Submitted:
08 July 2024
Posted:
09 July 2024
You are already at the latest version
Abstract
Keywords:
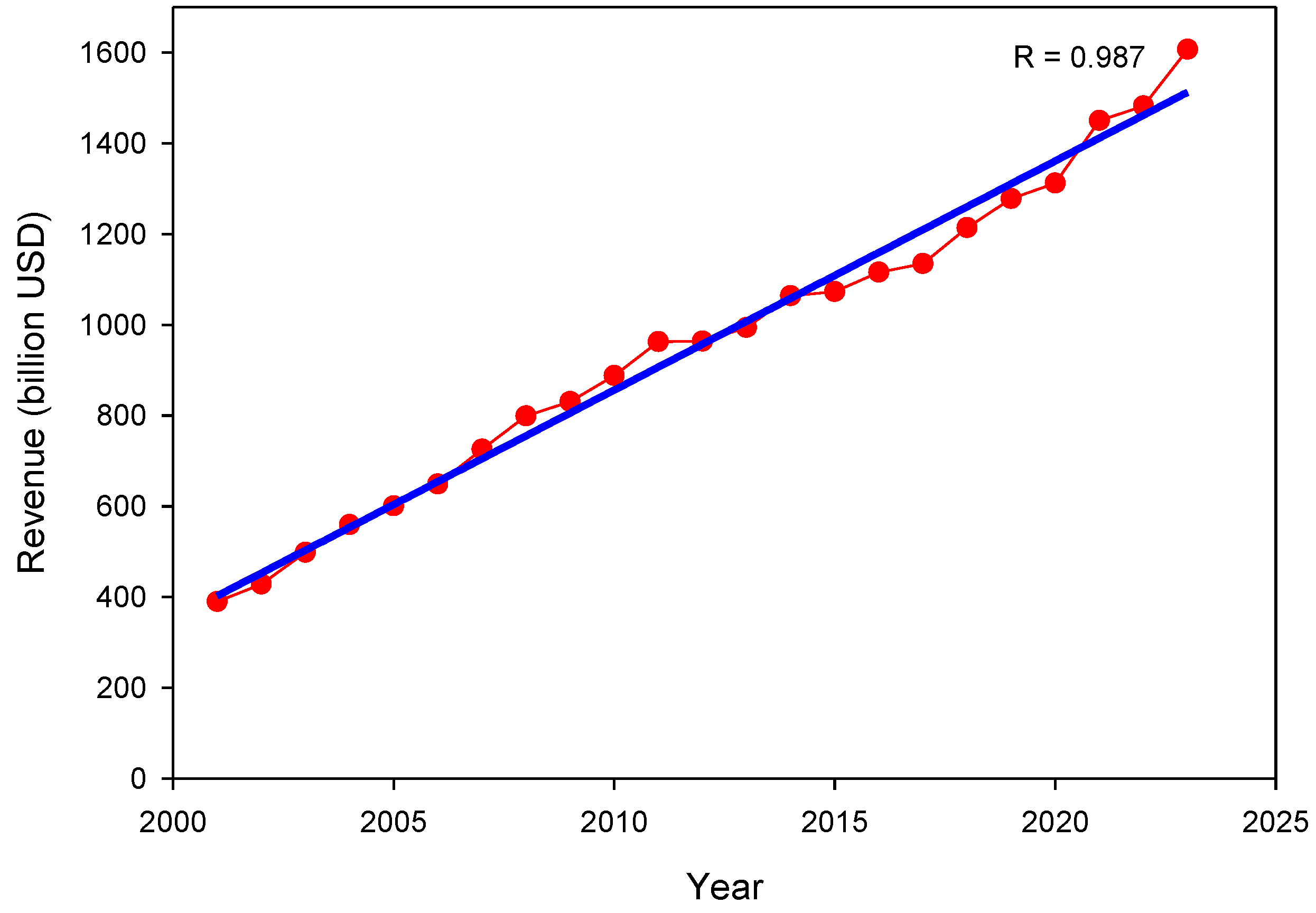
1. Introduction
2. Methodology
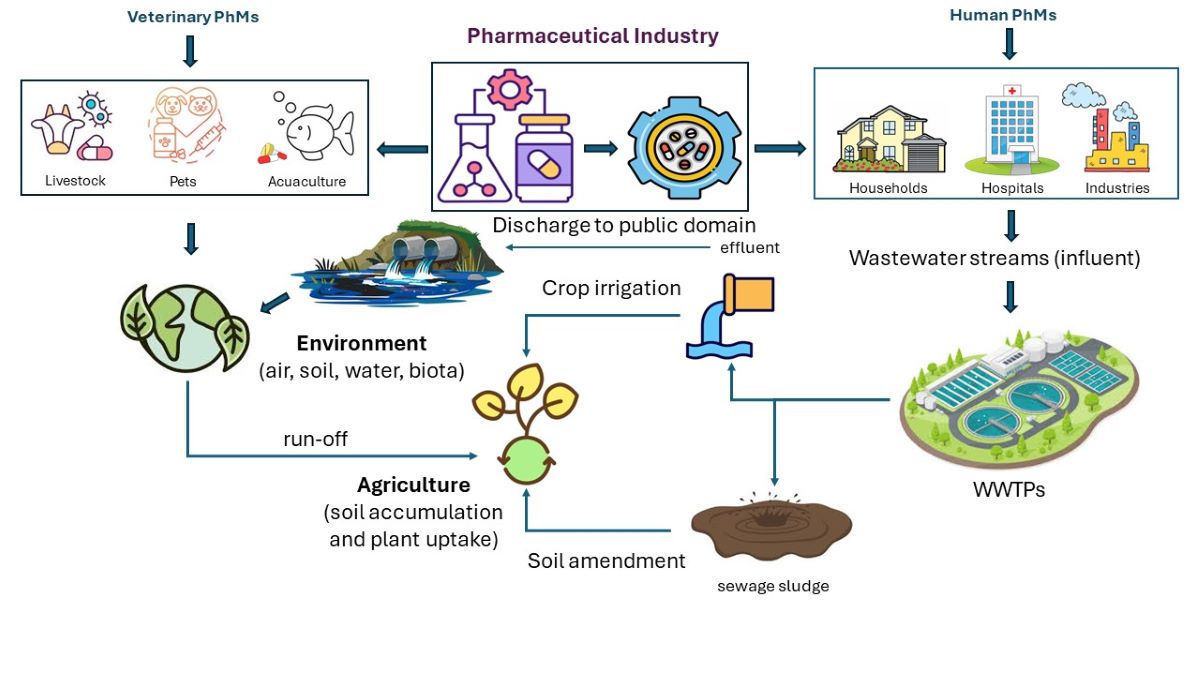
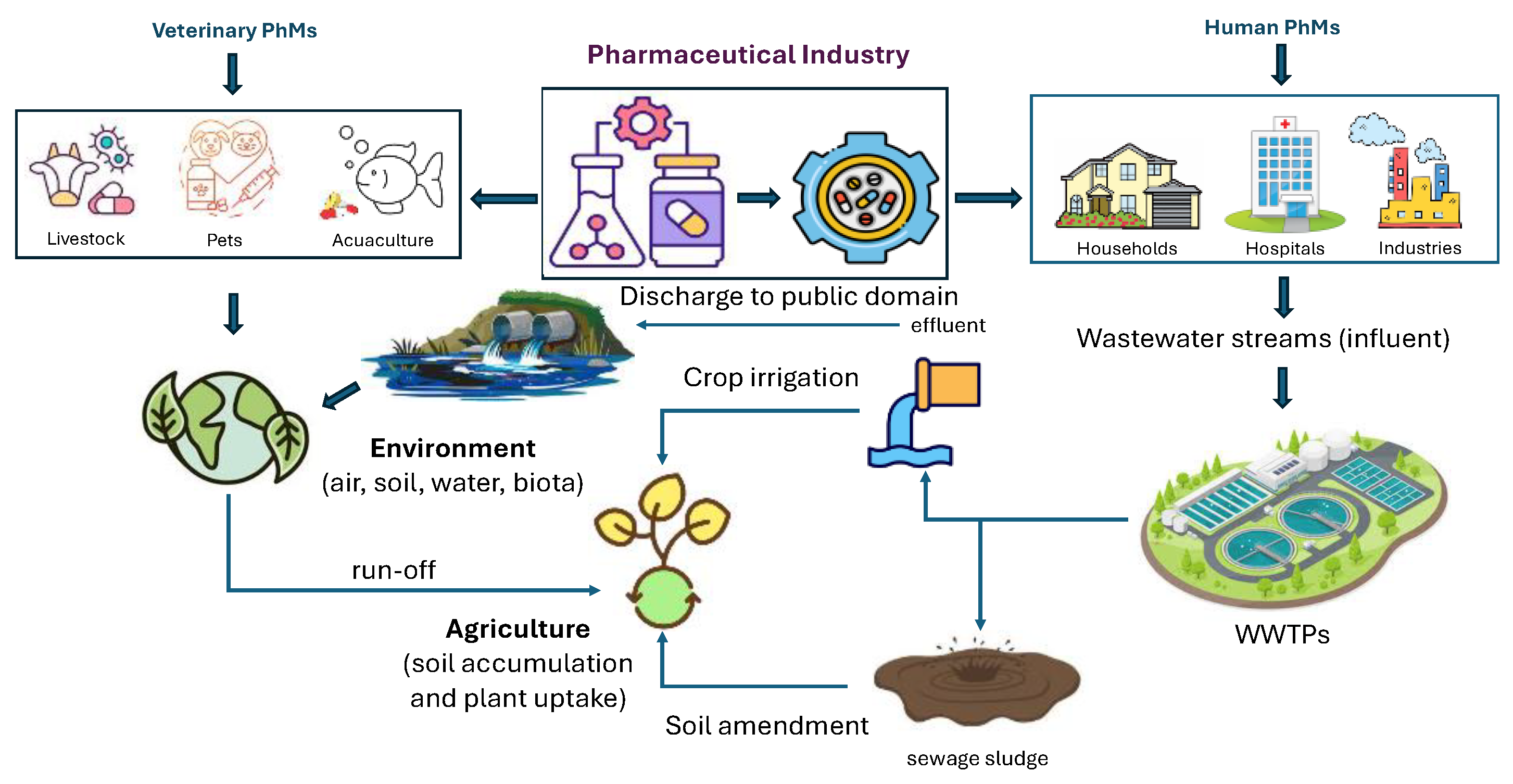
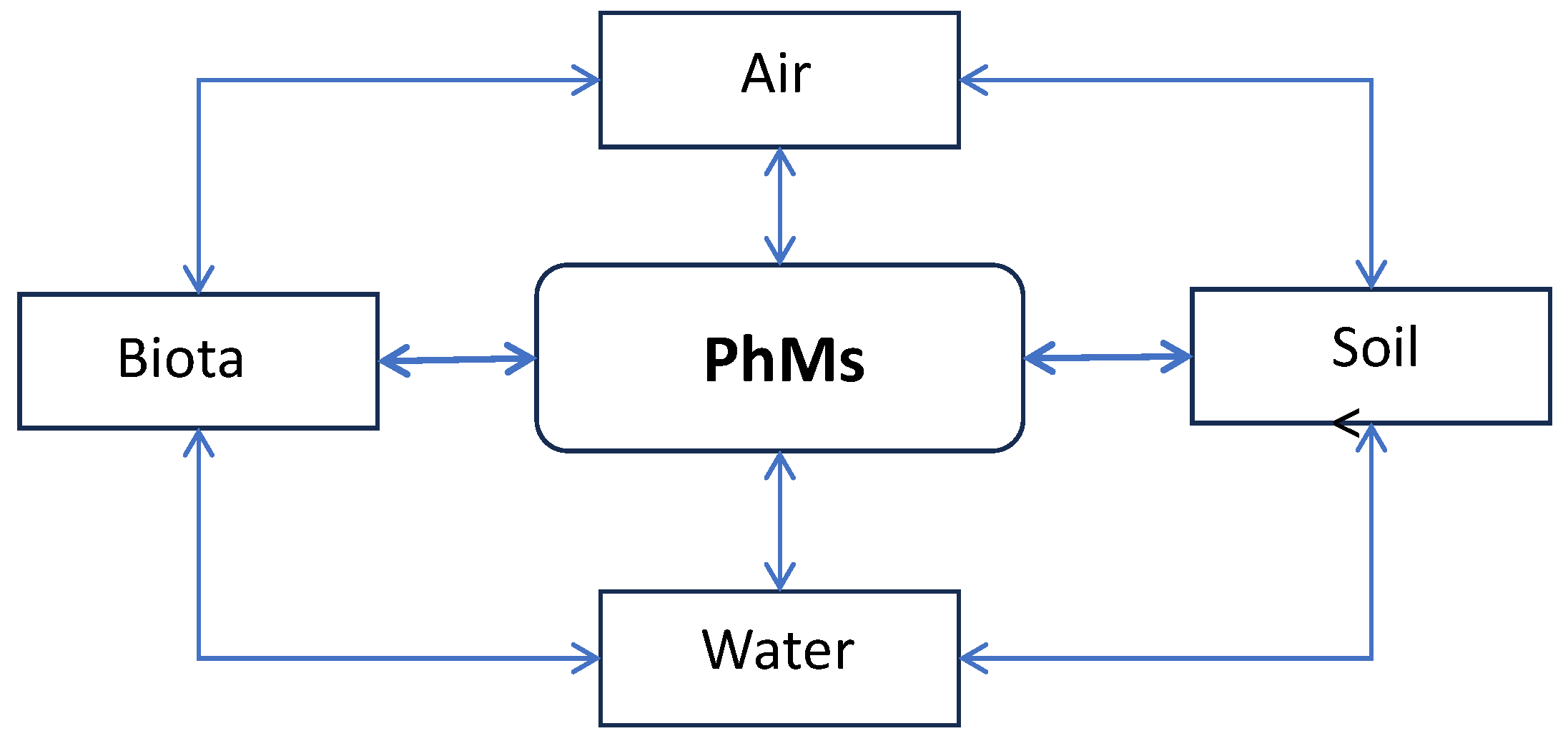
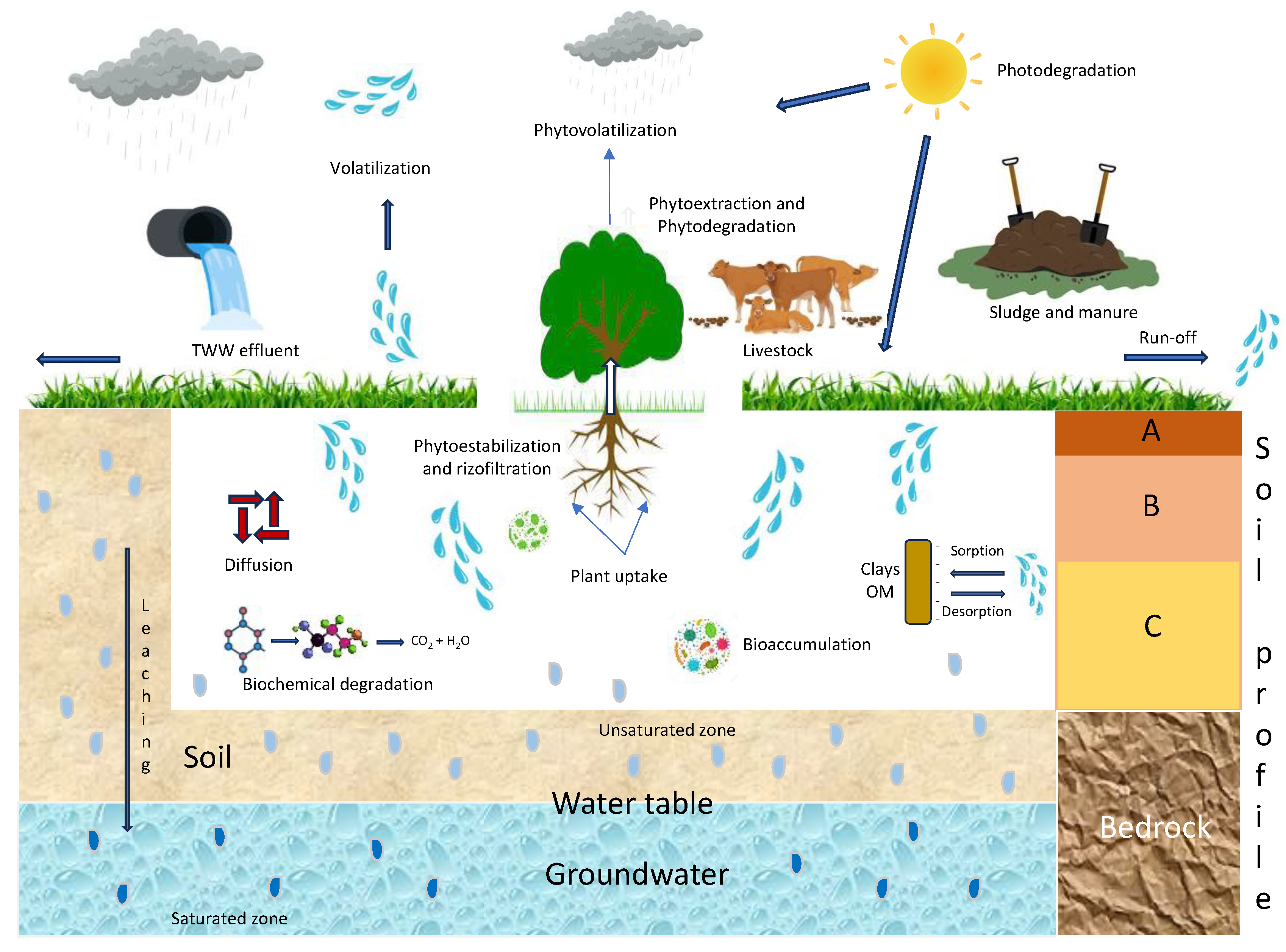
3. Sources of PhMs in the Environment
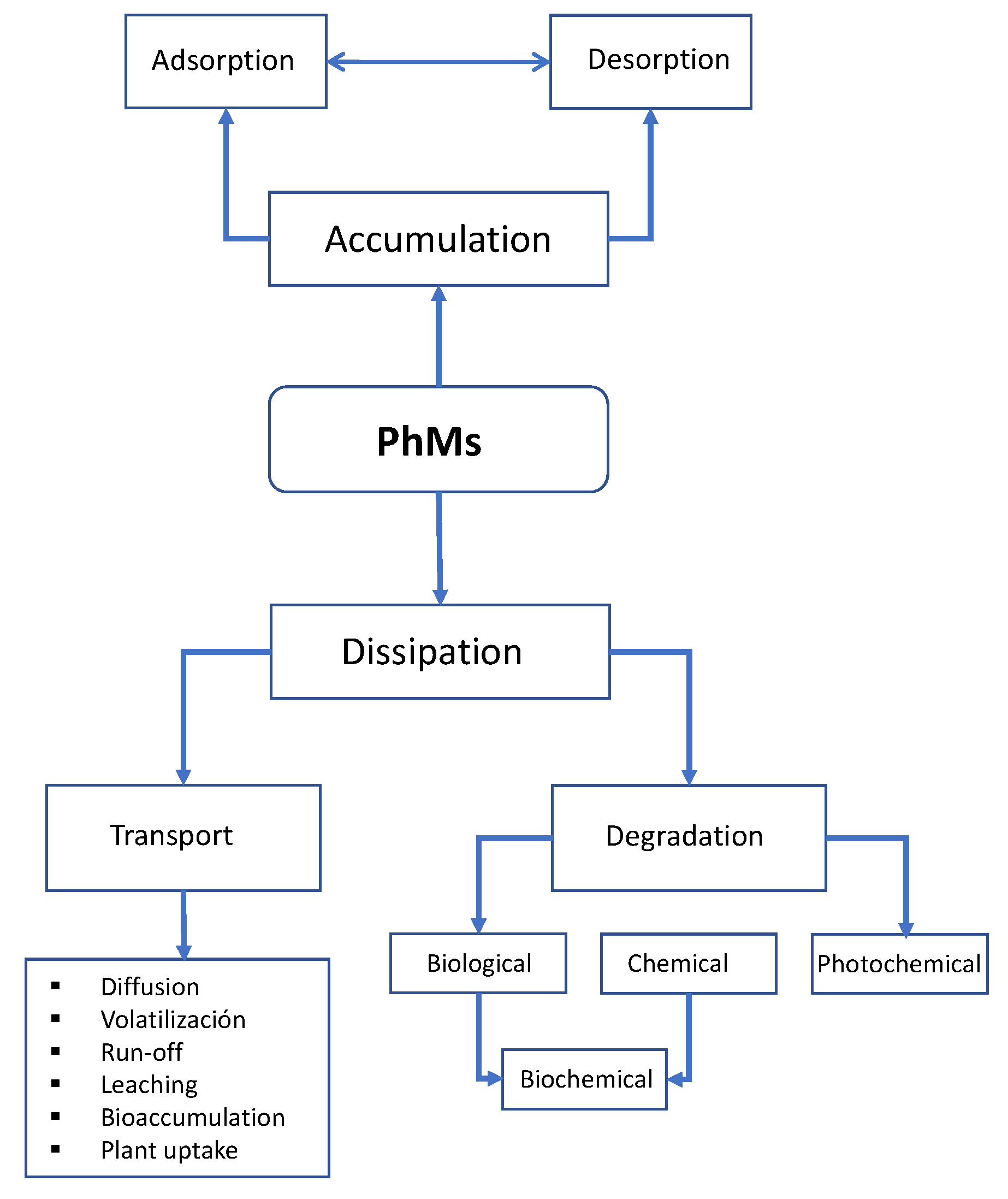
4. Behavior and Fate of PhMs in the Soil Environment
4.1. Adsorption-Desorption
4.2. Degradation
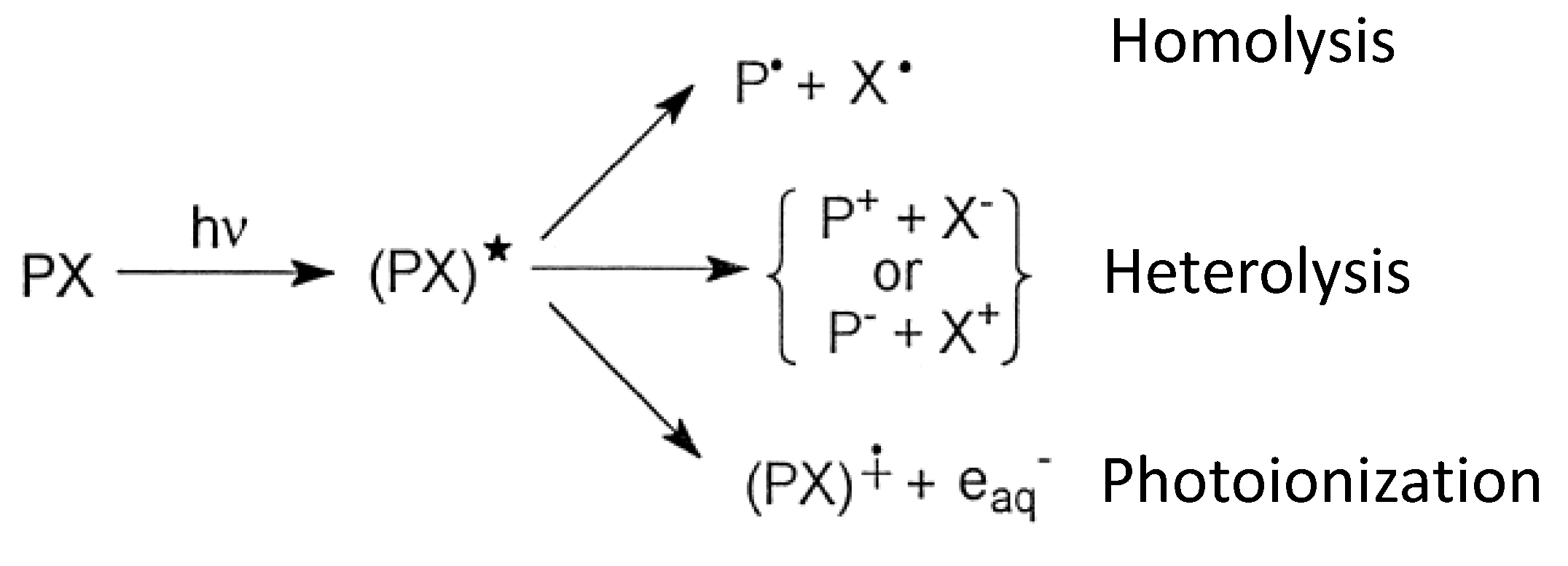
4.2.1. Photochemical Degradation
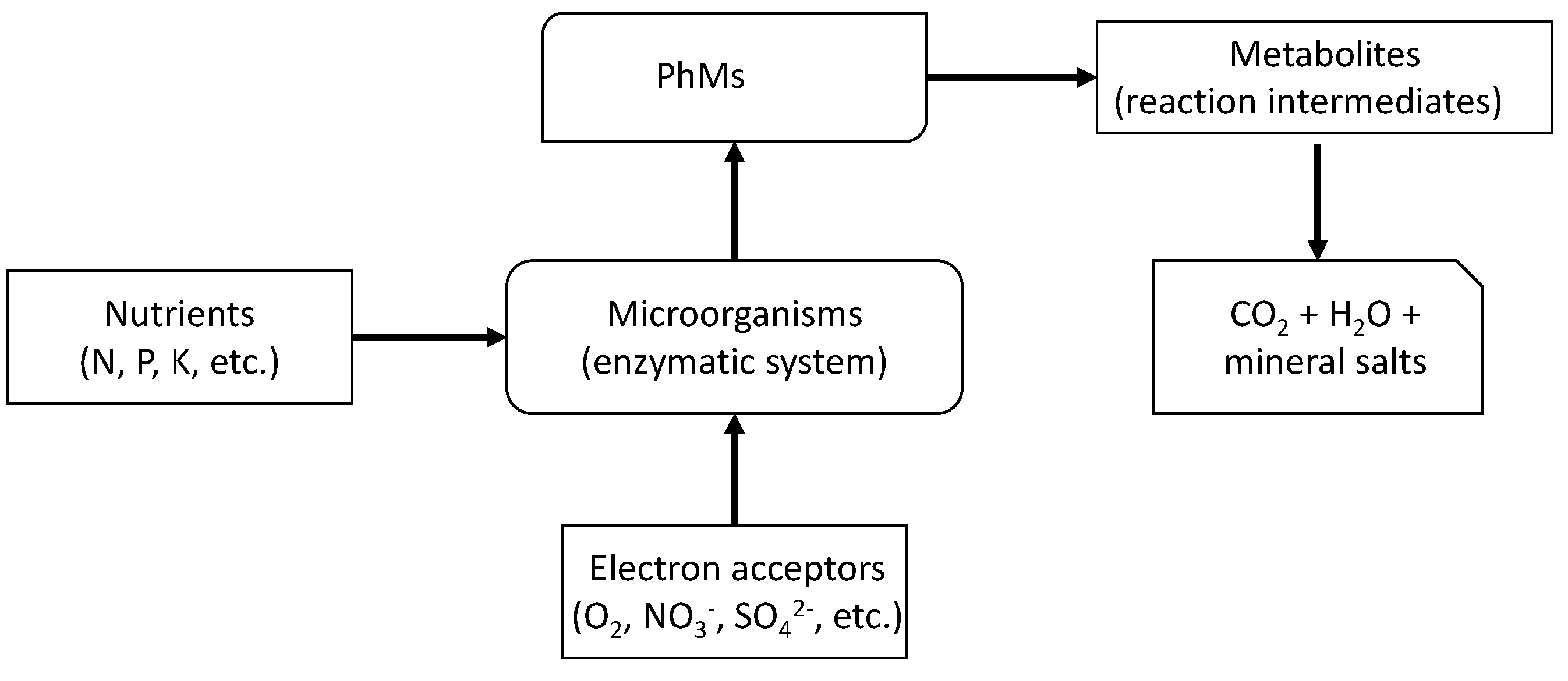
4.2.2. Biochemical Degradation
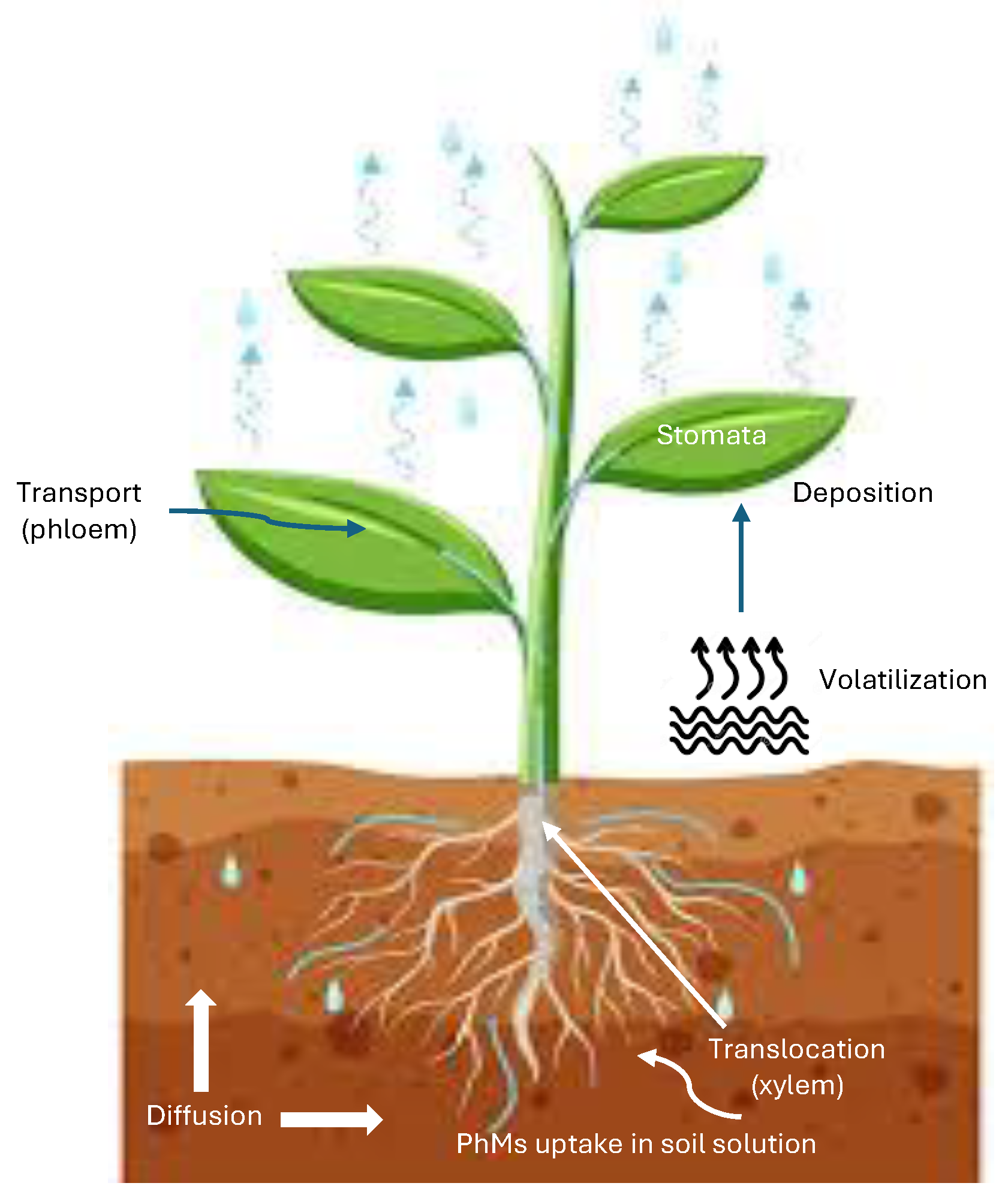
4.3. Movement (Transport) of PhMs in the Soil
4.3.1. Diffusion
4.3.2. Volatilization
4.3.3. Run-Off
4.3.4. Bioaccumulation
4.3.5. Plant Uptake
4.3.6. Leaching
5. Impact of PhMs on the Soil Health
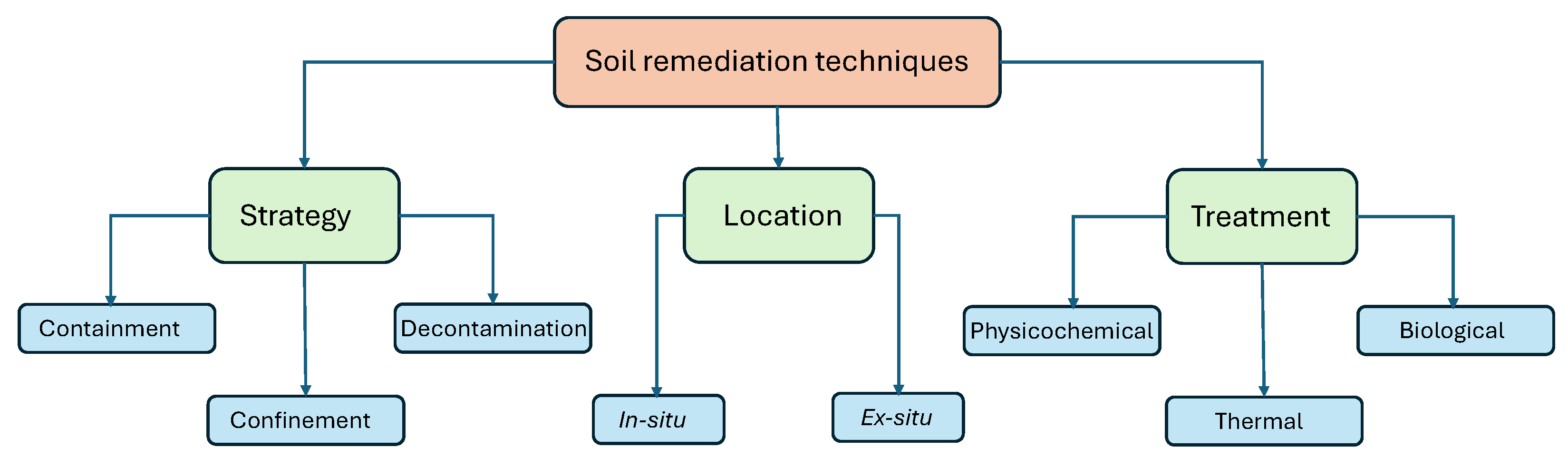
6. Remediation of PhMs-Polluted Soils
- Environmental characteristics. Topography, demography, hydrology and ecology of the contaminated area.
- Type of contaminant, concentration and toxicity. The type of pollutant (organic or inorganic) and its physicochemical characteristics provide us with the necessary information on the behavior of the pollutant in the soil and its greater or lesser persistence and hazardousness.
- Physicochemical properties and type of soil to be treated. Texture, structure, porosity, permeability, heterogeneity, pH, temperature, humidity and organic matter content are the parameters that determine the choice of one technique or another.
- Cost of the technique. The inherent uniqueness of each pollution event makes it difficult to rigorously compare the costs of different remediation techniques. The data on which we can rely are based on a treatment applied under specific conditions, and it can be very difficult to extrapolate to other conditions with a different contaminant, at a different concentration, and with a different type of soil. In addition, these costs can be expressed in different parameters/units (volume of soil treated, reduction of pollutant concentration, reduction of pollutant mobility, mass of pollutant removed or area treated), which increases this difficulty. In general, we can say that thermal techniques are the most expensive and biological techniques are the most economical.
