Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
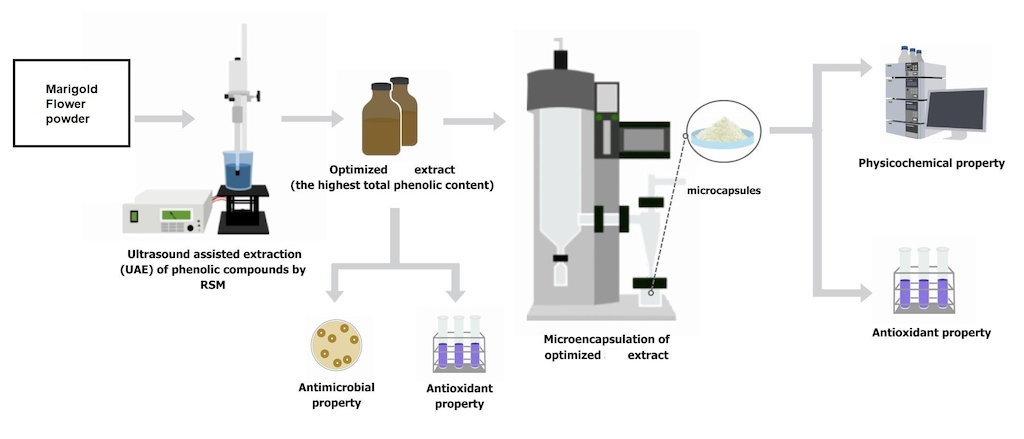
2.1. Sample Procurement and Preparation Marigold Flower Powder
2.2. Response Surface Methodology, Optimization, and Preparation of Marigold Flower Extract
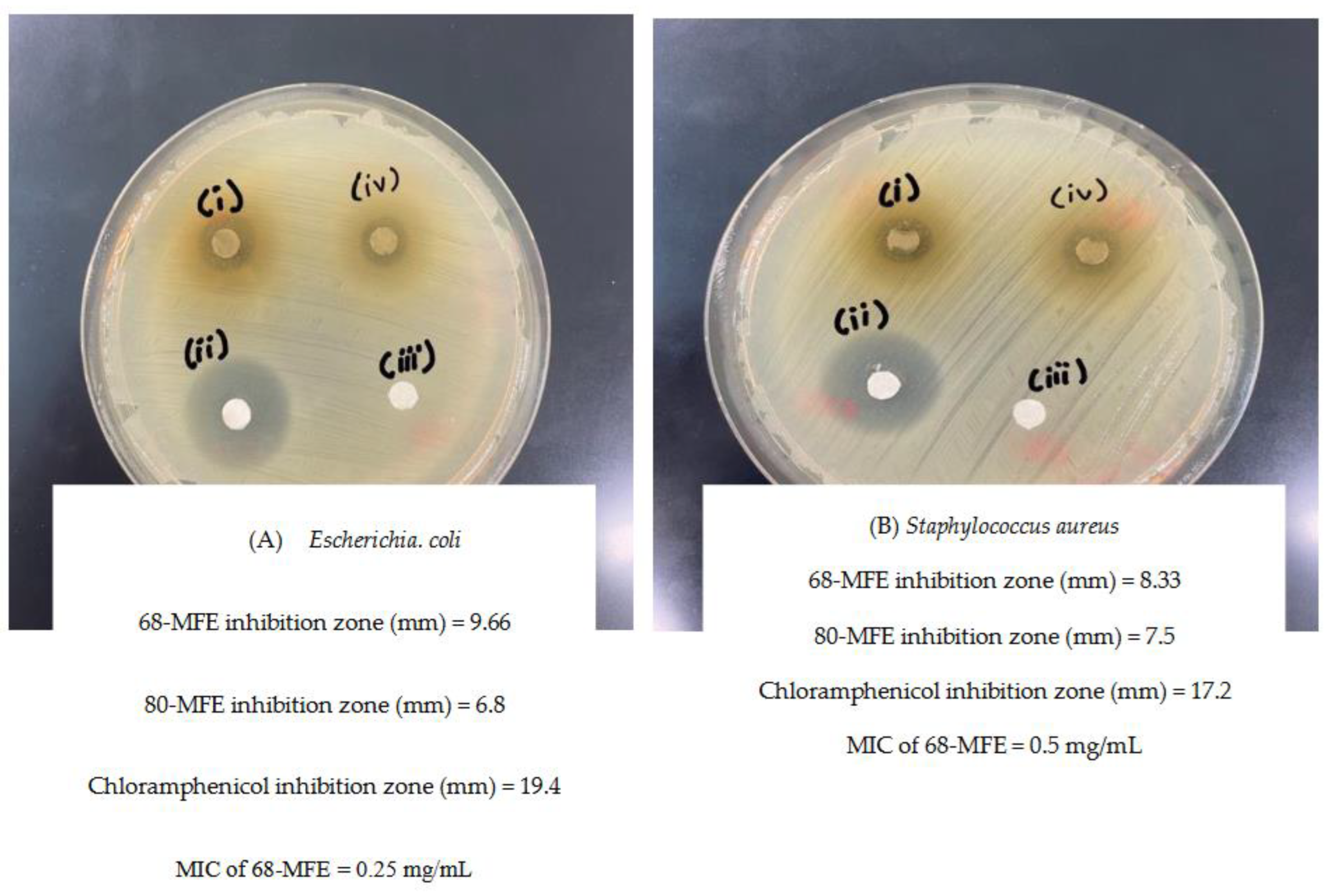
2.3. Antimicrobial Activity of RSM Optimized MFE
2.3.1. Growth Condition
2.3.2. Determination of Inhibition Zones of MFE by Minimum Inhibitory Concentration and Disk Diffusion Method
2.4. Microencapsulation of RSM Optimized MFE in Maltodextrin (MD) and Gum Arabic (GA)
| TPC (mg GAE/100g dry wt.) |
DPPH (mM Trolox/100 g dry wt.) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean square | P-value | Sum of squares | df | Mean squares | p-value | |
| Model | 5361.08 | 9 | 595.68 | 0.0001 | 2879.70 | 9 | 319.97 | 0.16 | |
| A | 45.44 | 1 | 45.44 | 0.17 | 24.72 | 1 | 24.72 | 0.69 | |
| B | 2992.49 | 1 | 2992.49 | 0.0001 | 634.57 | 1 | 634.57 | 0.08 | |
| C | 126.51 | 1 | 126.51 | 0.04 | 31.75 | 1 | 31.75 | 0.66 | |
| AB | 1.74 | 1 | 1.74 | 0.77 | 49.44 | 1 | 49.44 | 0.58 | |
| AC | 45.30 | 1 | 45.30 | 0.17 | 0.39 | 1 | 0.39 | 0.96 | |
| BC | 0.01 | 1 | 0.01 | 0.98 | 26.59 | 1 | 26.59 | 0.69 | |
