Submitted:
06 July 2024
Posted:
08 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
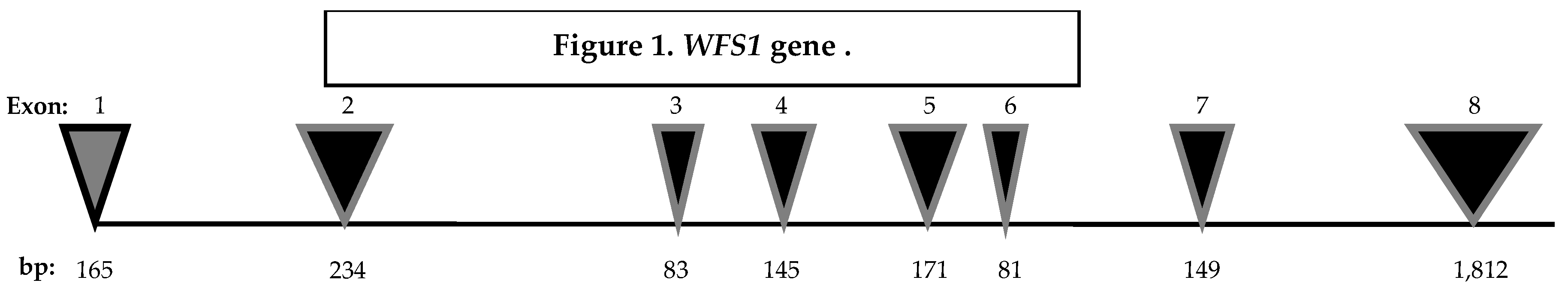
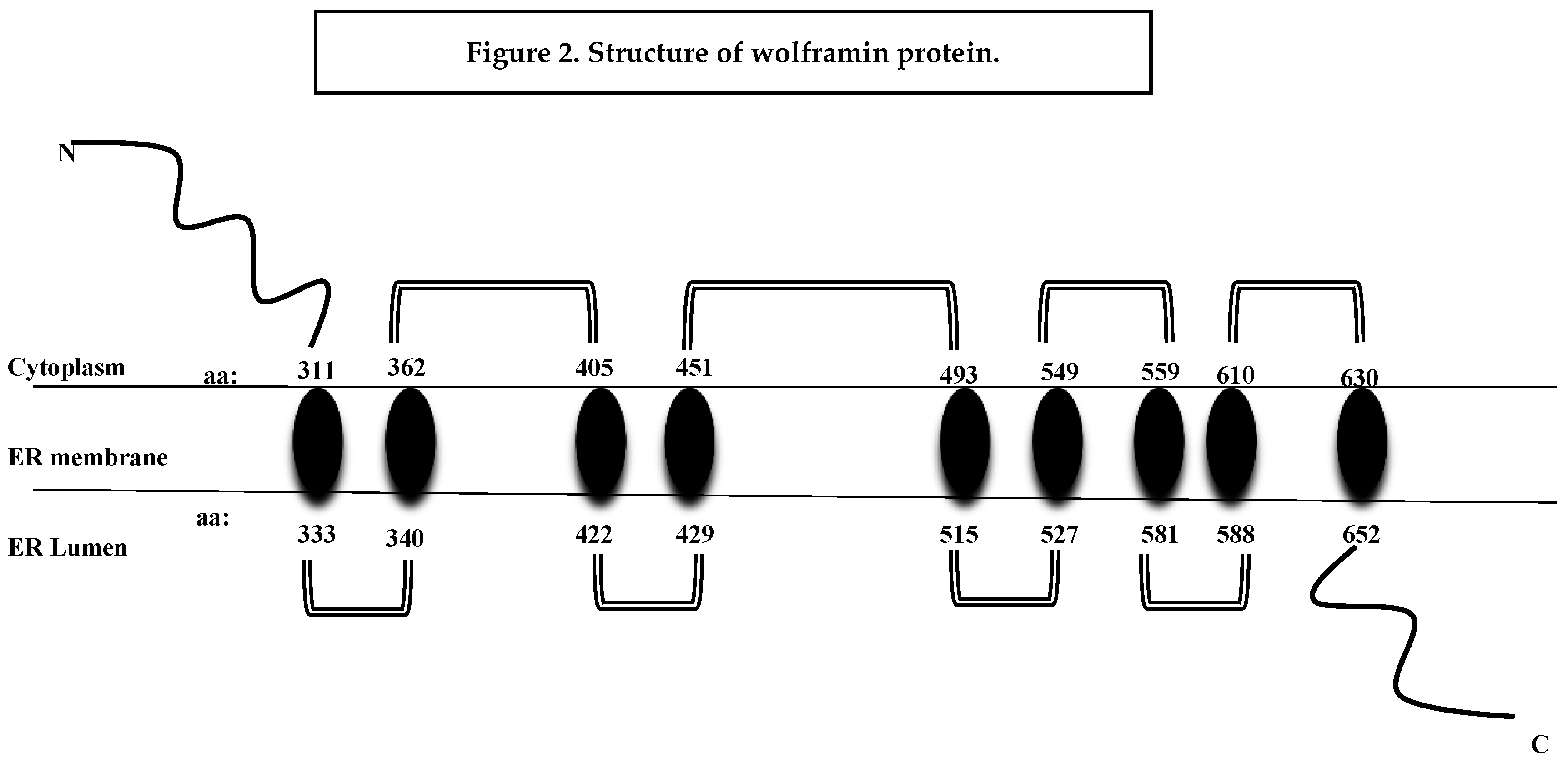
3. WFS1 Gene
4. Physiology and Pathophysiology of WS1
4.1. Wolframin and ER Stress
4.2. Wolframin, Calcium, and Mitochondria
4.3. Wolframin and Neurodevelopment
4.4. WFS1: Altered Neurodevelopment and Neurodegeneration.
4.5. Oligodendrocytes
4.6. Histopathological Alterations in Wolfram Syndrome 1
5. WFS1 and Neuropsychiatric Disorders
5.1. Psychiatric Disorders
6. Conclusion
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Inoue, H.; Tanizawa, Y.; Wasson, J.; Behn, P.; Kalidas, K.; Bernal-Mizrachi, E.; Mueckler, M.; Marshall, H.; Donis-Keller, H.; Crock, P.; et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat. Genet. 1998, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.M.; Hörtnagel, K.; Hofmann, S.; Gekeler, F.; Scharfe, C.; Rabl, W.; Gerbitz, K.D.; Meitinger, T. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum. Mol. Genet. 1998, 7, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- El-Shanti, H.; Lidral, A.C.; Jarrah, N.; Druhan, L.; Ajlouni, K. Homozygosity mapping identifies an additional locus for Wolfram syndrome on chromosome 4q. Am. J. Hum. Genet. 2000, 66, 1229–1236. [Google Scholar] [CrossRef]
- Takeda, K.; Inoue, H.; Tanizawa, Y.; Matsuzaki, Y.; Oba, J.; Watanabe, Y.; Shinoda, K.; Oka, Y. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum. Mol. Genet. 2001, 10, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, D.J.; Wagener, H.P. Diabetes Mellitus and Simple Optic Atrophy among Siblings: Report on Four Cases. Mayo Clin. Proc. 1938, 13, 13,715–718. [Google Scholar]
- Rigoli, L.; Caruso, V.; Salzano, G.; Lombardo, F. Wolfram Syndrome 1: From Genetics to Therapy. Int. J. Environ. Res. Public Health. 2022, 19, 3225. [Google Scholar] [CrossRef]
- Barrett, T.G.; Bundey, S.E. Wolfram (DIDMOAD) syndrome. J. Med. Genet. 1997, 34, 838–841. [Google Scholar] [CrossRef]
- Barrett, T.G.; Bundey, S.E.; Macleod, A.F. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995, 346, 1458–1463. [Google Scholar] [CrossRef]
- Rohayem, J.; Ehlers, C.; Wiedemann, B.; Holl, R.; Oexle, K.; Kordonouri, O.; Salzano, G.; Meissner, T. .; Burger, W.; Schober, E.; et al. Diabetes and neurodegeneration in Wolfram syndrome: a multicenter study of phenotype and genotype. Diabetes Care. 2011, 34, 1503–1510. [Google Scholar] [CrossRef]
- Chaussenot, A.; Bannwarth, S.; Rouzier, C.; Vialettes, B.; Mkadem, S.A.; Chabrol, B.; Cano, A.; Labauge, P.; Paquis-Flucklinger, V. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann. Neurol. 2011, 69, 501–508. [Google Scholar] [CrossRef]
