Submitted:
05 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Marine Peloids
1.2. Thalasso-Wellness: The Use of Seawater and Marine Peloids for Well-Being
1.3. Composition of Marine Peloids, Characterization, and Thermophysical Properties
1.4. Methods of Topical Application
1.5. Skin Biometrology Studies
2. Materials and Methods
3. Results and Discussion
3.1. Thermophysical Properties
3.2. Skin Hydration
5. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Microalgal Peloids for Cosmetic and Wellness Uses. Mar Drugs. 2021, 26, 19–666. [Google Scholar] [CrossRef]
- Gomes, C.; Carretero, M.I.; Pozo, M.; Maraver, F.; Cantista, P.; Armijo, F.; Legido, J.L.; Teixeira, F.; Rautureau, M.; Delgado, R. Peloids and pelotherapy: Historical evolution, classification and glossary. Appl. Clay Sci. 2013, 75, 28–38. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Y.; Li, H.; Jiao, Y.; Wang, Q.; Zhang, Y.; Ma, N.; Wang, W. Property of mud and its application in cosmetic and medical fields: a review. Environ Geochem Health. 2022, 44, 4235–4251. [Google Scholar] [CrossRef] [PubMed]
- Spilioti, E.; Vargiami, M.; Letsiou, S.; Gardikis, K.; Sygouni, V.; Koutsoukos, P.; Chinou, I.; Kassi, E.; Moutsatsou, P. Biological properties of mud extracts derived from various spa resorts. Environ Geochem Health. 2017, 39, 821–833. [Google Scholar] [CrossRef] [PubMed]
- PA, I.; Kakhetelidze, M.; Gabelaya, M.; Churadze, L. Cosmeceutical masks using therapeutic mud of Akhtala (Georgia) and products from plant materials. World J. Pharm. Res, 2020, 9, 189–194. [Google Scholar]
- Maccarone, M. C.; Magro, G.; Solimene, U.; Masiero, S. (2020). The effects of balneotherapy on human immune function: should baths and mud applications have a role during Covid-19 pandemic? Bulletin of Rehabilitation Medicine 2020, 97, 22–24. [Google Scholar] [CrossRef]
- Behroozian, S.; Svensson, S. L.; Davies, J.; Blaser, M. J. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. Mbio, 2016, 7, e01842–e01815. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Cantarini, L.; Guidelli, G. M.; Galeazzi, M. Mechanisms of action of spa therapies in rheumatic diseases: what scientific evidence is there? Rheumatol Int. 2011, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.I. Clays in pelotherapy. A review. Part II: Organic compounds, microbiology and medical applications. Appl. Clay Sci. 2020, 189, 105531. [Google Scholar] [CrossRef]
- Abu-Shakra M, Mayer A, Friger M, Harari M. Dead Sea mud packs for chronic low back pain. Isr Med Assoc J. 2014, 16, 574–577. [Google Scholar]
- Pestereva, N.M.; Khechumyan, A.F.; Udovenko, I.L.; Bekhterev, V.N. The application of climatic therapy in the health resorts of the Black Sea coast of the Caucasus: the current state-of-the-art and the prospects for the further development. Vopr Kurortol Fizioter Lech Fiz Kult. 2016, 93, 56–61. [Google Scholar] [CrossRef]
- Dokhnadze, T.D. The effect of rehabilitation with therapeutic Akhtala muds and electromagnetic radiation of millimeter range on biochemical indices in patients with post discectomy syndrome. Georgian Med News. 2011, 195, 65–70. (in Russian). [Google Scholar]
- Ionescu, E.V; Tica, I.; Oprea, C.; Iliescu, D.M.; Petcu, L.C.; Iliescu, M.G. ADIPONECTIN CORRELATION WITH BIOCLINICAL BENEFITS OF USING NATURAL THERAPEUTIC FACTORS IN KNEE OSTEOARTHRITIS. Acta Endocrinol (Buchar). 2017, 13, 308–313. [Google Scholar] [CrossRef] [PubMed]
