Submitted:
09 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
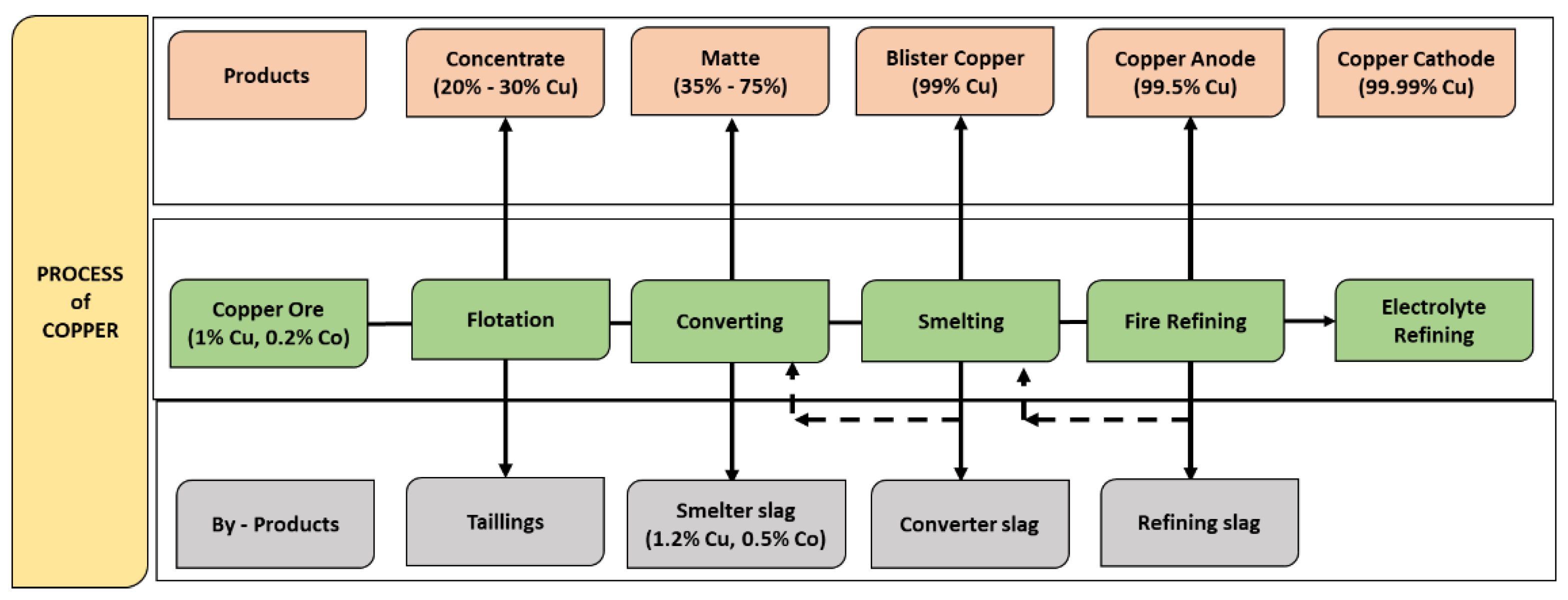
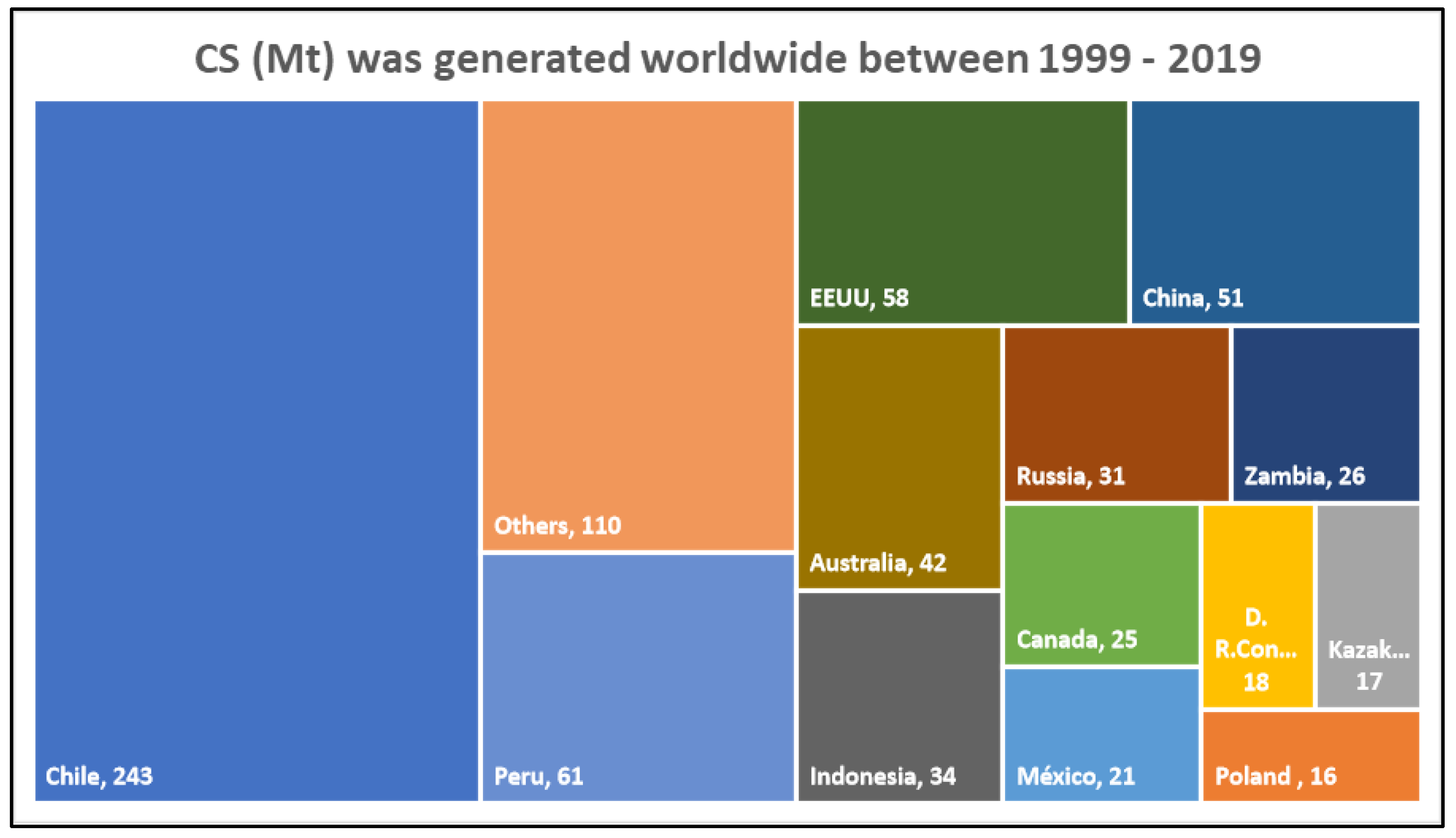
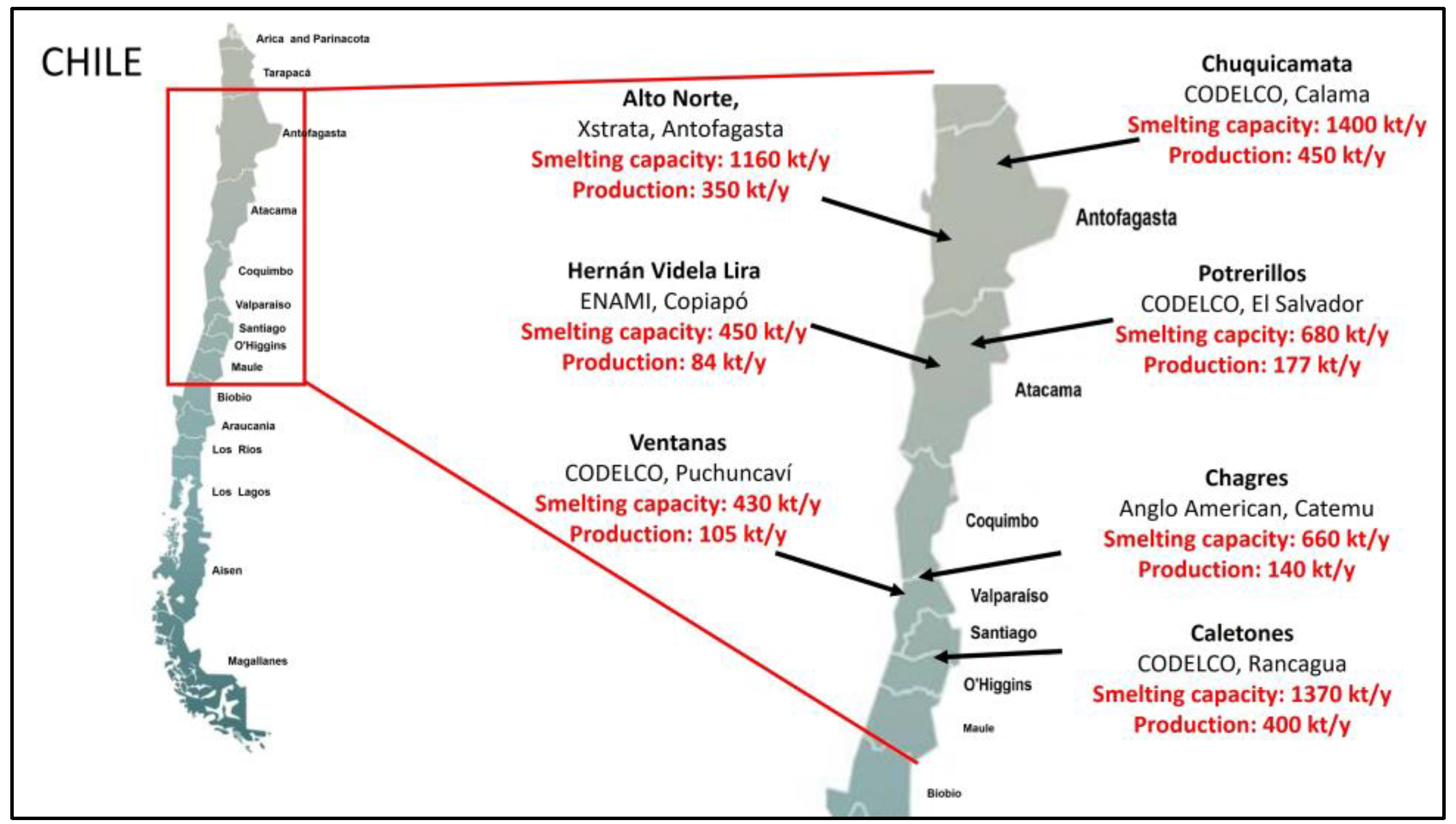
2. Production of Cupper Slag
2.1. Copper Slag
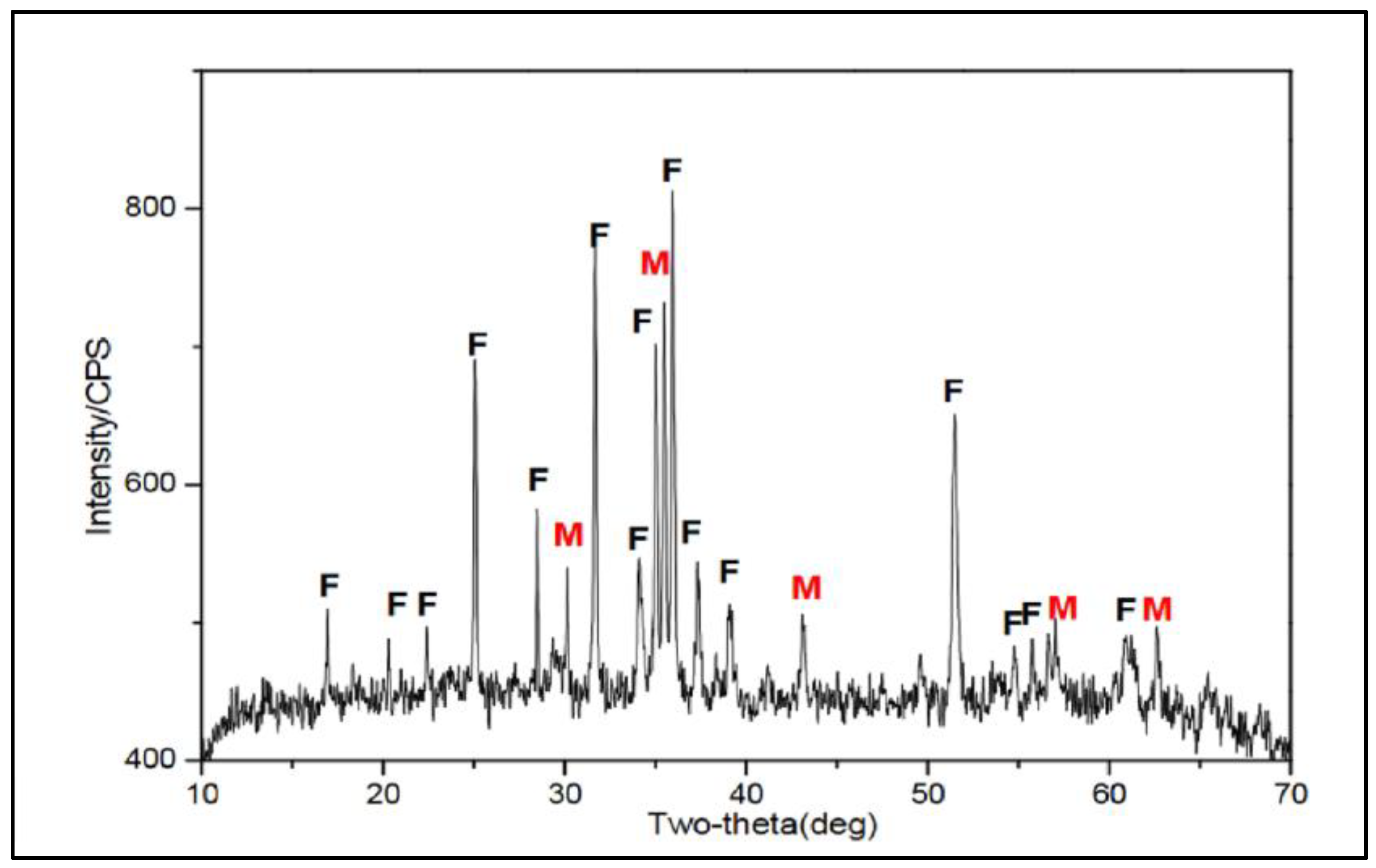
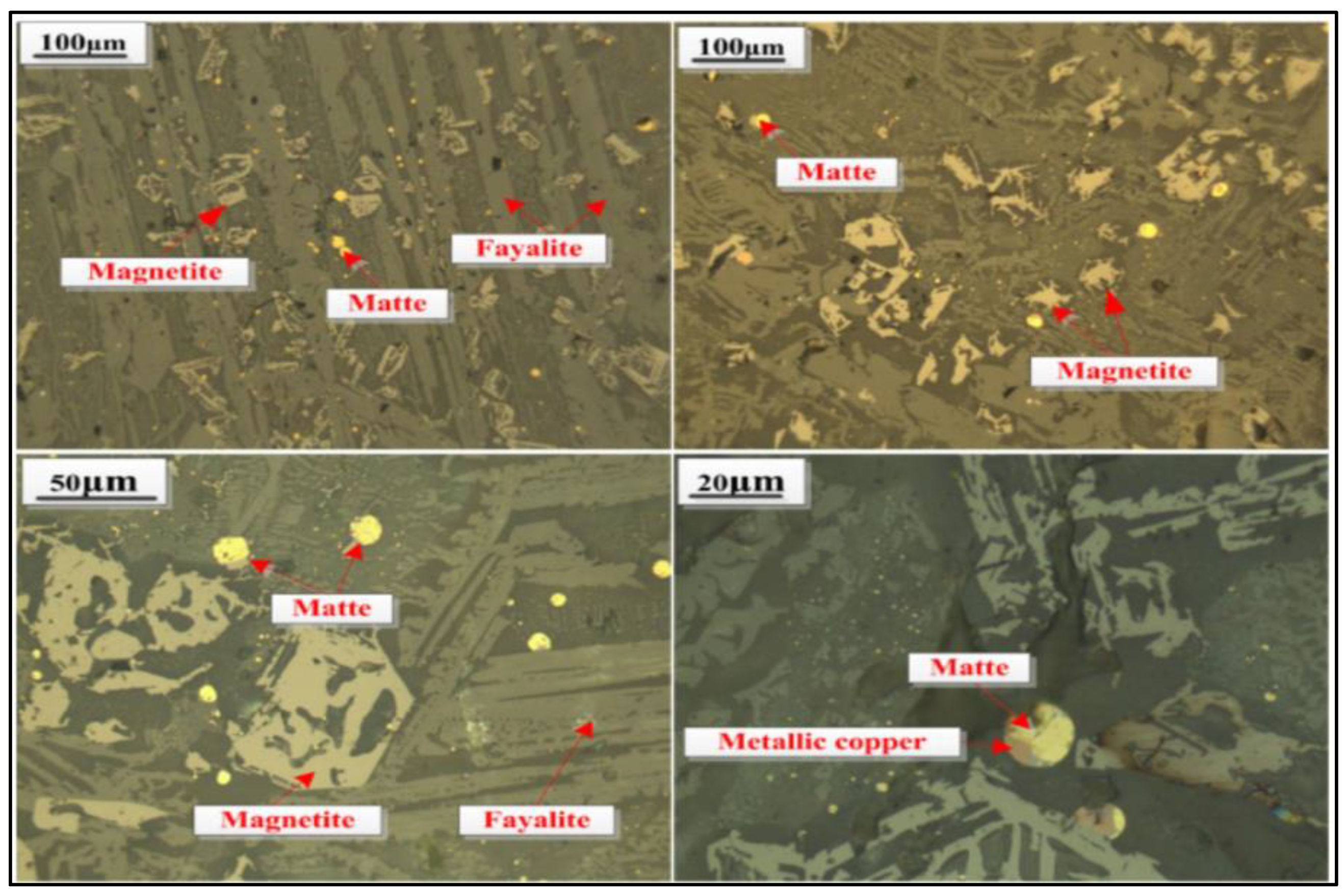
3. Chemical and Superficial Characterization of Cupper Slag
4. Semiconductor Electrodes as Photocatalysts
4.1. CS as Photocatalytic Material
4.2. Photocatalytic Mechanisms Applied to CS.
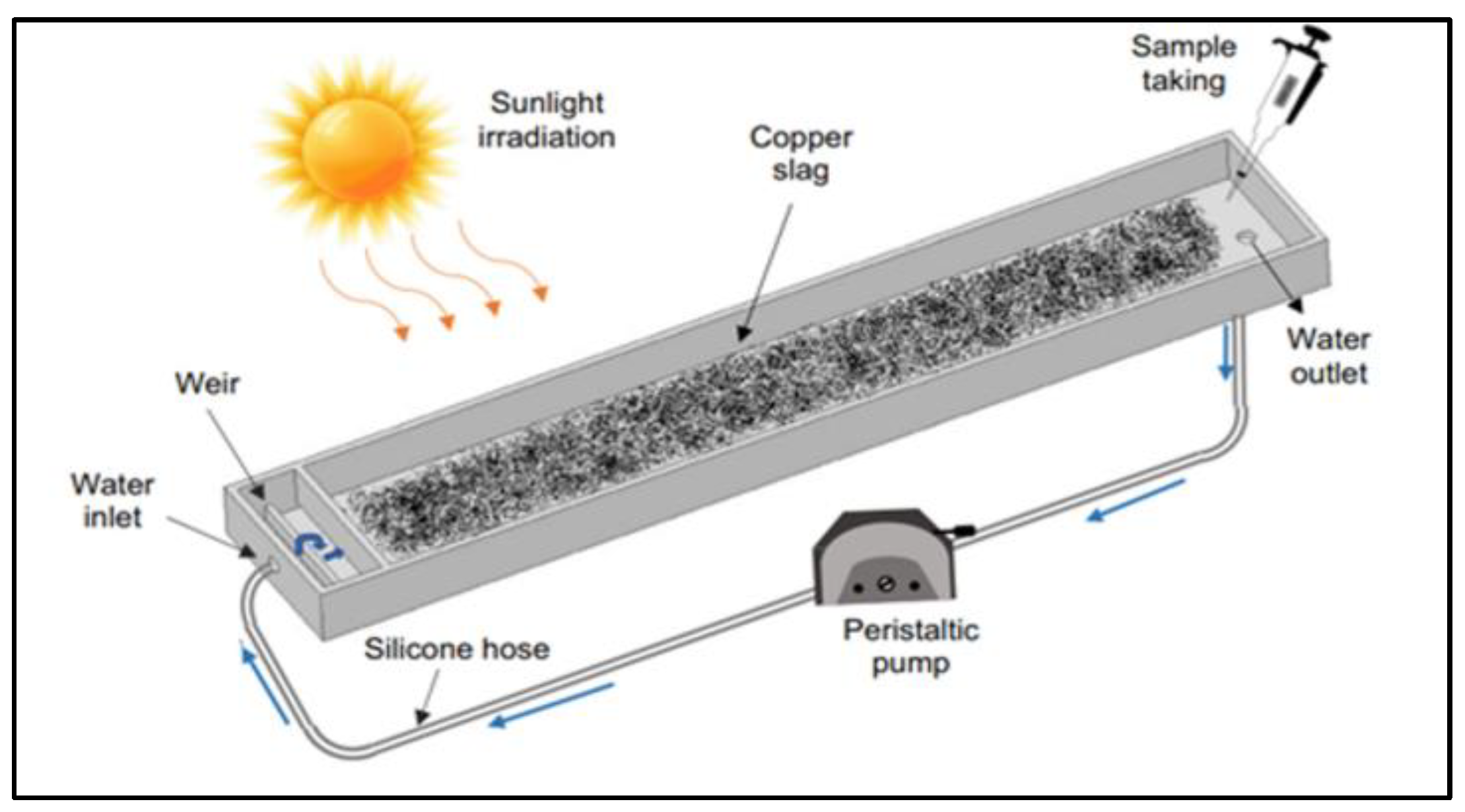
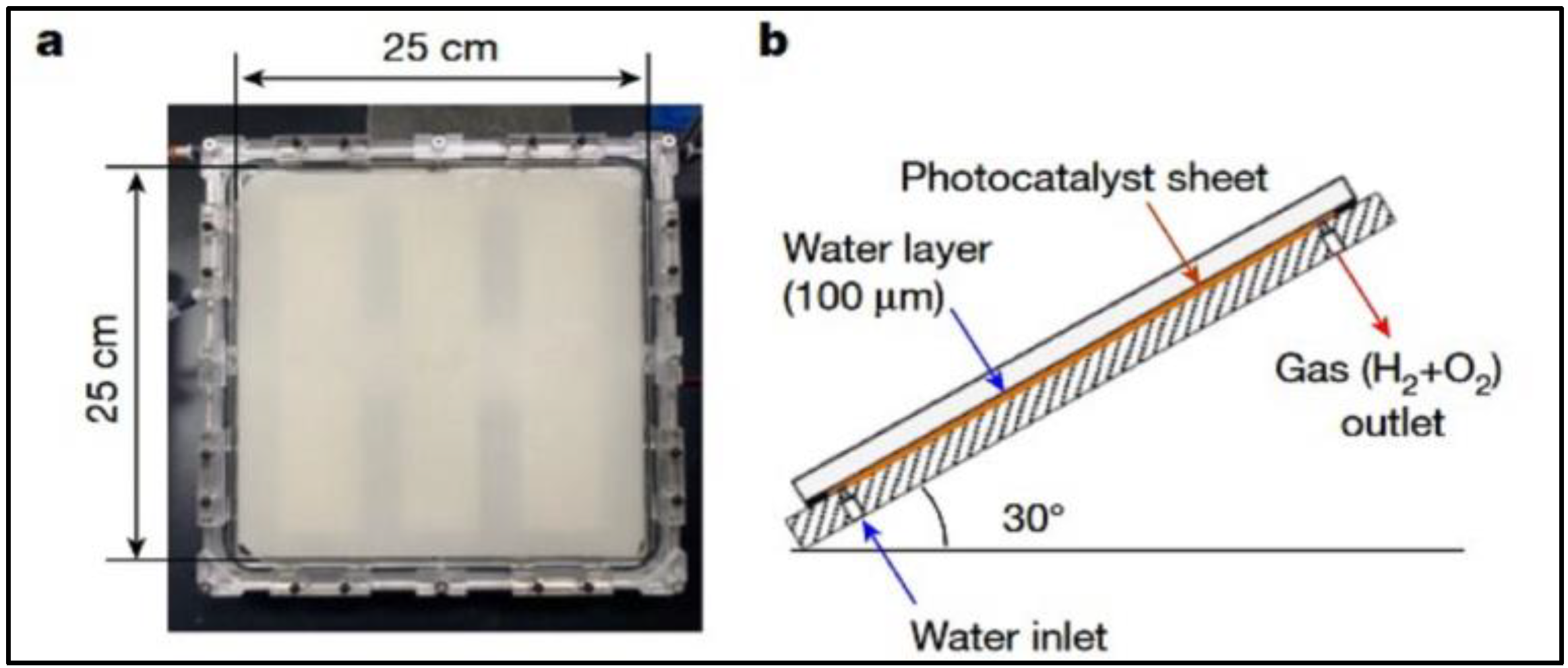
4.3. Photocatalytic Reactors for H2 Production
