1. Introduction
Hypophthalmichthys nobilis is an economically important fish species in China, widely distributed in the major water systems in the eastern part of China [
1].
H. nobilis is mainly cultured in the middle and lower reaches of the Yangtze River, and eastern and southern coastal areas of China, such as Hubei, Jiangxi, Guangdong, Hunan, Jiangsu and Anhui provinces [
2]. In the 1970s, the United States introduced
H. nobilis, and it is now widely distributed in the Mississippi River basin [
3].
H. nobilis is one of the important "Four Famous Domestic Fishes" in China, which is the main freshwater aquaculture fish, and the significant stocked fish and pond culture species, with high economic value, high protein and low-fat nutritional value, and is an important dietary source of fish [
1]. In recent years, with the impact of environmental pollution, engineering construction, and overfishing, the fishery resources in the Yangtze River have continued to decline. In order to better protect the aquatic resources in the Yangtze River and its related water bodies, the "Ten-Year Fishing Ban" was implemented from January 1, 2020, prohibiting the fishing of natural
H. nobilis resources in the main stream and important tributaries of the Yangtze River. As a result, the natural catch of
H. nobilis has decreased. Some traders, in pursuit of profit, are resorting to illegal fishing of
H. nobilis in the natural water bodies of the Yangtze River. Therefore, it is crucial for the management and accountability of illegal fishing activities to discriminate and trace the origin of
H. nobilis found in the market.
Currently, there are various methods for discriminating and tracing aquatic species from different sources, such as a single method based on individual morphological characteristics [
4], otolith morphology [
5], genetic sequences [
6], fatty acid composition [
7], isotope differences [
8], and element fingerprint analysis (EFA) [
9], or a combination of these methods for discrimination [
10,
11]. EFA has been proven to be a very useful technology for distinguishing biological species or populations and has been widely applied in studies related to the geographical tracing of aquatic species and safety assessments of aquatic products [
9,
12,
13,
14]. The elements in EFA can come from otoliths, whole body, and muscles, etc [
5,
15,
16]. In the past, otoliths EFA have commonly been used in various ecological studies of fish [
17,
18,
19,
20], especially in studies related to the life history [
21,
22]. This method typically requires complex sample preparation procedures and advanced instrument equipment [
23]. Given the different targets of application, difficulties in sample collection, and discrimination sensitivity of different methods. For
H. nobilis in this study, with its larger size reaching a maximum length of 146 cm, and typically around 60 cm in length, using whole body as samples poses challenges due to the large sample size [
1]. Obtaining otoliths from
H. nobilis is difficult due to its hard skull, which results in high processing and time costs. Therefore, to facilitate the determination of the source of unknown-origin
H. nobilis samples in market supervision, this study selected muscle tissue of
H. nobilis from different sources and analyzed their element fingerprint patterns to trace the origins of different
H. nobilis samples.
In recent years, EFA has been applied to the identification of illegally sourced aquatic products in the market and the differentiation of water products from different sources [
24,
25,
26], hence in this study, for the traceability determination of aquatic products in the market, a simpler method was chosen by using only a part of fish muscles instead of the whole body or small otoliths as experimental materials. The aim of this study is to distinguish
H. nobilis samples from different sources in the Yangtze River basin and its related lakes using EFA of muscle. By proposing a more convenient, cost-effective, and less sample-consuming method, the results of this study can guide the traceability discrimination of suspicious samples in market monitoring by collecting a small amount of local muscle samples, and are expected to provide technology and methods for the traceability and management of illegal fishing of this species in future market supervise.
2. Materials and Methods
2.1. Sampling Sites, Sample Collection, and Processing
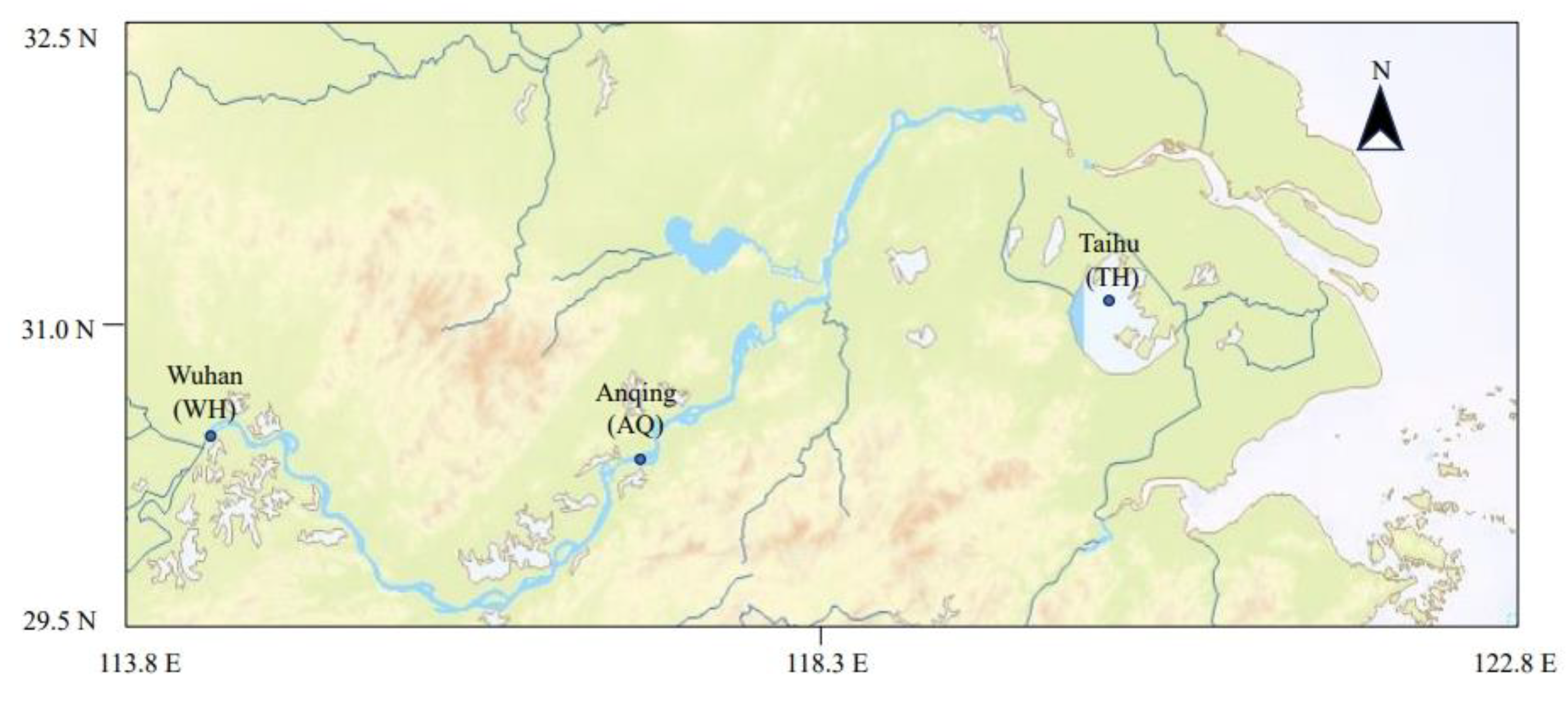
From September to December 2023, seventeen individuals of
H. nobilis were selected from three stations in the Yangtze River basin: Wuhan (WH), Anqing (AQ), and Taihu (TH) (
Figure 1,
Table 1). To authenticate the new source samples, five individuals were randomly selected from AQ as positive controls (PC-AQ). All samples were captured using gill nets. Upon boarding the ship, fish samples were labeled with sampling point coordinates and placed in plastic bags. The samples were then frozen at -20°C until processed in the laboratory.