- Immobilization or isolation of contaminants.
- Separation or extraction of contaminants.
- Destruction or transformation of pollutants.
- In-situ: When the treatment is carried out directly on the contaminated area, without the need to excavate the site. These are more economical techniques because the soil does not have to be excavated or transported. They have the disadvantage of requiring longer treatment times, the heterogeneous distribution of contaminants in the soil and the difficulty of verifying the effectiveness of the processes.
- Ex-situ: When a process (dredging, excavation, etc.) is necessary to remove (move/transport) the contaminated area before its treatment, which can take place on site or in a different place (off-site). Among the advantages of these techniques, we can mention shorter treatment times and greater uniformity in the soils to be treated, as they can be homogenized periodically. In contrast, they need some equipment to excavate the soil, which makes the process more expensive. In addition, there are risks associated with handling the material and possible exposure to the contaminant.
- Finally, depending on the type of treatment, three methods can be used:
- Bioremediation, which is a natural and environmentally friendly approach that uses microorganisms or plants to break down or neutralize contaminants in the soil. Microorganisms, such as bacteria and fungi, are able to metabolize contaminants and convert them into less harmful substances. Plants, in a process known as phytoremediation, can absorb and accumulate contaminants. For organic contaminants, including petroleum hydrocarbons, solvents, pesticides, and pharmaceuticals, bioremediation is effective.
- Chemical remediation, which involves the use of chemicals or chemical processes to treat soil contamination. The chemical composition of contaminants is altered to make them less toxic or immobile using techniques such as oxidation and reduction reactions. Soil washing, soil vapor extraction, and chemical oxidation are common chemical remediation methods. A wide range of persistent organic pollutants are amenable to these methods.
