Submitted:
09 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Big Question
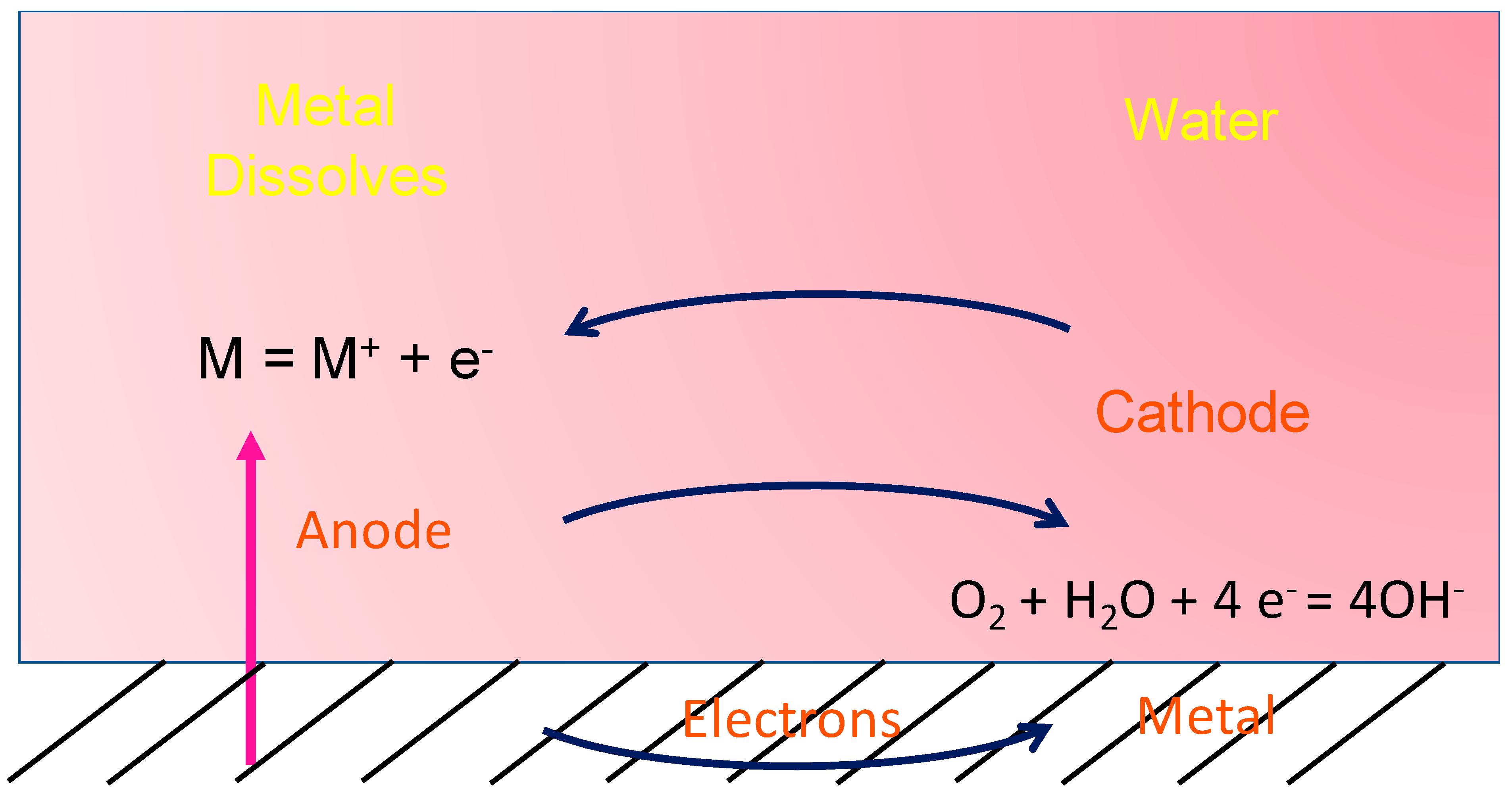
3. Corrosion Mechanism
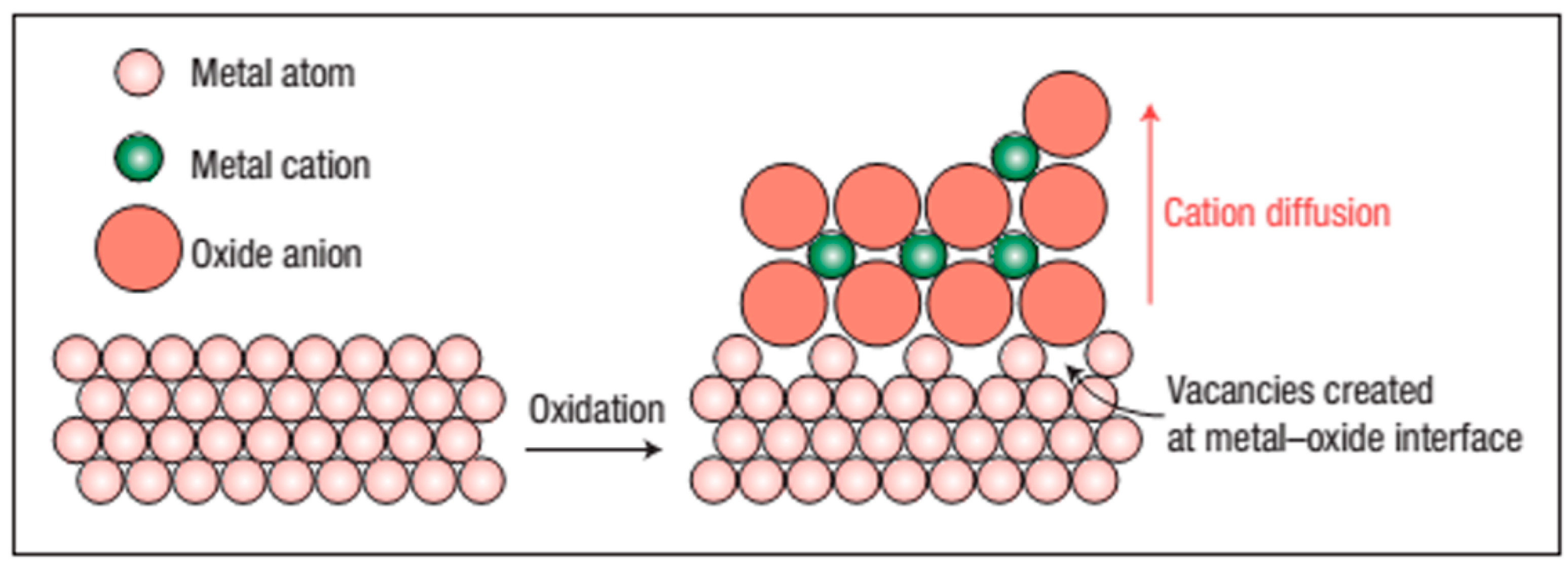
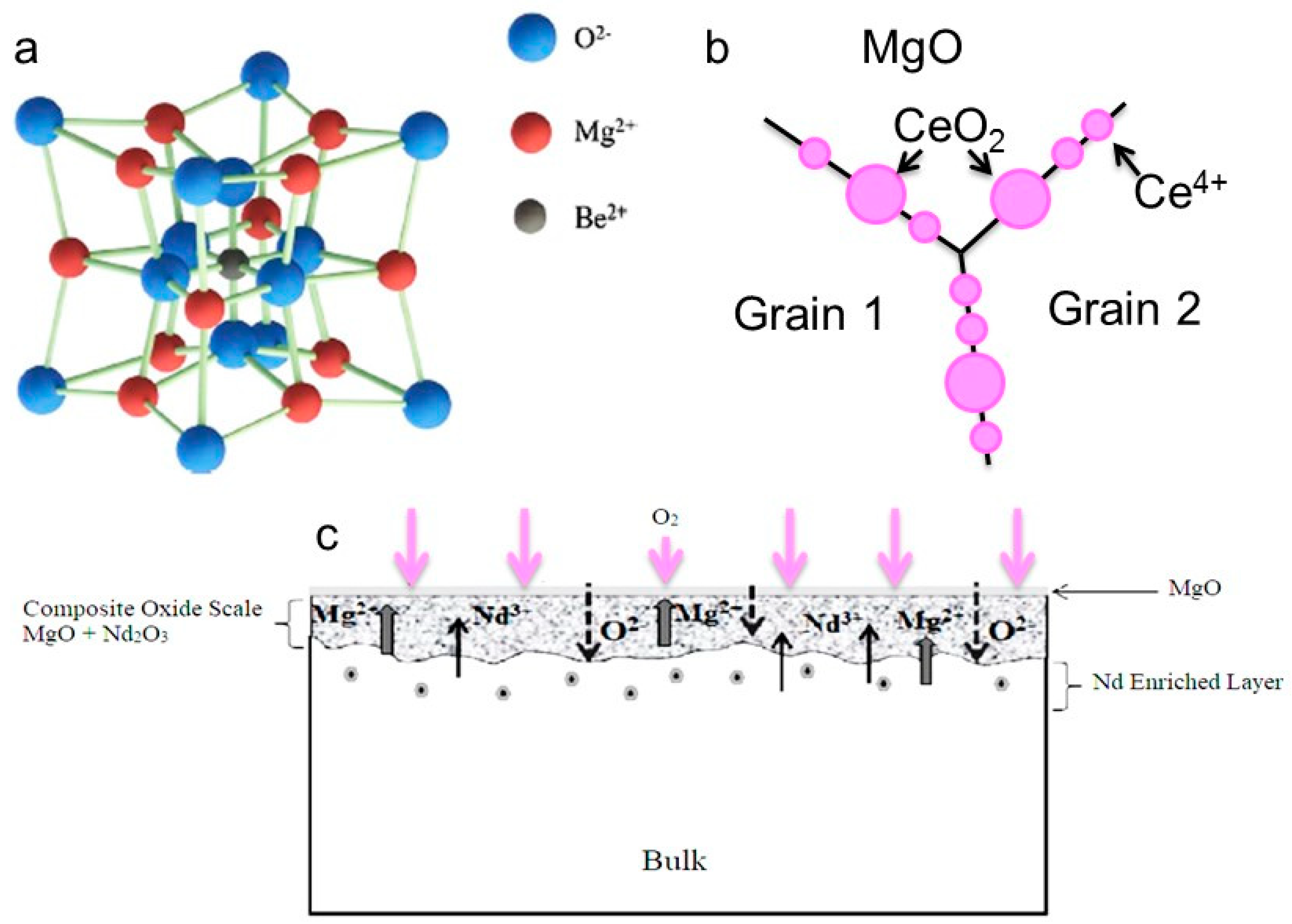
4. Why metal Diffuses Outwards and Oxygen Inward in the Alloy?
5. Quantum Approach in Understanding the Electron Transfer at Passive Interfaces
6. Metallic Additions in Alloys to Scale Up the Corrosion Resistance
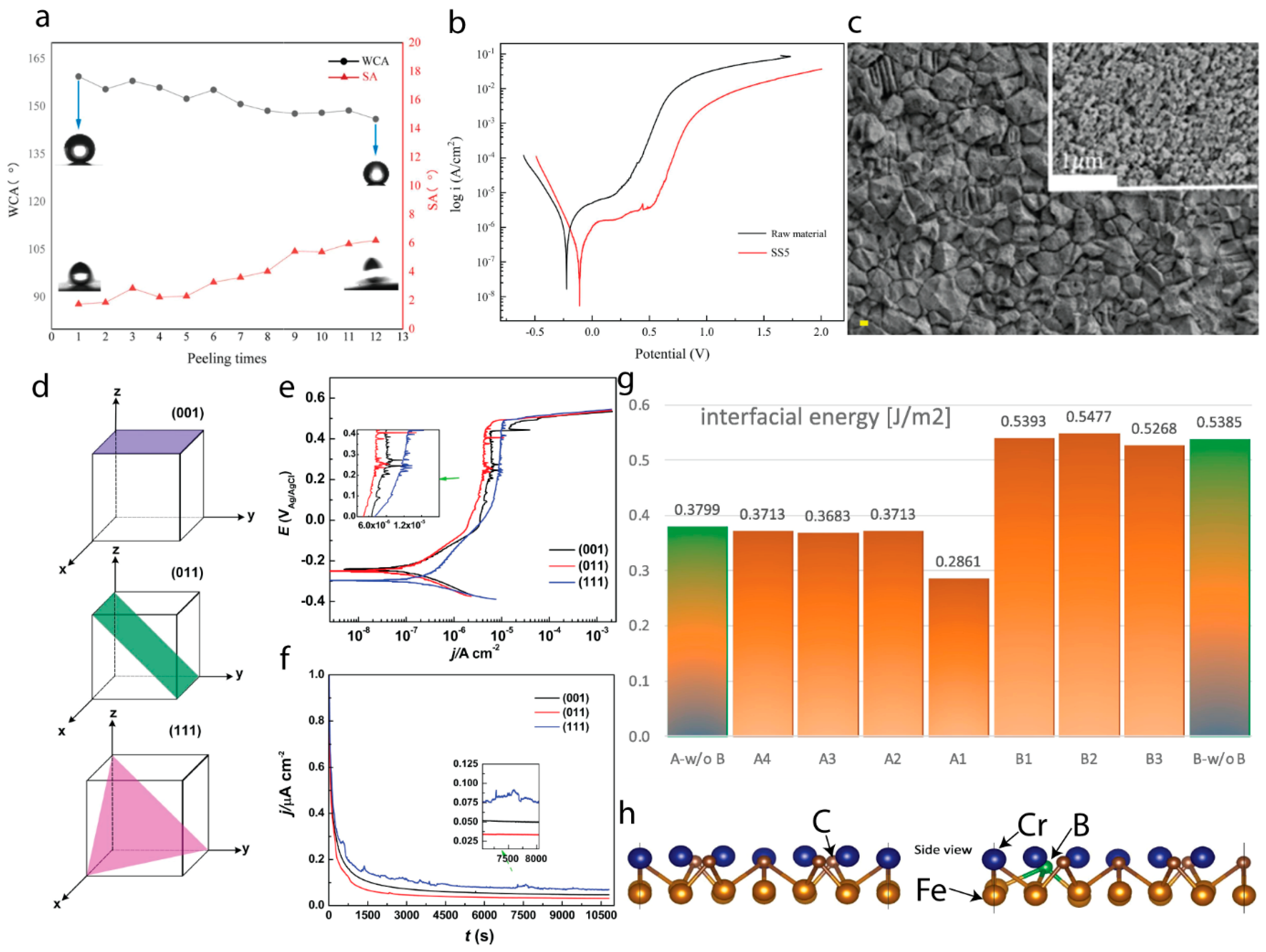
7. Role of Texture Control and Surface Energy in Corrosion Inhibition
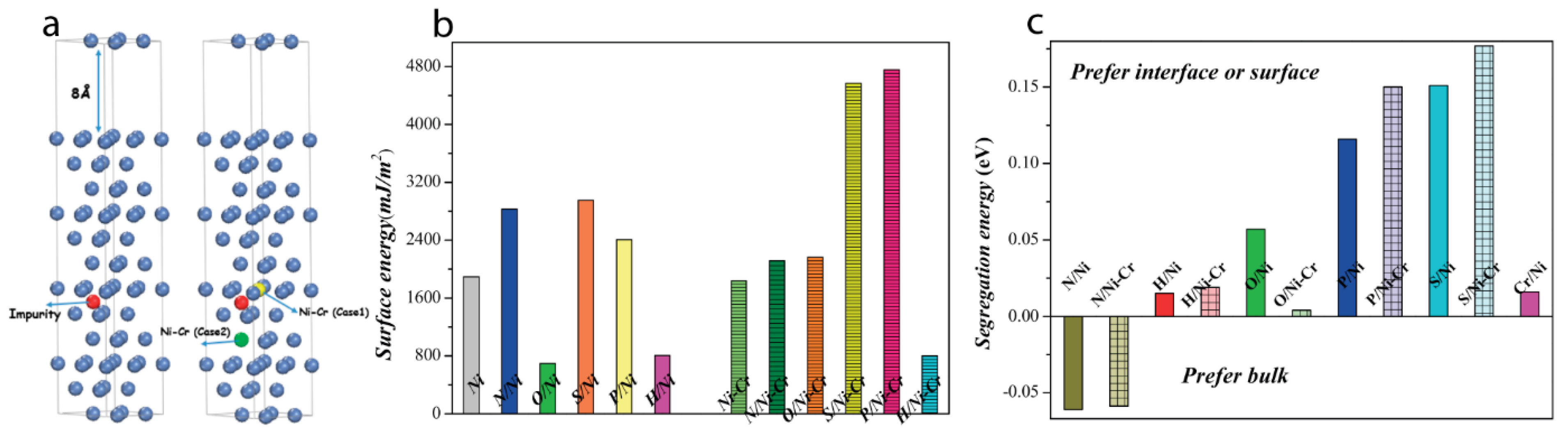
8. Atomistic Study of Corrosion
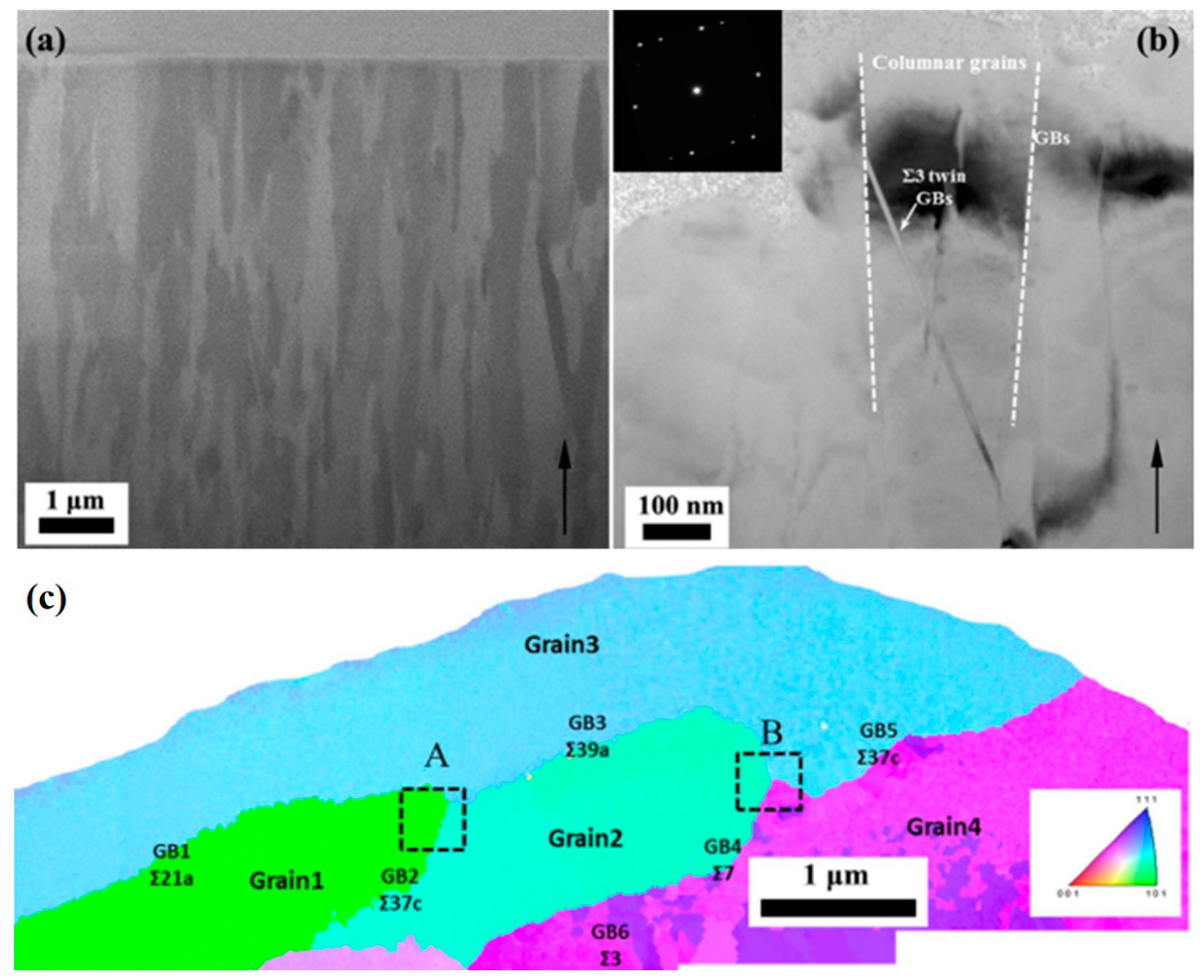
9. Corrosion and Sensitization control by Grain Boundary Engineering (GBE)
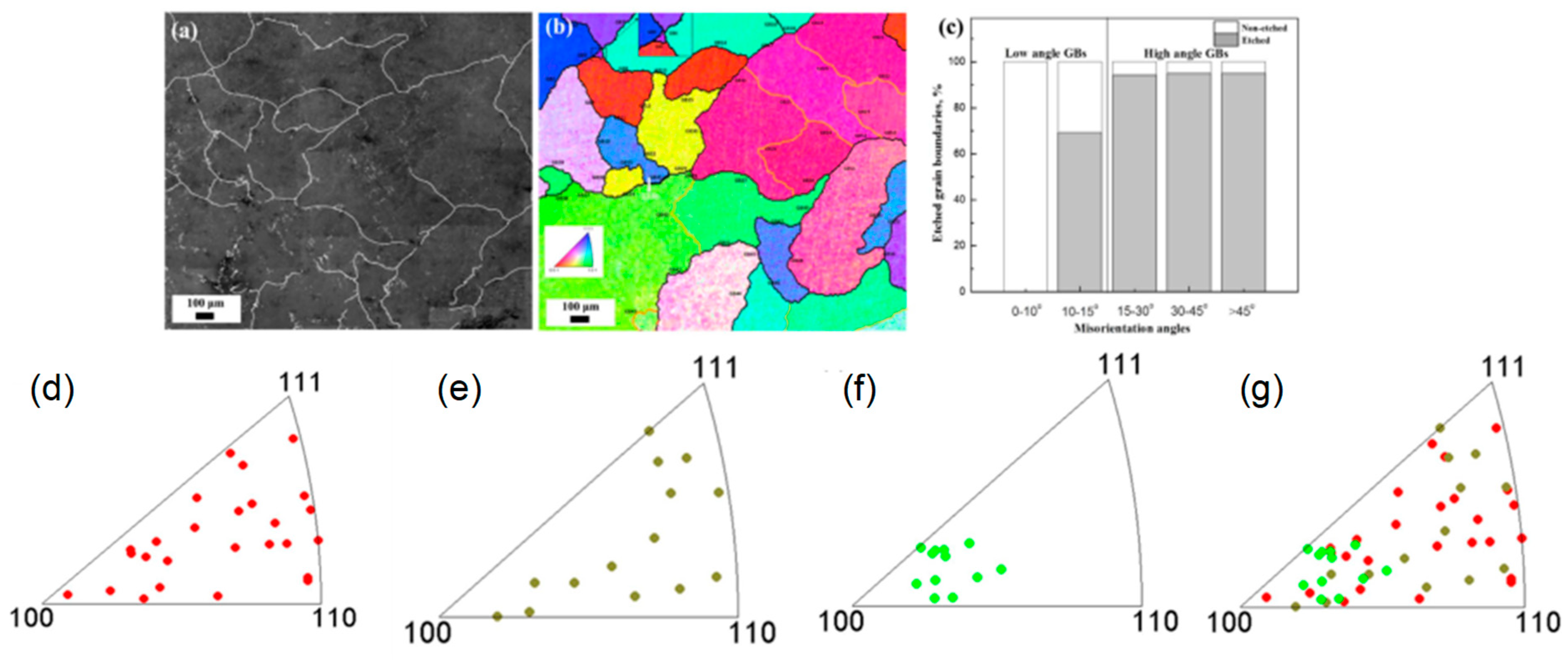
10. Effect of Grain Misorientation
10.1. Effect of GB Plane Orientations
11. The Emergence of New Material as a Guard against Corrosion
12. Present Challenges
13. Market Potentials of Protective Systems Used in High-Temperature Materials
14. Diverse Ideas in Research Do Wonder in Science
15. Outlook and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranganathan, S. Alloyed pleasures: multimetallic cocktails. Current science 2003, 85, 1404–1406. [Google Scholar]
- Ruzette, A.-V.; Leibler, L. Block copolymers in tomorrow's plastics. Nat. Mater. 2005, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, X.-x.; Kaub, T.; Martens, R.L.; Thompson, G.B. Grain Boundary Specific Segregation in Nanocrystalline Fe (Cr). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Sato, H. System evaluation for high temperature ultra supercritical steam power plants. Nihon Kikai Gakkai Ronbunshu, B Hen/Transactions of the Japan Society of Mechanical Engineers, Part B 2006, 72, 2570–2577. [Google Scholar] [CrossRef]
