Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Focused Question
2.2. Information Sources and Search Strategy
2.3. Study Selection
- in vitro studies involving C. albicans or other non-albicans Candida stains,
- animal studies involving C. albicans or other non-albicans Candida stains,
- RCTs involving patients with oral candidiasis or denture stomatitis,
- Candida elimination method used in vitro studies, in animal studies, and RCT was curcumin-mediated aPDT.
- case reports or case series,
- letters to the editor,
- historic reviews,
- reviews or systematic reviews,
- books and documents,
- duplicated publications or studies with the same ethical approval number,
- studies published in a non–English language,
- general medical applications,
- aPDT form not used as therapy,
- curcumin used not as a photosensitizer,
- other PS than curcumin was used,
- blue light used without PS,
- no Candida strains evaluated,
- endodontic, carious, or bone models, not related to oral candidiasis.
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment and Risk of Bias across Studies
- Was there a specific concentration of photosensitizer?
- Was the origin of the photosensitizer provided?
- Was an incubation time indicated?
- Were the light source parameters provided, such as type, wavelength, output power, fluence, and power density?
- Were clinical strains of Candida spp. used in the study?
- Was a negative control group included?
- Are numerical results (statistics)?
- No missing outcome data?
- Did the study include at least 10 patients per group?
- Was there a minimum 6-month follow-up period?
2.7. Data Extraction
- citation (first author and publication year),
- type of study,
- type of Candida strains used in the study,
- test/control groups,
- follow-up,
- outcomes,
- type and parameters of the light source,
- curcumin concentration,
- use of nanocarriers and additional substances, incubation, and irradiation time.
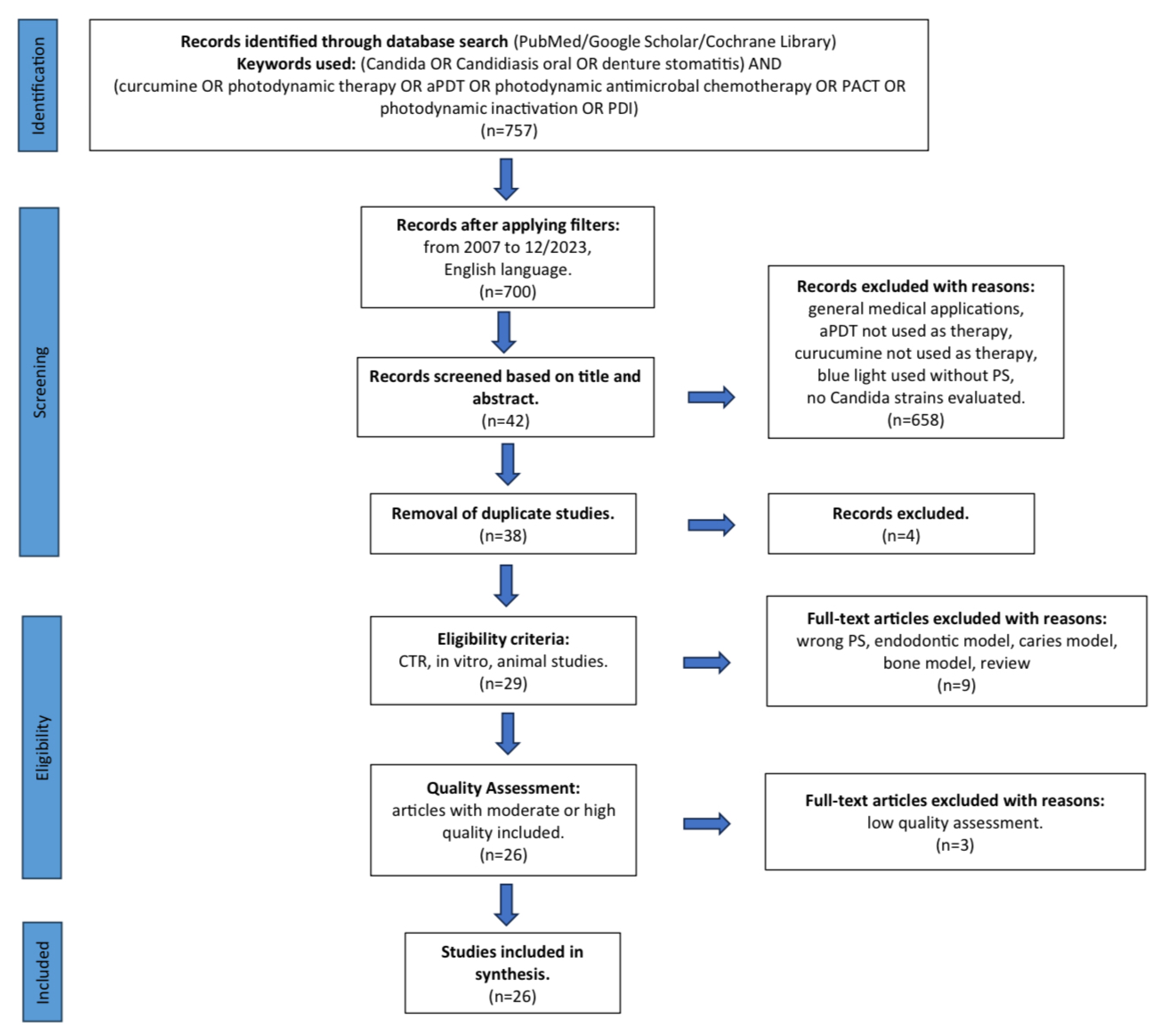
3. Results
3.1. Primary Outcome
3.2. Study Selection during Full-Text Analysis
3.3. Quality Assessment Presentation
3.4. Data Presentation
3.5. General Characteristics of the Included Studies
3.6. Characteristics of Light Sources Used in aPDT
3.7. Characterization of Curcumin Used as a Photosensitizer in aPDT.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence. 2022 Dec;13(1):89-121. [CrossRef] [PubMed] [PubMed Central]
- Mukaremera, L.; Lee, K.K.; Mora-Montes, H.M.; Gow, N.A.R. Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition. Front Immunol. 2017 Jun 7;8:629. [CrossRef] [PubMed] [PubMed Central]
- Akpan, A.; Morgan, R. Oral candidiasis, Postgraduate Medical Journal, Volume 78, Issue 922, August 2002, Pages 455–459. [CrossRef]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin Dermatol. 2016 Jul-Aug;34(4):487-94. Epub 2016 Mar 2. [CrossRef] [PubMed]
- Jordão, C.C.; Viana de Sousa, T.; Inêz Klein, M.; Mendonça Dias, L.; Pavarina, A.C.; Carmello, J.C. Antimicrobial photodynamic therapy reduces gene expression of Candida albicans in biofilms. Photodiagnosis Photodyn Ther. 2020 Sep;31:101825. Epub 2020 May 21. [CrossRef] [PubMed]
- Daliri, F.; Azizi, A.; Goudarzi, M.; Lawaf, S.; Rahimi, A. In vitro comparison of the effect of photodynamic therapy with curcumin and methylene blue on Candida albicans colonies. Photodiagnosis Photodyn Ther. 2019 Jun;26:193-198. Epub 2019 Mar 23. [CrossRef] [PubMed]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G.O. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules. 2018 Aug 19;23(8):2075. [CrossRef] [PubMed] [PubMed Central]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect Dis Clin North Am. 2021 Jun;35(2):389-413. [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; Berkow, E.L.; Castanheira, M.; Magobo, R.E.; Jabeen, K.; Asghar, R.J.; Meis, J.F.; Jackson, B.; Chiller, T.; Litvintseva, A.P. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis. 2017 Jan 15;64(2):134-140. Epub 2016 Oct 20. Erratum in: Clin Infect Dis. 2018 Aug 31;67(6):987. 10.1093/cid/ciy333. [CrossRef] [PubMed] [PubMed Central]
