Submitted:
05 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
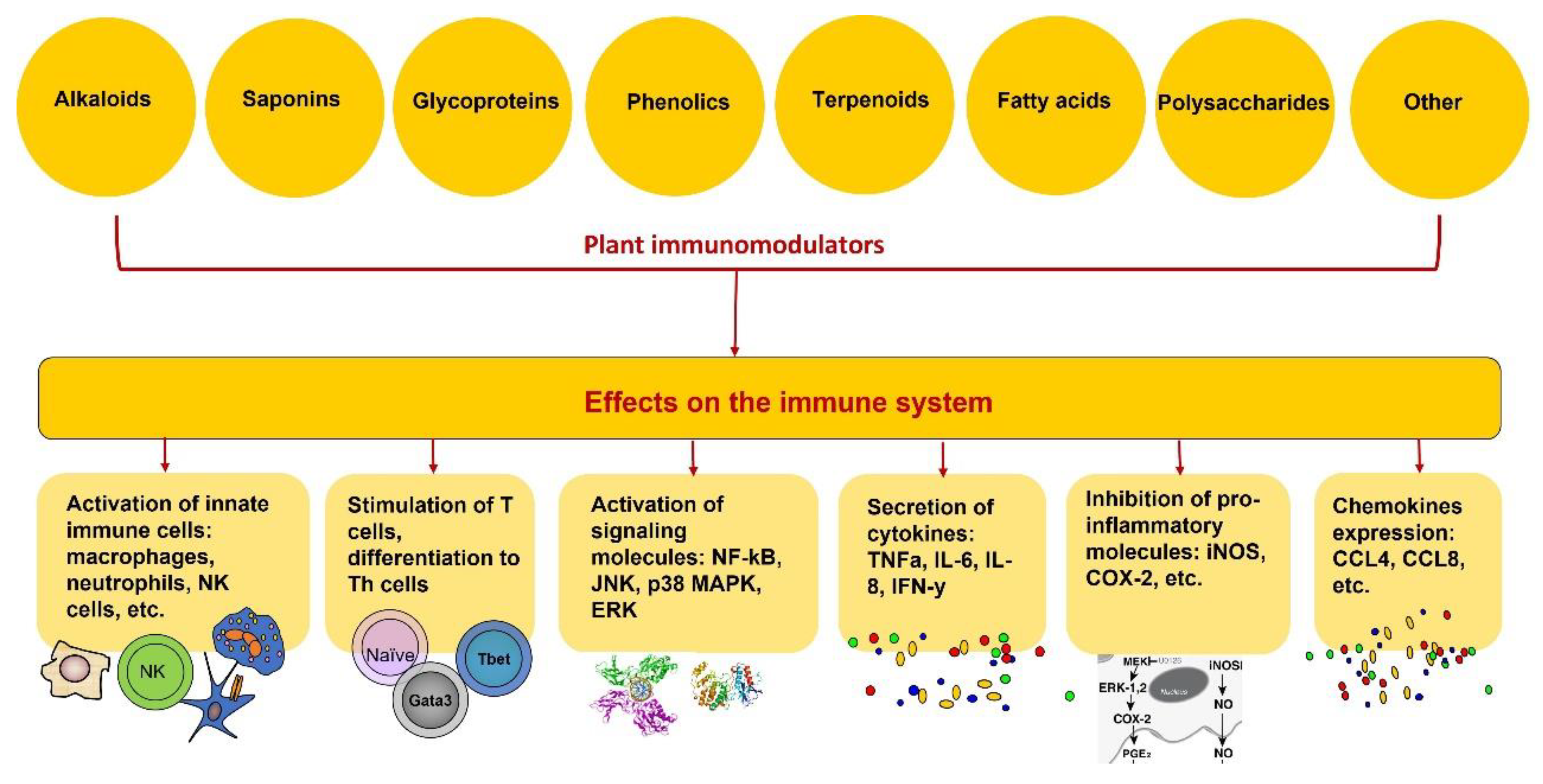
1. Introduction
2. Immunity, Immune System and Immunomodulators
3. Selected Medicinal Plants with Immunomodulatory Activity
4. Selected Plant Chemicals with Immunomodulatory Activity in Clinical Trials
4.1. Resveratrol
4.2. Curcumin
4.3. Quercetin
4.4. Capsaicin
4.5. Epigallocatechin-3-Gallate
4.6. Andrographolide
4.7. Genistein
4.8. Colchicin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puri A., Saxena R., Saxena R. P., Saxena K. C., Srivastava V., Tandon J. S. (1994). Immunostimulant activity of Nyctanthes arbor-tristis L. J. Ethnopharmacol. 42 31–37. [CrossRef]
- Liu L., Li Y. (2014). The unexpected side effects and safety of therapeutic monoclonal antibodies. Drugs Today 50 33–50. [CrossRef]
- Golan D. E. (2008). Principles of Pharmacology. The Pathophysiologic Basic of Drug Therapy, 2nd Edn Pennsylvania, PA: Lippincott Williams & Wilkins, 795–809.
- Hansel T. T., Kropshofer H., Singer T., Mitchell J. A., George A. J. (2010). The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 9 325–338. [CrossRef]
- Bartelds G. M., Krieckaert C. L., Nurmohamed M. T., Van Schouwenburg P. A., Lems W. F., Twisk J. W., et al. (2011). Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305 1460–1468. [CrossRef]
- Auffenberg C., Rosenthal L. J., Dresner N. (2013). Levamisole: a common cocaine adulterant with life-threatening side effects. Psychosomatics 54 590–593. [CrossRef]
- Oberlies N. H., Kroll D. J. (2004). Camptothecin and taxol: historic achievements in natural products research. J. Nat. Prod. 67 129–135. [CrossRef]
- Rakotoarivelo NH, Rakotoarivony F, Ramarosandratana AV, Jeannoda VH, Kuhlman AR, Randrianasolo A, Bussmann RW. Medicinal plants used to treat the most frequent diseases encountered in Ambalabe rural community, Eastern Madagascar. J Ethnobiol Ethnomed. 2015 Sep 15;11:68. [CrossRef]
- Mintah S., Asafo-Agyei T., Archer M., Atta-Adjei P., Boamah D., Kumadoh D., Appiah A., Ocloo A, Duah Boakye Y. and Agyare C., 2019, Medicinal Plants for Treatment of Prevalent Diseases, Ch 9 from Perveen, S., & Al-Taweel, A., Pharmacognosy - Medicinal Plants, IntechOpen, 978-1-83880-611-8. [CrossRef]
- Aschale Y, Wubetu M, Abebaw A, Yirga T, Minwuyelet A, Toru M. A Systematic Review on Traditional Medicinal Plants Used for the Treatment of Viral and Fungal Infections in Ethiopia. J Exp Pharmacol. 2021;13:807-815. [CrossRef]
- Jantan I, Ahmad W, Bukhari SN. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci. 2015 Aug 25;6:655. Erratum in: Front Plant Sci. 2018 Aug 13;9:1178. [CrossRef] [PubMed]
- Shukla, S., Bajpai, V.K. & Kim, M. Plants as potential sources of natural immunomodulators. Rev Environ Sci Biotechnol 13, 17–33 (2014). [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [CrossRef]
- Mohamed, S.I.A.; Jantan, I.; Haque, M.A. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int. Immunopharmacol. 2017, 50, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Nair A, Chattopadhyay D, Saha B. Chapter 17 – Plant-derived immunomodulators. In: New lokk to phytomedicine, Advancements in Herbal products as novel drugs Leads 2019; 435-499.