In the laboratory, all samples were thawed. Standard lengths were measured in centimeters, and fresh weights were measured in grams (±0.1 g). All samples were rinsed six times with Milli-Q water (Millipore Corp., USA), dissected with stainless steel surgical knives, and the dorsal muscles were cut into small pieces for thorough drying. After 24 hours of freeze-drying, the samples were ground into fine powder using a tissue grinder and immediately placed into a desiccator before analysis.
Each dry sample (0.5 ± 0.005 g) was placed in a digestion tube, 10 ml of purified HNO
3 (MOS Reagent, Sinopharm Chemical Reagent Co., Ltd., China) was added, and left to stand for 3 h. Then 2 ml of purified HClO
4 was added to each tube. Finally, an electric hot plate was used to digest all samples, and the fully digested samples were quantitatively transferred to a 100 ml calibration flask containing Milli-Q water [
15].
2.2. Elemental Analysis
Total concentrations of 20 elements in pre-digested samples were measured using the instruments and methods of ICP-OES (Agilent, 720ES) and ICP-MS (Agilent, 7700) [
27,
28,
29]: mercury (Hg), lead (Pb), chromium (Cr), cadmium (Cd), copper (Cu), zinc (Zn), nickel (Ni), arsenic (As), sodium (Na), magnesium (Mg), potassium (K), iron (Fe), aluminum (Al), manganese (Mn), molybdenum (Mo), strontium (Sr), barium (Ba), titanium (Ti), vanadium (V), and calcium (Ca). Calibration standards were prepared from multi-element mixed calibration standard reserve solutions (Cd, Pb, Cr, Cu, Zn, Ni, As, Mg, Fe, Al, Mn, Sr, Ba, Ti, V; Standard number: GSB 04-1767-2004; Hg, Standard number: GSB 04-1729-2004; Na, Standard number: GSB 04-1738-2004; K, Standard number: GSB 04-1733-2004; Ca, Standard number: GSB 04-1720-2004; Mo, Standard number: GSB 04-1737-2004; National Nonferrous Metals and Electronic Materials Analysis and Testing Center, National Standard (Beijing) Inspection and Certification Co., Ltd, China). All these analyses were repeated three times.
2.3. Data Analysis
In order to eliminate individual differences among different stations, the raw data on the content of the 19 elements were first divided by the content of Ca to obtain the data on the ratios of the 19 elements to Ca, which were expressed on this basis [
26]. One-way analysis of variance (ANOVA) was performed on the elemental ratios from different stations in order to test the differences between the groups at a significance level of α=0.05 for each element [
30]. The multivariate analysis models were then used to detect spatial patterns in these three stations [
31]. First, principal component analysis (PCA) was applied to detect the overall pattern of elemental variation. Stepwise linear discriminant analysis (LDA) was applied to develop a set of discriminant functions that were derived from the 19 elements ratios that were able to discriminate between different groups. The discriminative element ratios selected through stepwise discrimination are used for traceability discrimination of new source samples. Hierarchical cluster analysis (HCA) was applied to further explore the reasons for the elemental composition differences in muscles of
H. nobilis from different stations [
32,
33]. Statistical analysis was performed using the software IBM SPSS Statistics 25.0.
3. Results
3.1. Elemental Fingerprints Composition
Non-parametric tests were conducted on the 19 elements ratios in the muscle of
H. nobilis from different stations in the Yangtze River basin to analyze the differences of elemental composition among them. The results showed that there were significant differences (
P<0.05) in the 4 elements ratios (Hg/Ca, Pb/Ca, Al/Ca, Mn/Ca) in the muscle of
H. nobilis among the three groups, while the differences of the remaining 15 elements ratios were not significant (
P>0.05) (
Table 2).