- Arzmi, M.H.; Dashper, S.; McCullough, M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J Oral Pathol Med. 2019 Aug;48(7):546-551. Epub 2019 Jun 25. [CrossRef] [PubMed]
- Carmello, J.C.; Pavarina, A.C.; Oliveira, R.; Johansson, B. Genotoxic effect of photodynamic therapy mediated by curcumin on Candida albicans. FEMS Yeast Res. 2015 Jun;15(4):fov018. Epub 2015 Apr 20. [CrossRef] [PubMed]
- Finlay, P.M.; Richardson, M.D.; Robertson, A.G. A comparative study of the efficacy of fluconazole and amphotericin B in the treatment of oropharyngeal candidosis in patients undergoing radiotherapy for head and neck tumours. Br J Oral Maxillofac Surg. 1996 Feb;34(1):23-5. [CrossRef] [PubMed]
- Kanpittaya, K.; Teerakapong, A.; Morales, N.P.; Hormdee, D.; Priprem, A.; Weera-Archakul, W.; Damrongrungruang, T. Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans. Molecules. 2021 Apr 21;26(9):2405. [CrossRef] [PubMed] [PubMed Central]
- Labban, N.; Taweel, S.M.A.; ALRabiah, M.A.; Alfouzan, A.F.; Alshiddi, I.F.; Assery, M.K. Efficacy of Rose Bengal and Curcumin mediated photodynamic therapy for the treatment of denture stomatitis in patients with habitual cigarette smoking: A randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2021 Sep;35:102380. Epub 2021 Jun 1. [CrossRef] [PubMed]
- Andrade, M.C.; Ribeiro, A.P.; Dovigo, L.N.; Brunetti, I.L.; Giampaolo, E.T.; Bagnato, V.S.; Pavarina, A.C. Effect of different pre-irradiation times on curcumin-mediated photodynamic therapy against planktonic cultures and biofilms of Candida spp. Arch Oral Biol. 2013 Feb;58(2):200-10. Epub 2012 Nov 13. [CrossRef] [PubMed]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr Drug Deliv. 2023;20(5):441-456. [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol Res. 2008;163(1):1-12. Epub 2007 Nov 26. [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontol 2000. 2011 Feb;55(1):143-66. [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Tradit Complement Med. 2016 Jun 15;7(2):205-233. [CrossRef] [PubMed] [PubMed Central]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014 Oct 22;116(1):1-7. Epub 2014 Sep 6. [CrossRef] [PubMed]
- Tonnesen, H.H.; Arrieta, A.F.; Lerner, D. STUDIES ON CURCUMIN AND CURCUMINOIDS. 24. CHARACTERIZATION OF THE SPECTROSCOPIC PROPERTIES OF THE NATURALLY-OCCURRING CURCUMINOIDS AND SELECTED DERIVATIVES. Pharmazie 1995, 50, 689–693. [Google Scholar]
- Hussain, Z.; Thu, H.E.; Ng, S.F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf B Biointerfaces. 2017 Feb 1;150:223-241. Epub 2016 Nov 30. [CrossRef] [PubMed]
- Schamberger, B.; Plaetzer, K. Photofungizides Based on Curcumin and Derivates Thereof against Candida albicans and Aspergillus niger. Antibiotics (Basel). 2021 Oct 28;10(11):1315. [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; Glanville, J.; Grimshaw, J.M.; Hróbjartsson, A.; Lalu, M.M.; Li, T.; Loder, E.W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L.A.; Stewart, L.A.; Thomas, J.; Tricco, A.C.; Welch, V.A.; Whiting, P.; Moher, D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. [CrossRef] [PubMed] [PubMed Central]
- Watson, P.F.; Petrie, A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010 Jun;73(9):1167-79. [CrossRef] [PubMed]
- Higgins, J.; Savović, J.; Page, M.; Elbers, R.; Sterne, J. Assessing risk of bias in a randomized trial. In: Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; Chichester, UK: 2019. pp. 205–228.
