1. Introduction
Recent advancements in nanomedicine have led to the development of nanogels (NGs) for drug delivery, gene therapy, and nanotheranostics [
1,
2,
3]. NGs are polymeric nanomaterials that possess the advantages of both hydrogels and nanoparticles (NPs). Indeed, both hydrogels[
4] and nanogels are polymeric materials with a three-dimensional cross-linked structure (at the nanometer level) capable of retaining large amounts of water or other fluids without dissolving, and are often biocompatible, making them suitable for biomedical applications. Hydrogels can vary widely in size, from a few micrometres to several centimetres, and are used in a wide range of applications, such as medical devices, cosmetics, tissue engineering, and bandages. Their structure can be moulded into various shapes to suit different needs. On the other side, nanogels are defined by their sizes between few and few hundred nanometers, which allow for specific interactions at the cellular and tissue level. Moreover, like for NPs, the functionalization of their surfaces allows various moieties to be linked at high densities (because of the high surface-to-volume ratio of nanoparticles), and this can be used e.g., for targeted delivery and/or for modulating the protein corona in biofluids [
5]. Due to their inherent porosity, NGs can encapsulate very efficiently both hydrophilic and lipophilic payloads, protecting them from rapid renal clearance and from degradation (e.g., by hydrolysis or enzymatic degradation) during storage or blood circulation, increasing therefore their circulation half-life. Finally, they can be made responsive to specific external stimuli such as pH, temperature, or specific molecules by exhibiting changes in the gel volume and water content (“swelling”), colloidal stability, mechanical strength, and/or other physical/chemical properties;[
6] this enables controlled and precise release of drugs or therapeutics. For all these reasons, nanogels are indeed often used in advanced applications such as targeted drug delivery, biomedical imaging, and diagnostics. However, NGs share with nanoparticle a relatively low translation: e.g., despite extensive research in the last decades, only 15 nanoparticle-based pharmaceuticals for cancer treatment are currently on the market [
7]. The bottlenecks in NP translation include limited scalability, poor control over reaction parameters, extensive NP polydispersity, unsatisfactory batch-to-batch reproducibility, and large volumes of chemicals and therapeutics used. These issues affect encapsulation efficiency and release profiles, hindering optimal treatment performance [
8]. Moreover, NGs usually suffer of a low mechanical and biological stability under physiological conditions, due also to physical interactions, since they are often “softer” than other kinds of NPs.
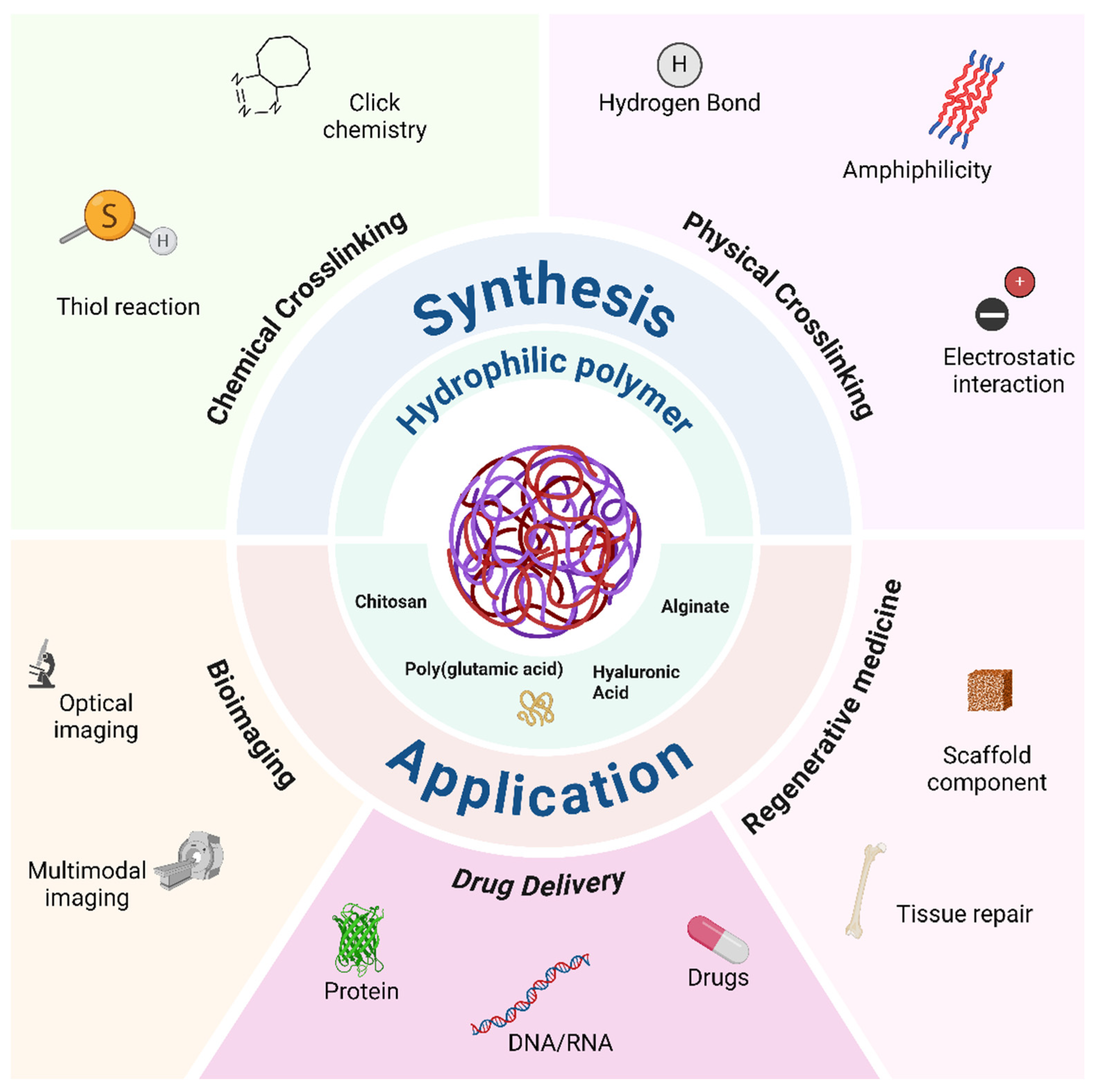
Nanogels can be composed of a variety of natural or synthetic polymers, depending on the specific applications and desired properties. Natural polymers include, e.g.,: (i) chitosan, derived from chitin, known for its biocompatibility, biodegradability and antimicrobial properties; (ii) alginate, extracted from algae, prized for its ability to form gels in the presence of divalent ions such as calcium; (iii) hyaluronic acid, a natural component of the extracellular matrix, chosen for its ability to retain water very efficiently. Among synthetic polymers, polyacrylamide is used for its ability to form strong gels and for the ease of derivatization due to the presence of the amine group, while poly 2-hydroxyethyl methacrylate (Poli(2-HEMA)), known for its biocompatibility and transparency, is often used in contact lenses and medical devices. Poly(N-isopropylacrylamide) (PNIPAM) is a thermoresponsive polymer useful for controlled drug release, featuring a lower critical solution temperature (LCST) of around 32°C in water: it forms temperature-sensitive gels that swell or shrink in response to temperature changes. Polyvinyl alcohol (PVA), chosen for its biocompatibility and solubility in water, forms tough films and gels,[
9] while poly(ethylene glycol) (PEG) is used to improve biocompatibility and reduce immunogenicity of nanogels. Polyglutamic acid (PGA), a biodegradable and biocompatible polymer, is utilized for its ability to enhance the stability and drug-loading capacity of nanogels [
10]. Nanogels are commonly classified according to the types of bonds involved in forming the polymer network, their stimulus-responsive capabilities[
11], routes of administration[
12] and applications[
13].
In this review, we will mainly focus on the synthesis methods of nanogels, highlighting their strengths and weaknesses; we will also describe synthesis protocols based on flow chemistry, in particular on microfluidics, since this can help in solving some of the bottlenecks for NPs translation in clinics mentioned above. In addition, we will illustrate the various applications of nanogels in different fields while also presenting a brief analysis of possible challenges for clinical translation (
Figure 1). Focusing mostly on relevant research from the past five years, this review aims to provide a comprehensive overview of the topic, highlighting current challenges and offering insights for researchers to overcome them.
2. Batch Synthesis
In the last several years, various techniques for creating nanogels have been discovered. NGs can be made via simultaneous polymerization and crosslinking or by polymerization first and subsequent crosslinking, depending on the used starting ingredients. There are several methods to synthesize them, such as ionic gelation, emulsion polymerization, precipitation polymerization, inverse nanoprecipitation, self-assembly and (micro)template-assisted polymerization [
14,
15]. For NGs, many polymerization processes can happen in an aqueous environment, due to the water solubility of most of the monomers and crosslinking agents used for the formation of nanogels.
Precipitation polymerization and reverse emulsion polymerization are the most widely used techniques based on simultaneous cross-linking and polymerization for creating nanogels. Precipitation polymerisation is a method in which soluble monomers are polymerised in a solvent in which the resulting polymer is insoluble. This process leads to the formation of polymer nanoparticles or nanogels through several key steps. Initially, the (multifunctional) monomers and a cross-linking agent are dissolved in a suitable solvent. A polymerisation is then initiated, usually by a thermal or chemical initiator, which leads to the formation of free radicals. These radicals initiate polymerisation of the monomers, and the cross-linking agent creates bonds between the polymer chains, forming a three-dimensional network. As polymerisation proceeds, the growing polymer chains become insoluble in the solvent and precipitate, forming nanogels. The size and structure of the nanogels can be controlled by adjusting the monomer concentration, the amount of crosslinking agent and the polymerisation conditions, such as temperature and time; in particular, controlled radical polymerization techniques, such as atom transfer radical polymerization (ATRP) and reversible addition–fragmentation chain transfer polymerization (RAFT), can slow the reaction rate to promote the formation of uniform particles [
13,
15,
16]. For instance, Ribovski
et al [
17]. used precipitation polymerization to produce fluorescently tagged PNIPAM nanogels, with the degree of polymer crosslinking determining the nanogels hardness. In another example, Kusmus
et al [
15]. described the development of versatile epoxide-functional precursor nanogels via controlled crosslinking polymerization; in a subsequent post-formation step, the epoxide moieties were functionalized with various amines, azides and thiols, as well as hydrolyzed to the corresponding diol.
Nanogels can also be synthesized using appropriate emulsification techniques with an oil-soluble emulsifier. Reverse emulsion polymerisation, also known as water-oil emulsion polymerisation, involves the dispersion of an aqueous phase containing monomers and a cross-linking agent in a continuous oil (or organic) phase (immiscible with water), with the aid of a surfactant. Polymerisation takes place within the dispersed water droplets, with the monomers reacting to form cross-linked polymer chains, leading to the formation of nanogels[
18]. The concentration of monomers and crosslinkers, the pH of the reaction medium, the choice of surfactant, and other parameters significantly influence the size of the nanogels formed during reverse emulsion polymerization. The drawbacks of this method include the use of an organic solvent as the reaction medium and the difficulty in purifying the resulting nanogels due to the presence of emulsifiers and co-emulsifiers.
While the simultaneous approach is widely adopted, nanogels can alternatively be synthesized through two sequential steps: polymerization and crosslinking. This sequential approach offers enhanced control over nanogel properties and functionalities, a topic that will be explored further in subsequent sections. In terms of post-polymerization crosslinking, nanogels are categorized into chemically crosslinked or physically self-assembled types: the first type rely on covalent bonds amongst different polymers, while the second one on non-covalent interactions amongst them. However, many nanogels incorporate both chemical and physical connections in their formation. Thus, this section will elaborate on these interactions and showcase examples of nanogels formed through these mechanisms. These diverse synthesis techniques provide versatile tools for tailoring nanogels with specific properties, making them highly adaptable for applications in drug delivery, diagnostics, and tissue engineering within the biomedical field.
2.1. Physical Methods
Physical methods for synthesizing nanogels rely on spontaneous interactions and arrangements of their components, resulting in rationalized, self-organized, and self-assembled supramolecular structures without covalent bonds. Despite having lower mechanical strength than covalently bonded systems, these physically constructed nanogels are favored because they do not require additional crosslinking agents or polymerization initiators, enhancing their safety and biocompatibility. The primary forces driving the formation of these entangled polymeric materials, including NGs, are often called host-guest interactions, and can include hydrogen bonds, electrostatic interactions, van der Waals forces, π-π stacking (interactions among aromatic rings), and hydrophobic interactions.
