Submitted:
08 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiology Spectrum 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Ballou, E.R.; Bates, S.; Bignell, E.M.; Borman, A.M.; Brand, A.C.; Brown, A.J.; Coelho, C.; Cook, P.C.; Farrer, R.A. The Pathobiology of Human Fungal Infections. Nature Reviews Microbiology 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
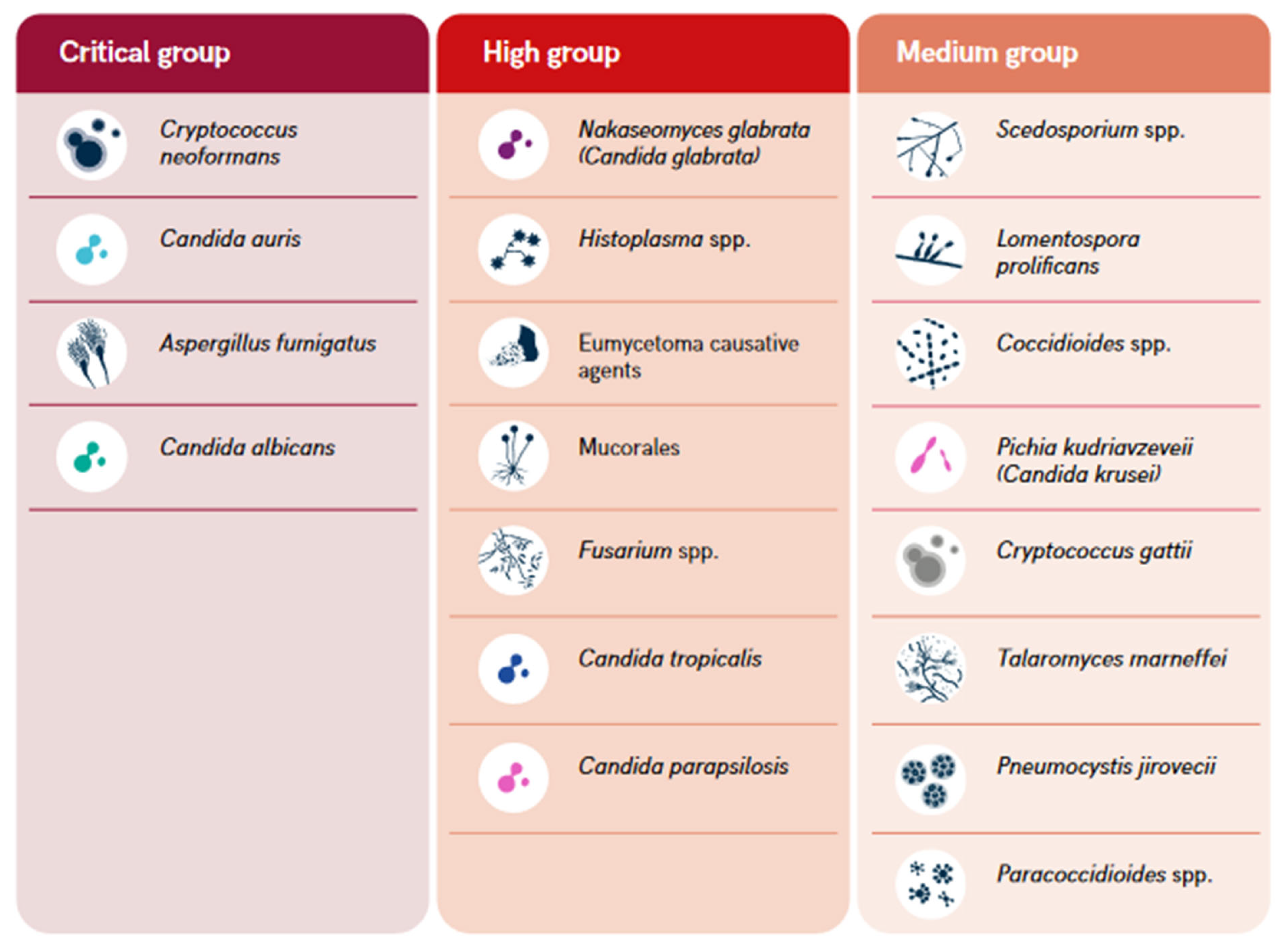
- The World Health Organization (WHO). WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization, 2022; ISBN 92-4-006024-3. [Google Scholar]
- Fisher, M.C.; Denning, D.W. The WHO Fungal Priority Pathogens List as a Game-Changer. Nat Rev Microbiol 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Casalini, G.; Giacomelli, A.; Antinori, S. The WHO Fungal Priority Pathogens List: A Crucial Reappraisal to Review the Prioritisation. The Lancet Microbe 2024, 0. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Ekeng, B.E.; Kibone, W.; Nsenga, L.; Olum, R.; Itam-Eyo, A.; Kuate, M.P.N.; Pebolo, F.P.; Davies, A.A.; Manga, M.; et al. Invasive Fungal Diseases in Africa: A Critical Literature Review. Journal of Fungi 2022, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wu, J.; Cheng, M.; Zhu, X.; Du, M.; Chen, C.; Liao, W.; Zhi, K.; Pan, W. Diagnosis of Invasive Fungal Infections: Challenges and Recent Developments. J Biomed Sci 2023, 30, 42. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. The Lancet Infectious Diseases 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.R.; Wu, J.J.; Huang, D.B.; Tyring, S.K. Subcutaneous Fungal Infections. Dermatologic Therapy 2004, 17, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Orefuwa, E.; Kamara, M.N.; Jiba, D.F.; Kamara, J.B.; Kpaka, S.; Denning, D.W. The Burden of Serious Fungal Infections in Sierra Leone: A National Estimate. Therapeutic Advances in Infection 2021, 8, 20499361211027996. [Google Scholar] [CrossRef]
