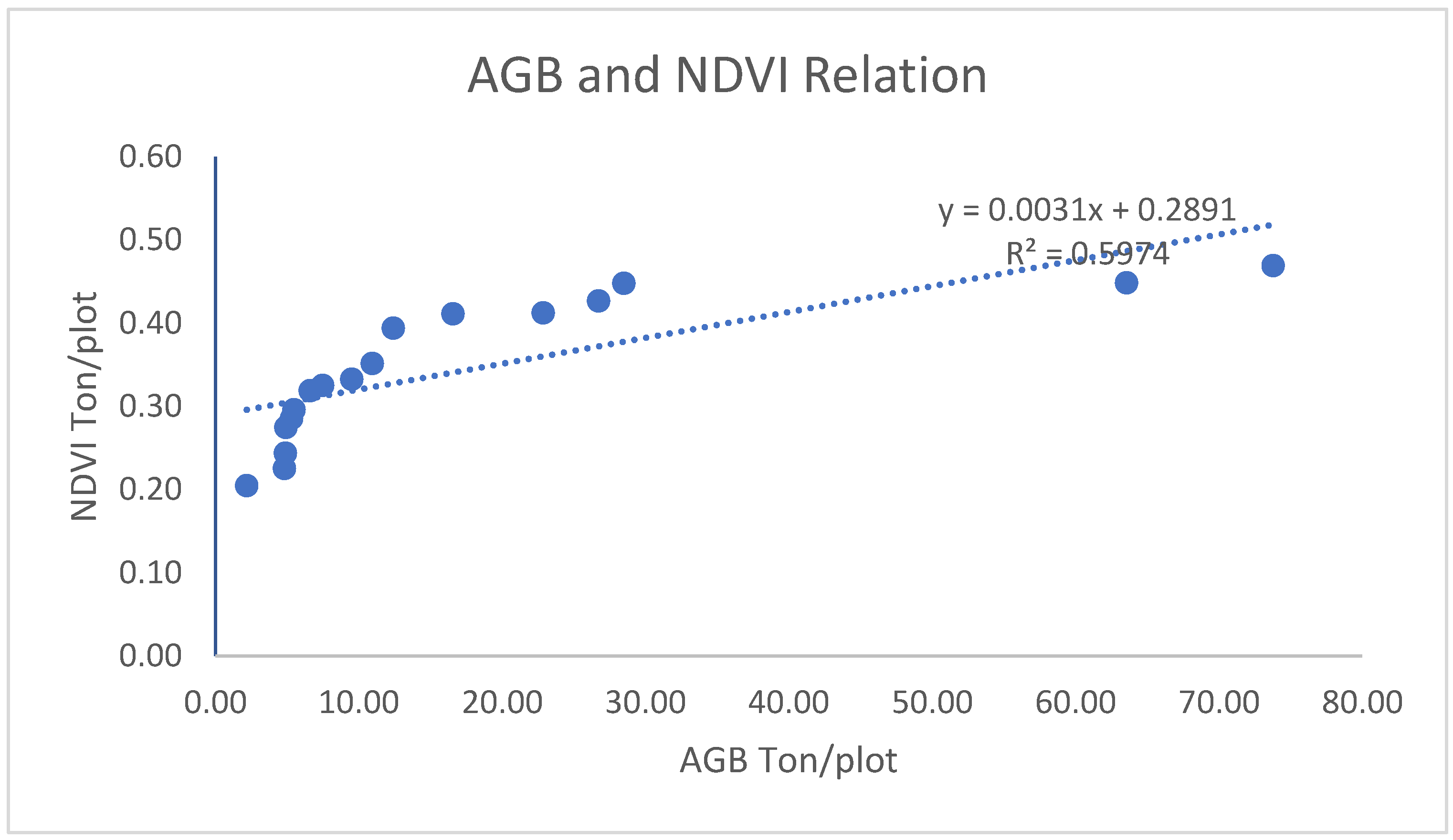
3.1.2.3. Correlation of AGB/AGC with ARVI
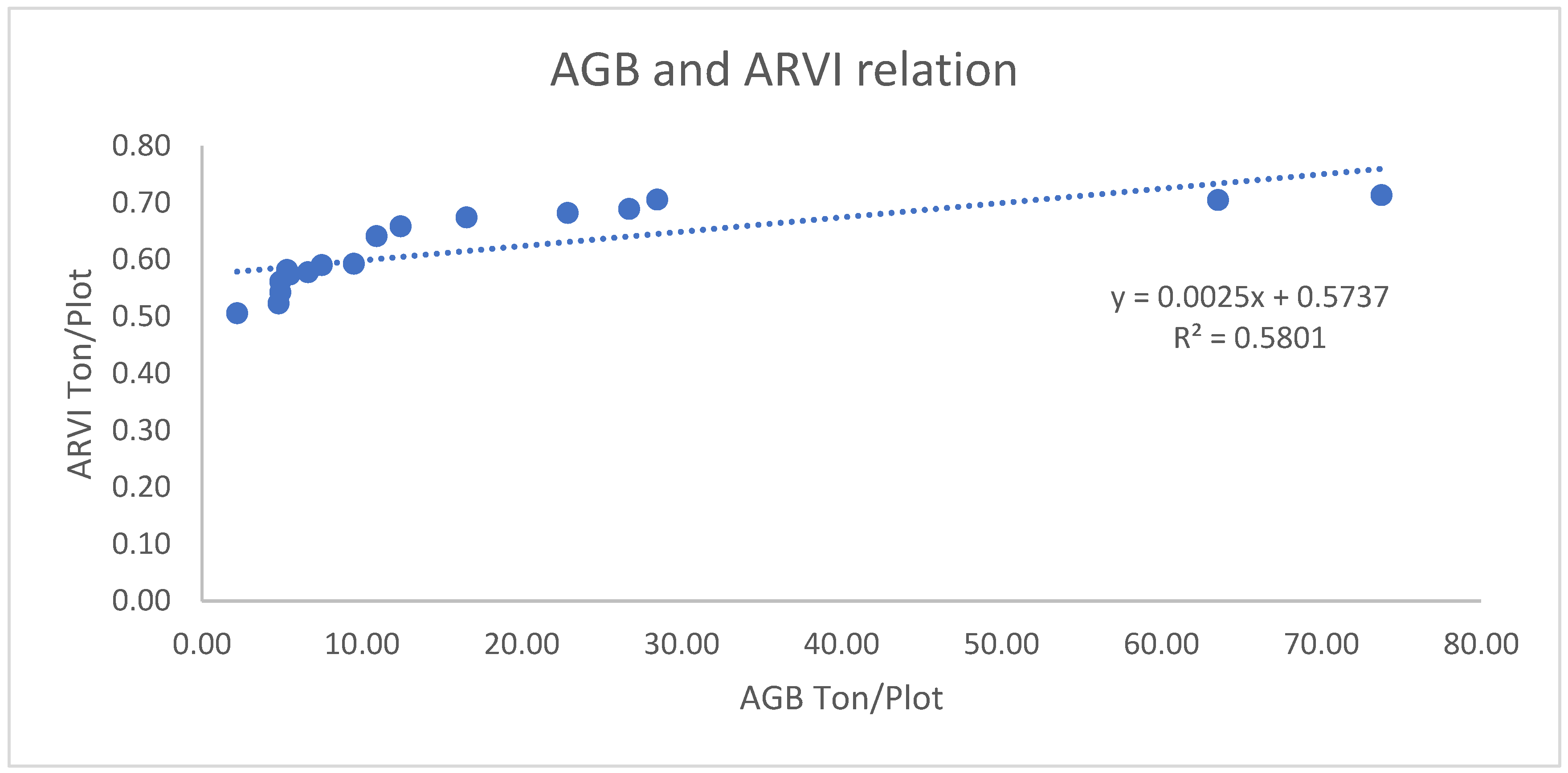
3.1.2.4. Correlation of AGB/AGC with NDII
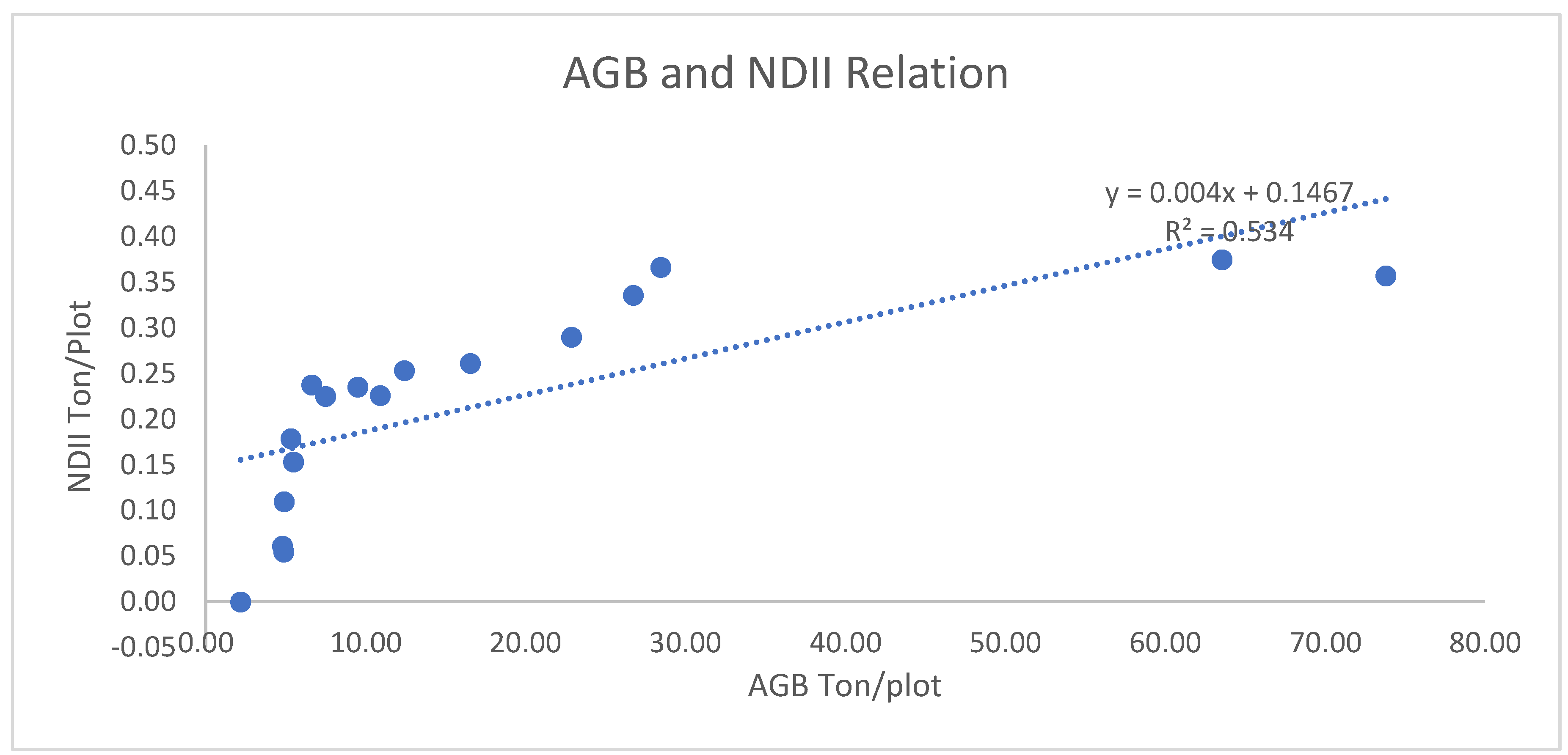
3.1.2.5. Correlation of AGB/AGC with GNDVI
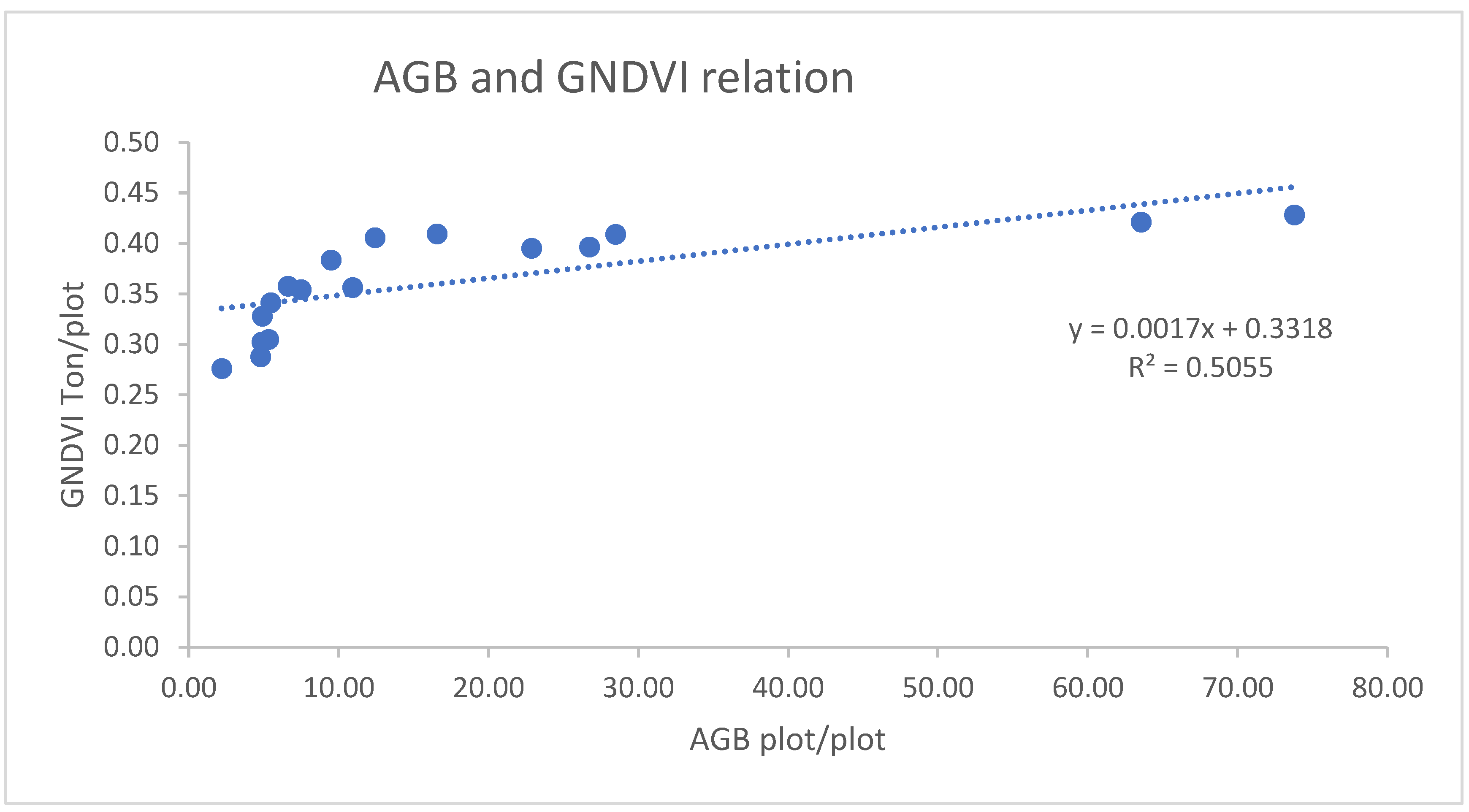
3.1.3. Modeling the relationship between AGC and VIs
| No | VIs | R | R2 | significance |
|---|---|---|---|---|
| 1 | ARVI | 0.76 | 0.58 | 0.00 |
| 2 | GNDVI | 0.71 | 0.51 | 0.00 |
| 3 | VDVI | 0.81 | 0.65 | 0.00 |
| 4 | NDII | 0.73 | 0.53 | 0.00 |
| 5 | NDVI | 0.77 | 0.60 | 0.00 |
| SUMMARY OUTPUT | |
| Regression Statistics | |
| Multiple R | 0.81 |
| R Square | 0.65 |
| Adjusted R Square | 0.63 |
| Standard Error | 12.68 |
| Observations | 17 |
| Coefficients | Standard Error | t Stat | P-value | Lower 95% | Upper 95% | Lower 95.0% | Upper 95.0% | |
| Intercept | 3.26 | 4.16 | 0.78 | 0.45 | -5.62 | 12.13 | -5.62 | 12.13 |
| VDVI | 57.24 | 89.08 | 5.27 | 0.00 | 279.43 | 659.18 | 279.43 | 659.18 |
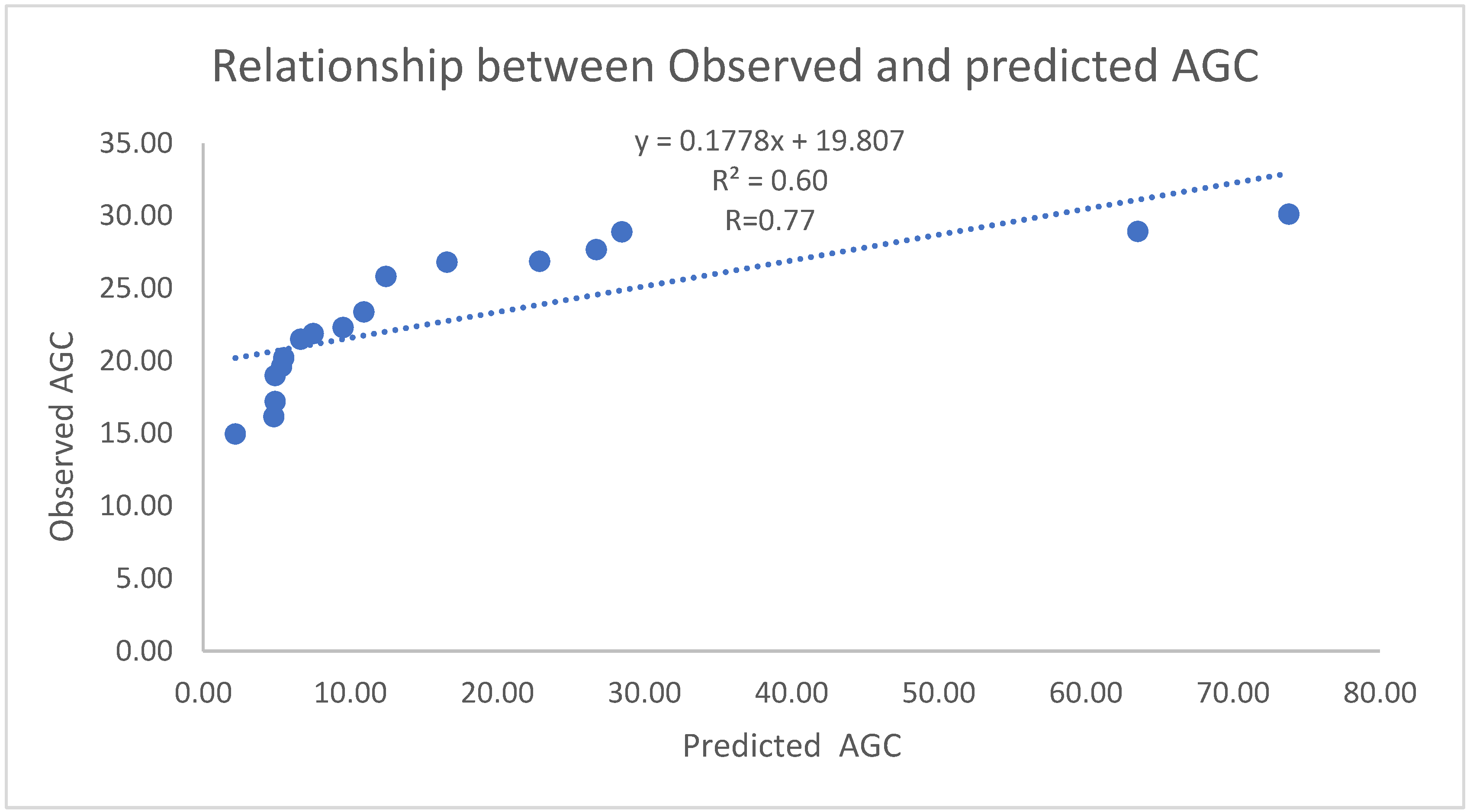