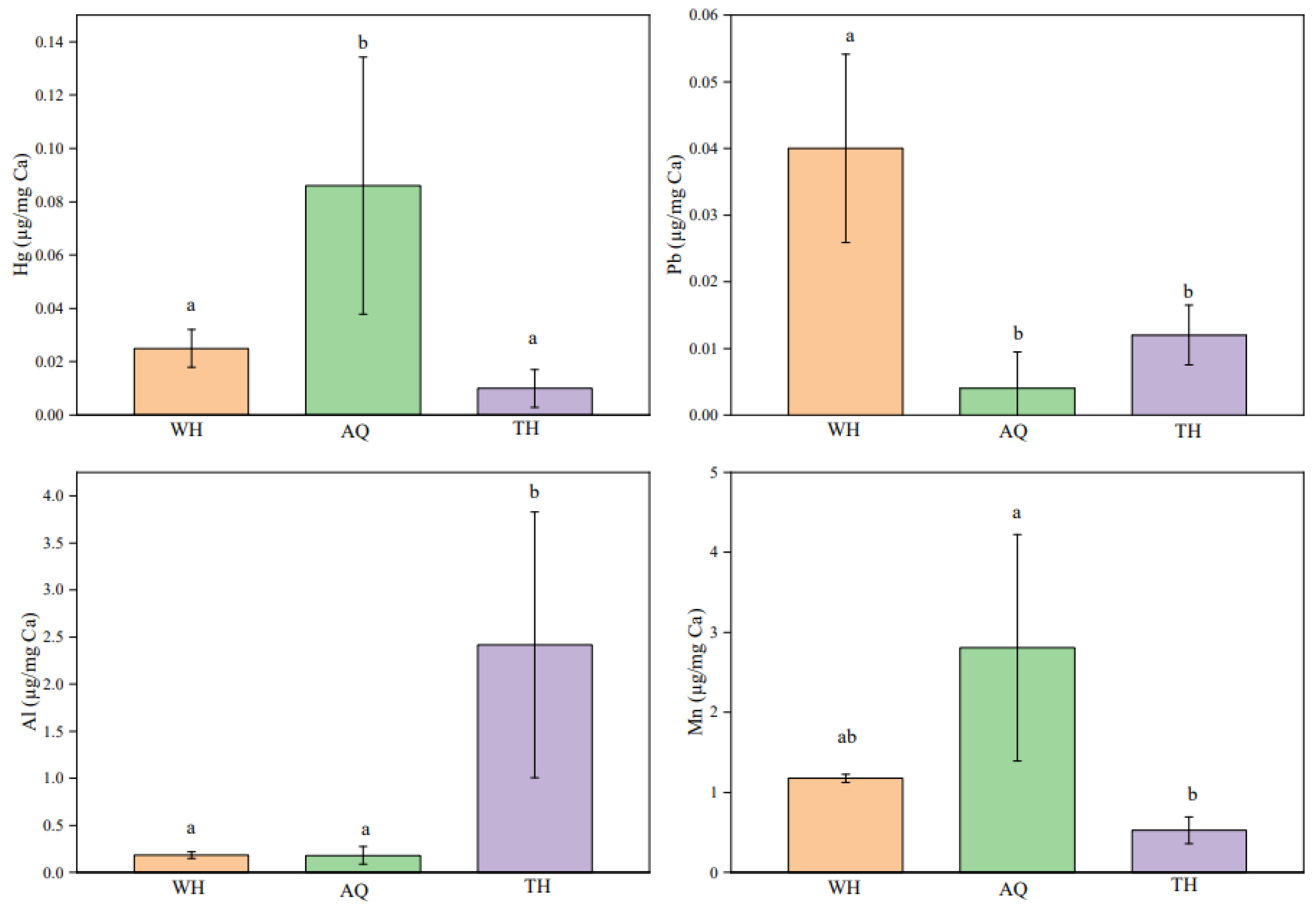
One-way ANOVA was performed on the 19 element ratios in the muscle of
H. nobilis to compare the differences in elemental composition among the three groups. Significant differences (
P<0.05) were observed in the ratios of two elements (Hg, Pb) between WH and AQ groups, two elements (Pb, Al) between the WH and TH groups, and three elements (Hg, Al, Mn) between AQ and TH groups. The four element ratios (Hg/Ca, Pb/Ca, Al/Ca, Mn/Ca) with significant differences identified among the three stations were selected for quantitative analysis (
Figure 2). Looking at the differences in element ratios among different groups, the Hg/Ca ratios in AQ was significantly higher than that in other groups, the Pb/Ca ratios in WH was significantly higher than that in other groups, and the Al/Ca ratios in TH was significantly higher than that in other groups (
P<0.05). Mn/Ca ratios in WH showed no significant difference with that in AQ and TH, respectively (
P>0.05). Therefore, the Hg/Ca, Pb/Ca, and Al/Ca ratios can be used as characteristic indicators for the AQ, WH, and TH groups, respectively.
3.2. Principal Component Analysis (PCA)
To screen and determine the main characteristic elemental indicators in the composition of
H. nobilis groups from different stations, PCA was performed on the ratios of 19 elements. A total of 5 principal components (PC1 to PC5) were derived (
Table 3).
As shown in
Table 3, the top 5 indicators contributing to PC1 were K, Zn, Na, Mg, and Mo, while the top 5 indicators contributing to PC2 were Cd, Ni, Cu, As, and Mn, and the top 3 indicators contributing to PC3 were Al, Cr, and Pb. It was observed that the top 5 elemental indicators in PC1 did not show significant differences among the samples from different stations. PC2 only showed a significant difference in the element ratios of Mn, between AQ and TH. On the other hand, Al in PC3 was significantly different between TH and WH, as well as TH and AQ, while Pb was significantly different between WH and AQ, as well as WH and TH (
Table 2,
