Submitted:
04 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Bromatological characterization
2.3. Quantification of Metabolites
2.4. Phenolic Profile
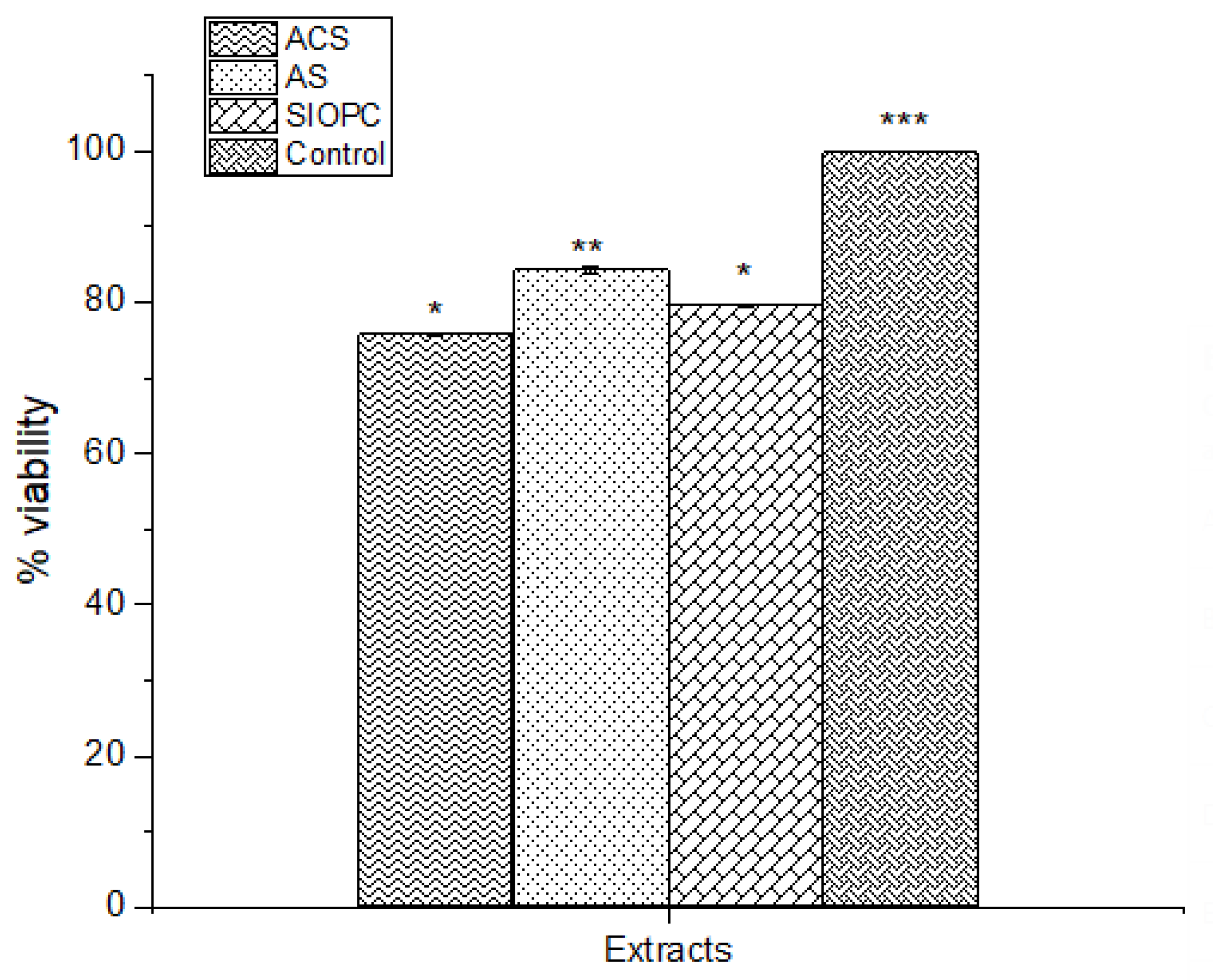
2.5. Cytotoxic Activity: Cell Viability Reduction of MTT in Leukocytes
2.6. Antioxidant Activity (AOX)