- Pathak S, Fialho J, Nandi D. Plant-based Immunomodulators and Their Potential Therapeutic Actions. J Explor Res Pharmacol. Published online: Aug 12, 2022. [CrossRef]
- Kumar S, Arya V, Kaur R, Ali Bhat Z, Gupta VK, Kumar V. A review of immunomodulators in the Indian traditional health care system. Journal of Microbiology, Immunology and Infection, 2012; 45 (3): 165-184. [ISSN 1684-1182. [CrossRef]
- Acharya P, Mohanty S, Mohanty M ImmunoProtective Role of Medicinal Herbs as Phytotherapeutic Drugs in Ayurveda – A Prospective Approach for Defending COVID19. J Nat Ayurvedic Med 2022; 6(2): 000342. [CrossRef]
- Huang S-C, Kao Y-H, Shih S-F, Tsai M-C, Lin C-S, Chen LW, Chuang Y-P, Tsui P-F, Ho L-J, Lai J-H, Chen S-J Epigallocatechin-3-gallate exhibits immunomodulatory effects in human primary T cells. Biochemical and Biophysical Research Communications 2021; 550: 70-76. [ISSN 0006-291X] (https://www.sciencedirect.com/science/article/pii/S0006291X21003594). [CrossRef]
- Alhazmi HA, Najmi A, Javed SA, Sultana S, Al Bratty B, Makeen HA, Meraya AM., Ahsan W, Mohan S, Taha MME, Khalid A. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Frontiers in Immunology 2021; 12. [ISSN 1664-3224] https://www.frontiersin.org/articles/10.3389/fimmu.2021.637553. [CrossRef]
- Singh N, Tailang M, Mehta.CS A review on herbal plants as immunomodulators. International Journal of Pharmaceutical Sciences and Research 2016; 7(9): 3602-3610 [E-ISSN: 0975-8232; P-ISSN: 2320-5148].
- Tharakan A, Shukla H, Benny IR, Tharakan M, George L, Koshy S. Immunomodulatory effect of Withania somnifera (Ashwagandha) Extract-A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. J Clin Med. 2021; 10(16): 3644. [CrossRef] [PubMed]
- Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci. 2015; 72(23): 4445-4460. [CrossRef] [PubMed]
- Saha S, Ghosh S. Tinospora cordifolia: One plant, many roles. Anc Sci Life. 2012; 31(4): 151-159. [CrossRef] [PubMed]
- Yates CR, Bruno EJ, Yates MED. Tinospora Cordifolia: A review of its immunomodulatory properties. J Diet Suppl. 2022; 19(2): 271-285. [CrossRef] [PubMed]
- Gupta PK, Chakraborty P, Kumar S, Singh PK, Rajan MGR, Sainis KB, Kulkarni S. G1-4A, a Polysaccharide from Tinospora cordifolia Inhibits the Survival of Mycobacterium tuberculosis by Modulating Host Immune Responses in TLR4 Dependent Manner. PLoS ONE 2016; 11(5): e0154725. [CrossRef]
- Wang R, Deng X, Gao Q, Wu X, Han L, Gao X, Zhao S, Chen W, Zhou R, Li Z, Bai C. Sophora alopecuroides L.: An ethnopharmacological, phytochemical, and pharmacological review. J Ethnopharmacol. 2020; 248: 112172. [CrossRef] [PubMed]
- Zhang R, Wang R, Zhao S, Chen D, Hao F, Wang B, Zhang J, Ma Y, Chen X, Gao X, Han L, Bai C. Extraction, Separation, Antitumor Effect, and Mechanism of Alkaloids in Sophora alopecuroides: A Review. Separations. 2022; 9(11): 380. [CrossRef]
- Zhang et al. 2022). Zhang L-H, Huang Y, Wang L-W, Xiao P-G. Several Compounds from Chinese Traditional and Herbal Medicine as Immunomodulators. Phytotherapy Research 1995; 9: 315-322.
- Dinesh P, Rasool M. Chapter 22. Herbal Formulations and Their Bioactive Components as Dietary Supplements for Treating Rheumatoid Arthritis. In: Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. Editor(s): Watson RR, Preedy VR (Second Edition), Academic Press, 2019, pp. 385-399. [ISBN 9780128138205;] (https://www.sciencedirect.com/science/article/pii/B9780128138205000222). [CrossRef]
- Zhu Y, Zhang L, Zhang X, Wu D, Chen L, Hu C, Wen C, Zhou J. Tripterygium wilfordii glycosides ameliorates collagen-induced arthritis and aberrant lipid metabolism in rats. Frontiers in Pharmacology 2022; 13. [ISSN=1663-9812] (https://www.frontiersin.org/articles/10.3389/fphar.2022.938849). [CrossRef]
- Coon JT, Ernst E. Panax ginseng. Drug-Safety 2002; 25, 323–344. [CrossRef]
- Shin J-Y, Song J-Y, Yun Y-S, Yang H-O, Rhee D-K, Pyo S. Immunostimulating Effects of Acidic Polysaccharides Extract of Panax Ginseng On Macrophage Function. Immunopharmacology and Immunotoxicology 2002; 24(3): 469-482. [CrossRef]
- Pham HNT, Vuong QV, Bowyer MC, Scarlett CJ. Phytochemicals Derived from Catharanthus roseus and Their Health Benefits. Technologies 2020; 8(4): 80. [CrossRef]
- Mahomoodally MF. Traditional medicines in Africa: an appraisal of ten potent african medicinal plants. Evid Based Complement Alternat Med. 2013; 2013: 617459. [CrossRef] [PubMed]
- Tawinwung S, Junsaeng D, Utthiya S, Khemawoot Ph. Immunomodulatory effect of standardized C. asiatica extract on a promotion of regulatory T cells in rats. BMC Complement Med Ther 2021; 21, 220. [ISSN: 2662-7671; [CrossRef]
- Batiha GE-S, Alqahtani A, Ojo OA, Shaheen HM, Wasef L, Elzeiny M, Ismail M, Shalaby M, Murata T, Zaragoza-Bastida A, Rivero-Perez N, Magdy Beshbishy A, Kasozi KI, Jeandet P, Hetta HF. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. International Journal of Molecular Sciences. 2020; 21(15): 5179. [CrossRef]
- Manayi A, Vazirian M, Saeidnia S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn Rev. 2015; 9(17): 63-72. [ PMCID: PMC4441164; [CrossRef] [PubMed]
- Pelvan E, Karaoğlu Ö, Fırat EÖ, Kalyon KB, Ros E, Alasalvar C. Immunomodulatory effects of selected medicinal herbs and their essential oils: A comprehensive review. Journal of Functional Foods 2022; 94, 105108. [ISSN 1756-4646; [CrossRef]
- Cosentino M, Bombelli R, Conti A, Maria C, Azzetti A, Bergamaschi A, Franca M, Lecchini S, Antioxidant properties and in vitro immunomodulatory effects of peppermint (Mentha x piperita l.) essential oils in human leukocytes. J. Pharm. Sci. & Res. 2009; 1(3): 33-43. (https://www.sciencedirect.com/science/article/pii/S1756464622001785).
