1. Introduction
Sarcopenia, characterized by the progressive loss of muscle mass and strength, significantly impacts the quality of life in older adults [
1]. The associated decline in muscle force production capacity is a crucial factor in the functional deterioration and onset of frailty in this population [
2]. These neuromuscular alterations are strong predictors of adverse outcomes such as falls [
3], hospitalizations [
4], loss of independence [
5], institutionalization, and ultimately, a reduction in life expectancy [
6]. Consequently, it is essential to optimize interventions, particularly targeting the neuromuscular system, to reduce dependency and prevent falls in older adults [
7].
Several studies have highlighted a significant correlation between decreased ankle plantar flexor (PF) force production capacities and functional abilities [
8,
9]. For instance, Cattagni et al. [
10] observed that within a group of 30 older adults, maximal torque of the PF was negatively correlated with the displacement of the center of pressure during static postural balance (r = 0.77). Furthermore, they demonstrated that 90% of individuals with maximal PF torque less than 3.1 Nm/kg had previously experienced falls, whereas 85% of those exceeding this value had not. Therefore, maximal PF force production capacity could be considered a predictive parameter for fall risk [
11]. However, the ability to avoid a fall depends not only on maximal force production capacity but also on motor response time, which reflects the speed to generate submaximal force, also known as the rate of force development (RFD) [
12,
13].
RFD, defined as the increase in force per unit of time, is calculated from the slope of the force-time curve (Δ force / Δ time) [
12,
13,
14]. RFD evaluations typically consider different phases of muscle contraction, including the early phase (0-50 ms) and the late phase (100-200 ms) [
13]. The early phase is largely related to the initiation of motor unit activation and their firing sequences, as well as intrinsic muscle characteristics, such as fiber composition and calcium dynamics [
15]. In contrast, the late phase relies on the ability to transmit the force produced by the contractile component through the parallel and series elastic components [
16]. Several studies consider RFD as a crucial predictor of functional capacities in older adults [
12,
17]. For example, Hester et al. [
12] demonstrated that RFD was the sole predictor of Timed Up and Go test performance in older adults [
18,
19,
20]. Improving RFD could therefore be a key objective in rehabilitation and training programs aimed at enhancing walking ability and reducing fall risks in older adults [
8].
Resistance training has been shown to improve muscle force production capacity in older adults [
21]. For instance, Walker et al. [
22] demonstrated improvements in maximal strength and quadriceps mass when exercises were performed at slow and controlled speeds. These results justify the feasibility [
23,
24] and necessity of integrating resistance exercises for older adults [
25,
26]. However, most interventions have advocated for slow-speed resistance exercises, consisting of 1 to 4 sets of about 8 to 15 repetitions at moderate to high loads, performed at least twice a week, in line with previous international recommendations [
27]. Recent studies and meta-analyses indicate that resistance training, including explosive training elements, is more effective for enhancing functional abilities compared to slow-speed resistance training [
28,
29]. High-speed resistance training involves performing the concentric muscle contraction of each exercise repetition as quickly as possible, demonstrating notable improvements in morphological and neural adaptations as well as functional performance capacities [
30]. For example, Cadore et al. [
23] showed significant improvements in muscle power production, strength, muscle cross-sectional area, and dual-task performance in frail institutionalized older adults after 12 weeks of high-speed resistance training. While the benefits of high-speed resistance training in developing muscle strength are generally recognized, no study, to our knowledge, has yet thoroughly examined the specific effects of explosive resistance training on improving both early and late RFD of PF in institutionalized older adults. These investigations could potentially provide crucial insights into the underlying mechanisms of muscle explosivity enhancement in this specific population and, by extension, the influence of these adaptations on walking speed, an essential parameter of physical function.
The objectives of our study were to: i) Compare the effects of high-velocity resistance training with slow-velocity training on neuromuscular and functional parameters, ii) Investigate the relationship between neuromuscular parameters of the PF and walking speed before training, iii) Analyze the relationship between changes in walking speed and changes in neuromuscular parameters after training. We hypothesize that high-velocity resistance training results in greater improvements in neuromuscular and functional parameters compared to slow-velocity resistance training. Additionally, we hypothesize that there is a significant correlation between improvements in RFD of the PF and increase in walking speed following the training period.
2. Materials and Methods
2.1. Study Design
This study was a prospective, controlled, single-blinded, randomized trial, with participants randomly assigned to either a high-speed resistance training group (GHS) or a low-speed resistance training group (GLS). Both groups underwent the same assessments before and after the intervention (3 sessions per week during 12 weeks) and performed the same resistance exercises. The distinction between the two groups lay in the execution speed: the GHS performed the exercises at high speed, while the GLS performed the exercises at low speed. This study adhered to the principles outlined in the Helsinki Declaration. Approval for the study protocol, patient information letter, and informed consent form was obtained from the local ethics committee of the Intercommunal Health Center of Sarthe et Loir.