- Siddig, E. e.; El Had Bakhait, O.; El nour Hussein Bahar, M.; Siddig Ahmed, E.; Bakhiet, S. m.; Motasim Ali, M.; Babekir Abdallah, O.; Ahmed Hassan, R.; Verbon, A.; van de Sande, W. w. j.; et al. Ultrasound-Guided Fine-Needle Aspiration Cytology Significantly Improved Mycetoma Diagnosis. Journal of the European Academy of Dermatology and Venereology 2022, 36, 1845–1850. [Google Scholar] [CrossRef]
- Siddig, E.E.; Ahmed, A.; Hassan, O.B.; Bakhiet, S.M.; Verbon, A.; Fahal, A.H.; van de Sande, W.W. Using a Madurella Mycetomatis-specific PCR on Grains Obtained via Non-invasive Fine-needle Aspirated Material Is More Accurate than Cytology. Mycoses 2023, 66, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Ekeng, B.E.; Kwizera, R.; Salmanton-García, J.; Kibone, W.; van Rhijn, N.; Govender, N.P.; Meya, D.B.; Osaigbovo, I.I.; Hamer, D.H.; et al. Fungal Diseases in Africa: Closing the Gaps in Diagnosis and Treatment through Implementation Research and Advocacy. Journal of Medical Mycology 2023, 33, 101438. [Google Scholar] [CrossRef] [PubMed]
- Siddig, E.E.; Ahmed, A.; Ali, Y.; Bakhiet, S.M.; Mohamed, N.S.; Ahmed, E.S.; Fahal, A.H. Eumycetoma Medical Treatment: Past, Current Practice, Latest Advances and Perspectives. Microbiology Research 2021, 12, 899–906. [Google Scholar] [CrossRef]
- Kozel, T.R.; Wickes, B. Fungal Diagnostics. Cold Spring Harb Perspect Med 2014, 4, a019299. [Google Scholar] [CrossRef] [PubMed]
- Organic Law Determining the Administrative Entities of the Republic of Rwanda; Official Gazette of the Republic Rwanda: Kigali, 2005.
