1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that affects the pediatric population, and its prevalence is rising globally. Classical approaches to ASD usually concentrate only on neuro-functional aspects. According to scientific studies, ASDs affect a variety of physiological systems, including the immune system, the sensory-motor system, and the gut-brain axis. Recent research suggests that connective tissue, which connects all bodily organs and systems, may have a role in the development of ASD and its associated co-morbidities. As a result, in autism, inadequate connection might cause increased sensitivity to environmental signals. The following interpretive model, known as the “connectivome theory,” considers variations in connective elements of shared mesodermal origin observed in various organs, as well as the evaluation and interpretation of ASDs by emphasizing somatic aspects and co-morbidities. Research has established a comorbidity and co-occurrence between genetic connective tissue disorders—like Ehlers-Danlos syndromes and hypermobility spectrum disorder—and autism within the same families [
1,
2]
Autoimmunity is one of the potential pathogenic causes for ASD, and it is receiving a lot of attention and being studied extensively. For over a decade, various groups have been examining the relationship between autoantibodies and ASD [
3]. Although ASD is not considered an autoimmune disease, self-reactive antibodies or autoantibodies against a variety of targets have been found in a subset of ASD patients. Furthermore, autoantibodies reactive to fetal brain proteins have been discovered in the prenatal stage of neurodevelopment, when they can be transmitted from mother to fetus via transplacental transfer [
4].
Autoantibodies can pass the blood-brain barrier and produce immune complexes that damage neurological tissue. Some autoantibodies could be regarded biological indicators for autism, and they may play a role in the pathology of this disorder [
5].
Recent evidence suggests that the nucleosome, a fundamental unit of chromatin and a natural byproduct of cell death, plays an important role in various autoimmune disorders because it is a primary target autoantigen for autoantibody-mediated tissue lesions [
6,
7].
Autoantibodies cause inflammation and injury to several organs, either directly or through the creation of immune complexes [
8]. The presence of anti-nuclear antibodies is the primary diagnostic test for systemic lupus erythematosus (SLE) and many others connective tissue diseases [
9]. The contributions of the primary participants in the pathogenic autoimmune response, namely, T cells, B cells, dendritic cells (DCs), and macrophages that are abnormally hyperactive in lupus, are dependent on elevated cyclooxygenase-2 (COX-2) expression and activity, which is similar to inflammatory cells in target organs.
Up-regulation of COX-2, and hence prostaglandin E2, is required for DCs survival, maturation, and activation [
10,
11], and DC hyperactivity in lupus results in immunogenic presentation of autoantigens [
12]. Not only T cells, but also DCs and macrophages in SLE rodent model constitutively hyper-expressed COX-2, and NF-κB activation, which is necessary for the functioning of these cells of the lupus immune system.
There is debate over the contribution of neuroinflammation and the immune system to the development of autism. Immune theories were not well supported until recently, but studying the relationship between immunological response and neuroinflammation may have significant clinical and therapeutic consequences. A ratio is typically defined as one variable divided by another. Ratios are commonly employed in biological sciences and are suggested for their higher predictive values than independent variables [
13]. In relation to this, it was interesting to find the relationship between COX-2/ prostaglandin E2 (PGE2) ratio as marker of inflammation and anti-nuclear antibodies as marker of abnormal immune response in the pathophysiology of ASD and its co-morbidities.
Abruzzo et al. [
14] emphasized the importance of employing receiver operating characteristic (ROC) curves as a good statistical tool for discovering biomarkers that are sufficiently sensitive and specific for early ASD diagnosis [
14]. Although more research is needed to determine their utility in predicting, evaluating, and assessing treatment approaches, ROC curves highlight the most statistically significant differences between patients and controls. The area under the curve (AUC) is a useful statistic for determining the predictive power of biomarkers. An effective way to evaluate the predictive power of biomarkers is to look at their AUC. A curve close to the diagonal (AUC = 0.5) has no diagnostic significance, whereas an AUC value close to 1.00 indicates a very good predictive marker. There is always a biomarker with appropriate specificity and sensitivity values when the AUC value is near 1.00 [
15]. When looking at potential biomarkers for ASD, high sensitivity suggests that most patients will have ASD diagnosed; high specificity, on the other hand, means that healthy people will hardly ever test positive for the variable being studied. Additionally, adding two different markers in ROC curve analysis typically boosted their specificity [
15] indicating that using a panel of variables rather than a single variable may be very beneficial as a diagnostic tool.
2. Materials and Methods
The ethical committee of medica collage, King Saud University accepted the study protocol in accordance with the most recent Declaration of Helsinki. The study recruited 35 autistic patients and 38 age and gender matched healthy controls. All subjects provided written informed consent through their parents and agreed to participate in the study. The study participants were enrolled at the ART Center (Autism Research & Treatment Center) clinic. The ART Center clinic sample population included children diagnosed with ASD. All study participants’ ASD diagnoses were confirmed using the Autism Diagnostic Interview-Revised (ADI-R), Autism Diagnostic Observation Schedule (ADOS), and 3DI (Developmental, Dimensional Diagnostic Interview) procedures. The study included autistic children aged two to twelve years old. All were simplex instances. All are negative for fragile x gene study. The control group was recruited from pediatric clinic at King Saud medical city whose mean age ranged from 2–14 years. Subjects were excluded from the investigation if they had dysmorphic features, or diagnosis of fragile or other serious neurological (e.g., seizures), psychiatric (e.g., bipolar disorder) or known medical conditions. All individuals were assessed for present and previous physical illnesses through a parental interview. Children with known endocrine, cardiovascular, lung, liver, kidney, or other medical conditions were excluded from the study. All of the patients and controls in the study ate a similar but not identical diet, and none of them followed a special high fat or fat restricted diet.