2.2. Recruitment
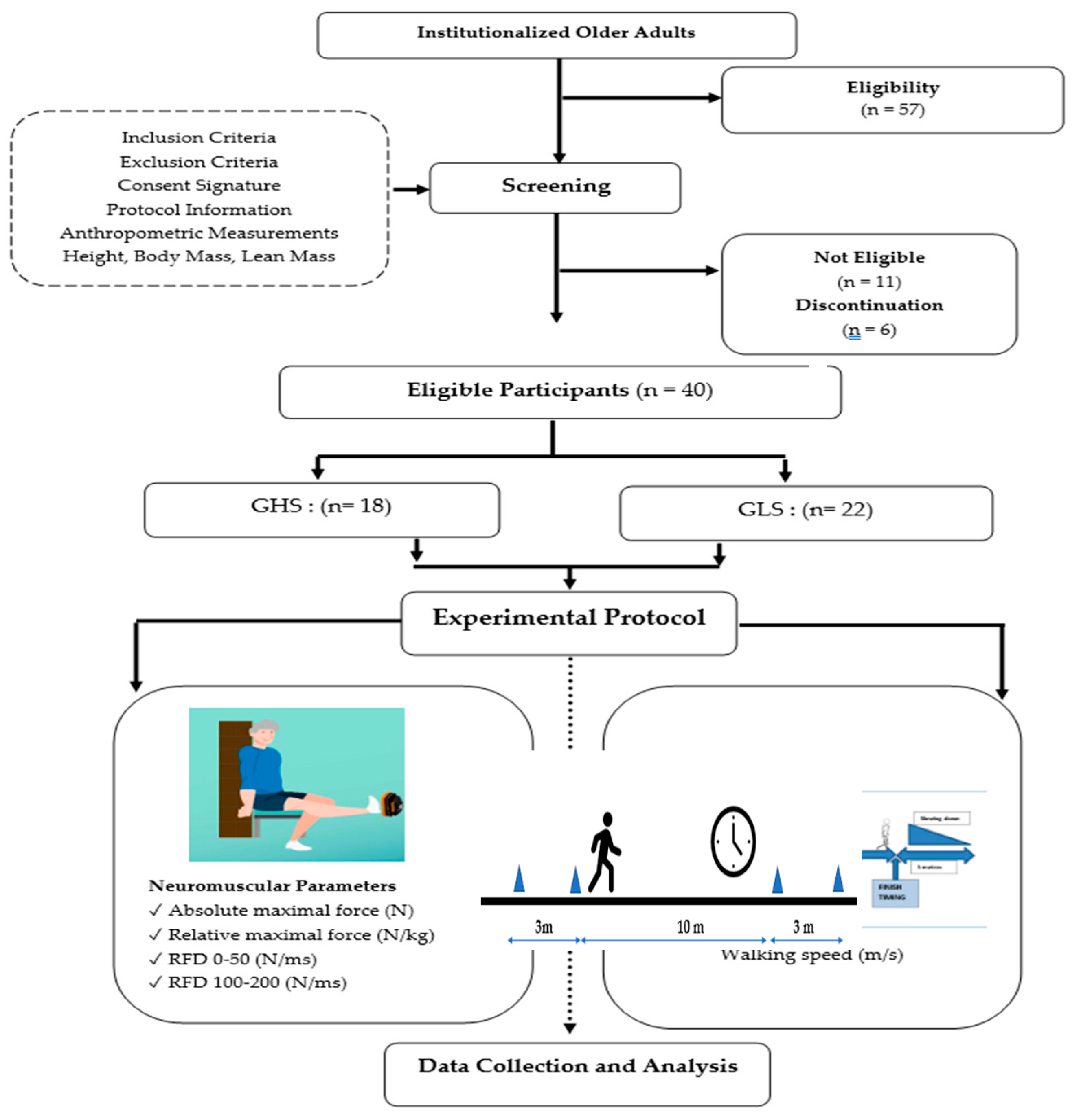
The recruitment process consisted of a three-week recruitment period, followed by a one-week screening phase (
Figure 1). Participants were recruited from a retirement home located in Sablé-sur-Sarthe (France) between January 15 and February 15, 2024. To be eligible, individuals had to be older than 65 years or, capable of walking without technical assistance (e.g., canes, walkers) from another person, and able to communicate verbally effectively with the research team. Individuals with neurological or cognitive disorders, severe cardiovascular diseases, significant musculoskeletal issues in the lower limbs, or those taking medications that could affect the tests were excluded. The eligibility of each participant was meticulously verified by the medical staff. Then, participants were randomized into one of two groups: the GHS and GLS. The randomization list was generated using a computer algorithm by an independent statistician. At the end of the screening process, the investigator, who was blinded to the treatment assignment, obtained a unique randomization number for each participant.
2.3. Evaluation Protocol
All assessments were conducted in a clinical examination room under consistent environmental conditions, supervised by a blinded evaluator who was unaware of the group affiliation of the participants. Prior to the assessments, participants received a standardized set of verbal instructions to ensure familiarity with the procedures.
2.3.1. Anthropometric Parameters
Body mass (BM) and height (H) of the participants were precisely measured using a digital floor scale and a wall-mounted stadiometer, respectively. Body mass index (BMI, in kg/m²) was subsequently calculated. Lean body mass (LBM) was measured using bioelectrical impedance analysis (Tanita; SC 24, Amsterdam, The Netherlands) [
31].
2.3.2. Gait Speed
Gait speed (m/s) was assessed over a 20-meter flat surface, with measurements taken between the 5th and 15th meters to exclude acceleration and deceleration phases [
32]. Participants were instructed to walk at their usual pace and attempt to reach their maximum speed between the 5th and 15th meters. A stopwatch was used to measure the time required to walk the 10-meter distance. The average walking speed (m/s) was then calculated using the following formula:
2.3.3. Neuromuscular Parameters
Neuromuscular parameters of PF of the dominant leg were assessed during maximal voluntary contractions (MVC) using a dynamometer (K-Force, Kinvent, Montpellier, France). Participants were seated on a chair, ensuring contact between their back, buttocks, and thighs with the chair while keeping their leg horizontally extended. They were instructed to push with the ends of their foot against the dynamometer [
31]. Two trials were conducted with a one-minute rest interval between them, and the maximum force of the PF (Fmax, N) from both trials was recorded. The relative maximum force (Fmax relative, N/kg) was calculated by normalizing the maximum force to the participant's body mass. The early RFD was calculated from the onset of each MVC to 50 ms (RFD 0 – 50), and the late RFD was calculated between 100 and 200 ms (RFD100 – 200). Both early and late RFD were derived from the linear slope of the force-time curve (Δ force / Δ time).