- Ruxin, J.; Negin, J. Removing the Neglect from Neglected Tropical Diseases: The Rwandan Experience 2008–2010. Global Public Health 2012, 7, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Ndoricyimpaye, E.L.; Obed, T.; Claude, H.J.; d’Amour, M.J.; Denyse, N.; Reverien, R. Candida Albicans Infection among HIV Positive and HIV Negative Women-Case Study at Butare University Teaching Hospital (CHUB), Southern Province of Rwanda. East Africa Science 2020, 2, 76–80. [Google Scholar] [CrossRef]
- Chrysostome, U.J.; Prudence, I.A.; Innocent, N.; Pierre, U.J.; Ezechiel, B.; Fabrice, U.; Julienne, M.; de Dieu, N.J.; Pacifique, M. Comparative Study of Candidiasis among Single and Married Women at Rwanda Military Hospital. Journal of Drug Delivery and Therapeutics 2024, 14, 59–63. [Google Scholar] [CrossRef]
- Jadin, J.B.; Vanderick, F.; Mbonyingabo, M. First Case of Histoplasma Duboisi in Rwanda. 1972. [Google Scholar]
- Dierckxsens, H.; Vanderick, F.; Vandepitte, J.; Ntabomvura, V. Premieres Observations d’histoplasmose à Histoplasma Capsulatum Au Rwanda. Ann Soc Belg Med Trop 1976, 56, 1–10. [Google Scholar] [PubMed]
- Raftopoulos, C.; Flament-Durand, J.; Coremans-Pelseneer, J.; Noterman, J. Intracerebellar Blastomycosis Abscess in an African Man. Clinical neurology and neurosurgery 1986, 88, 209–212. [Google Scholar] [CrossRef]
- Izimukwiye, A.I.; Mbarushimana, D.; Ndayisaba, M.C.; Bigirimana, V.; Rugwizangoga, B.; Laga, A.C. Cluster of Nasal Rhinosporidiosis, Eastern Province, Rwanda. Emerging Infectious Diseases 2019, 25, 1727. [Google Scholar] [CrossRef]
- Batungwanayo, J.; Taelman, H.; Lucas, S.; Bogaerts, J.; Alard, D.; Kagame, A.; Blanche, P.; Clerinx, J.; van de Perre, P.; Allen, S. Pulmonary Disease Associated with the Human Immunodeficiency Virus in Kigali, Rwanda. A Fiberoptic Bronchoscopic Study of 111 Cases of Undetermined Etiology. American journal of respiratory and critical care medicine 1994, 149, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Buginco, C. Dermatophytic Infection of the Scalp in the Region of Butare (Rwanda). International Journal of Dermatology 1983, 22, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Bugingo, G. [Causal agents of tinea of the scalp in the region of Butare (Rwanda)]. Ann Soc Belg Med Trop 1993, 73, 67–69. [Google Scholar] [PubMed]
- Rusuku, G.; Buruchara, R.A.; Gatabazi, M.; Pastor-Corrales, M.A. Occurrence and Distribution in Rwanda of Soilborne Fungi Pathogenic to the Common Bean. Plant disease 1997, 81, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A Global View on Fungal Infections in Humans and Animals: Opportunistic Infections and Microsporidioses. Journal of Applied Microbiology 2021, 131, 2095–2113. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449–20. [Google Scholar] [CrossRef] [PubMed]
- One Health: Fungal Pathogens of Humans, Animals, and Plants: Report on an American Academy of Microbiology Colloquium Held in Washington, DC, on October 18, 2017; American Academy of Microbiology Colloquia Reports; American Society for Microbiology: Washington, (DC), 2019.
