1. Introduction
Placing a nasogastric tube (NGT) into a critically ill patient admitted to an Intensive Care Unit (ICU) is a frequent nursing technique. The medical indications for NGT use are gastric emptying, abdominal tension reduction through stomach decompression, medication management, or gastric lavage procedures in poisoning cases. However, the main purpose of employing these tubes is usually for enteral nutrition (EN) administration [
1].
The latest guidelines related to critically ill patients’ nutrition from the European Society for Clinical Nutrition and Metabolism (ESPEN) [
2] and the American Society of Parenteral and Enteral Nutrition (ASPEN) [
3] recommend early EN initiation, during the first 48 hours after ICU admission whenever possible [
4]. In this sense, a European multinational study has recently concluded that providing suitable proteins and calories to critically ill patients admitted in an ICU for more than five days is related to better clinical outcomes [
5].
The NGT introduction technique is commonly performed by an intensive care nurse caring for a critically ill patient. As this procedure is performed blindly, it is essential to verify the tube position once this nursing technique is completed [
1]. Consequently, and without direct observation, possible complications related to NGT placement must be considered when performing this technique. Most complications are associated with locating the tube in the respiratory tract or the esophagus. In the first case, incidents implying the development of pneumothorax, pneumonia, chemical pneumonitis, and respiratory distress syndrome have been reported, sometimes leading to the patient’s death [
6,
7]. In the second case, an insufficiently introduced tube significantly increases the risk of bronchopulmonary aspiration, resulting in respiratory complications [
6,
7].
Regarding the methods to check proper NGT position, the “gold standard” or reference method is chest X-ray [
8,
9]. Most international guidelines strongly recommend this verification before using the tube [
10,
11]. These guidelines advise against epigastrium auscultation after air insufflation, which, despite being a widely disseminated method, does not allow to distinguish between respiratory, gastric, or intestinal locations [
12,
13]. It is also not advised to assume proper NGT position by merely inspecting gastric aspirates [
6]. Other less sensitive methods to verify the NGT position include measuring the pH of the gastric aspirate [
14], capnography [
15], or observing NGT introduction with an electromagnetic guide [
16].
Although X-ray is the most precise method to verify the position of a recently placed NGT [
11], it involves an undesirable effect, such as radiation accumulation in critically ill patients. For them, X-ray represents a strong ionizing source, potentially harmful to living tissues, with greater risk as the number of exposures increases [
17]. Therefore, in recent years the importance of using ultrasound as an alternative to X-ray in certain situations has been highlighted [
18].
Given its non-invasive nature, ultrasound presents some advantages over X-ray: it is innocuous, painless, and well-tolerated by patients. In addition, if the proper equipment is available, it is less expensive than X-ray, offering immediate access, which allows obtaining real-time images and, therefore, reducing the time for results achievement [
18].
Among the studies conducted to assess ultrasound as a method to verify NGT positioning, there are promising results in pediatric critical care [
19], prehospital critical care [
20], urgent and emergency care [
21,
22], hospitalized patients [
23], and admitted patients in ICU [
24,
25,
26], obtaining an ultrasound sensitivity varying from 85% to 100%. In this sense, it should be highlighted that physicians were the healthcare professionals who performed most of the ultrasounds in these studies. However, as NGT placement is considered a nursing technique [
1], nurses should be logically responsible for both NGT placement and its position verification.
To date, it has not been found sufficient evidence related to ultrasound use for verifying NGT positioning in intensive care nursing. Consequently, it is needed to assess the validity of ultrasound performed by an intensive care nurse when checking the NGT positioning in critically ill patients. Therefore, the objectives of our study were to analyze whether the ultrasound performed by an intensive care nurse is a valid method to verify the NGT’s correct positioning and to evaluate the degree of interobserver agreement between a nurse and an intensive care physician in the NGT visualization using ultrasound.
2. Materials and Methods
2.1. Study Design
A cross-sectional and observational pilot study was carried out to validate a diagnostic test [
26]. To control the methodological rigor and quality of this paper, the research and reporting methodology followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) [
27] criteria for cross-sectional studies.