2.4. Resistance Training Programs
The training protocol consisted of 36 sessions over 12 weeks, with a frequency of 3 sessions per week for both groups. Detailed instructions for each group are as follows (
Table 1):
The higher repetition range in the GHS was implemented to equalize work volume between groups and align with established resistance exercise guidelines [
25]. The primary distinctions between protocols were the percentage of 1RM (40% vs. 80%) and the velocity of the concentric movement phase. Each session concluded with a standardized 5-minute cool-down to facilitate recovery and minimize post-exercise muscle fatigue [
33]. This cool-down included light aerobic activity such as walking or cycling at a low intensity, followed by static stretching.
2.5. Statistical Analysis
The sample size was determined using the freeware G*Power (version 3.1.9.4) as outlined in the study by Ferhi et al. [
32]. For the power analysis, an ANOVA test was preselected, with the parameters set to control for a Type I error (alpha = 0.05) and a Type II error (beta = 0.60). Assuming a moderate estimated effect size (r = 0.35), the calculation indicated that a minimum of 40 participants would be required.
Statistical analysis was performed using Statistica Software 13.0 (StatSoft, Tulsa, OK, USA). The normality of the data sets and the homogeneity of variances were assessed using the Shapiro-Wilk and Levene's tests, respectively. The effects of time (pre- and post-training) and group (GHS and GLS) on neuromuscular and functional parameters, as well as their interaction, were tested using a two-factor ANOVA (group × time). Subsequently, a post-hoc analysis was conducted to determine significant inter- and intra-group effects. Finally, Pearson correlation analysis (r) was performed to examine the relationship between walking speed and the neuromuscular parameters of the PF before and after interventions. Results at baseline and after the intervention were presented as mean ± standard deviation. The significance level was set at p<0.05.
3. Results
3.1. Participants
A total of 57 volunteers were initially recruited for this study (
Figure 1). However, only 46 participants met our established eligibility criteria and were randomly assigned to either the GHS (n = 23) or the GLS (n = 23). Six individuals did not complete the study due to non-adherence to the protocol—five from the GHS group and one from the GLS group. The reasons for discontinuation included hospitalizations related to stroke, hip fracture, and ankle sprain for four participants, and personal reasons for two participants. Ultimately, a cohort of 40 participants successfully completed the study in its entirety (
Table 1), comprising the GHS (n = 18; age = 80.41 ± 10.12 years; BMI = 23.81 ± 3.45kg/m²) and the GLS (n = 22; age = 82.89 ± 5.32 years; BMI = 23.81 ± 3.45 kg/m²).
3.2. Anthropometric Parameters
No significant inter- or intra-group differences were observed between the GLS and GHS groups, both before and after training, across all anthropometric parameters presented in
Table 2.
3.3. Walking Speed
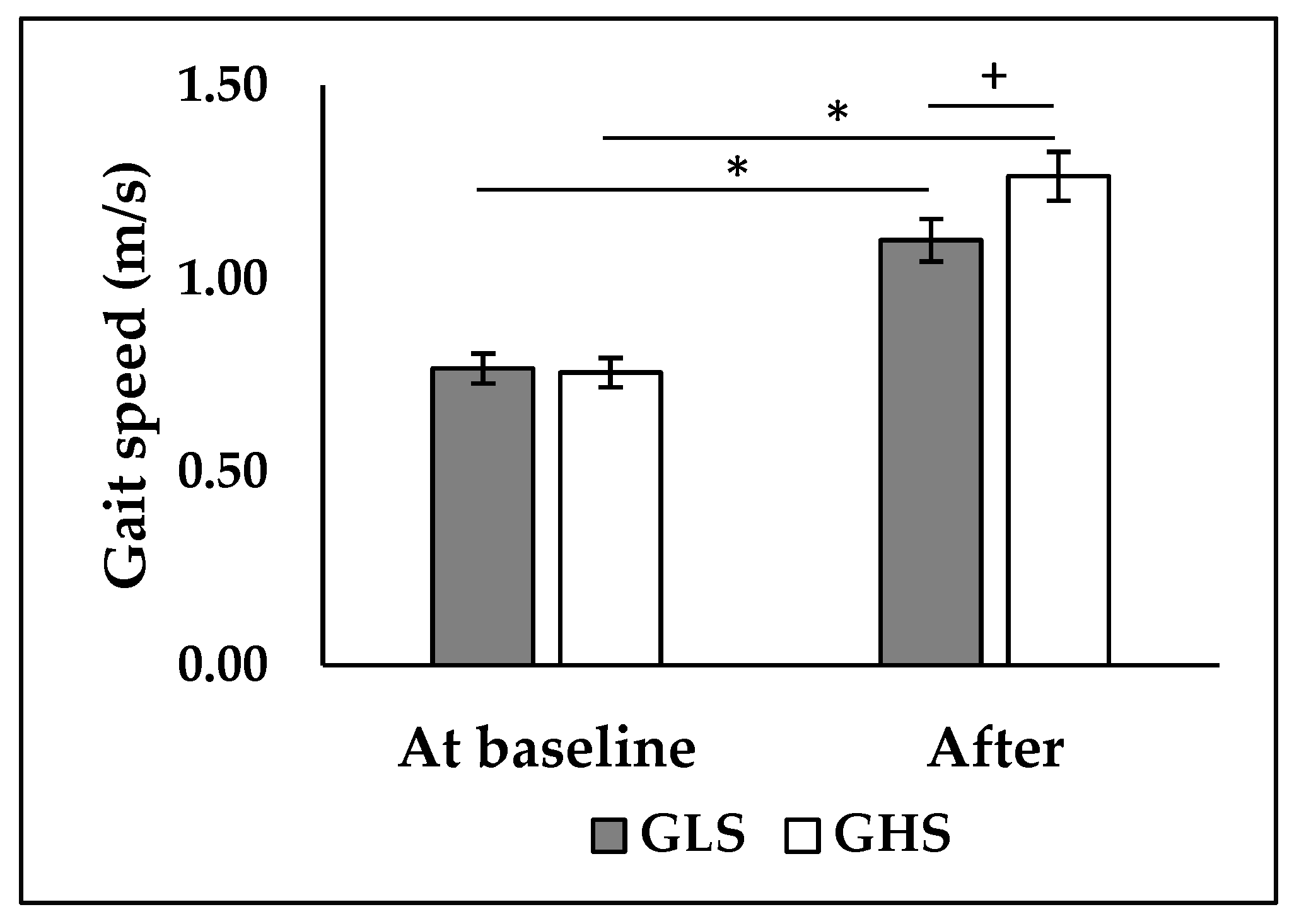
ANOVA analysis revealed a significant interaction between the effect of time and group on walking speed (p < 0.001; F = 11.6). Post-hoc analysis showed that there was no significant difference between the two groups before the intervention (
Figure 2). After the intervention, both the GHS and GLS showed a significant increase in walking speed (+63.2%, p < 0.001; +45.6%, p < 0.001, respectively). However, walking speed was higher in the GHS compared to the GLS (p < 0.05,
Figure 2).