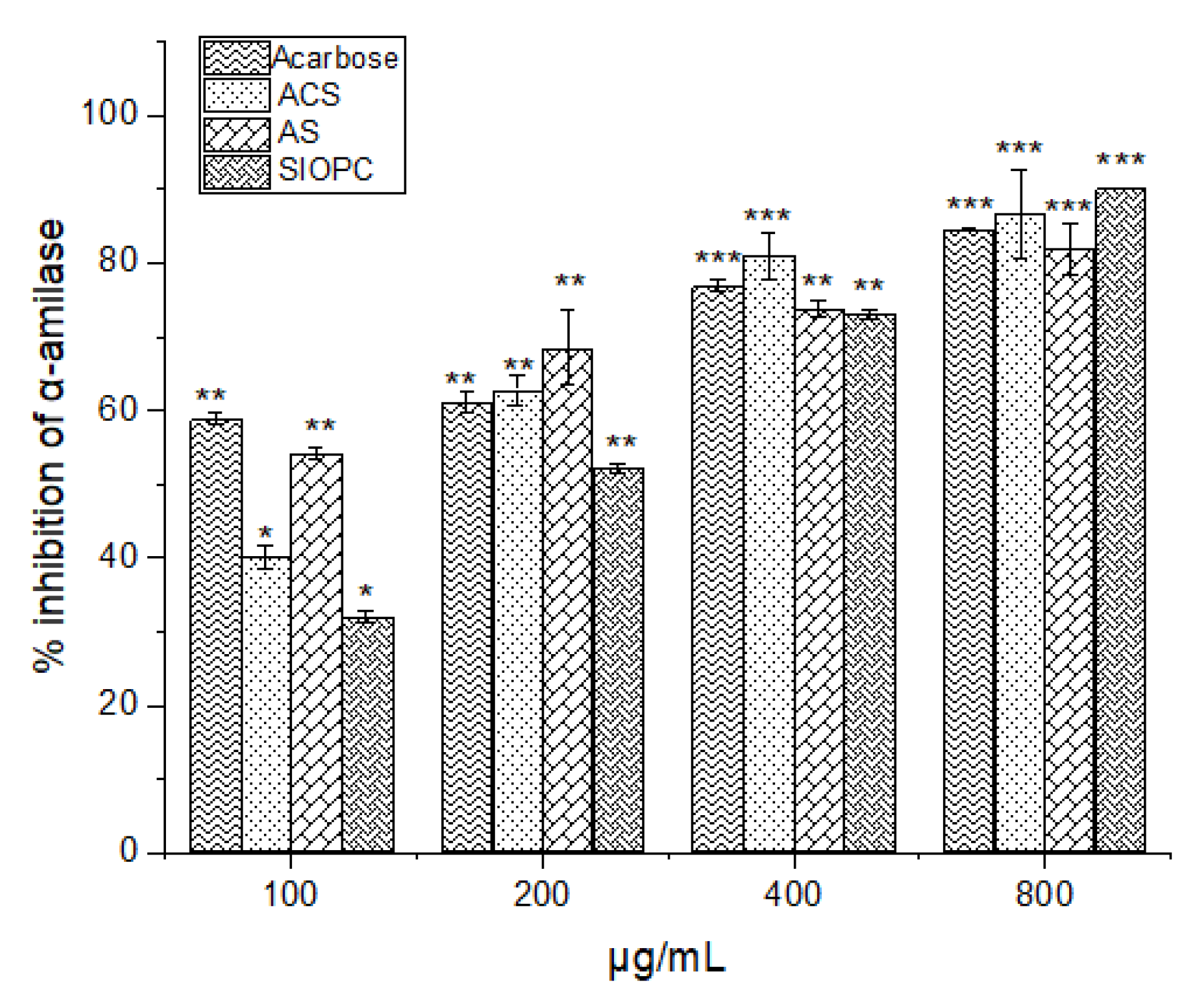
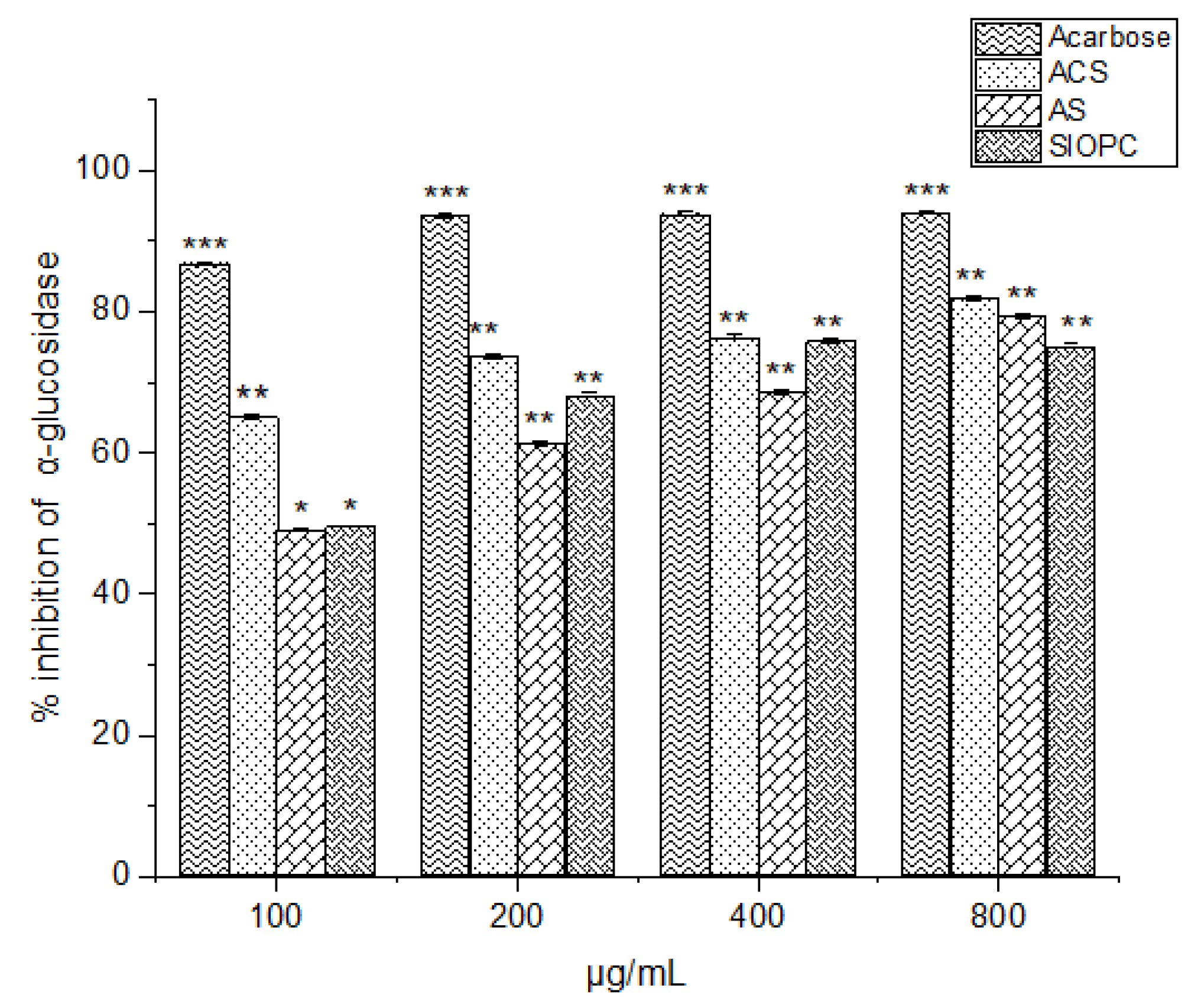
2.7. Hypoglycemic Activity
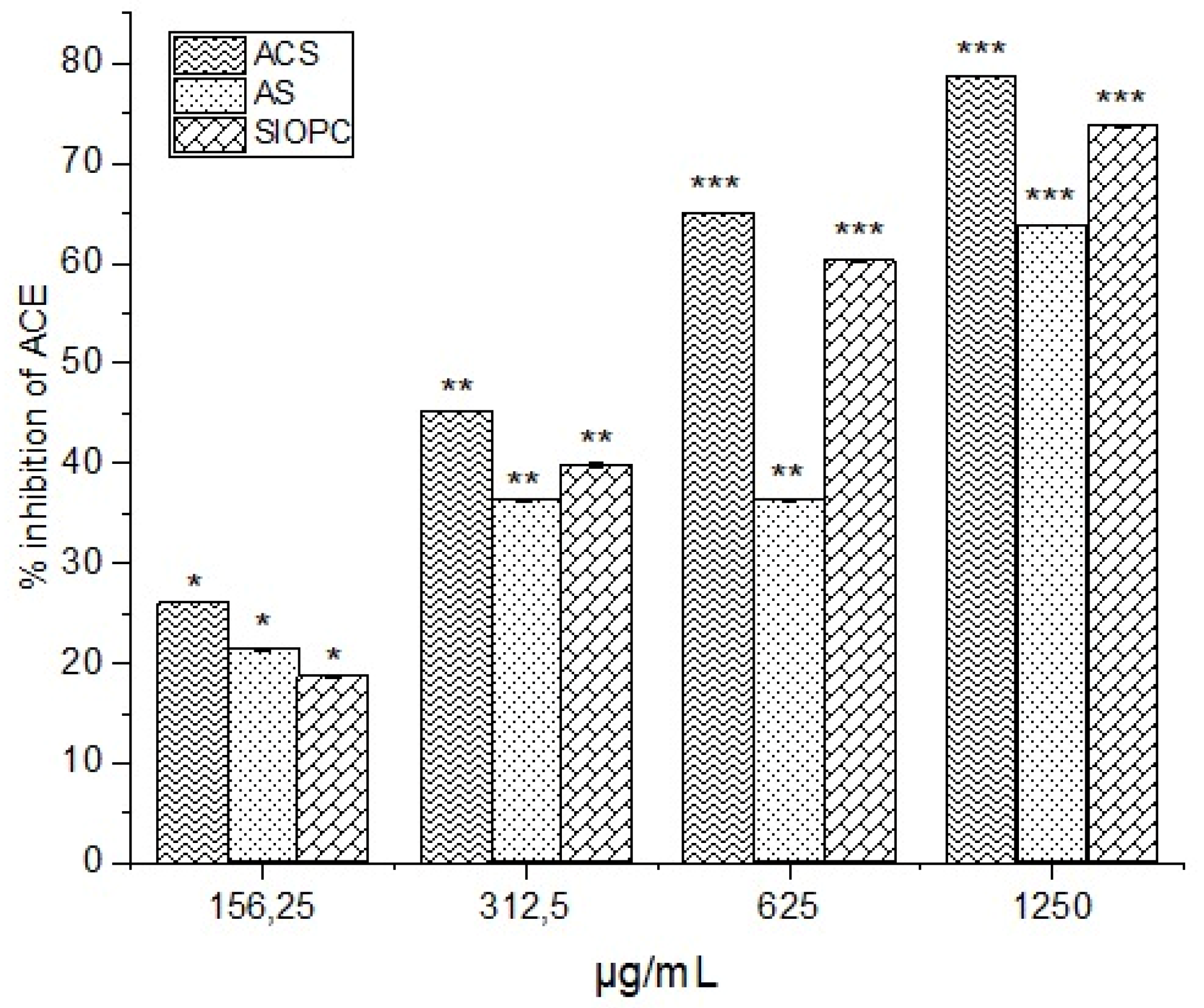
2.8. Inhibition of ACE In Vitro
3. Discussion
4. Materials and Methods
4.1. Extract Preparation
4.2. Nutritional Value and Mineral Element Composition
4.3. Characterization and Quantification of Metabolites
4.3.1. Analysis of Phenolic Profile
4.3.2. Qualitative Chemical
4.3.3. Quantification of Metabolites
- ○
- Carbohydrate Quantification: Total carbohydrates were quantified using the Antrone method[33]. An Antrone reagent was prepared by dissolving 0.2 g of Antrone in 100 mL of concentrated sulfuric acid (98%). An anhydrous glucose curve was used as the standard, and absorbance was measured at 625 nm.
- ○
- Total Carotenoids: Carotenoids were extracted using a hexane-acetone solution. β-carotene was used as a standard to create a calibration curve. Absorbance was measured at 450 nm.