- Physical remediation techniques that focus on the physical removal or isolation of contaminated soil from the surrounding environment. A common approach to physical remediation, particularly for localized contamination, is to excavate and remove contaminated soil. Soil capping is another method in which the contaminated soil is covered with a barrier to prevent further contamination of the soil. Physical remediation is often used for soils that have been contaminated with hazardous materials, such as asbestos or radioactive materials.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Noguera-Oviedo, K.; Aga, D. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass loads, source apportionment, and risk estimation of organic micropollutants from hospital and municipal wastewater in recipient catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Liu, G.; Hyotylainen, T.; Falandysz, J. Toxicology and environmental characteristics of emerging pollutants. Ecotoxicol. Environ. Saf. 2019, 181, e264. [Google Scholar] [CrossRef]
- Pérez-Carrera, E.; Hansen, M.; León, V.M.; Björklund, E.; Krogh, K.A.; Halling-Sørensen, B.; González-Mazo, E. Multiresidue method for the determination of 32 human and veterinary pharmaceuticals in soil and sediment by pressurized-liquid extraction and LC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 1173–1184. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Kumirska, J.; Borecka, M.; Caban, M.; Paszkiewicz, M.; Pazdro, K.; Stepnowski, P. Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J. Pharm. Biomed. Anal. 2016, 121, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Koba, O.; Kodesova, R.; Fedorova, G.; Kumar, V.; Grabic, R. Development of fast and robust multiresidual LC-MS/MS method for determination of pharmaceuticals in soils. Environ. Sci. Pollut. Res. 2016, 23, 14068–14077. [Google Scholar] [CrossRef]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Cacace, C.; Mottola, F.; Mezzina, A.; Brunetti, G. Validation of a modified QuEChERS method for the extraction of multiple classes of pharmaceuticals from soils. Chem. Biol. Technol. Agric. 2022, 9, e49. [Google Scholar] [CrossRef]