Table 3).
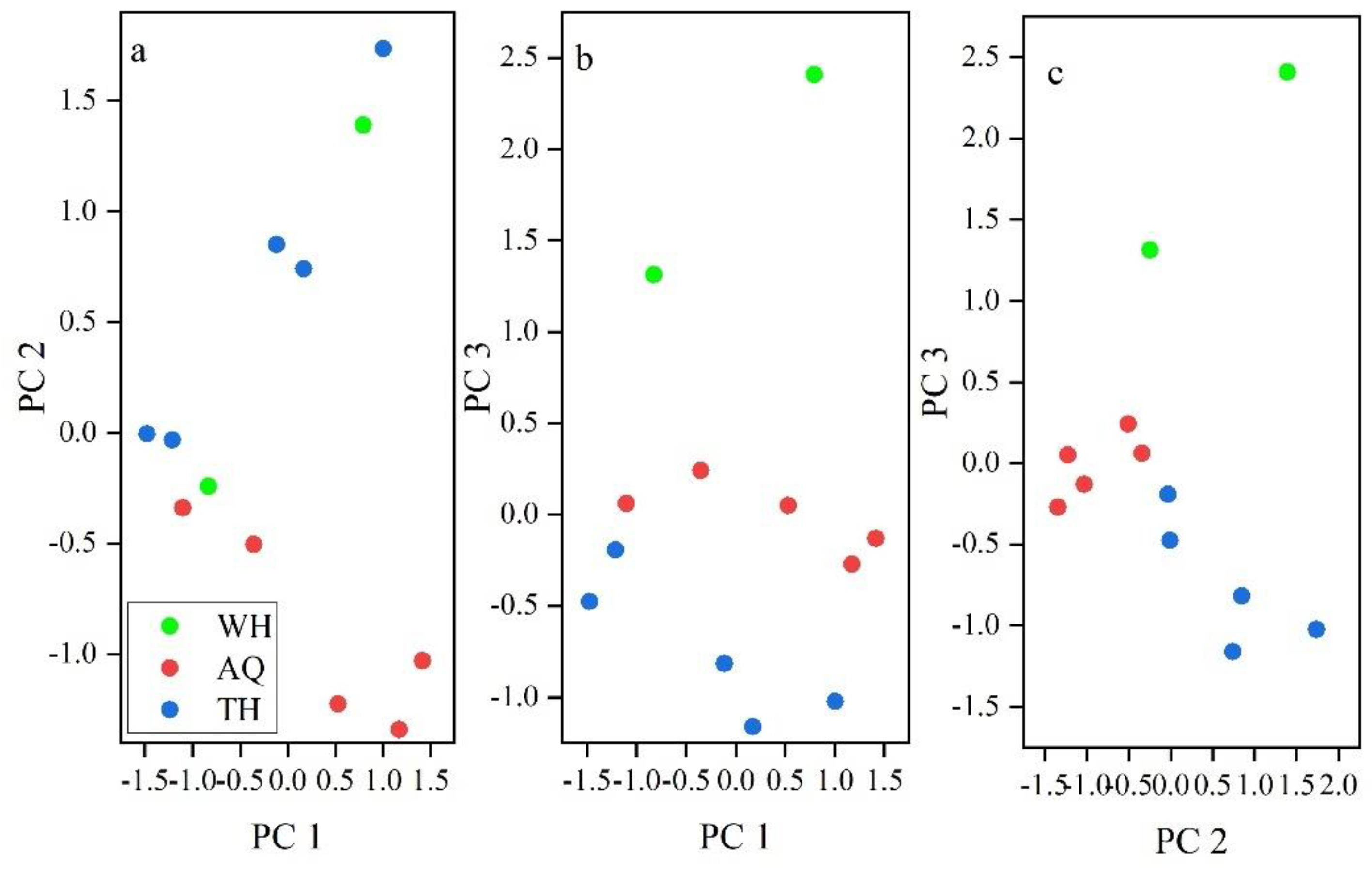
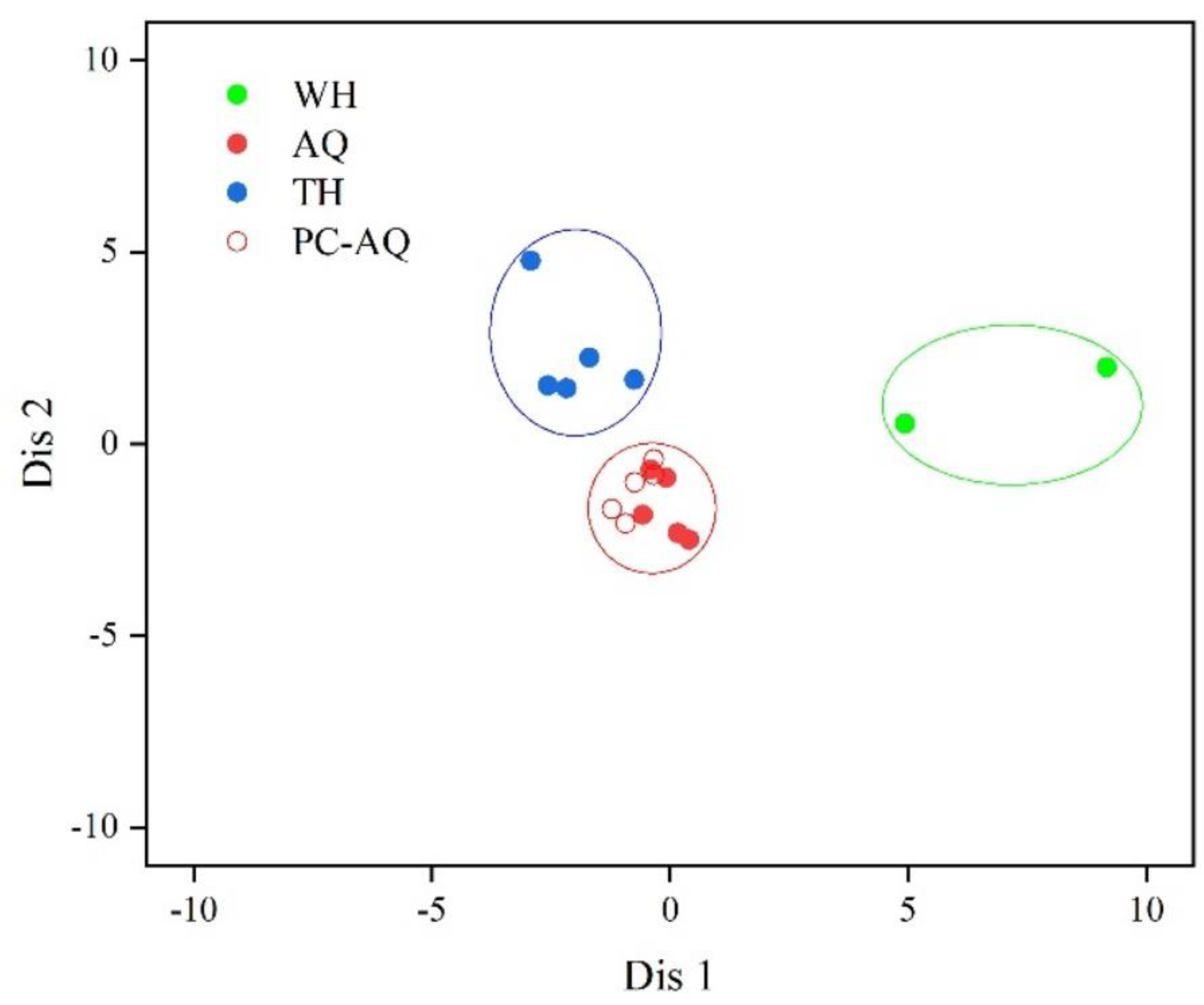
In the scatter plot formed by PC1 and PC2 (
Figure 3a), the main elemental indicators from different stations were close to each other, with only a few elemental indicators showing differences. As indicated by the main elemental indicators in PC2, Mn showed no significant difference between WH and AQ, as well as between WH and TH, so the scatter plot formed by PC1 and PC2, the elemental composition of WH merged with AQ and TH (
Figure 3a). As there were no significant differences for the main elemental indicators of Pb and Al in PC3 between AQ and the other groups, so the scatter plot formed by PC1 and PC3, the elemental composition in AQ was distributed between WH and TH (
Figure 3b). On the other hand, Mn in PC2 from AQ and TH, Pb in PC3 from WH and other two stations, and Al in PC3 from TH and other two stations showed significant differences. Therefore, on the scatter plot composed of PC 2 and PC 3, there are more differences in the elemental composition of the three groups, each of which is relatively concentrated and distinct (
Figure 3c). It can be seen that through the scatter plots formed by PC1 and PC2 as well as PC1 and PC3, the three groups could not be effectively distinguished as the main elements (K, Zn, Fe, Mg, Na) in PC1 showed no significant differences among the different groups, indicating overall similarity in the elemental composition of
H. nobilis muscle from different stations. However, through the scatter plots formed by PC2 and PC3, a preliminary differentiation of the three groups could be made, primarily because the main elements Mn in PC2, and Pb and Al in PC3 showed certain differences among the three groups. Especially in terms of the composition of minority elements in PC3, there are significant differences between Pb in WH, and Al in TH compared to the other two stations, indicating that the differences between samples from different stations are mainly manifested in the few characteristic elements in PC3.
