Submitted:
06 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
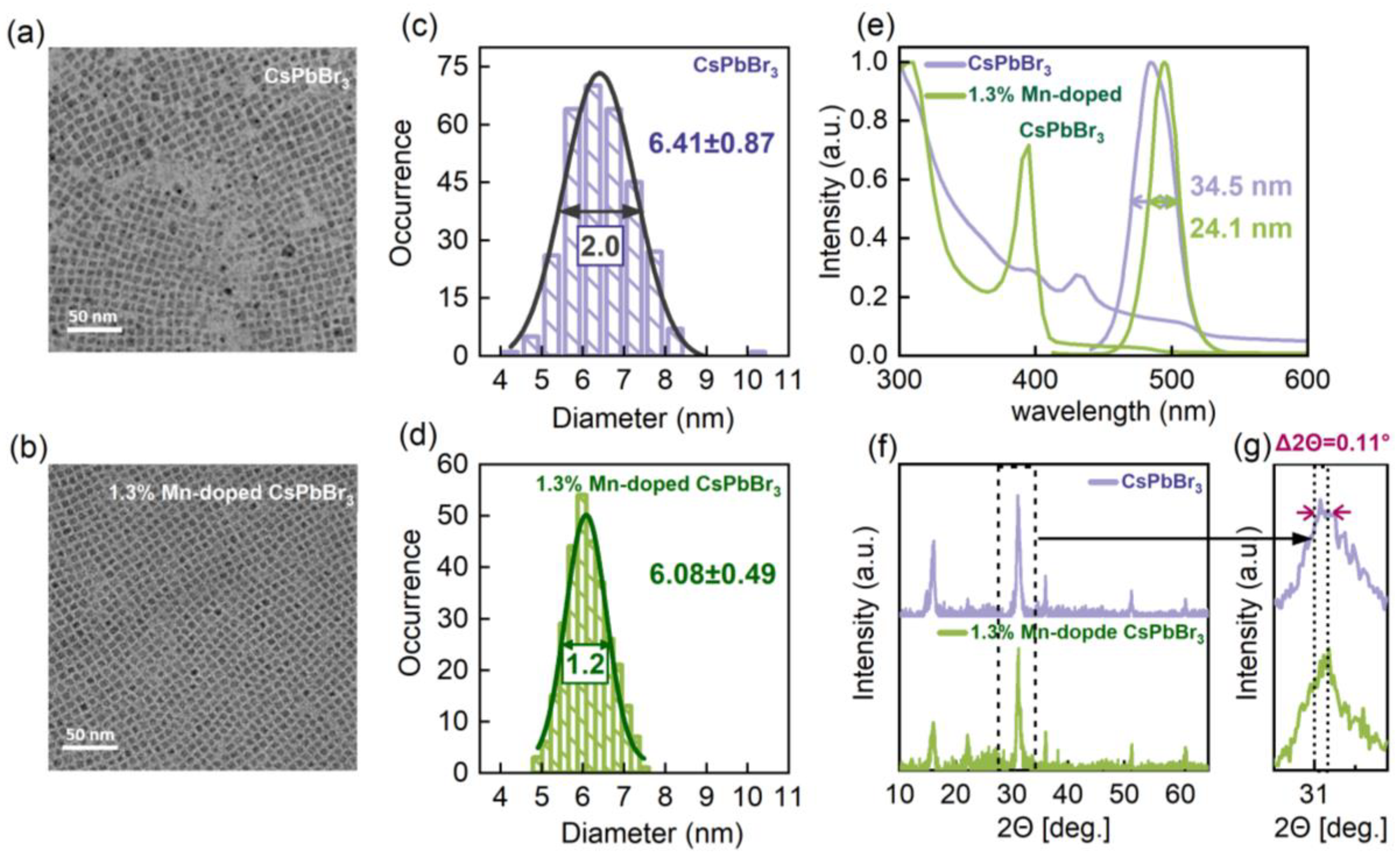
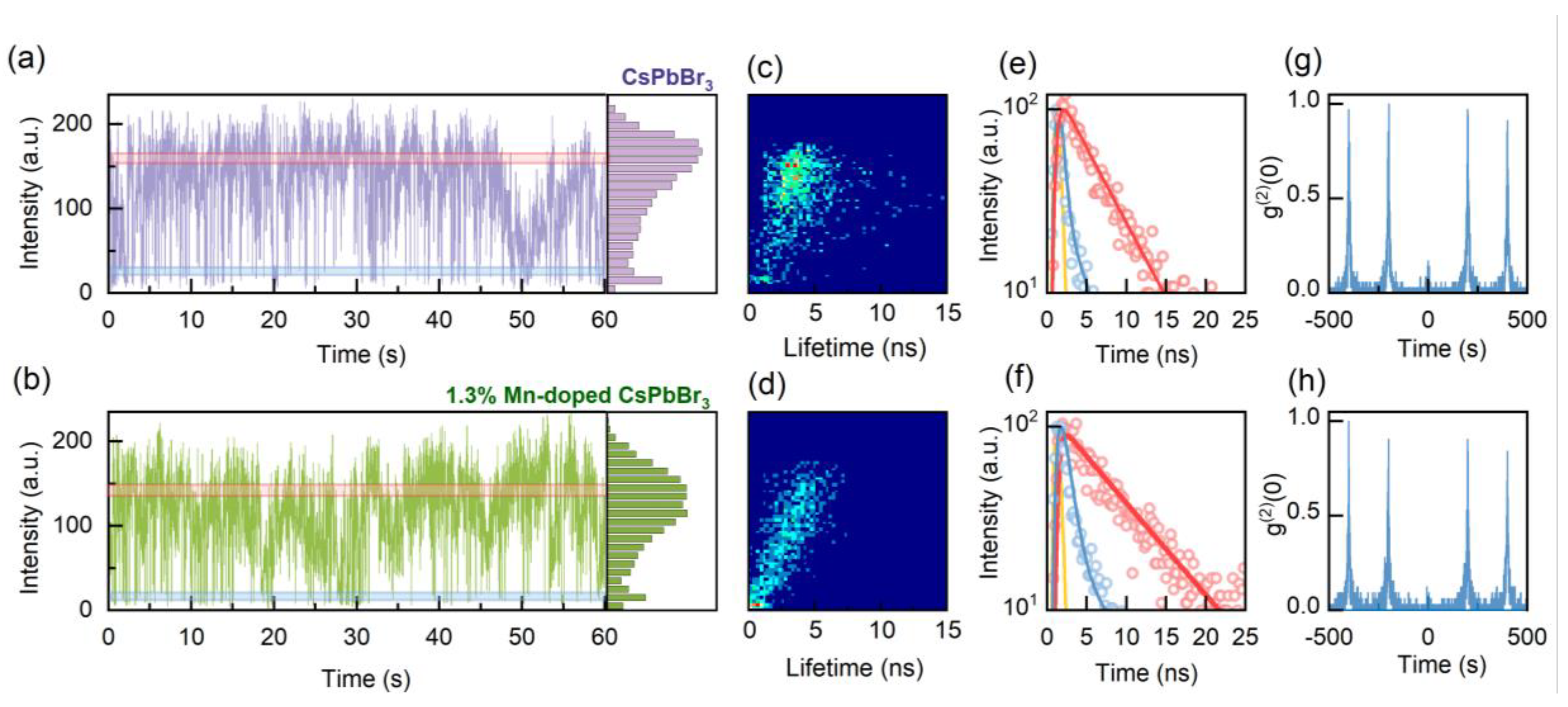
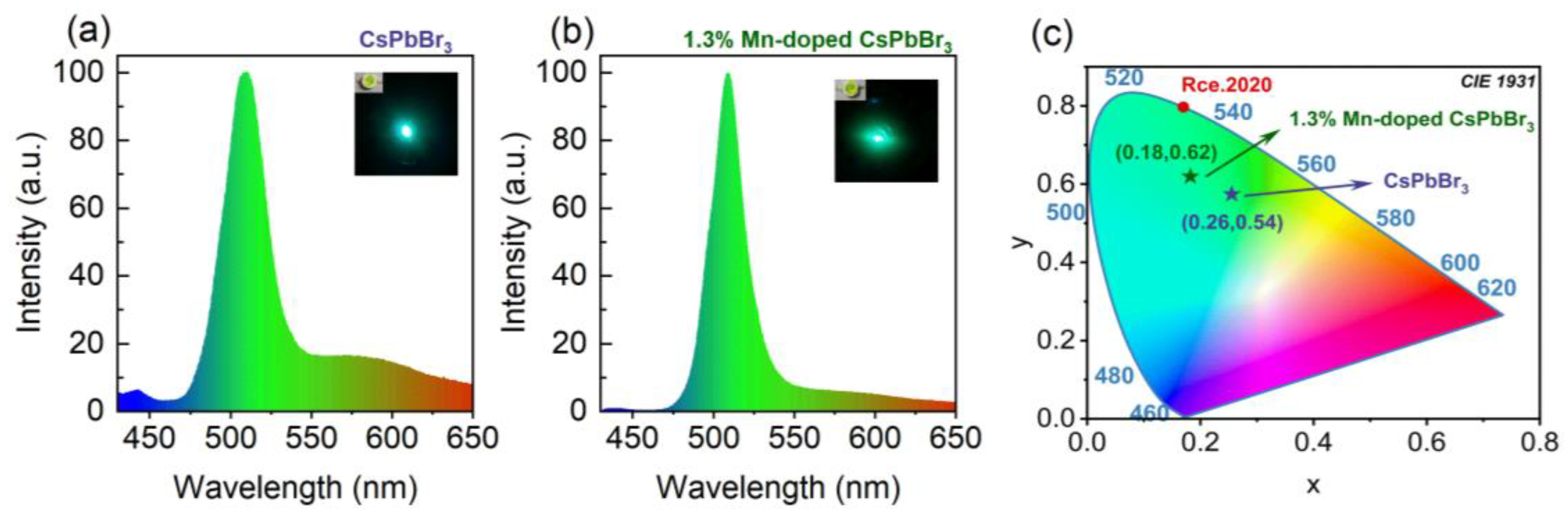
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.; Tang, S.; Luo, M.; Guo, Y.; Zheng, Y.; Lou, Y.; Zhao, Y. All-inorganic lead-free metal halide perovskite quantum dots: progress and prospects. Chem. Commun. 2021, 57, 7465–7479. [Google Scholar] [CrossRef]
- Wu, X.; Ji, H.; Yan, X.; Zhong, H. Industry outlook of perovskite quantum dots for display applications. Nat. Nanotechnol. 2022, 17, 813–816. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.; Gao, Y.; Chen, X.; Chen, R.; Qin, C.; Hu, J.; Wu, R.; Xiao, L.; Jia, S. Single quantum dot spectroscopy for exciton dynamics. Nano Res. 2024. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.; Zhang, G.; Yang, C.; Guo, W.; Chen, R.; Qin, C.; Gao, Y.; Biju, V.P.; Rogach, A.L.; et al. Excitons and biexciton dynamics in single CsPbBr3 perovskite quantum dots. J. Phys. Chem. Lett. 2018, 9, 6934–6940. [Google Scholar] [CrossRef]
- Li, Y.; Du, K.; Zhang, M.; Gao, X.; Lu, Y.; Yao, S.; Li, C.; Feng, J.; Zhang, H. Tunable ultra-uniform Cs4PbBr6 perovskites with efficient photoluminescence and excellent stability for high-performance white light-emitting diodes. J. Mater. Chem. 2021, 9, 12811–12818. [Google Scholar] [CrossRef]
- He, X.; Li, T.; Liang, Z.; Liu, R.; Ran, X.; Wang, X.; Guo, L.; Pan, C. Enhanced cyan photoluminescence and stability of CsPbBr3 quantum dots via surface engineering for white light-emitting diodes. Adv. Opt. Mater. 2024, 12, 2302726. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, S.; Zheng, H.; Wu, M.; Zhang, S.; Lan, J.; Li, W.; Fan, J. Structurally dimensional engineering in perovskite photovoltaics. Adv. Energy Mater. 2023, 13, 2300188. [Google Scholar] [CrossRef]