2.1. Biochemical Assays
2.1.1. Antinucleosome-Specific Antibodies
The ELISA technique (EUROIMMUN Medizinische Labordiagnostika AG, Germany) was used for this; samples were run in parallel on the same run with the same internal standards, randomly chosen, blinded, and with the expectation that any antibodies to highly purified human nucleosomes (antinucleosome-specific antibodies) present in diluted serum would bind in the microwells. Unbound serum antibodies are removed by washing the microwells. A conjugate/antibody/antigen complex is formed when the patient’s antibodies are immunologically linked to the horseradish peroxidase-conjugated anti-human IgG. Substrate is hydrolyzed to produce a blue hue when it is washed in the presence of bound conjugate. When an acid is added, the process is stopped and a yellow byproduct is produced. Photometrically, the intensity of this yellow color is measured at 450 nm. The quantity of color corresponds exactly to the number of IgG antibodies in the original sample. All samples were examined twice in two separate trials to maximize accuracy, evaluate inter-assay differences, and guarantee repeatability of the obtained outcomes (P > 0.05). There was no discernible interference or cross-reactivity.
2.1.2. Cyclooxygenase-2 (COX-2)
A quantitative sandwich enzyme-linked immunosorbent assay (ELISA) kit from CUSABIO (8400 Baltimore Avenue, Room 332 College Park, MD 20740) was used to measure the levels of COX-2. With a minimum detectable dosage of 0.31 ng/ml, the measurement was carried out in accordance with the instructions supplied by the manufacturer.
2.1.3. Prostaglandin E2