- Rando, T.A.; Horton, J.C.; Layzer, R.B. Wolfram syndrome: evidence of a diffuse neurodegenerative disease by magnetic resonance imaging. Neurology. 1992, 42, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Scolding, N.J.; Kellar-Wood, H.F.; Shaw, C.; Shneerson, J.M.; Antoun, N. Wolfram syndrome: hereditary diabetes mellitus with brainstem and optic atrophy. Ann. Neurol. 1996, 39, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Cur.r Diab. Rep. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Medlej, R.; Wasson, J.; Baz, P.; Azar, S.; Salti, I.; Loiselet, J.; Permutt, A.; Halaby, G. Diabetes mellitus and optic atrophy: a study of Wolfram syndrome in the Lebanese population. J. Clin. Endocrinol. Metab. 2004, 89, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Zmyslowska, A.; Borowiec, M.; Fichna, P.; Iwaniszewska, B.; Majkowska, L.; Pietrzak, I.; Szalecki, M.; Szypowska, A.; Mlynarski, W. Delayed recognition of Wolfram syndrome frequently misdiagnosed as type 1 diabetes with early chronic complications. Exp. Clin. Endocrinol. Diabetes 2014, 122, 35–38. [Google Scholar] [CrossRef]
- Lombardo, F.; Salzano, G.; Di Bella, C.; Aversa, T.; Pugliatti, F.; Cara, S.; Valenzise, M.; De Luca, F.; Rigoli, L. Phenotypical and genotypical expression of Wolfram syndrome in 12 patients from a Sicilian district where this syndrome might not be so infrequent as generally expected. J. Endocrinol. Invest. 2014, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Wolfram syndrome: important implications for pediatricians and pediatric endocrinologists. Pediat.r Diabetes. 2010, 11, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Fraser, F.C.; Gunn, T. Diabetes mellitus, diabetes insipidus, and optic atrophy. An autosomal recessive syndrome? J. Med. Genet. 1977, 14, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Tanabe, K.; Inoue, H.; Okuya, S.; Ohta, Y.; Akiyama, M.; Taguchi, A.; Kora, Y.; Okayama, N.; Yamada, Y.; et al. Wolfram syndrome in the Japanese population; molecular analysis of WFS1 gene and characterization of clinical features. PLoS One. 2014, 9, e106906. [Google Scholar] [CrossRef]
- Rigoli, L.; Lombardo, F.; Di Bella, C. Wolfram syndrome and WFS1 gene. Clin. Genet. 2011, 79, 103–117. [Google Scholar]
- Hofmann, S.; Philbrook, C.; Gerbitz, K.D.; Bauer, M.F. Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum. Mol. Genet. 2003, 12, 2003–2012. [Google Scholar] [CrossRef]
- Fonseca, S.G.; Fukuma, M.; Lipson, K.L.; Nguyen, L.X.; Allen, J.R.; Oka, Y.; Urano, F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J. Biol. Chem. 2005, 280, 39609–39615. [Google Scholar] [CrossRef]
- Ishihara, H.; Takeda, S.; Tamura, A.; Takahashi, R.; Yamaguchi, S.; Takei, D.; Yamada, T.; Inoue, H.; Soga, H.; Katagiri, H.; et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum. Mol. Genet. 2004, 13, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ishihara, H.; Tamura, A.; Takahashi, R.; Yamaguchi, S.; Takei, D.; Tokita, A.; Satake, C.; Tashiro, F. , Katagiri, H.; et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum. Mol. Genet. 2006, 15, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Manente, L.; Lucariello, A.; Baldi, G.; Fiore, P.; Laforgia, V.; Baldi, A.; Iannaccone, A.; De Luca, A. Localization and distribution of wolframin in human tissues. Front. Biosci. (Elite Ed) 2012, 4, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Hershey, T.; Lugar, H.M.; Shimony, J.S.; Rutlin, J.; Koller, J.M.; Perantie, D.C.; Paciorkowski, A.R.; Eisenstein, S.A.; Permutt, M.A.; Washington University Wolfram Study Group. Early brain vulnerability in Wolfram syndrome. PLoS One. 2012, 7, e40604. [Google Scholar] [CrossRef] [PubMed]