- But’eva, I.V. Climatic resources of the Dagestan shore of the Caspian Sea. Vopr Kurortol Fizioter Lech Fiz Kult. 1968, 33, 538–543. (In Russian) [Google Scholar] [PubMed]
- Komar, D.; Dolenec, T.; Dolenec, M.; Vrhovnik, P.; Lojen, S.; Belak, Ž.L.; Kniewald, G.; Šmuc, N.R. Physico-chemical and geochemical characterization of Makirina Bay peloid mud and its evaluation for potential use in balneotherapy (N Dalmatia, Republic of Croatia). Indian J. Tradit. Knowl. 2015, 14, 5–12. [Google Scholar]
- Mihelčić, G.; Kniewald, G.; Ivanišević, G.; Čepelak, R.; Mihelčić, V.; Vdović, N. Physico-chemical characteristics of the peloid mud from Morinje Bay (eastern Adriatic coast, Croatia): suitability for use in balneotherapy. Environ Geochem Health. 2012, 34, 91–198. [Google Scholar] [CrossRef]
- Glavaš, N.; Mourelle, M.L.; Gómez, C.P.; Legido, J.L.; Šmuc, N.R.; Dolenec, M.; Kovac, N. The mineralogical, geochemical, and thermophysical characterization of healing saline mud for use in pelotherapy. Appl. Clay Sci. 2017, 135, 119–128. [Google Scholar] [CrossRef]
- Bigovic, M.; Pantović, S.; Milašević, I.; Ivanović, L.; Djurović, D.; Slavić, V.; Popovic, M.; Vrvić, M.; Roganovic, M. Organic composition of Igalo Bay peloid (Montenegro). IJTK 2019, 18, 837–848. [Google Scholar]
- Özay, P.; Karagülle, M.; Kardeş, S.; Karagülle, M.Z. Chemical and mineralogical characteristics of peloids in Turkey. Environ Monit Assess. 2020, 192, 805. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.S.F.; Fernandes, J.V.; Fernandes, F.V.; Silva, J.B.P. Salt Mineral Water and Thalassotherapy. In Minerals Latu Sensu and Human Health, 1st ed.; C. Gomes, C. and M. Rautureau, M., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Pozo, M.; Carretero, M.I.; Maraver, F.; Pozo, E.; Gómez, I.; Armijo, F.; Martín-Rubí, J.A. Composition and physico-chemical properties of peloids used in Spanish spas: A comparative study. Appl. Clay Sci. 2013, 83, 270–279. [Google Scholar] [CrossRef]
- McGlue, M.M. , Ellis, G.S., and Cohen, A.S., 2015, Modern muds of Laguna Mar Chiquita (Argentina): Particle size and organic matter geochemical trends from a large saline lake in the thick-skinned Andean foreland. In: Paying Attention to Mudrocks: Priceless!; Larsen, D., Egenhoff, S.O., and Fishman, N.S., eds.; Geological Society of America Special Paper 515, p. 1–18. [CrossRef]
- da Silva, P. S. C.; Torrecilha, J. K.; Gouvea, P. F. D.; Maduar, M.F.; de Oliveira, S. M. B.; Scapin, M. A. Chemical and radiological characterization of Peruibe Black Mud. Appl. Clay Sci. 2015, 118, 221–230. [Google Scholar] [CrossRef]
- Piña-Leyte-Vidal, J.J.; González-Hernández, P.; Suárez-Muñoz, M.; Aguilar-Carrillo, J.; Cházaro-Ruíz, L.F.; Hernández-Mendoza, H.; Díaz Rizo, O.; Díaz López, C.; Melián-Rodríguez, C.; Martínez-Villegas, N. The sinks of rare earth elements in peloids from hydrothermal, estuarine, coastal, and saline formation environments from Cuba. Appl. Clay Sci. 2023, 242. [Google Scholar] [CrossRef]
- Lucchetta, M.C.; Monaco, G.; I Valenzi, V.; Russo, M.V.; Campanella, J.; Nocchi, S.; Mennuni, G.; Fraioli, A. The historical-scientific foundations of thalassotherapy: State of the art. Clin. Ter. 2008, 158, 533–541. (In Italian) [Google Scholar]