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- S. Nasirov, A. Girard, C. Peña, F. Salazar, and F. Simon, “Expansion of renewable energy in Chile: Analysis of the effects on employment,” Energy, vol. 226, p. 120410, Jul. 2021. [CrossRef]
- P. Del Río and C. P. Kiefer, “What will be the cost of renewable electricity generation technologies in the future?,” papers of the, pp. 34–250, 2022.
- G. Peñaranda, P. C. Vanegas, and M. Castañeda, “Carbon footprint calculation: Review key elements for the development of an opertional tool,” Seeds of knowledge magazine, vol. 2, no. 1, pp. 60–65, 2022.
- C. Moraga-Contreras et al., “Evolution of Solar Energy in Chile: Residential Opportunities in Arica and Parinacota,” Energies (Basel), vol. 15, no. 2, Jan. 2022. [CrossRef]
- C. Parrado, A. Girard, F. Simon, and E. Fuentealba, “2050 LCOE (Levelized Cost of Energy) projection for a hybrid PV (photovoltaic)-CSP (concentrated solar power) plant in the Atacama Desert, Chile,” Energy, vol. 94, pp. 422–430, Jan. 2016. [CrossRef]
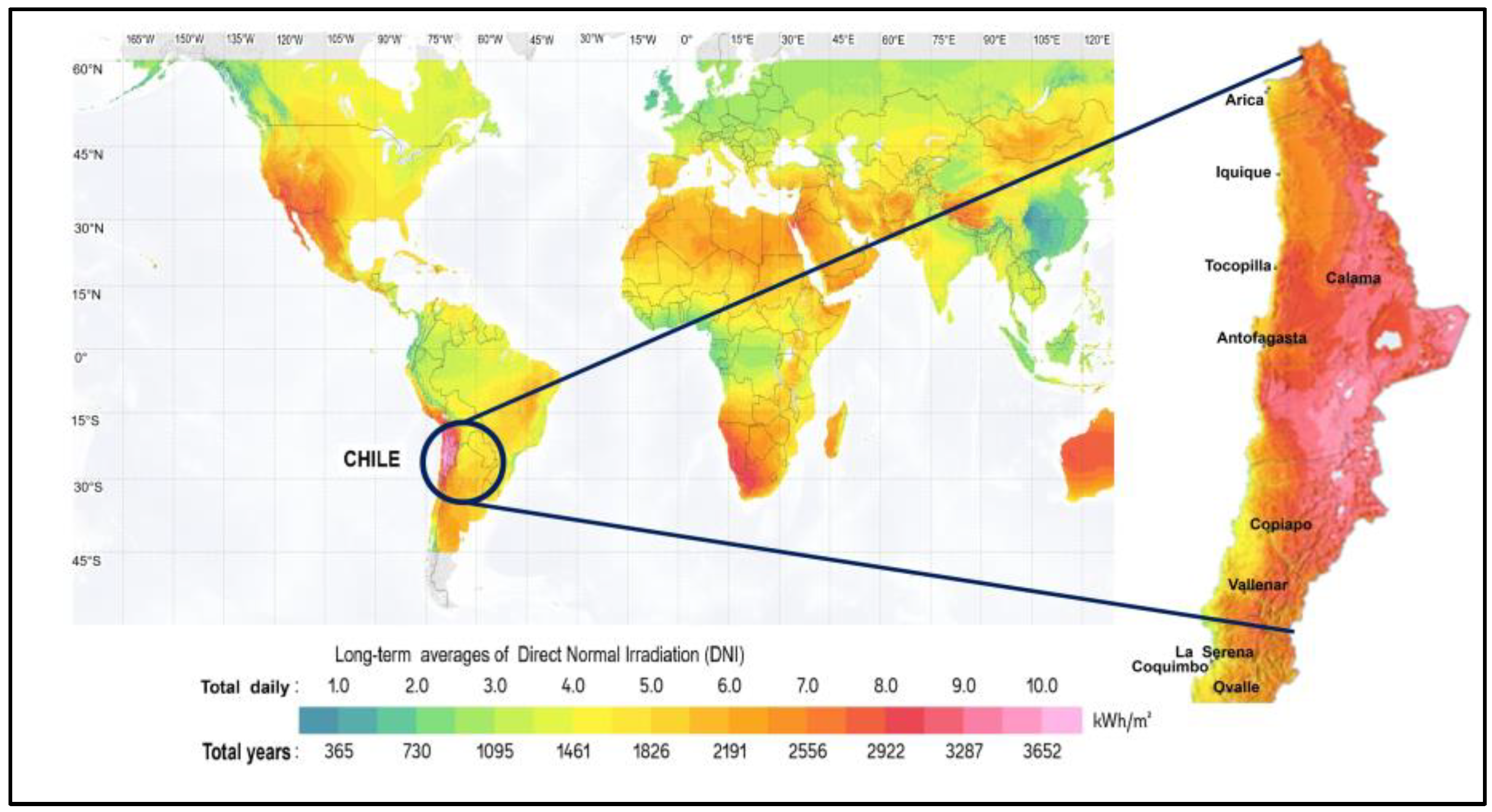
- Solargis, “Solar resource maps of World. © 2020 The World Bank, Source: Global Solar Atlas 2.0, Solar resource data: Solargis.,” © 2020 The World Bank, Source: Global Solar Atlas 2.0, Solar resource data: Solargis.