2.1.1. Electrostatic Interactions
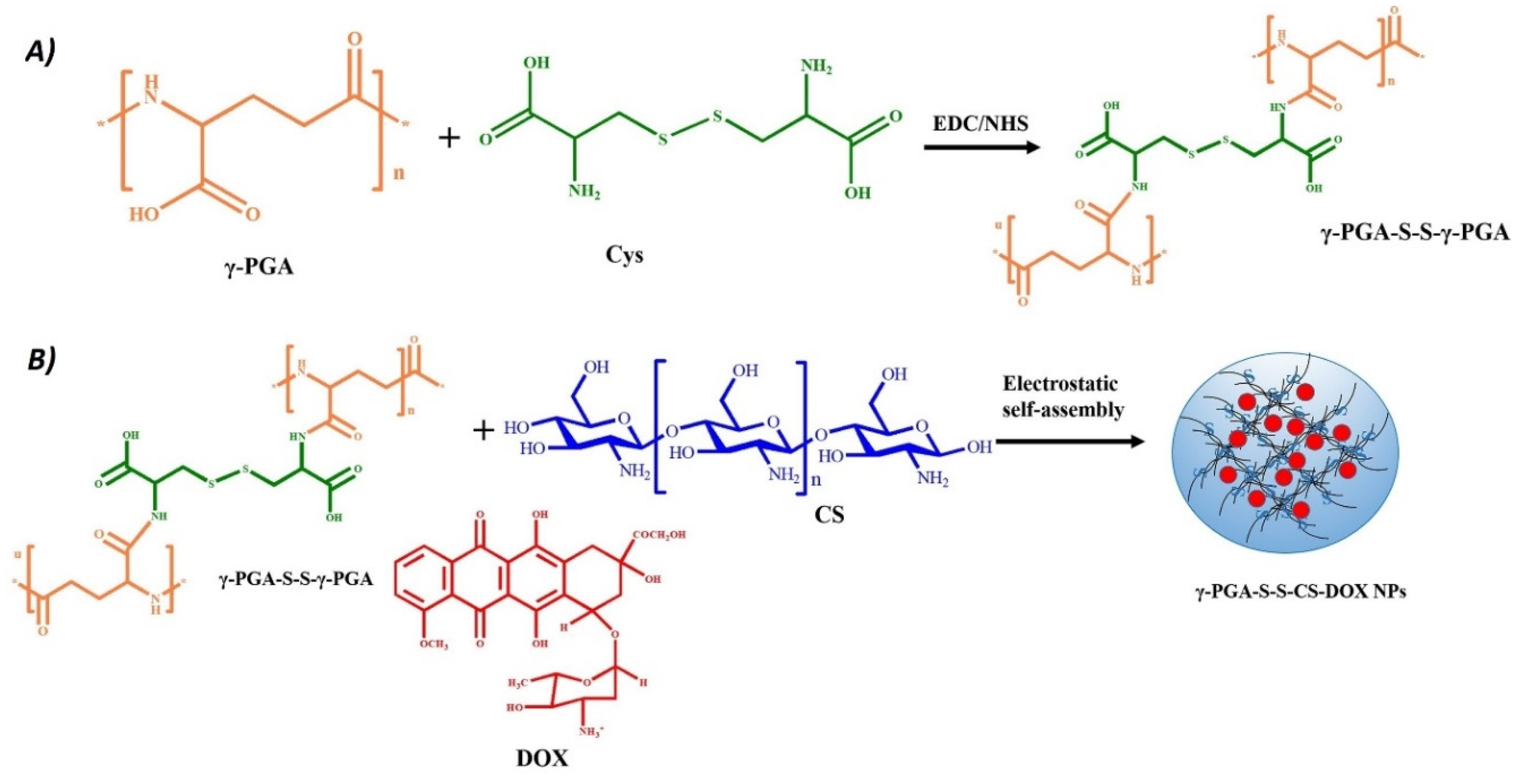
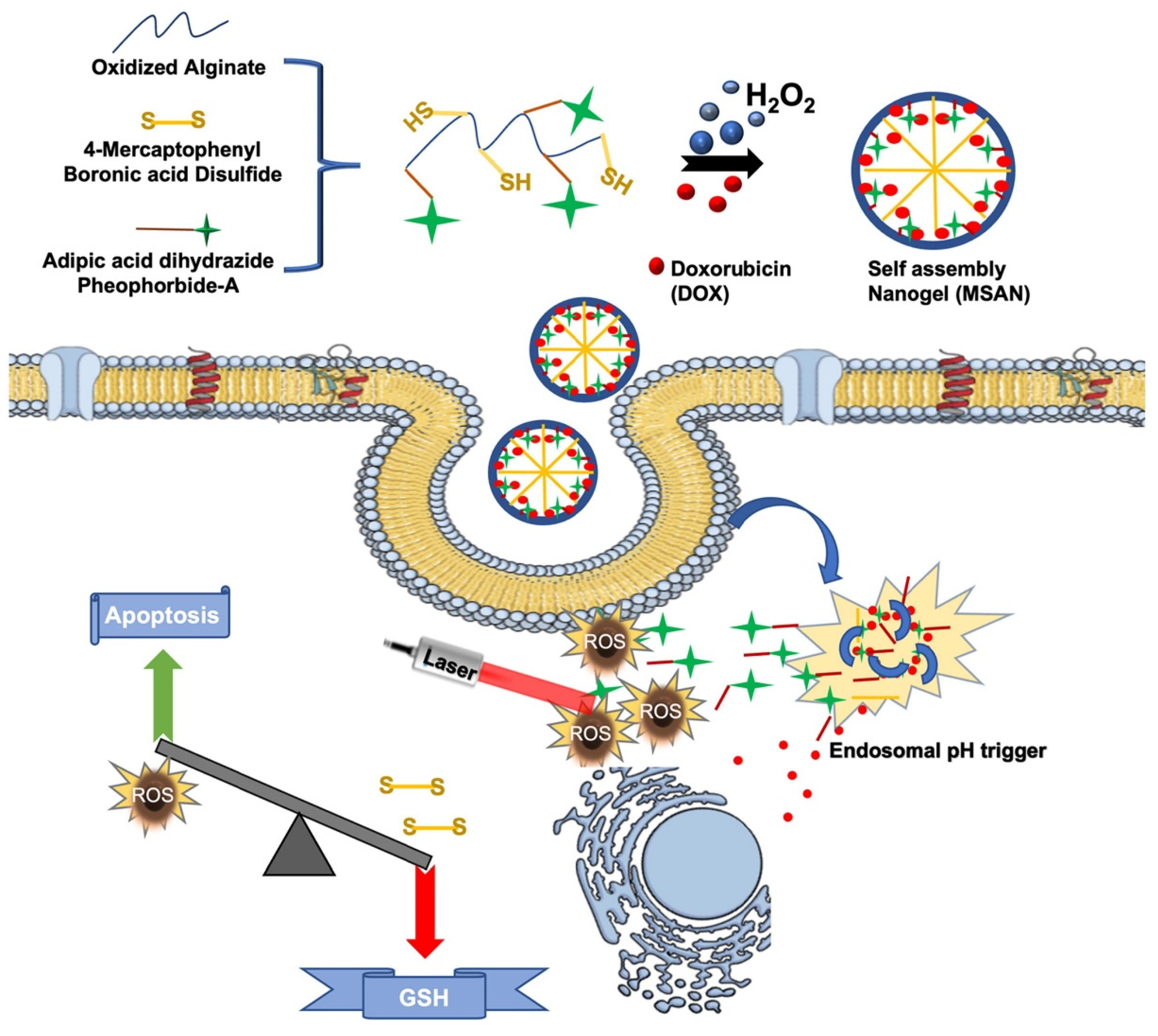
Nanogels with electrostatic interaction are created by the attraction between molecules with opposite charges, which facilitates their self-assembly and stability. These structures are typically constructed using polymers containing ionizable or ionic functional groups such as carboxylates, amines or quaternary ammonium ions. Polymers commonly used to create nanogels through electrostatic interactions include chitosan[
19], alginate[
20], hyaluronic acid (HA)[
21] and poly(glutamic acid) (PGA)[
22]. The synthesis of nanogels by physical cross-linking involves a simple and efficient procedure, usually conducted in an aqueous environment where the polymer chains are completely solvated and initially non-strongly interacting. When conditions are changed, e.g., by changing temperature, pH or ionic strength, the polymer chains begin to interact and form cross-links. For example, pH adjustment can protonate or deprotonate certain polymer functional groups, causing them to attract each other and form ionic cross-links. Another type of synthesis based on electrostatic interaction is ionic gelation, based on the use of polyelectrolytes forming cross-links in the presence of ions; it is a simple and rapid synthesis, but it is difficult to control given the very rapid assembly of polymers and crosslinkers, causing batch to batch variations in physiochemical properties such as particle size, polydispersity, surface charge and drug release profiles.
In general, electrostatic interactions cause the polymer chains to organize themselves into a nanoscale gel structure, effectively trapping water within the network. This straightforwardness not only simplifies production, but also makes the resulting nanogels ideal structures for biomedical applications, with reduced risk of toxic effects and an improved biological safety profile due to their synthesis without chemical cross-linking, which often requires non-biocompatible crosslinkers. One of the remarkable advantages of physically cross-linked nanogels is their adjustable size: by adjusting parameters such as polymer concentration, ionic strength, temperature and pH during synthesis, researchers can precisely control the size of nanogels[
23]. This tuning is crucial for adapting the properties of nanogels to the requirements of specific applications, such as drug delivery or tissue engineering. Furthermore, the ability to modulate the size of nanogels allows optimizing their pharmacokinetic profiles, cellular uptake and in vivo biodistribution. The electrostatically driven formation of nanogels allows for precise encapsulation of charged or polar drugs or biomolecules, which interacts with the charged groups in the nanogels components. This versatile system finds applications in encapsulating chemotherapeutic agents for improved treatment efficacy[
24], and more generally in theranostics[
25], where nanoparticles are formulated for simultaneous therapy and diagnostics. An example of synthesis of a drug-containing nanogel where also electrostatic self-assembly is involved is reported in
Figure 2.
2.1.2. Amphiphilic Properties
Polymers composed by hydrophobic and hydrophilic parts are called amphiphilic and can be used in the generation of amphiphilic nanogels. The synthesis of amphiphilic nanogels involves the dissolution of amphiphilic polymers in an aqueous solution, in which the hydrophilic segments interact with water and the hydrophobic segments tend to avoid it. By changing environmental conditions, such as temperature, pH or ionic strength, the polymers self-assemble, with the hydrophobic parts aggregating; often the structure of these kinds of nanoparticles is constituted by a hydrophobic core surrounded by a shell of hydrophilic segments. Amphiphilic nanogels offer many benefits. They possess the ability to swell in aqueous and organic media due to the mixture of hydrophilic and hydrophobic compounds. However, they exhibit a lower propensity to swell in water than nanogels consisting exclusively of hydrophilic polymers, consequently ensuring superior mechanical properties. In addition, they offer greater thermophysical and structural stability. In this formulation, the fundamental characteristics of the colloidal structure are driven by the primary structure of the polymer, including its composition, molecular weight, and branching. The type and abundance of hydrophobic groups along the polymer chains, which act as physical bonds, are particularly important, thus defining the network formation in the nanogel structure[
26]. Moreover, this formulation facilitates the encapsulation of poorly water-soluble payloads: the cargo loading capacity and release kinetics are set by the interaction between the hydrophobic cargo and the hydrophobic nanodomains[
27]. In addition, the flexible hydrophilic matrix allows to control the mechanical properties of the nanogel and to reduce the potential toxic effects of the hydrophobic groups. The regulation of these interactions can be achieved by varying the type and content of hydrophobic side groups on the polymer. This allows precise control over loading and release profiles[
28]. Consequently, the ability to finely tune the hydrophobicity of the nanogel, or the hydrophilic/hydrophobic balance, will open new therapeutic options for various administration routes[
29]. The amphiphilic structure offers the opportunity to regulate and tailor their interactions with biological systems; for example, Bewersdorff et al. produced amphiphilic nanogels with more or less idrophobic groups on their surface, and they were therefore able to control the protein corona formation and modulate interactions with biological barriers[
30]. Even if the self-assembly strategy offers considerable versatility, counting exclusively on physical bonds can be restrictive. To overcome this limitation, reactive groups can be incorporated into amphiphilic copolymers, facilitating covalent cross-linking after the self-assembly process (
Figure 3). This method stabilizes the properties of the nanoparticles, resulting in functional amphiphilic nanogels whose network characteristics can be precisely controlled by a combination of hydrophobic physical interactions and covalent cross-links. Covalent cross-linking of these self-assembled systems can be achieved through two main synthetic approaches. One incorporates all the necessary reactive groups directly into the amphiphilic copolymer. The other involves the reaction of the reactive copolymers with (bi)functional crosslinkers. These strategies, which employ crosslinking agents and chemical synthesis, will be discussed in detail in the following sections.
2.2. Chemical Methods
Covalent interactions in the synthesis of nanogels can provide essential stability and functional properties. Various chemical reactions are employed to form these bonds, including free radical polymerization, click chemistry, disulfide bond formation, and carboxyl-amine reactions. Each of these reactions offers distinct advantages in terms of specificity, efficiency, and stability, helping to ensure the integrity and functionality of the network under physiological conditions.