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-mediated Photodynamic Therapy for the treatment of oral infections-A review. Photodiagnosis Photodyn Ther. 2018 Mar;21:409-415. Epub 2018 Feb 22. [CrossRef] [PubMed]
- Ravazzi, T.P.Q.; de Jesus, I.M.; de Oliveira Santos, G.P.; Reis, T.A.; Rosa, L.P.; Rosa, F.C.S. The effects of antimicrobial photodynamic therapy (aPDT) with nanotechnology-applied curcumin and 450nm blue led irradiation on multi-species biofilms in root canals. Lasers Med Sci. 2023 Nov 6;38(1):254. [CrossRef] [PubMed]
- Pellissari, C.V.; Pavarina, A.C.; Bagnato, V.S.; Mima, E.G.; Vergani, C.E.; Jorge, J.H. Cytotoxicity of antimicrobial photodynamic inactivation on epithelial cells when co-cultured with Candida albicans. Photochem Photobiol Sci. 2016 May 11;15(5):682-90. Epub 2016 Apr 25. [CrossRef] [PubMed]
- Yasini, Z.; Roghanizad, N.; Fazlyab, M.; Pourhajibagher, M. Ex vivo efficacy of sonodynamic antimicrobial chemotherapy for inhibition of Enterococcus faecalis and Candida albicans biofilm. Photodiagnosis Photodyn Ther. 2022 Dec;40:103113. Epub 2022 Sep 10. [CrossRef] [PubMed]
- Dantas Lopes Dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.S. Curcumin-loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem Photobiol. 2021 Sep;97(5):1072-1088. Epub 2021 May 17. [CrossRef] [PubMed]
- da Silva, F.C.; Fernandes Rodrigues, P.L.; Santos Dantas Araújo, T.; Sousa Santos, M.; de Oliveira, J.M.; Pereira Rosa, L.; de Oliveira Santos, G.P.; de Araújo, B.P.; Bagnato, V.S. Fluorescence spectroscopy of Candida albicans biofilms in bone cavities treated with photodynamic therapy using blue LED (450 nm) and curcumin. Photodiagnosis Photodyn Ther. 2019 Jun;26:366-370. Epub 2019 May 4. [CrossRef] [PubMed]
- Reina, B.D.; Santezi, C.; Malheiros, S.S.; Calixto, G.; Rodero, C.; Victorelli, F.D.; Chorilli, M.; Dovigo, L.N. Liquid crystal precursor system as a vehicle for curcumin-mediated photodynamic inactivation of oral biofilms. J Biophotonics. 2023 Feb;16(2):e202200040. Epub 2022 Oct 13. [CrossRef] [PubMed]
- Soria-Lozano, P.; Gilaberte, Y.; Paz-Cristobal, M.P.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Aporta, J.; Pérez-Laguna, V.; García-Luque, I.; Revillo, M.J.; Rezusta, A. In vitro effect photodynamic therapy with differents photosensitizers on cariogenic microorganisms. BMC Microbiol. 2015 Sep 26;15:187. [CrossRef] [PubMed] [PubMed Central]
- Comeau, P.; Manso, A. A Systematic Evaluation of Curcumin Concentrations and Blue Light Parameters towards Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pharmaceutics. 2023 Nov 30;15(12):2707. [CrossRef] [PubMed] [PubMed Central]
- Sanitá, P.V.; Pavarina, A.C.; Dovigo, L.N.; Ribeiro, A.P.D.; Andrade, M.C.; Mima, E.G.O. Curcumin-mediated anti-microbial photodynamic therapy against Candida dubliniensis biofilms. Lasers Med Sci. 2018 May;33(4):709-717. Epub 2017 Nov 13. [CrossRef] [PubMed]
- Fonseca, L.L.; Durães, C.P.; Menezes, A.S.D.S.; Tabosa, A.T.L.; Barbosa, C.U.; Filho, A.P.S.; Souza, D.P.S.P.; Guimarães, V.H.D.; Santos, S.H.S.; de Paula, A.M.B.; Farias, L.C.; Guimarães, A.L.S. Comparison between two antimicrobial photodynamic therapy protocols for oral candidiasis in patients undergoing treatment for head and neck cancer: A two-arm, single-blind clinical trial. Photodiagnosis Photodyn Ther. 2022 Sep;39:102983. Epub 2022 Jun 27. [CrossRef] [PubMed]
- Mima, E.G.O.; Pavarina, A.C.; Jordão, C.C.; Vieira, S.M.; Dovigo, L.N. Curcuminoid-Mediated Antimicrobial Photodynamic Therapy on a Murine Model of Oral Candidiasis. J Vis Exp. 2023 Oct 27;(200). [CrossRef] [PubMed]
- Rocha, M.P.; Ruela, A.L.M.; Rosa, L.P.; Santos, G.P.O.; Rosa, F.C.S. Antimicrobial photodynamic therapy in dentistry using an oil-in-water microemulsion with curcumin as a mouthwash. Photodiagnosis Photodyn Ther. 2020 Dec;32:101962. Epub 2020 Aug 17. [CrossRef] [PubMed]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med Mycol. 2013 Apr;51(3):243-51. Epub 2012 Aug 31. [CrossRef] [PubMed]
- Trigo Gutierrez, J.K.; Zanatta, G.C.; Ortega, A.L.M.; Balastegui, M.I.C.; Sanitá, P.V.; Pavarina, A.C.; Barbugli, P.A.; Mima, E.G.O. Encapsulation of curcumin in polymeric nanoparticles for antimicrobial Photodynamic Therapy. PLoS One. 2017 Nov 6;12(11):e0187418. [CrossRef] [PubMed] [PubMed Central]
