Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Origin of Life, Evolution, and Biosystems
3. Stem Cells, Regenerating Cells, and Cancer Cells
4. Proteostasis, Proteasomes, and Peptides
5. Immune Responses to Cancers and the PHS Hypothesis
5.1. PHS Hypothesis
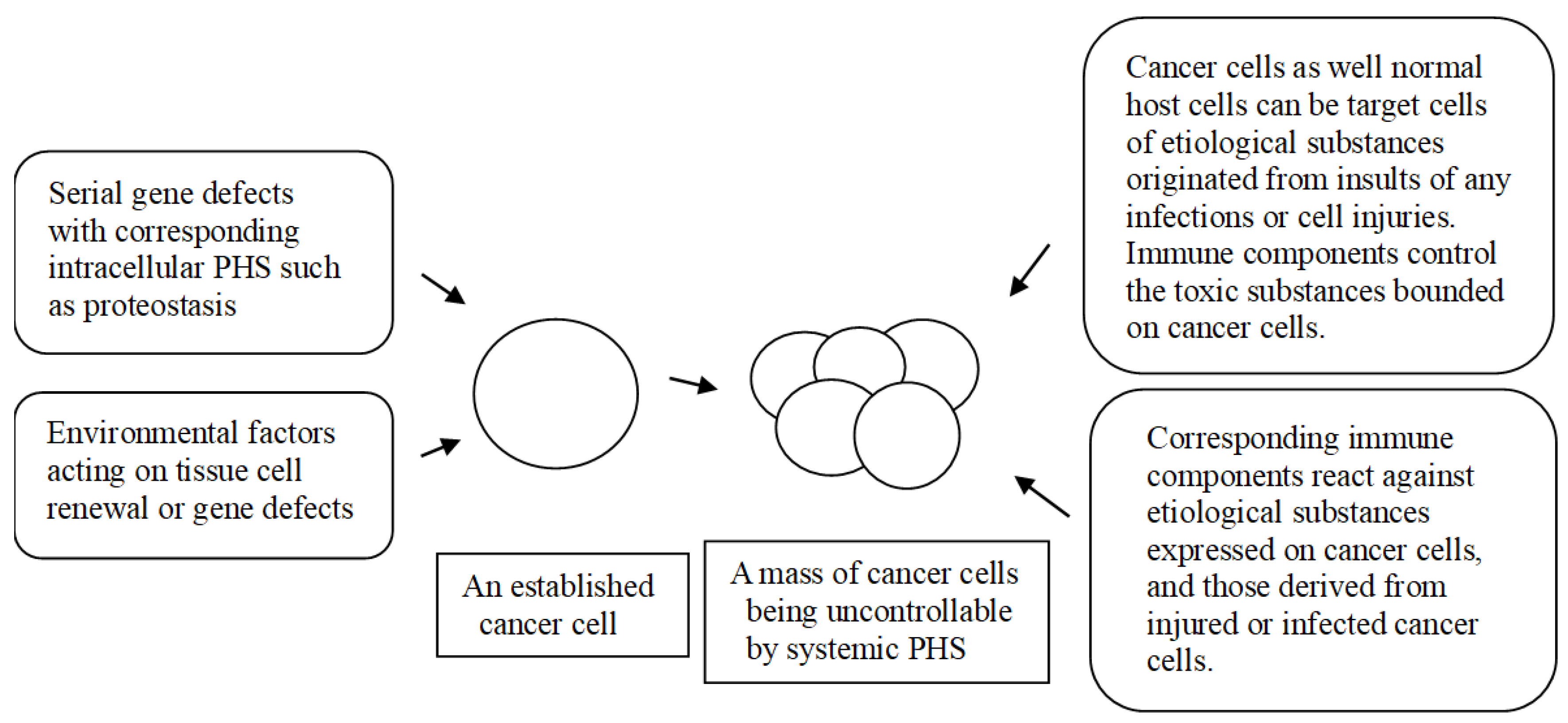
5.2. Oncogenesis in the PHS Hypothesis
6. MHCs, Cytokine Networks, and Communication of Immune Cells
7. Associated Factors in Cancers
7.1. Aging
7.2. Genetic and Epigenetic Factors
7.3. Microbiota and Infection
7.4. Environmental Factors
7.5. Host and Tumor Factors
8. Unresolved Issues in the Immunotherapeutic of Cancers
8.1. Histopathologic Findings and Tumor-Infiltrating Lymphocytes (TILs)
8.2. Immune Checkpoint Inhibitors (ICIs) and Autoimmune Diseases
8.3. Chimeric Antigen Receptor T Cell (CAR-T) Therapies
8.4. Therapeutic Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Gordon, S.; Imhof, B.A.; Núñez, G.; Bousso, P. Élie Metchnikoff (1845-1916): celebrating 100 years of cellular immunology and beyond. Nat. Rev. Immunol. 2016, 16, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Maman, S.; Witz, I.P. A history of exploring cancer in context. Nat. Rev. Cancer 2018, 18, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- Golstein. P.; Griffiths, G.M. An early history of T cell-mediated cytotoxicity. Nat. Rev. Immunol. 2018, 18, 527–535. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer. G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.A. TLRs and innate immunity. Blood 2009, 113, 1399–1407. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: a renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Rhim, J.-W.; Kang, J.-H. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med. J. 2012, 53, 262–275. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Rhim, J.-W.; Kang, J.-H. Hyperactive immune cells (T cells) may be responsible for acute lung injury in influenza virus infections: a need for early immune-modulators for severe cases. Med. Hypotheses 2011, 76, 64–69. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kang, J.-H.; Lee, K.-Y. Etiological and pathophysiological enigmas of severe coronavirus disease 2019, multisystem inflammatory syndrome in children, and Kawasaki disease. Clin. Exp. Pediatr. 2022, 65, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. A common immunopathogenesis mechanism for infectious diseases: the protein-homeostasis-system hypothesis. Infect. Chemother. 2015, 47, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. A unified pathogenesis for kidney diseases, including genetic diseases and cancers, by the protein-homeostasis-system hypothesis. Kidney Res. Clin. Pract. 2017, 36, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. Common immunopathogenesis of central nervous system diseases: the protein-homeostasis-system hypothesis. Cell Biosci. 2022, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Malaterre, C.; Jeancolas, C.; Nghe, P. The Origin of Life: What Is the Question? Astrobiology 2022, 22, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, X.; Wei, Y.; Cheng, Y.; Guo, Y.; Khudyakov, I.; et al. A widespread pathway for substitution of adenine by diaminopurine in phage genomes. Science 2021, 372, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014, 12, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Pross, A.; Pascal, R. The origin of life: what we know, what we can know and what we will never know. Open Biol. 2013, 3, 120190. [Google Scholar] [CrossRef]
- Valet, M.; Siggia, E.D.; Brivanlou, A.H. Mechanical regulation of early vertebrate embryogenesis. Nat. Rev. Mol. Cell. Biol. 2022, 23, 169–184. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, K.L. Hippo Signaling in Embryogenesis and Development. Trends Biochem. Sci. 2021, 46, 51–63. [Google Scholar] [CrossRef]