3.3. Discriminant Element Screening
LDA was conducted on the composition of 19 elemental ratios in
H. nobilis muscle from different stations in the Yangtze River basin, resulting in a discriminant equation with 4 elemental coefficient as independent variables, including 4 elemental ratios of Pb/Ca, Cr/Ca, Na/Ca and Al/Ca (
Table 4). From the discriminant coefficients of each element ratios, it can be seen that Pb/Ca had the largest coefficient in WH, while Al/Ca had the largest coefficient in TH, indicating that Pb and Al were the main differential elements among the three stations.
The results of the stepwise discriminant analysis showed an overall discriminant success rate of 100.00% among the 12 individuals of
H. nobilis from the three stations. The results of the cross-validation were consistent with the stepwise discriminant results (
Table S1). The three groups in the discriminant analysis scatterplot were scoped with circles, which showed that the elemental composition of
H. nobilis in the three groups can be clearly distinguished (
Figure S1).
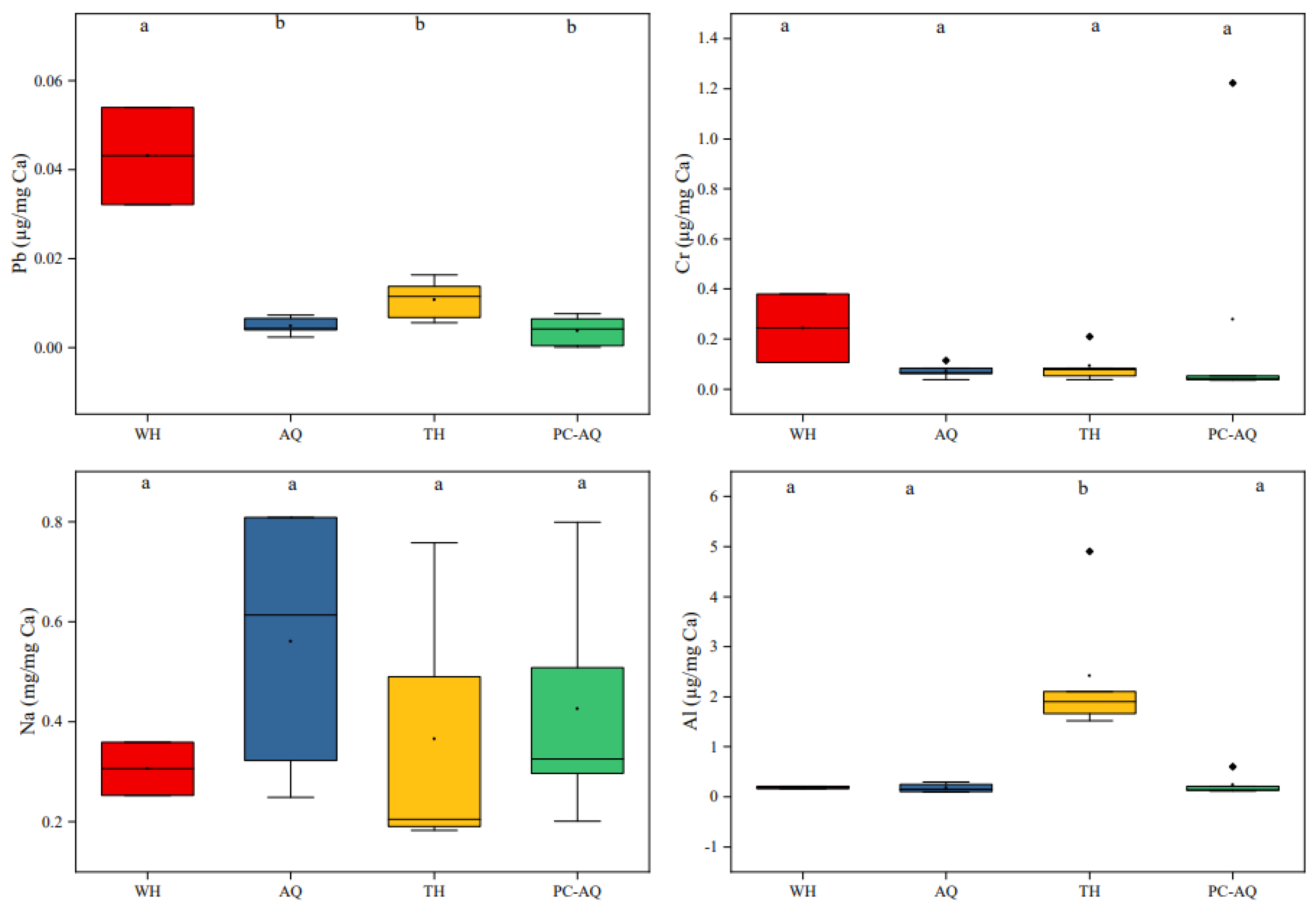
In order to further confirm whether the four selected discriminative element ratios can be used to determine the unknown source samples, the positive controls of PC-AQ, and the elements ratios from three stations of WH, AQ and TH were analyzed (
Figure 4). The Pb/Ca ratios in WH was significantly higher than that in other groups, the Al/Ca ratios in TH was significantly higher than that in other groups, the Cr/Ca and Na/Ca ratios showed no significant difference among these four groups. There was no significant difference of these four discriminative elements ratios between AQ and PC-AQ. It can be seen that the WH samples can be distinguished by Pb element ratios from these four groups, and the TH samples can be distinguished by Al element ratios, while the composition of the four discriminative elements between AQ and PC-AQ is relatively consistent, which is expected to identify and trace the source of samples from different sources by the composition differences of these four discriminative elements ratios.
3.4. Traceability and Verification Analysis
In order to further verify the source of the newly collected samples, LDA of the four discriminant elements ratios of
H. nobilis muscle from three stations in the Yangtze River basin and the PC-AQ group were performed, and scatter plots of discrimination (
Figure 5).
Figure 5 clearly showed that the PC-AQ group and AQ group are fused into one group, so PC-AQ group were included into the AQ group for further verification in the subsequent discriminant analysis, and the overall discriminant success rate of the total samples from AQ was obtained to be 100% (
Table 5). The results showed that, with the addition of the PC group, the overall discrimination success rate of 17
H. nobilis individuals from the three stations was still 100.00%. The results of cross verification were consistent with the results of stepwise discrimination (
Table 5). It can be seen that the new samples can be effectively traced by these four discriminant elements ratios that have been screened.