- R. Van De Krol, Y. Liang, and J. Schoonman, “Solar hydrogen production with nanostructured metal oxides,” J Mater Chem, vol. 18, no. 20, pp. 2311–2320, 2008. [CrossRef]
- M. K. T. Chee, B. J. Ng, Y. H. Chew, W. S. Chang, and S. P. Chai, “Photocatalytic Hydrogen Evolution from Artificial Seawater Splitting over Amorphous Carbon Nitride: Optimization and Process Parameters Study via Response Surface Modeling,” Materials, vol. 15, no. 14, Jul. 2022. [CrossRef]
- F. Orecchini, “The era of energy vectors,” Int J Hydrogen Energy, vol. 31, no. 14, pp. 1951–1954, Nov. 2006. [CrossRef]
- J. A. Okolie, B. R. Patra, A. Mukherjee, S. Nanda, A. K. Dalai, and J. A. Kozinski, “Futuristic applications of hydrogen in energy, biorefining, aerospace, pharmaceuticals and metallurgy,” International Journal of Hydrogen Energy, vol. 46, no. 13. Elsevier Ltd, pp. 8885–8905, Feb. 19, 2021. [CrossRef]
- S. I. Park, S. M. Jung, J. Y. Kim, and J. Yang, “Effects of Mono- and Bifunctional Surface Ligands of Cu–In–Se Quantum Dots on Photoelectrochemical Hydrogen Production,” Materials, vol. 15, no. 17, Sep. 2022. [CrossRef]
- Fujishima and K. Honda, “Electrochemical Photolysis of Water at a Semiconductor Electrode,” Nature, vol. 238, no. 5358, pp. 37–38, Jul. 1972. [CrossRef]
- M. A. Fox and M. T. Dulay, “Heterogeneous Photocatalysis,” 1993. [Online]. Available: https://pubs.acs.org/sharingguidelines.
- B. Lavand and Y. S. Malghe, “Visible light photocatalytic degradation of 4-chlorophenol using C/ZnO/CdS nanocomposite,” Journal of Saudi Chemical Society, vol. 19, no. 5, pp. 471–478, Sep. 2015. [CrossRef]
- J. Augustyński, B. D. Alexander, and R. Solarska, “Metal oxide photoanodes for water splitting,” Top Curr Chem, vol. 303, pp. 1–38, 2011. [CrossRef]
- T. Yui, Y. Tamaki, K. Sekizawa, and O. Ishitani, “Photocatalytic Reduction of CO2: From Molecules to Semiconductors,” 2011, pp. 151–184. [CrossRef]
- S. K. Bajpai, N. Chand, and V. Chaurasia, “Nano Zinc Oxide-Loaded Calcium Alginate Films with Potential Antibacterial Properties,” Food Bioproc Tech, vol. 5, no. 5, pp. 1871–1881, Jul. 2012. [CrossRef]
- Fujishima and K. Honda, “Electrochemical evidence for the mechanism of the primary stage of photosynthesis,” Bull Chem Soc Jpn, vol. 44, no. 4, pp. 1148–1150, 1971.
- D. S. M. Constantino, M. M. Dias, A. M. T. Silva, J. L. Faria, and C. G. Silva, “Intensification strategies for improving the performance of photocatalytic processes: A review,” Journal of Cleaner Production, vol. 340. Elsevier Ltd, Mar. 15, 2022. [CrossRef]
- L. Escobar-Alarcón and D. A. Solís-Casados, “Desarrollo de fotocatalizadores basados en TiO2 en forma de película delgada para la degradación de moléculas orgánicas en solución acuosa,” Mundo Nano. Revista Interdisciplinaria en Nanociencias y Nanotecnología, vol. 14, no. 26, pp. 1e–23e, Oct. 2020. [CrossRef]
- T. Takata and K. Domen, “Particulate Photocatalysts for Water Splitting: Recent Advances and Future Prospects,” ACS Energy Letters, vol. 4, no. 2. American Chemical Society, pp. 542–549, Feb. 08, 2019. [CrossRef]
- C. V. Montoya-Bautista, P. Acevedo-Peña, R. Zanella, and R.-M. Ramírez-Zamora, “Characterization and Evaluation of Copper Slag as a Bifunctional Photocatalyst for Alcohols Degradation and Hydrogen Production,” Top Catal, vol. 64, no. 1–2, pp. 131–141, 2021. [CrossRef]
- J. Portier, H. S. Hilal, I. Saadeddin, S. J. Hwang, M. A. Subramanian, and G. Campet, “Thermodynamic correlations and band gap calculations in metal oxides,” Progress in Solid State Chemistry, vol. 32, no. 3–4, pp. 207–217, 2004.
- F. Opoku, K. K. Govender, C. G. C. E. van Sittert, and P. P. Govender, “Recent progress in the development of semiconductor-based photocatalyst materials for applications in photocatalytic water splitting and degradation of pollutants,” Adv Sustain Syst, vol. 1, no. 7, p. 1700006, 2017.
- M. Mishra and D.-M. Chun, “α-Fe2O3 as a photocatalytic material: A review,” Appl Catal A Gen, vol. 498, pp. 126–141, 2015.
