Submitted:
05 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
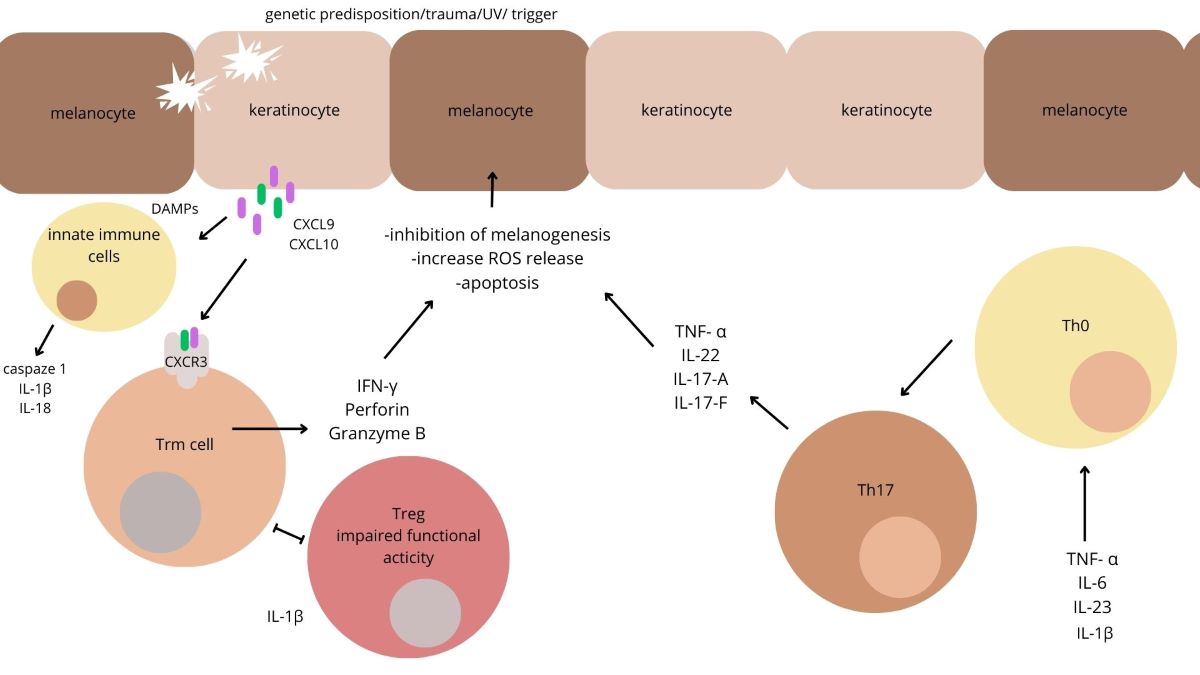
1. Introduction
1. IL-6
1.1. IL-6 Inhibitor
2. IL-15
2.1. IL-15 Inhibitors
3. TNF-α
3.1. TNF-α Inhibitors
4. IL-1β
5. IL-22
5.1. IL-22 Inhibitor
6. IL-17
1.1. IL-17A Inhibitors
7. IL-23
1.1. IL-23 Inhibitor
1.2. IL-12 and IL-23 Inhibitor
8. IFN-γ
8.1. JAK Inhibitors
8.1.1. Ruxolitinib
8.1.2. Tofacitinib
8.1.3. Baricitinib
8.1.4. Ritlecitinib
8.1.5. Ifidancitinib
8.1.6. Brepocitinib
8.1.7. Upadacitinib
8.1.8. Cerdulatinib
8.1.9. Delgocitinib
Other Perspectives
Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergqvist C, Ezzedine K. Vitiligo: A Review. Dermatology [Internet]. 2020 Nov 1 [cited 2023 Nov 5];236(6):571–92. Available from: https://pubmed.ncbi.nlm.nih.gov/32155629/.
- Ezzedine K, Grimes PE, Meurant JM, Seneschal J, Léauté-Labrèze C, Ballanger F, et al. Living with vitiligo: results from a national survey indicate differences between skin phototypes. Br J Dermatol [Internet]. 2015 Aug 1 [cited 2023 Nov 5];173(2):607–9. Available from: https://pubmed.ncbi.nlm.nih.gov/25892476/.
- Marchioro HZ, Silva de Castro CC, Fava VM, Sakiyama PH, Dellatorre G, Miot HA. Update on the pathogenesis of vitiligo. An Bras Dermatol [Internet]. 2022 Jul 1 [cited 2023 Nov 7];97(4):478. Available from: /pmc/articles/PMC9263675/.
- Iwanowski T, Kołkowski K, Nowicki RJ, Sokołowska-Wojdyło M. Etiopathogenesis and Emerging Methods for Treatment of Vitiligo. Int J Mol Sci [Internet]. 2023 Jun 1 [cited 2024 May 14];24(11). Available from: https://pubmed.ncbi.nlm.nih.gov/37298700/.
- Bergqvist C, Ezzedine K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J Dermatol [Internet]. 2021 Mar 1 [cited 2023 Oct 25];48(3):252–70. Available from: https://pubmed.ncbi.nlm.nih.gov/33404102/.
- Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet [Internet]. 2015 Jul 4 [cited 2023 Feb 8];386(9988):74–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25596811.
- Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. 2007 [cited 2023 Nov 15]; Available from: https://academic.oup.com/cei/article/148/1/32/6457759.
- Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med [Internet]. 2013 Feb 27 [cited 2024 May 14];5(174). Available from: https://pubmed.ncbi.nlm.nih.gov/23447019/.
- Steinbach K, Vincenti I, Merkler D. Resident-Memory T Cells in Tissue-Restricted Immune Responses: For Better or Worse? Front Immunol [Internet]. 2018 Nov 30 [cited 2024 May 14];9(NOV). Available from: https://pubmed.ncbi.nlm.nih.gov/30555489/.
- Boniface K, Jacquemin C, Darrigade AS, Dessarthe B, Martins C, Boukhedouni N, et al. Vitiligo Skin Is Imprinted with Resident Memory CD8 T Cells Expressing CXCR3. J Invest Dermatol [Internet]. 2018 Feb 1 [cited 2024 May 14];138(2):355–64. Available from: https://pubmed.ncbi.nlm.nih.gov/28927891/.
- Frisoli ML, Essien K, Harris JE. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu Rev Immunol [Internet]. 2020 Apr 26 [cited 2023 Nov 7];38:621–48. Available from: https://pubmed.ncbi.nlm.nih.gov/32017656/.
- Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, et al. Phototherapy for Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol [Internet]. 2017 Jul 1 [cited 2023 Nov 7];153(7):666. Available from: /pmc/articles/PMC5817459/.
- Yones SS, Palmer RA, Garibaldinos TM, Hawk JLM. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol [Internet]. 2007 May [cited 2023 Nov 7];143(5):578–84. Available from: https://pubmed.ncbi.nlm.nih.gov/17519217/.
- Parsad D, Kanwar AJ, Kumar B. Psoralen-ultraviolet A vs. narrow-band ultraviolet B phototherapy for the treatment of vitiligo. J Eur Acad Dermatol Venereol [Internet]. 2006 Feb [cited 2023 Nov 7];20(2):175–7. Available from: https://pubmed.ncbi.nlm.nih.gov/16441626/.
- Bhatnagar A, Kanwar AJ, Parsad D, De D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. Journal of the European Academy of Dermatology and Venereology [Internet]. 2007 May 1 [cited 2023 Nov 7];21(5):638–42. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1468-3083.2006.02035.x.
- Nicolaidou E, Antoniou C, Stratigos A, Katsambas AD. Narrowband ultraviolet B phototherapy and 308-nm excimer laser in the treatment of vitiligo: A review. J Am Acad Dermatol. 2009 Mar 1;60(3):470–7. [CrossRef]
- Esmat S, Hegazy RA, Shalaby S, Chu-Sung Hu S, Lan CCE. Phototherapy and Combination Therapies for Vitiligo. Dermatol Clin [Internet]. 2017 Apr 1 [cited 2023 Nov 7];35(2):171–92. Available from: https://pubmed.ncbi.nlm.nih.gov/28317527/.
- Taieb A, Alomar A, Böhm M, Dell’Anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol [Internet]. 2013 Jan [cited 2023 Nov 7];168(1):5–19. Available from: https://pubmed.ncbi.nlm.nih.gov/22860621/.
- Cavalié M, Ezzedine K, Fontas E, Montaudié H, Castela E, Bahadoran P, et al. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: a randomized, double blind, placebo-controlled study. J Invest Dermatol [Internet]. 2015 Apr 20 [cited 2023 Nov 7];135(4):970–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25521460/.
- Manga P, Elbuluk N, Orlow SJ. Recent advances in understanding vitiligo. F1000Res [Internet]. 2016 [cited 2023 Oct 28];5. Available from: /pmc/articles/PMC5017284/.
- Swope VB, Abdel-Malek Z, Kassem LM, Nordlund JJ. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol [Internet]. 1991 [cited 2024 Jan 3];96(2):180–5. Available from: https://pubmed.ncbi.nlm.nih.gov/1899443/.
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) [Internet]. 2012 Feb [cited 2024 Jan 1];122(4):143–59. Available from: https://pubmed.ncbi.nlm.nih.gov/22029668/.
- De A, Choudhary N, Sil A, Sarda A, Raja AHH. A Cross-Sectional Study of the Levels of Cytokines IL-6, TNF-α, and IFN-γ in Blood and Skin (Lesional and Uninvolved) of Vitiligo Patients and their Possible Role as Biomarkers. Indian J Dermatol [Internet]. 2023 [cited 2023 Oct 22];68(1):67. Available from: /pmc/articles/PMC10162755/.
- Karagün E, Baysak S. Levels of TNF-α, IL-6, IL-17, IL-37 cytokines in patients with active vitiligo. Aging Male [Internet]. 2020 [cited 2023 Nov 23];23(5):1487–92. Available from: https://pubmed.ncbi.nlm.nih.gov/33191834/.
- Abdallah M, El-Mofty M, Anbar T, Rasheed H, Esmat S, Al-Tawdy A, et al. CXCL-10 and Interleukin-6 are reliable serum markers for vitiligo activity: A multicenter cross-sectional study. Pigment Cell Melanoma Res [Internet]. 2018 Mar 1 [cited 2023 Oct 22];31(2):330–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29094481/.