3.4. Neuromuscular Parameters
ANOVA analysis revealed a significant interaction between the effects of time and group (
Table 3) on both absolute (p < 0.001; F = 1.77) and relative Fmax (p < 0.001; F = 14.6). Post-hoc analysis revealed a significant increase in both absolute and relative Fmax in the GHS (+20.5%, +21.1%, p < 0.001, respectively) and in the GLS (+16.2%, +17.1%; p < 0.001, respectively). The improvement in both absolute and relative Fmax was greater in GHS (p < 0.05). Additionally, Post-hoc analysis showed a significant increase in RFD 0-50 of 10% in the GLS (p < 0.05) and 20.1% (p < 0.05) in the GHS. The improvement in RFD 0-50 was greater in the GHS (p < 0.05). Moreover, RFD 100-200 improved only in the GHS (+20.4%, p < 0.05).
3.5. Relationship between Neuromuscular Parameters of the PF and Walking Speed
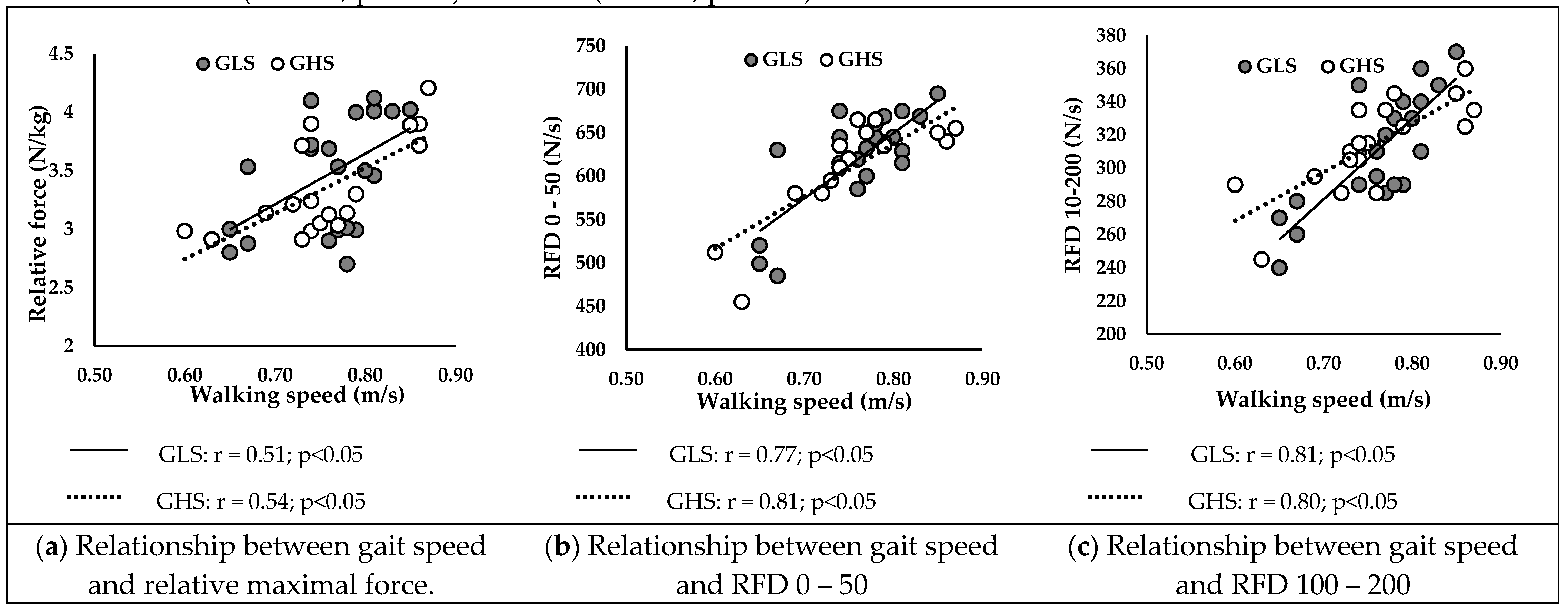
3.5.1. At Baseline
Figure 2 presents correlations between walking speed and neuromuscular parameters at baseline in GLS and GHS. Walking speed was positively correlated with relative Fmax (
Figure 2A) of GLS (r = 0.51; p < 0.05) and GHS (r = 0.54; p < 0.05).
Additionally, RFD 0-50 (
Figure 2B) and RFD 100-200 (
Figure 2C) were positively correlated with walking speed in GLS (r = 0.77; r = 0.81; p < 0.05, respectively) and in GHS (r = 0.81; r = 0.80; p < 0.05, respectively).