2.2. Study Setting
The study was conducted between February and June 2023 in the ICU of the tertiary-level “La Princesa” University Hospital in Madrid (Spain), which comprises 20 beds and attends mainly respiratory, cardiac, neurological, and post-surgical patients.
2.3. Participants
The study included critically ill adult patients with an NGT introduced by an intensive care nurse following a medical indication. The patients excluded were those who: 1) did not provide their written informed consent; 2) presented contraindications for blind NGT introduction; and 3) had a Body Mass Index (BMI) >35 (obesity class 2).
The participating patients were included consecutively. Ultrasound verifications of the NGT positioning were performed successively, first by an intensive care nurse and then by an intensive care physician.
2.4. Variables
The patients’ variables collected were gender, age, weight, height, BMI, and reason for admission. The variables related to NGT were medical indication, type, and caliber. Furthermore, other variables related to our study aims were collected, such as NGT direct and indirect visualization (yes/no) by the intensive care nurse and physician using ultrasound and the agreement degree between them visualizing the NGT through both methods.
2.5. Data Collection
Once the critically ill patient or their representative had fulfilled and signed the written informed consent form, both the intensive care nurse and the intensive care physician performed an abdominal ultrasound on each patient independently and immediately one after the other. Both healthcare professionals took all the measurements. Before starting the study, the intensive care nurse was trained during 4 training sessions in ultrasound, of which 2 were specifically focused on abdominal ultrasound. These practical training sessions were provided by the Radiology service of the participating hospital. The intensive care physician had received training in ultrasound during his medical education and routinely performed ultrasound in clinical procedures in critically ill patients.
The data were collected in an ad-hoc database, where all the study variables were detailed.
2.6. Technique to Perform the Ultrasound
The ultrasound available in the UCI (Philips CX50™) was used in this study. A 3-5 MHz convex probe was applied and an exploration routine with cross-sectional, longitudinal, and oblique cuts was followed, starting in the epigastrium. Initially, an attempt was performed through NGT direct visualization, whose ultrasound image appeared as a simple or double hyperechogenic line. Subsequently, another attempt was performed through NGT indirect visualization by injecting (as long as the NGT was not a newly introduced tube) a mix of 1 cm3 water and 30 cm3 air through the tube to verify indirect signs indicating that it was in the stomach. These signs consisted of the appearance of a moving mist with small hyperechogenic spots corresponding to air bubbles (“dynamic fogging” technique), which is used as an acceptance criterion for an NGT correctly placed in the stomach [
23,
25,29].
After performing NGT direct and indirect visualization using ultrasound, a chest X-ray was performed to verify proper NGT placement. Its position was checked and confirmed by another intensive care physician different from the one that had performed the ultrasound.
2.7. Data Analysis
The statistical analysis was performed using the IBM SPSS StatisticsTM software, version 25.0 for Windows (IBM Corp., Armonk, NY, USA). Mean values, standard deviations, frequencies, and percentages were calculated for the descriptive analysis.
To analyze the diagnostic precision of ultrasound as a valid method to verify the NGT positioning, its sensitivity and specificity were calculated, as well as positive and negative predictive values (PPV and NPV) obtained from the comparison with X-ray. The corresponding formulas from diagnostic test validity studies were used for their calculation: PPV (quotient between true positive rate [sensitivity] and false positive rate [1-specificity]) and NPV (quotient between false negative rate [1-sensitivity] and true negative rate [specificity]) [
26].
Finally, the interobserver agreement degree between the intensive care nurse and intensive care physician who performed ultrasounds was analyzed through Cohen’s Kappa index [30].
2.8. Ethical Considerations
This study was approved by the Research and Ethics Committee of the participating hospital (registration number: 5155). Furthermore, it was conducted according to the ethical principles for medical research of the International Declaration of Helsinki and the UNESCO Universal Declaration on Human Rights. In addition, our study complies with the requirements of Spanish Law 14/2007 on Biomedical Research and Law 41/2002 on Patients’ Autonomy. Each critically ill patient, or his/her representative, received the patient’s information sheet with a detailed explanation of the study, including the written informed consent form, allowing them to ask questions or solve their doubts.