- Mejías, C.; Arenas, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Green Assessment of Analytical Procedures for the Determination of Pharmaceuticals in Sewage Sludge and Soil. Crit. Rev. Anal. Chem. 2023, 3, 1–14. [Google Scholar] [CrossRef]
- Anand, U.; Adelodun, B.; Cabreros, C.; Kumar, P.; Suresh, S.; Dey, A.; Ballesteros, F.; Bontempi, E. Occurrence, transformation, bioaccumulation, risk and analysis of pharmaceutical and personal care products from wastewater: a review. Environ. Chem. Lett. 2022, 20, 3883–3904. [Google Scholar] [CrossRef]
- Mravcová, L.; Amrichová, A.; Navrkalová, J.; Hamplová, M.; Sedlář, M.; Gargošová, H. Z.; Fučík, J. Optimization and validation of multiresidual extraction methods for pharmaceuticals in Soil, Lettuce, and Earthworms. Environ. Sci. Pollut. Res 2024, 31, 33120–33140. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). European Chemicals Agency. 2022. Available online: https://echa.europa.eu/es/regulations/reach/understanding-reach (accessed on 20 May 2024).
- Jiang, J.-Q.; Zhou, Z.; Sharma, V.K. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in wastewater - A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Abdulrazaq, Y.; Abdulsalam, A.; Rotimi, A.L.; Abdulbasit, A. A.; Clifford, O.; Abdulsalam, O.A.; Racheal, O. N.; Joy, A.A.; Victor, F.O.; Johannes, Z.M.; Bilal, M.; Umar, S. Classification, Potential Routes and Risk of Emerging Pollutants/Contaminant. In Emerging Contaminants; Nuro, A., Ed.; IntechOpen: London, 2020; pp. 1–12. [Google Scholar]
- Revilla-Pacheco, C.; Terán-Hilares, R.; Colina-Andrade, G.; Mogrovejo-Valdivia, A.; Pacheco-Tanaka, D.A. Emerging contaminants, SARS-COV-2 and wastewater treatment plants, new challenges to confront: A short review. Bioresour. Technol. Rep 2021, 15, e100731. [Google Scholar] [CrossRef]
- Statista. Pharma and biotech M&A activity. 2024. Available online: https://www.statista.com/study/96092/biopharma-manda-deals/ (accessed on 29 May 2024).