3.1.4. Correlation of field AGB and Predicted AGB
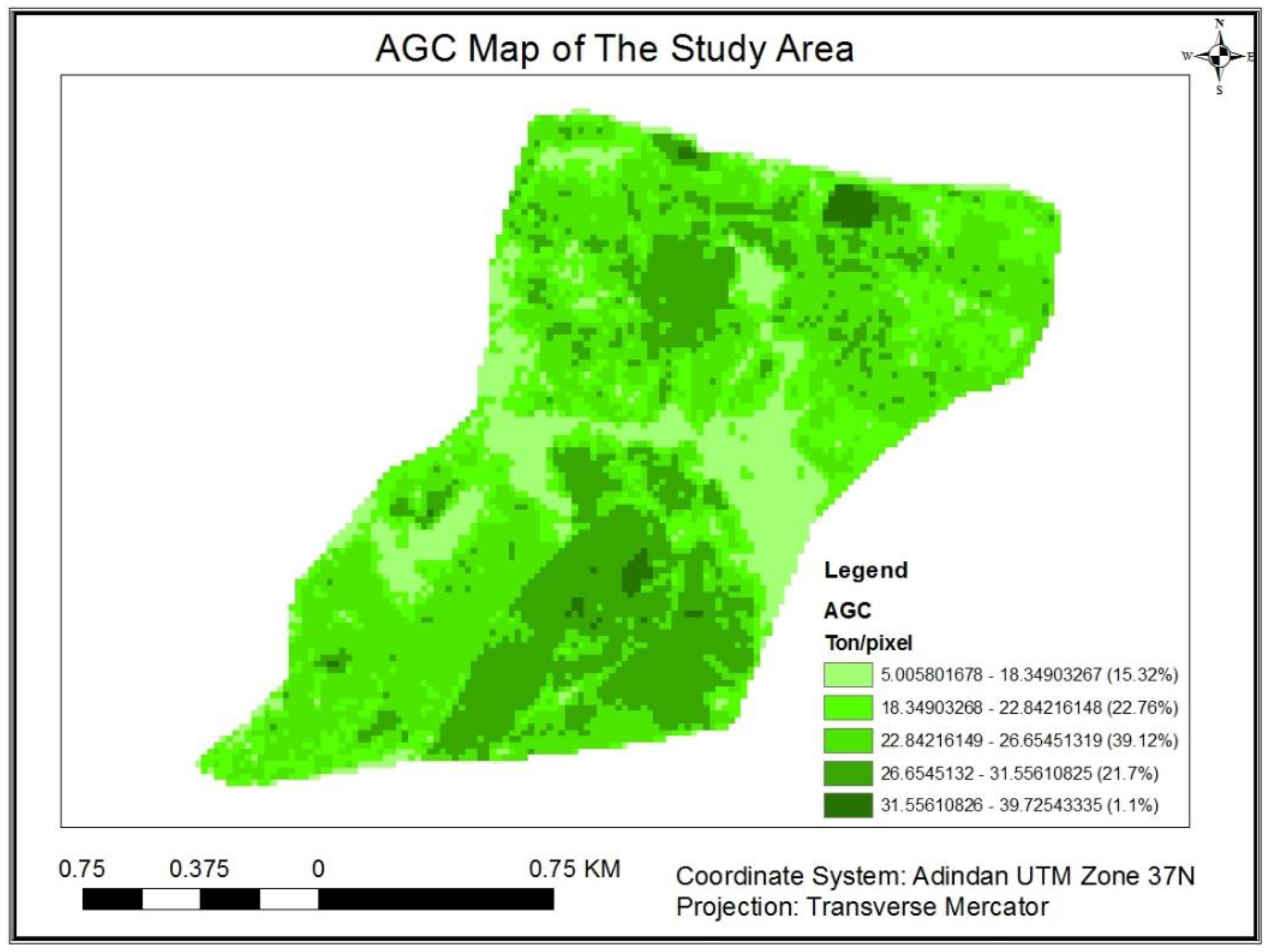
3.1.5. Above-Ground Carbon Stock distribution map
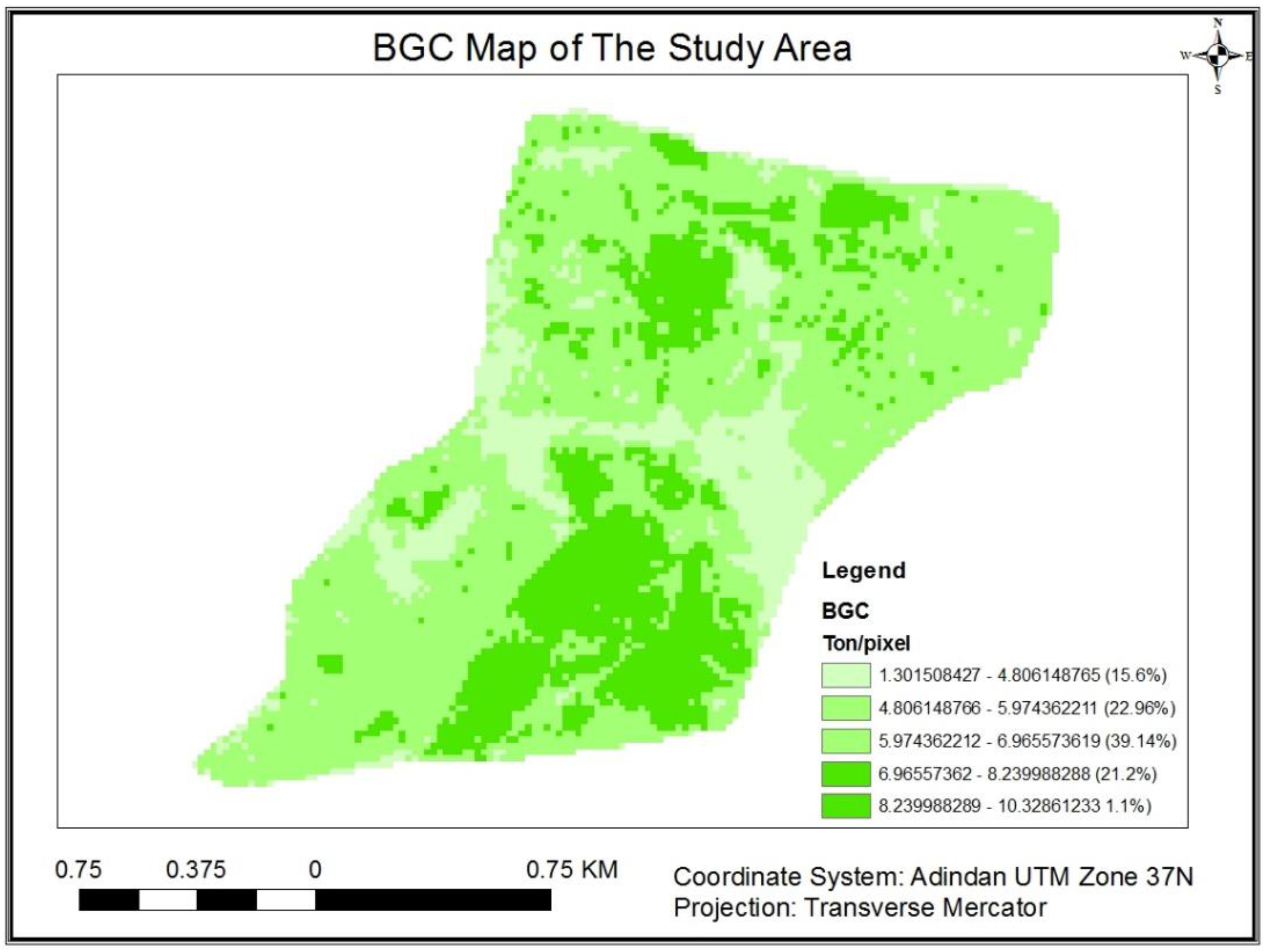
3.1.6. Below Ground Carbon Potential
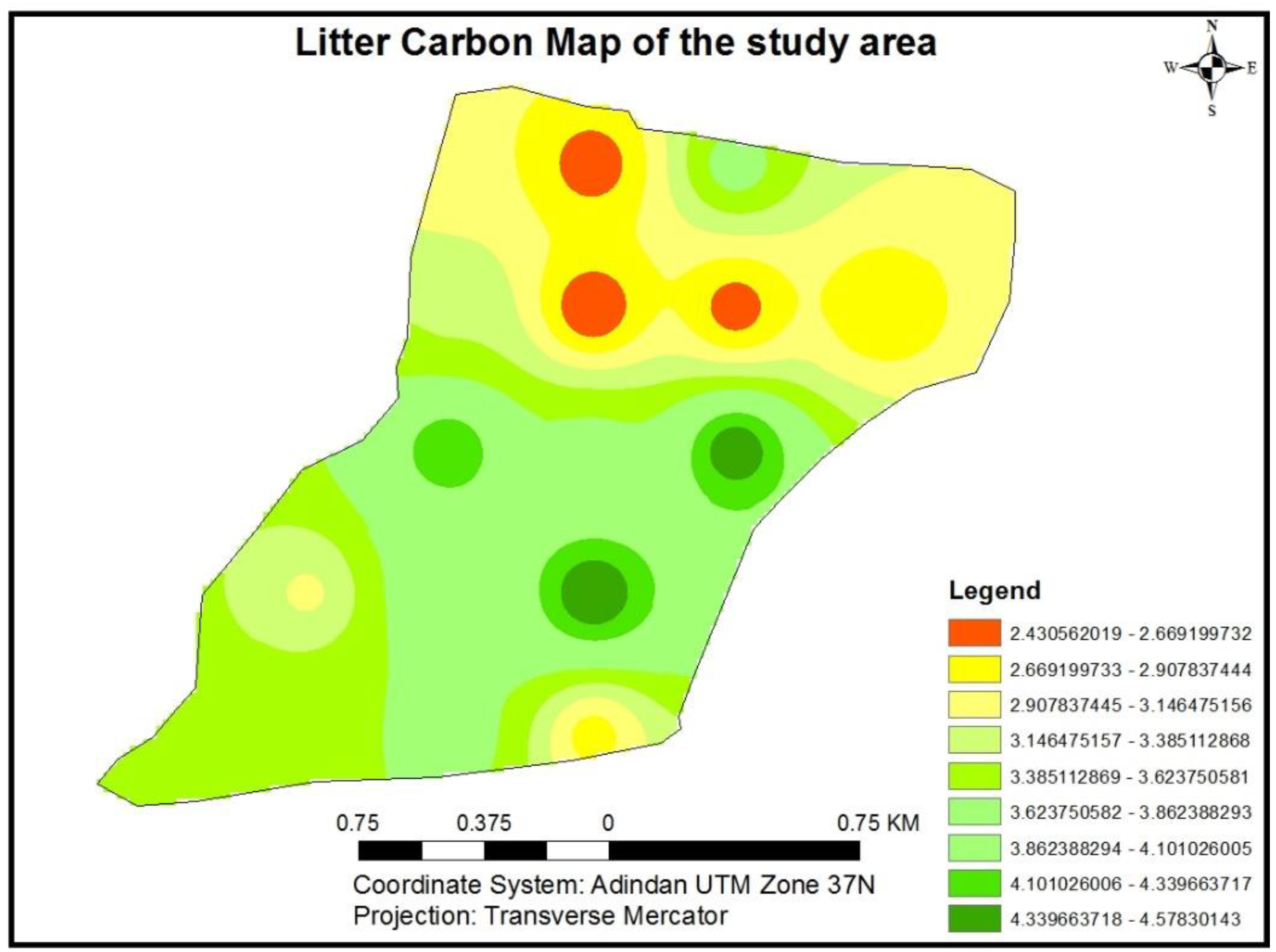
3.1.7. Litter Carbon Stock Potential
3.1.7. Estimation of Carbon Stocks in (SOC)
3.1.7.1. Bulk Density
3.1.7.2. Soil Organic Carbon
3.1.7.3. Soil Carbon Stock
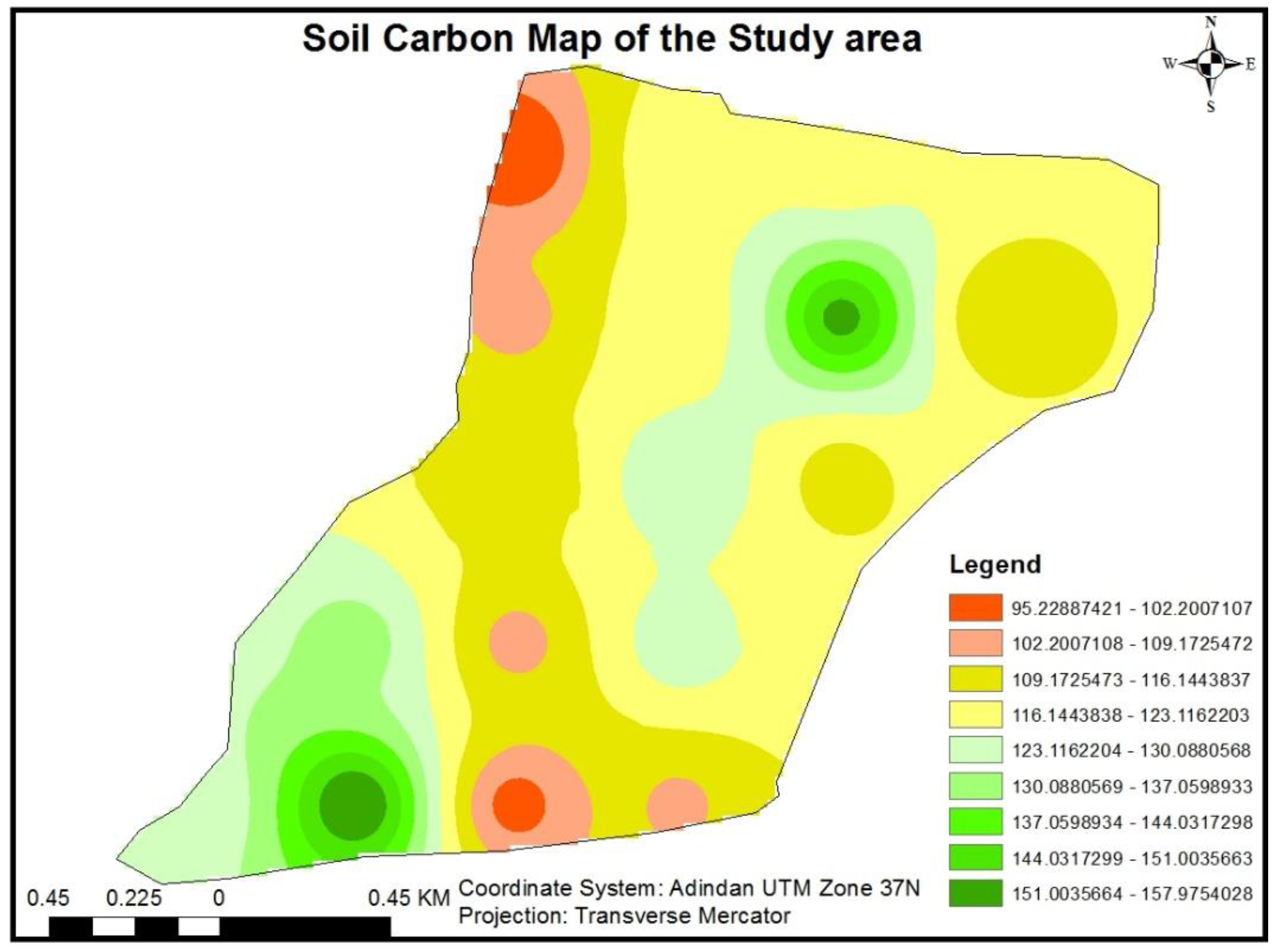
3.1.8. Total Carbon Stock of Yeraba state Forest
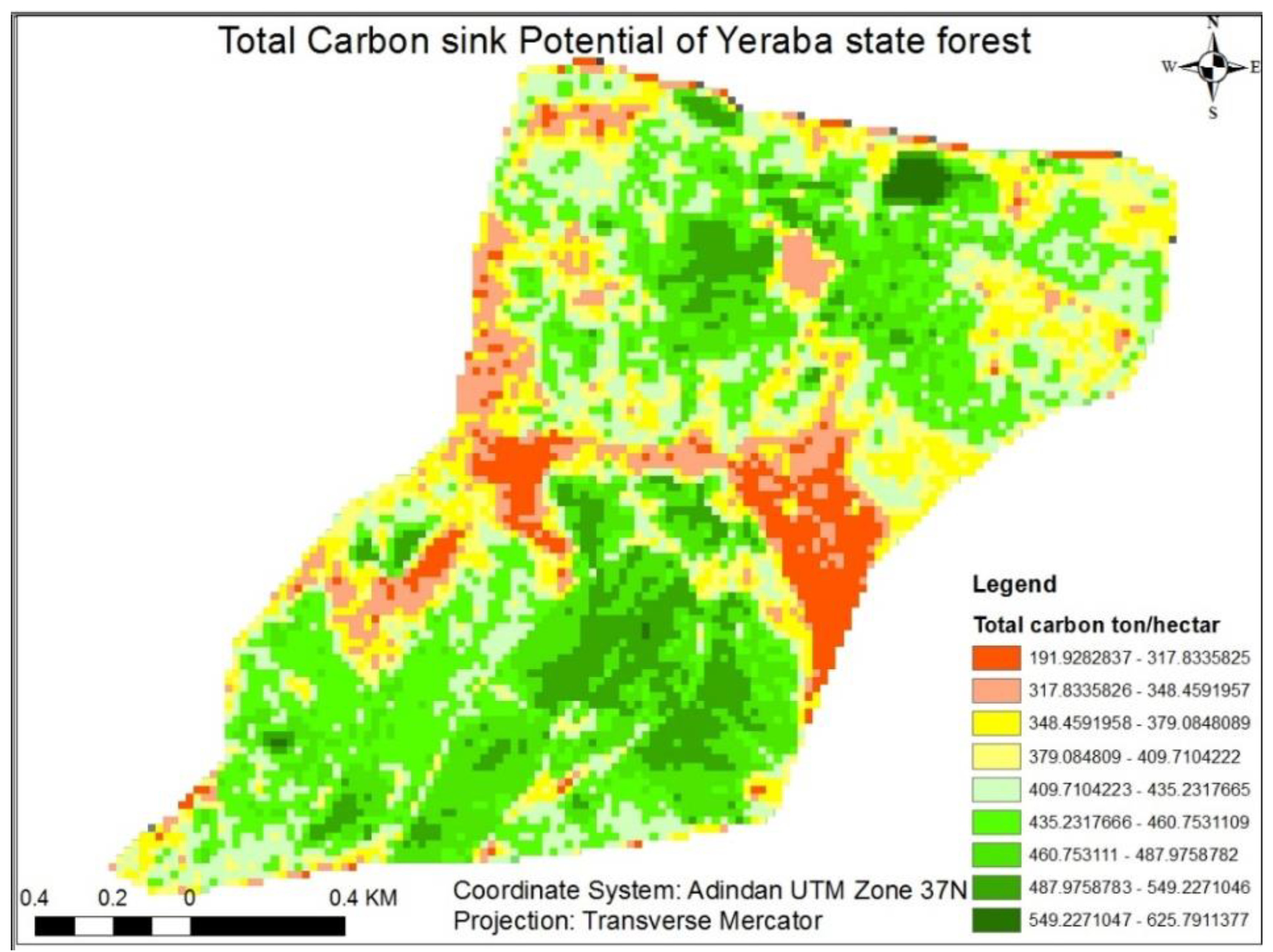
3.1.9. Influences of Environmental Factors on Carbon Stock
3.1.9.1. Variation of Carbon Stock along Altitudinal Gradient
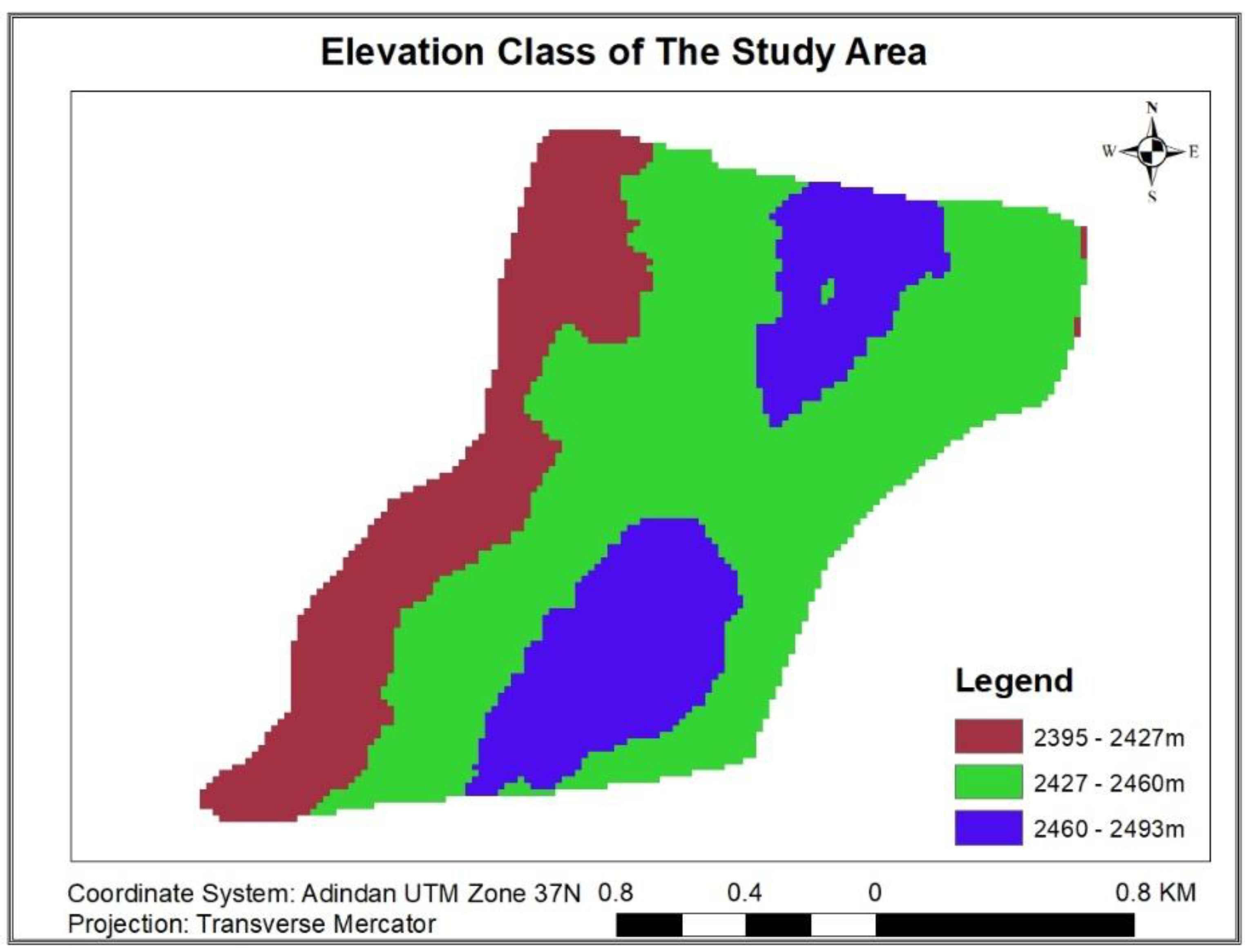
| Each Carbon pool statistical values with elevation class/ton | ||||||
| Elevation Class | MIN | MAX | RANGE | MEAN | STD | SUM |
| AGC | ||||||
| 2395 - 2427m | 6.71 | 34.64 | 27.93 | 21.88 | 3.75 | 38930.67 |
| 2427 - 2460m | 11.06 | 32.96 | 21.90 | 23.22 | 4.17 | 99346.66 |