- Zinsstag, J.; Hediger, K.; Osman, Y.M.; Abukhattab, S.; Crump, L.; Kaiser-Grolimund, A.; Mauti, S.; Ahmed, A.; Hattendorf, J.; Bonfoh, B.; et al. The Promotion and Development of One Health at Swiss TPH and Its Greater Potential. Diseases 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Mahmoud, I.; Eldigail, M.; Elhassan, R.M.; Weaver, S.C. The Emergence of Rift Valley Fever in Gedaref State Urges the Need for a Cross-Border One Health Strategy and Enforcement of the International Health Regulations. Pathogens 2021, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ali, Y.; Mohamed, N.S. Arboviral Diseases: The Emergence of a Major yet Ignored Public Health Threat in Africa. The Lancet Planetary Health 2020, 4, e555. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohamed, N.S.; Siddig, E.E.; Algaily, T.; Sulaiman, S.; Ali, Y. The Impacts of Climate Change on Displaced Populations: A Call for Actions. The Journal of Climate Change and Health 2021, 100057. [Google Scholar] [CrossRef]
- Ahmed, A.; Hemaida, M.A.; Hagelnur, A.A.; Eltigani, H.F.; Siddig, E.E. Sudden Emergence and Spread of Cutaneous Larva Migrans in Sudan: A Case Series Calls for Urgent Actions. IDCases 2023, 32, e01789. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Abubakr, M.; Sami, H.; Mahdi, I.; Mohamed, N.S.; Zinsstag, J. The First Molecular Detection of Aedes Albopictus in Sudan Associates with Increased Outbreaks of Chikungunya and Dengue. International Journal of Molecular Sciences 2022, 23, 11802. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Eldigail, M.; Elduma, A.; Breima, T.; Dietrich, I.; Ali, Y.; Weaver, S.C. First Report of Epidemic Dengue Fever and Malaria Co-Infections among Internally Displaced Persons in Humanitarian Camps of North Darfur, Sudan. International Journal of Infectious Diseases 2021, 108, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Siddig, E.E.; Mohamed, N.; Ahmed, A. Rift Valley Fever and Malaria Co-infection: A Case Report. Clin Case Rep 2023, 11, e7926. [Google Scholar] [CrossRef] [PubMed]
- Siddig, E.E.; Mohamed, N.S.; Ahmed, A. Severe Coinfection of Dengue and Malaria: A Case Report. Clinical Case Reports 2024, 12, e9079. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; EL-Sadig, S.M.; Siddig, E.E. Guillain–Barre Syndrome Associated with Hepatitis E Virus Infection: A Case Report. Clinical Case Reports 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Siddig, E.E.; Ahmed, A. When Parasites Stray from the Path: A Curious Case of Ectopic Cutaneous Schistosoma Haematobium. QJM: An International Journal of Medicine 2023, 116, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.T.H.; Abdelkhalig, R.E.; Hamid, E.; Ahmed, A.; Siddig, E.E. Recurrent Abdominal Wall Mass in a Hepatitis B-positive Male: An Unusual Case of Lumbar Mycetoma. Clinical Case Reports 2023, 11, e8275. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.T.H.; Abdelkhalig, R.E.; Hamid, E.; Ahmed, A.; Siddig, E.E. Unusual Manifestation of Cystic Mycetoma Lesions: A Case Report. Clinical Case Reports 2023, 11, e8054. [Google Scholar] [CrossRef]
- Ahmed, A.; Hagelnur, A.A.; Eltigani, H.F.; Siddig, E.E. Cutaneous Tuberculosis of the Foot Clinically Mimicking Mycetoma: A Case Report. Clin Case Rep 2023, 11, e7295. [Google Scholar] [CrossRef]
- Driemeyer, C.; Falci, D.R.; Oladele, R.O.; Bongomin, F.; Ocansey, B.K.; Govender, N.P.; Hoenigl, M.; Gangneux, J.P.; Lass-Flörl, C.; Cornely, O.A. The Current State of Clinical Mycology in Africa: A European Confederation of Medical Mycology and International Society for Human and Animal Mycology Survey. The Lancet Microbe 2022, 3, e464–e470. [Google Scholar] [CrossRef] [PubMed]
- Clinton Health Access Initiative (CHAI). The Road to Zero: Report on the Implementation of the Advanced HIV Disease Package of Care in Low- and Middle-Income Countries, 2022.