3. Results
23 critically ill adult patients participated in our study, 15 men (65.2%) and 8 women (34.8%) aged between 20 and 82 years (median=65; interquartile range=24). The response rate was 100%, with no refusals to participate. The main reason for admission was neurological (n=12; 52.2%), specifically stroke (n=8; 34.7%). Regarding the characteristics of the NGT placed, their main medical indication was enteral nutrition administration (n=18; 78.3%), most of them were made of polyurethane, and their caliber was mainly of 12 French gauge (n=13; 56.5%).
Table 1 shows all the characteristics of the participating critically ill patients and NGT placed.
Regarding the ultrasound discrimination capability to determine NGT presence or absence in the stomach,
Table 2 shows the validity analysis of the correct positioning of the NGT using ultrasound performed by the intensive care nurse and physician, and its comparison with the x-ray, including their corresponding sensitivity and specificity, PPV, and NPV values.
The X-ray confirmed proper NGT positioning in 20 cases (87.0%). Concerning the remaining cases, 1 NGT was found bent in the patient’s mouth and 2 NGTs were in the esophagus. Consequently, these 3 NGTs were replaced.
As for NGT indirect visualization through ultrasound, the intensive care nurse and physician reported that 7 NGTs (30.4%) were placed in the stomach, obtaining a sensitivity of 35.0% and specificity of 100%. However, through NGT indirect visualization using the “dynamic fogging” technique, the intensive care nurse verified that 17 NGTs (73.9%) were placed in the stomach, whereas the intensive care physician verified 18 NGTs (78.3%). Therefore, the sensitivity and specificity of the indirect visualization obtained by the intensive care nurse were 85.0% and 100%, respectively. These values were similar to values obtained by the intensive care physician (86.0% and 100% respectively) (
Table 2).
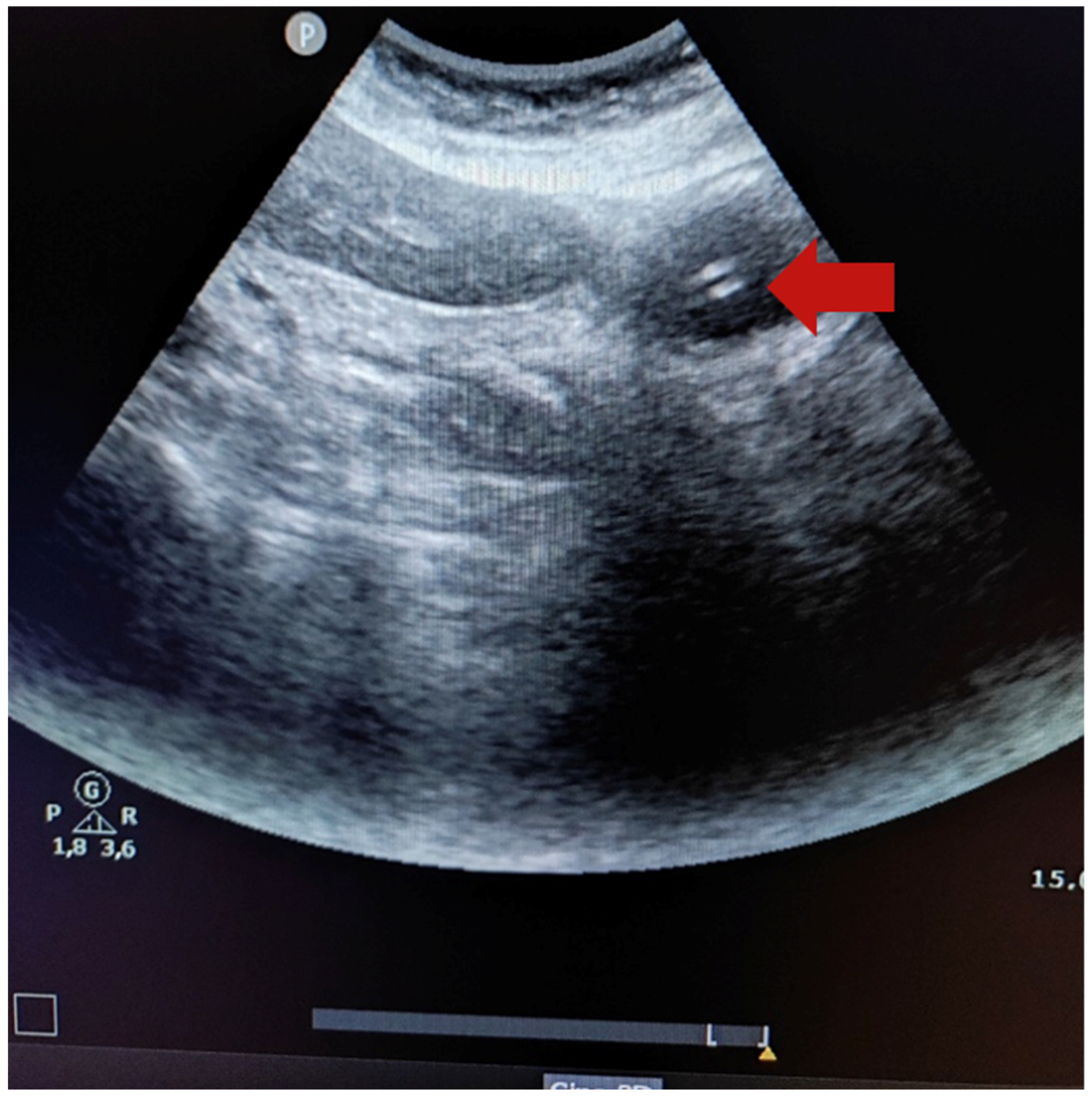
Figure 1 shows the ultrasound image of a 14-French gauge nasogastric tube placed in the stomach (the red arrow indicates two hyperechogenic parallel lines).