| A2 | 14.69 | 1 | 14.69 | 0.41 | 129.55 | 1 | 129.55 | 0.38 | |
| B2 | 2129.0 | 1 | 2129.0 | 0.0001 | 1901.32 | 1 | 1901.32 | 0.01 | |
| C2 | 44.24 | 1 | 44.24 | 0.17 | 3.11 | 1 | 3.11 | 0.89 | |
| Residual | 132.75 | 7 | 18.96 | 1040.41 | 7 | 148.63 | |||
| Lack of Fit | 116.64 | 3 | 38.88 | 0.03 | 663.45 | 3 | 221.15 | 0.21 | |
| Pure Error | 16.11 | 4 | 4.03 | 376.95 | 4 | 94.24 | |||
| Cor Total | 5493.84 | 16 | 3920.11 | 16 | |||||
| R2 | 0.97 | 0.73 | |||||||
| Adj-R2 | 0.94 | 0.39 | |||||||
|
FRAP (mM Trolox/100g dry wt.) |
TFC (mg QE/100g dry wt.) |
||||||||
| Source | Sum of squares | df |
Mean squares |
p-value | Sum of squares | df |
Mean squares |
p-value | |
| Model | 4.43+07 | 9 | 4.92+06 | 0.42 | 7147.90 | 9 | 794.21 | 0.04 | |
| A | 1.59+06 | 1 | 1.59+06 | 0.56 | 1073.51 | 1 | 1073.51 | 0.05 | |
| B | 2.49+07 | 1 | 2.49+07 | 0.04 | 3829.80 | 1 | 3829.80 | 0.0028 | |
| C | 2157.21 | 1 | 2157.21 | 0.98 | 87.20 | 1 | 87.20 | 0.52 | |
| AB | 4.87+05 | 1 | 4.87+05 | 0.74 | 127.21 | 1 | 127.21 | 0.44 | |
| AC | 800.5+54 | 1 | 800.5+54 | 0.97 | 84.96 | 1 | 84.96 | 0.52 | |
| BC | 6.05+06 | 1 | 6.05+06 | 0.27 | 363.47 | 1 | 363.47 | 0.21 | |