| 2460 - 2493m | 13.72 | 39.73 | 26.00 | 26.65 | 3.95 | 44671.82 |
| BGC | ||||||
| 2395 - 2427m | 1.75 | 9.01 | 7.26 | 5.69 | 0.97 | 10121.97 |
| 2427 - 2460m | 2.88 | 8.57 | 5.69 | 6.04 | 1.09 | 25830.13 |
| 2460 - 2493m | 3.57 | 10.33 | 6.76 | 6.93 | 1.03 | 11614.67 |
| LC | ||||||
| 2395 - 2427m | 2.75 | 4.30 | 1.55 | 3.38 | 0.34 | 6018.19 |
| 2427 - 2460m | 2.43 | 4.52 | 2.09 | 3.38 | 0.45 | 14471.04 |
| 2460 - 2493m | 2.54 | 4.58 | 2.04 | 3.57 | 0.47 | 5985.56 |
| SOC | ||||||
| 2395 - 2427m | 95.23 | 151.58 | 56.35 | 119.27 | 11.26 | 212057.59 |
| 2427 - 2460m | 104.15 | 157.98 | 53.83 | 120.28 | 8.03 | 514557.53 |
| 2460 - 2493m | 99.21 | 153.78 | 54.57 | 121.38 | 10.66 | 203429.42 |
| TC | ||||||
| 2395 - 2427m | 213.78 | 574.40 | 360.62 | 398.42 | 50.10 | 708385.25 |
| 2427 - 2460m | 262.04 | 540.99 | 278.94 | 416.19 | 53.11 | 1780063.00 |
| 2460 - 2493m | 296.73 | 625.79 | 329.06 | 460.79 | 47.75 | 772279.93 |
3.1.9.2. Distribution of Carbon Stock along Slope Gradient
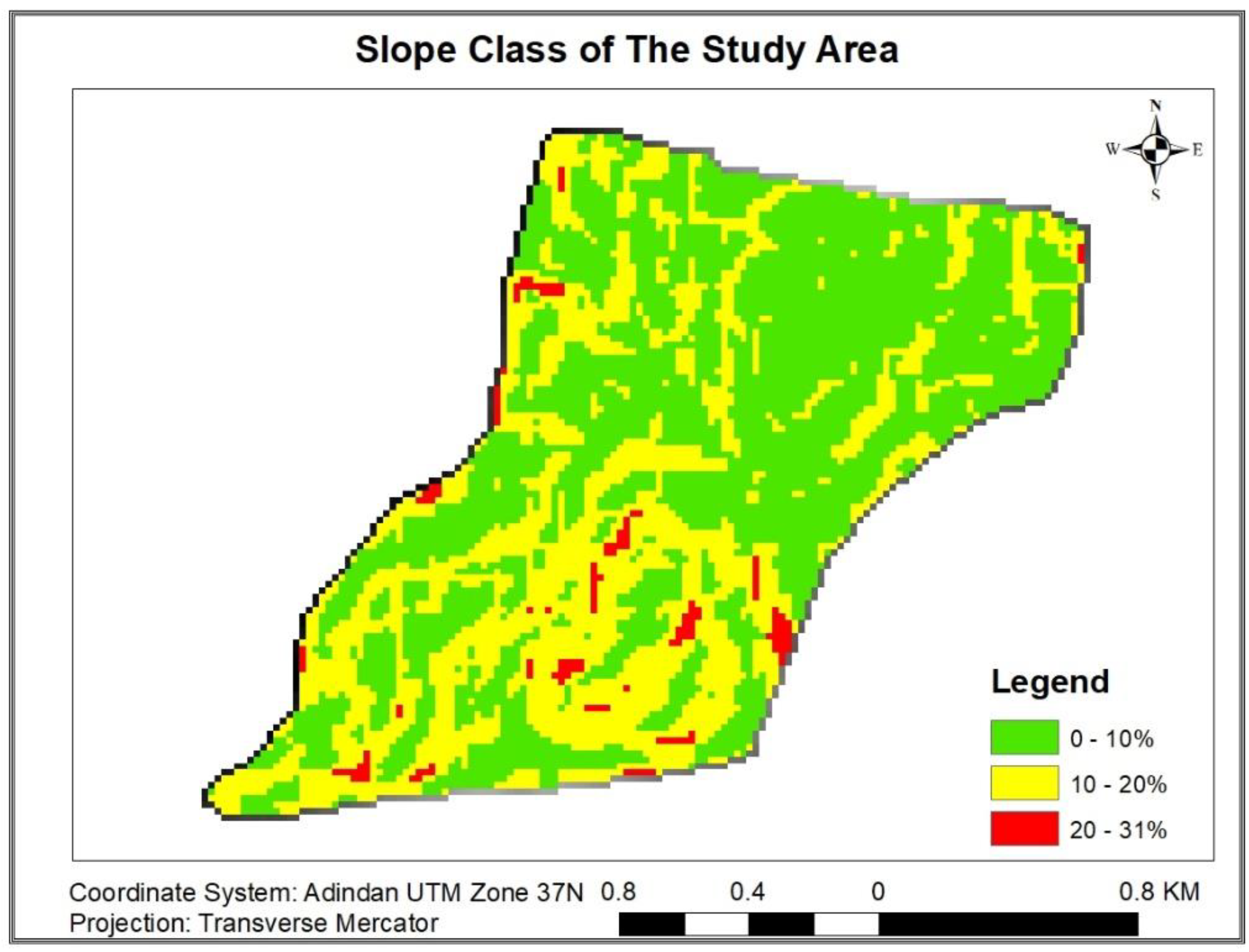