- Ahn, Y.; Bae, S.J.; Kim, M.; Cho, S.K.; Baik, S.; Lee, J.I.; Cha, J.E. Review of supercritical CO2 power cycle technology and current status of research and development. Nucl. Eng. Technol. 2015, 47, 647–661. [Google Scholar] [CrossRef]
- Singh, A.N.; Moitra, A.; Bhaskar, P.; Sasikala, G.; Dasgupta, A.; Bhaduri, A.K. Study of Aging-Induced Degradation of Fracture Resistance of Alloy 617 Toward High-Temperature Applications. Metall. Mater. Trans. A 2017, 48, 3269–3278. [Google Scholar] [CrossRef]
- Mohan, G.; Venkataraman, M.B.; Coventry, J. Sensible energy storage options for concentrating solar power plants operating above 600 °C. Renew. Sust. Energ. Rev. 2019, 107, 319–337. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sust. Energ. Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Kahraman, N.; Gulenc, B.; Findik, F. Corrosion and mechanical-microstructural aspects of dissimilar joints of Ti–6Al–4V and Al plates. Int. J. Impact Eng. 2007, 34, 1423–1432. [Google Scholar] [CrossRef]
- Degerbeck, J.; Gille, I. Crevice corrosion—A new crevice former. Corros. Sci. 1979, 19, 1113–1115. [Google Scholar] [CrossRef]
- Mazario, E.; Venegas, R.; Herrasti, P.; Alonso, M.C.; Recio, F.J. Pitting corrosion and stress corrosion cracking study in high strength steels in alkaline media. J. Solid State Electrochem. 2016, 20, 1223–1227. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Chen, Z.-y.; Luo, H.-f.; Teng, Z.; Zhao, Y.-l.; Ling, Z.-c. Corrosion resistances of metallic materials in environments containing chloride ions: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 377–410. [Google Scholar] [CrossRef]