2.2.1. Covalent Crosslinking Reaction
Nanogels formulated using covalent crosslinking techniques have stable chemical bonds between polymer chains forming a three-dimensional network that can retain water without dissolving. By selecting appropriate polymers and crosslinking agents, the properties of the nanogels can be tailored for specific functionalities, such as pH sensitivity, temperature responsiveness, or redox responsiveness. The incorporation of various functional groups during the crosslinking process also enhances the versatility of chemically crosslinked nanogels, making them suitable platforms for a wide range of biomedical applications. Both the choice of the starting materials and the particle formulation methods (e.g., microemulsion[
32], precipitation) can be tailored to effectively shape and stabilize the nanogel three-dimensional structure. In a study by Tian et al [
33]., the initial fabrication of a nanogel exploited a modified emulsion crosslinking technique: poly(ethylene glycol) diglycidyl ether acted as a long binding agent, linking hyaluronic acid (HA) chains within the emulsions to create a loose nanogel structure. Next, cystamine was introduced as an additional binding agent between HA chains via an EDC/NHS (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide) reaction to create a more compact nanogel. In this way, the nanogel became reactive to the glutathione (GSH) present in a tumor environment and in particular in the cytoplasm of cells: GSH breaks the disulfide bridge in the cystamine, reverting the nanogel to a loose structure with consequent release of the drug loaded within.
The formation of amide bonds, used e.g., for the reaction with cystamine mentioned above, is a common approach to chemical crosslinking: these bonds are typically formed from carboxyl and amine groups using carbodiimide chemistry or by generating activated esters. In the synthesis of nanogels, amide bonds can serve a dual role. They can act as crosslinking points within the nanogel network, contributing to its structural integrity. Moreover, they can function as linkers that facilitate the conjugation of drug molecules or other bioactive compounds to the nanogel structure. Apart from amide bonds, numerous other chemical bonds can be used for crosslinking in nanogel synthesis. These include disulfide bonds, which will be discussed later, and ester bonds formed by hydroxyl and carboxyl groups
2.2.2. Click Chemistry
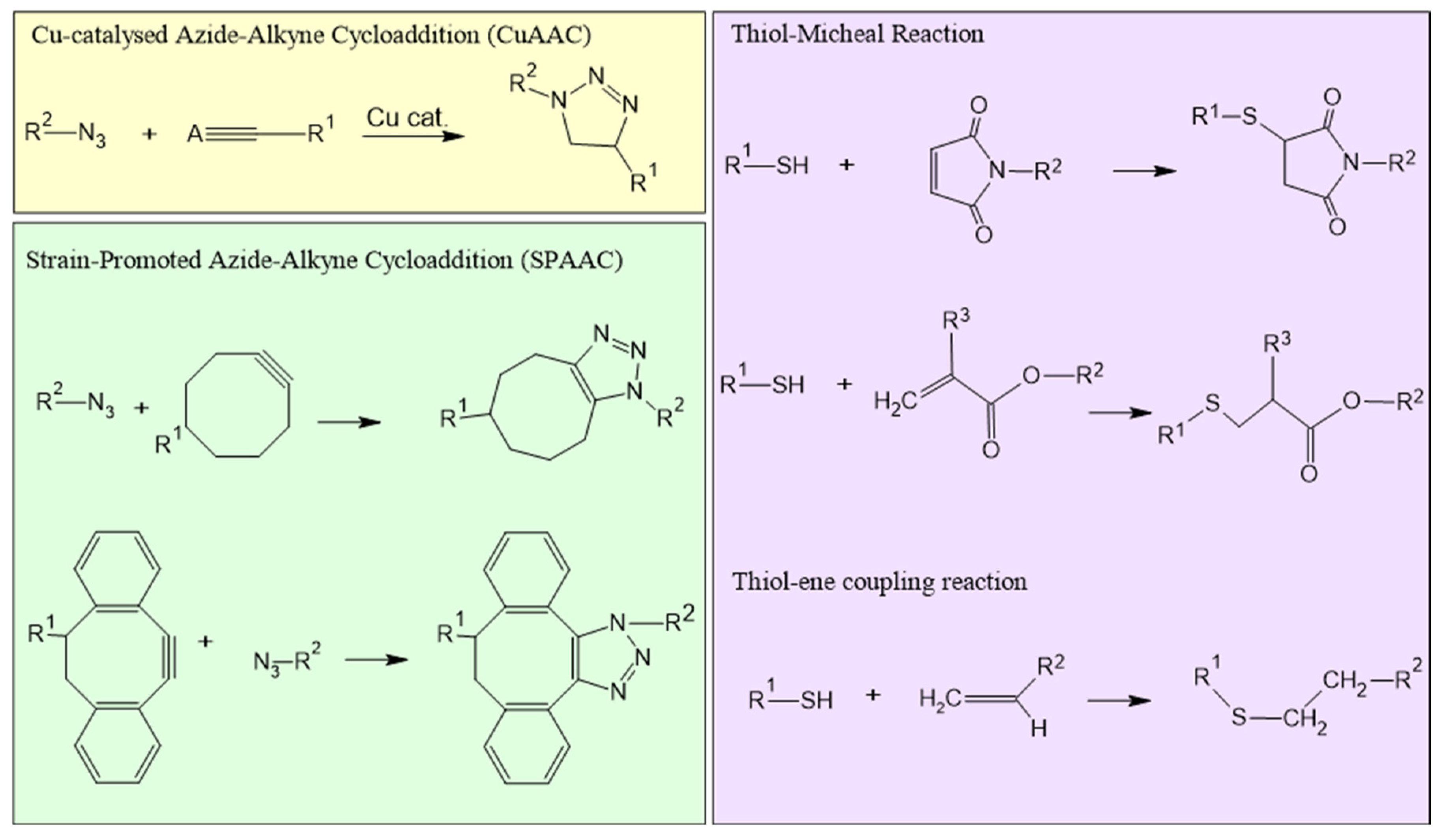
Click-chemistry (
Figure 4) involve reactions commonly used for joining two molecular entities of choice with very high chemical yield towards a single product; click-reactions are widely insensitive towards solvent parameters, oxygen, and water, and happen in mild (e.g., physiological) conditions[
34]. Typically, these reactions take place in an aqueous environment, ensuring compatibility with sensitive biological components. Furthermore, these reactions evolve according to a mechanism, called regiospecificity, whereby one of the possible functional isomers is generated preferentially, if not exclusively. Click chemistry is widely used in nanogel synthesis due to its efficiency, specificity and ability to form stable covalent bonds. This approach has significant advantages in nanogel production, including short reaction times, higher productivity and improved purity. These characteristics make click chemistry an ideal method for tailoring nanogels with precise control over their properties. Reactions such as azide-alkyne cycloaddition and thiol-ene reactions are particularly useful for cross-linking polymer networks within nanogels. Azide-alkynic cycloaddition, which includes variants such as Cu(I)-catalysed (CuAAC) and strain-promoted (SPAAC) reactions, involves the covalent binding of azide and alkynic groups to create 1,2,3-triazole bonds[
34]. CuAAC offers high specificity and fast kinetics under mild conditions; however, the application of CuAAC is somewhat limited by the potential cytotoxicity of copper ions and their ability to generate reactive oxygen species (ROS), which can damage biomolecules; sometimes, it is possible to mitigate these effects by removing the copper ions. Duro-Castano et al. optimised CuAAC coupling conditions in aqueous solution prior to the preparation of polyglutamic acid-based NGs to achieve higher cross-linking efficiency using the minimum amount of catalyst. Furthermore, they were able to establish washing protocols that used acidic conditions to protonate the carboxylic acid groups and thus hinder their complexation with the remaining copper ions[
35] (
Figure 5). Copper-free click reactions, including SPAAC, which utilize strained alkenes like dibenzocyclooctyne (DBCO), generally involve bioorthogonal cycloadditions, which allows them to take place within living systems without interfering with native biochemical processes and are therefore widely applied in nanogel synthesis due to their biocompatibility[
36]. For example, Nagel et al. reported a peptide-crosslinked nanogel in which dendritic polyglycerol (dPG) modified with bicyclononyne groups (BCN), to form dPG-BCN, was crosslinked by a matrix metalloproteinase (MMP)-sensitive peptide ligand modified with two azides[
37]. These reactions, together with thiol-ene coupling, disulfide exchange reactions and Michael reactions, which will be discussed in detail below, highlight their exceptional suitability for nanogel preparation.
2.2.3. Click-Like Reactions
Other reactions that are included in the category of “click chemistry” are thiol-click chemistry (
Figure 4), which covers various thiol-based reactions, such as thiol−alkene and thiol−alkyne reactions, Michael addition, and disulfide exchange. Thiol-based reactions are advantageous due to their moderate orthogonality, allowing them to proceed in the presence of various functional groups without interference. They are highly reactive and easy to perform, typically under mild conditions, and they generally produce high yields. Their efficiency, undemanding reaction conditions and high specificity make them particularly suitable for nanogel synthesis and other biomedical applications. In organosulfur chemistry, the thiol-ene reaction, also called alkene hydrothiolation, involves the reaction between a thiol (R-SH) and an alkene (R
2C=CR
2) to produce a thioether (R-S-R – note that “R” is a generic organic group, possibly different wherever it appears). Thiol-ene additions can occur through two mechanisms: free-radical additions and Michael-catalyzed additions. Free-radical additions can be triggered by light, heat or radical initiators, which generate thiyl radicals, while thiol-ene-Micheal addition is catalysed by a base or a nucleophile.
Thiol-ene-Michael addition is a significant member of the “click” chemistry family, which has many parallels with CuAAC and SPAAC reactions. However, a crucial difference is the natural presence of reactive groups. Azides and alkynes are absent in native biomolecules, allowing CuAAC to effectively functionalize only the particles in complex, living environments. In contrast, thiol-ene chemistry is very advantageous for conjugating or functionalizing colloids with biomacromolecules, such as proteins, due to the presence of thiols in cysteine-containing macromolecules. It can be used for the complete cross-linking of nanogels during their synthesis, but, to a reduced extent, also for the surface functionalization of nanogels. These functionalized nanogels can then be employed in various fields, including biosensing, bioimaging, drug delivery, and theranostics[
38,
39,
40]. Among the most efficient Michael-type additions, there are the reactions between thiols and maleimides. The primary driving forces for this reaction are the electron-withdrawing effect of the two adjacent activating carbonyl groups and the release of ring strain upon product formation. The reaction between maleimides and thiol-containing biological molecules has been employed since 1949 [
41]. However, it was not until 1980 that thiol-maleimide reactions were recognized as potential tools for nanocarrier functionalization. The maleimide-thiol reaction is widely used in functionalization protocols due to the high reactivity of maleimides under mild conditions, their selectivity for thiol groups at physiological pH and the stability of the resulting thioether bond under physiological conditions. In addition to surface functionalization applications, the thiol-maleimide reaction is also used in the synthesis and modification of nanogels for various biomedical purposes[
42,
43] (
Figure 5). E.g., Altinbasak et al. prepared a nanogel system cross-linked through the Michael thiol-maleimide addition reaction, which can be degraded in a reducing environment through a thiol-disulfide exchange reaction [
44]. A significant disadvantage in this reaction is the potential hydrolysis of maleimide groups in aqueous solutions, resulting in the formation of maleamic acid, which inhibits reaction with thiols. This secondary reaction can significantly reduce the degree of functionalization and negatively affect the properties of the resulting system overall. Disulfide cross-linking, a reaction similar to the other reactions discussed so far, is also a commonly used method in nanogel synthesis, which offers unique advantages for various applications. In this approach, disulfide bonds (-S-S-) are formed between polymer chains having thiol moieties, resulting in a three-dimensional network structure. In addition, the cleavable nature of disulfide bonds in response to certain stimuli, such as glutathione or reactive oxygen species (ROS),[
45,
46] enables the controlled release of encapsulated therapeutic agents within target cells or tissues. This responsiveness to environmental stimuli enhances the therapeutic efficacy and biocompatibility of disulfide-crosslinked nanogels, making them promising candidates for various biomedical applications[
47,
48,
49].