- Gutenbrunner, C.; Bender, T.; Cantista, P.; Karagülle, Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int. J. Biometeorol. 2010, 54, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Maraver, F.; Michan-Doña, A.; Morer, C.; Aguilera, L. Is thalassotherapy simply a type of climatotherapy? Int. J. Biometeorol. 2010, 55, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J. Sea Water as an Electrolyte. In: Chemistry of Marine Water and Sediments. Environmental Science; Gianguzza, A., Pelizzetti, E., Sammartano, S., eds. 2002. [Google Scholar] [CrossRef]
- Calin, M.R.; Radulescu, I.; Ion, A.C.; Capra, L.; Almasan, E.R. Investigations on chemical composition and natural radioactivity levels from salt water and peloid used in pelotherapy from the Techirghiol Lake, Romania. Environ Geochem Health. 2020, 42, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Almalty, A.M.; Alkhatib, H.S. The cutaneous effects of long-term use of Dead Sea mud on healthy skin: a 4-week study. Int J Dermatol. 2021, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, J.M.; Maraver, F. Salt water and skin interactions: New lines of evidence. Int. J. Biometeorol. 2018, 62, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Ma, X.; Yan, X.; Bao, X. The Biological Role of Dead Sea Water in Skin Health: A Review. Cosmetics 2023, 10, 21. [Google Scholar] [CrossRef]
- Bigovic, M.; Roganovic, M.; Milasevic, I.; Djurovic, D.; Slavic, V.; Kosovic, M.; Vlahovic, M.; Perovic, S.; Perovic, A.; Kastratovic, V.; Potpara, Z.; Martinovic, M.; Pantovic, S. Physico-chemical characterization of Igalo Bay peloid (Montenegro) and assessment of the pollution of potentially toxic elements in the sampling area. Farmacia, 2020, 68, 560–571. [Google Scholar] [CrossRef]
- Baricz, A.; Levei, E.A.; Șenilă, M.; Pînzaru, S.C.; Aluaş, M.; Vulpoi, A.; Filip, C.; Tripon, C.; Dădârlat, D.; Buda, D.M.; Dulf, F.V.; Pintea, A.; Cristea, A.; Muntean, V.; Keresztes, Z.G.; Alexe, M.; Banciu, H.L. Comprehensive mineralogical and physicochemical characterization of recent sapropels from Romanian saline lakes for potential use in pelotherapy. Sci Rep. 2021, 11, 18633. [Google Scholar] [CrossRef] [PubMed]
- Vreca, P.; Dolenec, T. Geochemical estimation of copper contamination in the healing mud from Makirina Bay, central Adriatic. Environ Int. 2005, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M. : Chrzanowski,W.; Rohanizadeh, R. Biomedical applications of cationic clay minerals. RSC Advances, 2015, 5, 29467–29481. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci, 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Centini, M.; Roberto Tredici, M.; Biondi, N.; Buonocore, A.; Facino, R.M.; Anselmi, C. Bioglea as a Source of Bioactive Ingredients: Chemical and Biological Evaluation. Cosmetics 2020, 7, 81. [Google Scholar] [CrossRef]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Demay, J.; Halary, S.; Knittel-Obrecht, A.; Villa, P.; Duval, C.; Hamlaoui, S.; Roussel, T.; Yéprémian, C.; Reinhardt, A.; Bernard, C.; et al. Anti-Inflammatory, Antioxidant, and Wound-Healing Properties of Cyanobacteria from Thermal Mud of Balaruc-Les-Bains, France: A Multi-Approach Study. Biomolecules 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M. Isolation and characterization of extremely halotolerant Bacillus species from Dead Sea black mud and determination of their antimicrobial and hydrolytic activities. Afr. J. Microbiol. Res. 2017, 11, 1303–1314. [Google Scholar]
- Al-Karablieh, N. Antimicrobial Activity of Bacillus Persicus 24-DSM Isolated from Dead Sea Mud. Open Microbiol. J.