- Santezi, C.; Reina, B.D.; de Annunzio, S.R.; Calixto, G.; Chorilli, M.; Dovigo, L.N. Photodynamic potential of curcumin in bioadhesive formulations: Optical characteristics and antimicrobial effect against biofilms. Photodiagnosis Photodyn Ther. 2021 Sep;35:102416. Epub 2021 Jun 29. [CrossRef] [PubMed]
- Marques Meccatti, V.; de Souza Moura, L.; Guerra Pinto, J.; Ferreira-Strixino, J.; Abu Hasna, A.; Alves Figueiredo-Godoi, L.M.; Campos Junqueira, J.; Marcucci, M.C.; de Paula Ramos, L.; Carvalho, C.A.T.; Pucci, C.R.; de Oliveira, L.D. Curcuma longa L. Extract and Photodynamic Therapy are Effective against Candida spp. and Do Not Show Toxicity In Vivo. Int J Dent. 2022 Jul 1;2022:5837864. [CrossRef] [PubMed] [PubMed Central]
- Al-Ghamdi, A.R.S.; Khanam, H.K.; Qamar, Z.; Abdul, N.S.; Reddy, N.; Vempalli, S.; Noushad, M.; Alqahtani, W.M.S. Therapeutic efficacy of adjunctive photodynamic therapy in the treatment of denture stomatitis. Photodiagnosis Photodyn Ther. 2023 Jun;42:103326. Epub 2023 Feb 10. [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.; Brunetti, I.L.; Costa, C.A.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem Photobiol. 2011 Jul-Aug;87(4):895-903. Epub 2011 Jun 13. [CrossRef] [PubMed]
- Dos Santos, D.D.L.; Besegato, J.F.; de Melo, P.B.G.; Junior, J.A.O.; Chorilli, M.; Deng, D.; Bagnato, V.S.; de Souza Rastelli, A.N. Effect of curcumin-encapsulated Pluronic® F-127 over duo-species biofilm of Streptococcus mutans and Candida albicans. Lasers Med Sci. 2022 Apr;37(3):1775-1786. Epub 2021 Oct 19. [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg Med. 2011 Nov;43(9):927-34. [CrossRef] [PubMed]
- Dias, L.M.; Klein, M.I.; Ferrisse, T.M.; Medeiros, K.S.; Jordão, C.C.; Bellini, A.; Pavarina, A.C. The Effect of Sub-Lethal Successive Applications of Photodynamic Therapy on Candida albicans Biofilm Depends on the Photosensitizer. J Fungi (Basel). 2023 Jan 13;9(1):111. [CrossRef] [PubMed] [PubMed Central]
- Trigo-Gutierrez, J.K.; Calori, I.R.; de Oliveira Bárbara, G.; Pavarina, A.C.; Gonçalves, R.S.; Caetano, W.; Tedesco, A.C.; Mima, E.G.O. Photo-responsive polymeric micelles for the light-triggered release of curcumin targeting antimicrobial activity. Front Microbiol. 2023 Apr 20;14:1132781. [CrossRef] [PubMed] [PubMed Central]
- Quishida, C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med Sci. 2016 Jul;31(5):997-1009. Epub 2016 Apr 28. [CrossRef] [PubMed]
- Damrongrungruang, T.; Panutyothin, N.; Kongjun, S.; Thanabat, K.; Ratha, J. Combined bisdemethoxycurcumin and potassium iodide-mediated antimicrobial photodynamic therapy. Heliyon. 2023 Jun 27;9(7):e17490. [CrossRef] [PubMed] [PubMed Central]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J Photochem Photobiol B. 2017 Aug;173:301-306. Epub 2017 Jun 8. [CrossRef] [PubMed]
- Hsieh, Y.H.; Zhang, J.H.; Chuang, W.C.; Yu, K.H.; Huang, X.B.; Lee, Y.C.; Lee, C.I. An in Vitro Study on the Effect of Combined Treatment with Photodynamic and Chemical Therapies on Candida albicans. Int J Mol Sci. 2018 Jan 24;19(2):337. [CrossRef] [PubMed] [PubMed Central]
- Merigo, E.; Conti, S.; Ciociola, T.; Fornaini, C.; Polonelli, L.; Lagori, G.; Manfredi, M.; Vescovi, P. Effect of different wavelengths and dyes on Candida albicans: In vivo study using Galleria mellonella as an experimental model. Photodiagnosis Photodyn Ther. 2017 Jun;18:34-38. Epub 2017 Jan 24. [CrossRef] [PubMed]
- Casu, C.; Orrù, G.; Scano, A. Curcumin/H2O2 photodynamically activated: an antimicrobial time-response assessment against an MDR strain of Candida albicans. Eur Rev Med Pharmacol Sci. 2022 Dec;26(23):8841-8851. [CrossRef] [PubMed]
- Leferman, C.E.; Stoica, L.; Tiglis, M.; Stoica, B.A.; Hancianu, M.; Ciubotaru, A.D.; Salaru, D.L.; Badescu, A.C.; Bogdanici, C.M.; Ciureanu, I.A.; Ghiciuc, C.M. Overcoming Drug Resistance in a Clinical C. albicans Strain Using Photoactivated Curcumin as an Adjuvant. Antibiotics (Basel). 2023 Jul 25;12(8):1230. [CrossRef] [PubMed] [PubMed Central]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Imamura, T.; Tatehara, S.; Ide, S.; Shirakawa, J.; Wakabayashi, N.; Satomura, K. Inhibitory effect of 405-nm blue LED light on the growth of Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin. Lasers Med Sci. 2022 Jun;37(4):2311-2319. Epub 2022 Jan 16. [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence. 2016 Jul 3;7(5):536-45. Epub 2016 Feb 24. [CrossRef] [PubMed] [PubMed Central]