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Fail. Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Zeng, L.; Shuang, S.; Guo, X.P.; Zhang, G.A. Erosion-corrosion of stainless steel at different locations of a 90° elbow. Corros. Sci. 2016, 111, 72–83. [Google Scholar] [CrossRef]
- Ige, O.O.; Umoru, L.E. Effects of shear stress on the erosion-corrosion behaviour of X-65 carbon steel: A combined mass-loss and profilometry study. Tribol. Int. 2016, 94, 155–164. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.A.; Guo, X.P. Erosion–corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Gleeson, B. Still plenty to explore. Nat. Mater. 2018, 17, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M. Peering below the surface. Nat. Mater. 2004, 3, 663–664. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Barnoush, A.; Johnsen, R. Materials and corrosion trends in offshore and subsea oil and gas production. npj Materials Degradation 2017, 1, 2. [Google Scholar] [CrossRef]
- McMillan, P.F. New materials from high-pressure experiments. Nat Mater 2002, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.D.; Tossey, B.M. High temperature oxidation of corrosion resistant alloys from machine learning. npj Materials Degradation 2021, 5, 38. [Google Scholar] [CrossRef]
- Oparaodu, K.O.; Okpokwasili, G.C. Comparison of Percentage Weight Loss and Corrosion Rate Trends in Different Metal Coupons from two Soil Environments. International Journal of Environmental Bioremediation & Biodegradation 2014, 2, 243–249. [Google Scholar]
- Chigondo, M.; Chigondo, F. Recent Natural Corrosion Inhibitors for Mild Steel: An Overview. J. Chem. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Muhammad, S. Corrosion protection with eco-friendly inhibitors. Advances in Natural Sciences: Nanoscience and Nanotechnology 2011, 2, 043001. [Google Scholar]