- van Gelder, M.M.; van Rooij, I.A.; Miller, R.K.; Zielhuis, G.A.; de Jong-van den Berg, L.T.; Roeleveld, N. Teratogenic mechanisms of medical drugs. Hum. Reprod. Update 2010, 16, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Verga Falzacappa, M.V.; Ronchini, C.; Reavie, L.B.; Pelicci, P.G. Regulation of self-renewal in normal and cancer stem cells. FEBS J. 2012, 279, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Li, D. Senescent Stem Cell Dysfunction and Age-Related Diseases. Stem Cells Dev. 2023, 32, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Cheng, L.; Du, J.; Peng, Y.; Allan, R.W.; Wei, L.; et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am. J. Surg. Pathol. 2010, 34, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, M.; Takahashi, K. Present and future challenges of induced pluripotent stem cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, M.; Wang, R. Identifying critical regulatory interactions in cell fate decision and transition by systematic perturbation analysis. J. Theor. Biol. 2024, 577, 111673. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, R.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; et al. Applications of single-cell sequencing in cancer research: progress and perspectives. J. Hematol. Oncol. 2021, 14, 91. [Google Scholar] [CrossRef]
- Samuel, N.; Hudson, T.J. Translating genomics to the clinic: implications of cancer heterogeneity. Clin. Chem. 2013, 59, 127–137. [Google Scholar] [CrossRef]
- Lejman, M.; Chałupnik, A.; Chilimoniuk, Z.; Dobosz, M. Genetic Biomarkers and Their Clinical Implications in B-Cell Acute Lymphoblastic Leukemia in Children. Int. J. Mol. Sci. 2022, 23, 2755. [Google Scholar] [CrossRef]
- Yoon, K.A.; Woo, S.M.; Kim, Y.H.; Kong, S.Y.; Lee, M.K.; Han, S.S.; et al. Comprehensive Cancer Panel Sequencing Defines Genetic Diversity and Changes in the Mutational Characteristics of Pancreatic Cancer Patients Receiving Neoadjuvant Treatment. Gut Liver 2019, 13, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, G.G.; Hipp, M.S.; Hartl, F.U. Functional Modules of the Proteostasis Network. Cold Spring Harb. Perspect. Biol. 2020, 12, a033951. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell. Biol. 2009, 10, 458–467. [Google Scholar] [CrossRef]
- Hommen, F.; Bilican, S.; Vilchez, D. Protein clearance strategies for disease intervention. J. Neural Transm. (Vienna) 2022, 129, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.V.; Karpov, V.L. Proteasomes and several aspects of their heterogeneity relevant to cancer. Front. Oncol. 2019, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Türker, F.; Cook, E.K.; Margolis, S.S. The proteasome and its role in the nervous system. Cell. Chem. Biol. 2021, 28, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Yang, C.; Castro, L.M.; Tashima, A.K.; Ferro, E.S.; Moir, R.D.; et al. Analysis of the yeast peptidome and comparison with the human peptidome. PLoS ONE 2016, 11, e0163312. [Google Scholar] [CrossRef] [PubMed]
- Lyapina, I.; Ivanov, V.; Fesenko, I. Peptidome: Chaos or Inevitability. Int. J. Mol. Sci. 2021, 22, 13128. [Google Scholar] [CrossRef]
- Nunes, A.T.; Annunziata, C.M. Proteasome inhibitors: structure and function. Semin. Oncol. 2017, 44, 377–380. [Google Scholar] [CrossRef]
- Hoyer, D.; Bartfai, T. Neuropeptides and neuropeptide receptors: drug targets, and peptide and non-peptide ligands: a tribute to Prof. Dieter Seebach. Chem. Biodivers. 2012, 9, 2367–2387. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1117. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Page`s, C.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Mohamed Khosroshahi, L.; Rokni, M.; Mokhtari, T.; Noorbakhsh, F. Immunology, immunopathogenesis and immunotherapeutics of COVID-19; an overview. Int. Immunopharmacol. 2021, 93, 07364. [Google Scholar] [CrossRef] [PubMed]