4. Discussion
In this study, based on the biological characteristics and ecological habits of
H. nobilis [
1], muscle tissue was selected as the research material to analyze the elemental composition in the muscle of
H. nobilis from different stations in the Yangtze River basin. The differential elemental composition of
H. nobilis muscle was preliminarily screened, and a few key discriminative elements were further screened through discriminant analysis methods for traceability and judgment of new source
H. nobilis samples. In this study, due to the large size of
H. nobilis, it is difficult to obtain whole body and otoliths [
1,
34,
35], as well as the presence of incomplete individuals in the market, and it is not suitable to obtain whole body and otolith samples. Therefore, compared to larger whole body and small otoliths, muscle tissue has a smaller sample size and easier sample acquisition, which can better meet the sampling needs of market regulation and is the best research materials.
There are various discrimination and identification methods for samples from different sources or populations, including individual morphology, otolith morphology, isotopes, fatty acids, molecular methods, etc [
4,
5,
6,
7,
8]. However, the above methods have some shortcomings in the discrimination and traceability analysis for different sources of
H. nobilis in this study. For example, using molecular analysis methods [
36] to identify population differences are not entirely effective due to the similarity for the same species. Traditional otolith microchemistry analysis can differentiate fish populations from different regions and is relatively stable and unaffected by external factors [
5]. However, this method is usually applied to the study of populations with different habitat records [
37,
38]. Moreover, the cost of otolith detection is high, and complicated pretreatment is needed [
39]. In contrast, EFA used in this study provides a more accurate indicator for distinguishing river sections based on the accumulation of elements in muscles from the environment during growth [
40]. Additionally, the cost of muscle element analysis is lower, requiring only simple pre-treatments [
12]. Therefore, based on the stability of element accumulation in muscles and the unique advantages of EFA in sample processing and analysis, this study selected this method to trace and determine the source of samples from different stations.
In order to further explore the reasons for the successful use of the muscle EFA method to trace and verify the sources of different
H. nobilis samples, the HCA was conducted on the identified 4 discriminative element ratios in the muscles of
H. nobilis from different stations. The results showed the minimum distance was 0.042 between WH and AQ, while the maximum distance was 5.005 between WH and TH (
Table S2). The cluster dendrogram showed that the difference between WH and AQ was relatively small, while TH exhibited greater differences from the other two stations (
Figure S2). It is possibly due to the fact that WH and AQ are sections of the Yangtze River that are open and interoperable with each other, with fewer differences, whereas TH is a relatively closed lake with greater differences from the other two stations (
Figure 1). For the reasons of the differences, we can discuss them through the elemental content characteristics of the watersheds. Some studies pointed out that Pb pollution is mainly distributed in the middle reaches of the Yangtze River [
41,
42], so the Pb contents in the muscle of
H. nobilis from WH in the middle reaches of the Yangtze River is greater than that from the other two stations. The reason for the higher Al content in TH may be the developed Al industry in Wuxi and the surrounding areas, with bauxite smelters and aluminum manufacturing plants around the TH watershed, discharging industrial wastewater with a high Al content [
43,
44]. The study of the source distribution and composition of heavy metals in the sediments of the lower Yangtze River and estuaries showed that the concentration of Hg in AQ section is relatively high compared to the other two stations [
45]. Through above analysis, it was found that the differences between samples from different sources are related to the ecological habits of
H. nobilis and the geographical distribution of the key elements composition in different stations.
Overall, in this study, the EFA method can be used to determine and trace the origin of H. nobilis from different sources. This is closely related to the geographical distribution characteristics of the sampling stations for H. nobilis in the Yangtze River basin, the biological and ecological habits of H. nobilis, and the stability of accumulated discriminative elements in H. nobilis muscles. On this basis, a series of methods such as, One-way ANOVA, PCA, LDA, and HCA in multivariate analysis were used to screen out a few key discriminant elements for traceability and validation of H. nobilis samples from different sources, ensuring the credibility and effectiveness of the discriminant results. At the same time, by selecting a small number of key discriminative elements from multiple elemental compositions and successfully applying them to the discriminative verification of new source samples, the simplicity of the analysis method and its operability in market supervision applications are ensured, which has important practical value.
5. Conclusions
This study utilized multidimensional statistical analysis to investigate the differences of 19 elements ratios in the muscle of H. nobilis from different stations in the Yangtze River basin. The study successfully identified the main elements in the muscle tissues from three stations and selected 4 differential elements for further analysis and verification, demonstrating the feasibility and effectiveness of using muscle element fingerprints to differentiate H. nobilis from different sources. The study preliminarily identified Al, Mn, Hg, Pb as the main differential elements in the muscle of H. nobilis from three stations, with Pb and Al being the primary elements used to distinguish H. nobilis from different sources. The results of this study provide technical support and theoretical basis for the source identification of H. nobilis from different stations in the Yangtze River basin.
Supplementary Materials
The following supporting information can be downloaded at The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Results of discriminant analysis for 19 element ratios in the muscle of H. nobilis from three stations in the Yangtze River basin; Table S2: Euclidean distance among 4 discriminant elements ratios in the muscles of H. nobilis from three stations in the Yangtze River basin. Figure S1: Scatter plot of scores based on the first two canonical discriminant functions for the 19 elements ratios in the muscles of H. nobilis from three stations in the Yangtze River basin; Figure S2: Clustering dendrogram for 4 discriminant elements ratios in the muscles of H. nobilis from three stations in the Yangtze River basin. Samples from AQ station include AQ group and PC-AQ group.
Author Contributions
Conceptualization and writing—original draft, C.S. and C.Y.; writing—review and editing, C.S., C.Y., F.Z. and H.T.; investigation and methodology, J.X. and X.H.; resources and funding acquisition, C.S., F.Z. and P.Z.; validation and supervision, H.T. and P. Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program Key Special Project (2022YFF0608203) and Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD14).
Institutional Review Board Statement
The experiments comply with current laws of China. All the samples in this study were obtained from legal commercial fisheries, and the samples were dead when they were obtained.
Data Availability Statement
The data presented in this study are available in the article. Further information is available upon request from the corresponding author.
Acknowledgments
The authors acknowledge researcher Yang, J. and associate researcher Jiang, T. from the Key Laboratory of Fishery Ecological Environment Assessment and Resource Conservation in the Middle and Lower Reaches of the Yangtze River, Chinese Academy of Fishery Sciences for providing the experimental materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G.; Zhao, F.; Feng, G.P.; Liu, J.Y.; Huang, X.R. Fishes of the Yangtze Estuary. Beijing, China Agriculture Press, 2018, 124-127.