- Randle, V. Grain boundary engineering: an overview after 25 years. Mater. Sci. Technol. 2010, 26, 253–261. [Google Scholar] [CrossRef]
- Randle, V. The measurement of grain boundary geometry; Institute of Physics Pub.: 1993.
- Randle, V. The coincidence site lattice and the ‘sigma enigma’. Mater. Charact. 2001, 47, 411–416. [Google Scholar] [CrossRef]
- Watanabe, T. Grain boundary engineering: historical perspective and future prospects. J. Mater. Sci. 2011, 46, 4095–4115. [Google Scholar] [CrossRef]
- Shimada, M.; Kokawa, H.; Wang, Z.J.; Sato, Y.S.; Karibe, I. Optimization of grain boundary character distribution for intergranular corrosion resistant 304 stainless steel by twin-induced grain boundary engineering. Acta Mater. 2002, 50, 2331–2341. [Google Scholar] [CrossRef]
- Lehockey, E.M.; Limoges, D.; Palumbo, G.; Sklarchuk, J.; Tomantschger, K.; Vincze, A. On improving the corrosion and growth resistance of positive Pb-acid battery grids by grain boundary engineering. J. Power Sources 1999, 78, 79–83. [Google Scholar] [CrossRef]
- Gabb, T.P.; Telesman, J.; Garg, A.; Lin, P.; Provenzano, V.; Heard, R.; Miller, H.M. Grain Boundary Engineering the Mechanical Properties of Allvac 718Plus™ Superalloy. Superalloy 718 and Derivatives 2010, 254–269. [Google Scholar]
- Moore, W.C. AQUA REGIA: PRELIMINARY PAPER. J. Am. Chem. Soc. 1911, 33, 1091–1099. [Google Scholar] [CrossRef]
- Karen, P. Oxidation State, A Long-Standing Issue. Angewandte Chemie (International Ed. in English) 2015, 54, 4716–4726. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of electrochemical corrosion; ASM international: 2000.
- Cao, G. Nanostructures and Nanomaterials:Synthesis, Properties and Applications; Imperial College Press: 2004.