- Yasunaga, M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Cancer Biol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Pelosi, A.C.; Scariot, P.P.M.; Garbuio, A.L.P.; Kraemer, M.B.; Priolli, D.G.; Masselli Dos Reis, I.G.; et al. A systematic review of exercise protocols applied to athymic mice in tumor-related experiments. Appl. Nutr. Metab. 2023, 48, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kamperschroer, C.; Frank, B.; Il, C.; Lebrecd, H.; Mitchell-Ryane, S.; Molinierf, B.; et al. Current approaches to evaluate the function of cytotoxic T-cells in non-human primates. J. Immunotoxicol. 2023, 20, 2176952. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Arlen, P.M.; Tsang, K.Y. Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy. Front. Immunol. 2023, 14, 1275904. [Google Scholar] [CrossRef]
- Rahman, T.; Das, A.; Abir, M.H.; Nafiz, I.H.; Mahmud, A.R.; Sarker, R.M.; et al. (2023) Cytokines and their role as immunotherapeutics and vaccine adjuvants: The emerging concepts. Cytokine 2023, 169, 156268. [Google Scholar] [CrossRef]
- Balkwil, I.F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Patel S (2018) Danger-associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr. Allergy Asthma Rep. 2018, 18, 63. [CrossRef]
- Meng, X.; Yerly, D.; Naisbitt, D.J. Mechanisms leading to T-cell activation in drug hypersensitivity. Curr. Opin.Allergy Clin. Immunol. 2018, 18, 317–324. [Google Scholar] [CrossRef]
- Deacy, A.M.; Gan, S.K.; Derrick, J.P. Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Front. Immunol. 2021, 12, 731845. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Rhim, J.-W.; Kang, J.-H. Immunopathogenesis of COVID-19 and early immunomodulators. Clin. Exp. Pediatr. 2020, 63, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. Int. J. Mol. Sci. E: pii. [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2012, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cel.l Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Kang, H.-M.; Lee, K.-Y. Pathophysiology of Virus Infections: The Protein-Homeostasis-System Hypothesis. Preprints 2023, 2023072131. [Google Scholar] [CrossRef]
- Smithers, D.W. An attack on cytologism. Lancet 1962, 1, 493–499. [Google Scholar] [CrossRef]
- Soto, A.M.; Sonnenschein, C. The cancer puzzle: Welcome to organicism. Prog. Biophys. Mol. Biol. 2021, 165, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hilden, J.M.; Dinndorf, P.A.; Meerbaum, S.O.; Sather, H.; Villaluna, D.; Heerema, N.A.; et al. ; Children’s Oncology Group. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children’s Oncology Group. Blood 2006, 108, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Drabent, P.; Fraitag, S. Malignant Superficial Mesenchymal Tumors in Children. Cancers (Basel) 2022, 14, 2160. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Reits, E.; Neefjes, J. Present yourself! By MHC class I and MHC class II molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef]
- Gruen, J.R.; Weissman, S.M. Human MHC class III and IV genes and disease associations. Front. Biosci. 2001, 6, D960–972. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.J. 3rd; Siew, S.; Kunz, H.W. Major histocompatibility complex (MHC)-linked genes affecting development. J. Exp. Zool. 1983, 228, 325–345. [Google Scholar] [CrossRef] [PubMed]
- McManigle, W.; Pavlisko, E.N.; Martinu, T. Acute cellular and antibody-mediated allograft rejection. Semin. Respir. Crit. Care Med. 2013, 34, 320–335. [Google Scholar] [CrossRef]
- Zinkernagel, R.M. The Nobel Lectures in Immunology. The Nobel Prize for Physiology or Medicine, 1996. Cellular immune recognition and the biological role of major transplantation antigens. Scand. J. Immunol. 1997, 46, 421–436. [Google Scholar] [PubMed]
- Robinette, M.L.; Colonna, M. Innate lymphoid cells and the MHC. HLA 2016, 87, 5–11. [Google Scholar] [CrossRef]