| Each Carbon pool statistical values along Slope gradient | |||||||
| Slope Class | COUNT | MIN | MAX | RANGE | MEAN | STD | SUM |
| AGC | |||||||
| 0 - 10% | 4323 | 12.23 | 39.73 | 27.50 | 23.61 | 4.44 | 102052.88 |
| 10 -20% | 2929 | 11.72 | 35.73 | 24.01 | 24.10 | 4.16 | 70591.83 |
| 20 -31% | 164 | 12.45 | 30.72 | 18.26 | 23.46 | 4.26 | 3847.12 |
| BGC | |||||||
| 0 - 10% | 4323 | 3.18 | 10.33 | 7.15 | 6.14 | 1.15 | 26533.75 |
| 10 -20% | 2929 | 3.05 | 9.29 | 6.24 | 6.27 | 1.08 | 18353.88 |
| 20 -31% | 164 | 3.24 | 7.99 | 4.75 | 6.10 | 1.11 | 1000.25 |
| LC | |||||||
| 0 - 10% | 4323 | 2.45 | 4.54 | 2.09 | 3.36 | 0.46 | 14535.31 |
| 10 -20% | 2929 | 2.43 | 4.58 | 2.15 | 3.51 | 0.41 | 10272.43 |
| 20 -31% | 164 | 2.85 | 4.56 | 1.71 | 3.68 | 0.39 | 603.47 |
| SOC | |||||||
| 0 - 10% | 4323 | 95.23 | 157.98 | 62.75 | 120.75 | 9.32 | 522010.54 |
| 10 -20% | 2929 | 96.48 | 156.41 | 59.92 | 119.87 | 9.61 | 351086.67 |
| 20 -31% | 164 | 97.95 | 151.78 | 53.83 | 120.10 | 11.95 | 19695.93 |
| TC | |||||||
| 0 - 10% | 4323 | 274.51 | 625.79 | 351.28 | 421.56 | 57.11 | 1822412.12 |