| A2 | 2.61+05 | 1 | 2.61+05 | 0.81 | 13.85 | 1 | 13.85 | 0.79 | |
| B2 | 7.89+06 | 1 | 7.89+06 | 0.21 | 1366.44 | 1 | 1366.44 | 0.03 | |
| C2 | 3.61+06 | 1 | 3.61+06 | 0.38 | 267.97 | 1 | 267.97 | 0.27 | |
| Residual | 2.92+07 | 7 | 4.18+06 | 1326.53 | 7 | 1326.53 | |||
| Lack of Fit | 2.89+07 | 3 | 9.62+06 | 0.0003 | 1040.66 | 3 | 346.89 | 0.08 | |
| Pure Error | 3.80+05 | 4 | 95124.65 | 285.86 | 4 | 71.47 | |||
| Cor Total | 7.36+07 | 16 | 8474.43 | 16 | |||||
| R2 | 0.60 | 0.84 | |||||||
| Adj-R2 | 0.09 | 0.64 | |||||||
|
Carotenoid content (mg carotenoid/ 100g dry wt.) |
|||||||||
| Source | Sum of squares | df |
Mean squares |
p-value | |||||
| Model | 1.89+05 | 9 | 20967.33 | 0.01 | |||||
| A | 2091.99 | 1 | 2091.99 | 0.38 | |||||
| B | 1582.19 | 1 | 1582.19 | 0.44 | |||||
| C | 17176.18 | 1 | 17176.18 | 0.03 | |||||
| AB | 3565.06 | 1 | 3565.06 | 0.26 | |||||
| AC | 5205.23 | 1 | 5205.25 | 0.18 | |||||
| BC | 5674.03 | 1 | 5674.03 | 0.16 | |||||
| A2 | 1.366+05 | 1 | 1.37+05 | 0.0001 | |||||
| B2 | 127.09 | 1 | 127.09 | 0.82 | |||||
| C2 | 21519.70 | 1 | 21519.70 | 0.02 | |||||
| Residual | 16505.75 | 7 | 2357.96 | ||||||