- Bubeník, J. Tumour MHC class I downregulation and immunotherapy (Review). Oncol. Rep. 2003, 10, 2005–2028. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Cron, R.Q. The Multifaceted Immunology of Cytokine Storm Syndrome. J. Immunol. 2023, 210, 1015–1024. [Google Scholar] [CrossRef]
- Ball, L.M.; Egeler, R.M. Acute GvHD: pathogenesis and classification. Bone Marrow Transplant. 2008, 41, S58–64. [Google Scholar] [CrossRef]
- Pedersen, S.J.; Maksymowych, W.P. The Pathogenesis of Ankylosing Spondylitis: an Update. Curr. Rheumatol. Rep. 2019, 21, 58. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, J.S. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med. Hypotheses 2006, 67, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Oh, J.H.; Rhim, J.W.; Lee, K.-Y. Correlation between elevated platelet count and immunoglobulin levels in the early convalescent stage of Kawasaki disease. Medicine (Baltimore) 2017, 96, e7583. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jurasz, P. The role of platelets in the tumor microenvironment: From solid tumors to leukemia. Biochim. Biophys. Acta 2016, 1863, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Jackaman, C.; Tomay, F.; Duong, L.; Abdol Razak, N.B.; Pixley, F.J.; Metharom, P.; et al. Aging and cancer: The role of macrophages and neutrophils. Ageing Res. Rev. 2017, 36, 105–116. [Google Scholar] [CrossRef]
- Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- King, M.C.; Marks, J.H.; Mandell, J.B.; New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Doan, J.V.; Ising, M.E.; Evans, N.R.; et al. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H. Mechanisms and impacts of chromosomal translocations in cancers. Front. Med. 2012, 6, 263–274. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: from mechanism to therapy. Cell 2019, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Ohtani, H.; Chakravarthy, A.; De Carvalho, D.D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer 2019, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Videtic Paska, A.; Konjevod, M.; Kouter, K.; Svob Strac, D.; Nedic Erjavec, G.; et al. Epigenetics of Alzheimer’s Disease. Biomolecules 2021, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Viatte, S.; Plant, D.; Raychaudhuri, S. Genetics and epigenetics of rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Liptáková, A.; Čurová, K.; Záhumenský, J.; Visnyaiová, K.; Varga, I.I. Microbiota of female genital tract - functional overview of microbial flora from vagina to uterine tubes and placenta. Physiol. Res. 2022, 71, S21–S33. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Salman, M.D.; Ma, M.J.; Gray, G.C. Animal influenza virus infections in humans: A commentary. Int. J. Infect. Dis. 2019, 88, 113–119. [Google Scholar] [CrossRef]
- Virgin, H.W. The virome in mammalian physiology and disease. Cell 2014, 157, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wergo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P.A. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef]
- Clinton, N.A. : Hameed, S.A.; Agyei, E.K.; Jacob, J.C.; Oyebanji, V.O.; Jabea, C.E. Crosstalk between the Intestinal Virome and Other Components of the Microbiota, and Its Effect on Intestinal Mucosal Response and Diseases. J. Immunol. Res. 2022, 2022, 7883945. [Google Scholar] [CrossRef]