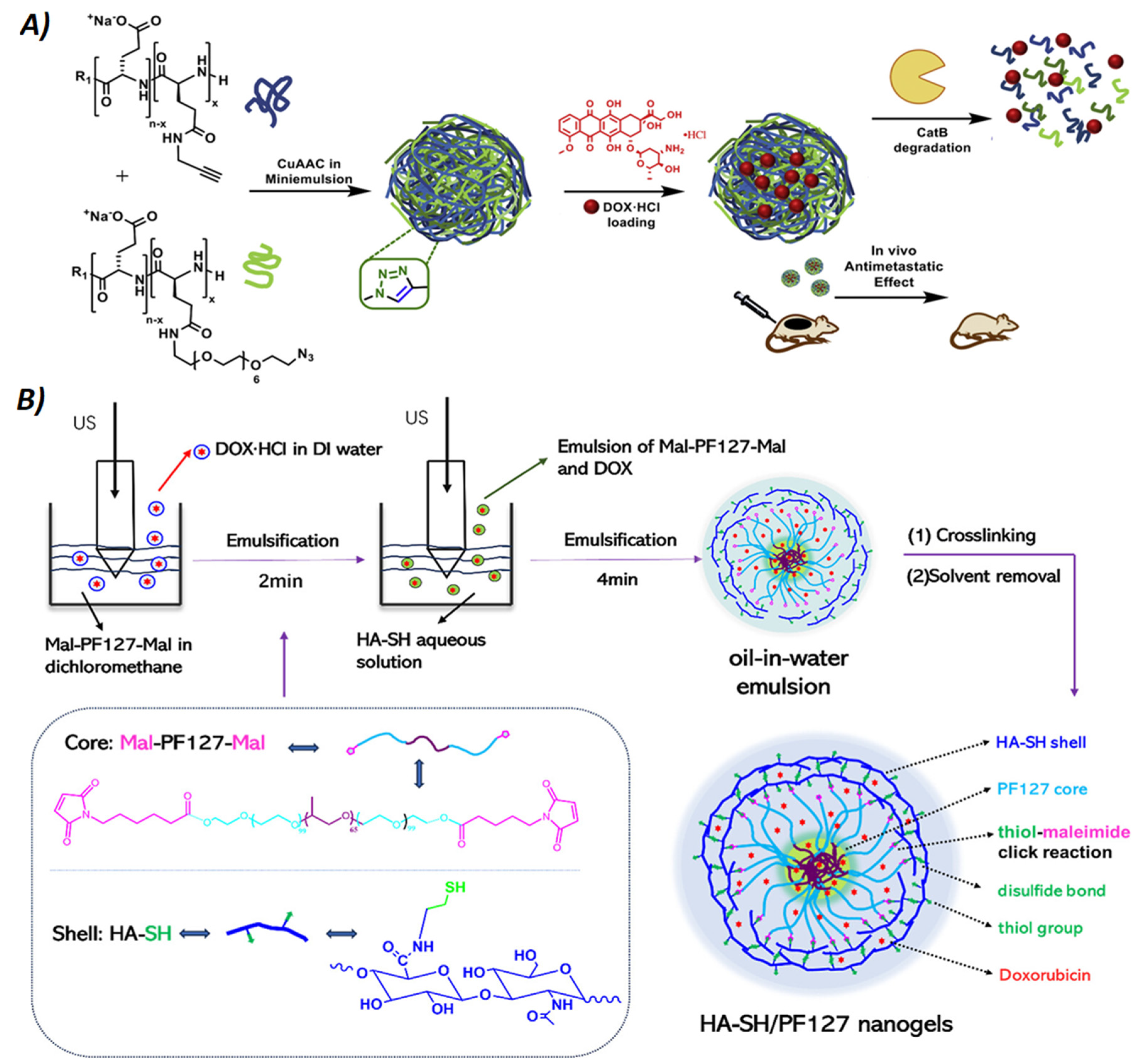
Figure 5.
A) Synthesis and mode of action of bioresponsive polyglutamic acid nanogels: nanogels are obtained by miniemulsion of azide- and alkyne-modified polyglutamic acid (blue and green filaments, respectively) cross-linked by click reactions (CuAAC) and then loaded with Doxorubicin (DOX). The image also shows the degradation mechanism mediated by Cathepsin B (CatB), a lysosomal enzyme that is overexpressed in the stroma of some types of tumors. Reprinted with permission from Ref. [
35]. B) Illustration of the preparation of mucoadhesive hyaluronic acid thiol (HA-SH)/pluronic acid (PF127) nanogels by thiol-maleimide click reactions. Using an ultrasonicator probe (US), nanogels were produced by a double emulsion technique: first by sonicating maleimide-modified PF127 with doxorubicin, and then by adding in the second emulsion hyaluronic acid with thiol. Reproduced from ref. [
43]. with permission from copyright © 2024, American Chemical Society.
Figure 5.
A) Synthesis and mode of action of bioresponsive polyglutamic acid nanogels: nanogels are obtained by miniemulsion of azide- and alkyne-modified polyglutamic acid (blue and green filaments, respectively) cross-linked by click reactions (CuAAC) and then loaded with Doxorubicin (DOX). The image also shows the degradation mechanism mediated by Cathepsin B (CatB), a lysosomal enzyme that is overexpressed in the stroma of some types of tumors. Reprinted with permission from Ref. [
35]. B) Illustration of the preparation of mucoadhesive hyaluronic acid thiol (HA-SH)/pluronic acid (PF127) nanogels by thiol-maleimide click reactions. Using an ultrasonicator probe (US), nanogels were produced by a double emulsion technique: first by sonicating maleimide-modified PF127 with doxorubicin, and then by adding in the second emulsion hyaluronic acid with thiol. Reproduced from ref. [
43]. with permission from copyright © 2024, American Chemical Society.
3. Flow Chemistry Synthesis
All the synthesis routes described above can be used with flow chemistry. In flow chemistry, also known as continuous processing or continuous flow chemistry, two or more streams of different reagents are pumped at specific flow rates into a chamber, tube, or microreactor. A reaction occurs, and the flow containing the resulting compound is collected at the outlet. To generate the final product, the solution can also be directed to subsequent loops of continuous reactors [
50,
51,
52]. This route offers significant benefits over traditional batch chemistry, including enhanced mass and heat transfer, improved safety and reproducibility, increased reaction efficiency, reduced waste, and better scalability [
53]. Due to the intrinsic characteristics of continuous-flow reactors, it is possible to exploit reaction conditions not achievable in batch processes, allowing for precise control over reaction conditions and real-time monitoring, resulting in high-quality products and streamlined processes. Advancements in 3D-printing flow setups and affordable electronic toolkits have made flow chemistry more accessible [
54]. Continuous manufacturing, often coupled with photochemistry and photocatalysis, improves performance and safety while reducing costs [
55,
56,
57]. Key challenges include solvent compatibilities and byproduct formation, necessitating inline analysis and purification strategies. Optimizing mass and heat transfer is crucial in flow chemistry: efficient mass transfer correlates with mixing efficiency, while heat transfer can be optimized by a high surface-to-volume ratio and large heat exchange surfaces in microchannels. Efficient heat transfer allows for isothermal or superheated conditions, improving chemical selectivity and safety. Small reactor volumes and precise reaction conditions provide, beside of better mass and heat transfer, also an increased safety; hazardous or impractical reactions under conventional conditions can be safely conducted in flow conditions [
58]. Flow chemistry enables the use of lower reagent amounts, reducing costs and environmental impact. Scale-up can be achieved through numbering up or sizing up, maintaining the benefits of microreactor environments [
59,
60].
3.1. Microfluidics Systems
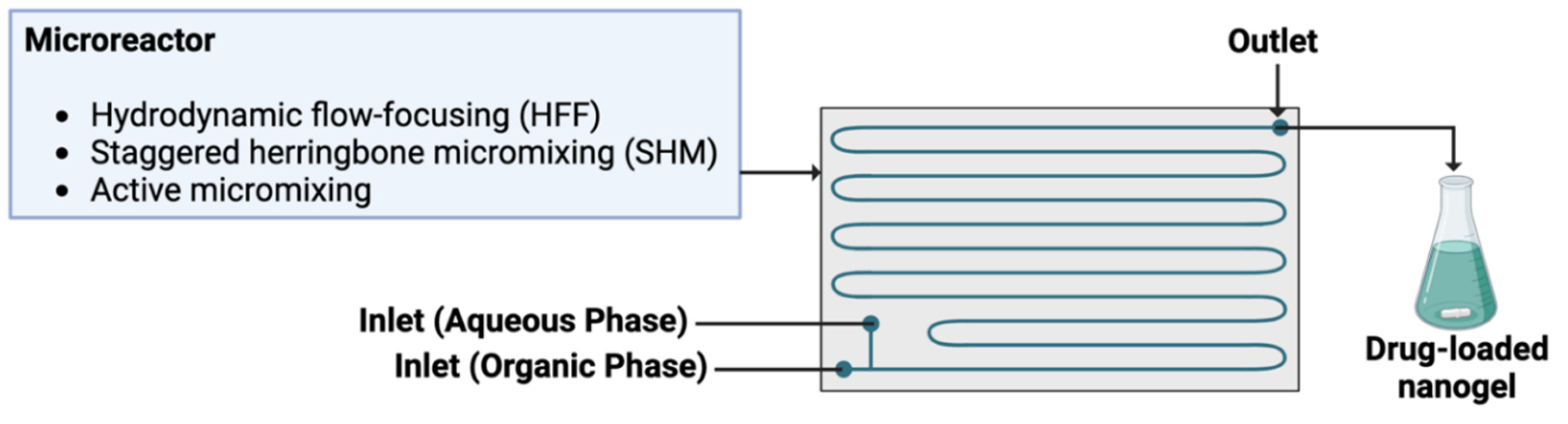
Several flow chemistry-based devices have been developed and optimized, and among them microfluidics stand out to be one of the most promising one. Microfluidics technology is based on fluidic circuits where the channels have lateral dimensions of ten to hundreds micrometers, and aims at miniaturizing the manufacturing nanoparticle production (
Figure 6). Compared to bulk synthesis, this approach leads to narrower size distribution, higher encapsulation efficiency, sustained drug release profiles, and highly controlled synthesis conditions. Moreover, in the photochemistry case, the small lateral dimensions of the microchannels allow a better and more homogeneous illumination even when molecules with high extinction coefficients are present. Additional benefits of microfluidics include low variation between batches, high throughput, reduced reagent volumes, low instrumental footprint, and scalable production yields. For these reasons, microfluidics is deemed a promising approach for the design of advanced micro- and nanoparticles and micro- and nanogels [
61]. Microfluidic approach can be employed for the production of several types of nanoparticles, from polymeric to lipidic to inorganic ones, by changing the microfluidic set-up and mixing parameters [
62]. E.g., for polymeric nanoparticles in general, several production strategies can be used exploiting microfluidics, like: (i) nanoprecipitation, with the mixing time between solvent and non-solvent phases more easily tweakable for a better control of nanoparticle nucleation and growth; (ii) self-assembly, which leverages the pH- or temperature-responsive behavior of amphiphilic derivatives; (iii) droplet-based methods, which adopts immiscible phases to create flowing micro-emulsion droplets that act as micro-reactors. While nanoprecipitation and self-assembly methods are prone to channel clogging due to undesired reagent interactions, droplet-based approaches provide rapid heat and mass transfer, enabling faster reaction kinetics and precise control over droplet size and composition [
63].
Common microfluidic designs are hydrodynamic flow focusing (HFF) and staggered herringbone micromixer (SHM) [
62]. HFF exploits the laminar flow regime typical of microfluidic platforms: a narrow stream of NP precursor (e.g., lipid or polymer dissolved in a solvent) flows parallel to an antisolvent (e.g., water or buffer) from two side channels. In this case, mixing occurs through diffusion due to the laminar flow [
64]. In contrast, SHM induces micromixing through chaotic advection caused by structures on the microchannel floor or walls, achieving shorter mixing times at lower flow rates, reducing therefore dilution and synthesis times [
65,
66]. Such passive mixing technologies can be coupled with active methodology to reduce the mixing time, control the nanoparticle size, and increase the throughput; active micromixing utilizes external energy sources to enhance mixing by disrupting the laminar flow regime, leading to faster homogenization and producing smaller NPs with narrower size distributions [
67,
68]. In this contest, ultrasound are noteworthy as they are generally employed to boost nucleation rates and significantly reducing NP mean size and polydispersity compared to other passive synthesis [
69,
70,
71].
However, the employment of microfluidics faces several challenges. A possible difficulty arises from the fact that many materials used for microfluidic devices, like polydimethylsiloxane (PDMS), swell upon contact with organic solvents, potentially adsorbing small molecules and affecting device structure and nanoparticle production efficiency [
72]. Moreover, the strong dependence of nanoparticle properties on multiple experimental factors calls for the application of a proper design of Experiments (DoE). DoE, and in particular response surface methodology (RSM), is meant to identify critical variables and their interactions in order to predict the most suitable experimental conditions while minimizing costs and reagent waste [
73,
74,
75].