- Ogaly HA, Eltablawy NA, Abd-Elsalam RM. Antifibrogenic Influence of Mentha piperita L. Essential Oil against CCl4-Induced Liver Fibrosis in Rats. Oxid Med Cell Longev. 2018; 4039753. [CrossRef] [PubMed]
- Kong CK, Low LE, Siew WS, Yap W-H, Khaw K-Y, Ming LCh, Mocan A, Goh B-H, Goh PH. Biological Activities of Snowdrop (Galanthus spp., Family Amaryllidaceae). Frontiers in Pharmacology 2021; 11: 552453. [ISSN=1663-9812] (https://www.frontiersin.org/articles/10.3389/fphar.2020.552453). [CrossRef]
- Matić S, Stanić S, Mihailović M, Bogojević D. Cotinus coggygria Scop.: An overview of its chemical constituents, pharmacological and toxicological potential. Saudi J Biol Sci. 2016; 23(4): 452-461. [CrossRef] [PubMed]
- Antal D, Ardelean F, Jijie R, Pinzaru I, Soica C, Dehelean C. Integrating Ethnobotany, Phytochemistry, and Pharmacology of Cotinus coggygria and Toxicodendron vernicifluum: What Predictions can be Made for the European Smoketree? Frontiers in Pharmacology 2021; 12. [ISSN 1663-9812] https://www.frontiersin.org/articles/10.3389/fphar.2021.662852. [CrossRef]
- Moutia M, Habti N, Badou A. In Vitro and In Vivo Immunomodulator Activities of Allium sativum L. Evid Based Complement Alternat Med. 2018; 2018: 4984659. [CrossRef] [PubMed]
- Uritu CM, Mihai CT, Stanciu GD, Dodi G, Alexa-Stratulat T, Luca A, Leon-Constantin MM, Stefanescu R, Bild V, Melnic S, Tamba BI. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res Manag. 2018 2018: 7801543. [CrossRef] [PubMed]
- Marc Vlaic RA, Mureșan V, Mureșan AE, Mureșan CC, Tanislav AE, Pușcaș A, Marţiș Petruţ GS, Ungur RA. Spicy and Aromatic Plants for Meat and Meat Analogues Applications. Plants (Basel). 2022 11(7): 960. [CrossRef] [PubMed]
- Park Y-G, Cho J-H, Choi J, Ju E-M, Adam GO, Hwang D-I, Lee J-H, An S-Y, Choi H-K, Park C-B, Oh H-G.Immunomodulatory effects of Curcuma longa L. and Carthamus tinctorius L. on RAW 264.7 macrophages and cyclophosphamide-induced immunosuppression C57BL/6 mouse models. Journal of Functional Foods 2022; 91,105000. [ISSN 1756-4646] (https://www.sciencedirect.com/science/article/pii/S1756464622000706). [CrossRef]
- Sunil MA, Sunitha VS, Radhakrishnan EK, Jyothis M. Immunomodulatory activities of Acacia catechu, a traditional thirst quencher of South India. J Ayurveda Integr Med. 2019 10(3): 185-191. [CrossRef] [PubMed]
- Sharififar F, Pournournohammadi S, Arabnejad M. Immunomodulatory activity of aqueous extract of Achiella wilhelmsii C. Koch in mice. Indian J Exp Biol 2009, 47: 668-671.
- Saeidnia S, Gohari A, Mokhber-Dezfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011;19(3):173-86. [PubMed]
- Rajanna M, Bharathi B, Shivakumar BR, Deepak M, Prashanth D’S, Prabakaran D, Vijayabhaskar T, Arun B. Immunomodulatory effects of Andrographis paniculata extract in healthy adults – An open-label study. Journal of Ayurveda and Integrative Medicine 2021; 12(3): 529-534. [ISSN 0975-9476] (https://www.sciencedirect.com/science/article/pii/S0975947621001121). [CrossRef]
- Intharuksa A, Arunotayanun W, Yooin W, Sirisa-ard P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27: 4479. [CrossRef]
- Bushmeleva K, Vyshtakalyuk A, Terenzhev D, Belov T, Parfenov A, Sharonova N, Nikitin E, Zobov V. Radical Scavenging Actions and Immunomodulatory Activity of Aronia melanocarpa Propylene Glycol Extracts. Plants 2021; 10(11): 2458. [CrossRef]
- Ho GT, Bräunlich M, Austarheim I, Wangensteen H, Malterud KE, Slimestad R, Barsett H. Immunomodulating activity of Aronia melanocarpa polyphenols. Int J Mol Sci. 2014; 15(7): 11626-11636. [CrossRef] [PubMed]
- Lewu FB, Grierson DS, Afolayan AJ. The leaves of Pelargonium sidoides may substitute for its roots in the treatment of bacterial infections. Biological Conservation 2006, 128 (4): 582-584. [ISSN 0006-3207] (https://www.sciencedirect.com/science/article/pii/S0006320705004374. [CrossRef]
- Çiğdem Y, Şeker KG, Bahadır AÖ, Küpeli AE, Hakan BT, Eduardo S-S, Michael A, Samira S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Frontiers in Pharmacology 2022; 13. ISSN=1663-9812. [CrossRef]
- Suciyati, SW, Adnyana, IK. Red ginger (Zingiber officinale Roscoe var rubrum): a review. Pharmacologyonline 2017; 2: 60-65. [ISSN: 1820-8620].
- Zakharchenko NS, Belous AS, Biryukova YK, Medvedeva OA, Belyakova AV, Masgutova GA, Trubnikova EV, Buryanov YI, Lebedeva AA. Immunomodulating and Revascularizing Activity of Kalanchoe pinnata Synergize with Fungicide Activity of Biogenic Peptide Cecropin P1. Journal of Immunology Research 2017; ID 3940743. [CrossRef]
- Coutinho MA, Muzitano MF, Cruz EA, Bergonzi MC, Kaiser CR, Tinoco LW, Bilia AR, Vincieric FF, Rossi-Bergmann B, Costa SS. Flowers from Kalanchoe pinnata are a rich source of T cell-suppressive flavonoids. Nat Prod Commun. 2012; 7(2): 175-178. [PubMed]
- Anil SM, Peeri H, Koltai H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Frontiers in Pharmacology 2022; 13. [ISSN=1663-9812; [CrossRef]
- Cruz-Chamorro I, Santos-Sánchez G, Bollati C, Bartolomei M, Li J, Arnoldi A, Lammi C. Hempseed (Cannabis sativa) Peptides WVSPLAGRT and IGFLIIWV Exert Anti-inflammatory Activity in the LPS-Stimulated Human Hepatic Cell Line. Journal of Agricultural and Food Chemistry 2022; 70 (2): 577-583. (https://pubs.acs.org/doi/full/10.1021/acs.jafc.1c07520). [CrossRef]
- Magcwebeba T, Swart P, Swanevelder S, Joubert E, Gelderblom W. Anti-Inflammatory Effects of Aspalathus linearis and Cyclopia spp. Extracts in a UVB/Keratinocyte (HaCaT) Model Utilising Interleukin-1α Accumulation as Biomarker. Molecules. 2016; 21(10): 1323. [CrossRef] [PubMed]
- Roza O, Lai W-C, Zupkó I, Hohmann J, Jedlinszki N, Chang F-R, Csupor D, Eloff JN. Bioactivity-guided isolation of phytoestrogenic compounds from Cyclopia genistoides by the pER8: GUS reporter system. South African Journal of Botany 2017; 110: 201-207. [ISSN 0254-6299] (https://www.sciencedirect.com/science/article/pii/S0254629915326727. [CrossRef]
- Kumar S, Malhotra R, Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 2010; 4(7): 58-61. [CrossRef] [PubMed]
- Noor-E-Tabassum, Das R, Lami MS, Chakraborty AJ, Mitra S, Tallei TE, Idroes R, Mohamed AA, Hossain MJ, Dhama K, Mostafa-Hedeab G, Emran TB. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid Based Complement Alternat Med. 2022; 2022: 8288818. [CrossRef] [PubMed]
- Oskoueian E, Abdullah N, Ahmad S, Saad WZ, Omar AR, Ho YW. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int J Mol Sci. 2011; 12(9): 5955-70. [CrossRef] [PubMed]
- Ramadan, M.F. Bioactive Phytochemicals from Jatropha (Jatropha curcas L.) Oil Processing Byproducts. In: Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-products. Reference Series in Phytochemistry. (2022). Ed(s Ramadan Hassanien MF. Springer, Cham. [CrossRef]
- An E-K, Hwang J, Kim S-J, Park H-B, Zhang W, Ryu J-H, You S, Jin J-O. Comparison of the immune activation capacities of fucoidan and laminarin extracted from Laminaria japonica. International Journal of Biological Macromolecules 2022; 208: 230-242. [ISSN 0141-8130] (https://www.sciencedirect.com/science/article/pii/S0141813022005931. [CrossRef]
- Yadav N, Shakya P, Kumar A, Gautam RD, Chauhan R, Kumar D, Kumar A, Singh S, Singh S. Investigation on pollination approaches, reproductive biology and essential oil variation during floral development in German chamomile (Matricaria chamomilla L.). Sci Rep 2022; 12: 15285. [CrossRef]
- Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011; 5(9): 82-95. [CrossRef] [PubMed]
- Janeczek M, Moy L, Lake EP, Swan J. Review of the Efficacy and Safety of Topical Mahonia aquifolium for the Treatment of Psoriasis and Atopic Dermatitis. J Clin Aesthet Dermatol. 2018; 11(12): 42-47. [PubMed]
- Andreicuț AD, Fischer-Fodor E, Pârvu AE, Ţigu AB, Cenariu M, Pârvu M, Cătoi FA, Irimie A. Antitumoral and Immunomodulatory Effect of Mahonia aquifolium Extracts. Oxid Med Cell Longev. 2019; 2019: 6439021. [CrossRef] [PubMed]
- Bharani SER., Asad M, Dhamanigi SS, Chandrakala GK. Immunomodulatory activity of methanolic extract of Morus alba linn. (mulberry) leaves. Pak J Pharm Sci 2010, 23(1): 63-68.