- D’Souza, S.; Lau, K.C.; Coffin, C.S.; Patel, T.R. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 5759–5783. [Google Scholar] [CrossRef] [PubMed]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-Associated Cancers and Related Diseases. N. Engl. J. Med. 2018, 378, 1029–1041. [Google Scholar] [CrossRef]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Kebudi, R.; Kiykim, A.; Sahin, M.K. Primary Immunodeficiency and Cancer in Children; A Review of the Literature. Curr. Pediatr. Rev. 2019, 15, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Vangipuram, R.; Tyring, S.K. AIDS-Associated Malignancies. Cancer Treat. Res. 2019, 177, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; Cortés-Macías, E.; Sánchez, G.; Collado, M.C. Gut Microbiota Development in Infants and Children. World Rev. Nutr. Diet 2022, 124, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Han, J.W.; Lee, J.S. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med. Hypotheses 2007, 69, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Fock, K.M. Clinical epidemiology of gastric cancer. Singapore Med. J. 2014, 55, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Eun, C.S. Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J. Int. Med. 2022, 37, 885–894. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kang, H.M.; Han, J.W.; Lee, K.-Y. A Presumed Etiology of Kawasaki Disease Based on Epidemiological Comparison With Infectious or Immune-Mediated Diseases. Front. Pediatr. 2019, 7, 202. [Google Scholar] [CrossRef]
- Shahin, O.A.; Chifotides, H.T.; Bose, P.; Masarova, L.; Verstovsek, S. Accelerated Phase of Myeloproliferative Neoplasms. Acta Haematol. 2021, 114, 484–499. [Google Scholar] [CrossRef]
- Cerquozzi, S.; Tefferi, A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer 2015, 5, e366. [Google Scholar] [CrossRef]
- Kil, H.R.; Yu, J.W.; Lee, S.C.; Rhim, J.-W.; Lee, K.-Y. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr. Rheumatol. Online J. 2017, 15, 60. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, Y.T.; Kang. H.M.; Suh, J.S.; Lee, K.-Y. Changes in clinical features in Henoch-Schönlein purpura during three decades: an observational study at a single hospital in Korea. Clin. Rheumatol. 2019, 38, 2811–1818. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; et al. The immune landscape of cancer. Immunity 2018, 48, 812–830. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Mullighan, C.G. Molecular markers in ALL: Clinical implications. Best Pract. Res. Clin. Haematol. 2020, 33, 101193. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Badoual, C.; Henderson, F.; Berland, L.; Hamila, M.; Long-Mira, E.; et al. Multiplexed immunohistochemistry for molecular and immune profiling in lung cancer-just about ready for prime-time? Cancers (Basel) 2019, 11, E283–118. [Google Scholar] [CrossRef]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Cheng, M.; Ma, J.; Chen, Y.; Zhang, J.; Zhao, W.; Zhang, J.; et al. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell. Transplant. 2011, 20, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Behrens, E.M. Hyperinflammation, rather than hemophagocytosis, is the common link between macrophage activation syndrome and hemophagocytic lymphohistiocytosis. Curr. Opin. Rheumatol. 2014, 26, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb). 2011, 91, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Arya, A.; Iams, W.; Cruz, M.R.; Chandra, S.; Choi, J.; et al. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.; Oronsky, B.; Reid, T.R. Commentary on oncolytic viruses: past, present, and future. J. Immunother. Cancer 2023, 11, e007905. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, L.; Mo, T.; Na, J.; Qian, Z.; Fan, D.; et al. Oncolytic viral vectors in the era of diversified cancer therapy: from preclinical to clinical. Clin. Transl. Oncol. 2022, 24, 1682–1701. [Google Scholar] [CrossRef] [PubMed]
- Klavdianou, K.; Melissaropoulos, K.; Filippopoulou, A.; Daoussis, D. Should We be Afraid of Immune Check Point Inhibitors in Cancer Patients with Pre-Existing Rheumatic Diseases? Immunotherapy in Pre-Existing Rheumatic Diseases. Mediterr. J. Rheumatol. 2021, 32, 218–226. [Google Scholar] [CrossRef]
- McColl, A.; Michlewska, S.; Dransfield, I.; Rossi, A.G. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. ScientificWorldJournal 2007, 7, 1165–1181. [Google Scholar] [CrossRef]