Finally, the agreement degree between the intensive care nurse and intensive care physician when observing the NGT in the stomach was calculated. Using a 5% significance level, the interobserver agreement degree reached a Cohen’s Kappa coefficient of 0.88, indicating a high degree of agreement between both professionals [30].
4. Discussion
Our study analyzed the validity of an ultrasound performed by an intensive care nurse as a method to verify the presence of an NGT in the stomach. The ultrasound sensitivity values were higher through indirect visualization than direct visualization (85% vs. 35%), obtaining the same specificity values in both methods (100% vs. 100%).
On one hand, for critically ill patients in which the NGT was not visualized in the stomach by the intensive care nurse using ultrasound and those who really did not have it there, the specificity was 100%. On the other hand, for critically ill patients in which the NGT was visualized in the stomach by her using ultrasound and those who really had the tube in its proper position, the sensitivity increased from 35.0% through NGT direct visualization to 85.0% through indirect visualization using the “dynamic fogging” technique [
23,
25,29]. This result is congruent with the study conducted by Duan et al. [31], which concluded that ultrasound sensitivity increased considerably to 92.2% and 100.0% whether ultrasound is combined introducing an air-water mix through the NGT. In this sense, Piton et al. [32] proposed introducing gastric aspirate through the NGT to visualize all the turbulences in the ultrasound image, allowing the verification of the proper NGT positioning.
Regarding the previous studies in which nurses performed ultrasounds, Tai et al. [
21] obtained a sensitivity of 52.2% and 88.4% for NGT direct and indirect visualization, respectively. Mak and Tam [33] obtained higher sensitivity values, 92.4% and 95.4% for NGT direct and indirect visualization, respectively. However, they only included home-care patients and the participating nurses were trained and supervised during 10 ultrasound assessments. Recently, Brotfain et al. [
26] concluded that nurses verified proper NGT positioning in the stomach using ultrasound in 78.0% of patients. Although this study did not provide any validity data, nurses received theoretical-practical training, and a supervision process was carried out during 50 ultrasounds before initiating the study.