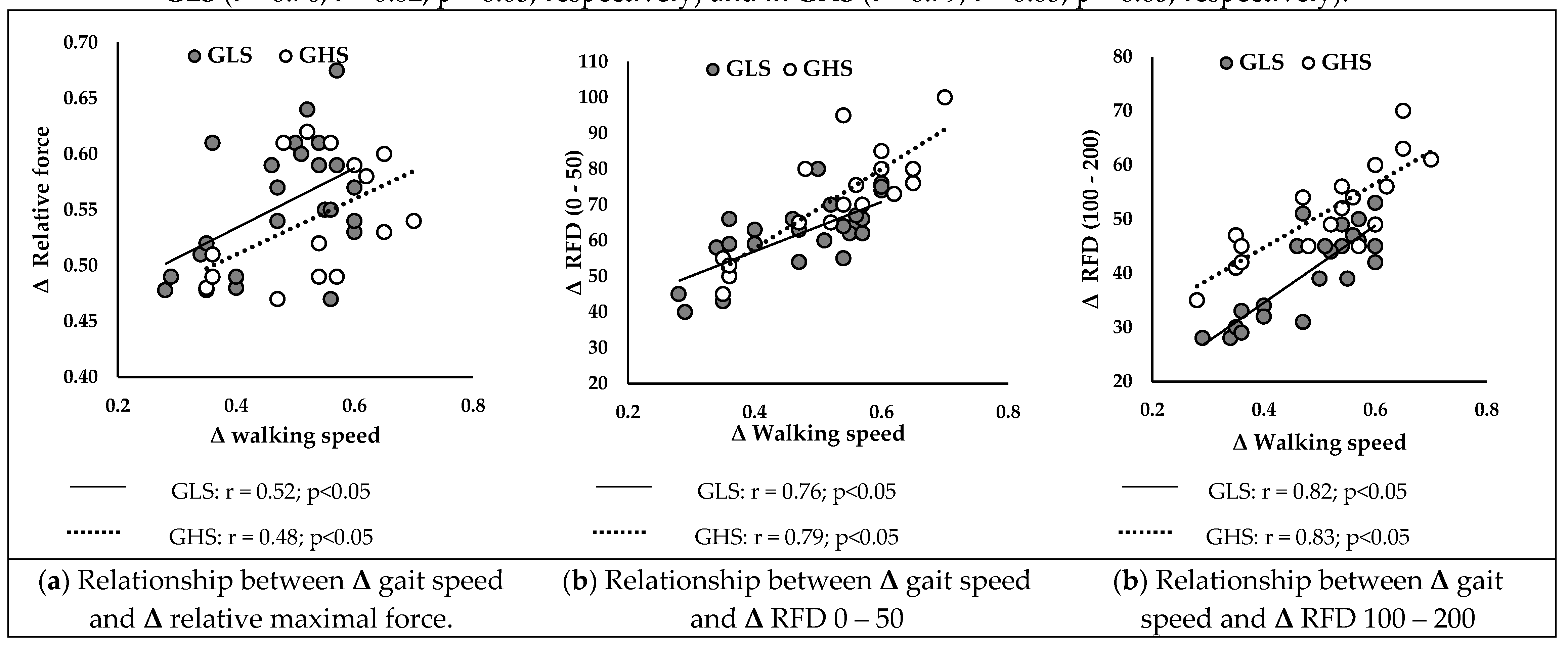
3.5.2. After the Intervention
Figure 3 presents correlations between ameliorations in walking speed and improvements in neuromuscular parameters in GLS and GHS.
Δ walking speed was positively correlated with
Δ relative Fmax (
Figure 1B) of GLS (r = 0.52; p < 0.05) and GHS (r = 0.48; p < 0.05). Additionally,
Δ RFD 0-50 (
Figure 2B) and
Δ RFD 100-200 (
Figure 2C) were positively correlated with
Δ walking speed in GLS (r = 0.76; r = 0.82; p < 0.05, respectively) and in GHS (r = 0.79; r = 0.83; p < 0.05, respectively).
4. Discussion
This study aimed to evaluate the effects of high-velocity versus low-velocity resistance training on the neuromuscular parameters of the PF, as well as to examine the relationships between improvements in these parameters and walking speed in institutionalized older adults. Our results revealed that both types of resistance training improved the neuromuscular capacities of the PF, but with different and specific adaptations. Low-velocity resistance training led to a more significant improvement in Fmax, while high-velocity training favored improvements in RFD. Furthermore, regardless of the type of training, improvement in walking speed were more strongly correlated RFD in both intervention groups.
The results of this study revealed that resistance training programs, regardless of contraction velocity, improved Fmax of PF. These findings are consistent with several studies highlighting possible neuromuscular adaptations related to resistance exercises, allowing for significant improvements after 12 weeks of muscle resistance training [
23] [
34,
35]. Specifically, our results showed a 21% improvement in relative Fmax in GLS and 16% in the GHS. These results align with the systematic review of Lopez et al. [
36] showing significant improvements in maximum knee extensor force ranged from 6.6% to 37.0%, after 12 weeks of resistance training in older adults. Several hypotheses can be proposed to explain the mechanisms of these neuromuscular adaptations to resistance training in older adults. Firstly, protein synthesis metabolism through the efficient transport of amino acids and growth factors (e.g., insulin-like growth factor-1 [IGF-1], hepatocyte growth factor [HGF], interleukin-6 [IL-6], and myostatin) to muscle fibers [
37]. These elements play a crucial role in regulating satellite cells, thereby supporting the repair and/or remodeling of neuromuscular adaptations, which is particularly essential after the age of 65 [
38]. Additionally, an increase in the cross-sectional area of the muscle, and consequently muscle mass, may also promote increased muscle force. Kryger et al. [
35] demonstrated a significant 22% increase in the number of type IIa muscle fibers of the knee extensor muscles in older adults, thus promoting muscle hypertrophy associated with improved Fmax. However, the results of this study did not reveal an increase in lean mass in the two groups after the intervention. It is possible that these subjects did not significantly improve muscle mass, but rather muscle quality through morphological adaptations, such as a decrease in fat infiltration [
23]. It is also possible that improvement in Fmax is related to specific neural adaptations, such as increased recruitment of fast-fiber motor units and improved nerve transmission [
39,
40,
41]. All these findings suggest that neuromuscular capacities in older adults can be effectively reversed through targeted adaptations induced by muscle resistance training.