- de Heredia, M.L.; Clèries, R.; Nunes, V. Genotypic classification of patients with Wolfram syndrome: insights into the natural history of the disease and correlation with phenotype. Genet. Med. 2013, 15, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Eiberg, H.; Hansen, L.; Kjer, B.; Hansen, T.; Pedersen, O.; Bille, M.; Rosenberg, T.; Tranebjaerg, L. Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J. Med. Genet. 2006, 43, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Rendtorff, N.D.; Lodahl, M.; Boulahbel, H.; Johansen, I.R.; Pandya, A.; Welch, K.O.; Norris, V.W.; Arnos, K.S.; Bitner-Glindzicz, M.; Emery, S.B. Identification of p.A684V missense mutation in the WFS1 gene as a frequent cause of autosomal dominant optic atrophy and hearing impairment. Am. J. Med. Genet. A. 2011, 155, 1298–1313. [Google Scholar] [CrossRef]
- Rigoli, L.; Aloi, C.; Salina, A.; Di Bella, C.; Salzano, G.; Caruso, R.; Mazzon, E.; Maghnie, M.; Patti, G.; D’Annunzio, G.; et al. Wolfram syndrome 1 in the Italian population: genotype-phenotype correlations. Pediatr. Res. 2020, 87, 456–462. [Google Scholar] [CrossRef]
- Zatyka, M.; Ricketts, C.; da Silva Xavier, G.; Minton, J.; Fenton, S.; Hofmann-Thiel, S.; Rutter, G.A.; Barrett, T.G. Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Hum Mol Genet. 2008, 17, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Kõks, S. Genomics of Wolfram Syndrome 1 (WFS1). Biomolecules. 2023, 13, 1346. [Google Scholar]
- Richard, E.M.; Brun, E.; Korchagina, J.; Crouzier, L.; Affortit, C.; Alves, S.; Cazevieille, C.; Mausset-Bonnefont, A.L.; Lenoir, M.; Puel, J.L.; et al. Wfs1E864K knock-in mice illuminate the fundamental role of Wfs1 in endocochlear potential production. Cell Death Dis. 2023, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, S.; Li, M.; Cai, X.; Liu, W.; Zhang, S.; Ma, Y.; Luo, Y.; Zhou, L.; Zhang, X.; et al. The genetic and clinical characteristics of WFS1 related diabetes in Chinese early onset type 2 diabetes. Sci Rep. 2023, 13, 9127. [Google Scholar] [CrossRef] [PubMed]
- Cryns, K.; Sivakumaran, T.A.; Van den Ouweland, J.M.; Pennings, R.J.; Cremers, C.W.; Flothmann, K.; Young, T.L.; Smith, R.J.; Lesperance, M.M.; Van Camp, G. Mutational spectrum of the WFS1 gene in Wolfram syndrome, nonsyndromic hearing impairment, diabetes mellitus, and psychiatric disease. Hum. Mutat. 2003, 22, 275–287. [Google Scholar] [CrossRef]
- Sequeira, A.; Kim, C.; Seguin, M.; Lesage, A.; Chawky, N.; Desautels, A.; Tousignant, M.; Vanier, C.; Lipp, O.; Benkelfat, C.; et al. Wolfram syndrome and suicide: Evidence for a role of WFS1 in suicidal and impulsive behavior. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 119B, 108–113. [Google Scholar] [PubMed]