- Sushama S, Dixit N, Gautam RK, Arora P, Khurana A, Anubhuti A. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of disease. J Cosmet Dermatol [Internet]. 2019 Feb 1 [cited 2023 Oct 22];18(1):337–41. Available from: https://pubmed.ncbi.nlm.nih.gov/29504235/.
- Kuet K, Goodfield M. Multiple halo naevi associated with tocilizumab. Clin Exp Dermatol [Internet]. 2014 [cited 2023 Oct 23];39(6):717–9. Available from: https://pubmed.ncbi.nlm.nih.gov/24986573/.
- Bunker CB, Manson J. Vitiligo remitting with tocilizumab. J Eur Acad Dermatol Venereol [Internet]. 2019 Jan 1 [cited 2023 Oct 22];33(1):e20. Available from: https://pubmed.ncbi.nlm.nih.gov/29888453/.
- Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood [Internet]. 2008 Nov 15 [cited 2023 Oct 23];112(10):3959–64. Available from: https://pubmed.ncbi.nlm.nih.gov/18784373/.
- Choong DJ, Tan E. Does tocilizumab have a role in dermatology? A review of clinical applications, its adverse side effects and practical considerations. Dermatol Ther. 2021 Jul 1;34(4).
- Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nature Reviews Immunology 2015 15:12 [Internet]. 2015 Nov 16 [cited 2024 May 16];15(12):771–83. Available from: https://www.nature.com/articles/nri3919.
- Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of Skin Resident Memory T Cells. Front Immunol [Internet]. 2020 Feb 3 [cited 2024 May 16];11:1. Available from: /pmc/articles/PMC7901930/.
- Chen X, Guo W, Chang Y, Chen J, Kang P, Yi X, et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8+ T cells activation via JAK-STAT pathway in vitiligo. Free Radic Biol Med [Internet]. 2019 Aug 1 [cited 2024 May 16];139:80–91. Available from: https://pubmed.ncbi.nlm.nih.gov/31078730/.
- Richmond JM, Strassner JP, Zapata LZ, Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Transl Med [Internet]. 2018 Jul 18 [cited 2024 May 16];10(450):7710. Available from: https://www.science.org/doi/10.1126/scitranslmed.aam7710.
- Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res [Internet]. 2013 Jul [cited 2024 Jan 1];62(7):641–51. Available from: https://pubmed.ncbi.nlm.nih.gov/23685857/.
- Ahmed R, Sharif D, Jaf M, Amin DM. Effect of TNF-α −308G/A (rs1800629) Promoter Polymorphism on the Serum Level of TNF-α Among Iraqi Patients with Generalized Vitiligo. Clin Cosmet Investig Dermatol [Internet]. 2020 [cited 2024 Jan 3];13:825. Available from: /pmc/articles/PMC7671505/.
- Qi F, Liu F, Gao L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front Immunol [Internet]. 2021 Nov 18 [cited 2023 Nov 3];12. Available from: /pmc/articles/PMC8636851/.
- Yang X, Yan L, Ha D, Qu L, Liu L, Tao Y. Changes in sICAM-1 and GM-CSF levels in skin tissue fluid and expression of IL-6, IL-17 and TNF-α in blood of patients with vitiligo. Exp Ther Med [Internet]. 2019 Nov 7 [cited 2023 Oct 21];17(1). Available from: https://pubmed.ncbi.nlm.nih.gov/30651813/.
- Wakabayashi T, Hosohata K, Oyama S, Inada A, Ueno S, Kambara H, et al. Comparison of Adverse Event Profiles of Tumor Necrosis Factor-Alfa Inhibitors: Analysis of a Spontaneous Reporting Database. Ther Clin Risk Manag [Internet]. 2020 [cited 2024 Jan 1];16:741. Available from: /pmc/articles/PMC7439489/.
- Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, Henning SW, et al. Tumour necrosis factor-a inhibition can stabilize disease in progressive vitiligo. British Journal of Dermatology [Internet]. 2015 [cited 2023 Oct 22]; Available from: https://academic.oup.com/bjd/article/173/3/641/6627485.
- Ramírez-Hernández M, Marras C, Martínez-Escribano JA. Infliximab-induced vitiligo. Dermatology [Internet]. 2005 [cited 2023 Oct 22];210(1):79–80. Available from: https://pubmed.ncbi.nlm.nih.gov/15604556/.
- Posada C, Flórez Á, Batalla A, Alcázar JJ, Carpio D. Vitiligo during Treatment of Crohn’s Disease with Adalimumab: Adverse Effect or Co-Occurrence? Case Rep Dermatol [Internet]. 2011 Jan [cited 2023 Oct 22];3(1):28–31. Available from: https://pubmed.ncbi.nlm.nih.gov/21931575/.
- Phan K, Charlton O, Smith SD. New onset vitiligo in a patient with hidradenitis suppurativa treated with adalimumab. Dermatol Ther [Internet]. 2020 May 1 [cited 2023 Oct 22];33(3). Available from: https://pubmed.ncbi.nlm.nih.gov/32239739/.
- Rigopoulos D, Gregoriou S, Larios G, Moustou E, Belayeva-Karatza E, Kalogeromitros D. Etanercept in the treatment of vitiligo. Dermatology [Internet]. 2007 Jun [cited 2023 Oct 22];215(1):84–5. Available from: https://pubmed.ncbi.nlm.nih.gov/17587849/.