A study kit from USCN Life Science (Wuhan, China) was used to quantify PGE2. This assay uses the competitive inhibition enzyme immunoassay method, in which a monoclonal antibody specific for human PGE2 was pre-coated on a microplate. Usually, PGE2 can be detected at a minimum of less than 1.78 pg/ml.
2.1.4. Statistical Analyses
The study evaluated data with IBM SPSS software version 22.0 (IBM Inc., Armonk, NY, USA). The Shapiro-Wilk Test was used to establish data normality in each group. The results were presented as the minimum, maximum, and median. The Mann-Whitney Test was used to compare two nonparametric groups, and p-values ≤ 0.05 indicated a significant difference. The Spearman rank correlation coefficient (R) was utilized to link various nonparametric variables. The ORs from logistic regression analysis show the correlation between biomarkers and clinical state in the combined ROC curve. ROC curves were created for all logistic regression models. A nonparametric method was used to compute the area under the curve (AUC) for each marker combination. In logistic regression, odds ratios larger than one signify “positive effects” since they raise the likelihood. Due to their tendency to lower the probabilities, those between 0 and 1 are referred to as “negative effects”. A ratio of odds of exactly one indicates “no association.” A ratio of odds cannot be smaller than zero.
3. Results
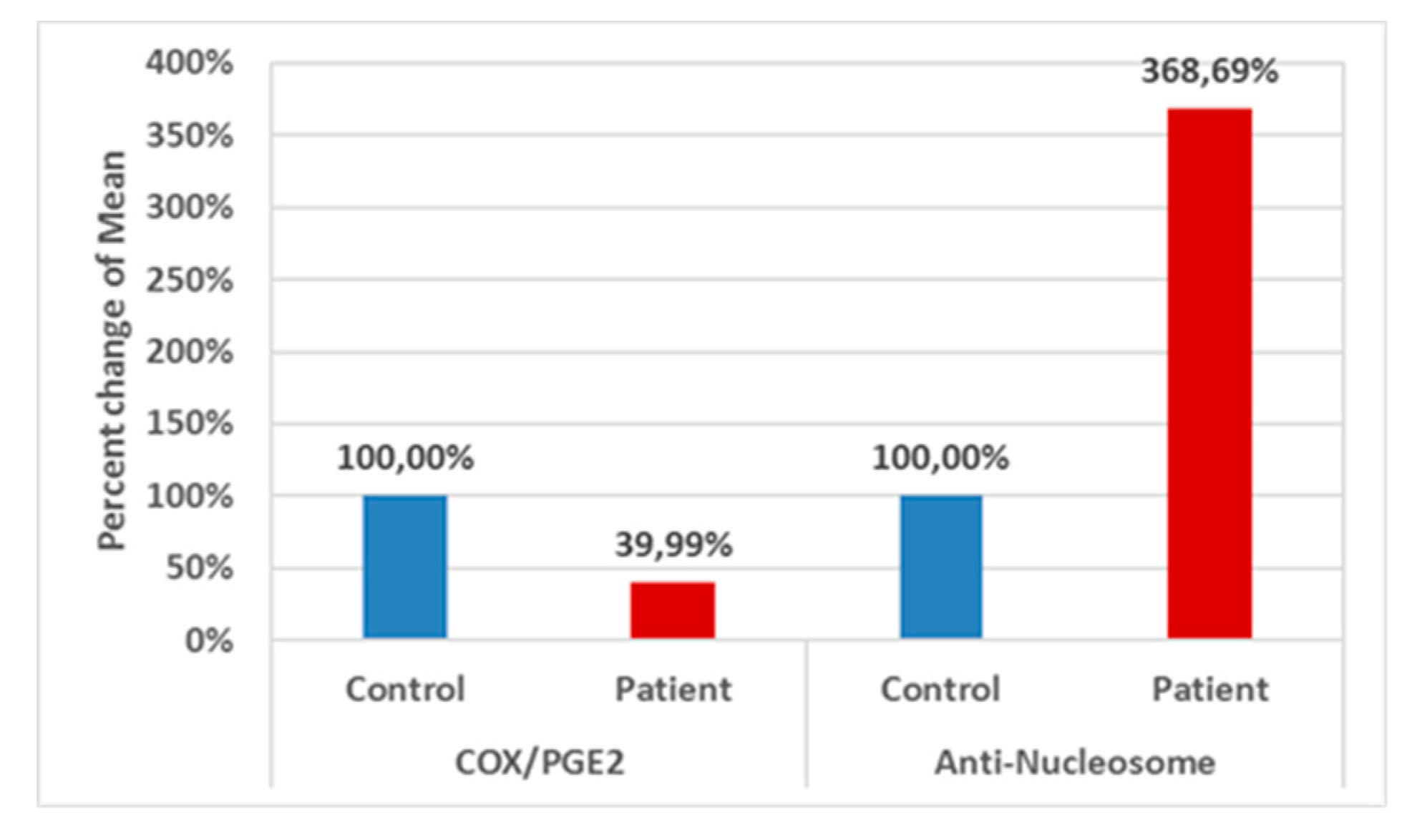
Table 1 and
Figure 1 describes comparison between the control group and patient group for each parameter using the Mann–Whitney Test (nonparametric data). A significant decrease of COX/PGE2 ratio (−60.01% lower) together with a significant increase of anti-Nucleosome autoantibodies (268.69% higher) values with