- Chen, L.J.; Dai, J.H.; Lin, J.D.; Mo, T.S.; Lin, H.P.; Yeh, H.C.; Chuang, Y.C.; Jiang, S.A.; Lee, C.R. Wavelength-tunable and highly stable perovskite-quantum-dot-doped lasers with liquid crystal lasing cavities. ACS Appl. Mater. Interfaces 2018, 10, 33307–33315. [Google Scholar] [CrossRef]
- Nguyen, T.; Tan, L.Z.; Baranov, D. Tuning perovskite nanocrystal superlattices for superradiance in the presence of disorder. J. Chem. Phys. 2023, 159, 204703. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Wu, W.; Huang, L.; Zhu, X.; Xie, Y.; Liu, H.; Zheng, B.; Liang, J.; Sun, X. Self-powered perovskite photodetector arrays with asymmetric contacts for imaging applications. Adv. Electron. Mater. 2023, 9, 2300106. [Google Scholar] [CrossRef]
- Pan, X.; Ding, L. Application of metal halide perovskite photodetectors. J. Semicond. 2022, 43, 020203. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Horder, J.; Sukhorukov, A.A.; Toth, M.; Neshev, D.N.; Aharonovich, I. Engineering quantum light sources with flat optics. Adv. Mater. 2024, 36, 2313589. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Yantara, N.; Tan, K.S.; Mathews, N.; Mhaisalkar, S.G. Perovskite nanostructures: leveraging quantum effects to challenge optoelectronic limits. Mater. Today 2020, 33, 122–140. [Google Scholar] [CrossRef]
- Lu, X.; Hou, X.; Tang, H.; Yi, X.; Wang, J. A high-quality CdSe/CdS/ZnS quantum-dot-based FRET aptasensor for the simultaneous detection of two different Alzheimer’s disease core biomarkers. Nanomaterials 2022, 12, 4031. [Google Scholar] [CrossRef]
- Chen, M.; Shen, G.; Guyot-Sionnest, P. Size distribution effects on mobility and intraband gap of HgSe quantum dots. J. Phys. Chem. C 2020, 124, 16216–16221. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Gao, Y.; Li, B.; Han, X.; Li, J.; Zhang, M.; Chen, Z.; Wei, Y.; Chen, R. Size-dependent photoluminescence blinking mechanisms and volume scaling of biexciton Auger recombination in single CsPbI3 perovskite quantum dots. J. Chem. Phys. 2024, 160, 174505. [Google Scholar] [CrossRef]