| Lack of Fit | 14275.60 | 3 | 4758.53 | 0.03 | |||||
| Pure Error | 2230.15 | 4 | 557.54 | ||||||
| Cor Total | 2.052+05 | 16 | |||||||
| R2 | 0.92 | ||||||||
| Adj-R2 | 0.82 | ||||||||
| Bioactive properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | TPC | p-values | TFC | p-values | DPPH | p-values | FRAP | p-values | Carotenoid | p-values |
| Intercept | 72.73 | 57.10 | 634.56 | 2932.63 | 287.79 | |||||
| Linear | ||||||||||
| A | 2.38 | 0.17 | 11.58 | 0.05 | 1.76 | 0.70 | -445.26 | 0.56 | -16.17 | 0.38 |
| B | 19.34 | <0.0001 | 21.88 | 0.0028 | 8.91 | 0.08 | 1764 | 0.04 | -14.06 | 0.44 |
| C | -3.98 | 0.04 | 3.30 | 0.52 | -1.99 | 0.66 | -16.42 | 0.98 | -46.34 | 0.03 |
| Cross product | ||||||||||
| AB | 0.66 | 0.77 | -5.64 | 0.44 | -3.52 | 0.58 | -348.95 | 0.74 | 29.85 | 0.26 |
| AC | -3.37 | 0.17 | -4.61 | 0.52 | -0.31 | 0.96 | 44.74 | 0.97 | 36.07 | 0.18 |
| BC | 0.051 | 0.98 | 9.53 | 0.21 | -2.58 | 0.69 | 1230.21 | 0.27 | 37.66 | 0.16 |
| Quadratic | ||||||||||
| A² | 1.87 | 0.41 | 1.81 | 0.79 | -5.55 | 0.38 | 249.10 | 0.81 | -180.13 | 0.0001 |
| B² | -22.49 | <0.0001 | -18.01 | 0.03 | -21.25 | 0.009 | 1368.89 | 0.21 | -5.49 | 0.82 |