P < 0.001 for both variables can be easily noticed.
Table 1 describes a comparison between the Control group and the Patient group for each parameter using Mann-Whitney Test (Non-parametric data).
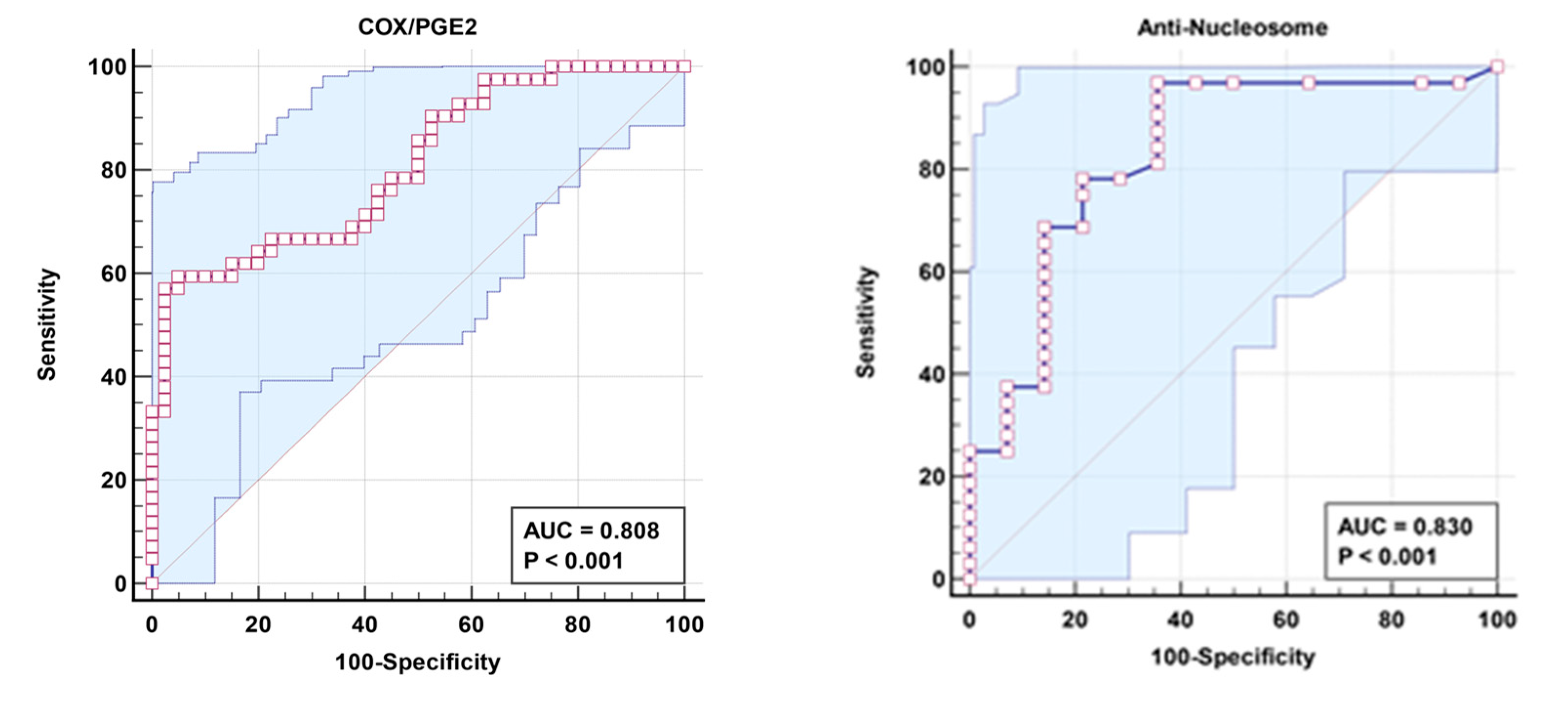
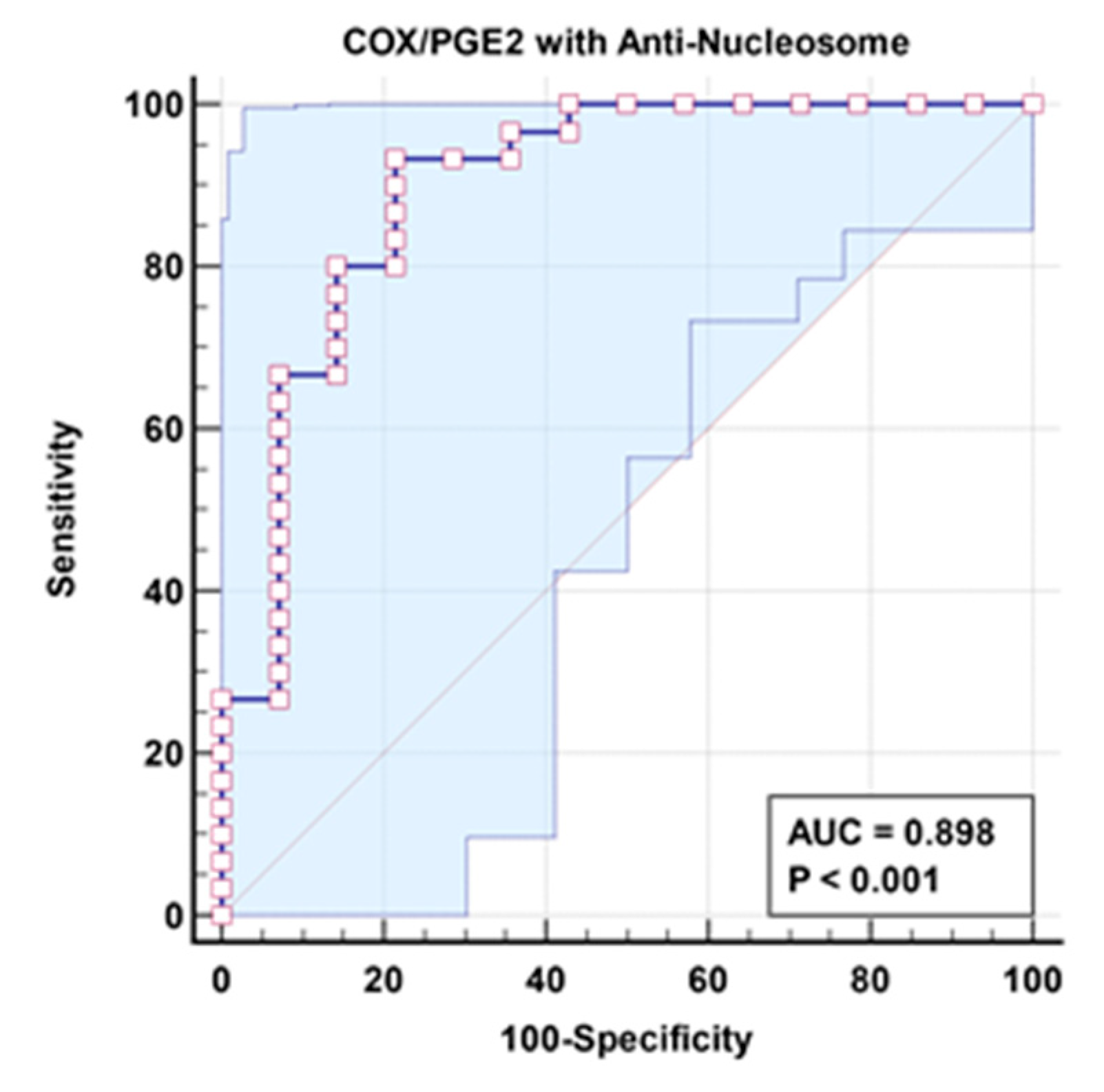
Table 2 demonstrates non-significant negative correlation between COX/PGE2 ratio and Anti-Nucleosome autoantibodies as markers of aberrant lipid metabolism and autoimmunity respectively. The remarkable increase in the independent AUC for COX/PGE2 and anti-nucleosome autoantibodies from 0.808 and 0.83, respectively (
Table 3 and
Figure 2), to 0.898 when combined (
Table 4 and
Figure 3) could easily demonstrate the integrative contribution of both signaling pathways as etiological mechanisms of ASD.
Table 4 describes Logistic Regression Test for Patient group as dependent variable, with (COX/PGE2 and Anti-Nucleosome) as independent variables using Enter method.
Table 5 presents the results of fitting the multivariate model including a test of significance for each predictor while controlling for the other variable. It proved that the effect of COX-2/ PGE2 and nucleosome autoantibodies as predictors of autism using standardized odds ratios (ORs) in the multivariate model differs in order and magnitude from that in the univariate model.
4. Discussion
Recent research reveals that ASD are caused by combinatorial molecular alterations that affect synapse and circuit activities. Defining the molecular alterations generated by ASD-linked risk factors is crucial for understanding ASD etiology and identifying viable diagnostic and treatment strategies [
16].