4.4. Bioactivities
4.4.1. Cytotoxicity: Cell Viability Measurement Using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide, a Tetrazole) Assay
4.4.2. Antioxidant Activity (AOX)
ABTS Radical Cation Decolorization Assay
DPPH Radical Cation Decolorization Assay
4.4.3. Hypoglycemic activity
Inhibitory Activity of α-Glucosidase Enzyme
Inhibitory Activity of α-Amylase Enzyme
4.4.4. Inhibition of ACE in vitro
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- B. A. Rojano, I. C. Zapata Vahos, A. F. Alzate Arbeláez, A. J. Mosquera Martínez, F. B. Cortés Correa, and L. Gamboa Carvajal, "Polifenoles y actividad antioxidante del fruto liofilizado de palma naidi (açai colombiano)(Euterpe oleracea Mart)," Revista Facultad Nacional de Agronomía Medellín, vol. 64, no. 2, pp. 6213-6220, 2011.
- I. A. Neri-Numa et al., "Evaluation of the antioxidant, antiproliferative and antimutagenic potential of araçá-boi fruit (Eugenia stipitata Mc Vaugh—Myrtaceae) of the Brazilian Amazon Forest," Food Research International, vol. 50, no. 1, pp. 70-76, 2013.
- L. Barros et al., "The powerful in vitro bioactivity of Euterpe oleracea Mart. seeds and related phenolic compounds," Industrial Crops and Products, vol. 76, pp. 318-322, 2015.
- P. S. Melo, M. M. Selani, R. H. Gonçalves, J. de Oliveira Paulino, A. P. Massarioli, and S. M. de Alencar, "Açaí seeds: An unexplored agro-industrial residue as a potential source of lipids, fibers, and antioxidant phenolic compounds," Industrial crops and products, vol. 161, p. 113204, 2021.
- Álvarez, A.; Jiménez, .; Méndez, J.; Murillo, E. Chemical and biological study of Eugenia Stipitata mc vaugh collected in the Colombian Andean region. Asian J. Pharm. Clin. Res. 2018, 11, 362–369. [CrossRef]
- R. Chirinos, G. Zuloeta, R. Pedreschi, E. Mignolet, Y. Larondelle, and D. Campos, "Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity," Food chemistry, vol. 141, no. 3, pp. 1732-1739, 2013.
- E. G. Torres Sánchez, B. Hernández-Ledesma, and L.-F. Gutiérrez, "Sacha inchi oil press-cake: physicochemical characteristics, food-related applications and biological activity," Food Reviews International, vol. 39, no. 1, pp. 148-159, 2023.
- Lima, R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Health from Brazilian Amazon food wastes: Bioactive compounds, antioxidants, antimicrobials, and potentials against cancer and oral diseases. Crit. Rev. Food Sci. Nutr. 2022, 63, 12453–12475. [CrossRef]
- Chaiyama, V.; Keawsompong, S.; LeBlanc, J.G.; LeBlanc, A.d.M.d.; Chatel, J.-M.; Chanput, W. Action modes of the immune modulating activities of crude mushroom polysaccharide from Phallus atrovolvatus. Bioact. Carbohydrates Diet. Fibre 2020, 23, 100216. [CrossRef]
- Araujo, N.M.P.; Arruda, H.S.; Farias, D.d.P.; Molina, G.; Pereira, G.A.; Pastore, G.M. Plants from the genus Eugenia as promising therapeutic agents for the management of diabetes mellitus: A review. Food Res. Int. 2021, 142, 110182. [CrossRef]
- V. M. Alves et al., "Provenient residues from industrial processing of açaí berries (Euterpe precatoria Mart): nutritional and antinutritional contents, phenolic profile, and pigments," Food Science and Technology, vol. 42, p. e77521, 2022.
- Rawdkuen, S.; Murdayanti, D.; Ketnawa, S.; Phongthai, S. Chemical properties and nutritional factors of pressed-cake from tea and sacha inchi seeds. Food Biosci. 2016, 15, 64–71. [CrossRef]
- D. Vásquez-Osorio, G. A. Hincapié-Llanos, M. Cardona, D. I. Jaramillo, and L. Vélez-Acosta, "Formulación de una colada empleando harina de Sancha Inchi (Plukenetia Volubilis L.) proveniente del proceso de obtención de aceite," Perspectivas en nutrición humana, vol. 19, no. 2, pp. 167-179, 2017.