- Crawford, J.; Zielinski, M.A.; Fisher, L.J.; Sutherland, G.R.; Goldney, R.D. Is there a relationship between Wolfram syndrome carrier status and suicide? Am. J. Med. Genet. 2002, 114, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Momin, I.D.; Rigler, J.; Chitrala, K.N. Analysis of Potential Biomarkers in Frontal Temporal Dementia: A Bioinformatics Approach. Int. J. Mol. Sci. 2023, 24, 14910. [Google Scholar] [CrossRef]
- Castell, L.; Le Gall, V.; Cutando, L.; Petit, C.P.; Puighermanal, E.; Makrini-Maleville, L.; Kim, H.R.; Jercog, D.; Tarot, P.; Tassou, A.; et al. Dopamine D2 receptors in WFS1-neurons regulate food-seeking and avoidance behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2024, 129, 110883. [Google Scholar] [CrossRef]
- Fonseca, S.G.; Ishigaki, S.; Oslowski, C.M.; Lu, S.; Lipson, K.L.; Ghosh, R.; Hayashi, E.; Ishihara, H.; Oka, Y.; Permutt, M.A.; et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest. 2010, 120, 744–755. [Google Scholar] [CrossRef]
- Osman, A.A.; Saito, M.; Makepeace, C.; Permutt, M.A.; Schlesinger, P.; Mueckler, M. Wolframin expression induces novel ion channel activity in endoplasmic reticulum membranes and increases intracellular calcium. J. Biol. Chem. 2003, 278, 52755–52762. [Google Scholar] [CrossRef] [PubMed]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells. 2019, 30, 501. [Google Scholar] [CrossRef]
- Binayi, F.; Fahanik-Babaei, J.; Salimi, M.; Eskandari, F.; Sahraei, M.; Ghorbani Ranjbary, A.; Ghasemi, R.; Hedayati, M.; Khodagholi, F.; Eliassi, A.; et al. Endoplasmic reticulum stress inhibition ameliorated WFS1 expression alterations and reduced pancreatic islets’ insulin secretion induced by high-fat diet in rats. Sci. Rep. 2023, 13, 1860. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011, 333, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.L.; Ghosh, R.; Urano, F. The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PLoS One. 2008, 3, e1648. [Google Scholar] [CrossRef] [PubMed]
- Samara, A.; Rahn, R.; Neyman, O.; Park, K.Y.; Samara, A.; Marshall, B.; Dougherty, J.; Hershey, T. Developmental hypomyelination in Wolfram syndrome: new insights from neuroimaging and gene expression analyses. Orphanet J. Rare Dis. 2019, 14, 279. [Google Scholar] [PubMed]
- Cagalinec, M.; Liiv, M.; Hodurova, Z.; Hickey, M.A.; Vaarmann, A.; Mandel, M.; Zeb, A.; Choubey, V.; Kuum, M.; Safiulina, D.; et al. Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol. 2016, 14, e1002511. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Fernandez-Checa, J.C.; Kaplowitz, N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014, 5, e989. [Google Scholar] [PubMed]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. The Unfolded Protein Response: At the Intersection between Endoplasmic Reticulum Function and Mitochondrial Bioenergetics. Front. Oncol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Zatyka, M.; Rosenstock, T.R.; Sun, C.; Palhegyi, A.M.; Hughes, G.W.; Lara-Reyna, S.; Astuti, D.; di Maio, A.; Sciauvaud, A.; Korsgen, M.E.; et al. Depletion of WFS1 compromises mitochondrial function in hiPSC-derived neuronal models of Wolfram syndrome. Stem Cell Reports. 2023, 18, 1090–1106. [Google Scholar] [CrossRef]