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.E.; Kim, K.H.; Kang, N.J. Beneficial Effects of Marine Algae-Derived Carbohydrates for Skin Health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.Y.; Lee, C.; Pan, J.L.; Wen, Z.H.; Huang, S.H.; Lan, C.W.; Liu, W.T.; Hour, T.C.; Hseu, Y.C.; Hwang, B.H.; Cheng, K.C.; Wang, H.M. Enriched Astaxanthin Extract from Haematococcus pluvialis Augments Growth Factor Secretions to Increase Cell Proliferation and Induces MMP1 Degradation to Enhance Collagen Production in Human Dermal Fibroblasts. Int J Mol Sci. 2016, 17, 955. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Galasso, C; Orefice, I; Nuzzo, G; Luongo, E; Cutignano, A; Romano, G; Brunet, C; Fontana, A; Esposito, F; Ianora, A. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchi, L.; Xu, Y.; Harvey, P. Stereoisomers of Colourless Carotenoids from the Marine Microalga Dunaliella salina. Molecules 2020, 25, 1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Lionti, J.; Ingegneri, M.; Burlando, B.; Cornara,L. ; Grillo, F.; Mastracci, L.; Trombetta,D. Xanthophyll-Rich Extract of Phaeodactylum tricornutum Bohlin as New Photoprotective Cosmeceutical Agent: Safety and Efficacy Assessment on In Vitro Reconstructed Human Epidermis Model. Molecules 2023, 28, 4190. [Google Scholar] [CrossRef] [PubMed]
- Legido, J.; Medina, C.; Mourelle, M.L.; Carretero, M.; Pozo, M. Comparative study of the cooling rates of bentonite, sepiolite and common clays for their use in pelotherapy. Appl. Clay Sci. 2007, 36, 148–160. [Google Scholar] [CrossRef]
- Carretero, M.I. Clays in pelotherapy. A review. Part I: Mineralogy, chemistry, physical and physicochemical properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Casás, L.; Pozo, M.; Gómez, C.P.; Pozo, E.; Bessières, L.; Plantier, F.; Legido, J.L. Thermal behavior of mixtures of bentonitic clay and saline solutions. Appl Clay Sci 2013, 72, 18–25. [Google Scholar] [CrossRef]