- X. Chen, S. Shen, L. Guo, and S. S. Mao, “Semiconductor-based photocatalytic hydrogen generation,” Chem Rev, vol. 110, no. 11, pp. 6503–6570, Nov. 2010. [CrossRef]
- M. Pelaez et al., “A review on the visible light active titanium dioxide photocatalysts for environmental applications,” Applied Catalysis B: Environmental, vol. 125. pp. 331–349, Aug. 21, 2012. [CrossRef]
- E. Mosquera, N. Carvajal, M. Morel, and C. Marín, “Fabrication of ZnSe nanoparticles: Structural, optical and Raman Studies,” J Lumin, vol. 192, pp. 814–817, Dec. 2017. [CrossRef]
- E. Mosquera, I. Del Pozo, and M. Morel, “Structure and red shift of optical band gap in CdO-ZnO nanocomposite synthesized by the sol gel method,” J Solid State Chem, vol. 206, pp. 265–271, 2013. [CrossRef]
- E. Mosquera, C. Rojas-Michea, M. Morel, F. Gracia, V. Fuenzalida, and R. A. Zárate, “Zinc oxide nanoparticles with incorporated silver: Structural, morphological, optical and vibrational properties,” Appl Surf Sci, vol. 347, pp. 561–568, Aug. 2015. [CrossRef]
- X. Guo, L. Liu, Y. Xiao, Y. Qi, C. Duan, and F. Zhang, “Band gap engineering of metal-organic frameworks for solar fuel productions,” Coordination Chemistry Reviews, vol. 435. Elsevier B.V., May 15, 2021. [CrossRef]
- H. Liang, F. Wang, L. Yang, Z. Cheng, Y. Shuai, and H. Tan, “Progress in full spectrum solar energy utilization by spectral beam splitting hybrid PV/T system,” Renewable and Sustainable Energy Reviews, vol. 141. Elsevier Ltd, May 01, 2021. [CrossRef]
- T. Huanosta-Gutiérrez, R. F. Dantas, R. M. Ramírez-Zamora, and S. Esplugas, “Evaluation of copper slag to catalyze advanced oxidation processes for the removal of phenol in water,” J Hazard Mater, vol. 213–214, pp. 325–330, Apr. 2012. [CrossRef]
- B. K. Mayer, D. Gerrity, B. E. Rittmann, D. Reisinger, and S. Brandt-Williams, “Innovative strategies to achieve low total phosphorus concentrations in high water flows,” Crit Rev Environ Sci Technol, vol. 43, no. 4, pp. 409–441, 2013. [CrossRef]
- R. García-Estrada, S. Arzate, and R.-M. Ramírez-Zamora, “Thiabendazole degradation by photo-NaOCl/Fe and photo-Fenton like processes, using copper slag as an iron catalyst, in spiked synthetic and real secondary wastewater treatment plant effluents,” Water Science and Technology, vol. 87, no. 3, pp. 620–634, 2023. [CrossRef]
- A. Morales-Pérez, R. García-Pérez, C. G. Tabla-Vázquez, and R. M. Ramírez-Zamora, “Simultaneous Hydrogen Production and Acetic Acid Degradation by Heterogeneous Photocatalysis using a Metallurgical Waste as Catalyst,” Top Catal, vol. 64, no. 1–2, pp. 17–25, Jan. 2021. [CrossRef]
- L. M. Herrera-Ibarra, R. M. Ramírez-Zamora, A. Martín-Domínguez, M. Piña-Soberanis, D. Schnabel-Peraza, and J. A. Bañuelos-Díaz, “Treatment of Textile Industrial Wastewater by the Heterogeneous Solar Photo-Fenton Process Using Copper Slag,” Top Catal, vol. 65, no. 9–12, pp. 1163–1179, Aug. 2022. [CrossRef]
- C. V. Montoya-Bautista, E. Avella, R. M. Ramírez-Zamora, and R. Schouwenaars, “Metallurgical wastes employed as catalysts and photocatalysts for water treatment: A review,” Sustainability (Switzerland), vol. 11, no. 9. MDPI, May 01, 2019. [CrossRef]
- M. Solís-López, A. Durán-Moreno, F. Rigas, A. A. Morales, M. Navarrete, and R. M. Ramírez-Zamora, “Assessment of Copper Slag as a Sustainable Fenton-Type Photocatalyst for Water Disinfection,” in Water Reclamation and Sustainability, Elsevier Inc., 2014, pp. 199–227. [CrossRef]
- B. Gorai, R. K. Jana, and Premchand, “Characteristics and utilisation of copper slag - A review,” Resour Conserv Recycl, vol. 39, no. 4, pp. 299–313, Nov. 2003. [CrossRef]
- W. Zhou, X. Liu, X. Lyu, W. Gao, H. Su, and C. Li, “Extraction and separation of copper and iron from copper smelting slag: A review,” J Clean Prod, p. 133095, 2022. [CrossRef]
- K. S. Barros, V. S. Vielmo, B. G. Moreno, G. Riveros, G. Cifuentes, and A. M. Bernardes, “Chemical composition data of the main stages of copper production from sulfide minerals in Chile: a review to assist circular economy studies,” Minerals, vol. 12, no. 2, p. 250, 2022. [CrossRef]
- T. C. Phiri, P. Singh, and A. N. Nikoloski, “The potential for copper slag waste as a resource for a circular economy: A review–Part I,” Miner Eng, vol. 180, p. 107474, 2022. [CrossRef]
- P. Nazer, S. Fuentes, O. Pavez, O. Varela, and O. Lanas, “Copper slag tiles. Innovation in clean production.,” Iberoam. J. Proj. Manag., vol. 3, no. 2, p. 12, 2012.
- Q. Zhai, R. Liu, C. Wang, X. Wen, X. Li, and W. Sun, “A novel scheme for the utilization of Cu slag flotation tailings in preparing internal electrolysis materials to degrade printing and dyeing wastewater,” J Hazard Mater, vol. 424, Feb. 2022. [CrossRef]
- C. González, R. Parra, A. Klenovcanova, I. Imris, and M. Sánchez, “Reduction of Chilean copper slags: A case of waste management project,” in Scandinavian Journal of Metallurgy, Apr. 2005, pp. 143–149. [CrossRef]
- Z. Guo, D. Zhu, J. Pan, and F. Zhang, “Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process,” J Clean Prod, vol. 187, pp. 910–922, Jun. 2018. [CrossRef]
- H. Shen and E. Forssberg, “An overview of recovery of metals from slags,” Waste Management, vol. 23, no. 10, pp. 933–949, 2003. [CrossRef]
- B. S. Kim, S. K. Jo, D. Shin, J. C. Lee, and S. B. Jeong, “A physico-chemical separation process for upgrading iron from waste copper slag,” Int J Miner Process, vol. 124, pp. 124–127, 2013. [CrossRef]
- Q. Jin and L. Chen, “A Review of the Influence of Copper Slag on the Properties of Cement-Based Materials,” Materials, vol. 15, no. 23, p. 8594, Dec. 2022. [CrossRef]
- Rohini and R. Padmapriya, “Properties of Bacterial Copper Slag Concrete,” Buildings, vol. 13, no. 2, p. 290, 2023. [CrossRef]
- W. Wu, W. Zhang, and G. Ma, “Mechanical properties of copper slag reinforced concrete under dynamic compression,” Constr Build Mater, vol. 24, no. 6, pp. 910–917, Jun. 2010. [CrossRef]
- C. Lavanya, A. S. Rao, and N. D. Kumar, “Study on Coefficient of Permeability of Copper slag when admixed with Lime and Cement,” IOSR Journal of Mechanical and Civil Engineering, vol. 7, no. 6, pp. 19–25, 2013.
- S. Salleh, M. G. Shaaban, H. B. Mahmud, J. Kang, and K. T. Looi, “Production of bricks from shipyard repair and maintenance hazardous waste,” International Journal of Environmental Science and Development, vol. 5, no. 1, p. 52, 2014. [CrossRef]
- S. Arivalagan, “Experimental study on the flexural behavior of reinforced concrete beams as replacement of copper slag as fine aggregate,” Journal of civil engineering and Urbanism, vol. 3, no. 4, pp. 176–182, 2013.
- P. S. Ambily, C. Umarani, K. Ravisankar, P. R. Prem, B. H. Bharatkumar, and N. R. Iyer, “Studies on ultra high performance concrete incorporating copper slag as fine aggregate,” Constr Build Mater, vol. 77, pp. 233–240, 2015. [CrossRef]
- R. Anudeep, K. V. Ramesh, and V. SowjanyaVani, “Study on mechanical properties of concrete with various slags as replacement to fine aggregate,” Concrete: Aggregate Replacement, 2015.
- Nazer, J. Payá, M. V. Borrachero, and J. Monzó, “Characterization of Chilean copper slag smelting nineteenth century,” Revista de Metalurgia, vol. 52, no. 4, p. 083, Dec. 2016. [CrossRef]
- W. Wu, W. Zhang, and G. Ma, “Optimum content of copper slag as a fine aggregate in high strength concrete,” Mater Des, vol. 31, no. 6, pp. 2878–2883, Jun. 2010. [CrossRef]
- D. Brindha and P. Sureshkumar, “Buckling strength of RCC columns incorporating copper slag as partial replacement of cement,” in Proceedings of National Conference on Emerging Trends in Civil Engineering, Tamil Nadu, India, 2010.
- Q. Zhang, B. Zhang, and D. Wang, “Environmental Benefit Assessment of Blended Cement with Modified Granulated Copper Slag,” Materials, vol. 15, no. 15, p. 5359, Aug. 2022. [CrossRef]
- L. Sun, Y. Feng, D. Wang, C. Qi, and X. Zeng, “Influence of CaO on physical and environmental properties of granulated copper slag: Melting behavior, grindability and leaching behavior,” Int J Environ Res Public Health, vol. 19, no. 20, p. 13543, 2022. [CrossRef]
- Pérez, Á. Villegas, M. Saldaña, R. I. Jeldres, J. González, and N. Toro, “Initial investigation into the leaching of manganese from nodules at room temperature with the use of sulfuric acid and the addition of foundry slag—Part II,” Sep Sci Technol, vol. 56, no. 2, pp. 389–394, Jan. 2021. [CrossRef]
- Y. Shi, Y. Wei, S. Zhou, B. Li, Y. Yang, and H. Wang, “Effect of B2O3 content on the viscosity of copper slag,” J Alloys Compd, vol. 822, p. 153478, May 2020. [CrossRef]
- S. Lewowicki and J. Rajczyk, “The usefulness of copper metallurgical slag as a micro-aggegate additive in mortars and concrete mixtures,” in 13. International conference on solid waste technology and management. Volume 2., 1997.
- Elizabeth Sangine, “U.S. Geological Survey Mineral commodity summaries Available online: ,” https://www.usgs.gov/centers/national-minerals-information-center.