- Jinich, A.; Rappoport, D.; Dunn, I.; Sanchez-Lengeling, B.; Olivares-Amaya, R.; Noor, E.; Even, A.B.; Aspuru-Guzik, A. Quantum Chemical Approach to Estimating the Thermodynamics of Metabolic Reactions. 2014, 4, 7022. [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Spalling failure of α-alumina films grown by oxidation: I. Dependence on cooling rate and metal thickness. Mater. Sci. Eng., A 2000, 278, 142–150. [Google Scholar] [CrossRef]
- Chen, L.; Yueming, L. Interface stress evolution considering the combined creep–plastic behavior in thermal barrier coatings. Mater. Design 2016, 89, 245–254. [Google Scholar] [CrossRef]
- Knipe, K.; Manero Ii, A.; Siddiqui, S.F.; Meid, C.; Wischek, J.; Okasinski, J.; Almer, J.; Karlsson, A.M.; Bartsch, M.; Raghavan, S. Strain response of thermal barrier coatings captured under extreme engine environments through synchrotron X-ray diffraction. 2014, 5, 4559. [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Kamrunnahar, M.; Bao, J.; Macdonald, D.D. Challenges in the theory of electron transfer at passive interfaces. Corros. Sci. 2005, 47, 3111–3139. [Google Scholar] [CrossRef]
- Gurney, R. The quantum mechanics of electrolysis. Proc. R. Soc. Lond. A 1931, 134, 137–154. [Google Scholar]
- Burstein, G.T. A hundred years of Tafel’s Equation: 1905–2005. Corros. Sci. 2005, 47, 2858–2870. [Google Scholar] [CrossRef]
- Liu, R.L.; Hurley, M.F.; Kvryan, A.; Williams, G.; Scully, J.R.; Birbilis, N. Controlling the corrosion and cathodic activation of magnesium via microalloying additions of Ge. Sci. Rep. 2016, 6, 28747. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Bazant, M.Z. Charge transfer kinetics at the solid–solid interface in porous electrodes. Nat. Commun. 2014, 5, 3585. [Google Scholar] [CrossRef] [PubMed]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Gabe, D. The centenary of Tafel's equation. Transactions of the IMF 2005, 83, 121–124. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochimica et Biophysica Acta (BBA) - Reviews on Bioenergetics 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Marcus, R. RA Marcus, J. Chem. Phys. 24, 966 (1956). J. Chem. Phys. 1956, 24, 966. [Google Scholar] [CrossRef]
- Marcus, R. On the theory of chemiluminescent electron-transfer reactions. J. Chem. Phys. 1965, 43, 2654–2657. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Ulstrup, J. Theory of electron transfer at electrified interfaces. Electrochim. Acta 2000, 45, 2339–2361. [Google Scholar] [CrossRef]
- Schmickler, W. A general model for electron-transfer reactions via electronic intermediate states. J. Electroanal. Chem. Interfacial Electrochem. 1982, 137, 189–198. [Google Scholar] [CrossRef]
- Esaki, L. Discovery of the tunnel diode. IEEE Trans. Electron Devices 1976, 23, 644–647. [Google Scholar] [CrossRef]
- Mizuta, H.; Tanoue, T. The physics and applications of resonant tunnelling diodes; Cambridge University Press: 2006; Volume 2.
- Schmickler, W.; Ulstrup, J. A theory of electron transfer reactions at film-covered metal electrodes. Chem. Phys. 1977, 19, 217–232. [Google Scholar] [CrossRef]
- Oksa, M.; Metsäjoki, J.; Kärki, J. Thermal Spray Coatings for High-Temperature Corrosion Protection in Biomass Co-Fired Boilers. J. Therm. Spray Technol. 2015, 24, 194–205. [Google Scholar] [CrossRef]
- Kang, Y.; Leng, X.; Zhao, L.; Bai, B.; Wang, X.; Chen, H. Review on the Corrosion Behaviour of Nickel-Based Alloys in Supercritical Carbon Dioxide under High Temperature and Pressure. Crystals 2023, 13, 725. [Google Scholar] [CrossRef]
- Cho, F.-Y.; Tung, S.-W.; Ouyang, F.-Y. High temperature oxidation behavior of high entropy alloy Al4Co3Cr25Cu10Fe25Ni33 in oxygen-containing atmospheres. Mater. Chem. Phys. 2022, 278, 125678. [Google Scholar] [CrossRef]
- Zhang, X.; Corrêa da Silva, C.; Liu, C.; Prabhakar, M.; Rohwerder, M. Selective oxidation of ternary Fe-Mn-Si alloys during annealing process. Corros. Sci. 2020, 174, 108859. [Google Scholar] [CrossRef]
- Li, L.; Guo, X. Microstructure and oxidation resistance of Ti modified Si-Mo coating on Nb-Si based ultrahigh temperature alloy. Corros. Sci. 2023, 220, 111293. [Google Scholar] [CrossRef]
- Reddy, G.M.S.; Prasad, C.D.; Shetty, G.; Ramesh, M.R.; Rao, T.N.; Patil, P. High-temperature oxidation behavior of plasma-sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ coatings on titanium alloy. Welding in the World 2022, 66, 1069–1079. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Materials Degradation 2017, 1, 15. [Google Scholar] [CrossRef]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Xie, X.; Li, N.; Liu, W.; Huang, S.; He, X.; Yu, Q.; Xiong, H.; Wang, E.; Hou, X. Research Progress of Refractory High Entropy Alloys: A Review. Chinese Journal of Mechanical Engineering 2022, 35, 142. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Oliveira, M.C.L.d.; Antunes, R.A. A review on corrosion of high entropy alloys: exploring the interplay between corrosion properties, alloy composition, passive film stability and materials selection. Materials Research 2022, 25, e20210442. [Google Scholar] [CrossRef]
- Godlewska, E.M.; Mitoraj-Królikowska, M.; Czerski, J.; Jawańska, M.; Gein, S.; Hecht, U. Corrosion of Al (Co) CrFeNi high-entropy alloys. Frontiers in Materials 2020, 7, 566336. [Google Scholar] [CrossRef]
- Liu, R.; Sun, H.; Jiang, X.; Liu, X.; Yan, W. Study of microstructure and corrosion resistance of FeCrAl-Gd alloys. Mater. Chem. Phys. 2023, 297, 127384. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Tylczak, J.H.; Gao, M.C.; Jablonski, P.D.; Detrois, M.; Ziomek-Moroz, M.; Hawk, J.A. Effect of Molybdenum on the Corrosion Behavior of High-Entropy Alloys CoCrFeNi2 and CoCrFeNi2Mo0. 25 under Sodium Chloride Aqueous Conditions. Advances in Materials Science and Engineering 2018, 2018. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. New studies on wear and corrosion behavior of laser cladding FeNiCoCrMox high entropy alloy coating: The role of Mo. Int. J. Refract. Met. Hard Mater. 2022, 102, 105721. [Google Scholar] [CrossRef]
- Mishra, A.; Shoesmith, D. The activation/depassivation of nickel–chromium–molybdenum alloys: An oxyanion or a pH effect—Part II. Electrochim. Acta 2013, 102, 328–335. [Google Scholar] [CrossRef]
- Hayes, J.; Gray, J.; Szmodis, A.; Orme, C. Influence of chromium and molybdenum on the corrosion of nickel-based alloys. Corrosion 2006, 62, 491–500. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, Q.; Jin, C.; Hua, K.; Wang, H. The determining role of Al addition on tribology properties and oxidation behavior at elevated temperatures of TiZrHfNb refractory high-entropy alloy. Mater. Charact. 2022, 189, 111921. [Google Scholar] [CrossRef]