- Rebelo, M.C. , Viseras, C., López-Galindo, A., Rocha, F., Silva, E.F. Rheological and thermal characterization of peloids made of selected Portuguese geological materials. Appl Clay Sci 2011, 52, 219–227. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Aydın, S. The physical and physicochemical properties of some Turkish thermal muds and pure clay minerals and their uses in therapy. Turk J Earth Sci 2017, 26, 26–395. [Google Scholar]
- Pozo, M.; Armijo, F.; Maraver, F.; Zuluaga, P.; Ejeda, J.M.; Corvillo, I. Variations in the Texture Profile Analysis (TPA) Properties of Clay/Mineral-Medicinal Water Mixtures for Pelotherapy: Effect of Anion Type. Minerals 2019, 9, 9–144. [Google Scholar] [CrossRef]
- Masiukovichi T, Murtazashvili T, Bakuridze A. DEVELOPMENT OF THE FORMULATION AND TECHNOLOGY OF HYDROGEL, CONTAINING ADJARA REGION SULFIDE SILT PELOID. Georgian Med News.
- Barhoumi, T.; Bekri-Abbes, I.; Srasra, E. Physicochemical characteristics and suitability of curative pastes made of Tunisian clay minerals and thermal waters for use in pelotherapy. C.R. Chimie, 2019, 22, 126e131. [Google Scholar] [CrossRef]
- Shahzad, Y.; Louw, R.; Gerber, M.; du Plessis, J. Breaching the skin barrier through temperature modulations. J Control Release. 2015, 202, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Heat: A Highly Efficient Skin Enhancer for Transdermal Drug Delivery. Front Bioeng Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Kilo, S.; Wick, J.; Mini Vijayan, S.; Göen, T.; Horch, R.E.; Ludolph, I.; Drexler, H. Impact of physiologically relevant temperatures on dermal absorption of active substances - an ex-vivo study in human skin. Toxicol In Vitro. 2020, 68, 104954. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Sassning, S.; Tsankov, N.; Fluhr, J.W. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009, 72, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Yazdi, S.J.; Baqersad, J. Mechanical modeling and characterization of human skin: A review. J Biomech, 1: 130, 1108. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L.; Legido, N. Innovación en el uso de microalgas en termalismo. Bol Soc Esp Hidrol Méd, 2016, 31, 53–64. (in Spanish). [Google Scholar] [CrossRef]