- M. A. Moram and M. E. Vickers, “X-ray diffraction of III-nitrides,” Reports on Progress in Physics, vol. 72, no. 3, 2009. [CrossRef]
- Y. Xue, Z. Guo, D. Zhu, J. Pan, Y. Wang, and R. Zhan, “Efficient utilization of copper slag in an innovative sintering process for Fe-Ni-Cu alloy preparation and valuable elements recovery,” Journal of Materials Research and Technology, vol. 18, pp. 3115–3129, May 2022. [CrossRef]
- T. C. Phiri, P. Singh, and A. N. Nikoloski, “The potential for copper slag waste as a resource for a circular economy: A review–Part II,” Miner Eng, vol. 172, p. 107150, 2021. [CrossRef]
- Z. Guo, D. Zhu, J. Pan, T. Wu, and F. Zhang, “Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag,” Metals (Basel), vol. 6, no. 4, Apr. 2016. [CrossRef]
- S. Duan et al., “Efficient photocatalytic hydrogen production from formic acid on inexpensive and stable phosphide/Zn3In2S6 composite photocatalysts under mild conditions,” Int J Hydrogen Energy, vol. 44, no. 39, pp. 21803–21820, Aug. 2019. [CrossRef]
- C. V. Montoya-Bautista et al., “Photocatalytic H2 Production and Carbon Dioxide Capture Using Metallurgical Slag and Slag-Derived Materials,” in Handbook of Ecomaterials, Springer International Publishing, 2018, pp. 1–19. [CrossRef]
- P. K. Gbor, V. Mokri, and C. Q. Jia, “Characterization of smelter slags,” J Environ Sci Health A Tox Hazard Subst Environ Eng, vol. 35, no. 2, pp. 147–167, 2000. [CrossRef]
- T. S. Gabasiane, G. Danha, T. A. Mamvura, T. Mashifana, and G. Dzinomwa, “Characterization of copper slag for beneficiation of iron and copper,” Heliyon, vol. 7, no. 4, Apr. 2021. [CrossRef]
- T. Chun, C. Ning, H. Long, J. Li, and J. Yang, “Mineralogical Characterization of Copper Slag from Tongling Nonferrous Metals Group China,” JOM, vol. 68, no. 9, pp. 2332–2340, Sep. 2016. [CrossRef]
- M. Sánchez and M. Sudbury, “Physicochemical characterization of copper slag and alternatives of friendly environmental management,” Journal of Mining and Metallurgy, Section B: Metallurgy, vol. 49, no. 2, pp. 161–168, 2013. [CrossRef]
- H. Tian et al., “Comprehensive review on metallurgical recycling and cleaning of copper slag,” Resources, Conservation and Recycling, vol. 168. Elsevier B.V., May 01, 2021. [CrossRef]
- X. Wang, D. Geysen, S. V. P. Tinoco, N. D’Hoker, T. Van Gerven, and B. Blanpain, “Characterisation of copper slag in view of metal recovery,” Transactions of the Institutions of Mining and Metallurgy, Section C: Mineral Processing and Extractive Metallurgy, vol. 124, no. 2, pp. 83–87, Jun. 2015. [CrossRef]
- R. Lori, A. Hassani, and R. Sedghi, “Investigating the mechanical and hydraulic characteristics of pervious concrete containing copper slag as coarse aggregate,” Constr Build Mater, vol. 197, pp. 130–142, Feb. 2019. [CrossRef]
- H. Zhang, C. Hu, W. Gao, and M. Lu, “Recovery of iron from copper slag using coal-based direct reduction: Reduction characteristics and kinetics,” Minerals, vol. 10, no. 11, pp. 1–17, Nov. 2020. [CrossRef]
- Y. Diaz-Rosero, L. González-Salcedo, and J. Diaz Rosero, “Caracterización de escoria de cobre secundaria y evaluación de su actividad puzolánica,” Informador Técnico, vol. 84, no. 2, Mar. 2020. [CrossRef]