It should be noted that calculations of predictive values in our study provided information about ultrasound safety. A PPV value of 100% was found in both direct and indirect visualization of the NGT, as well as low NPV values (19% and 50% for direct and indirect visualization, respectively). However, it is also noteworthy that most of the NGT used in our study were made of polyurethane and 12-French gauge. Except for a 14-French Salem NGT in a patient with abundant activated carbon in the stomach, the rest of the unobserved and well-positioned NGT corresponded to 12-French thin feeding tubes. On the contrary, Chenatia et al. [
20] used NGT of 14 and 18-French gauge, obtaining a sensitivity of 98.3%, but showing that the larger the NGT caliper, the better its visualization.
Furthermore, it should be highlighted the high interobserver agreement degree obtained between the intensive care nurse and intensive care physician (Cohen’s Kappa=0.88). We believe this result may contribute to the safe use and management of ultrasound by intensive care nurses, including ultrasound in their routine clinical practice, provided they are trained and receive regulated education and qualification.
Concerning the implications for the clinical practice of our study, incorporating ultrasound, and specifically using NGT direct visualization, as a method to verify proper NGT positioning by intensive care nurses would be useful to avoid the patients’ exposure to radiation sources. In addition, we believe it may constitute a method to monitor NGT positioning, following the recommendations from the American Association of Critical-Care Nurses (AACCN) [34], which recommends checking NGT positioning every 4 hours. This check would be complicated using X-ray owing to its undesirable radiation for critically ill patients. And even using other methods, such as gastric pH assessment, as it may hinder enteral nutrition administration in these patients. On the contrary, ultrasound represents a non-invasive method to verify NGT positioning and is less expensive than X-ray when the necessary equipment is available. Additionally, both performing and interpreting ultrasounds are faster than X-rays as they do not require moving any large equipment and an X-ray technician, or an intensive care physician to later interpret the X-ray. Therefore, using ultrasound in ICUs may positively impact critically ill patients’ health and reduce health-related costs (material and human) and waiting time required to use an NGT, allowing its use as soon as possible. In this sense, it would be advisable to not only have radiopaque NGTs available in ICUs but also echogenic NGTs, which may improve their visualization.
Although the high interobserver agreement was obtained, the training differences between the participating intensive care nurse and physician were a study limitation. On the other hand, as ultrasound is considered an operator-dependent technique34, it could limit our results. Consequently, it is needed to confirm our results by conducting more studies in intensive care nurses more trained in ultrasound. Another study limitation is related to the technological features of the ultrasound equipment available in the participating ICU, as not all ultrasound systems offer the same image quality and, therefore, the images obtained and their interpretation may be influenced.
5. Conclusions
The NGT indirect visualization through ultrasound could constitute a valid method for intensive care nurses to verify its proper positioning and could even become an alternative method to X-ray. However, the low sensitivity obtained for direct visualization suggests the need for better training and regulated qualification in ultrasound for intensive care nurses.
The agreement degree between the data obtained by the intensive care nurse and the intensive care physician was high. Consequently, ultrasounds could be properly performed by both healthcare professionals, requiring future studies to confirm this hypothesis.
Finally, the non-invasive nature of ultrasound and its easy accessibility evidence the suitability of this technique to expand its use in the routine clinical practice of intensive care nurses.
Author Contributions
Conceptualization, M.R.-G., O.A., J.A.S.-G., I.O.-S. and I.Z.-G.; methodology, M.R.-G., O.A., J.A.S.-G. and I.Z.-G.; formal analysis, M.R.-G., O.A., I.Z.-G.; investigation, M.R.-G. and J.A.S.-G.; data curation, M.R.-G., and O.A., J.A.S.-G.; writing—original draft preparation, M.R.-G., O.A. and I.Z.-G.; writing—review and editing, M.R.-G., O.A., J.A.S.-G., I.O.-S. and I.Z.-G.; supervision, M.R.-G., O.A., J.A.S.-G., I.O.-S. and I.Z.-G.; project administration, M.R.-G. and J.A.S.-G.; funding acquisition, M.R.-G., O.A., I.O.-S. and I.Z.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This project received the 2nd award for the best oral communication in the XLIX National Congress of the Spanish Society of Intensive Nursing and Coronary Units (Sociedad Española de Enfermería Intensiva y Unidades Coronarias, SEEIUC) held in Barcelona (Spain) in May 2024.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research and Ethics Committee of La Princesa University Hospital, Madrid, Spain (registration number: 5155).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank all critically ill patients and their representatives who participated in the study and provided us with the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aragonés Manzanares, R.; Rincón Ferrari, M.D. Manual de cuidados intensivos para Enfermería. Editorial Médica Panamericana: Madrid, Spain, 2020.