- Wang, D.; Wu; F.X.; Song; D.D.; Gao, H.Q. China Fisheries Statistical Yearbook, compiled by Fishery and Fisheries Administration of the Ministry of Agriculture and Rural Affairs, National Aquatic Technology Promotion Station and China Fisheries Society compile. China Agriculture Press, Beijing, 2023, 30.
- Farrington, H.L.; Edwards, C.E.; Guan, X.; Carr, M.R.; Baerwaldt, K.; Lance, R.F. Mitochondrial Genome Sequencing and Development of Genetic Markers for the Detection of DNA of Invasive Bighead and Silver Carp (Hypophthalmichthys nobilis and H. molitrix) in Environmental Water Samples from the United States. PLoS ONE 2015, 10, e0117803. [Google Scholar] [CrossRef]
- Kumar, R.; Jaiswar, A.K.; Sharma, R.; Prasad, L. Quantification of morphological variations among populations of Channa gachua (Hamilton, 1822) from different geographical locations in India. Indian J. Fish. 2020, 67, 114–119. [Google Scholar] [CrossRef]
- Milosevic, D.; Bigovic, M.; Mrdak, D.; Milasevic, I.; Piria, M. Otolith morphology and microchemistry fingerprints of European eel, Anguilla anguilla (Linnaeus, 1758) stocks from the Adriatic Basin in Croatia and Montenegro. Sci. Total Environ. 2021, 786, 147478. [Google Scholar] [CrossRef]
- Astorga, M.P.; Valenzuela, A.; Segovia, N.I.; Poulin, E.; Vargas-Chacoff, L.; Gonzalez-Wevar, C.A. Contrasting Patterns of Genetic Diversity and Divergence Between Landlocked and Migratory Populations of Fish, Evaluated Through Mitochondrial DNA Sequencing and Nuclear DNA Microsatellites. Front. Genet. 2022, 13, 854362. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Wang, W.X. Trace elemental and stable isotopic signatures to reconstruct the large-scale environmental connectivity of fish populations. Mar. Ecol. Prog. Ser. 2024, 730, 95–111. [Google Scholar] [CrossRef]
- Bai, S.Y.; Qin, D.L.; Chen, Z.X.; Wu, S.; Tang, S.Z.; Gao, L.; Wang, P. Geographic origin discrimination of red swamp crayfish Procambarus clarkia from different Chinese regions using mineral element analysis assisted by machine learning techniques. Food Control 2022, 138, 109047. [Google Scholar] [CrossRef]
- Ben Ghorbel, M.; Mejri, M.; Adjibayo, H.M.F.; Chalh, A.; Quignard, J.P.; Trabelsi, M.; Bouriga, N. Use of otolith microchemical and morphological analyses for stock discrimination of Sarpa salpa on two Tunisian islands, Djerba and Kerkennah. J. Mar. Biol. Assoc. UK 2024, 104, e33. [Google Scholar] [CrossRef]
- Ricardo, F.; Lopes, M.L.; Mamede, R.; Domingues, M.R.; da Silva, E.F.; Patinha, C.; Calado, R. Combined Use of Fatty Acid Profiles and Elemental Fingerprints to Trace the Geographic Origin of Live Baits for Sports Fishing: The Solitary Tube Worm (Diopatra neapolitana, Annelida, Onuphidae) as a Case Study. Animals 2024, 14, 1361. [Google Scholar] [CrossRef]
- Duarte, B.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Tanner, S.E.; Fonseca, V.F. Elemental fingerprinting of thornback ray (Raja clavata) muscle tissue as a tracer for provenance and food safety assessment. Food Control 2022, 133, 108592. [Google Scholar] [CrossRef]
- Mamede, R.; Duarte, I.A.; Cacador, I.; Tanner, S.E.; Silva, M.; Jacinto, D.; Fonseca, V.F.; Duarte, B. Elemental fingerprinting of sea urchin (Paracentrotus lividus) gonads to assess food safety and trace its geographic origin. J. Food Compos. Anal. 2022, 114, 104764. [Google Scholar] [CrossRef]
- Ricardo, F.; Mamede, R.; Bruzos, A.L.; Díaz, S.; Thébault, J.; da Silva, E.F.; Patinha, C.; Calado, R. Assessing the elemental fingerprints of cockle shells (Cerastoderma edule) to confirm their geographic origin from regional to international spatial scales. Sci. Total Environ. 2022, 814, 152304. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zhao, L.J.; Fan, Y.C.; Qu, X.C.; Liu, D.Y.; Guo, Z.L.; Wang, Y.H.; Liu, Q.; Chen, Y.S. Using whole body elemental fingerprint analysis to distinguish different populations of Coilia nasus in a large river basin. Biochem. System. Ecol. 2015, 60, 249–257. [Google Scholar] [CrossRef]
- Davis, R.P.; Boyd, C.E.; Godumala, R.; Mohan, A.B.C.; Gonzalez, A.; Duy, N.P.; Sasmita, P.G.; Ahyani, N.; Shatova, O.; Wakefield, J.; Harris, B.; McNevin, A.A.; Davis, D.A. Assessing the variability and discriminatory power of elemental fingerprints in whiteleg shrimp Litopenaeus vannamei from major shrimp production countries. Food Control 2022, 133, 108589. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Fréchet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Kingsford, M.J. Elemental fingerprints of otoliths of fish may distinguish estuarine 'nursery' habitats. Mar. Ecol. Prog. Ser. 2000, 201, 273–286. [Google Scholar] [CrossRef]
- D'Avignon, G.; Rose, G.A. Otolith elemental fingerprints distinguish Atlantic cod spawning areas in Newfoundland and Labrador. Fish. Res. 2013, 147, 1–9. [Google Scholar] [CrossRef]
- Avigliano, E.; Domanico, A.; Sánchez, S.; Volpedo, A.V. Otolith elemental fingerprint and scale and otolith morphometry in Prochilodus lineatus provide identification of natal nurseries. Fish. Res. 2017, 186, 1–10. [Google Scholar] [CrossRef]
- Coussau, L.; Robert, D.; Sirois, P. Spatiotemporal variability in otolith elemental fingerprint and the potential to determine deepwater redfish (Sebastes mentella) origins and migrations in the Estuary and Gulf of St. Lawrence, Canada. Fish. Res. 2023, 265, 106739. [Google Scholar] [CrossRef]
- Bassi, L.; Tremblay, R.; Morissette, O.; Sirois, P. Otolith elemental fingerprints reveal source-sink dynamics between two Greenland halibut nurseries in the St. Lawrence Estuary and Gulf. Mar. Ecol. Prog. Ser. 2024, 731, 217–229. [Google Scholar] [CrossRef]