- Bai, X.; Li, H.; Peng, Y.; Zhang, G.; Yang, C.; Guo, W.; Han, X.; Li, J.; Chen, R.; Qin, C.; et al. Role of aspect ratio in the photoluminescence of single CdSe/CdS dot-in-rods. J. Phys. Chem. C 2022, 126, 2699–2707. [Google Scholar] [CrossRef]
- Korepanov, O.; Kozodaev, D.; Aleksandrova, O.; Bugrov, A.; Firsov, D.; Kirilenko, D.; Mazing, D.; Moshnikov, V.; Shomakhov, Z. Temperature- and size-dependent photoluminescence of CuInS2 quantum dots. Nanomaterials 2023, 13, 2892. [Google Scholar] [CrossRef]
- Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise control of quantum confinement in cesium lead halide perovskite quantum dots via thermodynamic equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef]
- Larson, H.; Cossairt, B.M. Indium-poly (carboxylic acid) ligand interactions modify InP quantum dot nucleation and growth. Chem. Mater. 2023, 35, 6152–6160. [Google Scholar] [CrossRef]
- Paik, T.; Greybush, N.J.; Najmr, S.; Woo, H.Y.; Hong, S.V.; Kim, S.H.; Lee, J.D.; Kagan, C.R.; Murray, C.B. Shape-controlled synthesis and self-assembly of highly uniform upconverting calcium fluoride nanocrystals. Inorg. Chem. Front. 2024, 11, 278–285. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Li, S.; Jiang, F.; Jiang, P.; Liu, Y. Cu-deficient cuinse quantum dots for “turn-on” detection of adenosine triphosphate in living cells. ACS Appl. Nano Mater. 2021, 4, 6057–6066. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Park, S.; Radicchi, E.; Nunzi, F.; Mosconi, E.; De Angelis, F.; Brescia, R.; Rastogi, P.; Prato, M.; Manna, L. Nearly monodisperse insulator Cs4PbX6 (X= Cl, Br, I) nanocrystals, their mixed halide compositions, and their transformation into CsPbX3 nanocrystals. Nano Lett. 2017, 17, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Kim, Y.; Sihn, M.R.; Jeon, M.G.; Jeong, J.R.; Choi, J. Solubility-controlled room-temperature synthesis of cesium lead halide perovskite nanocrystals. ChemNanoMat 2020, 6, 1863–1869. [Google Scholar] [CrossRef]
- Manoli, A.; Papagiorgis, P.; Sergides, M.; Bernasconi, C.; Athanasiou, M.; Pozov, S.; Choulis, S.A.; Bodnarchuk, M.I.; Kovalenko, M.V.; Othonos, A. Surface functionalization of CsPbBr3 nanocrystals for photonic applications. ACS Appl. Nano Mater. 2021, 4, 5084–5097. [Google Scholar] [CrossRef]
- Yong, Z.; Guo, S.; Ma, J.; Zhang, J.; Li, Z.; Chen, Y.; Zhang, B.; Zhou, Y.; Shu, J.; Gu, J.; et al. Doping-enhanced short-range order of perovskite nanocrystals for near-unity violet luminescence quantum yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.; Zhang, G.; Yang, C.; Guo, W.; Han, X.; Bai, X.; Chen, R.; Qin, C.; Hu, J.; et al. The role of surface charges in the blinking mechanisms and quantum-confined stark effect of single colloidal quantum dots. Nano Res. 2022, 15, 7655–7661. [Google Scholar] [CrossRef]
- Li, B.; Gao, Y.; Wu, R.; Miao, X.; Zhang, G. Charge and energy transfer dynamics in single colloidal quantum dots/monolayer MoS2 heterostructures. Phys. Chem. Chem. Phys. 2023, 25, 8161–8167. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, S.; Li, Q.; Kershaw, S.V.; Tian, J.; Rogach, A.L. Thermally stable copper(II)-doped cesium lead halide perovskite quantum dots with strong blue emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef]
- Mir, W.J.; Mahor, Y.; Lohar, A.; Jagadeeswararao, M.; Das, S.; Mahamuni, S.; Nag, A. Postsynthesis doping of Mn and Yb into CsPbX3 (X = Cl, Br, or I) perovskite nanocrystals for downconversion emission. Chem. Mater. 2018, 30, 8170–8178. [Google Scholar] [CrossRef]