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; Preiser, J.C.; van Zanten, A.R.H.; Oczkowski, S.; Szczeklik, W.; Bischoff, S.C. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; Limketkai, B.N.; Nelson, K.K.; Powers, J.; Ronan, A.; Schober, N.; Strang, B.J.; Swartz, C.; Turner, J.; Tweel, L.; Walker, R.; Epp, L.; Malone, A.; ASPEN Enteral Nutrition Committee. When is enteral nutrition indicated? JPEN J. Parenter. Enteral Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef] [PubMed]
- Matejovic, M.; Huet, O.; Dams, K.; Elke, G.; Vaquerizo Alonso, C.; Csomos, A.; Krzych, L.J.; Tetamo, R.; Puthucheary, Z.; Rooyachers, O.; Tjäder, I.; Kuechenhoff, H.; Hartl, W.H.; Hiesmayr, M. Medical nutrition therapy and clinical outcomes in critically ill adults: a European multinational, prospective observational cohort study (EuroPN). Crit. Care. 2022, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.P.G.; Rigobello, M.C.G.; Silveira, R.C.C.P.; Gimenes, F.R.E. Nasogastric/nasoenteric tube-related adverse events: an integrative review. Rev. Lat. Am. Enfermagem 2021, 8, e3400. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.A.; Chase, D.M.; Coughlin, L.M.; Perry, E. Pulmonary complications of 9931 narrow-bore nasoenteric tubes during blind placement: A critical review. J. Parenter. Enter. Nutr. 2011, 35, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Manara, A.R. X-ray checks of NG tube position: a case for guided tube placement. Br. J. Radiol. 2021, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.M. Adult CCRN® certification review think in questions, learn by rationales (2nd ed.). Springer Publishing Company; New York, US, 2021.
- McClave, S.A.; Dibaise, J.K.; Mullin, G.E.; Martindale, R.G. ACG clinical guideline: Nutrition therapy in the adult hospitalized patient. Am. J. Gastroenterol. 2016, 111, 315–334. [Google Scholar] [CrossRef]
- Metheny, N.A.; Krieger, M.M.; Healey, F.; Meert, K.L. A review of guidelines to distinguish between gastric and pulmonary placement of nasogastric tubes. Heart Lung 2019, 48, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, A.M.; Halm, M.A. Feeding tube placement in adults: Safe verification method for blindly inserted tubes. Am. J. Crit. Care 2009, 18, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Judd, M. Confirming nasogastric tube placement in adults. Nursing 2020, 50, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rowat, A.M.; Graham, C.; Dennis, M. Study to determine the likely accuracy of pH testing to confirm nasogastric tube placement. BMJ Open Gastroenterol. 2018, 5, e000211. [Google Scholar] [CrossRef] [PubMed]
- Bloom, L.; Seckel, M.A. Placement of nasogastric feeding tube and postinsertion care review. AACN Adv. Crit. Care 2022, 33, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Luebbehusen, M.; Aguirre, L.; Cluff, J.; David, M.A.; Holly, V.; Linford, L.; Park, N.; Brunelle, R. Improved safety and efficacy of small-bore feeding tube confirmation using an electromagnetic placement device. Nutr. Clin. Pract. 2018, 33, 268–273. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Biomedical Imaging and Bioengineering. X-rays. Available online: https://www.nibib.nih.gov/science-education/science-topics/x-rays (accessed on 21 April 2024).