- Zmyslowska, A.; Kuljanin, M.; Malachowska, B.; Stanczak, M.; Michalek, D.; Wlodarczyk, A.; Grot, D.; Taha, J.; Pawlik, B.; Lebiedzińska-Arciszewska, M.; et al.; Stanczak, M ; Michalek, D.; Wlodarczyk, A.; Grot, D.; Taha, J.; Pawlik, B.; Lebiedzińska-Arciszewska, M.; et al. Multiomic analysis on human cell model of wolfram syndrome reveals changes in mitochondrial morphology and function. Cell Commun. Signal. 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, A.C.; Nierenberg, A.A. Mitochondrial Dysfunction: At the Core of Psychiatric Disorders? Biol. Psychiatry. 2018, 83, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Delprat, B.; Maurice, T.; Delettre, C. Wolfram syndrome: MAMs’ connection? Cell Death Dis. 2018, 9, 364. [Google Scholar] [PubMed]
- Eimre, M.; Kasvandik, S.; Ivask, M.; Kõks, S. Proteomic dataset of wolframin-deficient mouse heart and skeletal muscles. Data Brief. 2018, 21, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Kõks, S.; Overall, R.W.; Ivask, M.; Soomets, U.; Guha, M.; Vasar, E.; Fernandes, C.; Schalkwyk, L.C. Silencing of the WFS1 gene in HEK cells induces pathways related to neurodegeneration and mitochondrial damage. Physiol. Genomics. 2013, 45, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Elli, F.M.; Ghirardello, S.; Giavoli, C.; Gangi, S.; Dioni, L.; Crippa, M.; Finelli, P.; Bergamaschi, S.; Mosca, F.; Spada, A.; et al. A new structural rearrangement associated to Wolfram syndrome in a child with a partial phenotype. Gene. 2012, 509, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Lucariello, A.; Perna, A.; Sellitto, C.; Baldi, A.; Iannaccone, A.; Cobellis, L.; De Luca, A.; De Falco, M. Modulation of wolframin expression in human placenta during pregnancy: comparison among physiological and pathological states. Biomed. Res. Int. 2014, 2014, 985478. [Google Scholar] [CrossRef] [PubMed]
- Tekko, T.; Lilleväli, K.; Luuk, H.; Sütt, S.; Truu, L.; Örd, T.; Möls, M.; Vasar, E. Initiation and developmental dynamics of Wfs1 expression in the context of neural differentiation and ER stress in mouse forebrain. Int. J. Dev. Neurosci. 2014, 35, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lugar, H.M.; Koller, J.M.; Rutlin, J.; Marshall, B.A.; Kanekura, K.; Urano, F.; Bischoff, A.N.; Shimony, J.S.; Hershey, T.; Washington University Wolfram Syndrome Research Study Group. Neuroimaging evidence of deficient axon myelination in Wolfram syndrome. Sci. Rep. 2016, 6, 21167. [Google Scholar] [CrossRef]
- Miller, D.J.; Duka, T.; Stimpson, C.D.; Schapiro, S.J.; Baze, W.B.; McArthur, M.J.; Fobbs, A.J.; Sousa, A.M.; Sestan, N.; Wildman, D.E.; et al. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. U S A. 2012, 109, 16480–16485. [Google Scholar] [CrossRef]