The originality of the present study lies in its exploration of specific adaptations associated with the execution velocity of resistance exercises in older adults. Our results show that low-velocity resistance training led to a more significant improvement in Fmax (+8% in GLS), while high-velocity training favored improvements in early RFD (+9% in GHS) and late (+8% in GHS). Indeed, high-velocity exercise imposes high demands on the nervous system, resulting in significant improvements in neuromuscular function in older adults [
42,
43,
44]. In this context, several studies have emphasized that high-velocity training could partially reduce muscle activation deficit in older adults [
45,
46] with a superior effect on muscle power compared to low-velocity training [
47]. Furthermore, early RFD is primarily influenced by neural factors, such as the maximum discharge frequency of motor neurons [
15,
16]. The late RFD is predominantly influenced by the structural properties of the muscle-tendon complex and the effectiveness of force transmission through both parallel (e.g., cellular matrix) and series elastic elements (e.g., tendons) [
39,
48]. Consequently, we propose that the observed improvement in early RFD can be attributed to increased motor unit discharge rate and recruitment, reduced cortical inhibition, and enhanced nerve conduction velocity [
23,
49,
50,
51]. The improvement in late RFD could be linked to enhanced capacity of the muscle-tendon complex to transmit force through elastic elements and potential structural adaptations in the pennation angle and fiber number of the muscle [
52].
Our results revealed a significant improvement in walking speed for both GHS (+63.2%) and GLS (+45.6%), consistent with several studies showing similar effects of muscle resistance training on habitual and maximum walking speed [
36,
53]. These improvements, ranging from 5.5% to 20.4% and observed after short-term interventions (10-12 weeks), have been attributed to enhanced neuromuscular capacities of lower limb muscles [
23,
54]. However, in our study, the GHS showed a significantly superior improvement of 12% in walking speed compared to the GLS. Moreover, the improvement in walking speed was more strongly correlated with the RFD than with the improvement in Fmax (
Figure 3). Indeed, during walking, the time to develop force is limited, typically less than 300 ms. Superior gains in RFD, both in the early (0 – 50 ms) and late (100 – 200 ms) phases, allow for a rapid and efficient response, essential for maintaining balance and stability during walking [
39,
55]. Additionally, improved RFD enhances propulsion and braking during walking, crucial for speed and movement efficiency [
56]. These results suggest that improvements in RFD play a more decisive role in enhancing walking propulsion capacity than increases in Fmax. This underscores the importance of targeting these parameters in rehabilitation and training programs to optimize activities of daily living in older adults [
57,
58]. Moreover, an increased ability to rapidly develop force allows for better responses to situations of imbalance, such as during stumbling, and helps maintain equilibrium and stability while walking [
59]. Therefore, rehabilitation programs should include high-velocity resistance exercises to maximize neuromuscular and functional benefits in older adults.
Limitations and future perspectives
This study acknowledges several limitations that warrant consideration. Firstly, the small sample size and the inherent heterogeneity often observed in older adults may limit the generalizability of our conclusions. Moreover, our study primarily focused on the PF, . It would be important to investigate other muscle groups, especially those around the knee, which are also crucial for walking. An analysis of neuromuscular activities through electromyography would also be necessary to understand the underlying mechanisms of neural adaptations resulting from the two types of interventions.
5. Conclusions
The execution velocity of muscle resistance exercises induces different neuromuscular adaptations in older adults. High-velocity resistance training appears particularly effective in improving the ability to rapidly generate force, which is essential for many daily activities requiring explosive movements and quick responses. These results underscore the importance of including high-velocity resistance exercises, supported by regular assessments and adjustments based on individual progress, to maximize neuromuscular and functional benefits in older adults.
Author Contributions
Conceptualization, E.M. and W.M.; methodology, E.M.; software, J.J.; validation, P.P., A.R. and Y.C.; formal analysis, O.GC.; investigation, E.M.; resources, J.J.; data curation, O.GC.; writing—original draft preparation, E.M.; writing—review and editing, W.M.; visualization, P.P.; supervision, W.M.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the Intercommunal Health Center of Sarthe et Loir (protocol code 012, January 5, 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper” if applicable.
Data Availability Statement
The research was registered in the Pan African Clinical Trials Registry under the registration number PACTR202306912191110.