- B. M. Mercado-Borrayo, J. L. González-Chávez, R. M. Ramírez-Zamora, and R. Schouwenaars, “Valorization of Metallurgical Slag for the Treatment of Water Pollution: An Emerging Technology for Resource Conservation and Re-utilization,” Journal of Sustainable Metallurgy, vol. 4, no. 1. Springer Science and Business Media Deutschland GmbH, pp. 50–67, Mar. 01, 2018. [CrossRef]
- C. González, R. Parra, A. Klenovcanova, I. Imris, and M. Sánchez, “Reduction of Chilean copper slags: A case of waste management project,” in Scandinavian Journal of Metallurgy, Apr. 2005, pp. 143–149. [CrossRef]
- Manasse, M. Mellini, and C. Viti, “The copper slags of the Capattoli Valley, Campiglia Marittima, Italy,” European Journal of Mineralogy, vol. 13, no. 5, pp. 949–960, Sep. 2001. [CrossRef]
- R. Sáez, F. Nocete, J. M. Nieto, M. Á. Capitán, and S. Rovira, “The extractive metallurgy of copper from Cabezo Juré, Huelva, Spain: Chemical and mineralogical study of slags dated to the third millenium B.C,” Can Mineral, vol. 41, no. 3, pp. 627–638, 2003. [CrossRef]
- B. Zhang, T. Zhang, and C. Zheng, “Reduction Kinetics of Copper Slag by H2,” Minerals, vol. 12, no. 5, May 2022. [CrossRef]
- T. Kundu, S. Senapati, S. K. Das, S. I. Angadi, and S. S. Rath, “A comprehensive review on the recovery of copper values from copper slag,” Powder Technology, vol. 426. Elsevier B.V., Aug. 01, 2023. [CrossRef]
- Q. Ma et al., “Performance of copper slag contained mortars after exposure to elevated temperatures,” Constr Build Mater, vol. 172, pp. 378–386, May 2018. [CrossRef]
- Lasia, “Semiconductors and Mott-Schottky Plots,” in Electrochemical Impedance Spectroscopy and its Applications, Springer New York, 2014, pp. 251–255. [CrossRef]
- R. Gómez and T. Lana-Villarreal, “Tema 3. Conceptos de cinética electroquímica,” Corrosión, 2008.
- B. Tian, Q. Lei, B. Tian, W. Zhang, Y. Cui, and Y. Tian, “UV-driven overall water splitting using unsupported gold nanoparticles as photocatalysts,” Chemical Communications, vol. 54, no. 15, pp. 1845–1848, 2018.
- S. Nandy, S. A. Savant, and S. Haussener, “Prospects and challenges in designing photocatalytic particle suspension reactors for solar fuel processing,” Chemical Science, vol. 12, no. 29. Royal Society of Chemistry, pp. 9866–9884, Aug. 07, 2021. [CrossRef]
- C. D. Fernández-Solis et al., “Fundamentals of electrochemistry, corrosion and corrosion protection,” Soft Matter at Aqueous Interfaces, pp. 29–70, 2016. [CrossRef]
- E. McCafferty, “Validation of corrosion rates measured by the Tafel extrapolation method,” Corros Sci, vol. 47, no. 12, pp. 3202–3215, 2005.
- W. Halley, A. Schofield, and B. Berntson, “Use of magnetite as anode for electrolysis of water,” J Appl Phys, vol. 111, no. 12, 2012. [CrossRef]
- Y. J. Zhang and Q. Chai, “Alkali-activated blast furnace slag-based nanomaterial as a novel catalyst for synthesis of hydrogen fuel,” Fuel, vol. 115, pp. 84–87, 2014. [CrossRef]
- B. Tian, Q. Lei, W. Zhang, Y. Cui, Y. Tian, and B. Tian, “UV-driven overall water splitting using unsupported gold nanoparticles as photocatalysts,” Chemical Communications, vol. 54, no. 15, pp. 1845–1848, 2018. [CrossRef]
- C. Xu, P. Ravi Anusuyadevi, C. Aymonier, R. Luque, and S. Marre, “Nanostructured materials for photocatalysis,” Chemical Society Reviews, vol. 48, no. 14. Royal Society of Chemistry, pp. 3868–3902, Jul. 21, 2019. [CrossRef]