- Campanati A, Giuliodori K, Ganzetti G, Liberati G, Offidani AM. A patient with psoriasis and vitiligo treated with etanercept. Am J Clin Dermatol [Internet]. 2010 [cited 2023 Oct 22];11 Suppl 1(SUPPL. 1):46–8. Available from: https://pubmed.ncbi.nlm.nih.gov/20586509/.
- Bae JM, Kim M, Lee HH, Kim KJ, Shin H, Ju HJ, et al. Increased Risk of Vitiligo Following Anti-Tumor Necrosis Factor Therapy: A 10-Year Population-Based Cohort Study. Journal of Investigative Dermatology. 2018 Apr 1;138(4):768–74. [CrossRef]
- Li S, Kang P, Zhang W, Jian Z, Zhang Q, Yi X, et al. Activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in keratinocytes promotes cutaneous T-cell response in patients with vitiligo. J Allergy Clin Immunol [Internet]. 2020 Feb 1 [cited 2024 May 16];145(2):632–45. Available from: https://pubmed.ncbi.nlm.nih.gov/31756352/.
- Gu R, Shi Y, Huang W, Lao C, Zou Z, Pan S, et al. Theobromine mitigates IL-1β-induced oxidative stress, inflammatory response, and degradation of type II collagen in human chondrocytes. Int Immunopharmacol [Internet]. 2020 May 1 [cited 2024 May 16];82. Available from: https://pubmed.ncbi.nlm.nih.gov/32146317/.
- Bhardwaj S, Rani S, Srivastava N, Kumar R, Parsad D. Increased systemic and epidermal levels of IL-17A and IL-1β promotes progression of non-segmental vitiligo. Cytokine [Internet]. 2017 Mar 1 [cited 2024 May 16];91:153–61. Available from: https://pubmed.ncbi.nlm.nih.gov/28082234/.
- Marie J, Kovacs D, Pain C, Jouary T, Cota C, Vergier B, et al. Inflammasome activation and vitiligo/nonsegmental vitiligo progression. Br J Dermatol [Internet]. 2014 [cited 2024 May 16];170(4):816–23. Available from: https://pubmed.ncbi.nlm.nih.gov/24734946/.
- Cui D, Zhong F, Lin J, Wu Y, Long Q, Yang X, et al. Changes of circulating Th22 cells in children with hand, foot, and mouth disease caused by enterovirus 71 infection. Oncotarget [Internet]. 2016 Dec 21 [cited 2024 May 16];8(17):29370–82. Available from: https://www.oncotarget.com/article/14083/text/.
- Dong J, An X, Zhong H, Wang Y, Shang J, Zhou J. Interleukin-22 participates in the inflammatory process of vitiligo. Oncotarget [Internet]. 2017 Dec 12 [cited 2024 May 16];8(65):109161. Available from: /pmc/articles/PMC5752511/.
- Markota A, Endres S, Kobold S. Targeting interleukin-22 for cancer therapy. Hum Vaccin Immunother [Internet]. 2018 May 9 [cited 2024 Jun 3];14(8):2012. Available from: /pmc/articles/PMC6149728/.
- Singh RK, Lee KM, Vujkovic-Cvijin I, Ucmak D, Farahnik B, Abrouk M, et al. The role of IL-17 in vitiligo: A review. Autoimmun Rev [Internet]. 2016 Apr 1 [cited 2024 Jan 1];15(4):397–404. Available from: https://pubmed.ncbi.nlm.nih.gov/26804758/.
- Belpaire A, van Geel N, Speeckaert R. From IL-17 to IFN-γ in inflammatory skin disorders: Is transdifferentiation a potential treatment target? Front Immunol [Internet]. 2022 Jul 28 [cited 2023 Oct 25];13. Available from: /pmc/articles/PMC9367984/.
- Vaccaro M, Cannavò SP, Imbesi S, Cristani M, Barbuzza O, Tigano V, et al. Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol [Internet]. 2015 Jun 1 [cited 2023 Nov 21];54(6):672–4. Available from: https://pubmed.ncbi.nlm.nih.gov/25427848/.
- Bhardwaj S, Rani S, Srivastava N, Kumar R, Parsad D. Increased systemic and epidermal levels of IL-17A and IL-1β promotes progression of non-segmental vitiligo. Cytokine [Internet]. 2017 Mar 1 [cited 2023 Oct 21];91:153–61. Available from: https://pubmed.ncbi.nlm.nih.gov/28082234/.
- Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol [Internet]. 2011 Apr [cited 2023 Nov 15];36(3):292–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21198791/.
- Basak PY, Adiloglu AK, Ceyhan AM, Tas T, Akkaya VB. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol [Internet]. 2009 Feb [cited 2023 Nov 15];60(2):256–60. Available from: https://pubmed.ncbi.nlm.nih.gov/19022528/.
- Zhang L, Kang Y, Chen S, Wang L, Jiang M, Xiang L. Circulating CCL20: A potential biomarker for active vitiligo together with the number of Th1/17 cells. J Dermatol Sci [Internet]. 2019 Feb 1 [cited 2023 Oct 28];93(2):92–100. Available from: https://pubmed.ncbi.nlm.nih.gov/30655106/.
- Le Poole IC, Van Den Wijngaard RMJGJ, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol [Internet]. 1996 Apr [cited 2023 Oct 21];148(4):1219. Available from: /pmc/articles/PMC1861531/?report=abstract.