3.2. Nanogels Produced through Microfluidics
Various methods for producing NGs via microfluidic platforms have been explored:[
13,
16,
76] (i) chemical gelation, involving emulsifying low-viscosity monomer solutions, followed by photoinduced cross-linking or polymerization, resulting in mechanically strong microgels; however, without proper optimization, these particles may not be easily enzymatically cleaved or metabolized, and photo-irradiation can be harmful to encapsulated biological species. (ii) Gelation induced by temperature changes after emulsification: e.g., heated agarose solutions can be cooled to form micro- or nanogels; this approach has challenges in maintaining temperature differences across the microfluidic device and potential harm to bioactive species. (iii) Coalescence-induced gelation: this strategy involves the coalescence of biopolymer droplets with crosslinking agent droplets, as demonstrated with alginate hydrogel microbeads; the productivity depends on droplet collision probabilities. (iv) Reversible shear thinning: droplets are formed from shear-thinning polymers that restore their network structure after emulsification to form microgels. (v) Internal gelation: droplets contain a gelling polymer and a bound crosslinking agent; a compound in the continuous phase diffuses into the droplets, releasing the crosslinking agent and causing gelation; (vi) external gelation: droplets of gelling polymer are emulsified in a continuous phase containing a crosslinking agent. Diffusion of the agent into the droplets causes gelation.
These kinds of techniques were initially used to produce microgels, and some of the optimizations carried out in those cases, and the applications of those microgels, can be considered also for NGs. E.g., Zhao et al [
77]. developed an injectable scaffold using droplet-based microfluidic technology and photo-crosslinking to synthesize bone marrow stromal cell (BMSC)-laden microgels; with a similar technique, Feng et al [
78]. engineered multifunctional microgels with a precursor suspension containing kartogenin-loaded cyclodextrin nanoparticles (KGN@CD NPs), BMSCs, gelatin methacryloyl (GelMA), and phenylboronic acid-grafted methacrylate hyaluronic acid (HAMA-PBA). These microgels were then assembled using dynamic crosslinking between dopamine-modified hyaluronic acid (HA-DA) and phenylboronic acid groups on the surface of the microspheres. Upon injection into a cartilage defect, HA-DA facilitated adhesion to native tissue, and the microporous microgel assembly along with sustained KGN release promoted BMSC chondrogenesis and cartilage repair [
78]. Seiffert and Weitz[
79] used microfluidic devices to produce monodisperse poly(N-isopropylacrylamide) (pNIPAAm) microgels via a polymer-analogous crosslinking reaction, achieving higher crosslinking efficiency and greater homogeneity compared to classical free-radical crosslinking copolymerization techniques. Zhang et al [
61]. prepared droplets-formed sodium alginate biomicrogels (hydrogel microbeads derived from biopolymers), whose synthesis is usually done in two-stage: emulsification followed by gelation of the resulting droplets by chemical or physical crosslinking of the biopolymer. They generated stable alginate nanogels through Ca
2+-mediated crosslinking, after having compared external and internal gelation in their microfluidic preparation. They showed that internal gelation had a limited application in this field, as it did not allow control over morphology and the resulting microgels were soft and not colloidally stable. In contrast, external gelation produced stable microgels with a good control of the structure [
61].
Microfluidic synthesis of nanogels has demonstrated significant improvements in control and efficiency.[
16,
76,
80,
81,
82] E.g., Bazban Shotorbani et al [
83]. synthesized alginate nanogels via ionic gelation using hydrodynamic flow-focusing microchips, achieving precise size control and monodispersity. Mahmoudi et al [
84]. fine-tuned the flow rate ratio on microchips to form alginate nanogels, while Huang et al [
85]. created hyaluronic acid nanogels via photo-click cross-linking on a microchip platform. Majedi et al [
86]. utilized modified chitosan on a T-shaped microfluidic chip, and Chiesa et al [
87]. formed chitosan/sodium triphosphate (TPP) nanogels using a staggered herringbone micromixer.
Pessoa et al [
88]. used microfluidic microchips in order to attempt to mitigate fouling issues in the production of chitosan/ATP nanoparticles, while Whiteley and Ho[
89,
90] addressed these fouling challenges by creating a 3D flow-focusing profile on a coaxial flow reactor (CFR), producing nanoparticles with smaller size and higher monodispersity than 2D methods. They also tried to predict the interaction effects of process factors (component concentrations and flow ratio) on the size, PDI and encapsulation efficiency of the nanogels [
89,
90]. Giannitelli et al [
1]. synthesized hyaluronic acid (HA) and linear polyethyleneimine (LPEI)-based nanogels for controlled doxorubicin delivery using a pressure-actuated microfluidic chip. The formation of NGs was confirmed by nuclear magnetic resonance (NMR) and Fourier-transform infrared (FTIR) analyses. NG specimens were also evaluated in terms of size and morphology through dynamic light scattering (DLS), atomic force microscopy (AFM), scanning electron microscopy (SEM) and tunneling electron microscopy (TEM) analyses, in order to define a correlation between the active tuning of the flow focusing geometry and the physical features of the resulting nanoscaffolds. The optimized DOX-containing nanogels demonstrated significant antitumor and anti-metastatic effects in vivo [
1].
4. Stimuli Responsive Nanogel
Nanogels for biomedical applications require stability under many conditions but can also be engineered to adapt and respond to external signals by altering their chemical and physical properties. Nanogels can respond to a variety of stimuli, including physical (temperature, light, ultrasound, magnetic/electric fields, pressure), chemical (pH, redox potential), and biological (enzymes, specific biomolecules) ones. Stimulus-responsive nanogels are promising nanoformulations that by their selective response to environmental signals improve therapeutic precision, offering versatile platforms for targeted therapies and diagnostics and minimizing systemic toxicity. Continued research into optimizing these nanomaterials has the potential to realize revolutionizing biomedical technologies, while also advancing precision medicine and patient care. Other reviews have described in more detail the stimuli-response properties of nanogels,[
11,
91,
92] so only a brief introduction will be provided here.
Among the various stimuli, pH is one of the most commonly used in biomedical science: healthy tissues have a pH around 7.4, tumor tissues range from 6.5 to 7.0; within cells, the cytosol has a pH similar to blood (7.4), the Golgi apparatus is at about 6.4, endosomes range from 5.5 to 6.0, and lysosomes are the most acidic at 4.5 to 5.0. pH-reactive nanogels exploit these variations to deliver drugs in a targeted manner, minimizing off-target drug loss. These nanogels are typically synthesized by incorporating acidic[
93] or basic functional groups into the polymer backbone or using cross-linking molecules that degrade under specific pH conditions[
94]. This design enhances the swelling and degradation of the nanogel, promoting the release of the encapsulated cargo[
95].
Redox-responsive nanocarriers show great promise for delivering payloads within cells by utilizing the natural redox gradient between intracellular and extracellular environments to trigger the release of encapsulated substances. The antioxidant glutathione (GSH) primarily regulates these redox potentials, and its cytosolic concentration in cancer cells is significantly higher than in normal tissues. This difference highlights the importance of redox-responsive drug delivery systems for targeted therapies[
96]. Recent studies on nanogels have focused on incorporating disulfide-based crosslinking monomers to achieve precise control over degradation kinetics. This strategy optimizes the balance between crosslinking and the encapsulation/release of therapeutic agents, leading to the development of nanogels specifically aimed at intracellular release in antitumoral applications[
44,
97,
98,
99].
Recent years have seen significant advances in nanogel technology, particularly in the development of systems that can recognize and respond to multiple stimuli. This progress involves the integration of various response functions into a single platform, thereby improving the versatility, effectiveness, and/or specificity of nanogel-based therapies [
100,
101]. By incorporating two or more stimulus-responsive components within a single nanogel delivery system, researchers aim to achieve a higher level of precision in therapeutic applications[
102]. These sophisticated nanogels can dynamically adjust their behavior in response to a combination of environmental stimuli such as pH, temperature, redox potential, and light exposure, enabling therapeutic payloads to be released in a controlled manner [
103] (
Figure 7).
5. Application
As already mentioned, due to their unique structural and functional properties, nanogels have acquired significant attention in biomedical applications. Their biocompatibility and ability to encapsulate drugs, proteins and other biomolecules allow for targeted and controlled release, minimising side effects and improving treatment efficacy. As already mentioned, the possibility of stimuli-responsive nanogels allows drugs to be delivered in a specific manner. NGs Cellular uptake and biodistribution behavior can also be controlled by different types of surface functionality: factors such as polarity and surface charge impact on the hydrophilicity of the nanogel and on its blood-circulation time. In recent years, nanogels have seen enormous developments in terms of design, optimisation, functionalisation and application. Therefore, in this section we will mainly focus on recently published biomedical applications.
5.1. Nanogels for Drug Delivery
Nanogel-based drug delivery systems have been very efficient in precisely delivering drugs to their target sites, significantly reducing toxicity to surrounding healthy cells. This remarkable potential has led to extensive research into their application for the treatment of diseases with high morbidity and mortality rates, with the aim of improving traditional therapies and patients’ quality of life. Many nanogels have a high encapsulation efficiency and drug loading capacity, making them suitable for transporting both hydrophilic and hydrophobic drugs, including small molecules such as chemotherapeutic agents and inhibitors, as well as macromolecules such as proteins[
104], DNA or RNA[
105,
106]. Depending on the route of administration, nanogels encounter various physiological barriers during drug delivery. This necessitates specific properties in the nanogels used, such as mucoadhesivity and mucopenetrativity for mucosal routes, clearance-avoidance systems for intravenous routes, or sophisticated mechanisms capable of crossing the blood-brain barrier. Several reviews[
107,
108,
109] have examined these physiological barriers and the strategies of nanogels to overcome them, illustrating typical cases, and therefore we will not elaborate further on this topic. A big part of the research on nanogels for drug delivery focuses primarily on cancer therapy; nanogels designed for chemotherapeutic drug delivery play a crucial role in improving efficacy and reducing the side effects of cancer treatments. These particles are designed to encapsulate chemotherapeutic agents, protecting them from degradation and improving their delivery to tumor sites[
110,
111]. She et al [
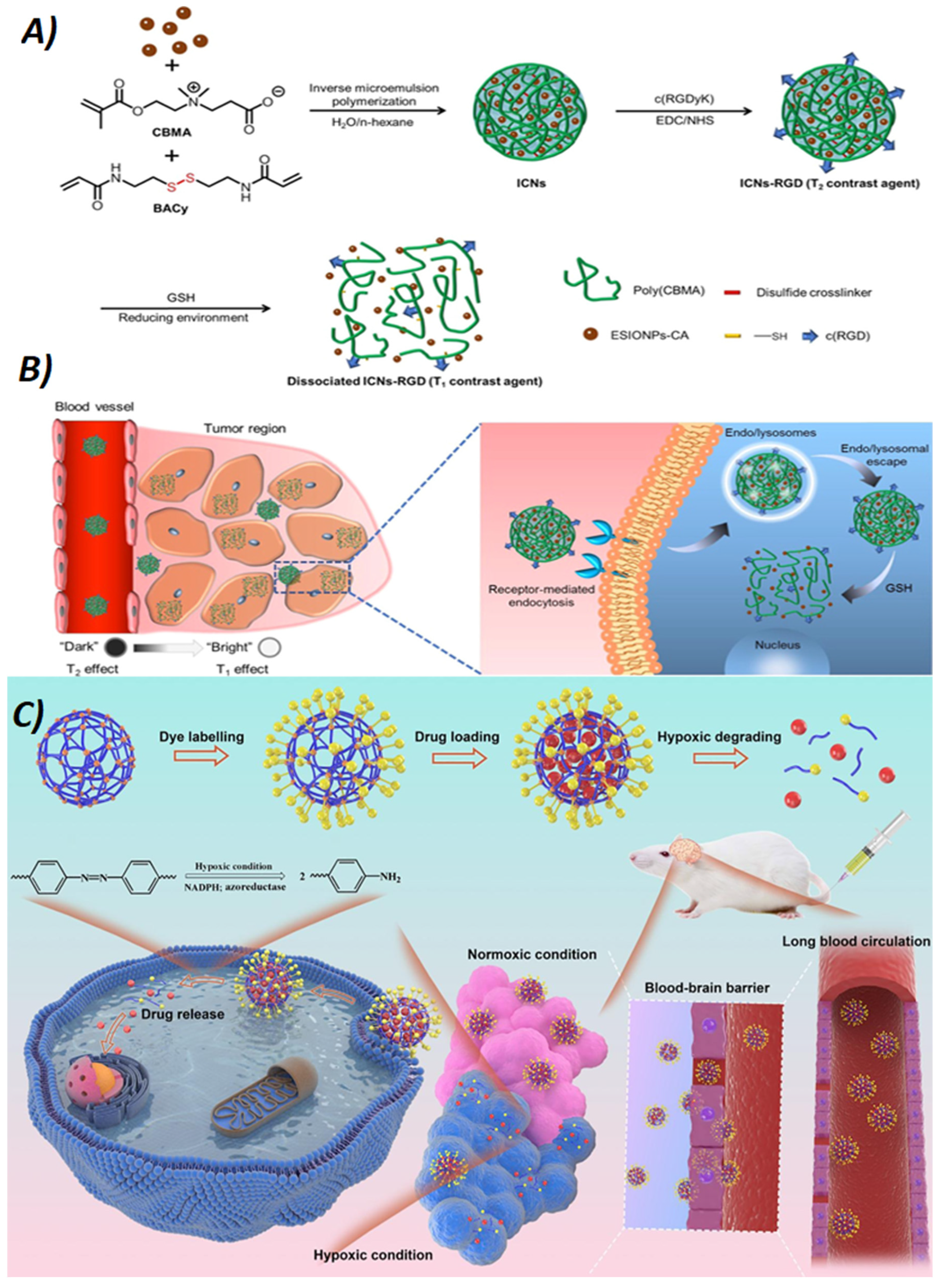
112]. developed a hypoxia-degradable zwitterionic phosphorylcholine nanogel (
HPMPC nanogel) using an azobenzene-based crosslinker (
Figure 8). This nanogel could degrade under hypoxic conditions, triggering the collapse of its structure and the rapid release of the drug doxorubicin (DOX) into tumor tissues. As a result, the nanogel showed prolonged accumulation in glioblastoma tissues and effectively inhibited the growth of this highly malignant tumor. However, the heterogeneity of tumors and underlying limitations of some anticancer drugs can lead to incomplete eradication of cancer cells when using a single compound for treatment, with possible tumor recurrence. In contrast, combining multiple chemotherapeutic agents with different mechanisms of action, i.e., using a “drug cocktails,” can synergistically increase therapeutic efficacy. As a result, there has recently been an intensification of loading nanogels with multiple drugs[
113,
114]. For example, Zhang and his colleagues[
115] designed a hyaluronic acid nanogel that can deliver doxorubicin due to its cationic nature. The nanogel uses cisplatin to cross-link its structure by binding it to the carboxyl (COOH) side-groups of hyaluronic acid. This cross-linking stabilizes the drug-loaded nanogel, preventing premature release during blood circulation. Besides focusing on specific diseases, many studies have created nanogel-based vectors for various applications, such as ocular administration; this innovative, noninvasive, and safer nanogel-based delivery method shows great potential for treating numerous conditions.