- Lin, C.H.; Chien, H.F.; Lin, M.H.; Chen, C.P.; Shen, M.; Chen, C.T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int J Mol Sci. 2018 Sep 1;19(9):2598. [CrossRef] [PubMed] [PubMed Central]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagnosis Photodyn Ther. 2018 Mar;21:182-189. Epub 2017 Dec 6. [CrossRef] [PubMed]
- Idrees, M.; Kujan, O. Curcumin is effective in managing oral inflammation: An in vitro study. J Oral Pathol Med. 2024 May 21. Epub ahead of print. [CrossRef] [PubMed]
- Lee, Y.S.; Chen, X.; Widiyanto, T.W.; Orihara, K.; Shibata, H.; Kajiwara, S. Curcumin affects function of Hsp90 and drug efflux pump of Candida albicans. Front Cell Infect Microbiol. 2022 Sep 27;12:944611. [CrossRef] [PubMed] [PubMed Central]
| Ordinal Number | Reason for Exclusion | Reference Number |
|---|---|---|
| 1 | Review | [27] |
| 2 | Endodontic model | [28] |
| 3 | Cytotoxicity testing PDT | [29] |
| 4 | Endodontic model | [30] |
| 5 | Caries model | [31] |
| 6 | Bone defect model | [32] |
| 7 | Caries model | [33] |
| 8 | Caries model | [34] |
| 9 | Caries model | [35] |
| Reference number | PS concentration | Origin of PS | Incubation time | Light source parameters | Clinical strains of Candida spp. | Negative control group | Numerical results available (statistics) | No missing outcome data | 10 patients per group | 6-month follow-up period | Total score 8/2 (RCT) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [5] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [6] | yes | yes | no | yes | no | yes | yes | no | - | - | 5/- |
| [7] | yes | no | yes | yes | no | yes | yes | yes | - | - | 6/- |
| [11] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [13] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [14] | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | 8/1 |
| [15] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [23] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [36] | yes | yes | yes | yes | yes | yes | yes | yes | - | - | 8/- |
| [37] | yes | yes | yes | yes | yes | no | yes | yes | yes | no | 7/1 |
| [38] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [39] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [40] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [41] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [42] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [43] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [44] | yes | no | yes | yes | yes | yes | yes | yes | yes | no | 7/1 |
| [45] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [46] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [47] | yes | yes | yes | yes | yes | yes | yes | no | - | - | 7/- |
| [48] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [49] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [50] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [51] | yes | yes | yes | yes | no | yes | yes | yes | - | - | 7/- |
| [52] | yes | yes | yes | yes | no | no | yes | yes | - | - | 6/- |
| [53] | yes | no | yes | yes | no | yes | yes | yes | - | - | 6/- |
| [54] | yes | yes | no | yes | no | yes | yes | yes | - | - | 6/- |
| [55] | no | yes | no | yes | yes | yes | yes | no | - | - | 5/- |
| [56] | yes | yes | no | yes | yes | yes | yes | no | - | - | 5/- |
| Reference number | Study design | Candida species | Study group | Outcomes |
|---|---|---|---|---|
| [5] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90028 Biofilm |
PS-L-, PS-L+37.5 J, PS-L+50 J, PS+L-40J, PS+L-80 J, PS+L+40/37.5 J, PS+L+40/50 J, PS+L+80/37.5 J, PS+L+80/50 J | aPDT 80/50 J promotes a greater reduction in the expression of C. albicans genes are associated with adhesion and biofilm formation and genes responsible for oxidative stress. |
| [7] | In vivo animal study, the tongues of mice infected with C. albicans | Reference strain C. albicans ATCC 90028 |
CUR+L+, CUR-L-, CUR+L-, AC+L-, AC+L+, CC+L-, CC+L-, NYS1, NYS4; C free CUR, AC anionic CUR, CC cationic CUR, NYS 100 000 IU 1 and 4 x daily | Free CUR shows a better photodynamic effect than NP-CUR in nanocarriers. APDT with free CUR results in tongue epithelial CK13 and CK14 expression like that observed in healthy mice, which was not observed with NYS. |