- Basdeo SA, Cluxton D, Sulaimani J, Moran B, Canavan M, Orr C, et al. Ex-Th17 (Nonclassical Th1) Cells Are Functionally Distinct from Classical Th1 and Th17 Cells and Are Not Constrained by Regulatory T Cells. The Journal of Immunology [Internet]. 2017 Mar 15 [cited 2024 Jan 1];198(6):2249–59. Available from: https://dx.doi.org/10.4049/jimmunol.1600737. [CrossRef]
- Speeckaert R, Mylle S, van Geel N. IL-17A is not a treatment target in progressive vitiligo. Pigment Cell Melanoma Res [Internet]. 2019 Nov 1 [cited 2023 Oct 28];32(6):842–7. Available from: https://pubmed.ncbi.nlm.nih.gov/31063266/.
- Aboobacker S, Kurn H, Aboud AM Al. Secukinumab. Turkderm Turkish Archives of Dermatology and Venereology [Internet]. 2023 Jun 20 [cited 2023 Nov 15];56:52–4. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537091/.
- Palazzo G. Resolution of post-adalimumab vitiligo with secukinumab in a patient with psoriasis vulgaris. Oxf Med Case Reports [Internet]. 2020 [cited 2023 Oct 21];2020:13–6. Available from: https://academic.oup.com/omcr/article/2020/1/omz134/5721283.
- Persechino S, Giordano D, Magri F, Persechino F, Lepore A, Verde R, et al. Single Case Vitiligo with Progressive Repigmentation during Secukinumab Treatment in a Patient with Psoriatic Arthritis: A Case Report. Case Rep Dermatol [Internet]. 2021 [cited 2023 Oct 21];13:209–15. Available from: www.karger.com/cde.
- Kim JC, Lee ES. Progression of Pre-Existing Vitiligo during Secukinumab Treatment for Psoriasis. Ann Dermatol [Internet]. 2023 May 1 [cited 2023 Oct 21];35(Suppl 1):S117–21. Available from: https://doi.org/10.5021/ad.21.078. [CrossRef]
- Nieto-Benito LM, Baniandrés-Rodríguez O. New-Onset Vitiligo During Treatment with Secukinumab: Report of Two Cases and Review of the Literature. Clin Drug Investig [Internet]. 2020 Nov 1 [cited 2023 Oct 21];40(11):1089–91. Available from: https://link.springer.com/article/10.1007/s40261-020-00964-w.
- Bouzid S, Hammami-Ghorbel H, Chamli A, Aounti I, Daly W, Kochbati S, et al. Secukinumab-induced vitiligo: A new case report and review of the literature. Therapie [Internet]. 2022 [cited 2023 Oct 21]; Available from: https://pubmed.ncbi.nlm.nih.gov/36566160/.
- Raimondo A, Guglielmi G, Marino C, Ligrone L, Lembo S. Hair whitening in a patient with psoriasis on adalimumab reversed after switching to ixekizumab. JAAD Case Rep [Internet]. 2021 May 1 [cited 2024 Jan 1];11:51–3. Available from: https://pubmed.ncbi.nlm.nih.gov/33912637/.
- Eker H, Kaya İslamoğlu ZG, Demirbaş A. Vitiligo development in a patient with psoriasis vulgaris treated with ixekizumab. Dermatol Ther. 2022 Apr 1;35(4.
- Marasca C, Fornaro L, Martora F, Picone V, Fabbrocini G, Megna M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol Ther [Internet]. 2021 Sep 1 [cited 2024 Jan 1];34(5). Available from: https://pubmed.ncbi.nlm.nih.gov/34436817/.
- Pathmarajah P, Benjamin-Laing Z, Abdurrahman M, Grunova A, Sinclair C. Generalized vitiligo in a psoriatic patient treated with ixekizumab. Dermatol Ther [Internet]. 2022 Dec 1 [cited 2024 Jan 1];35(12). Available from: https://pubmed.ncbi.nlm.nih.gov/36181252/.
- Maddur MS, Miossec P, Kaveri S V., Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol [Internet]. 2012 Jul [cited 2023 Nov 21];181(1):8–18. Available from: https://pubmed.ncbi.nlm.nih.gov/22640807/.
- Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med [Internet]. 2011 Nov [cited 2023 Nov 21];43(7):503–11. Available from: https://pubmed.ncbi.nlm.nih.gov/21585245/.
- Verstockt B, Salas A, Sands BE, Abraham C, Leibovitzh H, Neurath MF, et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol [Internet]. 2023 Jul 1 [cited 2023 Oct 22];20(7):433–46. Available from: https://pubmed.ncbi.nlm.nih.gov/37069321/.
- Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 Pathway and Inflammatory Diseases of the Intestines, Lungs, and Skin. https://doi.org/101146/annurev-pathol-011110-130318[Internet]. 2013 Jan 24 [cited 2023 Oct 22];8:477–512. Available from: https://www.annualreviews.org/doi/abs/10.1146/annurev-pathol-011110-130318.
- Jerjen R, Moodley A, Sinclair R. Repigmentation of acrofacial vitiligo with subcutaneous tildrakizumab. Australas J Dermatol [Internet]. 2020 Nov 1 [cited 2023 Oct 22];61(4):e446–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32441048/.