5.2. Nanogels for Bioimaging
Conventional imaging methods include various techniques used to visualize internal body structures for diagnosis, monitoring, and treatment of medical conditions. For example, computed tomography (CT) combines X-rays with computer technology to generate detailed three-dimensional images of organs, bones, and tissues, proving invaluable for visualizing anatomical details and evaluating trauma, tumors, and cardiovascular diseases. Magnetic resonance imaging (MRI) employs magnetic fields and radio waves to produce detailed images of soft tissues, brain, spinal cord, and joints, making it ideal for diagnosing neurological problems, musculoskeletal injuries, and central nervous system disorders. Despite their wide use and significant contributions to medical diagnostics, these methods have limitations. In fact, the low resolution and limited signal-to-noise ratio (SNR) of these imaging techniques have limited their wider application. Therefore, researchers have developed different contrast agents, i.e., substances that improve the visualization and definition of internal body structures by increasing the contrast between normal and abnormal tissues or between different types of tissues. This allows for clearer and more detailed images, aiding the diagnosis and monitoring of medical conditions. Gadolinium is a transition metal used as a contrast agent in imaging techniques such as magnetic resonance imaging (MRI). It is often bound to organic molecules to increase its solubility and stability in a biological environment, and sometimes to increase its concentration in certain tissues or cells. Due to its magnetic properties, gadolinium enhances the MRI-contrast between different tissues in the body, allowing more detailed and accurate images. Despite its effectiveness in improving diagnosis, it has been reported that gadolinium can accumulate in body tissues, especially in the brain, even in patients with normal renal function, although the extent and clinical implications of this phenomenon are still being studied and discussed. Among the various nanocarriers, contrast-agent-containing nanogels are particularly popular due to their high-water content and their ability to encapsulate a wide range of cargo[
116,
117] (
Figure 8). Kimura et al [
118]. developed ultra-small gelatin nanogels as contrast agents for MRI that cannot pass through the blood-brain barrier. They used a γ-radiation crosslinking technique for the formation of gelatin nanogels, and conjugated chelating agents to the protein nanogels to load gadolinium (Gd) and form gadolinium-coordinated gelatin nanogels (GdGN). In vivo studies confirmed the safety and effectiveness of GdGN as MRI contrast agents. GdGN were quickly eliminated via renal excretion within 90 minutes and did not cross the brain barriers. In their study, Shi[
119] and colleagues synthesized (AuNP)-loaded γ-polyglutamic acid (γ-PGA) NGs using a dual emulsion method for CT imaging of tumors. Initially, γ-PGA was activated with 1-ethyl-3-[3-(dimethylamino)propyl] carbodiimide hydrochloride (EDC) and then emulsified. Subsequently, polyethylenimine (PEI)-coated Au NPs [(Au0)200-PEI-NH2-mPEG]), synthesized by reduction of (HAuCl
4) with NaBH
4, were cross-linked in situ. In vivo CT imaging of the tumors showed that the γ-PGA-[(Au0)200-PEI-NH2-mPEG] nanoparticles effectively accumulated within the tumors and provided clear visualization of its site.
Multimodal imaging integrates multiple imaging techniques into a single procedure to provide complementary and detailed information about biological tissues. It combines modalities such as CT and MRI to enhance diagnostic sensitivity and specificity. This approach allows for a comprehensive assessment of anatomical structures and functional characteristics, aiding in early diagnosis, therapy monitoring, and understanding of pathophysiological processes. In the study of Sun et al [
120]., PEI, partially modified with polyethylene glycol (PEG), was used to encapsulate AuNPs and load gadolinium through chelation. These alginate nanogels (AGs) were obtained by a double emulsion process where the PEI-Au-Gd particles acted as crosslinkers to crosslink the alginate exploiting the activated carboxyl groups. The AG/PEI-Au-Gd nanogels exhibited higher T1 relaxivity in MRI and greater X-ray attenuation in CT compared to PEI-AuGd nanoparticles and conventional iodinated contrast agents. Due to their enhanced cellular uptake relative to PEI-Au-Gd nanoparticles, AG/PEI-Au-Gd nanogels represent a dual-mode MR/CT imaging system for improved visualization of tumor cells in vitro and enhanced imaging of tumors in vivo.
5.3. Nanogels for Regenerative Medicine
Nanogels have emerged as a promising tool in regenerative medicine, offering unique advantages due to their nanoscale size, high water content and versatile structure. For this purpose, these hydrophilic polymeric networks are designed to encapsulate and deliver bioactive molecules like growth factors, cytokines and genetic material, in a controlled and targeted manner. The gels ability to mimic the natural extracellular matrix and provide a supportive environment for cell growth and differentiation makes them ideal candidates for tissue engineering and regenerative therapies. This adaptability increases their potential to facilitate the repair and regeneration of damaged tissues and improve the integration of implanted biomaterials.
A field of application for these systems is bone tissue regeneration, where osteoclasts and osteoblasts handle bone resorption and formation, respectively; this is an intricate process involving other possible cell types and numerous intracellular and extracellular signaling pathways, including cytokines and growth factors [
121]. However, natural physiological mechanisms are insufficient for repairing large bone defects. The preferred treatment for critical bone defects is still autologous bone grafting, known for its osteoconductive (via bone fragments), osteoinductive (via growth factors), and osteogenic (via cells) properties. Nonetheless, this method often leads to chronic pain, infections, iatrogenic fractures, and suboptimal aesthetic outcomes. Nanocarriers can replicate the natural nanostructure of bone, potentially forming a precisely controlled porous microstructure that boosts osteoconduction[
122]. Nanogel formulations can serve as injectable carriers for systemic or localized delivery of drugs or genetic material or be integrated into scaffolds to accurately host and release active substances during tissue growth and to adjust the scaffold’s physical properties[
84,
123].
Another important application to consider is that of nanogels for wound healing. When the skin surface is damaged, timely restoration of its integrity becomes critical. The wound healing process takes place through stages of hemostasis, inflammation, proliferation, and remodeling. While minor wounds usually heal spontaneously in a few days, conditions such as diabetes, vascular insufficiency, or cancer can cause chronic wounds that resist normal healing. Delayed recovery of chronic wounds prolongs tissue regeneration, causing structural and functional damage. Due to their substantial water content, compatibility with natural extracellular matrices, ability to take desired shapes, and effective drug delivery capabilities, hydrogels have long been favored for wound healing. Nanogels inherit many of these advantages and have been designed for applications in managing bleeding and promoting wound healing[
124,
125,
126]. For example, Zhang et al [
127]. developed a novel nanocomposite consisting of chitin nanogels and rectorite (a mineral with hemostasis properties) for effective hemorrhage control. Chitin chains are intercalated into the rectorite, and vigorous mechanical agitation produces chitin nanogels. These nanogels assemble onto rectorite nanoplates through electrostatic interactions, forming a sandwich-like structure. In experimental models, the nanocomposite achieves hemostasis in 121 seconds in rat tail incisions and shows superior hemostatic activity compared with Celox, a commercial chitosan-based hemostatic, in rabbit artery injury models. The enhanced biocompatibility and hemostatic efficacy of the chitin/reptorite nanocomposite make it a promising and cost-effective option for hemorrhage management. In addition to the applications mentioned above, nanogels have also been studied for various other uses in regenerative medicine: cardiac repair[
128,
129], regeneration of ischemic limbs[
130], coating of biointerfaces[
131], etc. Despite the limited examples discussed in this review, extensive research in this area suggests that promising and encouraging applications of nanogels in regenerative medicine are likely to emerge in the near future.
6. Biocompatibility, Safety and Long-Term Stability
After introducing the various applications of nanogels, this chapter will address the potential challenges for their clinical translatability. Currently, there are no nanogel-based drugs or systems approved for clinical use. However, preclinical studies have shown promising results, indicating significant potential for their future clinical application. Current research efforts involving nanogel-based systems are focused on conducting in vitro and in vivo studies to validate their efficacy and safety in disease treatment. One of the biggest challenges for clinical translatability is reproducibility and scalability. As described in the chapter on synthetic methods, nanogel production involves a multitude of parameters and variables: different conditions and reaction settings produce nanogels of varying sizes, shapes, and behaviors, posing a significant challenge in obtaining reproducible results from batch to batch[
132]. This variability can affect the consistency of the nanogels’ performance, making it difficult to ensure uniformity in their therapeutic efficacy and safety. Overcoming these challenges is crucial for advancing nanogel-based systems towards clinical application, and this could be achieved also by the use of microfluidic systems as explained in section 4. In any case, it is crucial to implement regulatory guidelines that oversee the synthesis and development of nanogels, ensuring stringent adherence to uphold high standards of quality and safety. These guidelines would streamline the process of clinical translation, thereby facilitating the effective transition of nanogels from preclinical studies to clinical applications.
Another significant challenge associated with nanogel systems is their biocompatibility and safety in humans, which have yet to be fully explored. Potential immunogenicity may result from interactions between the nanogel and the drug delivered into the body, or from interactions with biological components, resulting in allergic, anaphylactic, or hypersensitivity reactions. However, accurately determining the immunotoxicity of nanogels through preclinical studies is difficult. Therefore, it is critical to use validated biocompatible materials during nanogel synthesis and conduct thorough investigations of potential toxicity to minimize immune responses. In addition, the potential toxicity of prolonged exposure to nanomaterials needs to be carefully examined because of their increased accumulation at disease sites. Nanogel-based carriers offer promising therapeutic potential but also present risks, which require further research into their safety, feasibility, and long-term stability as a treatment modality. Summarizing clinical studies on nanogels, it is notable that they are primarily administered through topical application, topical injection, or subcutaneous injection[
133]. The intravenous injection route, commonly used in preclinical research, has not yet been widely observed, likely due to significant challenges in addressing safety and to efficacy concerns with systemic administration. Furthermore, while clinical studies involving nanogels are increasing, most are currently in phase I or II trials,[
134,
135] indicating that their clinical translation still requires substantial advancement.
7. Conclusions and Future Perspectives
Nanogels, first developed in the late 1990s as part of polymer science and nanotechnology,[
2] have undergone significant evolution, becoming an integral part in modern nanomedicine studies. This review provides a concise exploration of nanogels, highlights their synthesis techniques and various biomedical applications. Nanogels represent a key advance in drug delivery, potentially capable of precise targeting and controlled release.
Even though there is still a significant journey to bring nanogels from bench to bedside for drug delivery applications, the results presented in literature offer a positive outlook for NGs as a novel drug delivery system: they hold promise for personalized therapies in regenerative medicine and contribute to advances in biomedical imaging. Despite these advances, several challenges hinder their widespread clinical application. Many current processes necessitate extreme pH and temperature conditions, as well as crosslinking agents that are not entirely biocompatible; to address these limitations, the use of peptide-based nanogels (PBNs) could serve as an innovative solution [
136,
137,
138]. Nanogels are still in the early stages of development and require extensive research to address issues such as reproducibility, scalability, efficient targeting, bioavailability, potential toxicity, and long-term stability. To facilitate the clinical transition of nanogels, future research should focus on developing scalable manufacturing methods and thoroughly investigating the benefits of nanogel-based therapies over traditional delivery methods. Standardizing manufacturing processes and ensuring batch consistency are critical for regulatory approval and clinical adoption; using flow chemistry approaches, especially based on microfluidics, could help in this aspect, but the research on quantifying the advantages of this approach for NGs production over conventional ones based on batch chemistry is still in its infancy. Addressing biocompatibility and long-term safety through rigorous preclinical and clinical studies is also imperative. Future studies should aim to establish reliable large-scale production techniques for nanogels without compromising their properties, preparing them for extensive clinical evaluation.