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses, OECD Studies on Water; OECD Publishing: Paris, 2019. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman Jr, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Wilikinson, J.L.; et al. Pharmaceutical pollution of the world’s rivers Proceedings of the National Academy of Sciences (PNAS). 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Nguyen, H.L.; Hung NT, Q.; La, D.D.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions. Sci. Total Environ. 2023, 899, e165323. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, N.; Topp, E.; Metcalfe, C.; Edwards, M.; Payne, M.; Kleywegt, S.; Rusell, P.; Lapen, D.R. Pharmaceutical and personal care products in groundwater, subsurface drainage, soil, and wheat grain, following a high single application of municipal biosolids to a field. Chemosphere 2012, 87, 194–203. [Google Scholar] [CrossRef]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef]
- bastos-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P. A. Monitoring the occurrence of pharmaceuticals in soils irrigated with reclaimed wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.C.; Soubrand, M.; Le Guet, T.; Le Floch, É.; Joussein, E.; Baudu, M.; Casellas, M. Occurrence, fate and environmental risk assessment of pharmaceutical compounds in soils amended with organic wastes. Geoderma 2020, 375, e114498. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Occurrence of the main metabolites of the most recurrent pharmaceuticals and personal care products in Mediterranean soils. J. Environ. Manag. 2021, 278, e111584. [Google Scholar] [CrossRef] [PubMed]
- Mejias, C.; Martin, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, J.A.; Coelho, M.R.; Martins, A.; Cardoso, E.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M. Occurrence of pharmaceutical active compounds in sewage sludge from two urban wastewater treatment plants and their potential behaviour in agricultural soils. Environ. Sci: Water Res. Technol. 2021, 7, 969–982. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, D.S. Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Filho, F.J.C.M.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. 2020, 705, e135568. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Mompelat, S.; Bot, B.L.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- EC. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directive 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. OJEC EC, L226, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013L0039&from=ES (accessed on 20 May 2024).
- EC. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. The European Green Deal. Brussels, COM (2019) 640 final. 2019. Available online: https://ec.europa.eu/info/sites/default/files/european-green-deal-communication_en.pdf (accessed on 22 May 2024).
- EC. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Chemicals Strategy for Sustainability. Towards a Toxic-Free Environment. Brussels, COM (2020) 667 final. 2020. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:f815479a-0f01-11eb-bc07-01aa75ed71a1.0003.02/DOC_1&format=PDF (accessed on 22 May 2024).
- EC. Communication from the Commission to the European Parliament, the Council and the European Economic and Social Committee. European Union Strategic Approach to Pharmaceuticals in the Environment. Brussels, COM (2019) 128 final. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52019DC0128&from=EN (accessed on 22 May 2024).
- EC. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Pharmaceutical Strategy for Europe, COM/2020/761 final. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0761 (accessed on 26 May 2024).
- EC. Proposal for a directive amending the water framework directive, the groundwater directive and the environmental quality standards directive. COM (2022) 540 final. 2022. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:d0c11ba6-55f8-11ed-92ed-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 23 May 2024).
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, e105966. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment—a review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Falahi OA, A.; Abdullah SR, S.; Hasan, H.A.; Othman, A.R.; Ewadh, H.M.; Kurniawan, S.B.; Imron, M.F. Occurrence of pharmaceuticals and personal care products in domestic wastewater, available treatment technologies, and potential treatment using constructed wetland: a review. Process Saf. Environ. Prot. 2022, 168, 1067–1088. [Google Scholar] [CrossRef]
- Silori, R.; Tauseef, S.M. A review of the occurrence of pharmaceutical compounds as emerging contaminants in treated wastewater and aquatic environments. Curr. Pharm. Anal. 2022, 18, 345–379. [Google Scholar]
- Nguyen, M.K.; Lin, C.; Bui, X.T.; Rakib MR, J.; Nguyen, H.L.; Truong, Q.M.; Hoang, H.-G.; Tran, H.T.; Malafaia, G.; Idris, A.M. Occurrence and fate of pharmaceutical pollutants in wastewater: Insights on ecotoxicity, health risk, and state–of–the-art removal. Chemosphere 2024, 354, e141678. [Google Scholar] [CrossRef]
- EC. Regulation 2020/741/EU of the European Parliament and of the Council of 25 May 2020 on minimum requirements for water reuse. OJEC 2020b, L177, 32–55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741&from=EN (accessed on 22 May 2024).
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the sorption and biotransformation of organic micropollutants in innovative biological wastewater treatment technologies. Sci. Total Environ 2018, 615, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García M, Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Gurke, R.; Eckert, H.; Kühn, K.; Fauler, J.; Cuniberti, G. Photocatalytic degradation of pharmaceuticals present in conventional treated wastewater by nanoparticle suspensions. J. Environ. Chem. Eng. 2016, 4, 287–292. [Google Scholar]
- Kårelid, V.; Larsson, G.; Björlenius, B. Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. J. Environ. Manag. 2017, 193, 491–502. [Google Scholar]
- Rodríguez-Narváez, O.M.; Peralta-Hernández, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Roccaro, P. Treatment processes for municipal wastewater reclamation: the challenges of emerging contaminants and direct potable reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 46–54. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions-A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, e100386. [Google Scholar] [CrossRef]
- Pesqueira, J.F.; Pereira, M.F.R.; Silva, A. Environmental impact assessment of advanced urban wastewater treatment technologies for the removal of priority substances and contaminants of emerging concern: a review. J. Clean. Prod. 2020, 261, e121078. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, AA. Simultaneous removal of antibiotics inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Proc. Eng. 2021, 42, e102089. [Google Scholar] [CrossRef]
- Hejna, M.; Kapúscinska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health, 2022, 19, e7717. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D. A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The persistence of a broad range of antibiotics during calve, pig and broiler manure storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Sundlof, S.F. Veterinary drugs residues: veterinary drugs – general. Encyclopedia of Food Safety, 2014, 3, 35–38. [Google Scholar]
- EMA. 2024. European public assessment reports: background and context. The European Medicines Agency. https://www.ema.europa.eu/en/medicines/what-we-publish-medicines-and-when/european-public-assessment-reports-background-and-context.
- Song, W.; Ding, Y.; Chiou, C.T.; Li, H. Selected veterinary pharmaceuticals in agricultural water and soil from land application of animal manure. J. Environ. Qual. 2010, 39, 1211–1217. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, e338. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An investigation of anticoccidial veterinary drugs as emerging organic contaminants in groundwater. Sci. Total Environ. 2020, 746, e141116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Li, X.; Ma, X.; Ru, S.; Qiu, T.; Lu, A. Veterinary antibiotics and estrogen hormones in manures from concentrated animal feedlots and their potential ecological risks. Environ. Res. 2021, 198, 110463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Barron, L.; Sturzenbaum, S. The transportation, transformation and (bio)accumulation of pharmaceuticals in the terrestrial ecosystem. Sci. Total Environ. 2021, 781, e146684. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Iglesias, I.; de la Torre, A. Prioritisation tool for targeting the monitoring of veterinary pharmaceuticals in soils at a national level: The case of Spain. Eur. J. Soil Sci. 2022, 73, e13268. [Google Scholar] [CrossRef]
- Monteiro, S.C.; Boxall, A.B. Occurrence and fate of human pharmaceuticals in the environment. Rev. Environ. Contam. Toxicol. 2010, 202, 53–154. [Google Scholar] [PubMed]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A. D.; Mansouri, K.; Baker, N. C.; Richard, A. M. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Chem. Inf. 2017, 9, e61. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Rakonjac, N.; van der Zee, S.E.; Wipfler, L.; Roex, E.; Kros, H. Emission estimation and prioritization of veterinary pharmaceuticals in manure slurries applied to soil. Sci. Total Environ. 2022, 815, e152938. [Google Scholar] [CrossRef]
- EU. Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 amending Directive 1999/31/EC on the landfill of waste. OJEU 2018, L150, 100–108. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L0850.
- EU 2020 European Union (Landfill) Regulations 2020, S.I. No. 321, 1-11. https://www.irishstatutebook.ie/eli/2020/si/321/made/en/pdf.