| C² | 3.24 | 0.17 | 7.98 | 0.27 | -0.86 | 0.89 | -925.42 | 0.38 | 71.49 | 0.02 |
2.5. Physiochemical Properties of MD and GA Microcapsules Loaded with MFE
2.5.1. Determination of Bioactive Compounds (TPC and TFC), Antioxidant Activity (DPPH and FRAP) and Carotenoids of MFE without and with Encapsulation
2.7. Statistical Analysis
3. Results and Discussion
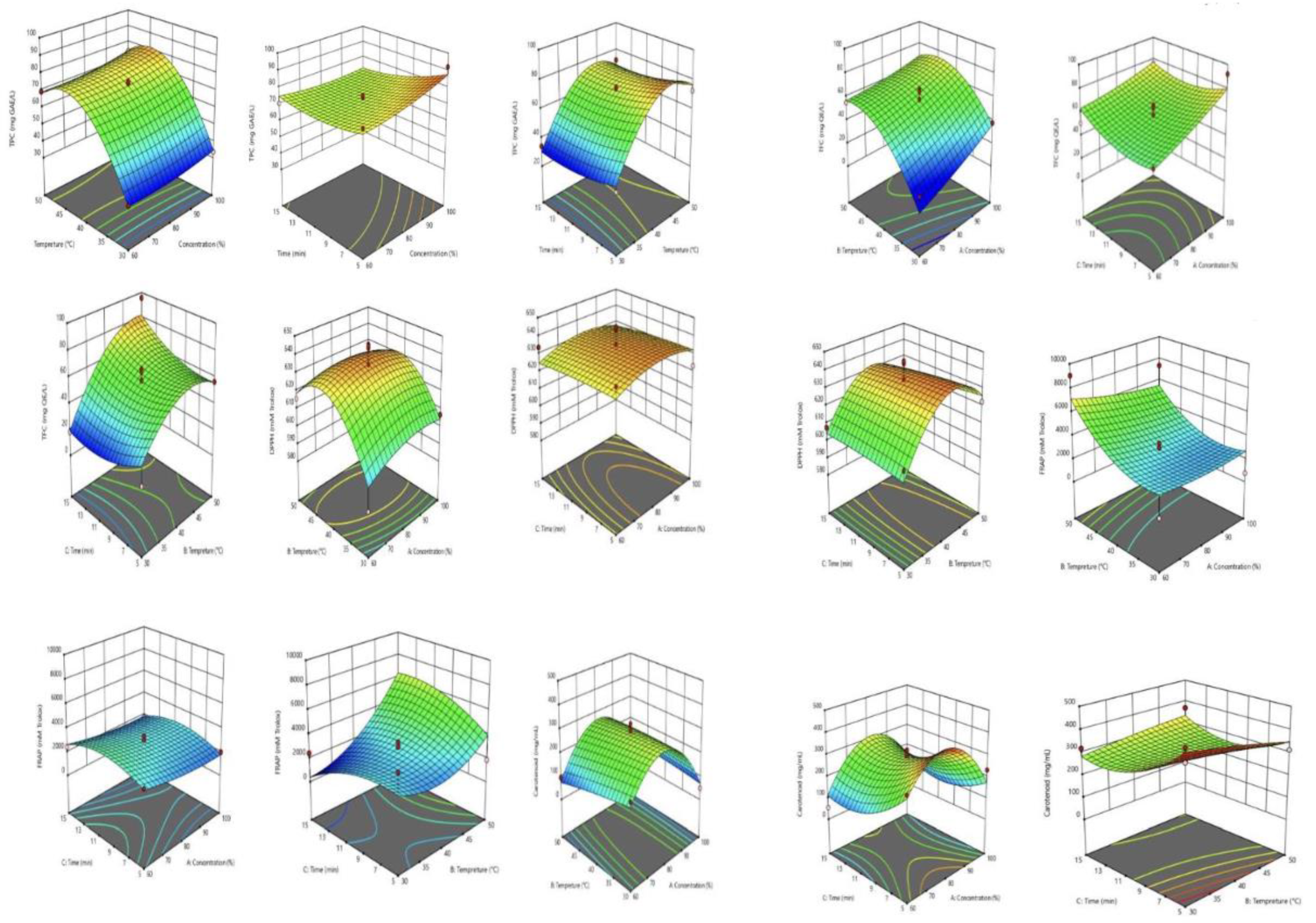
3.1. Optimization of Ultrasound-Assisted Extraction (UAE) of Bioactive Compounds from Marigold Flower Using RSM
3.2. Analysis of Bioactive Properties of MFE
3.3. Impact of Microencapsulation Process on Physicochemical Properties and Microstructure of MFE Microcapsules
3.3.1. Physicochemical Properties of GA and MD-Based MFE Microcapsules
3.3.2. Total Bioactive Analysis of GA and MD-Based MFE Microcapsules
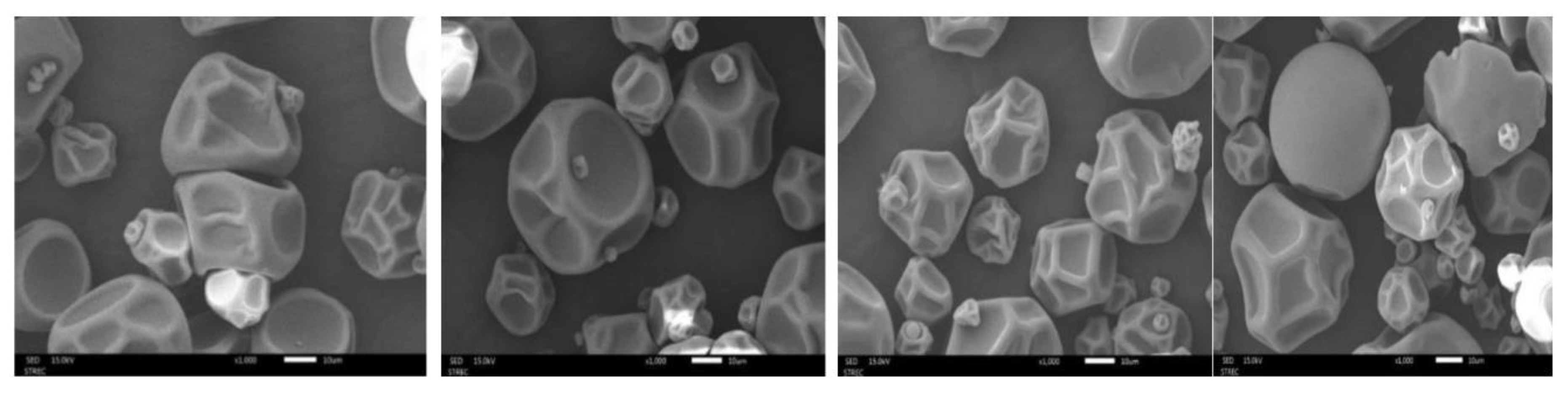
3.3.2. Microstructural Analysis of GA and MD-Based MFE Microcapsules
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janarny, G., Ranaweera, K., & Gunathilake, K. (2021). Antioxidant activities of hydro-methanolic extracts of Sri Lankan edible flowers. Biocatalysis and Agricultural Biotechnology, 35, 102081.