PGE2 is an endogenous lipid molecule that plays an important role in normal brain development. COX2 is the primary regulator of PGE2 production. Normal COX2/PGE2-mediated signaling is involved in fundamental brain functions such as dendritic spine formation, synaptic plasticity and memory and learning [
17,
18,
19]. Emerging clinical and molecular research provides persuasive evidence that aberrant COX2/PGE2 signaling is linked to ASD [
20]. It is well accepted that inflammation stimulates COX-2-catalyzed PGE2 production in CNS neuronal cells and that is related to inflammatory pain hypersensitivity as a well-known feature in ASD [
21,
22,
23]. Moreover, a link between aberrant COX2/PGE2 signaling and autistic-related behavior in (COX)-2− mice, adding to existing clinical and molecular data pointing to this pathway as a potential candidate for autism [
20].
The remarkable increase of independent COX2 and PGE2 [
24] together with the significant decrease of their ratio (COX2/PGE2) as marker of aberrant lipid metabolism signaling in autistic individuals compared to normal healthy controls (
Table 1 and
Figure 1) could be explained on the basis of altered COX-2 enzyme kinetics in ASD patients. It is interesting to mention that, as the Km (Michaelis constant) of an enzyme, relative to the concentration of its substrate under normal conditions permits prediction of whether or not the rate of formation of product will be affected by the availability of substrate, so the significantly lower COX-2/PGE2 ratio in ASD patients recorded in the current study, could help to suggest a COX-2 enzyme with remarkably lower Km and higher affinity to AA (Arachidonic acid) as substrate. This suggestion could find support by considering various abnormalities in key components of the COX2/PGE2 pathway due to both genetic and environmental influences which have been implicated in clinical studies on ASD [
25,
26]. For instance, increased ratios of AA to omega-3, decreased total AA and increased PGE2 levels previously reported in blood samples of human patients with ASD [
27,
28,
29].
The mechanisms underlying the remarkable increase of anti-nucleosome specific autoantibodies in ASD patients (
Table 1 and
Figure 1) remain unknown. Given the prevalence of nucleosomes in several autoimmune disorders, such as SLE [
30], it has been postulated that substantially accelerated rates of apoptosis [
31], as well as aberrant locations or faulty processing of apoptotic cells, may contribute to autoantibody formation [
32]. Nucleosomes may also stimulate lymphoproliferation and IgG synthesis in splenic B cells, as well as the generation of interleukin 6. This could result in polyclonal activation, which causes both specific (nucleosome-driven) and nonspecific antibody production [
30]. Altering these specified parameters confirms the role of apoptosis and neuroinflammation pathways in autism etiology [
33].
In an attempt to explain this combining signaling (
Table 4 and
Figure 3) and how it could be related to ASD clinical presentation, it was interesting to present that dysfunction of β-catenin (β-cat) as a component of Wnt signaling leads to impaired social interaction and increased repetitive behaviors, as well as altered expression levels of genes linked to ASD in humans [
16]. Previous research indicates that PGE2-dependent signaling can join with the canonical Wnt signaling pathway at the level of β-catenin via EP1-4 receptors (prostaglandin E2 receptor 1) [
34]. PGE2 regulates the effects of wnt signaling through cAMP/PKA activity, it may directly regulate β-catenin destruction by the nucleosome, and subsequent protein available for transcriptional activation which promotes gene expression and cell proliferation.
Based on the fact that excessive β-cat enhances dendritic branching, spine density, and synaptic function in cultured hippocampal neurons, indicating potential excitability imbalance, the remarkable decrease of COX-2/PGE2 and the increase of anti-nucleosome-autoantibodies reported in the present study could interrupt β-cat destructive mechanism, leading to accumulation of β-cat as contributor to glutamate excitotoxicity as an established etiological mechanism of ASD [
34,
35,
36]. Furthermore, it might be connected to abnormalities in connective tissues in ASD, which manifest as significantly elevated anti-nucleosome antibodies. The connectivome theory proposed by Zoccante et al. [
37] interprets the somatic comorbidities of ASD by taking into account differences in connective tissue components of shared mesodermal origin observed in different organs. Intestinal dysfunctions, malabsorption, and leaky gut syndrome are all conditions that may be connected to decreased intestinal connection. Furthermore, ASDs have a higher tendency to inflammatory events at the cutaneous and subcutaneous levels due to insufficient anti-inflammatory mediator release. ASD has also been linked to alveolar-capillary dysfunction, most notably interstitial inflammations, immune-mediated forms of allergic asthma, and bronchial hyperreactivity. This explanation could find support in previous study of Sherigar [
38] which reported that increased anti-nucleosome-autoantibodies is related to leaky gut as co-morbidity in individuals with ASD and other autoimmune disorders [
39].
Odds ratios larger than one in logistic regression represent “positive effects” because they enhance the likelihood. Those between 0 and 1 are referred to as “negative effects” because they tend to lower probabilities. A ratio of odds equal to one means “no association.” The ratio of odds must be smaller than zero. Based on this both the negative correlations presented in
Table 2 and the ORs presented in
Table 5 could help to confirm the contribution of increased anti-nucleosome-autoantibodies and the decreased COX-2/PGE2 in the etiology of ASD in positive and negative associations respectively.
Based on this, the significant alteration of COX-2/PGE2 and anti-nucleosome-autoantibodies (
Table 1), could be related to glutamate excitotoxicity as one of the major etiological mechanisms in ASD. Carlson et al [
40] hypothesized that the inducible isoform of the COX enzyme, known as COX-2, may mediate a significant relationship between neuroinflammation and glutamate-mediated excitotoxicity in neurological illness. According to their model, oligodendrocytes that produce COX-2 may be more vulnerable to glutamate-mediated excitotoxicity.