- Lin, C.-M.; Juan, C.-C.; Chang, C.-H.; Tsai, C.-W.; Yeh, J.-W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTiZr alloys. J. Alloys Compd. 2015, 624, 100–107. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, I.-J.; Kim, J.-G. Alloying effect of copper concentration on the localized corrosion of aluminum alloy for heat exchanger tube. Met. Mater. Int. 2017, 23, 708–714. [Google Scholar] [CrossRef]
- Seyeux, A.; Frankel, G.; Missert, N.; Unocic, K.; Klein, L.; Galtayries, A.; Marcus, P. ToF-SIMS imaging study of the early stages of corrosion in Al-Cu thin films. J. Electrochem. Soc. 2011, 158, C165–C171. [Google Scholar] [CrossRef]
- Larsen, M.H.; Walmsley, J.C.; Lunder, O.; Mathiesen, R.H.; Nisancioglu, K. Intergranular Corrosion of Copper-Containing AA6x xx AlMgSi Aluminum Alloys. J. Electrochem. Soc. 2008, 155, C550–C556. [Google Scholar] [CrossRef]
- Yan, H.Y.; Vorontsov, V.A.; Dye, D. Effect of alloying on the oxidation behaviour of Co–Al–W superalloys. Corros. Sci. 2014, 83, 382–395. [Google Scholar] [CrossRef]
- Giovanardi, C.; Hammer, L.; Heinz, K. Ultrathin cobalt oxide films on Ir (100)−(1× 1). Phys. Rev. B 2006, 74, 125429. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Selloni, A. Electronic structure and bonding properties of cobalt oxide in the spinel structure. Phys. Rev. B 2011, 83, 245204. [Google Scholar] [CrossRef]
- Tan, H.B.; Ke, K.; Ma, B.G.; Xiao, J. Effect and Solid Solution Mechanism of Co2O3 during C3S Formation. In Proceedings of the Advanced Materials Research; 2011; pp. 1587–1592. [Google Scholar]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: a review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Kangazian, J.; Shamanian, M. Micro-texture and corrosion behavior of dissimilar joints of UNS S32750 stainless steel/UNS N08825 Ni-based superalloy. Mater. Charact. 2019, 155, 109802. [Google Scholar] [CrossRef]
- Bateni, M.R.; Szpunar, J.A.; Wang, X.; Li, D.Y. The effect of wear and corrosion on internal crystalline texture of carbon steel and stainless steel. Wear 2005, 259, 400–404. [Google Scholar] [CrossRef]
- Fushimi, K.; Miyamoto, K.; Konno, H. Anisotropic corrosion of iron in pH 1 sulphuric acid. Electrochim. Acta 2010, 55, 7322–7327. [Google Scholar] [CrossRef]
- Kumar, B.R.; Singh, R.; Mahato, B.; De, P.; Bandyopadhyay, N.; Bhattacharya, D. Effect of texture on corrosion behavior of AISI 304L stainless steel. Mater. Charact. 2005, 54, 141–147. [Google Scholar] [CrossRef]
- Park, S.-A.; Kim, J.; He, Y.; Shin, K.; Yoon, J. Comparative study on the corrosion behavior of the cold rolled and hot rolled low-alloy steels containing copper and antimony in flue gas desulfurization environment. The Physics of Metals and Metallography 2014, 115, 1285–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, W.; Zhu, S.; Chen, G.; Wang, L.; Su, Y. Preparation of super-hydrophobic surface with micro-nano layered structure on 316 stainless steel by one-step wet chemical method. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 655, 130291. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, X.; Liu, E.; Yu, S.; Yin, X.; Wang, J.; Zhu, G.; Li, Q.; Li, J. Fabrication of superhydrophobic needle-like Ca-P coating with anti-fouling and anti-corrosion properties on AZ31 magnesium alloy. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 620, 126568. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Lei, Y.; Wang, Y.; Zhou, G.; Xu, C.; Rao, Y.; Wang, K. Preparation of intricate nanostructures on 304 stainless steel surface by SiO2-assisted HF etching for high superhydrophobicity. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 586, 124287. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl. Mater. Inter. 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Lei, X.W.; Hao, L.Y.; Han, S.X.; Yang, R.N.; Wang, N. The anisotropy electrochemical corrosion behavior of Ni-based single crystal superalloy on different crystal planes: An investigation from the film growth aspect. Applied Surface Science 2022, 576, 151785. [Google Scholar] [CrossRef]
- Zhang, L.; Ojo, O. Crystallographic orientation dependence of corrosion behavior of a single crystal nickel-based alloy. Metall. Mater. Trans. A 2018, 49, 295–304. [Google Scholar] [CrossRef]
- Yu, J.; Glazoff, M.V.; Capolungo, L.; Gao, M.C.; Ilevbare, G.O. Boron substitution induced FCC Fe/Cr23C6 interfacial strengthening: An ab initio study. Computational Materials Science 2023, 228, 112370. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V. Atomic level characterization in corrosion studies. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2017, 375, 20160414. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A. Molecular modeling of organic corrosion inhibitors: Calculations, pitfalls, and conceptualization of molecule–surface bonding. Corros. Sci. 2021, 193, 109650. [Google Scholar] [CrossRef]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; Lima-Neto, P.d. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Zuili, D.; Maurice, V.; Marcus, P. Surface structure of nickel in acid solution studied by in situ scanning tunneling microscopy. J. Electrochem. Soc. 2000, 147, 1393–1400. [Google Scholar] [CrossRef]
- Magnussen, O.M.; Scherer, J.; Ocko, B.M.; Behm, R.J. In Situ X-ray Scattering Study of the Passive Film on Ni(111) in Sulfuric Acid Solution. The Journal of Physical Chemistry B 2000, 104, 1222–1226. [Google Scholar] [CrossRef]
- Scherer, J.; Ocko, B.; Magnussen, O. Structure, dissolution, and passivation of Ni (111) electrodes in sulfuric acid solution: an in situ STM, X-ray scattering, and electrochemical study. Electrochim. Acta 2003, 48, 1169–1191. [Google Scholar] [CrossRef]
- Maurice, V.; Talah, H.; Marcus, P. A scanning tunneling microscopy study of the structure of thin oxide films grown on Ni (111) single crystal surfaces by anodic polarization in acid electrolyte. Surf. Sci. 1994, 304, 98–108. [Google Scholar] [CrossRef]
- Bouzoubaa, A.; Diawara, B.; Maurice, V.; Minot, C.; Marcus, P. Ab initio study of the interaction of chlorides with defect-free hydroxylated NiO surfaces. Corros. Sci. 2009, 51, 941–948. [Google Scholar] [CrossRef]
- Bouzoubaa, A.; Diawara, B.; Maurice, V.; Minot, C.; Marcus, P. Ab initio modelling of localized corrosion: Study of the role of surface steps in the interaction of chlorides with passivated nickel surfaces. Corros. Sci. 2009, 51, 2174–2182. [Google Scholar] [CrossRef]
- Maurice, V.; Klein, L.; Marcus, P. Atomic structure of metastable pits formed on nickel. Electrochem. Solid-State Lett. 2001, 4, B1–B3. [Google Scholar] [CrossRef]
- Seyeux, A.; Maurice, V.; Klein, L.; Marcus, P. In situ STM study of the effect of chloride on passive film on nickel in alkaline solution. J. Electrochem. Soc. 2006, 153, B453–B463. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.-H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Maurice, V.; Nakamura, T.; Klein, L.; Marcus, P. Initial stages of localised corrosion by pitting of passivated nickel surfaces studied by STM and AFM. Local Probe Techniques for Corrosion Research, EFC Publications.