| [11] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 18804 Planktonic cultures |
PS+L+, PS+L-, PS-L+, PS-L-, H2O2 10 mM | aPDT caused extensive DNA damage to C. albicans, which was not effectively repaired due to the inhibition caused by CUR. |
| [13] | In vitro study on a 6-well plate | Reference strain C. albicans ATCC 10231 Biofilm |
CUR+L+ (D10, D20, B10, B20, E110, E220, D10+E110, D10+E220, D10+Ti, D20+E110, D20+E220, D20+Ti, B10+E110, B10+E22, B10+Ti, B20+E110, B20+E220, B20+Ti, E110+Ti, E220+Ti, D10+E110+Ti, D10+E220+Ti, D20+E110+Ti, D20+E220+Ti, B10+E110+Ti, B10+E220+Ti, B20+E110+TI, B20+E220+Ti), NYS CUR-L- |
20 µM bisdemethoxycurcumin + erythrosine 110-220 µM + 10% titanium nanoxide tends to generate relatively large amounts of ROS and effectively inhibits Candida albicans without inducing cytotoxicity against normal human gingival fibroblasts. |
| [14] | A randomized controlled clinical trial with a 12-week follow-up | Oral candidiasis, prosthetic stomatitis; C. albicans, C. tropicalis, C. glabrata |
CUR+L+, NYS | CUR-mediated aPDT is as effective as topical NYS therapy in treating denture-induced stomatitis in cigarette smokers. |
| [15] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90028, C. glabrata ATCC 2001, C. dubliniensis CBS 7987 Planktonic cell solutions and biofilms |
PS+L+, PS-L+, PS+L-, PS-L- |
C. albicans - cell viability decreases proportionally regardless of concentration, best effect 20 min PIT and 40 µM CUR. C. glabrata - best effect 40 µM CUR, dependence on PIT unclear. C. dubliniensis - Groups irradiated for 4 min were concentration-dependent for extreme values (40 and 20 µM). In contrast, groups irradiated for 8 minutes were concentration and incubation time dependent. |
| [23] | In vitro study in Eppendorf tubes | Reference strain of C. albicans ATCC Mya 273 Planktonic form | CUR-L-, CUR-L+, CUR+L-, CUR+L+; 5µM 0/5/25min, 10µM 0/5/25µM, 20µM 0/5/25min, 50µM 0/5/25min (DMSO 5/10%) | CUR shows the best antimicrobial activity at a concentration of 50 µM without an incubation period regardless of the DMSO concentration. |
| [36] | In vitro study on a 96-well plate | Clinical strain C. dubliniensis CD6, CD7, CD8, reference strain CBS 7987 (control) Plankton cultures and biofilms | PS-L+, PS+L-5, PS+L-10, PS+L-20, PS-L-, PS+L+5, PS+L+10, PS+L+20 (for planctonic forms); PS-L+, PS+L-20, PS+L-30, PS+L-40, PS-L-, PS+L+20, PS+L+30, PS+L+40 (for biofilms) | The best therapeutic effect against plankton forms CUR 20 µM, and against biofilms CUR 40 µM. |
| [37] | Controlled, two-arm, parallel-group, single-blind clinical trial | Clinical strain, oral candidiasis, C. tropicalis, C. parapsilosis, C. krusei, C. glabrata |
MB, CUR | Curcumin at 80 µmol/L irradiated with an energy of 200 J/cm2 is associated with increased free radical generation. CUR was less effective than TBO. |
| [38] | In vivo study in a mice model of oral candidiasis | Reference strain C. albicans ATCC 90028 | CUR+L+20, CUR+L+40, CUR+L+80, CUR+L-20, CUR+L-40, CUR+L-80, CUR-L+, CUR-L- | Histological analysis of the tongues of mice treated with aPDT 80 µM CUR showed a reduced number of Candida cells that were confined to the stratum corneum and low inflammatory response. |
| [39] | In vitro test on silicone samples in a 24-well plates | Reference strain C. albicans ATCC 90028 Biofilm |
L-CUR-, L-CUR+, L+CUR-, L+CUR+ | The antimicrobial effect on C. albicans depends on the concentration of curcumin and the exposure time. The best results are obtained with 60 µg/mL curcumin and 30 min of irradiation. |
| [40] | In vivo study in a mice model of oral candidiasis | Reference strain C. albicans ATCC 90028 |
PS+L+20µM, PS+L+40µM, PS+L+80µM PS+L-20µM, PS+L-40µM, PS+L-80µM, PS-L+, PS-L- |
A curcumin concentration of 80 uM combined with LED light causes the greatest change in the number of C. albicans colonies. |
| [41] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90028 Biofilm, planktonic cultures |
CUR+L+ (free CUR, anionic CUR, cationic CUR), CUR+L-, CUR-L+, CUR-L- NL- (anionic and cationic nanoparticles without CUR) | Anionic CUR shows the lowest antibacterial photodynamic effect, cationic CUR was cytotoxic. |