| 10 -20% | 2929 | 277.16 | 575.52 | 298.36 | 427.05 | 52.57 | 1250816.17 |
| 20 -31% | 164 | 279.69 | 505.76 | 226.07 | 419.35 | 55.25 | 68773.12 |
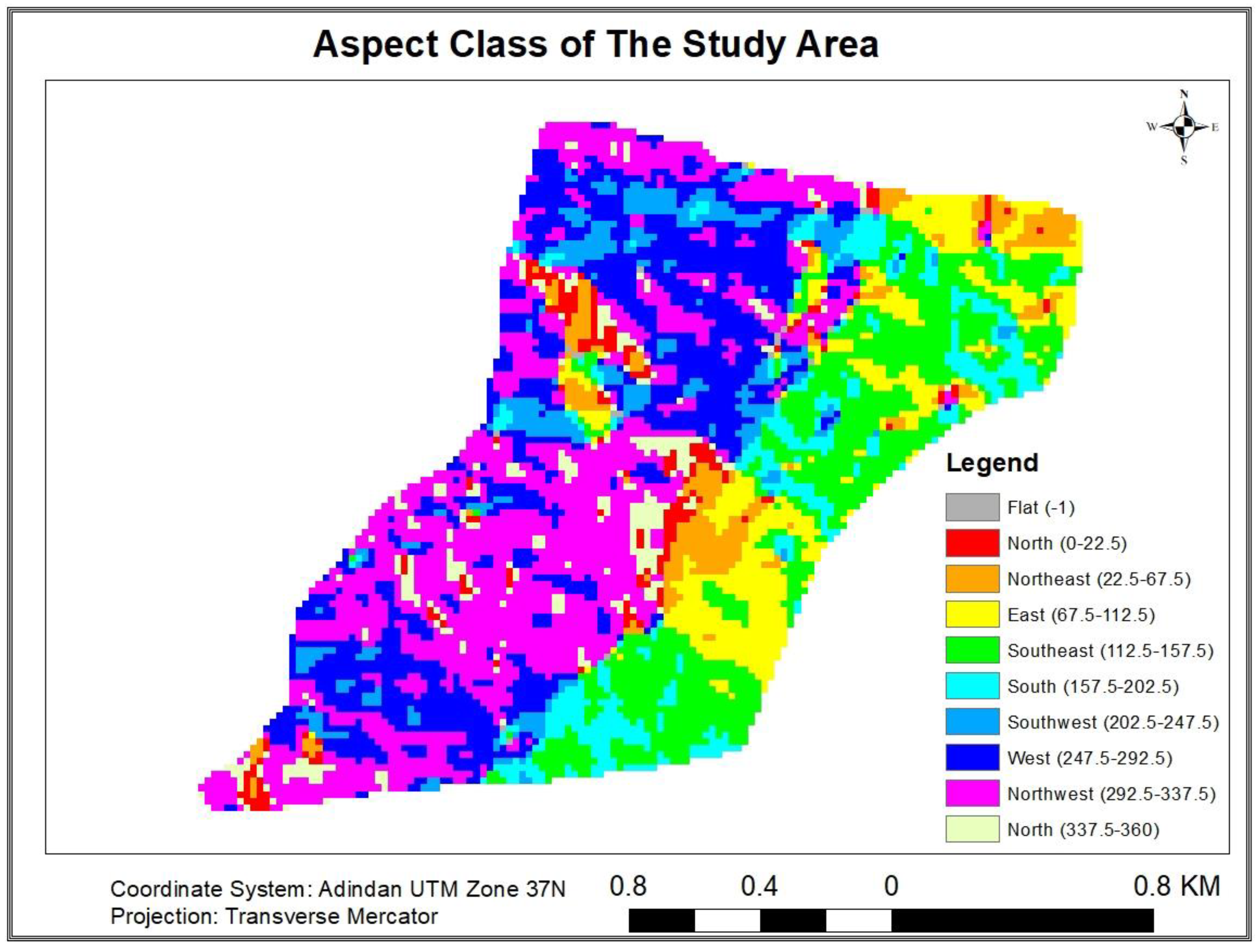
3.1.9.3. Distribution of Carbon Stock in the Aspects (Slope Facings)
3.2. Discussion
3.2.1. Woody Species Composition
3.2.2. DBH and Height Arrangement of Trees and their Contribution in the Studied Forest
3.2.3. Correlation of AGC with Derived Sentinel Vegetation Indices
3.2.4. Carbon Stock in Different Pools
4. Conculsions and Recommandetions
4.1. Conculsions
4.2. Recommendations
- ❖
- Estimating forest carbon stocks using optical images may be subject to the saturation problem because of the density of the forest. Furthermore, the canopy penetration capabilities of Sentinel2A and Landsat 5 images are inadequate. The effectiveness of carbon stock estimation for future studies may be improved by the use of LiDAR and Radar imageries that can tackle the saturation and canopy penetration problems.