Acknowledgments
We gratefully acknowledge the medical staff and administration of the intercommunal Health Center of Sarthe et Loir for their invaluable support and assistance during the course of this study. We also extend our heartfelt thanks to the patients who generously participated in this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yuan, S., Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. 2023, Jul;144:155533. [CrossRef]
- Dufour, A. B., Hannan, M. T., Murabito, J. M., Kiel, D. P., & McLean, R. R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The framingham study. J Gerontol A Biol Sci and Med Sci, 2023, 68(2), 168–174. [CrossRef]
- Landi, F., Liperoti, R., Russo, A., Giovannini, S., Tosato, M., Capoluongo, E., Bernabei, R., & Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin Nutr, 2012, 31(5), 652–658. [CrossRef]
- Cawthon, P. M.; Fox, K. M.; Gandra, S. R.; Delmonico, M. J.; Chiou, C. F.; Anthony, M. S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S. R.; Harris, T. B. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc, 2009, 57, 1411–1419. [CrossRef]
- Janssen, I.; Heymsfield, S. B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc, 2002, 50, 889–896. [CrossRef]
- Cruz-Jentoft, A. J.; Baeyens, J. P.; Bauer, J. M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F. C.; Michel, J. P.; Rolland, Y.; Schneider, S. M.; Topinková, E.; Vandewoude, M.; Zamboni, M. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [CrossRef]
- Coletti, C.; Acosta, G. F.; Keslacy, S.; Coletti, D. Exercise-mediated reinnervation of skeletal muscle in elderly people: An update. Eur. J. Transl. Myol. 2022, 32, 10416. [CrossRef]
- Bellumori, M.; Jaric, S.; Knight, C. A. Age-related decline in the rate of force development scaling factor. Motor Control, 2013, 17, 370–381. [CrossRef]
- Thompson, B. J.; Ryan, E. D.; Herda, T. J.; Costa, P. B.; Herda, A. A.; Cramer, J. T. Age-related changes in the rate of muscle activation and rapid force characteristics. Age 2014, 36, 839–849. [CrossRef]
- Cattagni, T.; Scaglioni, G.; Laroche, D.; Van Hoecke, J.; Gremeaux, V.; Martin, A. Ankle muscle strength discriminates fallers from non-fallers. Front Aging Neurosci, 2014, 6, 336. [CrossRef]
- Bohrer, R. C. D.; Pereira, G.; Beck, J. K.; Lodovico, A.; Rodacki, A. L. F. Multicomponent Training Program with High-Speed Movement Execution of Ankle Muscles Reduces Risk of Falls in Older Adults. Rejuvenation Res. 2019, 22, 43–50. [CrossRef]
- Hester, G. M.; Ha, P. L.; Dalton, B. E.; Vandusseldorp, T. A.; Olmos, A. A.; Stratton, M. T.; Bailly, A. R.; Vroman, T. M. Rate of Force Development as a Predictor of Mobility in Community-dwelling Older Adults. J Geriatr Phys Ther, 2021, 44, 74–81. [CrossRef]
- Olmos, A. A.; Stratton, M. T.; Ha, P. L.; Dalton, B. E.; VanDusseldorp, T. A.; Mangine, G. T.; Feito, Y.; Poisal, M. J.; Jones, J. A.; Smith, T. M.; Hester, G. M. Early and late rapid torque characteristics and select physiological correlates in middle-aged and older males. PLoS ONE 2020, 15. [CrossRef]
- Gerstner, G. R.; Thompson, B. J.; Rosenberg, J. G.; Sobolewski, E. J.; Scharville, M. J.; Ryan, E. D. Neural and Muscular Contributions to the Age-Related Reductions in Rapid Strength. Med Sci Sports Exerc, 2017, 49, 1331–1339. [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J, Appl, Physiol, 2008, 104, 739–746. [CrossRef]
- Andersen, L. L.; Aagaard, P. Influence of maximal muscle strength and intrinsic muscle contractile properties on contractile rate of force development. Eur, J, Appl, Physiol, 2006, 96, 46–52. [CrossRef]
- Clark, D. J.; Manini, T. M.; Fielding, R. A.; Patten, C.; Manuscript, A. Neuromuscular determinants of maximum walking speed in well-functioning older adults, Exp, Gerontol, 2013, 48, 358–363. [CrossRef]
- Ko, M.; Hughes, L.; Lewis, H. Walking speed and peak plantar pressure distribution during barefoot walking in persons with diabetes. Physiother, Res, Int, 2012, 17, 29–35. [CrossRef]
- Holmes, S. J.; Mudge, A. J.; Wojciechowski, E. A.; Axt, M. W.; Burns, J. Impact of multilevel joint contractures of the hips, knees and ankles on the Gait Profile score in children with cerebral palsy. Clin Biomech, 2018. [CrossRef]
- Tavoian, D.; Clark, B. C.; Clark, L. A.; Wages, N. P.; Russ, D. W. Comparison of strategies for assessment of rate of torque development in older and younger adults. Eur J Appl Physiol, 2023, 0123456789. [CrossRef]
- Boyd Foster-Burns, S. Sarcopenia and decreased muscle strength in the elderly woman: Resistance training as a safe and effective intervention. J Women Aging 1999, 11, 75–85. [CrossRef]
- Walker, S.; Peltonen, H.; Häkkinen, K. Medium-intensity, high-volume “hypertrophic” resistance training did not induce improvements in rapid force production in healthy older men. Age 2015, 37. [CrossRef]
- Cadore, E. L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr.) 2014, 36, 773–785. [CrossRef]
- Cadore, E. L.; Moneo, A. B. B.; Mensat, M. M.; Muñoz, A. R.; Casas-Herrero, A.; Rodriguez-Mañas, L.; Izquierdo, M. Positive effects of resistance training in frail elderly patients with dementia after long-term physical restraint. Age, 2014, 36, 801–811. [CrossRef]
- Cadore, E. L.; Pinto, R. S.; Reischak-Oliveira, Á.; Izquierdo, M. Explosive type of contractions should not be avoided during resistance training in elderly. Exp. Gerontol. 2018, 102, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M. S.; Cadore, E. L.; Dorgo, S.; Izquierdo, M.; Kraemer, W. J.; Peterson, M. D.; Ryan, E. D. Resistance training for older adults: Position statement from the national strength and conditioning association. J. Strength Cond. Res. 2019, 33, 2019–2052. [CrossRef]
- Garber, C. E.; Blissmer, B.; Deschenes, M. R.; Franklin, B. A.; Lamonte, M. J.; Lee, I. M.; Nieman, D. C.; Swain, D. P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Campillo, R.; Castillo, A.; de la Fuente, C. I.; Campos-Jara, C.; Andrade, D. C.; Álvarez, C.; Martínez, C.; Castro-Sepúlveda, M.; Pereira, A.; Marques, M. C.; Izquierdo, M. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp. Gerontol. 2014, 58, 51–57. [Google Scholar] [CrossRef]
- Tschopp, M.; Sattelmayer, M. K.; Hilfiker, R. Is power training or conventional resistance training better for function in elderly persons? A meta-analysis. Age Ageing 2011, 40, 549–556. [Google Scholar] [CrossRef]