- Wengner, A.M.; Scholz, A.; Haendler, B. Targeting DNA Damage Response in Prostate and Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8273. [Google Scholar] [CrossRef]
- Itani, M.; Goldman Gollan, Y.; Ezell, K.; Mohanna, M.; Sabbagh, S.; Mears, C.; et al. Thymoma and Myasthenia Gravis: An Examination of a Paraneoplastic Manifestation. Cureus 2023, 15, e34828. [Google Scholar] [CrossRef]
- Farina, A.; Villagrán-García, M.; Vogrig, A.; Zekeridou, A.; Muñiz-Castrillo, S.; Velasco, R.; et al. Neurological adverse events of immune checkpoint inhibitors and the development of paraneoplastic neurological syndromes. Lancet Neurol. 2024, 23, 81–94. [Google Scholar] [CrossRef]
- Rahmani, B.; Patel, S.; Seyam, O.; Gandhi, J.; Reid, I.; Smith, N.; et al. Current understanding of tumor lysis syndrome. Hematol. Oncol. 2019, 37, 537–347. [Google Scholar] [CrossRef]
- Ramesh, P.; Hui, H.TY.L.; Brownrigg, L.M.; Fuller, K.A.; Erber, W.N. Chimeric antigen receptor T-cells: Properties, production, and quality control. Int. J. Lab. Hematol. 2023, 5, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wu, X.; Zhang, Y.; Zeng. L.; Dong, Y.; Liu, R.; et al. Adverse events and efficacy of second-round CAR-T cell therapy in relapsed pediatric B-ALL. Eur. J. Haematol. 2024, 112, 75–82. [Google Scholar] [CrossRef]
- Sliwkowski, M.X.; Mellman, I. Antibody therapeutics in cancer. Science 2013, 341, 1192–1198. [Google Scholar] [CrossRef]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody-Drug Conjugates-A Tutorial Review. Molecules 2012, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Joo, W.D.; Visintin, I.; Mor, G. Targeted cancer therapy: are the days of systemic chemotherapy numbered? Maturitas 2013, 76, 308–314. [Google Scholar] [CrossRef] [PubMed]
| Immune effectors (or events) |
Main functions |
| Adaptive immune system | |
| T cells | Control of pathogenic peptides targeted to cancer cells or host cells via MHC-restricted (TCR-associated) and non-MHC-restricted manners |
| B cells | Control of pathogenic proteins targeted to cancer cells or host cells via MHC-restricted (BCR-associated) and non-MHC-restricted manners |
| Innate immune system | |
| Natural killer cells | Control of transformed cells such as virus-infected cells and tumor cells in MHC-non-restricted manner(?) |
| Tissue macrophage-linaeged cells | Antigen presentation to adaptive immune cells in MHC-restricted immune responses. Possible control of communications between cancers cells and host cells such as stromal cells in TMEs |
| Phagocytes (neutrophils and circulating monocyte/macrophages) | Control of large complex substances such as viruses, bacteria, parasite, and apoptotic & necrotic bodies associated with infected or injured cancer cells and normal cells |
| Mast cells, basophils, eosionphils | These cells are activated by substances that are associated with receptors on the cells and control the substances through inflammation involved in these cells |
| Unidentified innate immune components against small non-protein toxic materials | There are non-protein toxic or inflammation-inducing substances, including elements, monoamins, neuropeptides, LPS, RNAs, DNAs, chemicals, and biochemicals. TLR-associated immune responses, natural antibodies, and immun proteins and/or peptides control these diverse substances. The immune proteins, including PrP gene products and other amyloid proteins, control pathogenic monoamine metabolites or neuropeptides especially in CNS [14] |
| Production of alternative proteins in genetic diseases and cancers | The systemic and intracellular PHS control in part insults from a protein deficiency or malfunctional protein in organ tissues or within a cell |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