- Walia, A., Gupta, A. K., & Sharma, V. (2019). Role of bioactive compounds in human health. Acta Scientific Medical Sciences, 3, 25-33.
- Drabińska, N., Jeż, M., & Nogueira, M. (2021). Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants, 10, 1597. [CrossRef]
- Ahmad Shiekh, K., Odunayo Olatunde, O., Zhang, B., Huda, N., & Benjakul, S. (2021). Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chemistry, 359, 129976. [CrossRef]
- Spinelli, S. (2016). Study of microencapsulated bioactive compounds in food products.
- Kaimainen, M., Järvenpää, E., & Huopalahti, R. (2015). Enzyme-assisted oil extraction of lutein from marigold (Tagetes erecta) flowers and stability of lutein during storage.
- Ćetković, G., Djilas, S., Čanadanović-Brunet, J., & Tumbas Šaponjac, V. (2004). Antioxidant properties of marigold extracts. Food Research International, 37, 643-650. [CrossRef]
- Chauhan, A. S., Chen, C.-W., Singhania, R. R., Tiwari, M., Sartale, R. G., Dong, C.-D., & Patel, A. K. (2022). Valorizations of Marigold Waste for High-Value Products and Their Industrial Importance: A Comprehensive Review. Resources, 11(10).. [CrossRef]
- Priyanka, D., Shalini, T., & Navneet, V. K. (2013). A brief study on marigold (Tagetes species): a review. International Research Journal of Pharmacy, 4(1), 43-48.
- Usman, I., Hussain, M., Imran, A., Afzaal, M., Saeed, F., Javed, M., . . . A. Saewan, S. (2022). Traditional and innovative approaches for the extraction of bioactive compounds. International Journal of Food Properties, 25(1), 1215-1233. [CrossRef]
- Azmir, J., Zaidul, I. S. M., Rahman, M., Sharif, K., Mohamed, A., Sahena, F., . . . Omar, A. (2013). Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering, 117(4), 426-436.
- Otles, S. (2016). Handbook of food analysis instruments: CRC Press.
- Insang, S., Kijpatanasilp, I., Jafari, S., & Assatarakul, K. (2021). Ultrasound-assisted extraction of functional compound from mulberry (Morus alba L.) leaf using response surface methodology and effect of microencapsulation by spray drying on quality of optimized extract. Ultrasonics Sonochemistry, 105806.
- Calderón-Oliver, M., & Ponce-Alquicira, E. (2022). The Role of Microencapsulation in Food Application. Molecules, 27(5). [CrossRef]
- Šeregelj, V. N., Ćetković, G. S., Čanadanović-Brunet, J. M., Tumbas-Šaponjac, V. T., Vulić, J. J., & Stajčić, S. S. (2017). Extraction and encapuslation of bioactive compounds from carrots. Acta periodica technologica, 48, 261-273.
- Jafari, S., Karami, Z., Shiekh, K. A., Kijpatanasilp, I., Worobo, R. W., & Assatarakul, K. (2023). Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods, 12(2).. [CrossRef]
- Gonelimali, F. D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., & Hatab, S. R. (2018). Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Frontiers in Microbiology, 9.
- Ramakrishnan, Y., Adzahan, N. M., Yusof, Y. A., & Muhammad, K. (2018). Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technology, 328, 406-414. [CrossRef]
- Saénz, C., Tapia, S., Chávez, J., & Robert, P. J. F. c. (2009). Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). 114(2), 616-622.
- Sarabandi, K., Peighambardoust, S. H., Sadeghi, A., & Samaei, S. (2019). Effect of different carriers on microstructure and physical characteristics of spray dried apple juice concentrate. Journal of Food Science and Technology, 55, 3098–3109.