- Grajek K, Wawro A, Kokocha D. Bioactivity of Morus alba L. Extracts – An Overview. International Journal of Pharmaceutical Sciences and Research 2015; 6(8): 3110-3122. [CrossRef]
- Raudone L, Vilkickyte G, Pitkauskaite L, Raudonis R, Vainoriene R, Motiekaityte V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019; 24(5): 844. [CrossRef] [PubMed]
- Sánchez M, Ureña-Vacas I, González-Burgos E. Kumar PD, Gómez-Serranillos MP. The Genus Cetraria s. str.—A Review of Its Botany, Phytochemistry, Traditional Uses and Pharmacology. Molecules 2022, 27(15): 4990. [CrossRef]
- Dobros N, Zawada K, Paradowska K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022; 11(4): 711. [CrossRef]
- Takaoka M. Of the phenolic substrate of hellebore (Veratrum grandiflorum Loes. fil.). J Faculty Sci Hokkaido Imperial University. 1940;3:1-16.
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem 2002;50(11):3337 -3340; A. Rauf, M. Imran, S. Har, B. Ahmad, D.G. Peters, M.S. Mubarak, A comprehensive review of the health perspectives of resveratrol Food Funct., 8 (12) (2017), pp. 4284-4305. [CrossRef]
- M.V. Alvarez, M.R. Moreira, A. Ponce Antiquorum sensing and antimicrobial activity of natural agents with potential use in food J. Food Saf., 32 (3) (2012), pp. 379-387.
- S.M. Makwana, Study of Antibacterial Property of Plant Based Phenolic Compounds and Food Contact Materials Coated with Functionalized Nanoparticles, Dissertations & Theses, Gradworks, 2013;
- K.K. Abuamero, A.A. Kondkar, K.V. Chalam Resveratrol and ophthalmic diseases Nutrients, 8 (4) (2016), pp. 200-210.
- A.R. Oliveira, F.C. Domingues, S. Ferreira, The influence of resveratrol adaptation on resistance to antibiotics, benzalkonium chloride, heat and acid stresses of Staphylococcus aureus and Listeria monocytogenes, Food Control., 73 (Part B) (2017), pp. 1420-1425.
- J.A. Seukep, L.P. Sandjo, B.T. Ngadjui, V. Kuete Antibacterial and antibiotic-resistance modifying activity of the extracts and compounds from Nauclea pobeguinii against gram-negative multi-drug resistant phenotypes BMC Complement. Altern. Med., 16 (1) (2016), pp. 1-8.
- K. Szkudelska, T. Szkudelski Resveratrol, obesity and diabetes Eur. J. Pharmacol., 635 (1) (2010), pp. 1-8.
- J. Vanamala, L. Reddivari, S. Radhakrishnan, C. Tarver Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways BMC Cancer, 10 (1) (2010), p. 238.
- A. Anya, B. Sara Malka, M.Y. Kramer, N.S. Schwartz, M.K. Holz The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells J. Cell. Biochem., 116 (3) (2015), pp. 450-457).
- C. Meza-Torres, J.D. Hernández-Camacho, A.B. Cortés-Rodríguez, L. Fang, T. Bui Thanh, E. Rodríguez-Bies, P. Navas, G. López-Lluch Resveratrol regulates the expression of genes involved in CoQ synthesis in liver in mice fed with high fat diet, Antioxidants, 9 (5) (2020), p. 431(Basel, Switzerland)).
- S. Rotondo, G. Rajtar, S. Manarini, A. Celardo, D. Rotillo, G. Gaetano, De, V. Evangelista, C. Cerletti Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function Br. J. Pharmacol., 123 (8) (2010), pp. 1691-1699.
- Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol 2001;62(9):1299-1308;
- Holmes-McNary M, Baldwin AS. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res 2000;60(13):3477-3483.
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol 2000;164(12):6509-6519.
- Silva AM, Oliveira MI, Sette L, Almeida CR, Oliveira MJ, Barbosa MA, et al. Resveratrol as a natural anti-tumor necrosis factor-α molecule: implications to dendritic cells and their crosstalk with mesenchymal stromal cells. PLoS One 2014;9(3):e91406;
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol 2004;287(4):L774-L783.
- Eo SH, Kim SJ. Resveratrol-mediated inhibition of cyclooxygenase-2 in melanocytes suppresses melanogenesis through extracellular signal-regulated kinase 1/2 and phosphoinositide 3-kinase/Akt signalling. Eur J Pharmacol 2019;860:172586.
- Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep 2005;57(3):390-394.
- Ma C, Wang Y, Shen A, Cai W. Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155. Int J Mol Med 2017;39(1):231-237.
- Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci 2005;78(4):389-397.
- Zhang LX, Li CX, Kakar MU, Khan MS, Wu PF, Amir RM, Dai DF, Naveed M, Li QY, Saeed M, Shen JQ, Rajput SA, Li JH. Resveratrol (RV): A pharmacological review and call for further research. Biomed Pharmacother. 2021 Nov;143:112164. [CrossRef]
- Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des. 2013;19(34):6064-93. [CrossRef]
- Jang M, Cai L, Udeani GO, Slowing KV, et al. Cancer chemopreventive activity of resveratrol a natural product derived from grapes. Science 1997;275:218–20;
- Zhang F, Shi JS, Zhou H, Wilson B, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–77.
- Xia N, Daiber A, Habermeier A, Closs EI, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–54.
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–81.
- Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1 heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22.
- Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc (Min/+) mice. J Nutr. 2004;134:5–10;
- Zunino SJ, Storms DH, Newman JW, Pedersen TL, et al. Resveratrol given intraperitoneally does not inhibit the growth of high-risk t (4.1) acute lymphoblastic leukemia cells in a NOD/SCID mouse model. Int J Oncol. 2012;40:1277–84.
- Stakleff KS, Sloan T, Blanco D, Marcanthony S, et al. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac J Cancer Prev. 2012;13:1333–40.
- Huang JP, Huang SS, Deng JY, Chang CC, et al. Insulin and resveratrol act synergistically preventing cardiac dysfunction in diabetes, but the advantage of resveratrol in diabetics with acute heart attack is antagonized by insulin. Free Radic Biol Med. 2010;49:1710–21.
- Azorín-Ortuño M, Yañéz-Gascón MJ, Pallarés FJ, Rivera J, et al. A dietary resveratrol-rich grape extract prevents the developing of atherosclerotic lesions in the aorta of pigs fed an atherogenic diet. J Agric Food Chem. 2012;60:5609–20.
- Akar F, Uludag O, Aydin A, Aytekin YA, et al. High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats protective effect of resveratrol. Food Chem Toxicol. 2012;50:2135–41.
- Kumar A, Naidu PS, Seghal N, Padi SS. Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology. 2007;79:17–26.
- Mudò G, Mäkelä J, DiLiberto V, Tselykh TV, et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;7:1153–65.
- Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, Gupta SC. Health benefits of resveratrol: Evidence from clinical studies. Med Res Rev. 2019 Sep;39(5):1851-1891. [CrossRef]
- 116. Schraufstatter E, Bernt H. Antibacterial action of curcumin and related compounds. Nature. 1949;164(4167):456. [CrossRef]
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. [CrossRef]
- Aggarwal, B.B., Yuan, W., Li, S., & Gupta, S.C. (2013). Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Molecular Nutrition & Food Research, 57(9), 1529–1542. [CrossRef]
- Girisa, S., Kumar, A., Rana, V., Parama, D., Daimary, U.D., Warnakulasuriya, S., ... Kunnumakkara, A.B. (2021). From simple mouth cavities to complex oral mucosal disorders-curcuminoids as a promising therapeutic approach. ACS Pharmacology & Translational Science, 4(2), 647–665. [CrossRef]
- Shabnam, B., Harsha, C., Thakur, K.K., Khatoon, E., & Kunnumakkara, A.B. (2021). Chapter 7: Curcumin: A potential molecule for the prevention and treatment of inflammatory diseases. In The chemistry and bioactive components of turmeric (pp. 150–171). Piccadilly, London: The Royal Society of Chemistry.
- Sivani, B.M.; Azzeh, M.; Patnaik, R.; Pantea Stoian, A.; Rizzo, A.M.; Banerjee, Y. Reconnoitering the Therapeutic Role of Curcumin in Disease Prevention and Treatment: Lessons Learned and Future Directions. Metabolites 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhuang, Z.; Lu, Y.; Tao, T.; Zhou, Y.; Liu, G.; Wang, H.; Zhang, D.; Wu, L.; Dai, H. Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Front. Neurosci. 2019, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani KM, Mahesh VB, Brann DW. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem 2007;102(2):522-538.
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-B pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300; [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kawata, A.; Fujisawa, S. Expression of cyclooxygenase-2, nitric oxide synthase 2 and heme oxygenase-1 mRNA induced by bis-eugenol in RAW264. 7 cells and their antioxidant activity determined using the induction period method. In Vivo 2017, 31, 819–831;
- Bhaumik S., Jyothi M. D., Khar A. (2000). Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 483 78–82. [CrossRef]
- Surh Y. J., Chun K. S., Cha H. H., Han S. S., Keum Y. S., Park K. K., et al. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res. 481 243–268. [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Garufi, A.; Giorno, E.; Gilardini Montani, M.S.; Pistritto, G.; Crispini, A.; Cirone, M.; D’Orazi, G. p62/SQSTM1/Keap1/NRF2 axis reduces cancer cells death-sensitivity in response to Zn (II)–curcumin complex. Biomolecules 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Mou Y, Wen S, Li YX, Gao XX, Zhang X, Jiang ZY. Recent progress in Keap1-Nrf2 protein-protein interaction inhibitors. Eur J Med Chem. 2020 Sep 15;202:112532. [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin Prevents Osteoarthritis by Inhibiting the Activation of Inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed]
- Yabas, M.; Orhan, C.; Er, B.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Bhanuse, P.; Morde, A.A.; Padigaru, M.; et al. A Next Generation Formulation of Curcumin Ameliorates Experimentally Induced Osteoarthritis in Rats via Regulation of Inflammatory Mediators. Front. Immunol. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Nirvanashetty, S.; Parachur, V.A.; Mohanty, N.; Swain, T. A Randomized, Double Blind, Placebo Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Curene® versus Placebo in Reducing Symptoms of Knee OA. Biomed. Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis: A randomized trial. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef] [PubMed]
- Lev-Ari, S.; Strier, L.; Kazanov, D.; Elkayam, O.; Lichtenberg, D.; Caspi, D.; Arber, N. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology 2006, 45, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Gharbi, M.; Dierckxsens, Y.; Priem, F.; Marty, M.; Seidel, L.; Albert, A.; Heuse, E.; Bonnet, V.; Castermans, C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014, 14, 159. [Google Scholar] [CrossRef]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000;52(4):673-751.
- Ito T, Warnken SP, May WS. Protein synthesis inhibition by flavonoids: roles of eukaryotic initiation factor 2alpha kinases. Biochem Biophys Res Commun 1999;265(2):589-594.
- Ruiz PA, Braune A, Hölzlwimmer G, Quintanilla-Fend L, Haller D. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J Nutr 2007;137(5):1208-1215.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin. : from antioxidant to nutraceutical. Eur J Pharmacol 2008;585(2-3):325-337.
- Min Z, Yangchun L, Yuquan W, Changying Z. Quercetin inhibition of myocardial fibrosis through regulating MAPK signaling pathway via ROS. Pak J Pharm Sci 2019;32(3 Special):1355-1359.
- Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E., Anti -inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages, Mediators Inflamm 2007; 2007:45673.
- Kobuchi H, Roy S, Sen CK, Nguyen HG, Packer L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am J Physiol 1999;277(3):C403-C411.
- Ying B, Yang T, Song X, Hu X, Fan H, Lu X, et al. Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways. Mol Biol Rep 2009;36(7):1825-1832.
- Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, et al. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci 2003;74(6):709-721.
- Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, et al. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res 2010;61(4):288-297.
- Bungsu I, Kifli N, Ahmad SR, Ghani H, Cunningham AC. Herbal Plants: The Role of AhR in Mediating Immunomodulation. Front Immunol 2021;12:697663; Michalski J, Deinzer A, Stich L, Zinser E, Steinkasserer A, Knippertz I. Quercetin induces an immunoregulatory phenotype in maturing humans. dendritic cells. Immunobiology 2020;225(4):151929.
- Yu W, Zhu Y, Li H, He Y. Injectable Quercetin-Loaded Hydrogel with Cartilage-Protection and Immunomodulatory Properties for Articular Cartilage Repair. ACS Appl Bio Mater 2020;3(2):761-771.
- Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med 2019;145:146-160.
- Karimi A, Naeini F, Asghari Azar V, Hasanzadeh M, Ostadrahimi A, Niazkar HR, et al. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine 2021;86:153567.
- R. A. Rifaai, N.F. El-Tahawy, and S. E. Ali, “Effect of quercetin on the endocrine pancreas of the experimentally induced diabetes in male albino rats: a histological and immunohistochemical study,” Journal of Diabetes & Metabolism, vol. 3, p. 3, 2012.
- H. E. Eitah, Y.A. Maklad, N.F. Abdelkader, A.A. Gamal El Din, M.A. Badawi, and S. A. Kenawy, “Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats,” Toxicology and Applied Pharmacology, vol. 365, pp. 30–40, 2019.
- Yi H, Peng H, Wu X, Xu X, Kuang T, Zhang J, Du L, Fan G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxid Med Cell Longev. 2021 Jun 23;2021:6678662. [CrossRef]
- Cesare Bonezzi, Amedeo Costantini, Giorgio Cruccu, Diego M.M. Fornasari, Vittorio Guardamagna, Vincenzo Palmieri, Enrico Polati, Pierangelo Zini & Anthony H Dickenson (2020), Capsaicin 8% dermal patch in clinical practice: an expert opinion, Expert Opinion on Pharmacotherapy, 21:11, 1377-1387. [CrossRef]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389(6653):816-824 ; Yang F, Zheng J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017;8(3):169-177.
- O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev 2012;64(4):939-971.
- Haanpää M, Treede RD. Capsaicin for neuropathic pain: linking traditional medicine and molecular biology. Eur Neurol 2012;68(5):264-275.
- Sanz-Salvador L, Andrés-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem 2012;287(23):19462-19471.
- Kim CS, Kawada T, Kim BS, Han IS, Choe SY, Kurata T, et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal 2003;15(3):299-306.
- Li T, Wang G, Hui VCC, Saad D, de Sousa Valente J, La Montanara P, et al. TRPV1 feed-forward sensitisation depends on COX2 upregulation in primary sensory neurons. Sci Rep 2021;11(1):3514.