- Tufa, T.B.; Bongomin, F.; Fathallah, A.; Cândido, A.L.S.; Hashad, R.; Abdallaoui, M.S.; Nail, A.A.; Fayemiwo, S.A.; Penney, R.O.; Orefuwa, E.; Denning, D.W. Access to the World Health Organization-recommended essential diagnostics for invasive fungal infections in critical care and cancer patients in Africa: a diagnostic survey. Journal of infection and public health 2023, 16, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
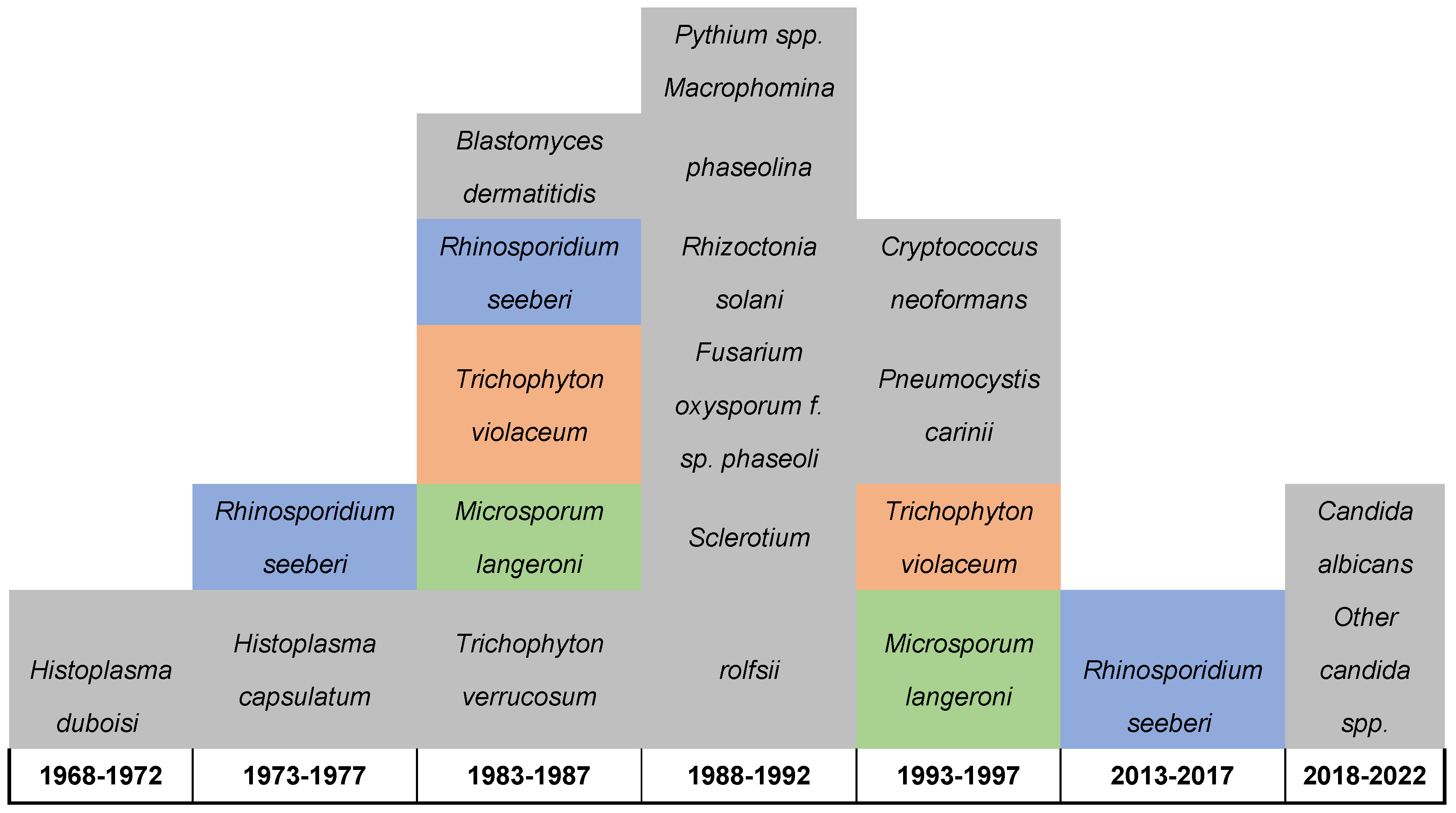
| # | Fungal Name | Site of isolations | Year | Diagnostic tool used | District | Reference |
|---|---|---|---|---|---|---|
| 1 |
Candida albicans Other candida spp. |
Vagina | 2021-2022 | Culture technique | Kicukiro District | 19 |
| 2 |
Candida albicans Other candida spp., |
Vagina | 2020 | Culture technique | Huye District | 18 |
| 3 | Rhinosporidium seeberi | Nasal | 2016 | Histopathology | Kirehe District | 23 |
| 4 | Rhinosporidium seeberi | Nasal | 2014-2015 | Histopathology | Gatsibo District | 23 |
| 5 | Cryptococcus neoformans | Respiratory and Brain | 1994 | Culture and histopathology | Kigali District | 24 |
| 6 | Pneumocystis carinii | Respiratory | 1994 | Cytology | Kigali District | 24 |
| 7 | Trichophyton violaceum | Scalp | 1993 | Direct microscope and culture | Butare Distirct | 26 |
| 8 | Microsporum langeroni | Scalp | 1993 | Direct microscope and culture | Butare Distirct | 26 |
| 9 | Pythium spp. | Bean | 1989-1990 | Symptoms, colony characteristics, reproductive structures, and pathogenicity assessments | Gikongoro; Butare; Gitarama; Kigali; Byumba; Ruhengeri; Gisenyi and Kibungo | 27 |
| 10 |
Macrophomina phaseolina |