- C. V. Montoya-Bautista et al., “Photocatalytic H2 Production and Carbon Dioxide Capture Using Metallurgical Slag and Slag-Derived Materials,” in Handbook of Ecomaterials, Springer International Publishing, 2018, pp. 1–19. [CrossRef]
- S. Y. Arzate-Salgado, A. A. Morales-Pérez, M. Solís-López, and R. M. Ramírez-Zamora, “Evaluation of metallurgical slag as a Fenton-type photocatalyst for the degradation of an emerging pollutant: Diclofenac,” in Catalysis Today, Elsevier B.V., May 2016, pp. 126–135. [CrossRef]
- J. H. Kim, D. Hansora, P. Sharma, J. W. Jang, and J. S. Lee, “Toward practical solar hydrogen production-an artificial photosynthetic leaf-to-farm challenge,” Chemical Society Reviews, vol. 48, no. 7. Royal Society of Chemistry, pp. 1908–1971, Apr. 07, 2019. [CrossRef]
- C. C. Lo, C. W. Huang, C. H. Liao, and J. C. S. Wu, “Novel twin reactor for separate evolution of hydrogen and oxygen in photocatalytic water splitting,” Int J Hydrogen Energy, vol. 35, no. 4, pp. 1523–1529, Feb. 2010. [CrossRef]
- Dincer, “Green methods for hydrogen production,” in International Journal of Hydrogen Energy, Jan. 2012, pp. 1954–1971. [CrossRef]
- E. Borges, S. Navarro, H. de Paz Carmona, and P. Esparza, “Natural Volcanic Material as a Sustainable Photocatalytic Material for Pollutant Degradation under Solar Irradiation,” Materials, vol. 15, no. 11, Jun. 2022. [CrossRef]
- Li, R. Chen, Q. Liao, X. Zhu, G. Wang, and D. Wang, “High surface area optofluidic microreactor for redox mediated photocatalytic water splitting,” in International Journal of Hydrogen Energy, Elsevier Ltd, Nov. 2014, pp. 19270–19276. [CrossRef]
- H. Nishiyama et al., “Photocatalytic solar hydrogen production from water on a 100-m2 scale,” Nature, vol. 598, no. 7880, pp. 304–307, Oct. 2021. [CrossRef]
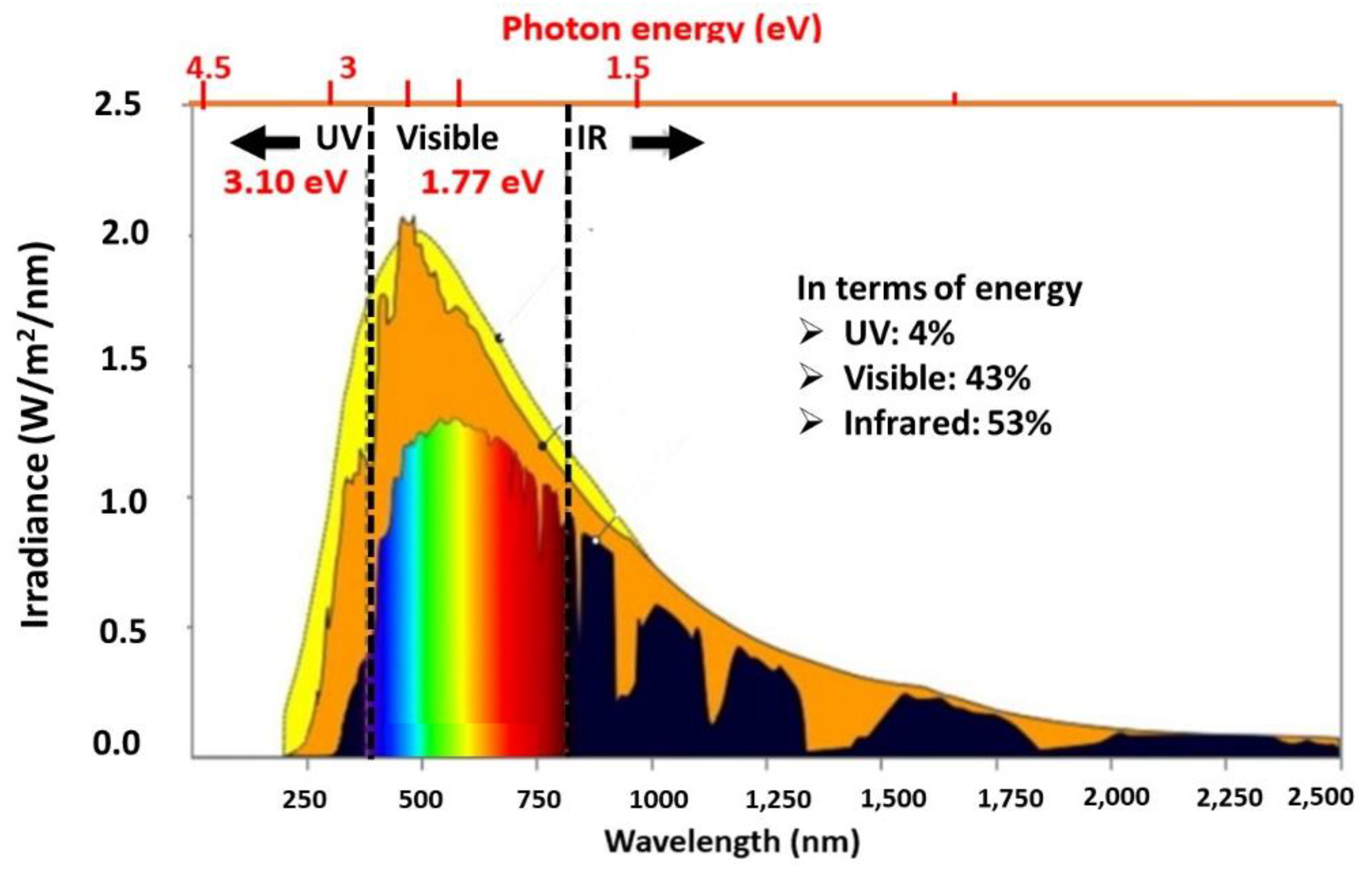
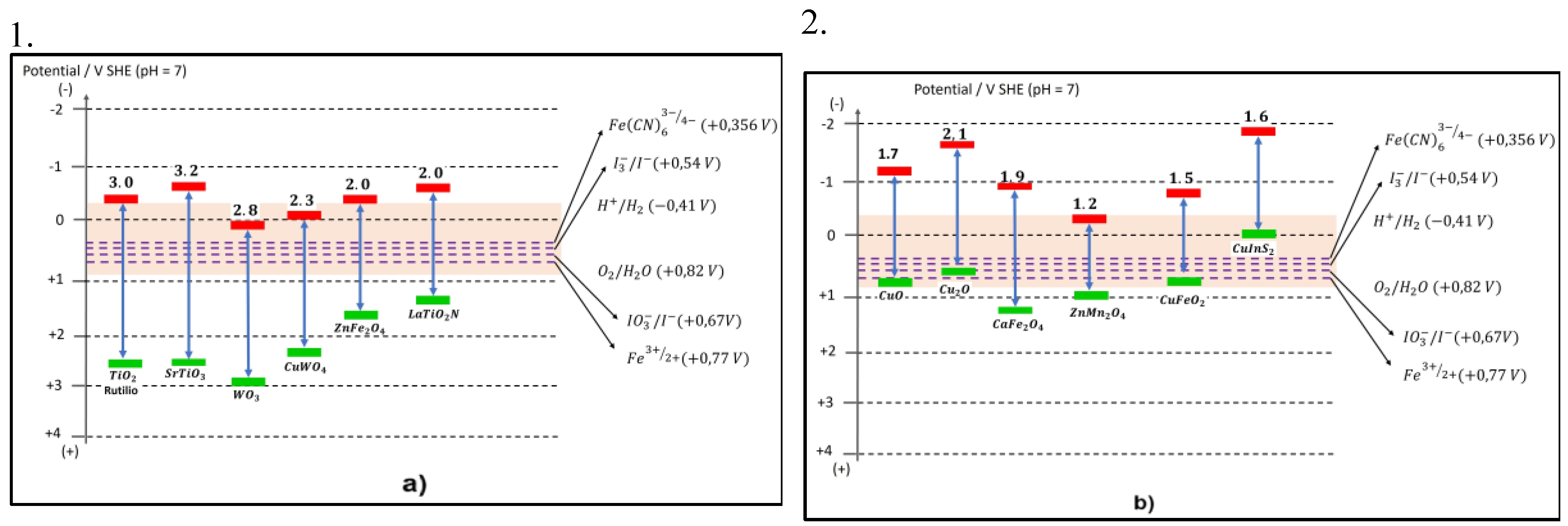
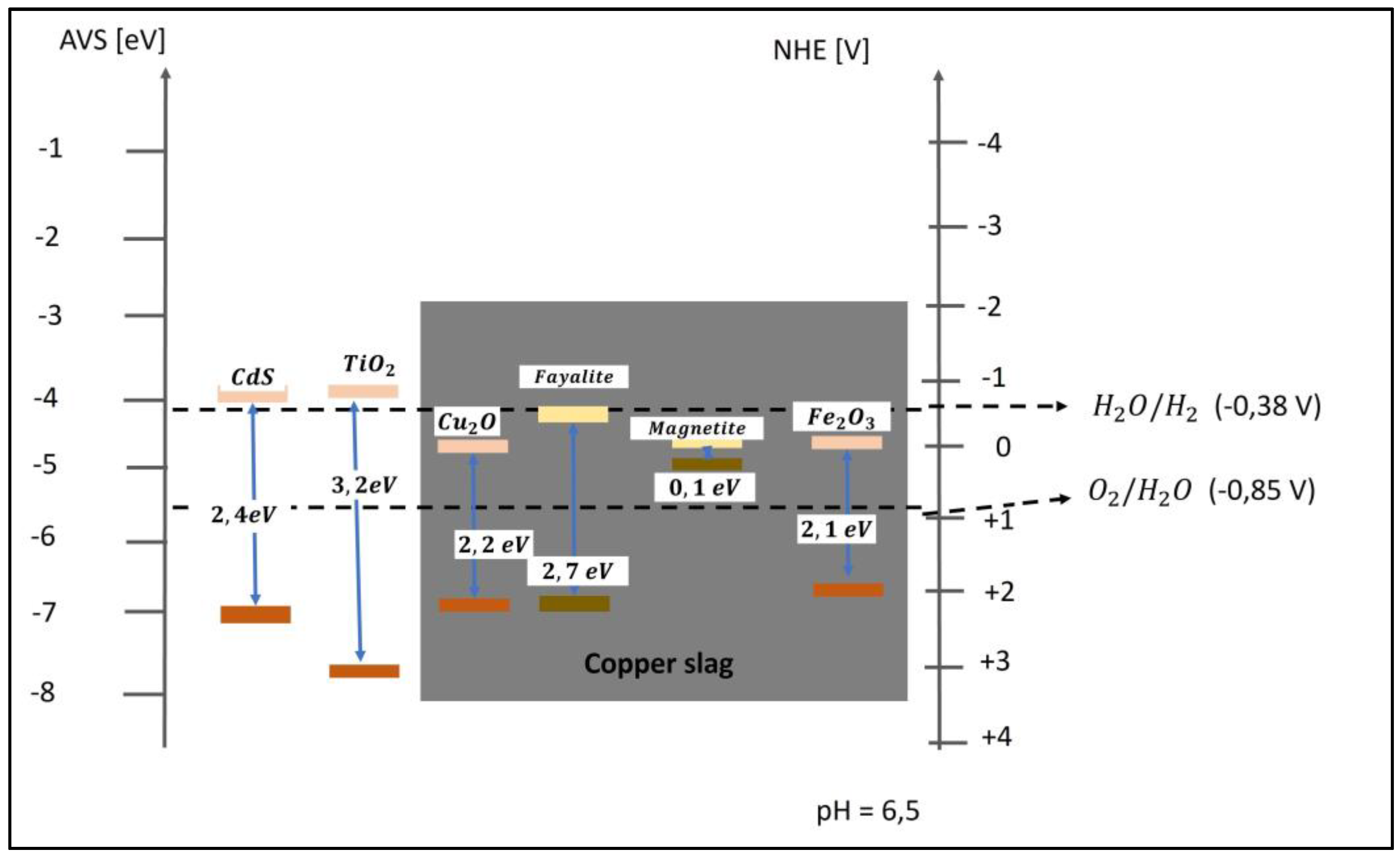
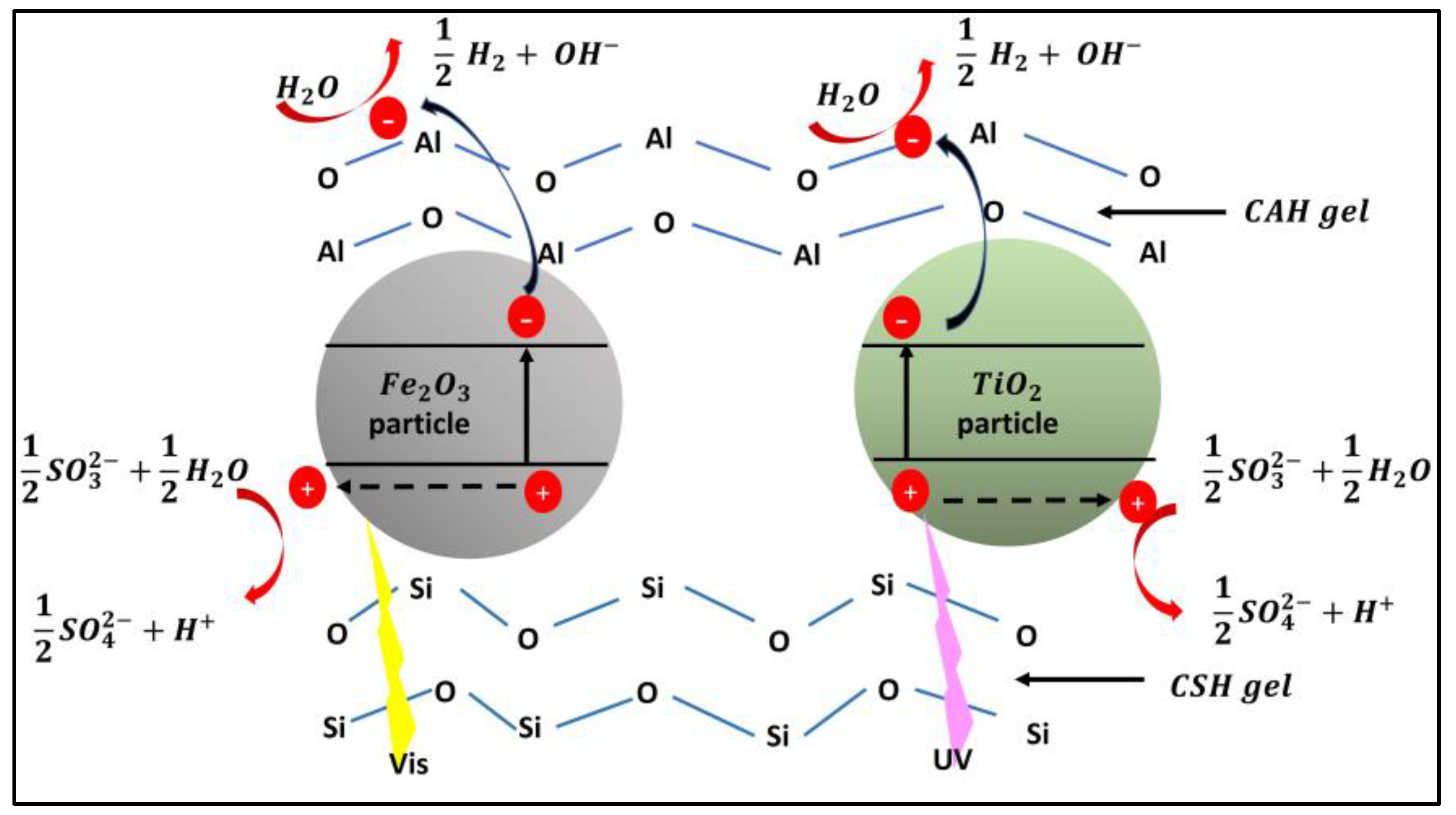
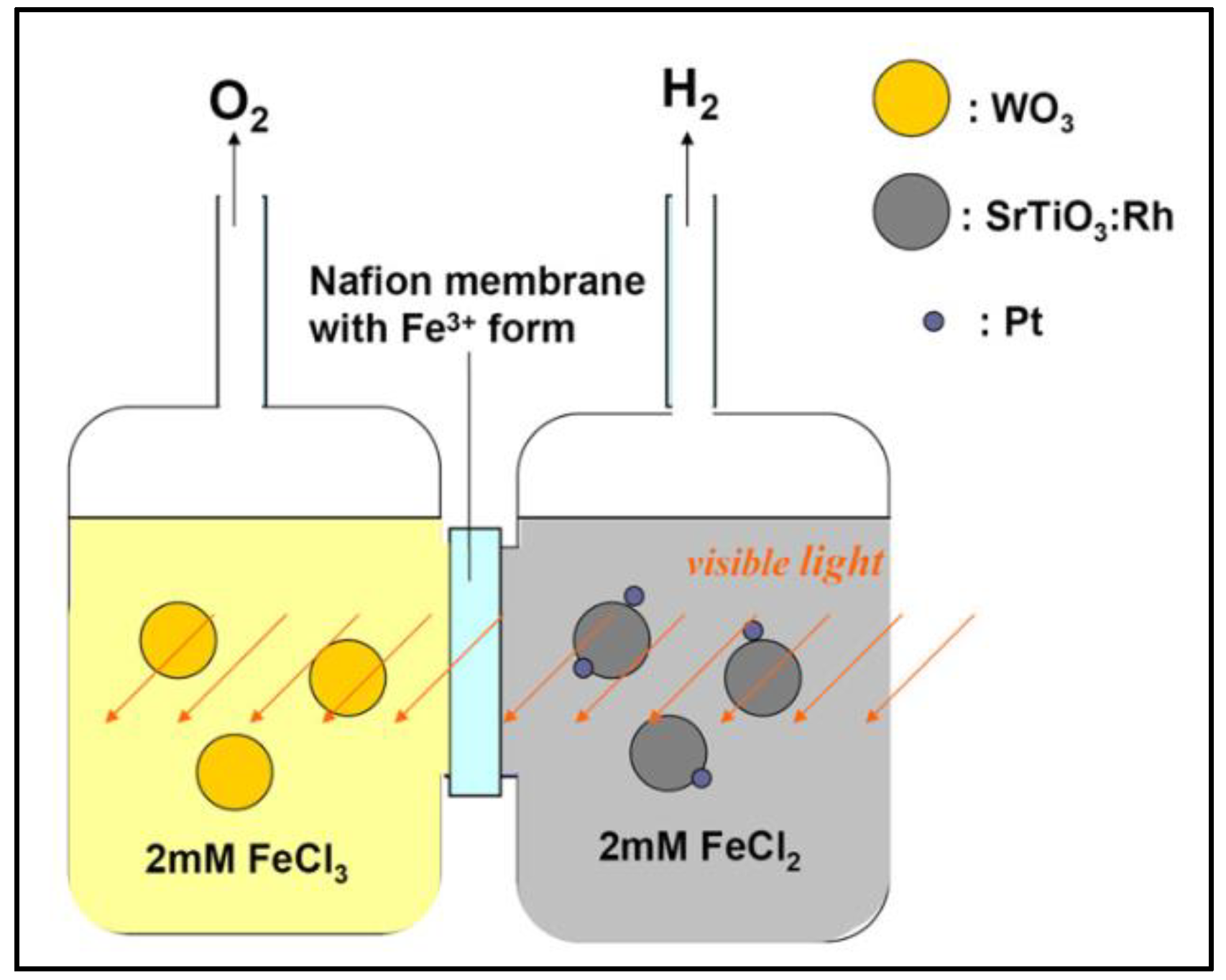


| Photocatalyst material | Band gap [eV] |
wavelength (nm) | Ref. |
|---|---|---|---|
| ZnO TiO2 (anatase) TiO2 (rutile) Bi2O3 Copper Slag (CS) Fe2SiO4 WO3 BiVO4 CdS Fe2O3 α-Fe2O3 CdSe |
3.4 3.2 3.0 2.9 2.75 2.7 2.7 2.4 2.4 2.3 1.9 1.7 |
365 380 414 427 450 --- 460 --- 497 565 600 730 |
[16,17] [18,19] [19] [20] [21,22] [22,23] [24] [16] [20,25] [20,23] [24] [20] |
| Property | Value |
|---|---|
| Unit weight (T/m3) Absorption Bulk density (T/m3) Conductivity (µs/cm) Specific gravity Hardness (Moh) Moisture (ppm) Abrasion loss (%) Internal friction angle |
2,8 – 3,8 0,13 2,3 – 2,6 500 2,8 – 3,8 6 – 7 < 5 2,4 – 10 40 - 53 |
| Property | Description | Ref. |
|---|---|---|
|
Particle shape |
Angular Irregular Multifaceted |
[52,53,54] [55,56] [57] |
|
Surface Texture |
Glassy Smooth Granular Rough |
[54,56,58,59]. [50,52,57,59]. [60,61,62] [54] |
| Color | Black Blackish grey Brown with green, red, or black tint |
[54,56,59,63]. [57,64]. [58,65]. |
| Components | SiO2 FeO Fe2O3 CaO Al2O3 MgO K2O S MnO TiO2 P2O5 Na2O |
| (wt%) | 33,18 32,15 31,93 6,21 5,88 2,02 1,53 1,19 0,42 0,40 0,39 0,06 |
| Elements | Ca Al Zn Ag Mg Cu Pb Co As Ti Ni Mn Sb Mo |
| (wt%) | 3,05 2,59 1,83 1,67 1,36 1,19 0,94 0,48 0,38 0,28 0,24 0,22 0,10 0,06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