- Abdallat, G.A.; Salameh, E.; Shteiwi, M.; Bardaweel, S. Pharmaceuticals as emerging pollutants in the reclaimed wastewater used in irrigation and their effects on plants, soils, and groundwater. Water, 2022, 14, e1560. [Google Scholar] [CrossRef]
- de Santiago-Martín, A.; Meffe, R.; Teijón, G.; Hernández, V. M.; López-Heras, I.; Alonso, C. A.; Arenas-Romasanta, M.; de Bustamante, I. Pharmaceuticals and trace metals in the surface water used for crop irrigation: Risk to health or natural attenuation? Sci. Total Environ. 2020, 705, e135825. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Papadavid, G.; Dalias, P.; Fotopoulos, V.; Michael, C.; Bayona, J.M.; Piña, B.; Fatta-Kassinos, D. Ranking of crop plants according to their potential to uptake and accumulate contaminants of emerging concern. Environ. Res. 2019, 170, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Insight into the distribution of pharmaceuticals in soil-water-plant systems. Water Res. 2019, 152, 38–46. [Google Scholar] [CrossRef]
- Cucina, M.; Ricci, A.; Zadra, C.; Pezzolla, D.; Tacconi, C.; Sordi, S.; Gigliotti, G. Benefits and risks of long-term recycling of pharmaceutical sewage sludge on agricultural soil. Sci. Total Environ. 2019, 695, e133762. [Google Scholar] [CrossRef] [PubMed]
- Bolesta, W.; Głodniok, M.; Styszko, K. From sewage sludge to the soil—transfer of pharmaceuticals: a review. Int. J. Environ. Res. Pub. Health 2022, 19, e10246. [Google Scholar] [CrossRef]
- Aydın, S.; Ulvi, A.; Bedük, F.; Aydın, M.E. Pharmaceutical residues in digested sewage sludge: Occurrence, seasonal variation and risk assessment for soil. Sci. Total Environ. 2022, 817, e152864. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil-A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, M.D.; Santos, J.L.; Aparicio, I.; Alonso, E. Distribution and temporal evolution of pharmaceutically active compounds alongside sewage sludge treatment. Risk assessment of sludge application onto soils. J. Environ. Manag. 2012, 102, 18–25. [Google Scholar] [CrossRef]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids—soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef]
- Bourdat-Deschamps, M.; Ferhi, S.; Bernet, N.; Feder, F.; Crouzet, O.; Patureau, D.; Montenach, D.; Moussard, G.D.; Mercier, V.; Benoit, P.; et al. Fate and impacts of pharmaceuticals and personal care products after repeated applications of organic waste products in long-term field experiments. Sci. Total Environ. 2017, 607, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Mejías, C.; Santos, J.L.; Aparicio, I.; Alonso, E. Pharmaceuticals and their main metabolites in treated sewage sludge and sludge-amended soil: availability and sorption behaviour. Molecules, 2021, 26, e5910. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, A.; Cela-Dablanca, R.; Nebot, C.; Rodríguez-López, L.; Santás-Miguel, V.; Arias-Estévez, M.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E. Occurrence of nine antibiotics in different kinds of sewage sludge, soils, corn and grapes after sludge spreading. Span. J. Soil Sci. 2022, 12, e10741. [Google Scholar] [CrossRef]
- Gworek, B.; Kijeńska, M.; Wrzosek, J.; Graniewska, M. Pharmaceuticals in the soil and plant environment: a review. Water Air Soil Pollut. 2021, 232, 145. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the environment: Plant uptake, translocation, bioaccumulation, and human health risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Gavrilescu, M. Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 2005, 5, 497–526. [Google Scholar] [CrossRef]
- Kodešová, R.; Kočárek, M.; Klement, A.; Golovko, O.; Koba, O.; Fér, M.; Nikodem, A.; Vondráčková, L.; Jakšik, O.; Grabic, R. An analysis of the dissipation of pharmaceuticals under thirteen different soil conditions. Sci. Total Environ. 2016, 544, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Malchi, T.; Carter, L.J.; Li, H.; Gan, J.; Chefetz, B. Pharmaceutical and Personal Care Products: From Wastewater Treatment into Agro-Food Systems. Environ. Sci.Technol. 2019, 53, 14083–14090. [Google Scholar] [CrossRef]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere, 2008, 73, 1335–1343. [Google Scholar] [CrossRef]
- Lin, K.; Gan, J. Sorption and degradation of wastewater associated non-steroidal anti-inflammatory drugs and antibiotics in soils. Chemosphere 2011, 83, 240–246. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, X.; Xu, B.; Peng, D.; Guo, X. Sorption of pharmaceuticals and personal care products on soil and soil components: Influencing factors and mechanisms. Sci. Total Environ. 2021, 753, e141891. [Google Scholar] [CrossRef]
- Rouquerol, F., Rouquerol, J., Sing, K. S. W. 1999. Adsorption by powders and porous solids. Principles, methodology and applications. Academic Press, London.
- OECD. 2000. Guidelines for testing of chemicals, No 106, adsorption–desorption using a batch equilibrium method. Organization for Economic Cooperation and Development (OECD), Paris.
- US EPA. 2008. Fate, Transport and Transformation Test Guidelines OPPTS 835.1230 Adsorption/Desorption (Batch Equilibrium). Washington DC.
- Karickhoff, S.W.; Brown, D.S.; Scott, T.A. Sorption of hydrophobic pollutants on natural sediments. Water Res. 1979, 13, 421–428. [Google Scholar] [CrossRef]
- Wu, X.; Ernst, F.; Conkle, J. L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef]
- Carter, L. J.; Harris, E.; Williams, M.; Ryan, J. J.; Kookana, R. S.; Boxall, A. B. A. Fate and uptake of pharmaceuticals in soil-plant systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef]
- Xu, J.; Chen, W.; Wu, L.; Chang, A.C. Adsorption and degradation of ketoprofen in soils. J. Environ. Qual. 2009, 38, 1177–1182. [Google Scholar] [CrossRef]
- Tang, T.; Shi, T.; Li, D.; Xia, J.; Hu, Q.; Cao, Y. Adsorption properties and degradation dynamics of endocrine-disrupting chemical levonorgestrel in soils. J. Agric. Food Chem. 2012, 60, 3999–4004. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Paz, A.; Tadmor, G.; Malchi, T.; Blotevogel, J.; Borch, T.; Polubesova, T.; Chefetz, B. Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: Sorption, leaching and plant uptake. Chemosphere 2016, 160, 22–29. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Zhang, W. Influence of manure as a complex mixture on soil sorption of pharmaceuticals-studies with selected chemical components of manure. Int. J. Environ. Res. Public Health, 2023, 20, e6154. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals' sorptions relative to properties of thirteen different soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef]
- Kočárek, M.; Kodešová, R.; Vondráčková, L.; Golovko, O.; Fér, M.; Klement, A.; Nikodem, A.; Jakšík, O.; Grabic, R. Simultaneous sorption of four ionizable pharmaceuticals in different horizons of three soil types. Environ. Pollut. 2016, 218, 563–573. [Google Scholar] [CrossRef]
- Wu, L.; Bi, E. Sorption of ionic and neutral species of pharmaceuticals to loessial soil amended with biochars. Environ. Sci. Pollut. Res. 2019, 26, 35871–35881. [Google Scholar] [CrossRef]
- Maszkowska, J.; Wagil, M.; Mioduszewska, K.; Kumirska, J.; Stepnowski, P.; Białk-Bielińska, A. Thermodynamic studies for adsorption of ionizable pharmaceuticals onto soil. Chemosphere 2014, 111, 568–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Price, G.W.; Jamieson, R.; Burton, D.; Khosravi, K. Sorption and desorption of selected non-steroidal anti-inflammatory drugs in an agricultural loam textured soil. Chemosphere 2017, 174, 628–637. [Google Scholar] [CrossRef]
- Carter, L.J.; Wilkinson, J.L.; Boxall AB, A. Evaluation of existing models to estimate sorption coefficients for ionisable pharmaceuticals in soils and sludge. Toxics 2020, 8, e13. [Google Scholar] [CrossRef]
- Li, J.; Wilkinson, J.L.; Boxall, A.B. Use of a large dataset to develop new models for estimating the sorption of active pharmaceutical ingredients in soils and sediments. J. Hazard. Mat. 2021, 415, e125688. [Google Scholar] [CrossRef]
- Li, J.; Carter, L.J.; Boxall, A.B. Evaluation and development of models for estimating the sorption behaviour of pharmaceuticals in soils. J. Hazard. Mat. 2020, 392, e122469. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.; Zhuang, J. The fate and impact of pharmaceuticals and personal care products in agricultural soils irrigated with reclaimed water. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1379–1408. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Wu, L. Sorption and degradation of pharmaceuticals and personal care products (PPCPs) in soils. Environ. Sci. Pollut. Control Ser. 2013, 20, 4261–4267. [Google Scholar] [CrossRef]
- Hong, H.; Liu, C.; Li, Z. Chemistry of soil-type dependent soil matrices and its influence on behaviors of pharmaceutical compounds (PCs) in soils. Heliyon 2023, 9, e22931. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.C.; Boxall, A.B. Factors affecting the degradation of pharmaceuticals in agricultural soils. Environ. Toxicol. Chem. 2009, 28, 2546–2554. [Google Scholar] [CrossRef]
- Salvia, M. V.; Experton, J.; Geandel, C.; Cren-Olivé, C.; Vulliet, E. Fate of pharmaceutical compounds and steroid hormones in soil: study of transfer and degradation in soil columns. Environ. Sci. Pollut. Res. 2014, 21, 10525–10535. [Google Scholar] [CrossRef]
- Boesten, J.J.T.I.; Aden, K.; Beigel, C.; Beulke, S.; Dust, M.; Dyson, J.S.; Fomsgaard, I.S.; Jones, R.L.; Karlsson, S.; van der Linden, A.M.A.; Richter, O.; Magrans, J.O.; Soulas, G. Guidance Document on Estimating Persistence and Degradation Kinetics From Environmental Fate Studies on Pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference Sanco/10058/2005 version 2.0. 2006. [Google Scholar]
- OECD. 2000. Guidelines for testing of chemicals, No 307, aerobic and anaerobic transformation in soil. Organization for Economic Cooperation and Development (OECD). París.
- US EPA. 2008. Fate, Transport and Transformation Test Guidelines OPPTS 835.6100 Terrestrial Field Dissipation. Washington DC.