Author Contributions
“Conceptualization, P.M., B.T., S.L.; methodology, P.M., B.T., S.L.; investigation, P.M.; data curation, P.M.; writing—original draft preparation, P.M.; writing—review and editing, P.M., B.T., S.L.; visualization, P.M.; supervision, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the European Union Next-GenerationEU through the PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR - MISSIONE 4 COMPONENTE 2) within the National Quantum Science and Technology Institute (NQSTI – INVESTIMENTO 1.3; PE_00000023) and the Tuscany Health Ecosystem (THE - INVESTIMENTO 1.5; ECS_00000017).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Giannitelli SM, Limiti E, Mozetic P, et al. Droplet-based microfluidic synthesis of nanogels for controlled drug delivery: tailoring nanomaterial properties via pneumatically actuated flow-focusing junction. Nanoscale. 2022;14(31):11415-11428. [CrossRef]
- Vinogradov S, Batrakova E, Kabanov A. Poly(ethylene glycol)-polyethyleneimine NanoGel(TM) particles: Novel drug delivery systems for antisense oligonucleotides. Colloids Surfaces B Biointerfaces. 1999;16(1-4):291-304. [CrossRef]
- Vinogradov S V., Bronich TK, Kabanov A V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54(1):135-147. [CrossRef]
- Peppas NA, ed. Hydrogels in Medicine and Pharmacy. CRC Press; 2019. [CrossRef]
- Santi M, Maccari G, Mereghetti P, et al. Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization. Bioconjug Chem. 2017;28(2):471-480. [CrossRef]
- Raemdonck K, Demeester J, De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5(4):707-715. [CrossRef]
- Bhatia SN, Chen X, Dobrovolskaia MA, Lammers T. Cancer nanomedicine. Nat Rev Cancer. 2022;22(10):550-556. [CrossRef]
- Todaro B, Moscardini A, Luin S. Pioglitazone-Loaded PLGA Nanoparticles: Towards the Most Reliable Synthesis Method. Int J Mol Sci. 2022;23(5):2522. [CrossRef]
- Moshe H, Davizon Y, Menaker Raskin M, Sosnik A. Novel poly(vinyl alcohol)-based amphiphilic nanogels by non-covalent boric acid crosslinking of polymeric micelles. Biomater Sci. 2017;5(11):2295-2309. [CrossRef]
- Zhang Y, Song W, Lu Y, et al. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules. 2022;12(5):636. [CrossRef]
- Hajebi S, Rabiee N, Bagherzadeh M, et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1-18. [CrossRef]
- Zhao Q, Zhang S, Wu F, et al. Rational Design of Nanogels for Overcoming the Biological Barriers in Various Administration Routes. Angew Chemie Int Ed. 2021;60(27):14760-14778. [CrossRef]
- Mauri E, Giannitelli SM, Trombetta M, Rainer A. Synthesis of Nanogels: Current Trends and Future Outlook. 2021;7(2):36. Accessed July 3, 2024. https://www.mdpi.com/2310-2861/7/2/36/htm.
- Li C, Obireddy SR, Lai WF. Preparation and use of nanogels as carriers of drugs. Drug Deliv. 2021;28(1):1594-1602. [CrossRef]
- Kusmus DNM, van Veldhuisen TW, Khan A, Cornelissen JJLM, Paulusse JMJ. Uniquely sized nanogels via crosslinking polymerization. RSC Adv. 2022;12(45):29423-29432. [CrossRef]
- Pandey T, Sharma A, Sandhu A, Pandey V. Controlled polymerization in microfluidics: Advancement in nanogel synthesis. SPE Polym. 2024;5(2):113-126. [CrossRef]
- Ribovski L, de Jong E, Mergel O, et al. Low nanogel stiffness favors nanogel transcytosis across an in vitro blood–brain barrier. Nanomedicine Nanotechnology, Biol Med. 2021;34:102377. [CrossRef]
- Peres LB, dos Anjos RS, Tappertzhofen LC, et al. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur Polym J. 2018;101:341-349. [CrossRef]
- Ay Şenyiğit Z, Coşkunmeriç N, Çağlar EŞ, et al. Chitosan-bovine serum albumin-Carbopol 940 nanogels for mupirocin dermal delivery: ex-vivo permeation and evaluation of cellular binding capacity via radiolabeling. Pharm Dev Technol. 2021;26(8):852-866. [CrossRef]
- Zhang H-F, Ma L, Su F, et al. pH and reduction dual-responsive feather keratin - sodium alginate nanogels with high drug loading capacity for tumor-targeting DOX delivery. Polym Test. 2021;103:107375. [CrossRef]
- Liu Y, Chen D, Zhang A, et al. Composite inclusion complexes containing hyaluronic acid/chitosan nanosystems for dual responsive enrofloxacin release. Carbohydr Polym. 2021;252:117162. [CrossRef]
- Ren D, Chen P, Zheng P, Xu Z. pH/redox dual response nanoparticles with poly-γ-glutamic acid for enhanced intracellular drug delivery. Colloids Surfaces A Physicochem Eng Asp. 2019;577:412-420. [CrossRef]
- Rusu AG, Chiriac AP, Nita LE, Rosca I, Rusu D, Neamtu I. Self-Assembled Nanocarriers Based on Modified Chitosan for Biomedical Applications: Preparation and Characterization. Polymers (Basel). 2020;12(11):2593. [CrossRef]
- Radeva L, Zaharieva MM, Spassova I, et al. Biopolymeric Nanogel as a Drug Delivery System for Doxorubicin—Improved Drug Stability and Enhanced Antineoplastic Activity in Skin Cancer Cells. Pharmaceuticals. 2024;17(2):186. [CrossRef]
- Veloso SRS, Marta ES, Rodrigues P V., et al. Chitosan/Alginate Nanogels Containing Multicore Magnetic Nanoparticles for Delivery of Doxorubicin. Pharmaceutics. 2023;15(9):2194. [CrossRef]
- Thünemann AF, Gruber A, Klinger D. Amphiphilic Nanogels: Fuzzy Spheres with a Pseudo-Periodic Internal Structure. Langmuir. 2020;36(37):10979-10988. [CrossRef]
- Cho S-H, Hong JH, Noh Y-W, Lee E, Lee C-S, Lim YT. Raspberry-like poly(γ-glutamic acid) hydrogel particles for pH-dependent cell membrane passage and controlled cytosolic delivery of antitumor drugs. Int J Nanomedicine. 2016;Volume 11:5621-5632. [CrossRef]
- López-Iglesias C, Markovina A, Nirmalananthan-Budau N, Resch-Genger U, Klinger D. Optically monitoring the microenvironment of a hydrophobic cargo in amphiphilic nanogels: influence of network composition on loading and release. Nanoscale. 2024;16(19):9525-9535. [CrossRef]
- Gruber A, Işık D, Fontanezi BB, Böttcher C, Schäfer-Korting M, Klinger D. A versatile synthetic platform for amphiphilic nanogels with tunable hydrophobicity. Polym Chem. 2018;9(47):5572-5584. [CrossRef]
- Bewersdorff T, Gruber A, Eravci M, Dumbani M, Klinger D, Haase A. Amphiphilic nanogels: influence of surface hydrophobicity on protein corona, biocompatibility and cellular uptake. Int J Nanomedicine. 2019;Volume 14:7861-7878. [CrossRef]
- Kertsomboon T, Chirachanchai S. Amphiphilic biodegradable co-networks of Poly(butylene succinate)-Poly(ethylene glycol) chains for nano-gelation via Click chemistry and its potential dispersant for multi-walled carbon nanotubes. Polym Degrad Stab. 2020;179:109266. [CrossRef]
- Muñana-González S, Veloso-Fernández A, Ruiz-Rubio L, Pérez-Álvarez L, Vilas-Vilela JL. Covalent Cross-Linking as a Strategy to Prepare Water-Dispersible Chitosan Nanogels. Polymers (Basel). 2023;15(2):434. [CrossRef]
- Tian Y, Tian R, Chen L, et al. Redox-Responsive Nanogel with Intracellular Reconstruction and Programmable Drug Release for Targeted Tumor Therapy. Macromol Rapid Commun. 2019;40(8). [CrossRef]
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chemie Int Ed. 2001;40(11):2004-2021. [CrossRef]
- Duro-Castano A, Sousa-Herves A, Armiñán A, et al. Polyglutamic acid-based crosslinked doxorubicin nanogels as an anti-metastatic treatment for triple negative breast cancer. J Control Release. 2021;332:10-20. [CrossRef]
- Choi H, Kwon M, Choi HE, Hahn SK, Kim KS. Non-Invasive Topical Drug-Delivery System Using Hyaluronate Nanogels Crosslinked via Click Chemistry. Materials (Basel). 2021;14(6):1504. [CrossRef]
- Nagel G, Sousa-Herves A, Wedepohl S, Calderón M. Matrix Metalloproteinase-sensitive Multistage Nanogels Promote Drug Transport in 3D Tumor Model. Theranostics. 2020;10(1):91-108. [CrossRef]
- Lee S, Woo C, Ki CS. Pectin nanogel formation via thiol-norbornene photo-click chemistry for transcutaneous antigen delivery. J Ind Eng Chem. 2022;108:159-169. [CrossRef]
- Wang J, Wang X, Yan G, Fu S, Tang R. pH-sensitive nanogels with ortho ester linkages prepared via thiol-ene click chemistry for efficient intracellular drug release. J Colloid Interface Sci. 2017;508:282-290. [CrossRef]
- Zhang Y, Andrén OCJ, Nordström R, et al. Off-Stoichiometric Thiol-Ene Chemistry to Dendritic Nanogel Therapeutics. Adv Funct Mater. 2019;29(18). [CrossRef]
- FRIEDMANN E, MARRIAN DH, SIMON-REUSS I. ANTIMITOTIC ACTION OF MALEIMIDE AND RELATED SUBSTANCES. Br J Pharmacol Chemother. 1949;4(1):105-108. [CrossRef]
- Aktan B, Chambre L, Sanyal R, Sanyal A. “Clickable” Nanogels via Thermally Driven Self-Assembly of Polymers: Facile Access to Targeted Imaging Platforms using Thiol–Maleimide Conjugation. Biomacromolecules. 2017;18(2):490-497. [CrossRef]
- Li F, Chen X, He Y, Peng Z. Mucoadhesive Thiolated Hyaluronic Acid/Pluronic F127 Nanogel Formation via Thiol–Maleimide Click Reaction for Intravesical Drug Delivery. ACS Appl Bio Mater. 2024;7(3):1976-1989. [CrossRef]
- Altinbasak I, Kocak S, Sanyal R, Sanyal A. Redox-responsive nanogels for drug-delivery: thiol–maleimide and thiol–disulfide exchange chemistry as orthogonal tools for fabrication and degradation. Polym Chem. 2023;14(34):3897-3905. [CrossRef]
- Sui B, Wang M, Cheng C, et al. Nanogel-Facilitated Protein Intracellular Specific Degradation through Trim-Away. Adv Funct Mater. 2021;31(30). [CrossRef]
- Campea MA, Lofts A, Xu F, Yeganeh M, Kostashuk M, Hoare T. Disulfide-Cross-Linked Nanogel-Based Nanoassemblies for Chemotherapeutic Drug Delivery. ACS Appl Mater Interfaces. 2023;15(21):25324-25338. [CrossRef]
- Chen D, Zhu X, Tao W, et al. Regulation of pancreatic cancer microenvironment by an intelligent gemcitabine@nanogel system via in vitro 3D model for promoting therapeutic efficiency. J Control Release. 2020;324:545-559. [CrossRef]
- Yang Y, Zhu H, Wang J, Fang Q, Peng Z. Enzymatically Disulfide-Crosslinked Chitosan/Hyaluronic Acid Layer-by-Layer Self-Assembled Microcapsules for Redox-Responsive Controlled Release of Protein. ACS Appl Mater Interfaces. 2018;10(39):33493-33506. [CrossRef]
- Elkassih SA, Kos P, Xiong H, Siegwart DJ. Degradable redox-responsive disulfide-based nanogel drug carriers via dithiol oxidation polymerization. Biomater Sci. 2019;7(2):607-617. [CrossRef]
- Wegner J, Ceylan S, Kirschning A. Flow Chemistry – A Key Enabling Technology for (Multistep) Organic Synthesis. Adv Synth Catal. 2012;354(1):17-57. [CrossRef]
- Plutschack MB, Pieber B, Gilmore K, Seeberger PH. The Hitchhiker’s Guide to Flow Chemistry. Chem Rev. 2017;117(18):11796-11893. [CrossRef]
- Breen CP, Nambiar AMK, Jamison TF, Jensen KF. Ready, Set, Flow! Automated Continuous Synthesis and Optimization. Trends Chem. 2021;3(5):373-386. [CrossRef]