- Pradeep, K.R.; Viswanatha, R. Doped or not doped? importance of the local structure of Mn (II) in Mn doped perovskite nanocrystals. Mater. Res. Bull. 2021, 141, 111374. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Yun, R.; Yang, H.; Sun, W.; Zhang, L.; Liu, X.; Zhang, X.; Li, X. Recent advances on Mn2+-doping in diverse metal halide perovskites. Laser Photonics Rev. 2023, 17, 2200524. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, H.; Sun, C.; Yin, C.; Lv, B.; Zhang, C.; Yu, W.W.; Wang, X.; Zhang, Y.; Xiao, M. Superior optical properties of perovskite nanocrystals as single photon emitters. ACS Nano 2015, 9, 12410–12416. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, G.; Li, B.; Yang, C.; Guo, W.; Bai, X.; Huang, P.; Chen, R.; Qin, C.; Hu, J. Blinking mechanisms and intrinsic quantum-confined stark effect in single methylammonium lead bromide perovskite quantum dots. Small 2020, 16, 2005435. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Y.; Hou, X.; Zhang, M.; Zhang, G.; Li, B.; Guo, W.; Han, X.; Bai, X.; Li, J. Conversion of photoluminescence blinking types in single colloidal quantum dots. Small 2023, 20, 2309134. [Google Scholar] [CrossRef] [PubMed]
- Podshivaylov, E.A.; Kniazeva, M.A.; Tarasevich, A.O.; Eremchev, I.Y.; Naumov, A.V.; Frantsuzov, P.A. A quantitative model of multi-scale single quantum dot blinking. J. Mater. Chem. 2023, 11, 8570–8576. [Google Scholar] [CrossRef]
- Yuan, G.; Gómez, D.E.; Kirkwood, N.; Boldt, K.; Mulvaney, P. Two mechanisms determine quantum dot blinking. ACS Nano 2018, 12, 3397–3405. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, G.; Yang, C.; Li, Z.; Chen, R.; Qin, C.; Gao, Y.; Huang, H.; Xiao, L.; Jia, S. Fast recognition of single quantum dots from high multi-exciton emission and clustering effects. Opt. Express 2018, 26, 4674. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, S.; Li, Q.; Kershaw, S.V.; Tian, J.; Rogach, A.L. Thermally stable copper (II)-doped cesium lead halide perovskite quantum dots with strong blue emission. J. Phys. Chem. Lett. 2019, 10, 943–952. [Google Scholar] [CrossRef]
- Nair, G.; Zhao, J.; Bawendi, M.G. Biexciton quantum yield of single semiconductor nanocrystals from photon statistics. Nano Lett. 2011, 11, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tang, J.; Zhang, G.; Li, B.; Yang, C.; Chen, R.; Qin, C.; Hu, J.; Zhong, H.; Xiao, L.; et al. Photoluminescence blinking and biexciton Auger recombination in single colloidal quantum dots with sharp and smooth core/shell interfaces. J. Phys. Chem. Lett. 2021, 12, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Bae, W.K.; Padilha, L.A.; Pietryga, J.M.; Klimov, V.I. Effect of the core/shell interface on Auger recombination evaluated by single-quantum-dot spectroscopy. Nano Lett. 2014, 14, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Liang, B.; Jiang, C.; Wang, S.; Bi, H.; Wang, Y. Narrow band organic emitter for pure green solution-processed electroluminescent devices with CIE coordinate y of 0.77. Adv. Opt. Mater. 2024, 12, 2400490. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Yu, Y.; Zhao, F.; Wang, Y.; Liao, L. Advances in solution-processed blue quantum dot light-emitting diodes. Nanomaterials 2023, 13, 1695. [Google Scholar] [CrossRef]
- Kumar, S.; Jagielski, J.; Kallikounis, N.; Kim, Y.; Wolf, C.; Jenny, F.; Tian, T.; Hofer, C.J.; Chiu, Y.; Stark, W.J.; et al. Ultrapure green light-emitting diodes using two-dimensional formamidinium perovskites: achieving recommendation 2020 color coordinates. Nano Lett. 2017, 17, 5277–5284. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