| [42] | In vitro study on a 96-well plate. In situ biofilm study in the oral cavity (volunteers wore palatal appliances containing enamel samples to establish dental biofilms in situ; study on a 24-well plate) | Reference strain C. albicans ATCC 90028 Biofilm |
CUR+L+ (CUR-LCP, CUR-CHIH, CUR-ME, CUR-S), CUR+L-, CUR-L+, CUR-L- | CUR-S is the only formulation that can significantly reduce the viability of the biofilm after photodynamic treatment. |
| [43] | In vitro study on a 24-well plate | Reference strain C. albicans ATCC 18804, C. tropicalis ATCC 13803 Planktonic cultures |
CUR-L- (saline), N (nystatin), C. longa+L-, CUR+L-, CUR-L+, CUR+L+ |
The isolated curcumin longa extract and photodynamic therapy with CUR have antifungal activity against C. albicans and C. tropicalis and no toxicity to the invertebrate model G. mellonella. |
| [44] | Randomized controlled clinical trial with 2-month follow-up | Prosthetic stomatitis; C. albicans, C. krusei |
Group I (antifungal gel therapy), Group II (aPDT CUR + antifungal gel) | CUR-mediated aPDT is an effective treatment method for reducing the mycological burden on the palate mucosa and denture surfaces, as well as improving salivary pro-inflammatory cytokine levels in patients with denture-related stomatitis. |
| [45] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90028 Planktonic cultures, biofilm |
PS+L+, PS+L-, PS-L-, PS-L+ | Highest therapeutic efficacy 20 µM CUR, 5.28 J/cm2, 20 min incubated time. |
| [46] | In vitro study on a 96-well plate | Reference strain of C. albicans ATCC 18804 Planktonic forms and biofilm |
PS-L-, PS-L+, CHX, NYS, PS-D+L-, PS-D+L+, PS+D+L-, PS+D+L+, PS-M+L-, PS-M+L+, PS+M+L-, PS+M+L+ | CUR-Plu shows a lower reduction than CUR-DMSO. Multispecies biofilm shows greater resistance than monospecies. CUR-Plu can be considered a stable and effective method for controlling biofilm within a short time after synthesis. |
| [47] | In vitro study on a 96-well plate | Clinical isolates C. albicans Ca1, Ca2, Ca3, Ca4, Ca5; C. glabrata Cg1, Cg2, Cg3, Cg4, Cg5; C. tropicails Ct1, Ct2, Ct3, Ct4, Ct5 Planktonic cell solution and biofilm |
PS+L+, PS+L-, PS-L+, PS-L- | The greatest reduction in the activity of C. albicans, C. tropicalis, and C. glabrata using 40 µM CUR and 18 J/cm2. |
| [48] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90028 Planktonic cultures and biofilms |
CUR+L+, CUR+L-, CUR-L+, CUR-L- | C. albicans planktonic cultures are susceptible to subsequent applications of sublethal aPDT doses via CUR. Sublethal aPDT CUR may have made C. albicans cells more resistant to therapy. |
| [49] | In vitro study on a 96-well plate | Reference strain C. albicans SC 5314 Planktonic cultures and biofilms |
CUR-L-, F+CUR-L-, P+CUR-L-, M+CUR-L-, F+CUR+L-, P+CUR+L-, M+CUR+L-, CUR+L-, CUR-UL+, CUR-BL+, M+CUR-UL+, M+CUR+UL+, M+CUR+BL+, F+CUR+BL+, P+CUR+BL+, CUR+BL+, M+CUR+UL+BL+ | CUR-loaded F127 micelles bound to blue light caused biofilm photoinactivation. |
| [50] | In vitro test on acrylic samples in a 24-well plate | Reference strain C. albicans ATCC 90028, C. glabrata ATCC 2001 Biofilm |
PS+L+(24h), PS+L-(24h), PS+L+(48h), PS+L-(48h), PS-L+(24h), PS-L+(48h), PS-L-(24h), PS-L-(48h) | 24- and 48-hour biofilms are susceptible to CUR-mediated aPDT at concentrations assessed at 37.5 J/cm2. |
| [51] | In vitro study on a 6-well plate | Reference strain C. albicans ATCC 10231 Biofilm |
CUR20+L+, CUR40+L+, CUR80+L+, KI+L+, CUR20+KI+L+, CUR40+KI+L+, CUR80+KI+L+, Nystatin, Phosphate-buffered saline | 40 uM bisdemethoxycurcumin + 100 mM KI combined with blue light can effectively reduce C. albicans biofilm after 6 hours with efficacy comparable to NYS. |
| [52] | In vitro test on Petri dishes | Reference strain C. albicans ATCC 10231 Cells in water mixture, cells on agar surface |
PS+L+, PS+L- | C. albicans in an aqueous mixture are inhibited at any light dose, on the agar surface at 96 J/cm2, spore soaking had no significant effect on cell number reduction. |
| [53] | In vitro study on a 96-well plate | Reference strain C. albicans ATCC 90029 Planktonic cell solutions, adherent cultures |
PS+L+, PS+L+ 208 µM fluconazole |