- Ball, P. A sense of dislocations. Nat. Mater. 2015, 14, 968–968. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.H.; Li, C.X.; Han, P.D. Synergetic effects of impurities and alloying element Cr on oxidation and dissolution corrosion of Ni (111) surfaces: A DFT study. Chinese Journal of Physics 2019, 61, 1–7. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsurekawa, S.; Zhao, X.; Zuo, L. The Coming of Grain Boundary Engineering in the 21st Century. In Microstructure and Texture in Steels: and Other Materials, Haldar, A., Suwas, S., Bhattacharjee, D., Eds.; Springer London: London, 2009; pp. 43–82. [Google Scholar]
- Watanabe, T. An approach to grain-boundary design for strong and ductile polycrystals. Res Mechanica 1984, 11, 47–84. [Google Scholar]
- Randle, V.; Brown, A. Development of grain misorientation texture, in terms of coincident site lattice structures, as a function of thermomechanical treatments. Philos. Mag. A 1989, 59, 1075–1089. [Google Scholar] [CrossRef]
- Cheng, Y.; Jin, Z.H.; Zhang, Y.W.; Gao, H. On intrinsic brittleness and ductility of intergranular fracture along symmetrical tilt grain boundaries in copper. Acta Mater. 2010, 58, 2293–2299. [Google Scholar] [CrossRef]
- Hossein Nedjad, S.; Nili Ahmadabadi, M.; Furuhara, T. Correlation between the intergranular brittleness and precipitation reactions during isothermal aging of an Fe–Ni–Mn maraging steel. Mater. Sci. Eng., A 2008, 490, 105–112. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. 2016, 6, 26870. [CrossRef]
- Yamaura, S.-i.; Tsurekawa, S.; Watanabe, T. The Control of Oxidation-Induced Intergranular Embrittlement by Grain Boundary Engineering in Rapidly Solidified Ni-Fe Alloy Ribbons. Mater. Trans. 2003, 44, 1494–1502. [Google Scholar] [CrossRef]
- Kobayashi, S.; Maruyama, T.; Tsurekawa, S.; Watanabe, T. Grain boundary engineering based on fractal analysis for control of segregation-induced intergranular brittle fracture in polycrystalline nickel. Acta Mater. 2012, 60, 6200–6212. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsurekawa, S.; Watanabe, T. A new approach to grain boundary engineering for nanocrystalline materials. Beilstein J. Nanotechnol. 2016, 7, 1829. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tsurekawa, S.; Fujii, H.; Kanno, T. The control of texture and grain boundary microstructure by magnetic annealing. In Proceedings of the Mater. Sci. Forum; 2005; pp. 1151–1158. [Google Scholar]
- Bettayeb, M.; Maurice, V.; Klein, L.H.; Lapeire, L.; Verbeken, K.; Marcus, P. Nanoscale Intergranular Corrosion and Relation with Grain Boundary Character as Studied In Situ on Copper. J. Electrochem. Soc. 2018, 165, C835–C841. [Google Scholar] [CrossRef]
- Yang, F.-q.; Xue, H.; Zhao, L.-y.; Fang, X.-R.; Zhang, H.-b. Effects of Crystal Orientation and Grain Boundary Inclination on Stress Distribution in Bicrystal Interface of Austenite Stainless Steel 316L. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N. Effect of grain size on corrosion: a review. Corrosion 2010, 66, 075005–075005. [Google Scholar] [CrossRef]
- Polmear, I. Light alloys- Metallurgy of the light metals//((Book)). London and New York, Edward Arnold, 1989, 288.
- Shahabi-Navid, M.; Esmaily, M.; Svensson, J.-E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.-G. NaCl-induced atmospheric corrosion of the MgAl alloy AM50-the influence of CO2. J. Electrochem. Soc. 2014, 161, C277–C287. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R. Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Jones, R.; Baer, D.; Danielson, M.; Vetrano, J. Role of Mg in the stress corrosion cracking of an Al-Mg alloy. Metall. Mater. Trans. A. 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Zhang, R.; Gupta, R.; Davies, C.; Hodge, A.; Tort, M.; Xia, K.; Birbilis, N. The influence of grain size and grain orientation on sensitization in AA5083. Corrosion 2015, 72, 160–168. [Google Scholar]
- Choi, D.-H.; Ahn, B.-W.; Quesnel, D.J.; Jung, S.-B. Behavior of β phase (Al 3 Mg 2) in AA 5083 during friction stir welding. Intermetallics 2013, 35, 120–127. [Google Scholar] [CrossRef]
- Walczak, M.S.; Morales-Gil, P.; Belashehr, T.; Kousar, K.; Lozada, P.A.; Lindsay, R. Determining the Chemical Composition of Corrosion Inhibitor/Metal Interfaces with XPS: Minimizing Post Immersion Oxidation. Journal of visualized experiments: JoVE.
- Wang, Y.; Gupta, R.; Sukiman, N.; Zhang, R.; Davies, C.; Birbilis, N. Influence of alloyed Nd content on the corrosion of an Al–5Mg alloy. Corros. Sci. 2013, 73, 181–187. [Google Scholar] [CrossRef]
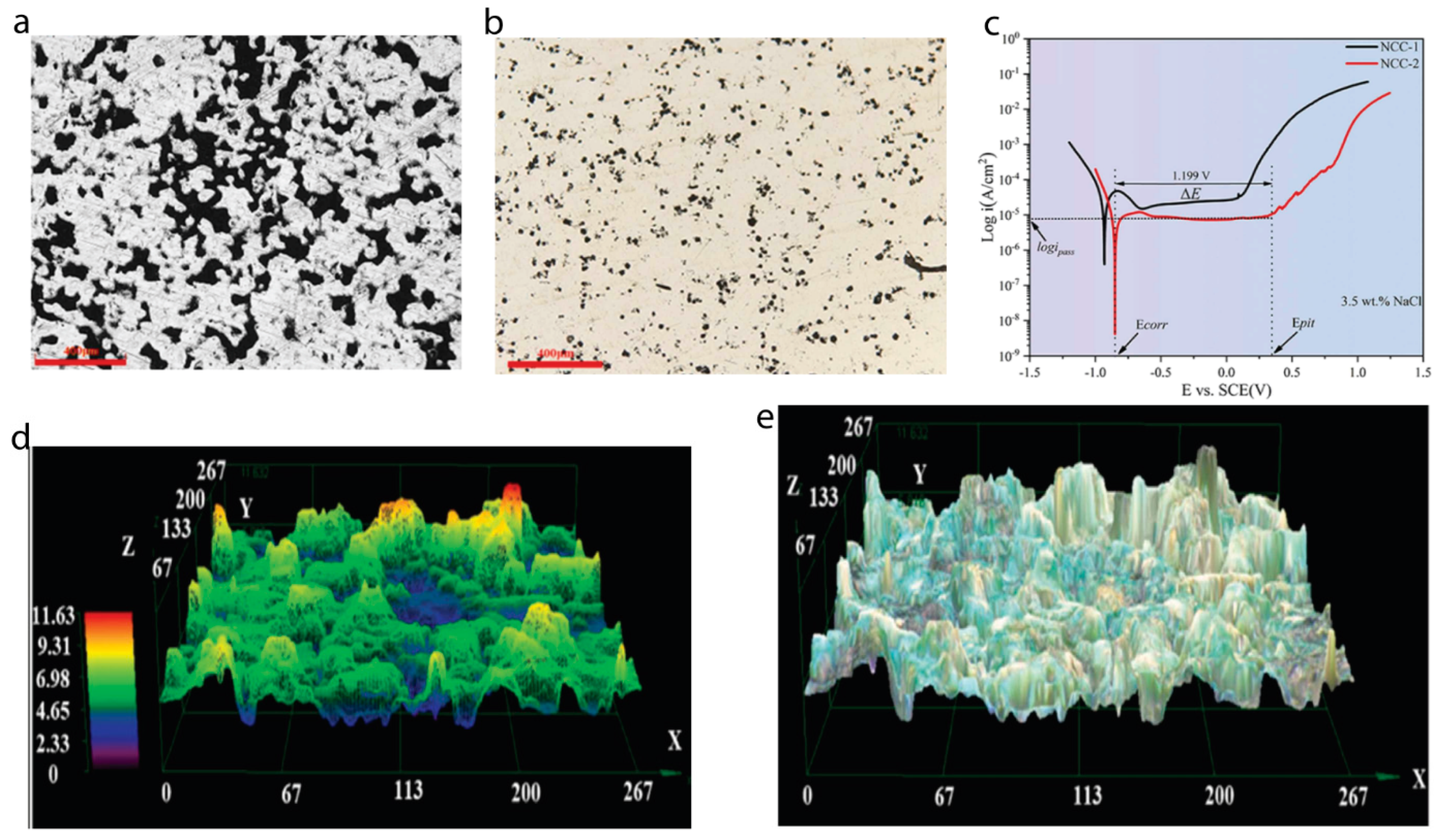
- Zhang, Y.; Xiao, Z.; Zhao, Y.; Li, Z.; Xing, Y.; Zhou, K. Effect of thermo-mechanical treatments on corrosion behavior of Cu-15Ni-8Sn alloy in 3.5 wt% NaCl solution. Mater. Chem. Phys. 2017, 199, 54–66. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Oguocha, I.; Adigun, O.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al–Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Shi, P.; Hu, R.; Zhang, T.; Yuan, L.; Li, J. Grain boundary character distribution and its effect on corrosion of Ni–23Cr–16Mo superalloy. Mater. Sci. Technol. 2017, 33, 84–91. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsurekawa, S. The control of brittleness and development of desirable mechanical properties in polycrystalline systems by grain boundary engineering. Acta Mater. 1999, 47, 4171–4185. [Google Scholar] [CrossRef]
- Hickman, J.; Mishin, Y. Extra variable in grain boundary description. Phys. Rev. Mater. 2017, 1, 010601. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. Sci. Rep. 2016, 6, 26870. [Google Scholar] [CrossRef] [PubMed]
- Homer, E.R.; Patala, S.; Priedeman, J.L. Grain boundary plane orientation fundamental zones and structure-property relationships. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Davenport, A.J.; Yuan, Y.; Ambat, R.; Connolly, B.J.; Strangwood, M.; Afseth, A.; Scamans, G.M. Intergranular corrosion and stress corrosion cracking of sensitised AA5182. In Proceedings of the Mater. Sci. Forum; 2006; pp. 641–646. [Google Scholar]
- Kaigorodova, L. The effect of grain-boundary structure formation on β-precipitation in aged Al-Mg alloys. In Proceedings of the Mater. Sci. Forum; 1999; pp. 477–480. [Google Scholar]
- D’Antuono, D.S.; Gaies, J.; Golumbfskie, W.; Taheri, M. Grain boundary misorientation dependence of β phase precipitation in an Al–Mg alloy. Scripta Mater. 2014, 76, 81–84. [Google Scholar] [CrossRef]
- Brandon, D. The structure of high-angle grain boundaries. Acta Metall. 1966, 14, 1479–1484. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. Sci. Rep. 2016, 6, 26870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Polyakov, M.N.; Mecklenburg, M.; Kassner, M.E.; Hodge, A.M. The role of grain boundary plane orientation in the β phase precipitation of an Al–Mg alloy. Scripta Mater. 2014, 89, 49–52. [Google Scholar] [CrossRef]
- Zhao, Y. The role of nanotwins and grain boundary plane in the thermal, corrosion, and sensitization behavior of nanometals; University of Southern California: 2014.