- Casás, L.; Legido, J.; Pozo, M.; Mourelle, L.; Plantier, F.; Bessières, D. Specific heat of mixtures of bentonitic clay with sea water or distilled water for their use in thermotherapy. Thermochim. Acta 2011, 524, 68–73. [Google Scholar] [CrossRef]
- Lago, A.; Rivas, M.G.; José Luis Legido, J.L.; Pérez-Iglesias, T. Study of static permittivity and density of the systems {(n-nonane plus monoglyme or diglyme)} at various temperatures. J. Chem. Thermodyn. 2009, 41, 257–264. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.; Casanova, C.; Páramo, A.R.; Barbés, B.; Legido, J.L.; Piñeiro, M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.; Lugo, L.; Legido, J.; Piñeiro, M. Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res Lett 2011, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Legido, J.L.; Paz Andrade, M.I.; Arias, I.; Casás, L.M. , 2011. Microcalorimetric study on the growth and metabolism of Pseudomonas aeruginosa. J Therm Anal Calorim 2011, 105, 651–655. [Google Scholar] [CrossRef]
- Verdes, P.V.; Mato, M.M.; Paz Andrade, M.I.; Legido, J.L. , Contribution to study of the thermodynamics properties of mixtures containing 2-methoxy-2-methylpropane, alkanol, alkane. J Chem Thermodyn 2014, 73, 224–231. [Google Scholar] [CrossRef]
- de Melo, M.O.; Maia Campos, P.M.B.G. Application of biophysical and skin imaging techniques to evaluate the film-forming effect of cosmetic formulations. Int. J. Cosmet. Sci. 2019, 41, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Bare, A.; Clarys, P. In vitro calibration of the capacitance method (Comeometer CM 825) and conductance method (Skicon-200) for the evaluation of the hydration state of the skin. Skin Res Technol. 1997, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.O.; Maia Campos, P.M.B.G. Characterization of oily mature skin by biophysical and skin imaging techniques. Skin Res Technol. 2018, 24, 386–395. [Google Scholar] [CrossRef] [PubMed]