- ❖
- Research findings, such as socioeconomic, ethnobotanical, and in-depth ecological studies in relation to various environmental aspects, such as soil type and characteristics, should aid in the development and management of the Forest. In contrast, basic and applied research into the soil seed bank, seed physiology, population dynamics, the biology and ecology of endangered species, as well as the forest as a whole, should be done to fill the gaps in this effort.
References
- Allan, J., Mitchell, T., Harborne, N., Bohm, L., & Crane-Robinson, C. (1986). Roles of H1 domains in determining higher order chromatin structure and H1 location. Journal of Molecular Biology, 187(4), 591–601. [CrossRef]
- Alves, L. F., Vieira, S. A., Scaranello, M. A., Camargo, P. B., Santos, F. A. M., Joly, C. A., & Martinelli, L. A. (2010). Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). Forest Ecology and Management, 260(5), 679–691. [CrossRef]
- Anderson-Teixeira, K. J., Herrmann, V., Morgan, R. B., Bond-Lamberty, B., Cook-Patton, S. C., Ferson, A. E., Muller-Landau, H. C., & Wang, M. M. H. (2021). Carbon cycling in mature and regrowth forests globally. Environmental Research Letters, 16(5). [CrossRef]
- Andrew, R. M. (2019, November 20). Global CO2 emissions from cement production, 1928-2018. Earth System Science Data; Copernicus GmbH. [CrossRef]
- Assefa, G., Mengistu, T., Getu, Z., & Zewdie, S. (2013). Training manual on: Forest carbon pools and carbon stock assessment in the context of SFM and REDD+. January, 74.
- Ayanu, Y. Z., Conrad, C., Nauss, T., Wegmann, M., & Koellner, T. (2012). Quantifying and mapping ecosystem services supplies and demands: a review of remote sensing applications. Environmental Science & Technology, 46(16), 8529–8541. [CrossRef]
- Azene, B. tesema. (2007). Useful trees and shrubs of Ethiopia: Identification, Propagation and Management for 17 Agroclimatic Zones. Relma, 559. https://books.google.com/books/about/Useful_Trees_and_Shrubs_of_Ethiopia.html?id=15UfAQAAIAAJ.
- Barstow, T. J. (2019). Understanding near infrared spectroscopy and its application to skeletal muscle research. Journal of Applied Physiology, 126(5), 1360–1376. [CrossRef]
- Berhanu, M., Jabasingh, S. A., & Kifile, Z. (2017). Expanding sustenance in Ethiopia based on renewable energy resources – A comprehensive review. Renewable and Sustainable Energy Reviews, 75, 1035–1045. [CrossRef]
- Bodo, T., Gbidum, B., Kemetonye, G., & Seomoni, J. (2021). Deforestation and Habitat Loss : Human Causes , Consequences and Possible Solutions. 04(02), 22–30.
- Brockerhoff, E. G., Barbaro, L., Castagneyrol, B., Forrester, D. I., Gardiner, B., González-Olabarria, J. R., Lyver, P. O. B., Meurisse, N., Oxbrough, A., Taki, H., Thompson, I. D., van der Plas, F., & Jactel, H. (2017). Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodiversity and Conservation, 26(13), 3005–3035. [CrossRef]
- Caviglia-Harris, J. L., Toomey, M., Harris, D. W., Mullan, K., Bell, A. R., Sills, E. O., & Roberts, D. A. (2015). Detecting and interpreting secondary forest on an old Amazonian frontier. Journal of Land Use Science, 10(4), 442–465. [CrossRef]
- Chaminé, H. I., Pereira, A. J. S. C., Teodoro, A. C., & Teixeira, J. (2021). Remote sensing and GIS applications in earth and environmental systems sciences. SN Applied Sciences, 3(12). [CrossRef]
- Chave, J., Réjou-Méchain, M., Búrquez, A., Chidumayo, E., Colgan, M. S., Delitti, W. B. C., Duque, A., Eid, T., Fearnside, P. M., Goodman, R. C., Henry, M., Martínez-Yrízar, A., Mugasha, W. A., Muller-Landau, H. C., Mencuccini, M., Nelson, B. W., Ngomanda, A., Nogueira, E. M., Ortiz-Malavassi, E., … Vieilledent, G. (2014). Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biology, 20(10), 3177–3190. [CrossRef]
- Chen, L., Ren, C., Zhang, B., Wang, Z., & Xi, Y. (2018). Estimation of forest above-ground biomass by geographically weighted regression and machine learning with sentinel imagery. Forests, 9(10). [CrossRef]
- Cui, X., Zhao, K., Zhou, Z., & Huang, P. (2021). Examining the uncertainty of carbon emission changes: A systematic approach based on peak simulation and resilience assessment. Environmental Impact Assessment Review, 91. [CrossRef]
- Das, B., Bordoloi, R., Deka, S., Paul, A., Pandey, P. K., Singha, L. B., Tripathi, O. P., Mishra, B. P., & Mishra, M. (2021). Above ground biomass carbon assessment using field, satellite data and model based integrated approach to predict the carbon sequestration potential of major land use sector of Arunachal Himalaya, India. Carbon Management, 12(2), 201–214. [CrossRef]
- Das, S., & Singh, T. P. (2012). Correlation analysis between biomass and spectral vegetation indices of forest ecosystem. International Journal of Engineering Research & Technology, 1(5). [CrossRef]
- Dayathilake, D. D. T. L., Lokupitiya, E., & Wijeratne, V. P. I. S. (2020). Estimation of aboveground and belowground carbon stocks in urban freshwater wetlands of Sri Lanka. Carbon Balance and Management, 15(1). [CrossRef]
- De Cáceres, M., Martín-Alcón, S., González-Olabarria, J. R., & Coll, L. (2019). A general method for the classification of forest stands using species composition and vertical and horizontal structure. Annals of Forest Science, 76(2). [CrossRef]
- Domke, G. M., Perry, C. H., Walters, B. F., Woodall, C. W., Russell, M. B., & Smith, J. E. (2016). Estimating litter carbon stocks on forest land in the United States. Science of the Total Environment, 557–558, 469–478. [CrossRef]
- Ekoungoulou, R., Liu, X., Loumeto, J. J., Ifo, S. A., Bocko, Y. E., Koula, F. E., & Niu, S. (2014). Tree Allometry in Tropical Forest of Congo for Carbon Stocks Estimation in Above-Ground Biomass. Open Journal of Forestry, 04(05), 481–491. [CrossRef]
- Fang, H., Ji, B., Deng, X., Ying, J., Zhou, G., Shi, Y., Xu, L., Tao, J., Zhou, Y., Li, C., & Zheng, H. (2018). Effects of topographic factors and aboveground vegetation carbon stocks on soil organic carbon in Moso bamboo forests. Plant and Soil, 433(1–2), 363–376. [CrossRef]
- Figueres, C., Schellnhuber, H. J., Whiteman, G., Rockström, J., Hobley, A., & Rahmstorf, S. (2017). Three years to safeguard our climate. Nature, 546(7660), 593–595. [CrossRef]
- Forests, T. S. of the W. (2020). The State of the World’s Forests 2020. The State of the World’s Forests 2020. [CrossRef]
- Gedefaw, M., Soromessa, T., & Belliethathan, S. (2014). Forest Carbon Stocks in Woody Plants of Tara Gedam Forest: Implication for Climate Change Mitigation. Science, Technology and Arts Research Journal, 3(1), 101. [CrossRef]
- Giardino, A. P., & Lyn, M. A. (2021). Utilization Management, Case Management, and Care Coordination. Medical Quality Management, 139–175. [CrossRef]
- Gibbs, H. K., Brown, S., Niles, J. O., & Foley, J. A. (2007). Monitoring and estimating tropical forest carbon stocks: Making REDD a reality. Environmental Research Letters, 2(4). [CrossRef]
- Gitelson, A. A., Kaufman, Y. J., & Merzlyak, M. N. (1996). Use of a green channel in remote sensing of global vegetation from EOS- MODIS. Remote Sensing of Environment, 58(3), 289–298. [CrossRef]
- Giweta, M. (2020). Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. Journal of Ecology and Environment, 44(1). [CrossRef]
- Gómez-Guerrero, A., & Doane, T. (2018). The Response of Forest Ecosystems to Climate Change. 35, 185–206. [CrossRef]
- Graves, R. A., Haugo, R. D., Holz, A., Nielsen-Pincus, M., Jones, A., Kellogg, B., Macdonald, C., Popper, K., & Schindel, M. (2020). Potential greenhouse gas reductions from Natural Climate Solutions in Oregon, USA. PLoS ONE, 15(4). [CrossRef]
- Grundel, I., Christenson, N., & Dahlström, M. (2022). Identifying interests and values in forest areas through collaborative processes and landscape resource analysis. Forest Policy and Economics, 142. [CrossRef]
- Gubena, A. F., & Teshome, S. (2017). Variations in Forest Carbon Stocks Along Environmental Gradients in Egdu Forest of Oromia Region , Variations in Forest Carbon Stocks Along Environmental Gradients in Egdu Forest of Oromia Region , Ethiopia : Im. 6(January), 1–8. [CrossRef]
- H/MICHAEL, T. (2015). CARBON STOCK OF METTI MOUNTAIN FOREST-WECHECHA, WEST OROMIA, ETHIOPIA TSEGAYE. 1–83.