This can find support through considering the fact that offspring born to female SLE patients had a higher frequency of cognitive problems than healthy controls [
41,
42,
43]. While antibodies cannot pass through the mature blood–brain barrier into the adult brain, they can pass through the immature blood–brain barrier in developing embryos [
44]. Research has demonstrated that certain kinds of anti-DNA antibodies can bind to fetal neural receptors, leading to excitotoxicity which kill neurons [
44,
45,
46].
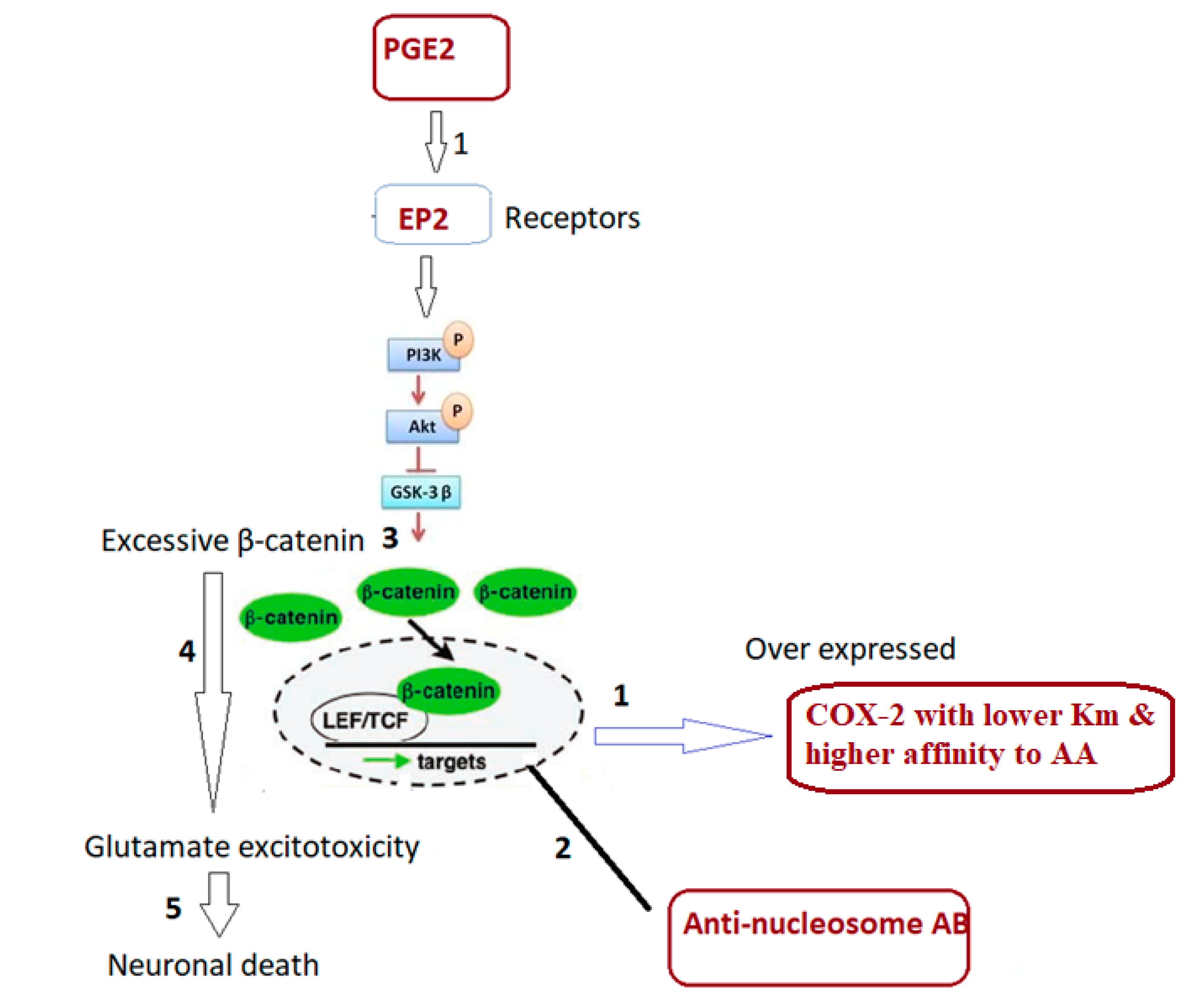
Within the context of canonical Wnt signaling, β-catenin is phosphorylated at particular sites by glycogen synthase kinase-3β (GSK-3β), which causes β-catenin to become ubiquitinated and degrade [
47]. Under the presence of aberrant COX-2/PGE2 ratio, excessive β-catenin accumulation together with elevated anti-nucleosome autoantibodies could lead to neuronal death in response to glutamate excitotoxicity (
Figure 4).
5. Conclusions
The COX2/PGE2 ratio, when combined with anti-nucleosome autoantibodies, has a higher predictive power than direct assessment of PGE2 levels and COX-2 enzyme activity [
24]. ASD patients with an abnormal COX-2/PGE2 signaling pathway have a significantly lower ratio, despite higher levels of independent variables [
24]. The increased AUC of combined ROC of COX/PGE2 and anti-nucleosome auto-antibodies along with their proven roles in impaired lipid metabolism, autoimmunity, and glutamate excitotoxicity as the disorder’s three etiological mechanisms (illustrated in
Figure 4) raise the possibility that these variables could be used to early diagnose ASD and certain co-morbidities seen in ASD patients.