- Fischer BS, Qin D, Kim K, McDonald TV. Capsaicin inhibits Jurkat T-cell activation by blocking calcium entry current I(CRAC). J Pharmacol Exp Ther 2001;299(1):238-246.
- Zhang J, Nagasaki M, Tanaka Y, Morikawa S. Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk Res 2003;27(3):275-283.
- Nevius E, Srivastava PK, Basu S. Oral ingestion of Capsaicin, the pungent component of chili pepper, enhances a discreet population of macrophages and confers protection from autoimmune diabetes. Mucosal Immunol 2012;5(1):76-86.
- Viveros-Paredes JM, Puebla-Pérez AM, Gutiérrez-Coronado O, Macías-Lamas AM, Hernández-Flores G, Ortiz-Lazareno PC, et al. Capsaicin attenuates immunosuppression induced by chronic stress in BALB/C mice. Int Immunopharmacol 2021;93:107341.
- Singh B. N., Shankar S., Srivastava R. K. (2011). Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 82 1807–1821. [CrossRef]
- Yang H, Landis-Piwowar K, Chan TH, Dou QP. Green tea polyphenols as proteasome inhibitors: implication in chemoprevention. Curr Cancer Drug Targets 2011;11(3):296-306.
- Zhou Y, Tang J, Du Y, Ding J, Liu JY. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumour Biol 2016;37(7):8555-8566.
- Chen BH, Hsieh CH, Tsai SY, Wang CY, Wang CC. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci Rep 2020;10(1):5163.
- Muraoka K, Shimizu K, Sun X, Tani T, Izumi R, Miwa K, et al. Flavonoids exert diverse inhibitory effects on the activation of NF-kappaB. Transplant Proc 2002;34(4):1335-1340.
- Joo SY, Song YA, Park YL, Myung E, Chung CY, Park KJ, et al. Epigallocatechin-3-gallate Inhibits LPS-Induced NF-κB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver 2012;6(2):188-196).
- Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (-)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J 2001;15(11):2022-2024.
- Shih LJ, Lin YR, Lin CK, Liu HS, Kao YH. Green tea (-)-epigallocatechin gallate induced growth inhibition of human placental choriocarcinoma cells. Placenta 2016;41:1-9.
- Hara Y, Fujino M, Adachi K, Li XK. The reduction of hypoxia-induced and reoxygenation-induced apoptosis in rat islets by epigallocatechin gallate. Transplant Proc 2006;38(8):2722-2725.
- Yu HN, Ma XL, Yang JG, Shi CC, Shen SR, He GQ. Comparison of effects of epigallocatechin-3-gallate on hypoxia injury to human umbilical vein, RF/6A, and ECV304 cells induced by Na(2)S(2)O(4). Endothelium 2007;14(4-5):227-23.
- Gu JJ, Qiao KS, Sun P, Chen P, Li Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Eur Rev Med Pharmacol Sci 2018;22(14):4557-4563.
- Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, et al. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol 2004;173(9):5794-5800 118.
- Wang J, Ren Z, Xu Y, Xiao S, Meydani SN, Wu D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am J Pathol 2012;180(1):221-234.
- Byun JK, Yoon BY, Jhun JY, Oh HJ, Kim EK, Min JK, et al. Epigallocatechin-3-gallate ameliorates both obesity and autoinflammatory arthritis aggravated by obesity by altering the balance among CD4+ T-cell subsets. Immunol Lett 2014;157(1-2):51-59.
- Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett 2011;139(1-2):7-13.
- Sadava D., Whitlock E., Kane S. E. (2007). The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem. Biophys. Res. Commun. 360 233–237. [CrossRef]
- Bandele O. J., Osheroff N. (2008). (-)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem. Res. Toxicol. 21 936–943. [CrossRef]
- Lee W. J., Shim J. Y., Zhu B. T. (2005). Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 68 1018–1030.
- Cai Y, Kurita-Ochiai T, Hashizume T, Yamamoto M. Green tea epigallocatechin-3-gallate attenuates Porphyromonas gingivalis-induced atherosclerosis. Pathog Dis 2013;67(1):76-83.
- Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, et al. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo 2013;27(5):627-634.
- Farooqi AA, Attar R, Sabitaliyevich UY, Alaaeddine N, de Sousa DP, Xu B, Cho WC. The Prowess of Andrographolide as a Natural Weapon in the War against Cancer. Cancers (Basel). 2020 Aug 4;12(8):2159. [CrossRef]
- Maiti K., Gantait A., Mukherjee K., Saha B., Mukherjee P. K. (2006). Therapeutic potentials of andrographolide from Andrographis paniculata: a review. J. Nat. Remed. 6 1–13.
- Qin L. H., Kong L., Shi G. J., Wang Z. T., Ge B. X. (2006). Andrographolide inhibits the production of TNF-alpha and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinases. Biol. Pharm. Bull. 29 220–224. [CrossRef]
- Rajagopal, S. Rajagopal S., Kumar R. A., Deevi D. S., Satyanarayana C., Rajagopalan R. (2003). Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 3 147–158. [CrossRef]
- Chiou W. F., Chen C. F., Lin J. J. (2000). Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 129 1553–1560. [CrossRef]
- Lee K. C., Chang H. H., Chung Y. H., Lee T. Y. (2011). Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 135 678–684. [CrossRef]
- Islam MT. Andrographolide, a New Hope in the Prevention and Treatment of Metabolic Syndrome. Front Pharmacol. 2017 Aug 23;8:571. [CrossRef]
- Corbett J. A., Kwon G., Marino M. H., Rodi C. P., Sullivan P. M., Turk J., et al. (1996). Tyrosine kinase inhibitors prevent cytokine-induced expression of iNOS and COX-2 by human islets. Am. J. Physiol. 270 C1581–C1587).
- Mccabe M. J., Jr., Orrenius S. (1993). Genistein induces apoptosis in immature human thymocytes by inhibiting topoisomerase-II. Biochem. Biophys. Res. Commun. 194 944–950. [CrossRef]
- Si H., Liu D. (2007). Phytochemical genistein in the regulation of vascular function: new insights. Curr. Med. Chem 14 2581–2589. [CrossRef]
- Lee Y. W., Lee W. H. (2008). Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J. Nutr. Biochem. 19 819–825. [CrossRef]
- Wang J., Zhang Q., Jin S., He D., Zhao S., Liu S. (2008). Genistein modulate immune responses in collagen-induced rheumatoid arthritis model. Maturitas 59 405–412. [CrossRef]
- Wang X., Chen S., Ma G., Ye M., Lu G. (2005). Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 16 267–270. [CrossRef]
- Yalniz M., Bahcecioglu I. H., Kuzu N., Poyrazoglu O. K., Bulmus O., Celebi S., et al. (2007). Preventive role of genistein in an experimental non-alcoholic steatohepatitis model. J. Gastroenterol. Hepatol. 22 2009–2014. [CrossRef]
- Seibel J., Molzberger A. F., Hertrampf T., Laudenbach-Leschowski U., Diel P. (2009). Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur. J. Nutr. 48 213–220. [CrossRef]
- Bhattacharyya B., Panda D., Gupta S., Banerjee M. (2008). Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 28 155–183. [CrossRef]
- Stanton R. A., Gernert K. M., Nettles J. H., Aneja R. (2011). Drugs that target dynamic microtubules: a new molecular perspective. Med. Res. Rev. 31 443–481. [CrossRef]
- Imazio M, Bobbio M, Cecchi E, Demarie D, Demichelis B, Pomari F, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005;112(13):2012-2016.