Bean | 1989-1990 | Symptoms, colony characteristics, reproductive structures, and pathogenicity assessments | Gikongoro; Butare; Gitarama; Kigali; Byumba; Ruhengeri; Gisenyi and Kibungo | 27 |
| 11 | Rhizoctonia solani | Bean | 1989-1990 | Symptoms, colony characteristics, reproductive structures, and pathogenicity assessments | Gikongoro; Butare; Gitarama; Kigali; Byumba; Ruhengeri; Gisenyi and Kibungo | 27 |
| 12 | Fusarium oxysporum f. sp. phaseoli | Bean | 1989-1990 | Symptoms, colony characteristics, reproductive structures, and pathogenicity assessments | Gikongoro; Butare; Gitarama; Kigali; Byumba; Ruhengeri; Gisenyi and Kibungo | 27 |
| 13 |
Sclerotium rolfsii |
Bean | 1989-1990 | Symptoms, colony characteristics, reproductive structures, and pathogenicity assessments | Gikongoro; Butare; Gitarama; Kigali; Byumba; Ruhengeri; Gisenyi and Kibungo | 27 |
| 14 | Blastomyces dermatitidis | Cerebellar | 1986 | Immunology, Histopathology | Kigali district | 22 |
| 15 | Rhinosporidium seeberi | Conjunctiva | 1986 | Histopathology | NA | 23 |
| 16 | Trichophyton violaceum | Scalp | 1983 | Direct microscopic | Butare Distirct | 25 |
| 17 | Microsporum langeroni | Scalp | 1983 | Direct microscopic | Butare Distirct | 25 |
| 18 | Trichophyton verrucosum | Scalp | 1983 | Direct microscopic | Butare Distirct | 25 |
| 19 | Rhinosporidium seeberi | Nasal | 1975-1977 | Histopathology | NA | 23 |
| 20 | Histoplasma capsulatum | NA | 1976 | NA | NA | 21 |
| 21 | Histoplasma duboisi | Disseminated infection | 1972 | Culture technique | Butaro District | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