- Zhou, X.; Yu, X.-x.; Kaub, T.; Martens, R.L.; Thompson, G.B. Grain Boundary Specific Segregation in Nanocrystalline Fe(Cr). 2016, 6, 34642. [CrossRef]
- Klostermann, J. The concept of the habit plane and the phenomenological theories of the martensite transformation. J. Less Common Met. 1972, 28, 75–94. [Google Scholar] [CrossRef]
- Baur, A.P.; Cayron, C.; Logé, R.E. {225}(γ) habit planes in martensitic steels: from the PTMC to a continuous model. Sci. Rep. 2017, 7, 40938. [Google Scholar] [CrossRef]
- Rusakov, G.M.; Lobanov, M.L.; Redikultsev, A.A.; Kagan, I.V. Retention of the Twinning Σ3 Misorientation in the Process of Lattice Transformation during Cold Rolling of a Fe3 Pct Si Single Crystal. Metall. Mater. Trans. A. 2011, 42, 1435–1438. [Google Scholar] [CrossRef]
- Wood, R.M.; Entwisle, A.R. Martensite habit planes in Fe-Mn-C alloys. Metal Science 1976, 10, 72–76. [Google Scholar] [CrossRef]
- Dai, C.; Balogh, L.; Yao, Z.; Daymond, M.R. The habit plane of 〈a〉-type dislocation loops in α-zirconium: an atomistic study. Philos. Mag. 2017, 97, 944–956. [Google Scholar] [CrossRef]
- Cayron, C. Continuous atomic displacements and lattice distortions during martensitic transformations in fcc-bcc-hcp systems. arXiv preprint arXiv:1502.07152, arXiv:1502.07152 2015.
- Zhang, Y.-W. Mechanical properties: Nanotwins only. Nat Nano 2012, 7, 551–552. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, W.; Qiu, D.; Zhang, M.-X.; Pan, F. Grain refinement of Ca addition in a twin-roll-cast Mg–3Al–1Zn alloy. Mater. Chem. Phys. 2012, 133, 611–616. [Google Scholar] [CrossRef]
- Jeong, J.; Im, J.; Song, K.; Kwon, M.; Kim, S.K.; Kang, Y.-B.; Oh, S.H. Transmission electron microscopy and thermodynamic studies of CaO-added AZ31 Mg alloys. Acta Mater. 2013, 61, 3267–3277. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Czerwinski, F. Overcoming Barriers. Advanced Materials & Processes.
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Medved, J.; Mrvar, P.; Vončina, M. Oxidation resistance of cast magnesium alloys. Oxid. Met. 2009, 71, 257–270. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.-X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Czerwinski, F. Oxidation characteristics of magnesium alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
- Weimin, Z.; Yong, S.; Haipeng, L.; Chunyong, L. The effects of some elements on the igniting temperature of magnesium alloys. Materials Science and Engineering: B 2006, 127, 105–107. [Google Scholar] [CrossRef]
- Czerwinski, F. The early stage oxidation and evaporation of Mg–9% Al–1% Zn alloy. Corrosion Science 2004, 46, 377–386. [Google Scholar] [CrossRef]
- Tan, Q.; Mo, N.; Jiang, B.; Pan, F.; Atrens, A.; Zhang, M.-X. Oxidation resistance of Mg–9Al–1Zn alloys micro-alloyed with Be. Scripta Mater. 2016, 115, 38–41. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Lü, Y.; Ding, W.; Zhu, Y.; Zhai, C.; Lu, C.; Xu, X. Behavior of surface oxidation on molten Mg–9Al–0.5 Zn–0.3 Be alloy. Mater. Sci. Eng., A 2001, 301, 154–161. [Google Scholar] [CrossRef]
- Inoue, S.-i.; Yamasaki, M.; Kawamura, Y. Formation of an incombustible oxide film on a molten Mg-Al-Ca alloy. Corros. Sci. 2017, 122, 118–122. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The high temperature oxidation and ignition behavior of Mg–Nd alloys part I: The oxidation of dilute alloys. J. Alloys Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, L.; Liu, L.; Du, K.; Wang, D. Effect of doping aluminum and yttrium on high-temperature oxidation behavior of Ni-11Fe-10Cu alloy. J. Rare Earth 2016, 34, 1139–1147. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E.-h. Influence of yttrium element on the corrosion behaviors of Mg–Y binary magnesium alloy. Journal of Magnesium and Alloys 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Fan, Q.; Yu, H.; Wang, T.; Liu, Y. Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings 2018, 8, 264. [Google Scholar] [CrossRef]
- Fan, Q.; Peng, X.; Yu, H.; Jiang, S.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Azarmehr, S.A.; Shirvani, K.; Schütze, M.; Galetz, M. Microstructural evolution of silicon-platinum modified aluminide coatings on superalloy GTD-111. Surf. Coat. Technol. 2017, 321, 455–463. [Google Scholar] [CrossRef]
- Mortazavi, N.; Geers, C.; Esmaily, M.; Babic, V.; Sattari, M.; Lindgren, K.; Malmberg, P.; Jönsson, B.; Halvarsson, M.; Svensson, J.E.; et al. Interplay of water and reactive elements in oxidation of alumina-forming alloys. Nat. Mater. 2018, 17, 610–617. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Wu, J.; Wang, C.; Hou, Z.; Tang, Y.; Liu, Z.; Ouyang, X. Improvement of corrosion resistance of Ni-based alloy by adding 5 wt.% rhenium. Mater. Today Commun. 2023, 35, 106387. [Google Scholar] [CrossRef]
- Eswarappa Prameela, S.; Pollock, T.M.; Raabe, D.; Meyers, M.A.; Aitkaliyeva, A.; Chintersingh, K.-L.; Cordero, Z.C.; Graham-Brady, L. Materials for extreme environments. Nat. Rev. Mater. 2023, 8, 81–88. [Google Scholar] [CrossRef]
- Thompson, N.G.; Yunovich, M.; Dunmire, D. Cost of corrosion and corrosion maintenance strategies. Corros. Rev. 2007, 25, 247–262. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. npj Materials Degradation 2017, 1, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