- Domenech, X.; Peral, J. Química Ambiental de sistemas terrestres. Reverté, Barcelona. 2006. [Google Scholar]
- Burrows, H. D.; Santaballa, J. A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B: Biol. 2002, 67, 71–108. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils -a review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Picó, Y.; Andreu, V. Fluoroquinolones in soil—risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils -a review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Thiele-Brun, S.; Peters, D. Photodegradation of pharmaceutical antibiotics on slurry and soil surfaces. Landbauforschung Volkenrode 2007, 57, 13–23. [Google Scholar]
- Microbial biodegradation and bioremediation. Elsevier, Amsterdam. Das, S. (Ed.) 2014. [Google Scholar]
- Solliec, M.; Roy-Lachapelle, A.; Gasser, M.O.; Coté, C.; Généreux, M.; Sauvé, S. Fractionation and analysis of veterinary antibiotics and their related degradation products in agricultural soils and drainage waters following swine manure amendment. Sci. Total Environ. 2016, 543, 524–535. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Fasani, E.; Albini, A. Sunlight-induced degradation of soil-adsorbed veterinary antimicrobials marbofloxacin and enrofloxacin. Chemosphere 2012, 86, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Nazhakaiti, P.; Tsutsui, H.; Urase, T. Aerobic and anaerobic biological degradation of pharmaceutically active compounds in rice paddy soils. Appl. Sci. 2019, 9, e2505. [Google Scholar] [CrossRef]
- Carr, D.L.; Morse, A.N.; Zak, J.C.; Anderson, T.A. Biological degradation of common pharmaceuticals and personal care products in soils with high water content. Water Air Soil Pollut. 2011, 217, 127–134. [Google Scholar] [CrossRef]
- Grossberger, A.; Hadar, Y.; Borch, T.; Chefetz, B. Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated wastewater. Environ. Pollut. 2014, 185, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Lee, D.S. Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Laurila, T.; Vuorinen, V.; Divinski, S. V. Thermodynamics, diffusion and the Kirkendall effect in solids. Springer Cham. NY. 2014. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman Jr, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. K.; Sanghvi, G.; Yadav, M.; Padhiyar, H.; Christian, J.; Singh, V. Fate of pesticides in agricultural runoff treatment systems: Occurrence, impacts and technological progress. Environ. Res. 2023, 237, e117100. [Google Scholar] [CrossRef] [PubMed]
- Kinney, C.A.; Furlong, E.T.; Kolpin, D.W.; Burkhardt, M.R.; Zaugg, S.D.; Werner, S.L.; Bossio, J.P.; Benotti, M.J. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ. Sci. Technol. 2008, 42, 1863–1870. [Google Scholar] [CrossRef]
- Armitage, J.A.; Gobas, P.C. A terrestrial food-chain bioaccumulation model for POPs. Environ. Sci. Technol. 2007, 41, 4019–4025. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Czajkowski, K.P. Uptake of pharmaceutical and personal care products by soybean plants from soils applied with biosolids and irrigated with contaminated water. Environ. Sci. Technol. 2010, 44, 6157–6161. [Google Scholar] [CrossRef]
- Holling, C.S.; Bailey, J.L.; Heuvel, B.V.; Kinney, C.A. Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-amended soils. J. Environ. Monit. 2012, 14, 3029–3036. [Google Scholar] [CrossRef]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Sridhar, B.M. Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil. Ecotoxicol. Environ. Saf. 2012, 85, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kodešová, R.; Klement, A.; Golovko, O.; Fér, M.; Kočárek, M.; Nikodem, A.; Grabic, R. Soil influences on uptake and transfer of pharmaceuticals from sewage sludge amended soils to spinach. J. Environ. Manag. 2019, 250, e109407. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Guo, M. Soil–plant transfer of pharmaceuticals and personal care products. Curr. Pollut. Rep. 2021, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Characterization of plant accumulation of pharmaceuticals from soils with their concentration in soil pore water. Environ. Sci. Technol. 2022, 56, 9346–9355. [Google Scholar] [CrossRef] [PubMed]
- Dickman, R.A.; Brunelle, L.D.; Kennedy, B.C.; Noe-Hays, A.; Love, N.G.; Aga, D.S. Increasing accuracy of field-scale studies to investigate plant uptake and soil dissipation of pharmaceuticals. Anal. Methods 2021, 13, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Grimon, V.; Fernandez-Vera, J.R.; Hernandez-Moreno, J.M.; Guedes-Alonso, R.; Estévez, E.; Palacios-Diaz MD, P. Soil and water management factors that affect plant uptake of pharmaceuticals: A case study. Water 2022, 14, e1886. [Google Scholar] [CrossRef]
- De Mastro, F.; Brunetti, G.; De Mastro, G.; Ruta, C.; Stea, D.; Murgolo, S.; De Ceglie, C.; Mascolo, G.; Sannino, F.; Cocozza, C.; Traversa, A. Uptake of different pharmaceuticals in soil and mycorrhizal artichokes from wastewater. Environ. Sci. Pollut. Res. 2023, 30, 33349–33362. [Google Scholar] [CrossRef]
- Stando, K.; Korzeniewska, E.; Felis, E.; Harnisz, M.; Bajkacz, S. Uptake of pharmaceutical pollutants and their metabolites from soil fertilized with manure to parsley tissues. Molecules 2022, 27, e4378. [Google Scholar] [CrossRef]
- Menacherry, S. P. M.; Kodešová, R.; Švecová, H.; Klement, A.; Fér, M.; Nikodem, A.; Grabic, R. Selective accumulation of pharmaceutical residues from 6 different soils by plants: a comparative study on onion, radish, and spinach. Environ. Sci. Pollut. Res. 2023, 30, 54160–54176. [Google Scholar] [CrossRef]
- Trapp, S.; Legind, C.N. Uptake of organic contaminants from soil into vegetables and fruits. In: Dealing with contaminated sites: From theory towards practical application, Springer. NY. pp. 369–408.
- Hurtado, C.; Domínguez, C.; P_erez-Babace, L.; Cañameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Preciado, D.; Renault, Q.; Matamoros, V.; Cañameras, N.R.; Bayona, J.M. Uptake of organic emergent contaminants in spath and lettuce: an in vitro experiment. J. Agric. Food Chem. 2012, 60, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley. Pest. Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Fate of diclofenac in wetland mesocosms planted with Scirpus validus. Eco. Eng. 2012, 49, 59–64. [Google Scholar] [CrossRef]
- Calderón-Preciado, D.; Matamoros, V.; Savé, R.; Muñoz, P.; Biel, C.; Bayona, J. Uptake of microcontaminants by crops irrigated with reclaimed water and groundwater under real field greenhouse conditions. Environ. Sci. Pollut. Res. 2013, 20, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Bartha, B.; Huber, C.; Harpaintner, R.; Schröder, P. Effects of acetaminophen in Brassica juncea, L. Czern.: investigation of uptake, translocation, detoxification, and the induced defense pathways. Environ. Sci. Pollut. Res. 2010, 17, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Paltiel, O.; Fedorova, G.; Tadmor, G.; Kleinstern, G.; Maor, Y.; Chefetz, B. Human exposure to wastewater-derived pharmaceuticals in fresh produce: a randomized controlled trial focusing on carbamazepine. Environ. Sci. Technol 2016, 50, 4476–4482. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Conkle, J.L.; Ernst, F.; Gan, J. Treated wastewater irrigation: uptake of pharmaceutical and personal care products by common vegetables under field conditions. Environ. Sci. Technol. 2014, 48, 11286–11293. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: a review. Sci Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef]
- Prosser, R.; Sibley, P. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar] [CrossRef]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of root vegetables with treated wastewater: evaluating uptake of pharmaceuticals and the associated human health risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Cisneros, B.J. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Czajkowski, K.P.; Ames, A. Dissipation and leaching potential of selected pharmaceutically active compounds in soils amended with biosolids. Arch. Environ. Contam. Toxicol. 2010, 59, 343–351. [Google Scholar] [CrossRef]
- Bondarenko, S.; Gan, J.; Ernst, F.; Green, R.; Baird, J.; McCullough, M. Leaching of pharmaceuticals and personal care products in turfgrass soils during recycled water irrigation. J. Environ. Qual. 2012, 41, 1268–1274. [Google Scholar] [CrossRef]
- Borgman, O.; Chefetz, B. Combined effects of biosolids application and irrigation with reclaimed wastewater on transport of pharmaceutical compounds in arable soils. Water Res. 2013, 47, 3431–3443. [Google Scholar] [CrossRef]
- Vassilis, L.D.; George, B.C.; Charalampos, P.G.; Athina, P.V.; Xanthippos, K.N. Mobility of pharmaceutical compounds in the terrestrial environment: adsorption kinetics of the macrocyclic lactone eprinomectin in soils. Chemosphere 2016, 144, 1201–1206. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.; Zhuang, J. The surface-pore integrated effect of soil organic matter on retention and transport of pharmaceuticals and personal care products in soils. Sci. Total Environ. 2017, 599, 42–49. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, Y.; Zhang, J.; Yang, Q.; Li, G.; Zhang, D. Impacts of irrigation water sources and geochemical conditions on vertical distribution of pharmaceutical and personal care products (PPCPs) in the vadose zone soils. Sci. Total Environ. 2018, 626, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.N.; Popova, I.E.; Hammel, J.E.; Morra, M.J. Transport of potential manure hormone and pharmaceutical contaminants through intact soil columns. J. Environ. Qual. 2019, 48, 47–56. [Google Scholar] [CrossRef]
- Wojsławski, J.; Białk-Bielińska, A.; Stepnowski, P.; Dołżonek, J. Leaching behavior of pharmaceuticals and their metabolites in the soil environment. Chemosphere 2019, 231, 269–275. [Google Scholar] [CrossRef]
- Levine, A.J.; Bean, E.Z.; Hinz, F.O.; Wilson, P.C.; Reisinger, A.J. Leaching of select per-/poly-fluoroalkyl substances, pharmaceuticals, and hormones through soils amended with composted biosolids. J. Environ. Manag. 2023, 343, e118185. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Navarro, G. An overview on the environmental behavior of pesticide residues in soils. Span. J. Agric. Res. 2007, 5, 357–375. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Vela, N.; El Aatik, A.; Navarro, S. Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In: Larramendy, M., Soloneski, S. (Eds.), Pesticides - Use and Misuse and their Impact in the Environment. IntechOpen, London. pp. 45–71. 2019. [Google Scholar]
- OECD. Guidelines for testing of chemicals, No 312, leaching in soil columns. Organization for Economic Cooperation and Development (OECD). Organization for Economic Cooperation and Development (OECD). Paris. 2002. [Google Scholar]
- US EPA. Fate, Transport and Transformation Test Guidelines OPPTS 835.1240 Leaching Studies. Washington DC. 2008. [Google Scholar]
- Briceño, G.; Palma, G.; Durán, N. Influence of organic amendment on the biodegradation and movement of pesticides. Crit. Rev. Environ. Sci. Technol. 2007, 37, 233–271. [Google Scholar] [CrossRef]
- Ros, M.; Hernández, M.T.; García, C. Soil microbiota activity after restoration of a semiarid soil by organic amendments. Soil. Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- EC.2023. Proposal for a Directive of the European Parliament and of the Council on Soil Monitoring and Resilience (Soil Monitoring Law). European Commission. COM (2023) 416 final. Brussels. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52023PC0416.