- Chirinos, R.; Aquino, M.; Pedreschi, R.; Campos, D. Optimized Methodology for Alkaline and Enzyme-Assisted Extraction of Protein from Sacha Inchi (Plukenetia volubilis) Kernel Cake. J. Food Process. Eng. 2016, 40. [CrossRef]
- Torres-Sánchez, E.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake. Foods 2023, 12, 2401. [CrossRef]
- D. C. V. Osorio, J. D. J. Ramírez, G. A. H. Llanos, and L. M. V. Acosta, "Desarrollo de galletas empleando harina de Sacha Inchi (Plukenetia volubilis l.) obtenida de la torta residual," UGCiencia, vol. 23, pp. 101-113, 2017.
- S. Vélez Pérez, "Exploración de la sacha inchi (Plukenetia volubilis) como fuente de proteína para uso en nutrición animal en Colombia," Universidad EAFIT, 2013.
- F. F. de Araújo et al., "Chemical characterization of Eugenia stipitata: A native fruit from the Amazon rich in nutrients and source of bioactive compounds," Food Research International, vol. 139, p. 109904, 2021.
- H. Escalante, J. Orduz, H. Zapata, M. Cardona, and M. Ortega, "Atlas Del Potencial Energético de La Biomasa Residual En Colombia; Universidad Industrial de Santander: Bucaramanga, Colombia, 2011," Google Scholar.
- da Cunha, F.A.B.; Waczuk, E.P.; Duarte, A.E.; Barros, L.M.; Elekofehinti, O.O.; Matias, E.F.F.; da Costa, J.G.M.; Sanmi, A.A.; Boligon, A.A.; da Rocha, J.B.T.; et al. Cytotoxic and antioxidative potentials of ethanolic extract of Eugenia uniflora L. (Myrtaceae) leaves on human blood cells. Biomed. Pharmacother. 2016, 84, 614–621. [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [CrossRef]
- A. E. Martin and P. A. Montgomery, "Acarbose: An α-glucosidase inhibitor," American journal of health-system pharmacy, vol. 53, no. 19, pp. 2277-2290, 1996.
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [CrossRef]
- Li, H.; Yang, J.; Wang, M.; Ma, X.; Peng, X. Studies on the inhibition of α-glucosidase by biflavonoids and their interaction mechanisms. Food Chem. 2023, 420, 136113. [CrossRef]
- G. F. de Bem et al., "Antidiabetic effect of Euterpe oleracea Mart.(açaí) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction," PLoS One, vol. 13, no. 6, p. e0199207, 2018.
- Pallerla, P.; Posani, H. IN-VITRO ANTIDIABETIC ACTIVITY OF STANDARDIZED ACAIBERRY EXTRACT. J. Adv. Sci. Res. 2022, 13, 41–45. [CrossRef]
- A. G. Schauss, "Advances in the study of the health benefits and mechanisms of action of the pulp and seed of the Amazonian palm fruit, Euterpe oleracea Mart., known as “Açai”," Fruits, Vegetables, and Herbs, pp. 179-220, 2016.
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae 2022, 8, 344. [CrossRef]
- S. Suwanangul, M. A. Alashi, R. E. Aluko, W. Tochampa, and K. Ruttarattanamongkol, "Inhibition of α-amylase, α-glucosidase and pancreatic lipase activities in vitro by sacha inchi (Plukenetia volubilis L.) protein hydrolysates and their fractionated peptides," Maejo International Journal of Science & Technology, vol. 15, no. 1, 2021.
- J. Harborne, "Methods of plant analysis," in Phytochemical methods: a guide to modern techniques of plant analysis: Springer, 1973, pp. 1-32.
- P. Temviriyanukul et al., "The Effect of Sacred Lotus (Nelumbo nucifera) and Its Mixtures on Phenolic Profiles, Antioxidant Activities, and Inhibitions of the Key Enzymes Relevant to Alzheimer’s Disease," Molecules, vol. 25, no. 16, p. 3713, 2020.