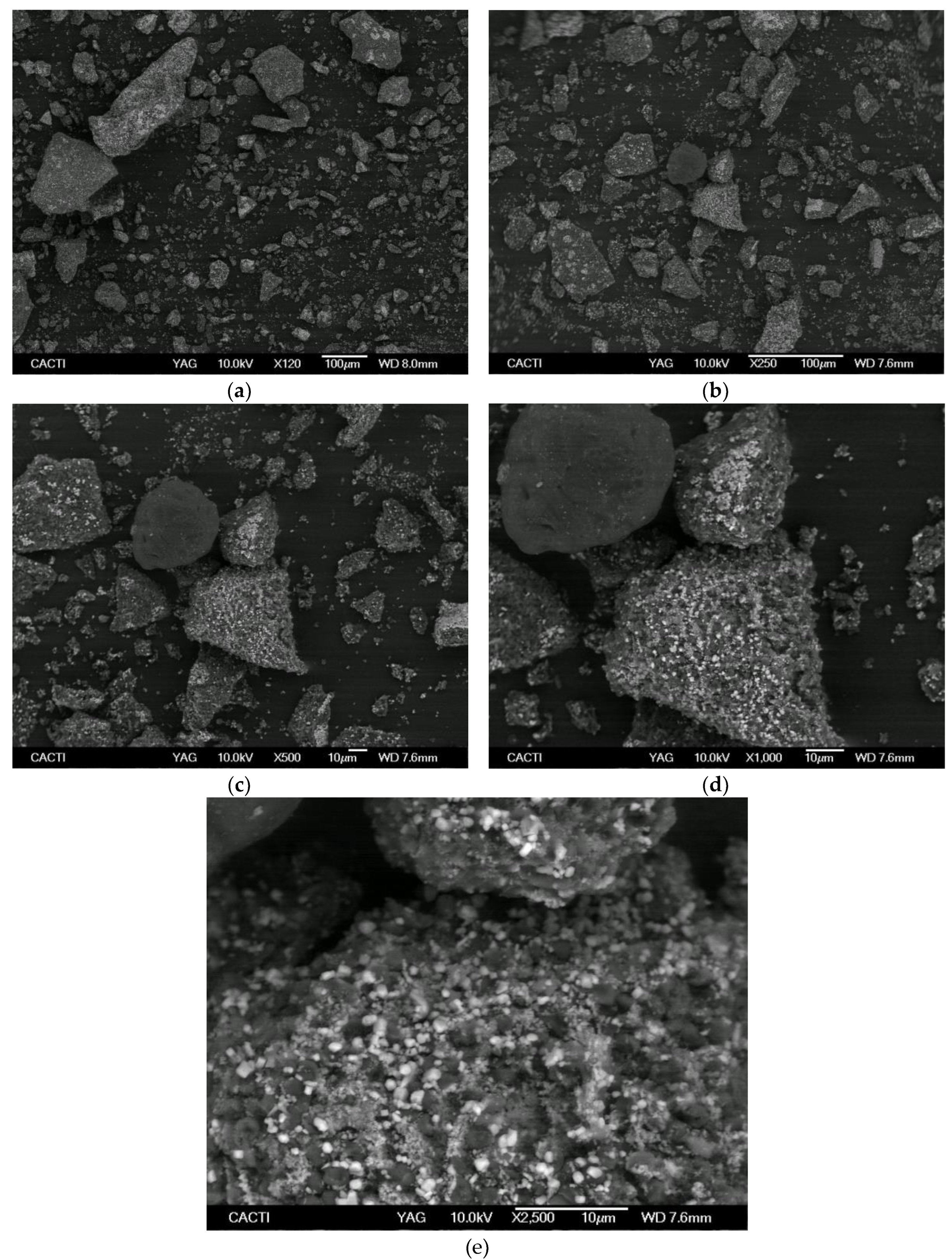
| Area / Country | Peloid | Reference |
|---|---|---|
| Israel, Jordan | Dead Sea | [10] |
| Black Sea | Athala mud | [11,12] |
| Romanian salted lakes | Techirghiol lake | [13] |
| Russian coasts and salted lakes | Bugaz Liman | [14] |
| Adriatic coast | Makirina Bay Morinja Bay Sečovlje Salina Igalo Bay |
[15] [16] [17] [18] |
| Turkey | Tuz Gölü | [19] |
| Portugal | Cale do Oiro Porto Santo |
[20] |
| Spain | Lo Pagán | [21] |
| Argentina | Mar Chiquita | [22] |
| Brazil | Peruíbe | [23] |
| Cuba | Santa Lucia | [24] |
| H | C | N | Na | Mg | Al | Si | P | S | Cl | K | Ca | Mn | Fe | Cu | Zn | Br | Sr | Ba | *o.c. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 5.57 | 34.5 | 5.35 | 2.6 | 0.829 | 0.0075 | 0.017 | 3.06 | 0.25 | 6.31 | 4.48 | 4.36 | 0.0077 | 0.0724 | 0.003 | 0.015 | 0.0046 | 0.394 | 0.016 | 32,15 |
| Sample | % Distilled water | % Seawater | % Bentonite | % Nannochloropsis sp |
|---|---|---|---|---|
| M1 | 0 | 60 | 20 | 20 |
| M2 | 85 | 0 | 7.5 | 7.5 |
| Sample | T (K) | pH | ρ kg/m3 | cp J/kg K | λ W/m K | σ m2/s (107) | η mPa s |
|---|---|---|---|---|---|---|---|
| Seawater | 298.15K | 7.8 | 1023.5 | 3980 | 0.60 | 1.47 | 0.932 |
| 308.15K | 7.6 | 1020.2 | 3990 | 0.62 | 1.52 | 0.731 | |
| Distilled water | 298.15K | 7.0 | 997.0 | 4170 | 0.61 | 1.47 | 0.997 |
| 308.15K | 6.8 | 994.0 | 4160 | 0.62 | 1.50 | 0.792 |
| Sample | T (K) | pH | ρ kg/m3 | cp J/kg K | λ W/m K | σ m2/s (107) | η Pa s |
|---|---|---|---|---|---|---|---|
| M1 | 298.15K | 8.0 | 1290 | 2800 | 0.66 | 1.83 | 12.9 |
| 308.15K | 7.9 | 1280 | 2810 | 0.67 | 1.86 | 11.5 | |
| M2 | 298.15K | 8.6 | 1120 | 3700 | 0.63 | 1.52 | 14.5 |
| 308.15K | 8.5 | 1110 | 3710 | 0.65 | 1.58 | 13.8 |
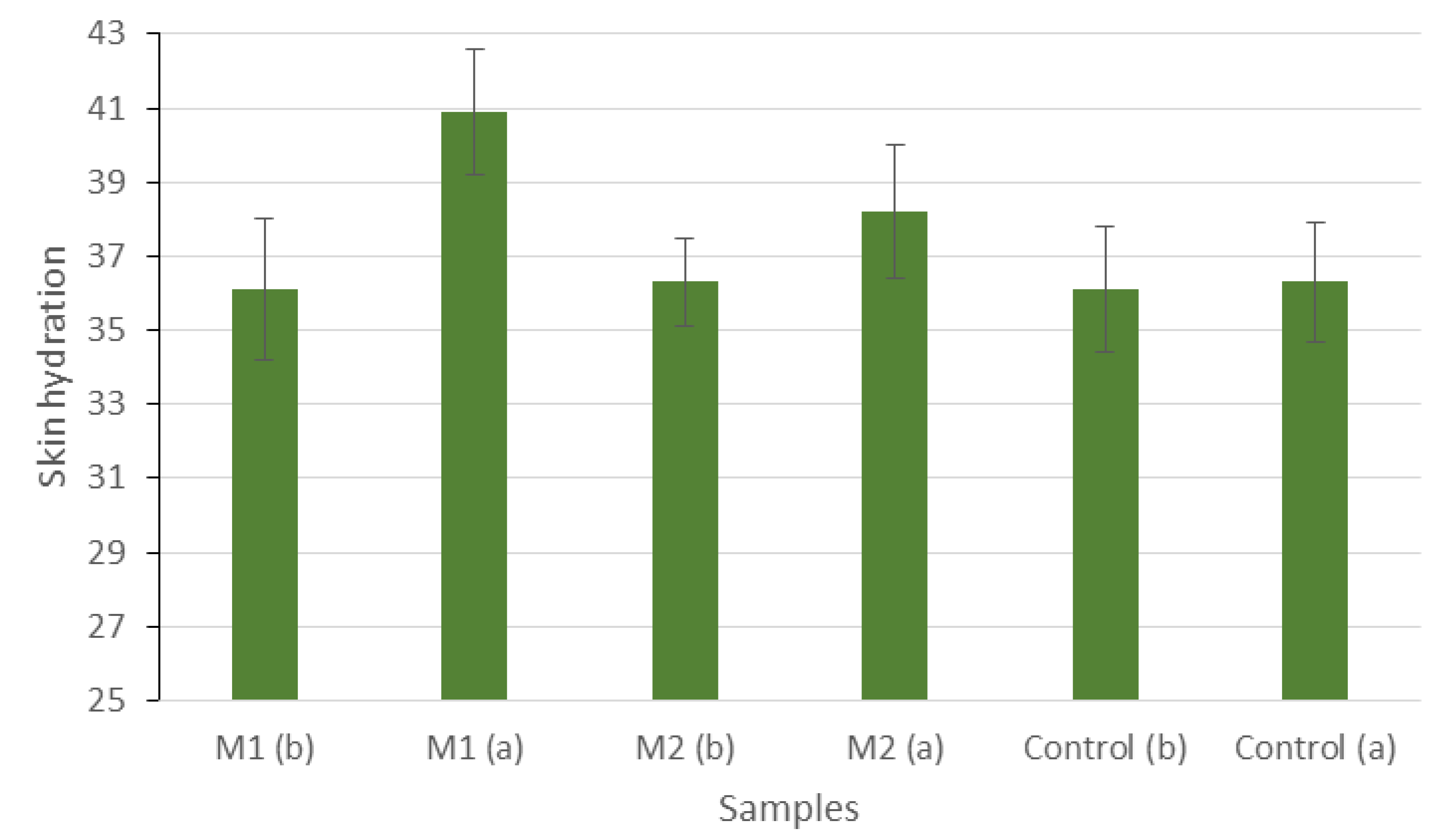
| M1 (before) | M1 (after) | M2 (before) | M2 (after) | Control (before) | Control (after) | |
|---|---|---|---|---|---|---|
| Mean | 36.1 | 40.9 | 36.3 | 38.2 | 36.1 | 36.3 |
| Error | 1.9 | 1.7 | 1.2 | 1.8 | 1.7 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