Fluconazole eliminates the yeast form, CUR-aPDT the biofilm. Fluconazole + CUR-aPDT eliminates the growth and virulence of C. albicans. |
| [54] | In vitro test on solid medium plates and in Eppendorf tubes | Reference strain C. albicans SC 5314 Planktonic cell solution, adherent cultures |
PS+L+, PS+L-, PS-L+, PS-L- | 100% inhibition of C. albicans growth at any fluence. |
| Reference number | Light source | Wavelength (nm) | Energy density (Fluence) (J/cm2) | Power output (mW) | Irradiation Time | Spot size / Fiber surface area (cm2) |
|---|---|---|---|---|---|---|
| [5] | LED | 450 | 37.5, 50 | 30 | 21, 27 min | - |
| [7] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5 | 75 | 7 min | 0,196 |
| [11] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5 | 89.2 | 7 min | 0.196 |
| [13] | Dental lamp (VALO Ortho Cordless, South Jordan, UT, USA) | 395-480 | 72 | 3200 | 27 sek | 0.747 |
| [14] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5 (denture) 122 (palate) | 260 | 26 min (denture), 20 min (palate) | 0.196 |
| [15] | LED (Institute of Physics Sao Carlos, São Paulo, Brasil) | 455 | 5.28 | 22 | 4 min | - |
| [23] | LED self-made | 435 | 15.8 | - | 60 min | - |
| [36] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 5.28 | 22 | 4 min | 0.196 |
| [37] | Blue LED (New Dent s/n) | 480 | 200 | 480 | 90 sek | 0.216 |
| [38] | LED (University Sao Paulo, Sao Carlos, SP, Brasil) | 455 | 37.5 | 89,2 | 7 min | - |
| [39] | LED (Biotable RGB, MMOptics, Sao Carlos, SP, Brasil) | 430 | 10.8, 32.4 | 18 | 10, 30 min | - |
| [40] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5 | 89.2 | 7 min | 0.196 |
| [41] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 43.2 | 33.58 | 20 min | 0.196 |
| [42] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 450 (440-460) | 18 | 22 | 14 min | 0.196 |
| [43] | Prototype device based on LEDs (Biotable Irrad/LED) | 450 ± 5 | 10, 25 | 110 | 91, 228 sek | - |
| [44] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37,5 (denture) 122 (palate) | 260 | 26 min (denture), 20 min (palate) | 0.196 |
| [45] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5, 1.32, 2.64, 3.96, 5.28, 6.60, 13.20, 26.40 | 22 | 29 min, 1, 2, 3, 4, 5, 10, 20 min | 0.196 |
| [46] | LED (Biotable RGB, MMOptics, Sao Carlos, SP, Brasil) | 460 | 15 | - | 11 min 36 sek | - |
| [47] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 5.28, 18, 25.5, 37.5 | 22 | n.a | 0.196 |
| [48] | LED (Biotable 3.4, Sao Carlos, Brasil) | 450 | 18 | 47 | 6.38 min | - |
| [49] | LED (Biotable RGB, MMOptics, Sao Carlos, SP, Brasil) | 455 | 33.84 | 47 | 12 min | - |
| [50] | LED (LXHL-PR09, Luxeon III Emitter, Lumileds Lighting, San Jose, California, USA) | 455 (440-460) | 37.5 | 22 | 29 min | 0.196 |
| [51] | Dental lamp (Elipar DeepCure-L Curing Light, 3M, Singapore) | 450 ± 30 | 90 | 950 | 95 sek | 0.785 |
| [52] | Xenon arc lamp (Polilight, PL 500, Rofin Australia Pty Ltd., Victoria, Australia) | 370-680; 420 | 24, 48, 72, 96, 240, 360 | 500 000 | 2, 4, 6, 8, 20, 30 min | - |
| [53] | LED self-made | 430 | 9 | - | 30 min | - |
| [54] | Diode laser blue-violet | 405 | 10, 20, 30 | - | 50, 100, 150 sek | 0.2 |
| Reference number | Incubation time (in minutes) | Concentration/s of PS used |
|---|---|---|
| [5] | 20 | 40, 80 µM |
| [7] | 20 | 260 µM |
| [11] | 20 | 2,5 µM |
| [13] | 15 | 10, 20 µM |
| [14] | 30 | 5 µg/mL |
| [15] | 1, 5, 10, 20 | 5, 10, 20, 30, 40 µM |
| [23] | 0, 5, 25 | 5, 10, 20, 50 µM CUR + 5% DMSO / 10% DMSO; 10, 50, 100 µM SA-CUR12a |
| [36] | 20 | 5, 10, 20 µM (for planktonic form), 20, 30, 40 µM (for biofilms) |
| [37] | 1 | 80 µM |
| [38] | 20 | 20, 40, 80 µM |
| [39] | 5 | 30, 60 µg/mL |
| [40] | 20 | 20, 40, 80 µM |
| [41] | 40 | 130 µM |
| [42] | 5 | 20, 40, 60, 80 µM |
| [43] | 20 | 100 mg/mL CUR longa / 200 µg/mL CUR |
| [44] | 30 | 5 µg |
| [45] | 5, 20 | 0.005; 0.01; 0.05; 0.1; 0.5; 1; 5; 10; 20 µM |
| [46] | 5 | 270 µM |
| [47] | 20 | 5, 10, 20 µM |
| [48] | 20 | 40 µM |
| [49] | 20 | 63 µM |
| [50] | 20 | 80, 100, 120 µM |
| [51] | 20 | 20, 40, 80 µM bisdemethoxycurcumin + 100 µM KI |
| [52] | 10, 20, 30 | 100-1000 µM, 800 µM |
| [53] | 20 | 1, 5, 10, 20, 40, 80 µM |
| [54] | n.a. | 100 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