- Dimler, R.J.; Schaefer, W.C.; Wise, C.S.; Rist, C.E. Quantiative Paper Chromatography of D-Glucose and Its Oligosaccharides. Anal. Chem. 1952, 24, 1411–1415. [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid Determination of Polyphenols and Vitamin C in Plant-Derived Products. J. Agric. Food Chem. 2005, 53, 1370–1373. [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and Antiproliferative Activities of Raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [CrossRef]
- Van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [CrossRef]
- C. G. Conde, X. Y. Rueda, and G. G. S. Patiño, "Evaluación de la actividad antioxidante del aceite esencial foliar de Calycolpus moritzianus y Minthostachys mollis de Norte de Santander," Bistua: Revista de la Facultad de Ciencias Básicas, vol. 10, no. 1, pp. 12-23, 2012.
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [CrossRef]
- P. Thangaraj, "Anti-diabetic Activity," in Pharmacological Assays of Plant-Based Natural Products, T. Parimelazhagan Ed. Cham: Springer International Publishing, 2016, pp. 139-143.
| Seeds | ACS | AS | SIOPC |
|---|---|---|---|
| % Ash | 2,3 | 0,82 | 3,2 |
| %Crude protein | 5,04 | 4,96 | 8,1 |
| Ethereal extract | 4,43 | 0,16 | 33,9 |
| % Crude fiber | 48,9 | 2,27 | 13,5 |
| % Nitrogen | 0,5 | 0,78 | 1,3 |
| % Ca | 0,21 | 0,16 | 0,57 |
| % Mg | 0,03 | 2,27 | 0,17 |
| Na (mg/kg) | <0,03 | 1,02 | 75,5 |
| % K | 0,10 | 0,01 | 0,43 |
| Fe (mg/kg) | 22,7 | 20,2 | 34,5 |
| Cu (mg/kg) | 6,0 | 0,07 | 7,6 |
| Mn (mg/kg) | 141,2 | 21,6 | 7,4 |
| Zn (mg/kg) | 16,0 | 14,0 | 26,4 |
| B (mg/kg) | <0,03 | 5,51 | 31,3 |
| % P | 0,08 | 13,5 | 0,5 |
| % S | 0,04 | <0,03 | 0,05 |
| Carbohydrates μg glucose/100 mg dry weight | 45,39 ± 0,1 | 13,80 ± 0,1 | 21,07 ± 0,1 |
|
Polyphenols μg gallic acid/100 mg dry weight |
99,32 ± 8,87 | 155,88 ± 6,12 | 110 ± 2,61 |
|
Flavonoids μg quercetin/100 mg dry weight |
74,77 ± 1,29 | 41,68 ± 8,13 | 92,11 ± 4,52 |
|
ß- Carotenoids μg ß-carotene/100 mg dry weight |
0,31 ± 1,2 | 43,66 ± 1,5 | 0,38 ± 1,3 |
| Phenolic compounds | ACS | AS | SIOPC |
|---|---|---|---|
| Concentrations (µg/100 g DW) | |||
| Catechin | 1.78 ± 0.14a | 90.02 ± 2.51ª | 101.79 ± 7.11ª |
| Quercetin | 2.11 ± 0.23b | ND | 84.08 ± 2.35b |
| Myricetin | 4.56 ± 0.11c | ND | ND |
| Kaempferol | 34.90 ± 1.02d | ND | ND |
| Caffeic acid | 12.93 ± 1.47e | 10.57 ± 1.05b | 35.37 ± 6.02c |
| Syringic acid | ND | ND | ND |
| p-Coumaric acid | ND | 38.27 ± 1.13c | 127.64 ± 4.43d |
| Ferulic acid | 0.83 ± 0.37f | ND | 148.42 ± 8.26e |
| Seeds | DPPH | ABTS |
|---|---|---|
| IC50 | IC50 | |
| ACS | 52,37 ± 1,4a | 21,31 ± 1,6b |
| AS | 70,78 ± 1,8b | 13,09 ± 1,7a |
| SIOPC | 7212,85 ± 2,4c | 858,24 ± 2,5c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