- Straight, C. R.; Lindheimer, J. B.; Brady, A. O.; Dishman, R. K.; Evans, E. M. Effects of Resistance Training on Lower-Extremity Muscle Power in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2016, 46, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Magtouf, E.; Chortane, S. G.; Chortane, O. G.; Boyas, S.; Beaune, B.; Durand, S.; Maktouf, W. Influence of Concurrent Exercise Training on Ankle Muscle Activation during Static and Proactive Postural Control on Older Adults with Sarcopenic Obesity: A Multicenter, Randomized, and Controlled Trial. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2779–2794. [CrossRef]
- Ferhi, H.; Gaied Chortane, S.; Durand, S.; Beaune, B.; Maktouf, W. Effects of Physical Activity Program on Body Composition, Physical Performance, and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity: Randomized Controlled Trial. Healthcare 2023, 11, 2294. [CrossRef]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined effects of aging and obesity on postural control, muscle activity and maximal voluntary force of muscles mobilizing ankle joint. J. Biomech. 2018, 79, 198–206. [CrossRef]
- Serra-Rexach, J. A.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: a randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Kryger, A. I.; Andersen, J. L. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [CrossRef]
- Lopez, P.; Pinto, R. S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E. L. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Brightwell, C. R.; Phalen, D. E.; McKenna, C. F.; Lane, S. J.; Porter, C.; Volpi, E.; Rasmussen, B. B.; Fry, C. S. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp. Gerontol. 2019, 127, 110723. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J. P.; Joanisse, S.; Snijders, T.; Ivankovic, V.; Baker, S. K.; Phillips, S. M.; Parise, G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J. Cachexia Sarcopenia Muscle 2016, 7, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Unhjem, R.; Lundestad, R.; Fimland, M. S.; Mosti, M. P.; Wang, E. Strength training-induced responses in older adults: attenuation of descending neural drive with age. Age 2015, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonsen, E. B.; Andersen, J. L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, J.; Baudry, S. Maximal discharge rate of motor units determines the maximal rate of force development during ballistic contractions in human. Front. Hum. Neurosci. 2014, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A. P.; Miller, M. E.; Rejeski, W. J.; Hutton, S. L.; Kritchevsky, S. B. Lower extremity muscle function after strength or power training in older adults. J. Aging Phys. Act. 2009, 17, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Reid, K. F.; Callahan, D. M.; Carabello, R. J.; Phillips, E. M.; Frontera, W. R.; Fielding, R. A. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin. Exp. Res. 2014, 20, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S. P.; Gibson, K. A comparison of high-speed power training and traditional slow-speed resistance training in older men and women. J. Strength Cond. Res. 2010, 24, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.; Bautmans, I. The influence of strength training on muscle activation in elderly persons: a systematic review and meta-analysis. Exp. Gerontol. 2014, 58, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Hvid, L. G.; Justesen, L.; Christensen, U.; Neergaard, K.; Simonsen, L.; Ortenblad, N.; Magnusson, S. P.; Kjaer, M.; Aagaard, P. Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. 2009, 107, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, M.; Machado, S. N.; Nogueira, W.; Scales, R.; Veloso, J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur. J. Appl. Physiol. 2007, 99, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yan, E.; He, L.; Wang, Y.; Xiang, Y.; Zhang, P.; Liu, X.; Yin, J. Dietary supplementation with lauric acid improves aerobic endurance in sedentary mice via enhancing fat mobilization and glyconeogenesis. J. Nutr. 2023, 153, 3207–3219. [CrossRef]
- Gabriel, D. A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006, 36, 133–149. [CrossRef]
- Christie, A.; Kamen, G. Cortical inhibition is reduced following short-term training in young and older adults. Age 2014, 36, 749–758. [CrossRef]
- Balducci, S.; Iacobellis, G.; Parisi, L.; Di Biase, N.; Calandriello, E.; Leonetti, F.; Fallucca, F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J. Diabetes Complications 2006, 20, 216–223. [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S. A.; Ferrucci, L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 2014, 6, 208. [CrossRef]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J. Clin. Med. 2021, 10, 1630. [CrossRef]
- Lee, N. K.; Son, S. M.; Nam, S. H.; Kwon, J. W.; Kang, K. W.; Kim, K. Effects of progressive resistance training integrated with foot and ankle compression on spatiotemporal gait parameters of individuals with stroke. J. Phys. Ther. Sci. 2013, 25, 1235–1237. [CrossRef]
- Clark, D. J.; Reid, K. F.; Patten, C.; Phillips, E. M.; Ring, S. A.; Wu, S. S.; Fielding, R. A. Does quadriceps neuromuscular activation capability explain walking speed in older men and women? Exp. Gerontol. 2014, 55, 49–53. [CrossRef]
- Peterson, C. L.; Kautz, S. A.; Neptune, R. R. Braking and propulsive impulses increase with speed during accelerated and decelerated walking. Gait Posture 2011, 33, 562–567. [CrossRef]
- Jaque, C.; Véliz, P.; Ramirez-Campillo, R.; Moran, J.; Gentil, P.; Cancino, J. High-speed bodyweight resistance training improves functional performance through maximal velocity in older females. J. Aging Phys. Act. 2021, 29, 659–669. [CrossRef]
- Coelho-Júnior, H. J.; Uchida, M. C. Effects of low-speed and high-speed resistance training programs on frailty status, physical performance, cognitive function, and blood pressure in prefrail and frail older adults. Front. Med. 2021, 8, 702436. [CrossRef]
- Bårdstu, H. B.; Andersen, V.; Fimland, M. S.; Raastad, T.; Saeterbakken, A. H. Muscle strength is associated with physical function in community-dwelling older adults receiving home care: a cross-sectional study. Front. Public Health, 2022, 25, 10:856632. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).