- Imazio M, Brucato A, Cemin R, Ferrua S, Belli R, Maestroni S, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med 2011;155(7):409-414;
- Imazio M., Brucato A., Cemin R., Ferrua S., Maggiolini S., Beqaraj F., et al. (2013). A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 369 1522–1528. [CrossRef]
- Deftereos S., Giannopoulos G., Kossyvakis C., Efremidis M., Panagopoulou V., Kaoukis A., et al. (2012). Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J. Am. Coll. Cardiol. 60 1790–1796. [CrossRef]
- Imazio, M. Imazio M., Trinchero R., Brucato A., Rovere M. E., Gandino A., Cemin R., et al. (2010). Colchicine for the prevention of the post-pericardiotomy syndrome (COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur. Heart J. 31 2749–2754. [CrossRef]
- Perico N, Ostermann D, Bontempeill M, Morigi M, Amuchastegui CS, Zoja C, et al. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol 1996;7(4):594-601.
- Titus RG. Colchicine is a potent adjuvant for eliciting T cell responses. J Immunol 1991;146(12):4115-4119.
- Weng JH, Koch PD, Luan HH, Tu HC, Shimada K, Ngan I, et al. Colchicine acts selectively in the liver to induce hepatokines that inhibit myeloid cell activation. Nat Metab 2021;3(4):513-522.
- Li C, Yang CW, Ahn HJ, Kim WY, Park CW, Park JH, et al. Colchicine decreases apoptotic cell death in chronic cyclosporine nephrotoxicity. J Lab Clin Med. 2002;139(6):364–371.
- Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, et al. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28(Suppl 5):S53–S57.
- Lee FY, Lu HI, Zhen YY, Leu S, Chen YL, Tsai TH, et al. Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur J Pharm Sci. 2013;50(3–4):372–384.
- Nuki G. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr Rheumatol Rep 2008;10(3):218-227; Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev 2011;31(3):443-481.
- Lin W. C., Lin J. Y. (2011). Berberine down-regulates the Th1/Th2 cytokine gene expression ratio in mouse primary splenocytes in the absence or presence of lipopolysaccharide in a preventive manner. Int. Immunopharmacol. 11 1984–1990. [CrossRef]
- Son D. J., Akiba S., Hong J. T., Yun Y. P., Hwang S. Y., Park Y. H., et al. (2014). Piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting cyclooxygenase-1 activity: different mechanisms of action are involved in the inhibition of platelet aggregation and macrophage inflammatory response. Nutrients 6 3336–3352. [CrossRef]
- Zhao F., Nozawa H., Daikonnya A., Kondo K., Kitanaka S. (2003). Inhibitors of nitric oxide production from hops (Humulus lupulus L.). Biol. Pharm. Bull. 26 61–65. [CrossRef]
- Zhang B., Liu Z. Y., Li Y. Y., Luo Y., Liu M. L., Dong H. Y., et al. (2011). Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur. J. Pharm. Sci. 44 573–579. [CrossRef]
- Hamalainen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. (2007). Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007:45673. [CrossRef]
- Chen C. Y., Peng W. H., Tsai K. D., Hsu S. L. (2007). Luteolin suppresses inflammation-associated gene expression by blocking NF-κB and AP-1 activation pathways in mouse alveolar macrophages. Life Sci. 81 1602–1614. [CrossRef]
- Kang H.-K., Ecklund D., Liu M., Datta S. K. (2009). Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res. Ther. 11 R59. [CrossRef]
- Kang S. R., Park K. I., Park H. S., Lee D. H., Kim J. A., Nagappan A., et al. (2011). Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 129 1721–1728. [CrossRef]
- Chandrashekar N., Selvamani A., Subramanian R., Pandi A., Thiruvengadam D. (2012). Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol. Appl. Pharmacol. 261 10–21. [CrossRef]
- Lee W., Ku S.-K., Bae J.-S. (2015). Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 38 110–125. [CrossRef]
- Yoo H., Ku S.-K., Baek Y.-D., Bae J.-S. (2014). Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflam. Res. 63 197–206. [CrossRef]
- Liu X., Mei Z., Qian J., Zeng Y., Wang M. (2013). Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen. Res. 8 3203 10.3969/j.issn.1673-5374.2013.34.004.
- Vaillancourt F., Silva P., Shi Q., Fahmi H., Fernandes J. C., Benderdour M. (2011). Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell. Biochem. 112 107–117. [CrossRef]
- Youn J., Lee J. S., Na H. K., Kundu J. K., Surh Y. J. (2009). Resveratrol and piceatannol inhibit iNOS expression and NF-κB activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 61 847–854. [CrossRef]
- Andújar I., Recio M. C., Bacelli T., Giner R. M., Rios J. L. (2010). Shikonin reduces oedema induced by phorbol ester by interfering with IκBα degradation thus inhibiting translocation of NF-κB to the nucleus. Br. J. Pharmacol. 160 376–388. [CrossRef]
- Brinker A. M., Ma J., Lipsky P. E., Raskin I. (2007). Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68 732–766. [CrossRef]
- Kannaiyan R., Shanmugam M. K., Sethi G. (2011). Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 303 9–20. [CrossRef]
- Wu C. J., Wang Y. H., Lin C. J., Chen H. H., Chen Y. J. (2011). Tetrandrine down-regulates ERK/NF-κB signaling and inhibits activation of mesangial cells. Toxicol In Vitro 25 1834–184. [CrossRef]
- Kim S. Y., Moon K. A., Jo H. Y., Jeong S., Seon S. H., Jung E., et al. (2012). Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol. Cell Biol. 90 441–448. 10.1038/icb.2011.60; Stefanska J., Pawliczak R. (2008). Apocynin: molecular aptitudes. Mediators Inflamm. 2008:106507. [CrossRef]
- Ammon H. P. (2006). Boswellic acids in chronic inflammatory diseases. Planta Med. 72 1100–1116. [CrossRef]
- Khanna K, Kohli SK, Kaur R, Bhardwaj A, Bhardwaj V, Ohri P, Sharma A, Ahmad A, Bhardwaj R, Ahmad P. Herbal immune-boosters: Substantial warriors of pandemic Covid-19 battle. Phytomedicine. 2021 May;85:153361. Epub 2020 Oct 3. [CrossRef] [PubMed]
| Botanical name/Family. | Source countries | Part used | Bioactive-chemical constituent | Biological activity | Reference |
|---|---|---|---|---|---|
| Acacia catechu/ Fabaceae | India, East Africa | leaves, bark | flavonoids (quercetin, catechin, epicatechin) | antioxidant, immunomodulatory, hypoglycaemic | [17,35,49] |
| Achillea millefolium/Compositae | Northern Hemisphere | whole plant | Flavonoids, alkaloids, polyacetylenes, coumarins, triterpenes, lactones | anti-inflammatory, antispasmodic, antipyretic, diuretic | [50,51] |
| Andrographis paniculata/Acanthaceae | India, Sri Lanka | whole plant, leaves, stems, roots | diterpenoids (andrographolide), lactones, flavonoids, polysaccharides | immunomodulatory, hepatoprotective, antispasmodic, anticancer, anti-inflammatory, antiviral, anti-proliferative, anti-platelet | [52,53] |
| Aronia melanocarpa/ Rosaceae | North Amerika | fruits, bark, leaves | flavonoids (procyanidins anthocyanins), catechins, phenolic acids, ascorbic acid |
immunomodulatory, anti-inflammation, antioxidant, gastroprotective, hepatoprotective, antiproliferative, cardiovascular-protective, antioxidants | [20,54,55] |