- Khan, S.; Schilling, H.T.; Khan, M.A.; Patel, D.K.; Maslen, B.; Miyan, K. Stock delineation of striped snakehead, Channa striata using multivariate generalised linear models with otolith shape and chemistry data. Sci. Rep. 2021, 11, 8158. [Google Scholar] [CrossRef] [PubMed]
- Mamede, R.; Santos, A.; Díaz, S.; da Silva, E.F.; Patinha, C.; Calado, R.; Ricardo, F. Elemental fingerprints of bivalve shells (Ruditapes decussatus and R. philippinarum) as natural tags to confirm their geographic origin and expose fraudulent trade practices. Food Control 2022, 135, 108785. [Google Scholar] [CrossRef]
- Mamede, R.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Canada, P.; Ré, P.; Tanner, S.E.; Fonseca, V.F.; Duarte, B. Elemental Fingerprinting of Wild and Farmed Fish Muscle to Authenticate and Validate Production Method. Foods 2022, 11, 3081. [Google Scholar] [CrossRef] [PubMed]
- Mamede, R.; Santos, A.; da Silva, E.F.; Patinha, C.; Calado, R.; Ricardo, F. New evidence of fraudulent mislabeling and illegal harvesting of Manila clams (philippinarum) through elemental fingerprints of their shells and chemometric analyses. Food Control 2024, 163, 110501. [Google Scholar] [CrossRef]
- Orlowski, G.; Niedzielski, P.; Karg, J.; Proch, J. Colour-assisted variation in elytral ICP-OES-based ionomics in an aposematic beetle. Sci Rep 2020, 10, 22262. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, F.; Gornati, G.; Biancolillo, A.; D'Archivio, A.A. ICP-OES analysis coupled with chemometrics for the characterization and the discrimination of high added value Italian Emmer samples. J. Food Compos. Anal. 2021, 98, 103842. [Google Scholar] [CrossRef]
- Lossow, K.; Schlörmann, W.; Tuchtenhagen, M.; Schwarz, M.; Schwerdtle, T.; Kipp, A.P. Measurement of trace elements in murine liver tissue samples: Comparison between ICP-MS/MS and TXRF. J. Trace Elem. Med. Bio. 2023, 78, 127167. [Google Scholar] [CrossRef]
- Güven, G. Testing the equality of treatment means in one-way ANOVA: Short-tailed symmetric error terms with heterogeneous variances. Hacet. J. Math. Stat. 2022, 51, 1736–1751. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, F.; Yang, Q.L. Origin traceability of peanut kernels based on multi-element fingerprinting combined with multivariate data analysis. J. Sci. Food Agr. 2020, 100, 4040–4048. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists. BKS Press, 2011.
- Legendre, P.; Legendre, L. Numerical Ecology, Elsevier, 2012.
- Anderson, C.L.; Whitledge, G.W.; Rude, N.P.; Lamer, J.T. Using Otolith Chemistry to Determine Early Life Environments and Movement of the Emerging Bigheaded Carp Population in Pools 16-19 of the Upper Mississippi River. N. Am. J. Fish. Manage. 2023, 43, 126–140. [Google Scholar] [CrossRef]
- Whitledge, G.W.; Knights, B.; Vallazza, J.; Larson, J.; Weber, M.J.; Lamer, J.T.; Phelps, Q.E.; Norman, J.D. Identification of Bighead Carp and Silver Carp early-life environments and inferring Lock and Dam 19 passage in the Upper Mississippi River: insights from otolith chemistry. Biol. Invasions 2019, 21, 1007–1020. [Google Scholar] [CrossRef]
- Domínguez-Contreras, J.F.; Jiménez-Rosenberg, S.P.A.; Reguera-Rouzaud, N.; Sánchez-Velasco, L.; Díaz-Viloria, N. Domínguez-Contreras, J.F.; Jiménez-Rosenberg, S.P.A.; Reguera-Rouzaud, N.; Sánchez-Velasco, L.; Díaz-Viloria, N. Molecular identification and first morphological description of the Colorado snapper Lutjanus colorado (Perciformes: Lutjanidae) larvae. J. Fish Biol. 2023, 102, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, H.B.; Hu, Y.H.; Chen, X.B.; Yang, J. Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry. Fishes 2022, 7, 147. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Yang, J. Otolith microchemistry and microsatellite DNA provide evidence for divergence between estuarine tapertail anchovy (Coilia nasus) populations from the Poyang Lake and the Yangtze River Estuary of China. Reg. Stud. Mar. Sci. 2022, 56, 102649. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Feng, G.; Yang, J.; Zhao, F.; Shen, C.; Song, C.; Jiang, T. Otolith Microchemistry Assessment: Evidence of Migratory Coilia Nasus of Yangtze River Living in the Shengsi Sea Area. Fishes 2022, 7, 7040172. [Google Scholar] [CrossRef]
- Lehel, J.; Plachy, M.; Palotás, P.; Bartha, A.; Budai, P. Possible Metal Burden of Potentially Toxic Elements in Rainbow Trout (Oncorhynchus mykiss) on Aquaculture Farm. Fishes 2024, 9, 9070252. [Google Scholar] [CrossRef]
- Liu, P.C.; Ma, Z.H.; Wei, P.G.; Zhao, Y.X.; Liu, H.L. Progress of Researches on Heavy Metal Pollution Characteristics and Comprehensive Prevention and Control in the Yangtze River Basin. Ecol. Environ. Monitor. Three Gorge 2018, 3, 33–37. [Google Scholar]
- Guo, J.; Wang, K.; Yu, Q.; Duan, X.; Liu, S.; Chen, D. Pollution characteristics of the heavy metals and their potential ecological risk assessment in nearshore sediments of the middle reaches of the Yangtze River. Acta Sci. Circum. 2021, 41, 4625–4636. [Google Scholar]
- Pan, L.; Zhang, R.B.; Pan, Z.X.; Xi, D.G.; Wang, L.Y. Process for Deep Nitrogen and Phosphorus Removal of Tail water from Sewage Treatment Facilities in the Taihu Lake Basin. China Environ. Protec. Ind. 2020, 50–52. [Google Scholar]
- Wu, K.Q.; Meng, Y.H.; Gong, Y.; Wu, L.L.; Liu, W.W.; Ding, X.L. Drinking water elements constituent profiles and health risk assessment in Wuxi, China. Environ. Monit. Assess. 2022, 194, 106. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.M.; Hu, W.Y.; Tian, K.; Huang, B.; Zhao, Y.C. Comparison of heavy metals in riverine and estuarine sediments in the lower Yangtze River: Distribution, sources, and ecological risks. Environ. Techno. Inno. 2023, 30, 103076. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).