- Takeda, K.; Inoue, H.; Tanizawa, Y.; Matsuzaki, Y.; Oba, J.; Watanabe, Y.; Shinoda, K.; Oka, Y. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum. Mol. Genet. 2001, 10, 477–484. [Google Scholar] [CrossRef]
- Kawano, J.; Fujinaga, R.; Yamamoto-Hanada, K.; Oka, Y.; Tanizawa, Y.; Shinoda, K. Wolfram syndrome 1 (Wfs1) mRNA expression in the normal mouse brain during postnatal development. Neurosci. Res. 2009, 64, 213–230. [Google Scholar] [CrossRef]
- Li, L.; Venkataraman, L.; Chen, S.; Fu, H. Function of WFS1 and WFS2 in the Central Nervous System: Implications for Wolfram Syndrome and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2020, 118, 775–783. [Google Scholar] [CrossRef]
- Dennis, E.L.; Thompson, P.M. Reprint of: Mapping connectivity in the developing brain. Int. J. Dev. Neurosci. 2014, 32, 41–57. [Google Scholar] [CrossRef]
- Abramov, A.Y. The brain-from neurodevelopment to neurodegeneration. FEBS J. 2022, 289, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Lugar, H.M.; Koller, J.M.; Rutlin, J.; Eisenstein, S.A.; Neyman, O.; Narayanan, A.; Chen, L.; Shimony, J.S.; Hershey, T. Evidence for altered neurodevelopment and neurodegeneration in Wolfram syndrome using longitudinal morphometry. Sci Rep. 2019, 9, 6010. [Google Scholar] [CrossRef] [PubMed]
- Ghirardello, S.; Dusi, E.; Castiglione, B.; Fumagalli, M.; Mosca, F. Congenital central diabetes insipidus and optic atrophy in a Wolfram newborn: is there a role for WFS1 gene in neurodevelopment? J. Pediatr. 2014, 40, 76. [Google Scholar] [CrossRef] [PubMed]
- Hadidy, A.M.; Jarrah, N.S.; Al-Till, M.I.; El-Shanti, H.E.; Ajlouni, K.M. Radiological findings in Wolfram syndrome. Saudi Med. J. 2004, 25, 638–641. [Google Scholar]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef]
- Southwood, C.M.; Garbern, J.; Jiang, W.; Gow, A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002, 36, 585–596. [Google Scholar] [CrossRef]
- Hilson, J.B.; Merchant, S.N.; Adams, J.C.; Joseph, J.T. Wolfram syndrome: a clinicopathologic correlation. Acta Neuropathol. 2009, 118, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Becker, L.; Deck, J. Evidence of widespread axonal pathology in Wolfram syndrome. Acta Neuropathol. 1999, 98, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Genís, D.; Dávalos, A.; Molins, A.; Ferrer, I. Wolfram syndrome: a neuropathological study. Acta Neuropathol. 1997, 93, 426–429. [Google Scholar] [CrossRef]
- Pickett, K.A.; Duncan, R.P.; Hoekel, J.; Marshall, B.; Hershey, T.; Earhart, G.M.; Washington University Wolfram Study. Early presentation of gait impairment in Wolfram Syndrome. Orphanet J. Rare Dis. 2012, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Crock, P.A.; King, B.R.; Meldrum, C.J.; Scott, R.J. Phenotype-genotype correlations in a series of wolfram syndrome families. Diabetes Care. 2004, 27, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.N.; Reiersen, A.M.; Buttlaire, A.; Al-Lozi, A.; Doty, T.; Marshall, B.A.; Hershey, T.; Washington University Wolfram Syndrome Research Group. Selective cognitive and psychiatric manifestations in Wolfram Syndrome. Orphanet J. Rare Dis. 2015, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, S.; Li, M.; Cai, X.; Liu, W.; Zhang, S.; Ma, Y.; Luo, Y.; Zhou, L.; Zhang, X.; et al. The genetic and clinical characteristics of WFS1 related diabetes in Chinese early onset type 2 diabetes. Sci. Rep. 2023, 13, 9127. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Zielinski, M.A.; Fisher, L.J.; Sutherland, G.R.; Goldney, R.D. Is there a relationship between Wolfram syndrome carrier status and suicide? Am. J. Med. Genet. 2002, 114, 343–346. [Google Scholar] [CrossRef]
- Swift, R.G.; Polymeropoulos, M.H.; Torres, R.; Swift, M. Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol. Psychiatry. 1998, 3, 86–91. [Google Scholar] [CrossRef]
- Shrestha, P.; Mousa, A.; Heintz, N. Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors. Elife 2015, 15, e08752. [Google Scholar] [CrossRef]
- Luuk, H.; Plaas, M.; Raud, S.; Innos, J.; Sütt, S.; Lasner, H.; Abramov, U.; Kurrikoff, K.; Kõks, S.; Vasar, E. Wfs1-deficient mice display impaired behavioural adaptation in stressful environment. Behav. Brain Res. 2009, 198, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Altpere, A.; Raud, S.; Sütt, S.; Reimets, R.; Visnapuu, T.; Toots, M.; Vasar, E. Mild stress induces brain region-specific alterations of selective ER stress markers’ mRNA expression in Wfs1-deficient mice. Behav Brain Res. 2018, 352, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Licis, A.; Davis, G.; Eisenstein, S.A.; Lugar, H.M.; Hershey, T. Sleep disturbances in Wolfram syndrome. Orphanet J Rare Dis. 2019, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C.; Kenkare, J.D.; Schramm, C.M. An adolescent with Wolfram syndrome and central sleep apnea. J. Clin. Sleep Med. 2024, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Reiersen, A.M.; Noel, J.S.; Doty, T.; Sinkre, R.A.; Narayanan, A.; Hershey, T. Psychiatric Diagnoses and Medications in Wolfram Syndrome. Scand. J. Child. Adolesc. Psychiat.r Psychol. 2022, 10, 63–174. [Google Scholar] [CrossRef]
- Hao, H.; Song, L.; Zhang, L. Wolfram syndrome 1 regulates sleep in dopamine receptor neurons by modulating calcium homeostasis. PLoS Genet. 2023, 19, e1010827. [Google Scholar] [CrossRef]
| Groups of mutations | Localization of mutations | Type of mutations | Type of alterations of wolframin |
|---|---|---|---|
| type 1 | before exone 8 | nonsense and frameshift | complete deletion |
| type 2 | aa 1-aa 670 aa 701-aa 890 | missense nonsense | complete degradation |
| type 3 | after exon 8 and before aa700 | nonsense | expression of a defective or shorter protein |
| after exon 8 | frameshift | ||
| aa 671-aa 700 | missense |
| Class | functional alterations | |
|---|---|---|
| A | A1 | wolframin depletion due to WFS1 mRNA degradation |
| A2 | wolframin depletion due to mRNA and protein degradation | |
| A3 | wolframin depletion due to protein degradation | |
| B | reduced expression of a defective wolframin | |
| C | expression of a defective wolframin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