| Pelargonium sidoides/ Geraniaceae | South Africa | roots, shoot, leaves | coumarins, phenols | immunomodulatory, antibacterial | [35,56] |
|
Zingiber officinale/ Zingiberaceae (ginger) |
Asia | roots, leaves | phenolic acid (eugenol, gingerols, shogaols, paradols) lactons, terpenes | immunostimulation, antimicrobial, antioxidant, analgesic, anti-inflammatory, anticancer, antihypertensive | [57,58] |
| Kalanchoe pinnata/Crassulaceae | Madagascar | leaves, flowers | flavonoid glycosides (quercitrin), bufadienolides, lectins, polyphenols | immunosuppressive, antifungal, antimicrobial, antiviral, wound healing (antiscar), anti-inflammatory | [59,60] |
|
Camellia sinensis/Theaceae (green tea) |
China, India, Nepal | leaves | catechins (epigallocatechin-3-gallate, epigallocatechin, epicatechin), triterpenoids, saponins | immunomodulatory, antioxidant, antiviral, anticancer, antifungal activities. | [20] |
| Cannabis sativa/Cannabaceae | Central Asia, widely cultivated around the world | leaves, seeds, inflorescence | cannabinoid (cannabidiol, cannabigerol, Δ9-tetrahydrocannabinol), terpenes, flavonoids | anti-inflammatory, immunosuppressive, neuroprotective, antioxidant | [61,62] |
| Capsicum species/ Solanaceae | Central and South America | fruits | provitamin A, vitamins (E, C) carotenoids, phenolic compounds ( capsaicinoids, luteolin, quercetin) | antioxidant, antimicrobial, antiseptic, anticancer, counterirritant, antioxidant, immunomodulator | [37] |
|
Cyclopia genistoides/Fabaceae (Honeybush) |
South Africa | flowers, leaves, stems | phenols, flavones, flavanones isoflavones, xanthones (mangiferin), coumestans, catechins (epigallocatechin-3-gallate), benzaldehyde derivates, phytoestrogens | immunomodulatory, anti-inflammatory, antioxidant, anti-proliferative, anticancer, cytoprotective | [63,64] |
| Euphorbia hirta/Euphorbiaceae | India, Australia | herb, leaves, roots | flavanoid glycoside, phenolic acids, alkaloids | anticancer, antioxidant, antibacterial, antifungal, antimalarial, anti-inflammatory, antiasthmatic | [17,54] |
| Ginkgo biloba/ Ginkgoaceae | China | leaves, seeds | flavonoids, terpenoids, alkylphenols, anthocyanidins, lignans, polyprenols polysaccharides, 4′-o-methylpyridoxine | immunomodulatory, antioxidants, anti-inflammation, anticancer, antidiabetic, antilipidemic, antimicrobial, anti-lipid peroxidation, antiplatelet, hepatoprotective, neuroprotective | [66] |
| Jatropha curcas/Euphorbiaceae | Mexico, Central America, Brazil | leaves, roots, stems | phenolics, flavonoids, sterols, saponins, phorbol esters, cyclic peptides, lignans, alkaloids, coumarins, terpenes | anti-inflammatory, antimicrobial, antioxidant | [67,68] |
|
Lycium barbarum/Solanaceae (Goji berry) |
China, Asia, Europe | fruits, leaves, roots | polysaccharides, scopoletin, carotenoids, flavonoids, vitamins | antioxidant, antiviral, anticancer, anti-inflammatory, cardioprotective | [20,69] |
| Matricaria chamomilla/ Asteraceae | Southeast Europe | flowers | terpenoids (α-bisabolol, chamazulene), flavonoids sesquiterpenes, coumarins, polyacetylenes | immunomodulatory, antioxidant, anti- inflammatory antiseptic, antispasmodic | [70,71] |
| Mahonia aquifolium/Berberidacea | Eastern Asia, North and Central America | leaves, bark | alkaloids, phenolics, flavonoids, quinones, vitamins, coumarins | anti-inflammatory, antifungal, antimicrobial, antiproliferative, hepatoprotective, analgesic antioxidant | [72,73] |
| Morus alba/Moraceae | Central and Eastern Asia, Caucasus, widely cultivated around the world | fruits, leaves, bark | flavonoids, anthocyanins, saponins, alkaloids, tannins, phenolic acids, anthocyanins, ascorbic acid, β-carotene | anticancer, antimicrobial, antidiabetic, immunomodulatory, cardioprotective, hepatoprotective, hypocholesterolaemic, | [17,74,75] |
| Vaccinium vitis-idaea/Ericaceae | Baltic countries (Europe), Russia, Canada | leaves, fruits | phenolic, arbutin, flavonol glycosides, proanthocyanidins | antioxidant | [76] |
| Cetraria islandica/ Parmeliaceae | Europe, North America | seeds, fruits, roots, leaves, stems, | dibenzofuranos, depsidones, fatty acids (lichesterinic acid, protolichesterinic acids), depsides, terpenes | immunomodulatory, antioxidant, cytotoxic, genotoxic, antigenotoxic antimicrobial, anticancer, antidiabetic, anti-inflammatory | [77] |
| Lavandula angustifolia/ Lamiaceae | Europe | stems, flowers | terpenes, polyphenols (rosmarinic acid, caffeic acid, lavandufurandiol, lavandunat), coumarins, flavonoids (apigenin, luteolin glycosides, catechin) | immunomodulatory, antioxidant, anti-inflammatory, analgesic, antibacterial | [78] |
| Chemical c compounds/molecules |
Mechanism | Clinical trials (number) |
Reference |
|---|---|---|---|
| Berberine | Regulate T- cells cytokines TNF-α, IL-2 and IL-4 production. | 84 | [220] |
| Piperine | Reduce IL-1β, IL-6, and TNF-α; regulate expression of COX-2, NOS-2, and NF-κB. | 28 | [221] |
| Xanthohumol | Inhibit NO production | 10 | [222] |
| Matrine | Reduced reactive oxygen species inflammatory mediators and myeloperoxidase and maleic dialdehyde activity |
2 | [223] |
| Daidzein | Decreases TNF-α, IL-1β, MCP-1, NO, and iNOS | 24 | [224] |
| Luteolin | Reduce secretion of INF-γ, IL-6, COX-2 and ICAM-1 Block heat shock protein 90 activity. |
18 | [225] |
| Apigenin | Downregulate expression of IL-1α, TNF-α, IL-8, COX-2 and iNOS; Decreased response of Th1 and Th17 cells. |
12 | [226] |
| Nobiletin | Inhibit COX-2 and iNOS expression by blocking NF-κB and MAPK signaling | 1 | [227] |
| Baicalein | Inhibit expression of iNOS, COX-2, TNF-α, IL-1β, PGE2, and TNF-α by regulating NF-κB and ER-dependent pathway. | 1 | [228,229] |
| Kaempferol | Reduce iNOS and COX-2 by suppressing STAT-1, NF-kappa B, and AP-1. Decrease expression of ICAM-1, VCAM-1 and MCP-1. | 5 | [224] |
| Rutin | Suppress production of TNF-α, IL-6. Activation of NF-κB and leukocyte migration. |
34 | [230] |
| Puerarin | Inhibit NF-κB and activation of STAT3. | 8 | [231] |
| Thymoquinone | Inhibit IL-1β, TNF-α, MMP-13, COX-2, and PGE2, MAPK p38, ERK1/2, and NF-kBp65. | 8 | [232] |
| Piceatannol | Inhibit iNOS expression and NF-kB, ERK, and STAT3. | 1 | [233] |
| Shikonin | Inhibit NF-κB activity and Th1 cytokines expression and induce Th2 cytokines. | 2 | [234] |
| Oleanolic acid | Reduce the level of IL-1α, IL-6, and TNF-α and adenosine deaminase activity. | 4 | [235] |
| Triptolide | Inhibits lymphocyte activation, IL-2, iNOS, TNF-α, COX-2, IFN-γ, NF-kB, NFAT, and STAT3. | 25 | [235] |
| Celastrol | Inhibit IL-2, iNOS, TNF-α, COX-2, adhesion molecules and topoisomerase II. | 2 | [236] |
| Tetrandrine | Regulates ERK/NF-κB signaling and inhibits activation of mesangial cells | 2 | [237] |
| Apocynin | Inhibit NADPH oxidase and suppress pro-inflammatory cytokines, CD4+ and CD8+T cells production. |
8 | [239] |
| 11-keto-β-boswellic acid | Decrease IL-1, IL-2, IL-4, IL-6, and IFN-γ | 1 | [240] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