- Hairiah, K., Sitompul, S., van Noordwijk, M., & Palm, C. (2001). Methods of sampling for sampling above and below-ground organic pools. IC-SEA Report No. 6: Modelling Global Change Impacts on the Soil Environment., 1–31.
- Hamilton, S. E., & Friess, D. A. (2018). Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nature Climate Change 2018 8:3, 8(3), 240–244. [CrossRef]
- Haslwanter, T. (2022). Linear Regression Models. 229–263. [CrossRef]
- Houghton, R. A. (2005). Aboveground Forest Biomass and the Global Carbon Balance. Global Change Biology, 11(6), 945–958. [CrossRef]
- Hunt, E. R., & Rock, B. N. (1989). Detection of changes in leaf water content using Near- and Middle-Infrared reflectances. Remote Sensing of Environment, 30(1), 43–54. [CrossRef]
- ICRAF Database - Wood Density. (n.d.). Retrieved June 15, 2023, from http://db.worldagroforestry.org//wd/genus/Acacia.
- IPCC. (2006). Good Practice Guidance for Land Use, Land-Use Change and Forestry. 590.
- Islam, M., Deb, G. P., & Rahman, M. (2017). Forest fragmentation reduced carbon storage in a moist tropical forest in Bangladesh: Implications for policy development. Land Use Policy, 65, 15–25. [CrossRef]
- Jandl, R., Lindner, M., Vesterdal, L., Bauwens, B., Baritz, R., Hagedorn, F., Johnson, D. W., Minkkinen, K., & Byrne, K. A. (2007). How strongly can forest management influence soil carbon sequestration?: Review. Geoderma, 137(3–4), 253–268. [CrossRef]
- Kebede, T. A. (2021). FACTORS AFFECTING LOCAL FARMERS ADOPTION OF EUCALYPTUS WOODLOT IN JAMMA DISTRICT, SOUTH WOLLO ZONE, AMHARA REGIONAL STATE OF ETHIOPIA. EPRA International Journal of Research & Development (IJRD), 251–256. [CrossRef]
- Kindler, E. (2016). A comparison of the concepts: Ecosystem services and forest functions to improve interdisciplinary exchange. Forest Policy and Economics, 67, 52–59. [CrossRef]
- Kothari. (2004). Research methdology method and technique. In NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS (Vol. 4, Issue 1). NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS.
- Loew, A., Bell, W., Brocca, L., Bulgin, C. E., Burdanowitz, J., Calbet, X., Donner, R. V., Ghent, D., Gruber, A., Kaminski, T., Kinzel, J., Klepp, C., Lambert, J. C., Schaepman-Strub, G., Schröder, M., & Verhoelst, T. (2017). Validation practices for satellite-based Earth observation data across communities. Reviews of Geophysics, 55(3), 779–817. [CrossRef]
- Ma, H., Mo, L., Crowther, T. W., Maynard, D. S., van den Hoogen, J., Stocker, B. D., Terrer, C., & Zohner, C. M. (2021). The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nature Ecology and Evolution, 5(8), 1110–1122. [CrossRef]
- Machado, R. R., Conceição, S. V., Leite, H. G., De Souza, A. L. D., & Wolff, E. (2015). Evaluation of forest growth and carbon stock in forestry projects by system dynamics. Journal of Cleaner Production, 96, 520–530. [CrossRef]
- Majasalmi, T., & Rautiainen, M. (2016). The potential of Sentinel-2 data for estimating biophysical variables in a boreal forest: A simulation study. Remote Sensing Letters, 7(5), 427–436. [CrossRef]
- Maleika, W. (2020). Inverse distance weighting method optimization in the process of digital terrain model creation based on data collected from a multibeam echosounder. Applied Geomatics, 12(4), 397–407. [CrossRef]
- Maleki, K., & Kiviste, A. (2015). Effect of sample plot size and shape on estimates of structural indices: A case study in mature silver birch (Betula pendula Roth) dominating stand in Järvselja. Forestry Studies, 63, 130–150. [CrossRef]
- Marigi, S. N. (2017). Climate Change Vulnerability and Impacts Analysis in Kenya. American Journal of Climate Change, 06(01), 52–74. [CrossRef]
- MarshetTefera, & TeshomeSoromessa. (2015). Carbon Stock Potentials of Woody Plant Species in Biheretsige and Central Closed Public Parks of Addis Ababa and Its Contribution to Climate Change Mitigation. Journal of Environment and Earth Science, 5(13), 1–15.
- McRoberts, R. E., Tomppo, E. O., Vibrans, A. C., & Freitas, J. V. de. (2013). Design considerations for tropical forest inventories. Pesquisa Florestal Brasileira, 33(74), 189–202. [CrossRef]
- Metz, B., Meyer, L., & Bosch, P. (2007). Climate change 2007 mitigation of climate change. Climate Change 2007 Mitigation of Climate Change, 9780521880114, 1–861. [CrossRef]
- Mitsch, W. J., Bernal, B., Nahlik, A. M., Mander, Ü., Zhang, L., Anderson, C. J., Jørgensen, S. E., & Brix, H. (2013). Wetlands, carbon, and climate change. Landscape Ecology, 28(4), 583–597. [CrossRef]
- Mondal, I., & Singh, K. H. (2022). Fluid substitution in NMR T2 distribution and resistivity independent saturation computation using synthetic capillary pressure data. Petroleum Research. [CrossRef]
- Mooser, A., Ulmer, S., Blaum, K., Franke, K., Kracke, H., Leiteritz, C., Quint, W., Rodegheri, C. C., Smorra, C., & Walz, J. (2014). Direct high-precision measurement of the magnetic moment of the proton. Nature 2014 509:7502, 509(7502), 596–599. [CrossRef]
- Mucheye, G., & Yemata, G. (2020). Species composition, structure and regeneration status of woody plant species in a dry Afromontane forest, Northwestern Ethiopia. Cogent Food and Agriculture, 6(1). [CrossRef]
- Muluken, N. B., Teshome, S., & Eyale, B. (2015). Carbon stock in Adaba-Dodola community forest of Danaba District, West-Arsi zone of Oromia Region, Ethiopia: An implication for climate change mitigation. Journal of Ecology and The Natural Environment, 7(1), 14–22. [CrossRef]
- Nigussie, W. (2016). Carbon Stock and Its Variations along Environmental Factors in Gendo Moist Montane Forest, East Wollega Zone, Western Ethiopia.
- Nijnik, M., & Bizikova, L. (2008). Responding to the Kyoto Protocol through forestry: A comparison of opportunities for several countries in Europe. Forest Policy and Economics, 10(4), 257–269. [CrossRef]
- Ontl, T. A., Janowiak, M. K., Swanston, C. W., Daley, J., Handler, S., Cornett, M., Hagenbuch, S., Handrick, C., McCarthy, L., & Patch, N. (2020). Forest Management for Carbon Sequestration and Climate Adaptation. Journal of Forestry, 118(1), 86–101. [CrossRef]
- Pan, Y., Birdsey, R. A., Phillips, O. L., & Jackson, R. B. (2013). The structure, distribution, and biomass of the world’s forests. Annual Review of Ecology, Evolution, and Systematics. 44(1): 593-622., 44(1), 593–622. [CrossRef]
- Pandey, P. C., & Sharma, L. K. (2021). Advances in remote sensing for natural resource monitoring. Advances in Remote Sensing for Natural Resource Monitoring, 1–480. [CrossRef]
- Pearson, T., Walker, S., & Brown, S. (2005). Sourcebook for Land use, Land-use change and forestry projects. Winrock International and the BioCarbon Fund of the World Bank 57 (2005), 21(3), 64. http://wbcarbonfinance.org/docs/Background_LULUCF_Sourcebook_compressed.pdf.
- Powers, J. S., Montgomery, R. A., Adair, E. C., Brearley, F. Q., Dewalt, S. J., Castanho, C. T., Chave, J., Deinert, E., Ganzhorn, J. U., Gilbert, M. E., González-Iturbe, J. A., Bunyavejchewin, S., Grau, H. R., Harms, K. E., Hiremath, A., Iriarte-Vivar, S., Manzane, E., De Oliveira, A. A., Poorter, L., … Lerdau, M. T. (2009). Decomposition in tropical forests: A pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. Journal of Ecology, 97(4), 801–811. [CrossRef]
- Proistosescu, C., & Wagner, G. (2020). Uncertainties in Climate and Weather Extremes Increase the Cost of Carbon. One Earth, 2(6), 515–517. [CrossRef]
- Pugh, T. A. M., Lindeskog, M., Smith, B., Poulter, B., Arneth, A., Haverd, V., & Calle, L. (2019). Role of forest regrowth in global carbon sink dynamics. Proceedings of the National Academy of Sciences of the United States of America, 116(10), 4382–4387. [CrossRef]
- Reddy, C. S. (2021). Remote sensing of biodiversity: what to measure and monitor from space to species? Biodiversity and Conservation, 30(10), 2617–2631. [CrossRef]
- Sahle, M. (2011). Estimating and Mapping of Carbon Stocks based on Remote Sensing, GIS and Ground Survey in the Menagesha Suba State Forest, Ethiopia. 128.