Limitations
A disadvantage of the present study is the imperfect sample size and variations in the number of evaluated samples caused by insufficient blood withdrawn samples.
Author Contributions
Conceptualization, A.E.-A.; methodology, H.A.A., A.B.B. and L.Y.A.-A.; software, A.B.B.; validation, A.E.-A., H.A.A. and L.Y.A.-A.; formal analysis, A.B.B.; investigation, H.A.A.; resources, H.A.A.; data curation, L.Y.A.-A., A.B.B. and H.A.A.; writing—original draft preparation, A.E.-A.; writing—review and editing, A.B.B. and H.A.A.; visualization, H.A.A.; supervision, A.E.-A.; funding acquisition, H.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia (number RSP-2024R341).
Institutional Review Board Statement
This work was approved by the ethics committee of King Khalid Hospital, King Saud University (approval number: 11/2890/IRB) on 13 January 2019.
Informed Consent Statement
Written informed consent was obtained from the parents of all participants recruited for the study according to the guidelines of the ethics committee.
Data Availability Statement
The original contributions presented in this work are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (number RSP-2024R341), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baeza-Velasco, C., Grahame, R., and Bravo, J.F. (2017). A connective tissue disorder may underlie ESSENCE problems in childhood. Research in Developmental Disabilities 60, 232-242.
- Piedimonte, C. , Penge, R., Morlino, S., Sperduti, I., Terzani, A., Giannini, M.T., Colombi, M., Grammatico, P., Cardona, F., and Castori, M. (2018). Exploring relationships between joint hypermobility and neurodevelopment in children (4–13 years) with hereditary connective tissue disorders and developmental coordination disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 177, 546-556.
- Zou, T. , Liu, J., Zhang, X., Tang, H., Song, Y., and Kong, X. (2020). Autoantibody and autism spectrum disorder: A systematic review. Research in Autism Spectrum Disorders 75, 101568.
- Mazón-Cabrera, R., Vandormael, P., and Somers, V. (2019). Antigenic targets of patient and maternal autoantibodies in autism spectrum disorder. Frontiers in immunology 10, 1474.
- Elamin, N.E., and Al-Ayadhi, L.Y. (2014). Brain autoantibodies in autism spectrum disorder. Biomarkers in medicine 8, 345-352.
- Amoura, Z. , Piette, J.C., Bach, J.F., and Koutouzov, S. (1999). The key role of nucleosomes in lupus. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 42, 833-843.
- Mohan, C. , Liu, F., Xie, C., and Williams Jr, R. (2001). Anti-subnucleosome reactivities in systemic lupus erythematosus (SLE) patients and their first-degree relatives. Clinical & Experimental Immunology 123, 119-126.
- Yap, D.Y. , and Lai, K.N. (2015). Pathogenesis of renal disease in systemic lupus erythematosus—the role of autoantibodies and lymphocytes subset abnormalities. International journal of molecular sciences 16, 7917-7931.
- Didier, K. , Bolko, L., Giusti, D., Toquet, S., Robbins, A., Antonicelli, F., and Servettaz, A. (2018). Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Frontiers in immunology 9, 541.
- Baratelli, F. , Krysan, K., Heuzé-Vourc’h, N., Zhu, L., Escuadro, B., Sharma, S., Reckamp, K., Dohadwala, M., and Dubinett, S.M. (2005). PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. Journal of leukocyte biology 78, 555-564.
- Fogel-Petrovic, M. , Long, J.A., Knight, D.A., Thompson, P.J., and Upham, J.W. (2004). Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunology and cell biology 82, 47-54.
- Kalled, S.L. , Cutler, A.H., and Burkly, L.C. (2001). Apoptosis and altered dendritic cell homeostasis in lupus nephritis are limited by anti-CD154 treatment. The Journal of Immunology 167, 1740-1747.
- Atchley, W.R., and Anderson, D. (1978). Ratios and the statistical analysis of biological data. Systematic Zoology 27, 71.
- Abruzzo, P.M. , Ghezzo, A., Bolotta, A., Ferreri, C., Minguzzi, R., Vignini, A., Visconti, P., and Marini, M. (2015). Perspective biological markers for autism spectrum disorders: advantages of the use of receiver operating characteristic curves in evaluating marker sensitivity and specificity. Disease markers 2015, 329607.
- Metz, C.E. (1978). Basic principles of ROC analysis. In Seminars in nuclear medicine, Volume 8. (Elsevier), pp. 283-298.
- Alexander, J.M. , Pirone, A., and Jacob, M.H. (2020). Excessive β-catenin in excitatory neurons results in reduced social and increased repetitive behaviors and altered expression of multiple genes linked to human autism. Frontiers in synaptic neuroscience 12, 14.
- Chen, C. , Magee, J.C., and Bazan, N.G. (2002). Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. Journal of neurophysiology 87, 2851-2857.
- Chen, C. , and Bazan, N.G. (2005). Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins & other lipid mediators 77, 65-76.
- Sang, N., and Chen, C. (2006). Lipid signaling and synaptic plasticity. The Neuroscientist 12, 425-434.
- Wong, C.T. , Bestard-Lorigados, I., and Crawford, D.A. (2019). Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes, Brain and Behavior 18, e12506.
- Vardeh, D., Wang, D., Costigan, M., Lazarus, M., Saper, C.B., Woolf, C.J., FitzGerald, G.A., and Samad, T.A. (2009). COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. The Journal of clinical investigation 119, 287-294.