- Syers, M.K.; Hamblin, A.; Pushparjah, E. Indicators and thresholds for the evaluation of sustainable land management. Can. J. Soil Sci. 1995, 75, 423–428. [Google Scholar] [CrossRef]
- Aon, M.A.; Colaneri, A.C. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. App. Soil Ecol. 2001, 18, 225–270. [Google Scholar] [CrossRef]
- Liu, F.; Ying, G.G.; Tao, R.; Zhao, J.L.; Yang, J.F.; Zhao, L.F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar] [CrossRef]
- Gutiérrez, I.R.; Watanabe, N.; Harter, T.; Glaser, B.; Radke, M. Effect of sulfonamide antibiotics on microbial diversity and activity in a Californian Mollic Haploxeralf. J. Soils Sed. 2010, 10, 537–544. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Ying, G.G.; Luo, Z.; Feng, H. Changes in functional diversity of soil microbial community with addition of antibiotics sulfamethoxazole and chlortetracycline. Appl. Microbiol. Biotechnol. 2012, 95, 1615–1623. [Google Scholar] [CrossRef]
- Ding, G.-C.; Radl, V.; Schloter-Hai, B.; Jechalke, S.; Heuer, H.; Smalla, K.; et al. Dynamics of Soil Bacterial Communities in Response to Repeated Application of Manure Containing Sulfadiazine. PLoS ONE 2014, 9, e92958. [Google Scholar] [CrossRef]
- Jechalke, S.; Focks, A.; Rosendahl, I.; Groeneweg, J.; Siemens, J.; Heuer, H.; Smalla, K. Structural and functional response of the soil bacterial community to application of manure from difloxacin-treated pigs. FEMS Microbiol. Ecol. 2014, 87, 77–88. [Google Scholar] [CrossRef]
- Cycón, M.; Borymski, S.S.S.; Zołnierczyk, B.; Piotrowska-Seget, Z.; Cycon, M.; Borymski, S.S.S.; Zolnierczyk, B.; Piotrowska-Seget, Z. Variable effects of non-steroidal anti-inflammatory drugs (NSAIDs) on selected biochemical processes mediated by soil microorganisms. Front. Microbiol. 2016, 7, 1–19. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Muñiz, S.; Val, J.; Navarro, E. Effects of 18 pharmaceuticals on the physiological diversity of edaphic microorganisms. Sci. Total Environ. 2017, 595, 441–450. [Google Scholar] [CrossRef]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—degradation and their impact on microbial activity and diversity. Front. Microbiol 2019, 10, e338. [Google Scholar] [CrossRef]
- Frkova, Z.; Vystavna, Y.; Koubová, A.; Kotas, P.; Grabicová, K.; Grabic, R.; Kodesova, R.; Chroňáková, A. Microbial responses to selected pharmaceuticals in agricultural soils: microcosm study on the roles of soil, treatment and time. Soil Biol. Biochem. 2020, 149, e107924. [Google Scholar] [CrossRef]
- Ugrina, M.; Jurić, A. Current trends and future perspectives in the remediation of polluted water, soil and air-a review. Processes 2023, 11, e3270. [Google Scholar] [CrossRef]
- Keesstra, S.; Nunes, J.; Novara, A.; Finger, D.; Avelar, D.; Kalantari, Z.; Cerdà, A. The superior effect of nature-based solutions in land management for enhancing ecosystem services. Sci. Total Environ. 2018, 610, 997–1009. [Google Scholar] [CrossRef]
- Reineke, W.; Schlömann, M. Biotechnology and Environmental Protection. In Environmental Microbiology. Springer, Berlin. pp. 551–587. 2023. [Google Scholar]
- Nathanail, C.P.; Bakker, L.M.; Bardos, P.; Furukawa, Y.; Nardella, A.; Smith, G.; Smith, J.W.N.; Goetsche, G. Towards an international standard: The ISO/DIS 18504 standard on sustainable remediation. Remediation J. 2017, 28, 9–15. [Google Scholar] [CrossRef]
- Harclerode, M.; Ridsdale, D.R.; Darmendrail, D.; Bardos, P.; Alexandrescu, F.; Nathanail, P.; Pizzol, L.; Rizzo, E. Integrating the social dimension in remediation decision-making: State of the practice and way forward. Remediation J. 2015, 26, 11–42. [Google Scholar] [CrossRef]
- Scullion, J. Remediating polluted soils. Naturwiss 2006, 93, 51–65. [Google Scholar] [CrossRef]
- Ghahari, S.; Ghahari, S.; Ghahari, S.; Nematzadeh, G.; Sarma, H. Integrated remediation approaches for selected pharmaceutical and personal care products in urban soils for a sustainable future. Energ. Ecol. Environ. 2021, 7, 439–452. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Hassani, A.; Vaccari, M.; Franzetti, A.; Prasad, S.; Formicola, F.; Rosatelli, A.; Rehman, M.Z.U.; Mohanakrishna, G.; Ganachari, S.V.; Aminabhavi, T.M.; Rtimi, S. Emerging technologies for the removal of pesticides from contaminated soils and their reuse in agriculture. Chemosphere 2024, 362, e142433. [Google Scholar] [CrossRef]
| Therapeutic class | Compound | Structure | Log KOW | pKa | Solubility (H2O) (mg L-1) |
|---|---|---|---|---|---|
| Analgesics and, anti-inflammatories | Acetylsalicylic acid | 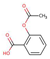 |
1.2 | 3.5 | 4600 |
| Dicoflenac | 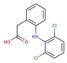 |
4.5 | 4.1 | 2.4 | |
| Ibuprofen | 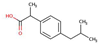 |
4.0 | 4.9 | 21 | |
| Naproxen | 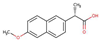 |
3.2 | 4.1 | 16 | |
| Antibiotics | AzithromycinMC | 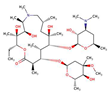 |
4.0 | 8.7 | 7.1 |
| ClarithromycinMC | 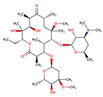 |
3.2 | 9.0 | 0.3 | |
| ErythromycinMC | 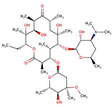 |
3.1 | 8.9 | 1.4 | |
| AmoxicillinPN | 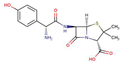 |
0.9 | 4.4 | 3430 | |
| SulfamethoxazoleSF | 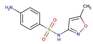 |
0.9 | 3.8 | 610 | |
| CiprofloxacinQN | 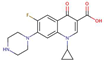 |
0.3 | 6.1 | 3x104 | |
| OxytetracyclineTC | 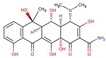 |
-0.9 | 3.3 | 313 | |
| Antiepileptics | Carbamazepine | 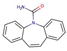 |
2.5 | 2.3 | 17.7 |
| Antimicrobials | Triclosan | 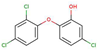 |
4.8 | 8.7 | 1.0 |
| Antineoplastic agents | Cyclophosphamide | 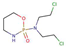 |
0.63 | 7.6 | 4.0x104 |
| β-blockers | Atenolol | 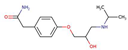 |
0.16 | 9.4 | 1.3x104 |
| Propanolol | 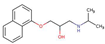 |
3.5 | 9.4 | 23 | |
| Hormones | 17α-ethinylestradiol | 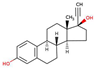 |
3.7 | 12.2 | 4.3x10-5 |
| Progesterone | 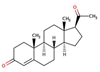 |
3.9 | 9.8 | 8.8 | |
| Testosterone | 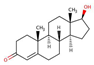 |
3.3 | 9.7 | 23 | |
| Illicit drugs | Benzoylecgonine (cocaine metabolite) | 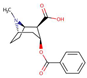 |
1.6 | 4.4 | 3820 |
| Tetrahydrocannabinol (THC-COOH) (cannabinoid) | 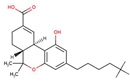 |
4.2 | 4.2 | Very low | |
| Lipid regulators | Lovastatin | 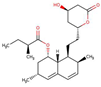 |
4.3 | - | 2.1 |
| Clofibrate | 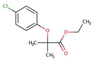 |
3.6 | - | Very low | |
| SSRIs | Fluoxetine | 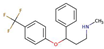 |
4.0 | - | 3.5 |
| Paroxetine | 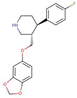 |
3.9 | - | 8.5 |
| Parameter | Values | Comments |
|---|---|---|
| Water solubility (mg L-1) | < 50 | Low |
| 50-500 | Moderate | |
| > 500 | High | |
| Hydrolysis (TD50, días) | < 30 | Nonpersistent |
| 30-100 | Moderately persistent | |
| 100-365 | Persistent | |
| > 365 | Very persistent | |
| Vapor pressure (PV, mPa) | < 5 | Low volatility |
| 5-10 | Moderately volatile | |
| < 10 | Highly volatile | |
| Henry’s Law Constant (H, Pa m3 mol-1) | > 102 | Volatile |
| 10-1 - 102 | Moderately volatile | |
| < 10-1 | Nonvolatile | |
| Octanol-water partition coefficient (log KOW) | < 2.7 | Low bioaccumulation |
| 2.7-3.0 | Moderate bioaccumulation | |
| > 3 | High bioaccumulation | |
| Organic carbon partition coefficient (log KOC, ml g-1) | < 1.2 | Very mobile |
| 1.2-1.9 | Mobile | |
| 1.9-2.7 | Moderately mobile | |
| 2.7-3.6 | Slightly mobile | |
| > 3.6 | Nonmobile | |
| Dissociation constant (pKa) | pH < pKa | Neutral state |
| pH > pKa | Negative charge | |
| GUS Index | > 2.8 | High leachability |
| 2.8-1.8 | Transition state | |
| < 1.8 | Low leachability |
| Model | Equation | Parameters | Endpoints |
|---|---|---|---|
| SFO | 2 (C0, k) | ||
| FOMC | 3 (C0, α, β) | ||
| DFOP | 4 (C1, C2, k1, k2) | DTx values can only be calculated via an iterative procedure | |
| FOSB |
when t ≤ tb when t > tb |
4 (C0, k1, k2, tb) |
when DTx ≤ tb when DTx > tb |
| H | 3 (C0, k, a) | DTx values can only be calculated via an iterative procedure |
| Treatment | Technique | Application | |
|---|---|---|---|
| Removal | Physicochemical | Aeration | In situ |
| Whasing | Ex situ | ||
| Dragging | In situ | ||
| Adsorption | In situ | ||
| (Photo)chemical oxidation | In situ | ||
| Electrokinetic treatment | In situ | ||
| Biological | Bio-augmentation and bio-stimulation | In situ | |
| Biopiles | Ex situ | ||
| Composting | Ex situ | ||
| Phytoremediation | In situ | ||
| Landfarming | Ex situ | ||
| Bioventing | In situ | ||
| Natural attenuation | In situ | ||
| Enzymatic degradation | In situ | ||
| Biosorption | In situ | ||
| Thermal | Incineration | Ex situ | |
| Thermal desorption | Ex situ | ||
| Plasma | In situ | ||
| Solar | Solarization | In situ | |
| Biosolarization | In situ | ||
| Containment and confinement | Barriers | In situ | |
| Deep sealing | In situ | ||
| Solidifying injection | In situ | ||
| Vitrification | In situ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