- Miyoshi J, Matsuura M, Hisamatsu T. Safety evaluation of ustekinumab for moderate-to-severe ulcerative colitis. Expert Opin Drug Saf [Internet]. 2022 [cited 2024 Jan 1];21(1):1–8. Available from: https://pubmed.ncbi.nlm.nih.gov/34511011/.
- Rawal S, Kianian S, Guo W, Marquez J, Ayasse M, Siamas KA, et al. Alternative uses of ustekinumab for non-indicated dermatological conditions: a systematic review. Arch Dermatol Res [Internet]. 2022 Aug 1 [cited 2023 Oct 21];314(6):503–14. Available from: https://pubmed.ncbi.nlm.nih.gov/34156549/.
- Elkady A, Bonomo L, Amir Y, Vekaria AS, Guttman-Yassky E, York N. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. [cited 2023 Oct 21]. [CrossRef]
- L MB, K B, G C, A K, F M, H M, et al. New-onset vitiligo and progression of pre-existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol [Internet]. 2017 [cited 2023 Oct 19];31(1). Available from: https://pubmed.ncbi.nlm.nih.gov/27291924/.
- Anthony N, Bourneau-martin D, Ghamrawi S, Lagarce L, Babin M, Briet M. Drug-induced vitiligo: a case/non-case study in Vigibase® , the WHO pharmacovigilance database. Fundam Clin Pharmacol [Internet]. 2020 Dec 1 [cited 2023 Oct 19];34(6):736–42. Available from: https://pubmed.ncbi.nlm.nih.gov/32246859/.
- Montilla Francisco Gó mez-García Pedro J Gó mez-Arias Jesú Gay-Mimbrera Jorge Hernández-Parada Beatriz Isla-Tejera Juan Ruano AM. Scoping Review on the Use of Drugs Targeting JAK/ STAT Pathway in Atopic Dermatitis, Vitiligo, and Alopecia Areata. Dermatol Ther (Heidelb) [Internet]. 2019 [cited 2023 Nov 5];9. Available from: https://doi.org/10.6084/.
- Tang Q, Sousa J, Echeverria D, Fan X, Hsueh YC, Afshari K, et al. RNAi-based modulation of IFN-γ signaling in skin. Molecular Therapy [Internet]. 2022 Aug 8 [cited 2023 Nov 5];30(8):2709. Available from: /pmc/articles/PMC9372319/.
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med [Internet]. 2014 Feb 12 [cited 2023 Nov 15];6(223). Available from: https://pubmed.ncbi.nlm.nih.gov/24523323/.
- Liu H, Wang Y, Le Q, Tong J, Wang H. The IFN-γ-CXCL9/CXCL10-CXCR3 axis in vitiligo: pathological mechanism and treatment. Eur J Immunol [Internet]. 2023 Nov 8 [cited 2023 Nov 15]; Available from: https://pubmed.ncbi.nlm.nih.gov/37937817/.
- Maouia A, Sormani L, Youssef M, Helal AN, Kassab A, Passeron T. Differential expression of CXCL9, CXCL10, and IFN-γ in vitiligo and alopecia areata patients. Pigment Cell Melanoma Res [Internet]. 2017 Mar 1 [cited 2024 May 14];30(2):259–61. Available from: https://pubmed.ncbi.nlm.nih.gov/27863059/.
- Yu HS, Chang KL, Yu CL, Li HF, Wu MT, Wu CS, et al. Alterations in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligo. J Invest Dermatol [Internet]. 1997 [cited 2024 May 16];108(4):527–9. Available from: https://pubmed.ncbi.nlm.nih.gov/9077486/.
- Rashighi M, Harris JE. Interfering with the IFN-γ/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann Transl Med [Internet]. 2015 Dec 1 [cited 2024 Jan 4];3(21). Available from: https://pubmed.ncbi.nlm.nih.gov/26734651/.
- Nada HR, El Sharkawy DA, Elmasry MF, Rashed LA, Mamdouh S. Expression of Janus Kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res [Internet]. 2018 Jan 1 [cited 2023 Nov 3];310(1):39–46. Available from: https://pubmed.ncbi.nlm.nih.gov/29127481/.
- Abdel Motaleb AA, Tawfik YM, El-Mokhtar MA, Elkady S, El-Gazzar AF, ElSayed SK, et al. Cutaneous JAK Expression in Vitiligo. J Cutan Med Surg [Internet]. 2021 Mar 1 [cited 2023 Nov 3];25(2):157–62. Available from: https://pubmed.ncbi.nlm.nih.gov/33174479/.
- Boukhedouni N, Martins C, Darrigade AS, Drullion C, Rambert J, Barrault C, et al. Type-1 cytokines regulate MMP-9 production and E-cadherin disruption to promote melanocyte loss in vitiligo. JCI Insight [Internet]. 2020 Jun 4 [cited 2024 May 14];5(11). Available from: https://pubmed.ncbi.nlm.nih.gov/32369451/.
- Rosmarin D, Passeron T, Pandya AG, Grimes P, Harris JE, Desai SR, et al. Two Phase 3, Randomized, Controlled Trials of Ruxolitinib Cream for Vitiligo. N Engl J Med [Internet]. 2022 Oct 20 [cited 2023 Nov 5];387(16):1445–55. Available from: https://pubmed.ncbi.nlm.nih.gov/36260792/.