- Giuliano, F. , and Warner, T.D. (2002). Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. Journal of Pharmacology and experimental therapeutics 303, 1001-1006.
- Failla, M.D. , Gerdes, M.B., Williams, Z.J., Moore, D.J., and Cascio, C.J. (2020). Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain reports 5, e861.
- El-Ansary, A., Hassan, W.M., Qasem, H., and Das, U.N. (2016). Identification of biomarkers of impaired sensory profiles among autistic patients. PloS one 11, e0164153.
- Wong, C., and Crawford, D.A. (2014). Lipid signalling in the pathology of autism spectrum disorders. Comprehensive guide to autism 18, 1259-1283.
- Wong, C.T., Wais, J., and Crawford, D.A. (2015). Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. European Journal of Neuroscience 42, 2742-2760.
- Bell, J.G., Miller, D., MacDonald, D.J., MacKinlay, E.E., Dick, J.R., Cheseldine, S., Boyle, R.M., Graham, C., and O’Hare, A.E. (2010). The fatty acid compositions of erythrocyte and plasma polar lipids in children with autism, developmental delay or typically developing controls and the effect of fish oil intake. British Journal of Nutrition 103, 1160-1167. [CrossRef]
- Jory, J. (2016). Abnormal fatty acids in Canadian children with autism. Nutrition 32, 474-477.
- El-Ansary, A.K., Ben Bacha, A.G., and Al-Ayadhi, L.Y. (2011). Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids in health and disease 10, 1-9.
- Lorenz, H.M. , Grünke, M., Hieronymus, T., Herrmann, M., Kühnel, A., Manger, B., and Kalden, J.R. (1997). In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 40, 306-317.
- Amoura, Z. , Piette, J.C., Chabre, H., Cacoub, P., Papo, T., Wechsler, B., Bach, J.F., and Koutouzov, S. (1997). Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus. Correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 40, 2217-2225.
- Herrmann, M. , Voll, R.E., Zoller, O.M., Hagenhofer, M., Ponner, B.B., and Kalden, J.R. (1998). Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis & Rheumatism 41, 1241-1250.
- El-Ansary, A., and Al-Ayadhi, L. (2012). Neuroinflammation in autism spectrum disorders. Journal of neuroinflammation 9, 1-9.
- Buchanan, F.G. , and DuBois, R.N. (2006). Connecting COX-2 and Wnt in cancer. Cancer cell 9, 6-8.
- Yu, X. , and Malenka, R.C. (2003). β-catenin is critical for dendritic morphogenesis. Nature neuroscience 6, 1169-1177.
- Okuda, T. , Yu, L.M., Cingolani, L.A., Kemler, R., and Goda, Y. (2007). β-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proceedings of the National Academy of Sciences 104, 13479-13484.
- Zoccante, L. , Ciceri, M.L., Gozzi, L.A., Gennaro, G.D., and Zerman, N. (2022). The “connectivome theory”: a new model to understand autism spectrum disorders. Frontiers in Psychiatry 12, 794516.
- Sherigar, D.K. (2019). ORAL PAPERS FINAL. Indian Journal of Psychiatry 61, S452-S520.
- Kharrazian, D., Herbert, M., and Lambert, J. (2023). The relationships between intestinal permeability and target antibodies for a spectrum of autoimmune diseases. International journal of molecular sciences 24, 16352. [CrossRef]
- Carlson, N.G. , Rojas, M.A., Redd, J.W., Tang, P., Wood, B., Hill, K.E., and Rose, J.W. (2010). Cyclooxygenase-2 expression in oligodendrocytes increases sensitivity to excitotoxic death. Journal of neuroinflammation 7, 1-11.
- Ross, G. , Sammaritano, L., Nass, R., and Lockshin, M. (2003). Effects of mothers’ autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Archives of pediatrics & adolescent medicine 157, 397-402.
- Neri, F., Chimini, L., Bonomi, F., Filippini, E., Motta, M., Faden, D., Lojacono, A., Rebaioli, C.B., Frassi, M., and Danieli, E. (2004). Neuropsychological development of children born to patients with systemic lupus erythematosus. Lupus 13, 805-811.
- Lee, J.Y., Huerta, P.T., Zhang, J., Kowal, C., Bertini, E., Volpe, B.T., and Diamond, B. (2009). Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nature medicine 15, 91-96.
- DeGiorgio, L.A. , Konstantinov, K.N., Lee, S.C., Hardin, J.A., Volpe, B.T., and Diamond, B. (2001). A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature medicine 7, 1189-1193.
- Kowal, C., DeGiorgio, L.A., Lee, J.Y., Edgar, M.A., Huerta, P.T., Volpe, B.T., and Diamond, B. (2006). Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proceedings of the National Academy of Sciences 103, 19854-19859.
- Wang, L., Zhou, D., Lee, J., Niu, H., Faust, T.W., Frattini, S., Kowal, C., Huerta, P.T., Volpe, B.T., and Diamond, B. (2012). Female mouse fetal loss mediated by maternal autoantibody. Journal of Experimental Medicine 209, 1083-1089. [CrossRef]
- McCubrey, J.A. , Steelman, L.S., Bertrand, F.E., Davis, N.M., Abrams, S.L., Montalto, G., D’Assoro, A.B., Libra, M., Nicoletti, F., and Maestro, R. (2014). Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 28, 15-33.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).