- Biswas, A. K., Sahoo, J., & Chatli, M. K. (2011). A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT - Food Science and Technology, 44(8), 1809-1813. [CrossRef]
- Brand-Williams, W., Cuvelier, M.E., & Burset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft & Technologie - Food Science and Technology, 28(1), 25-30.
- Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239(1), 70-76.
- Air, M. B. P. U. B. (2018). Total phenolic, flavonoid content and antioxidant activity of Clinacanthus nutans leaves by water-based ultrasonic assisted extraction. Malaysian Journal of Analytical Sciences, 22(4), 659-666.
- Yıkmış, S. (2019). Optimization of uruset apple vinegar production using response surface methodology for the enhanced extraction of bioactive substances. Foods, 8(3), 107.
- Wen, C., Zhang, J., Zhang, H., Dzah, C. S., Zandile, M., Duan, Y., . . . Luo, X. (2018). Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrasonics Sonochemistry, 48, 538-549.
- Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., . . . Martins, N. (2019). The Therapeutic Potential of Apigenin. International Journal of Molecular Sciences, 20(6).. [CrossRef]
- Liyana-Pathirana, C. M., & Shahidi, F. (2006). Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. Journal of the Science of Food and Agriculture, 86(3), 477-485.
- Sungthong, B., & Phadungkit, M. (2015). Anti-tyrosinase and DPPH radical scavenging activities of selected Thai herbal extracts traditionally used as skin toner. Pharmacognosy Journal, 7(2).
- Umair, M., Jabbar, S., Nasiru, M. M., Lu, Z., Zhang, J., Abid, M., . . . Zhao, L. (2021). Ultrasound-assisted extraction of carotenoids from carrot pomace and their optimization through response surface methodology. Molecules, 26(22), 6763.
- Jiao, S., Li, Y., Wang, Z., Sun-Waterhouse, D., Waterhouse, G. I., Liu, C., & Wang, X. (2020). Optimization of enzyme-assisted extraction of bioactive-rich juice from Chaenomeles sinensis (Thouin) Koehne by response surface methodology. Journal of Food Processing and Preservation, 44(9), e14638.
- De Zoysa, M. H. N., Rathnayake, H., Hewawasam, R. P., & Wijayaratne, W. M. D. G. B. J. I. j. o. m. (2019). Determination of in vitro antimicrobial activity of five Sri Lankan medicinal plants against selected human pathogenic bacteria. 2019.
- Breijyeh, Z., Jubeh, B., & Karaman, R. (2020). Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules, 25(6). [CrossRef]
- Kalušević, A., et al. (2017). "Microencapsulation of anthocyanin-rich black soybean coat extract by spray drying using maltodextrin, gum Arabic and skimmed milk powder." Journal of Microencapsulation, 34(5), 475-487.
- Pitalua, E., et al. (2010). "Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material." Food and Bioproducts Processing, 88(2-3), 253-258.
- Cano-Higuita, D. M., Vélez, H. A. V., & Telis, V. R. N. (2015). Microencapsulation of turmeric oleoresin in binary and ternary blends of gum Arabic, maltodextrin and modified starch. Ciência e Agrotecnologia, 39, 173-182.
- Bernstein, A., & Noreña, C. P. Z. (2015). Encapsulation of red cabbage (Brassica oleracea L. var. capitata L. f. rubra) anthocyanins by spray drying using different encapsulating agents. Brazilian Archives of Biology and Technology, 58, 944-952.