- Salas, E. A. L., Subburayalu, S. K., Slater, B., Dave, R., Parekh, P., Zhao, K., & Bhattacharya, B. (2021). Assessing the effectiveness of ground truth data to capture landscape variability from an agricultural region using Gaussian simulation and geostatistical techniques. Heliyon, 7(7). [CrossRef]
- Sha, Z., Bai, Y., Li, R., Lan, H., Zhang, X., Li, J., Liu, X., Chang, S., & Xie, Y. (2022). The global carbon sink potential of terrestrial vegetation can be increased substantially by optimal land management. Communications Earth & Environment 2022 3:1, 3(1), 1–10. [CrossRef]
- Sibanda, M., Mutanga, O., & Rouget, M. (2015). Examining the potential of Sentinel-2 MSI spectral resolution in quantifying above ground biomass across different fertilizer treatments. ISPRS Journal of Photogrammetry and Remote Sensing, 110, 55–65. [CrossRef]
- Smith, C. C., Healey, J. R., Berenguer, E., Young, P. J., Taylor, B., Elias, F., Espírito-Santo, F., & Barlow, J. (2021). Old-growth forest loss and secondary forest recovery across Amazonian countries. Environmental Research Letters, 16(8). [CrossRef]
- Soromessa, T., & Eshete, A. (2022). Carbon storage of selected church forests in northern Ethiopia: Implications for climate change mitigation. European Journal of Agriculture and Forestry Research, 10(1), 25–48. [CrossRef]
- Sousa, A. M. O., Gonçalves, A. C., Mesquita, P., & Marques da Silva, J. R. (2015). Biomass estimation with high resolution satellite images: A case study of Quercus rotundifolia. ISPRS Journal of Photogrammetry and Remote Sensing, 101, 69–79. [CrossRef]
- Srikrishnan, V., Guan, Y., Tol, R. S. J., & Keller, K. (2022). Probabilistic projections of baseline twenty-first century CO2 emissions using a simple calibrated integrated assessment model. Climatic Change, 170(3–4). [CrossRef]
- Stubenrauch, J., Ekardt, F., Hagemann, K., & Garske, B. (2022). Potential and Limits of Forest Ecosystems on Climate and Biodiversity Protection and Implications for the Legislative Process. 91–113. [CrossRef]
- Subedi, B., Rana, E., Nepal, A., & Bhattarai, S. (2010). Forest Carbon Stock Measurement: Guidelines for measuring carbon stocks in community-managed forests. https://www.academia.edu/1098241/Forest_Carbon_Stock_Measurement_Guidelines_for_measuring_carbon_stocks_in_community_managed_forests.
- Taye, F. A., Folkersen, M. V., Fleming, C. M., Buckwell, A., Mackey, B., Diwakar, K. C., Le, D., Hasan, S., & Ange, C. Saint. (2021). The economic values of global forest ecosystem services: A meta-analysis. Ecological Economics, 189. [CrossRef]
- Tomppo, E., Gschwantner, T., Lawrence, M., & McRoberts, R. E. (2010). National Forest Inventories : pathways for common reporting. National Forest Inventories: Pathways for Common Reporting, 1–612. [CrossRef]
- Tura, T. T., Argaw, M., & Eshetu, Z. (2013). Estimation of carbon stock in church forests: Implications for managing church forest to help with carbon emission reduction. Climate Change Management, 403–414. [CrossRef]
- Vanino, S., Nino, P., De Michele, C., Falanga Bolognesi, S., D’Urso, G., Di Bene, C., Pennelli, B., Vuolo, F., Farina, R., Pulighe, G., & Napoli, R. (2018). Capability of Sentinel-2 data for estimating maximum evapotranspiration and irrigation requirements for tomato crop in Central Italy. Remote Sensing of Environment, 215, 452–470. [CrossRef]
- Vasiljevic, N., & Gavrilovic, S. (2019). Cultural Ecosystem Services. 1–10. [CrossRef]
- Verrelst, J., Schaepman, M. E., Koetz, B., & Kneubühler, M. (2008). Angular sensitivity analysis of vegetation indices derived from CHRIS/PROBA data. Remote Sensing of Environment, 112(5), 2341–2353. [CrossRef]
- Waqar Ahmed Khan, R., Shaheen, H., & Awan, S. N. (2021). Biomass and soil carbon stocks in relation to the structure and composition of Chir Pine dominated forests in the lesser Himalayan foothills of Kashmir. Carbon Management, 12(4), 429–437. [CrossRef]
- Wolde, B. M., Kelbessa, E., & Soromessa, T. (2014). Forest Carbon Stocks in Woody Plants of Arba Minch Ground Water Forest and its Variations along Environmental Gradients. Science, Technology and Arts Research Journal, 3(2), 141. [CrossRef]
- Xue, J., & Su, B. (2017). Significant remote sensing vegetation indices: A review of developments and applications. Journal of Sensors, 2017. [CrossRef]
- Yitebitu, M. (2010). Yitebitu MOGES | National REDD+ Coordinator | National REDD+ Secretariat | Research profile. https://www.researchgate.net/profile/Yitebitu-Moges.
- Zhao, Y., Liu, Z., & Wu, J. (2020). Grassland ecosystem services: a systematic review of research advances and future directions. Landscape Ecology, 35(4), 793–814. [CrossRef]
- Zhu, L. P., Li, L., Li, R., & Zhu, L. X. (2011). Model-free feature screening for ultrahigh-dimensional data. Journal of the American Statistical Association, 106(496), 1464–1475. [CrossRef]
- Zhuo, Z., Chen, Q., Zhang, X., Chen, S., Gou, Y., Sun, Z., Huang, Y., & Shi, Z. (2022). Soil organic carbon storage, distribution, and influencing factors at different depths in the dryland farming regions of Northeast and North China. CATENA, 210, 105934. [CrossRef]
| Each Carbon pool statistical values along aspect variation | |||||||
| Aspect Class | COUNT | MIN | MAX | RANGE | MEAN | STD | SUM |
| AGC | |||||||
| North (0-22.5) | 941 | 13.09 | 39.30 | 26.21 | 24.22 | 4.01 | 22789.92 |
| Northeast (22.5-67.5) | 347 | 13.77 | 39.73 | 25.95 | 23.29 | 4.39 | 8081.37 |
| East (67.5-112.5) | 652 | 12.81 | 39.42 | 26.61 | 23.96 | 4.34 | 15621.87 |
| Southeast (112.5 - 157.5) | 1049 | 13.65 | 38.90 | 25.25 | 23.97 | 4.33 | 25148.79 |
| South (157.5-202.5) | 1106 | 12.86 | 38.55 | 25.69 | 24.00 | 4.25 | 26548.40 |
| Southwest (202.5-247.5) | 781 | 13.07 | 38.18 | 25.11 | 23.68 | 4.30 | 18492.21 |
| West (247.5-292.5) | 651 | 12.23 | 39.22 | 26.99 | 23.88 | 4.64 | 15543.93 |
| Northwest (292.5-337.5) | 1105 | 12.68 | 39.11 | 26.43 | 23.76 | 4.32 | 26253.56 |
| North (337.5-360) | 423 | 12.42 | 39.52 | 27.10 | 23.81 | 4.75 | 10073.71 |
| BGC | |||||||
| North (0-22.5) | 941 | 3.40 | 10.22 | 6.82 | 6.30 | 1.04 | 5925.38 |
| Northeast (22.5-67.5) | 347 | 3.58 | 10.33 | 6.75 | 6.06 | 1.14 | 2101.16 |
| East (67.5-112.5) | 652 | 3.33 | 10.25 | 6.92 | 6.23 | 1.13 | 4061.69 |
| Southeast (112.5-157.5) | 1049 | 3.55 | 10.12 | 6.57 | 6.23 | 1.13 | 6538.68 |
| South (157.5-202.5) | 1106 | 3.34 | 10.02 | 6.68 | 6.24 | 1.10 | 6902.58 |
| Southwest (202.5-247.5) | 781 | 3.40 | 9.93 | 6.53 | 6.16 | 1.12 | 4807.97 |
| West (247.5-292.5) | 651 | 3.18 | 10.20 | 7.02 | 6.21 | 1.21 | 4041.42 |
| Northwest (292.5-337.5) | 1105 | 3.30 | 10.17 | 6.87 | 6.18 | 1.12 | 6825.92 |
| North (337.5-360) | 423 | 3.23 | 10.28 | 7.05 | 6.19 | 1.23 | 2619.16 |
| LC | |||||||
| North (0-22.5) | 941 | 2.47 | 4.58 | 2.11 | 3.39 | 0.47 | 3187.11 |
| Northeast (22.5-67.5) | 347 | 2.48 | 4.53 | 2.04 | 3.39 | 0.44 | 1177.74 |
| East (67.5-112.5) | 652 | 2.53 | 4.47 | 1.93 | 3.42 | 0.45 | 2232.40 |
| Southeast (112.5-157.5) | 1049 | 2.45 | 4.47 | 2.03 | 3.39 | 0.44 | 3560.57 |
| South (157.5-202.5) | 1106 | 2.45 | 4.51 | 2.06 | 3.44 | 0.43 | 3804.26 |
| Southwest (202.5-247.5) | 781 | 2.43 | 4.54 | 2.11 | 3.43 | 0.46 | 2680.44 |
| West (247.5-292.5) | 651 | 2.44 | 4.57 | 2.13 | 3.42 | 0.46 | 2228.05 |
| Northwest (292.5-337.5) | 1105 | 2.45 | 4.57 | 2.13 | 3.49 | 0.44 | 3861.01 |
| North (337.5-360) | 423 | 2.47 | 4.55 | 2.08 | 3.45 | 0.45 | 1459.89 |
| SC | |||||||
| North (0-22.5) | 941 | 95.65 | 157.60 | 61.95 | 120.78 | 10.00 | 113650.46 |
| Northeast (22.5-67.5) | 347 | 99.27 | 154.69 | 55.42 | 119.80 | 9.49 | 41571.57 |
| East (67.5-112.5) | 652 | 99.98 | 156.23 | 56.24 | 120.06 | 8.28 | 78281.59 |
| Southeast (112.5-157.5) | 1049 | 97.76 | 157.23 | 59.47 | 121.39 | 9.64 | 127342.39 |
| South (157.5-202.5) | 1106 | 97.68 | 157.55 | 59.86 | 120.96 | 9.90 | 133785.82 |
| Southwest (202.5-247.5) | 781 | 95.25 | 157.98 | 62.72 | 119.45 | 8.94 | 93288.99 |
| West (247.5-292.5) | 651 | 96.00 | 157.13 | 61.12 | 119.68 | 8.72 | 77913.60 |
| Northwest (292.5-337.5) | 1105 | 95.27 | 157.63 | 62.36 | 121.00 | 10.10 | 133699.80 |
| North (337.5-360) | 423 | 100.16 | 153.04 | 52.88 | 119.51 | 8.84 | 50552.12 |
| TC | |||||||
| North (0-22.5) | 941 | 287.72 | 620.17 | 332.45 | 429.32 | 51.38 | 403990.59 |
| Northeast (22.5-67.5) | 347 | 296.00 | 625.79 | 329.79 | 416.64 | 55.46 | 144574.59 |
| East (67.5-112.5) | 652 | 284.38 | 621.83 | 337.45 | 425.38 | 55.47 | 277349.56 |
| Southeast (112.5-157.5) | 1049 | 293.28 | 614.81 | 321.54 | 426.86 | 55.65 | 447777.69 |
| South (157.5-202.5) | 1106 | 283.16 | 609.98 | 326.82 | 426.85 | 54.35 | 472099.97 |
| Southwest (202.5-247.5) | 781 | 284.67 | 605.63 | 320.96 | 421.22 | 54.91 | 328971.22 |
| West (247.5-292.5) | 651 | 274.51 | 619.17 | 344.67 | 423.96 | 59.10 | 275995.20 |
| Northwest (292.5-337.5) | 1105 | 278.94 | 618.39 | 339.45 | 423.85 | 55.47 | 468355.60 |
| North (337.5-360) | 423 | 279.66 | 623.70 | 344.03 | 423.03 | 60.25 | 178940.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