- Howell MD, Kuo FI, Smith PA. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front Immunol. 2019 Oct 9;10:490313.
- Study Results | A Study to Evaluate the Mechanism of Action of Ruxolitinib Cream in Subjects With Vitiligo (TRuE-V MOA) | ClinicalTrials.gov [Internet]. [cited 2024 Jan 1]. Available from: https://clinicaltrials.gov/study/NCT04896385?tab=results.
- Tavoletti G, Avallone G, Conforti C, Roccuzzo G, Maronese CA, Mattioli MA, et al. Topical ruxolitinib: A new treatment for vitiligo. Journal of the European Academy of Dermatology and Venereology [Internet]. 2023 Nov 1 [cited 2023 Nov 5];37(11):2222–30. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/jdv.19162.
- Ajayi S, Becker H, Reinhardt H, Engelhardt M, Zeiser R, von Bubnoff N, et al. Ruxolitinib. Recent Results Cancer Res [Internet]. 2018 [cited 2023 Nov 18];212:119–32. Available from: https://pubmed.ncbi.nlm.nih.gov/30069628/.
- Phan K, Phan S, Shumack S, Gupta M. Repigmentation in vitiligo using janus kinase (JAK) inhibitors with phototherapy: systematic review and Meta-analysis. Journal of Dermatological Treatment [Internet]. 2022 [cited 2023 Nov 5];33(1):173–7. Available from: https://www.tandfonline.com/doi/abs/10.1080/09546634.2020.1735615.
- Janus Kinase and Tyrosine Kinase Inhibitors in Dermatology [Internet]. [cited 2023 Nov 5]. Available from: https://www.skintherapyletter.com/dermatology/janus-tyrosine-kinase-inhibitors-review/.
- Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol [Internet]. 2017 Oct 1 [cited 2023 Nov 5];77(4):675-682.e1. Available from: https://pubmed.ncbi.nlm.nih.gov/28823882/.
- Craiglow BG, King BA. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol [Internet]. 2015 Oct 1 [cited 2023 Nov 5];151(10):1110–2. Available from: https://pubmed.ncbi.nlm.nih.gov/26107994/.
- Joshipura D, Plotnikova N, Goldminz A, Deverapalli S, Turkowski Y, Gottlieb A, et al. Importance of light in the treatment of vitiligo with JAK-inhibitors. J Dermatolog Treat [Internet]. 2018 Jan 2 [cited 2023 Nov 5];29(1):98–9. Available from: https://pubmed.ncbi.nlm.nih.gov/28581823/.
- Kim SR, Heaton H, Liu LY, King BA. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol [Internet]. 2018 Mar 1 [cited 2023 Nov 5];154(3):370–1. Available from: https://pubmed.ncbi.nlm.nih.gov/29387870/.
- Zhang J, Qi F, Dong J, Tan Y, Gao L, Liu F. Application of Baricitinib in Dermatology. J Inflamm Res [Internet]. 2022 [cited 2023 Nov 5];15:1935. Available from: /pmc/articles/PMC8939862/.
- Dong J, Huang X, Ma LP, Qi F, Wang SN, Zhang ZQ, et al. Baricitinib is Effective in Treating Progressing Vitiligo in vivo and in vitro. Dose Response [Internet]. 2022 Apr 1 [cited 2023 Nov 5];20(2). Available from: https://pubmed.ncbi.nlm.nih.gov/35663493/.
- Diotallevi F, Gioacchini H, De Simoni E, Marani A, Candelora M, Paolinelli M, et al. Vitiligo, from Pathogenesis to Therapeutic Advances: State of the Art. Int J Mol Sci [Internet]. 2023 Mar 1 [cited 2024 Jan 18];24(5). Available from: https://pubmed.ncbi.nlm.nih.gov/36902341/.
- Ezzedine K, Peeva E, Yamaguchi Y, Cox LA, Banerjee A, Han G, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: A randomized phase 2b clinical trial. J Am Acad Dermatol [Internet]. 2023 Feb 1 [cited 2024 Jan 4];88(2):395–403. Available from: http://www.jaad.org/article/S0190962222029899/fulltext.
- Feng Y, Lu Y. Advances in vitiligo: Update on therapeutic targets. Front Immunol [Internet]. 2022 Aug 31 [cited 2024 Jan 18];13. Available from: /pmc/articles/PMC9471423/.
- Sardana K, Bathula S, Khurana A. Which is the Ideal JAK Inhibitor for Alopecia Areata – Baricitinib, Tofacitinib, Ritlecitinib or Ifidancitinib - Revisiting the Immunomechanisms of the JAK Pathway. Indian Dermatol Online J [Internet]. 2023 Jul 1 [cited 2024 Jan 18];14(4):465. Available from: /pmc/articles/PMC10373824/.
- Liu H, Wang Y, Le Q, Tong J, Wang H. The IFN-γ-CXCL9/CXCL10-CXCR3 axis in vitiligo: Pathological mechanism and treatment. Eur J Immunol [Internet]. 2024 Apr 1 [cited 2024 May 17];54(4):2250281. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/eji.202250281.
- Su X, Luo R, Ruan S, Zhong Q, Zhuang Z, Xiao Z, et al. Efficacy and tolerability of oral upadacitinib monotherapy in patients with recalcitrant vitiligo. J Am Acad Dermatol [Internet]. 2023 Dec 1 [cited 2024 Mar 11];89(6):1257–9. Available from: https://pubmed.ncbi.nlm.nih.gov/37516357/.
- Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J Pharmacol Exp Ther [Internet]. 2014 Dec 1 [cited 2024 May 17];351(3):538–48. Available from: https://pubmed.ncbi.nlm.nih.gov/25253883/.
- Ma J, Xing W, Coffey G, Dresser K, Lu K, Guo A, et al. Cerdulatinib, a novel dual SYK/JAK kinase inhibitor, has broad anti-tumor activity in both ABC and GCB types of diffuse large B cell lymphoma. Oncotarget [Internet]. 2015 Dec 12 [cited 2024 May 14];6(41):43881. Available from: /pmc/articles/PMC4791274/.
- Yagi K, Ishida Y, Otsuka A, Kabashima K. Two cases of vitiligo vulgaris treated with topical Janus kinase inhibitor delgocitinib. Australas J Dermatol [Internet]. 2021 Aug 1 [cited 2024 May 14];62(3):433–4. Available from: https://pubmed.ncbi.nlm.nih.gov/33667323/.
- Skurkovich S, Skurkovich B, Kelly J. Anticytokine therapy, particularly anti-IFN-gamma, in Th1-mediated autoimmune diseases. Expert Rev Clin Immunol [Internet]. 2005 May [cited 2024 May 17];1(1):11–25. Available from: https://pubmed.ncbi.nlm.nih.gov/20477651/.
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol [Internet]. 2012 [cited 2024 May 16];132(7):1869–76. Available from: https://pubmed.ncbi.nlm.nih.gov/22297636/.
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med [Internet]. 2014 Feb 12 [cited 2024 May 17];6(223). Available from: https://pubmed.ncbi.nlm.nih.gov/24523323/.
- Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. J Invest Dermatol [Internet]. 2017 Apr 1 [cited 2024 May 17];137(4):982–5. Available from: https://pubmed.ncbi.nlm.nih.gov/28126463/.
- Nowroozpoor Dailami K, Hosseini A, Rahmatpour Rokni G, Saeedi M, Morteza-Semnani K, Sadeghi Z, et al. Efficacy of topical latanoprost in the treatment of eyelid vitiligo: A randomized, double-blind clinical trial study. Dermatol Ther [Internet]. 2020 Jan 1 [cited 2024 Jun 24];33(1). Available from: https://pubmed.ncbi.nlm.nih.gov/31758835/.
- Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol [Internet]. 2015 Jan 1 [cited 2024 Jun 25];151(1):42–50. Available from: https://pubmed.ncbi.nlm.nih.gov/25230094/.
- Elrewiny EM, Shawky A, Mohamed SFF, Ammar AM, Mansour M, Rageh MA. Intralesional methotrexate in the treatment of localized vitiligo: A pilot study. Australasian Journal of Dermatology [Internet]. 2023 Aug 1 [cited 2024 Jun 25];64(3):e207–11. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/ajd.14071.
| Drug | Target | Effect |
|---|---|---|
| Tocilizumab [27,28,29] | IL-6 receptor | Lack of effectiveness, new vitiligo lesions (case reports) |
| anti-CD122 [33] | IL-15 | Repigmentation of vitiligo lesions (mice model) |
| IL-15 monoclonal antibody (AMG 714) [34] | IL-15 | Ongoing phase IIa clinical trial |
| Adalimumab Infliximab Etanercept [38,39,40,41,42,43,44,45] |
TNF- alpha | Increased risk of new-onset vitiligo, controversial therapeutic results (case reports, cohort study) |
| - | IL-1β | - |
| IL-22 neutralizing antibody [53] | IL-22 | - |
| Ustekinumab [79,80,81] | IL-12 and IL-23 | New vitiligo lesions, controversial therapeutic results (case reports, case/non-case study) |
| Secukinumab [64,65,66,67,68,69] Ixekizumab [71,72,73] |
IL-17A | Appearance of new vitiligo lesions (case reports) |
| Tildrakizumab [78] | IL-23 | Insufficient studies (case report) |
| Ruxolitinib [96,97,98,99] | JAK1/2 | Good clinical response, repigmentation of vitiligo lesions (approved by FDA and EMA in adults and adolescents from 12 years of age with non-segmental vitiligo) |
| Tofacitinib [102,103,104] | JAK1/2/3 | Repigmentation of vitiligo lesions, nbUVB may increase clinical effect (case reports, retrospective case series) |
| Baricitinib [36,105,106] | JAK1/2 | Repigmentation of vitiligo lesions, nbUVB may increase clinical effect (phase II clinical trial) |
| Ritlecitinib [108,110] |
JAK3/TEC | Repigmentation of vitiligo (phase III clinical trial) |
| Ifidancitinib [109,110] | JAK1/3 | Repigmentation of vitiligo lesions (phase II clinical trial) |
| Brepocytinib [111] | JAK1/TYK | No results (phase II clinical trial) |
| Upadacitinib [112] | JAK1 | Repigmentation of vitiligo lesions (phase III clinical trial) |
| Cerdulatynib [113,114] | JAK1/3 | No results (phase II clinical trial) |
| Delgocitinib [115] | JAK1/2/3, TYK2 | Good clinical response (case reports) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