| Independent Variables | Independent Variable Codes | Level | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Ethanol concentration (%) | A | 60 | 80 | 100 |
| Extraction temperature (◦C) | B | 30 | 40 | 50 |
| Ultrasonication time (min) | C | 5 | 10 | 15 |
| Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Concentration (%) | Temperature (°C) | Time (min) | TPC (mg GAE/100g dry wt.) | TFC (mg QE/100g dry wt.) | DPPH (mM Trolox/100g dry wt.) | FRAP (mM Trolox/100g dry wt.) | Carotenoid content (mg carotenoid/ 100g dry wt.) |
| 1 | 80 | 50 | 15 | 72.38 | 95.73 | 610.50 | 4627.37 | 368.23 |
| 2 | 100 | 30 | 10 | 33.59 | 37.83 | 606.75 | 750.74 | 49.51 |
| 3 | 80 | 40 | 10 | 71.28 | 66.30 | 624.25 | 3364.21 | 288.62 |
| 4 | 80 | 40 | 10 | 70.29 | 47.68 | 625.50 | 2995.79 | 266.23 |
| 5 | 100 | 40 | 15 | 69.75 | 76.19 | 621.44 | 1722.11 | 137.96 |
| 6 | 80 | 40 | 10 | 74.14 | 49.36 | 643.62 | 2501.05 | 323.17 |
| 7 | 80 | 30 | 5 | 34.69 | 17.45 | 609.25 | 4585.26 | 414.67 |
| 8 | 80 | 40 | 10 | 72.71 | 57.83 | 644.87 | 2869.47 | 294.69 |
| 9 | 80 | 50 | 5 | 72.71 | 56.72 | 622.37 | 1869.47 | 312.94 |
| 10 | 100 | 50 | 10 | 74.14 | 55.73 | 627.37 | 7364.21 | 79.36 |
| 11 | 60 | 40 | 5 | 79.19 | 48.37 | 634.25 | 2880.00 | 292.48 |
| 12 | 100 | 40 | 5 | 91.94 | 92.14 | 623.31 | 1995.79 | 231.12 |
| 13 | 80 | 40 | 10 | 75.20 | 64.30 | 634.56 | 2932.63 | 266.23 |
| 14 | 60 | 50 | 10 | 69.31 | 55.24 | 615.81 | 9048.42 | 95.12 |
| 15 | 60 | 40 | 15 | 70.46 | 50.85 | 633.62 | 2427.37 | 55.04 |
| 16 | 60 | 30 | 10 | 31.39 | 14.78 | 581.12 | 1039.16 | 184.68 |
| 17 | 80 | 30 | 15 | 34.14 | 18.33 | 607.69 | 2422.32 | 319.30 |
| Treatments | ||||
|---|---|---|---|---|
| Parameters | 20% GAM (1:2) |
20% GAM (1:3) |
45% MDM (1:1) |
45% MDM (1:2) |
| Yield (%) | 53.59±0.70d | 56.15±0.73c | 61.15±0.58b | 79.20±0.56a |
| Moisture content (%) | 4.42±0.18a | 3.45±0.13b | 4.35±0.13a | 3.19±2.05b |
| Water activity | 0.12±0.03ab | 0.15±0.07a | 0.12±0.03ab | 0.10±0.04b |
| Color values | ||||
| L* | 61.46±1.55c | 58.16±3.81d | 73.18±1.51a | 71.27±1.39b |
| a* | -4.47±0.17b | -3.17±0.13a | -5.99±0.00c | -5.23±0.11c |
| b* | 30.45±1.59a | 25.09±1.53b | 24.46±0.61c | 19.02±0.73d |
| Encapsulation efficiency (%) | 80.79±1.12b | 80.49±1.85b | 88.00±1.15a | 78.05±1.04c |
| Solubility (%) | 88.41±2.91bc | 92.73±3.20ab | 89.31±1.31b | 92.98±4.22a |
| TPC (mg GAE/100g dry wt.) | 627.91±24.86a | 508.13±9.52b | 435.24±8.32c | 304.10±7.06d |
| TFC (mg QE/100g dry wt.) | 389.56±9.58a | 376.73±10.37b | 282.90±1.53c | 209.87±4.60d |
| Carotenoid content (mg carotenoid/ 100g dry wt.) | 208.45±2.36a | 162.75±1.69b | 55.22±1.94c | 44.62±2.63d |
| DPPH (mM Trolox/100 g dry wt.) | 1756.66±28.99a | 1469.70±31.02b | 1217.83±22.68c | 794.50±15.61d |
| FRAP (mM Trolox/100g dry wt.) | 4837.89±27.85a | 4308.07±26.49b | 3076.49±16.08c | 2665.96±21.91d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