- Poggio, G.A.; Mariano, J.; Gipar, L.A.; Ucar, M.E. La ecografía primero: ¿Por qué, cómo y cuándo? Revista Argentina de Radiología 2017, 81, 192–203. [Google Scholar] [CrossRef]
- Atalay, Y.O.; Aydin, R.; Ertugrul, O.; Gul, S.B.; Polat, A.V.; Paksu, M.S. Does bedside sonography effectively identify nasogastric tube placements in pediatric critical care patients? Nutr. Clin. Pract. 2016, 31, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Chenaitia, H.; Brun, P.M.; Querellou, E.; Leyral, J.; Bessereau, J.; Aimé, C.; Bouaziz, R.; Georges, A.; Louis, F.; WINFOCUS Group France. Ultrasound to confirm gastric tube placement in prehospital management. Resuscitation 2012, 83, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Lau, W.S.; Chan, P.Y.; Ng, S.Y.; Lam, Y.C.; Mak, H.T.; Mak, Y.T. Nurse performed ultrasonography in confirming the position of nasogastric tube in the emergency department: a prospective single group diagnostic. Hong Kong J. Emerg. Med. 2016, 23, 340–349. [Google Scholar] [CrossRef]
- Kim, H.M.; So, B.H.; Jeong, W.J.; Choi, S.M.; Park, K.N. The effectiveness of ultrasonography in verifying the placement of a nasogastric tube in patients with low consciousness at an emergency center. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Mumoli, N.; Vitale, J.; Pagnamenta, A.; Mastroiacovo, D.; Cei, M.; Pomero, F.; Giorgi-Pierfranceschi, M.; Giuntini, L.; Porta, C.; Capra, R.; Mazzone, A.; Dentali, F. Bedside abdominal ultrasound in evaluating nasogastric tube placement: a multicenter, prospective, cohort study. Chest 2021, 159, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- McMullen, C.D.; Anstey, C.; Garrett, P.; Moore, J. Nasogastric tube placement under sonographic observation: A comparison study of ultrasound and chest radiography in mechanically ventilated patients. Aust. Crit. Care 2022, 35, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.; Vezzali, N. 4-Point ultrasonography to confirm the correct position of the nasogastric tube in 114 critically ill patients. J. Ultrasound 2017, 20, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Erblat, A.; Luft, P.; Elir, A.; Gruenbaum, B.F.; Livshiz-Riven, I.; Koyfman, A.; Fridrich, D.; Koyfman, L.; Friger, M.; Grivnev, A.; Zlotnik, A.; Klein, M. Nurse-performed ultrasound assessment of gastric residual volume and enteral nasogastric tube placement in the general intensive care unit. Intensive Crit. Care Nurs. 2022, 69, 103183. [Google Scholar] [CrossRef] [PubMed]
- Pita Fernández, S.; Pértegas Díaz, S. Guía: Pruebas diagnósticas: Sensibilidad y especificidad. Cad. Aten. Primaria 2003, 10, 120–124. Available online: https://www.fisterra.com/formacion/metodología-investigacion/pruebas-diagnosticas-sensibilidad-especificidad/ (accessed on 6 May 2024).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Chenaitia, H.; Lablanche, C.; Pradel, A. 2-Point Ultrasonography to confirm correct position of the gastric tube in prehospital setting. Mil. Med. 2014, 179, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Abraira, V. El índice kappa. SEMERGEN 2001, 27, 247–249. [Google Scholar] [CrossRef]
- Duan, M.; Chen, X.; Qin, X.; Liang, Q.; Dong, W.; Zhang, Y.; Lin, J. A review of location methods of nasogastric tube in critically ill patients. Open J. Nurs. 2020, 10, 943–951. [Google Scholar] [CrossRef]
- Piton, G.; Parel, R.; Delabrousse, E.; Capellier, G. Echography for nasogastric tube placement verification. Eur. J. Clin. Nutr. 2017, 71, 669–670. [Google Scholar] [CrossRef]
- Mak, M.Y.; Tam, G. Ultrasonography for nasogastric tube placement verification: an additional reference. Br. J. Community Nurs. 2020, 25, 328–334. [Google Scholar] [CrossRef] [PubMed]
- American Association of Critical Care Nurses. AACN practice alert: Initial and ongoing verification of feeding tube placement in adults. Crit. Care Nurse 2017, 37, 100. Available online: https://www.aacn.org/clinical-resources/practice-alerts/initial-and-ongoing-verification-of-feeding-tube-placement-in-adults (accessed on 25 April 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).