- Capaldo L, Wen Z, Noël T. A field guide to flow chemistry for synthetic organic chemists. Chem Sci. 2023;14(16):4230-4247. [CrossRef]
- Monia Kabandana GK, Zhang T, Chen C. Emerging 3D printing technologies and methodologies for microfluidic development. Anal Methods. 2022;14(30):2885-2906. [CrossRef]
- Dinter R, Willems S, Nissalk T, Hastürk O, Brunschweiger A, Kockmann N. Development of a microfluidic photochemical flow reactor concept by rapid prototyping. Front Chem. 2023;11:1244043. [CrossRef]
- Sohrabi S, Keshavarz Moraveji M, Iranshahi D. A review on the design and development of photocatalyst synthesis and application in microfluidic reactors: Challenges and opportunities. Rev Chem Eng. 2020;36(6):687-722. [CrossRef]
- Sun AC, Steyer DJ, Allen AR, Payne EM, Kennedy RT, Stephenson CRJ. A droplet microfluidic platform for high-throughput photochemical reaction discovery. Nat Commun. 2020;11(1):1-6. [CrossRef]
- Rebrov E V., Duinkerke SA, de Croon MHJM, Schouten JC. Optimization of heat transfer characteristics, flow distribution, and reaction processing for a microstructured reactor/heat-exchanger for optimal performance in platinum catalyzed ammonia oxidation. Chem Eng J. 2003;93(3):201-216. [CrossRef]
- Dong Z, Wen Z, Zhao F, Kuhn S, Noël T. Scale-up of micro- and milli-reactors: An overview of strategies, design principles and applications. Chem Eng Sci X. 2021;10:100097. [CrossRef]
- Vladisavljević GT, Khalid N, Neves MA, et al. Industrial lab-on-a-chip: Design, applications and scale-up for drug discovery and delivery. Adv Drug Deliv Rev. 2013;65(11-12):1626-1663. [CrossRef]
- Zhang H, Tumarkin E, Sullan RMA, Walker GC, Kumacheva E. Exploring microfluidic routes to microgels of biological polymers. Macromol Rapid Commun. 2007;28(5):527-538. [CrossRef]
- Liu Y, Yang G, Hui Y, et al. Microfluidic Nanoparticles for Drug Delivery. Small. 2022;18(36):2106580. [CrossRef]
- Bandulasena M V., Vladisavljević GT, Benyahia B. Droplet-based microfluidic method for robust preparation of gold nanoparticles in axisymmetric flow focusing device. Chem Eng Sci. 2019;195:657-664. [CrossRef]
- Baby T, Liu Y, Middelberg APJ, Zhao CX. Fundamental studies on throughput capacities of hydrodynamic flow-focusing microfluidics for producing monodisperse polymer nanoparticles. Chem Eng Sci. 2017;169:128-139. [CrossRef]
- Williams MS, Longmuir KJ, Yager P. A practical guide to the staggered herringbone mixer. Lab Chip. 2008;8(7):1121-1129. [CrossRef]
- Hadjigeorgiou AG, Boudouvis AG, Kokkoris G. Thorough computational analysis of the staggered herringbone micromixer reveals transport mechanisms and enables mixing efficiency-based improved design. Chem Eng J. 2021;414:128775. [CrossRef]
- Hessel V, Löwe H, Schönfeld F. Micromixers - A review on passive and active mixing principles. In: Chemical Engineering Science. Vol 60. Pergamon; 2005:2479-2501. [CrossRef]
- Bayareh M, Ashani MN, Usefian A. Active and passive micromixers: A comprehensive review. Chem Eng Process - Process Intensif. 2020;147:107771. [CrossRef]
- Delacour C, Stephens DS, Lutz C, Mettin R, Kuhn S. Design and Characterization of a Scaled-up Ultrasonic Flow Reactor. Org Process Res Dev. 2020;24(10):2085-2093. [CrossRef]
- Dong Z, Udepurkar AP, Kuhn S. Synergistic effects of the alternating application of low and high frequency ultrasound for particle synthesis in microreactors. Ultrason Sonochem. 2020;60:104800. [CrossRef]
- Udepurkar AP, Clasen C, Kuhn S. Emulsification mechanism in an ultrasonic microreactor: Influence of surface roughness and ultrasound frequency. Ultrason Sonochem. 2023;94:106323. [CrossRef]
- Shakeri A, Khan S, Didar TF. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip. 2021;21(16):3053-3075. [CrossRef]
- Choi JW, Kim YJ, Lee JM, Choi JH, Choi JW, Chung BG. Droplet-based Synthesis of Homogeneous Gold Nanoparticles for Enhancing HRP-based ELISA Signals. Biochip J. 2020;14(3):298-307. [CrossRef]
- Rebollo R, Oyoun F, Corvis Y, et al. Microfluidic Manufacturing of Liposomes: Development and Optimization by Design of Experiment and Machine Learning. ACS Appl Mater Interfaces. 2022;14(35):39736-39745. [CrossRef]
- Gholinejad M, Jabari Moghadam A, Mousavi Shaegh SA, Miri AK. Multifactorial analysis of ion concentration polarization for microfluidic preconcentrating applications using response surface method. Phys Fluids. 2020;32(7):72012. [CrossRef]
- Zoratto N, Montanari E, Viola M, et al. Strategies to load therapeutics into polysaccharide-based nanogels with a focus on microfluidics: A review. Carbohydr Polym. 2021;266:118119. [CrossRef]
- Zhao X, Liu S, Yildirimer L, et al. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv Funct Mater. 2016;26(17):2809-2819. [CrossRef]
- Feng Q, Li D, Li Q, et al. Dynamic Nanocomposite Microgel Assembly with Microporosity, Injectability, Tissue-Adhesion, and Sustained Drug Release Promotes Articular Cartilage Repair and Regeneration. Adv Healthc Mater. 2022;11(8):2102395. [CrossRef]
- Seiffert S, Weitz DA. Controlled fabrication of polymer microgels by polymer-analogous gelation in droplet microfluidics. Soft Matter. 2010;6(14):3184-3190. [CrossRef]
- Salvati B, Santagapita P, Perullini M. Exploring the conditions to generate alginate nanogels. J Sol-Gel Sci Technol. 2022;102(1):142-150. [CrossRef]
- Su M, Zhu Y, Chen J, et al. Microfluidic synthesis of manganese-alginate nanogels with self-supplying H2O2 capability for synergistic chemo/chemodynamic therapy and boosting anticancer immunity. Chem Eng J. 2022;435:134926. [CrossRef]
- Montalbo RCK, Wu MJ, Tu HL. One-step flow synthesis of size-controlled polymer nanogels in a fluorocarbon microfluidic chip. RSC Adv. 2024;14(16):11258-11265. [CrossRef]
- Bazban-Shotorbani S, Dashtimoghadam E, Karkhaneh A, Hasani-Sadrabadi MM, Jacob KI. Microfluidic Directed Synthesis of Alginate Nanogels with Tunable Pore Size for Efficient Protein Delivery. Langmuir. 2016;32(19):4996-5003. [CrossRef]
- Mahmoudi Z, Mohammadnejad J, Razavi Bazaz S, et al. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydr Polym. 2020;229:115551. [CrossRef]
- Huang K, He Y, Zhu Z, et al. Small, Traceable, Endosome-Disrupting, and Bioresponsive Click Nanogels Fabricated via Microfluidics for CD44-Targeted Cytoplasmic Delivery of Therapeutic Proteins. ACS Appl Mater Interfaces. 2019;11(25):22171-22180. [CrossRef]
- Majedi FS, Hasani-Sadrabadi MM, Hojjati Emami S, et al. Microfluidic assisted self-assembly of chitosan based nanoparticles as drug delivery agents. Lab Chip. 2013;13(2):204-207. [CrossRef]
- Chiesa E, Greco A, Riva F, et al. Staggered herringbone microfluid device for the manufacturing of chitosan/TPP nanoparticles: Systematic optimization and preliminary biological evaluation. Int J Mol Sci. 2019;20(24):6212. [CrossRef]
- Pessoa ACSN, Sipoli CC, De La Torre LG. Effects of diffusion and mixing pattern on microfluidic-assisted synthesis of chitosan/ATP nanoparticles. Lab Chip. 2017;17(13):2281-2293. [CrossRef]
- Whiteley Z, Ho HMK, Gan YX, et al. Microfluidic synthesis of protein-loaded nanogels in a coaxial flow reactor using a design of experiments approach. Nanoscale Adv. 2021;3(7):2039-2055. [CrossRef]
- Whiteley Z, Massaro G, Gkogkos G, et al. Microfluidic production of nanogels as alternative triple transfection reagents for the manufacture of adeno-associated virus vectors. Nanoscale. 2023;15(12):5865-5876. [CrossRef]
- Chakroborty S, Nath N, Mahal A, et al. Stimuli-responsive nanogels: A smart material for biomedical applications. J Mol Liq. 2024;403:124828. [CrossRef]
- Kumar N, Singh S, Sharma P, Kumar B, Kumar A. Single-, Dual-, and Multi-Stimuli-Responsive Nanogels for Biomedical Applications. Gels. 2024;10(1):61. [CrossRef]
- Wang X, Zheng Y, Xue Y, et al. pH-sensitive and tumor-targeting nanogels based on ortho ester-modified PEG for improving the in vivo anti-tumor efficiency of doxorubicin. Colloids Surfaces B Biointerfaces. 2021;207:112024. [CrossRef]
- Liu L, Luan S, Zhang C, et al. Encapsulation and pH-responsive release of bortezomib by dopamine grafted hyaluronate nanogels. Int J Biol Macromol. 2021;183:369-378. [CrossRef]
- Rashidzadeh H, Tabatabaei Rezaei SJ, Danafar H, Ramazani A. Multifunctional pH-responsive nanogel for malaria and cancer treatment: Hitting two targets with one arrow. J Drug Deliv Sci Technol. 2022;76:103740. [CrossRef]
- Dabas R, Kamaly N. Redox-Responsive Nanogels for Precision Protein Delivery. Eur Polym J. 2024;215:113183. [CrossRef]
- Laradji AM, Kolesnikov A V., Karakoçak BB, Kefalov VJ, Ravi N. Redox-Responsive Hyaluronic Acid-Based Nanogels for the Topical Delivery of the Visual Chromophore to Retinal Photoreceptors. ACS Omega. 2021;6(9):6172-6184. [CrossRef]
- Peng H, Huang X, Melle A, Karperien M, Pich A. Redox-responsive degradable prodrug nanogels for intracellular drug delivery by crosslinking of amine-functionalized poly(N-vinylpyrrolidone) copolymers. J Colloid Interface Sci. 2019;540:612-622. [CrossRef]
- Wang K-S, Jin Y-F, Tong Q-S, et al. A Redox-responsive Prodrug Nanogel of TLR7/8 Agonist for Improved Cancer Immunotherapy. Chinese J Polym Sci. 2023;41(1):32-39. [CrossRef]
- Macchione MA, Bedoya DA, Rivero-Buceta E, Botella P, Strumia MC. Mesoporous Silica and Oligo (Ethylene Glycol) Methacrylates-Based Dual-Responsive Hybrid Nanogels. Nanomaterials. 2022;12(21):3835. [CrossRef]
- Yang L, Ling J, Wang N, et al. Delivery of doxorubicin by dual responsive carboxymethyl chitosan based nanogel and in vitro performance. Mater Today Commun. 2022;31:103781. [CrossRef]
- Gray DM, Town AR, Niezabitowska E, Rannard SP, McDonald TO. Dual-responsive degradable core–shell nanogels with tuneable aggregation behaviour. RSC Adv. 2022;12(4):2196-2206. [CrossRef]
- Pillarisetti S, Vijayan V, Rangasamy J, Bardhan R, Uthaman S, Park I-K. A multi-stimuli responsive alginate nanogel for anticancer chemo-photodynamic therapy. J Ind Eng Chem. 2023;123:361-370. [CrossRef]
- Jiang T, Ma Y, Xu X, et al. Enzyme-instructed hybrid nanogel/nanofiber oligopeptide hydrogel for localized protein delivery. Acta Pharm Sin B. 2021;11(7):2070-2079. [CrossRef]
- Huang X, Wu G, Liu C, et al. Intercalation-Driven Formation of siRNA Nanogels for Cancer Therapy. Nano Lett. 2021;21(22):9706-9714. [CrossRef]
- Ding F, Huang X, Gao X, et al. A non-cationic nucleic acid nanogel for the delivery of the CRISPR/Cas9 gene editing tool. Nanoscale. 2019;11(37):17211-17215. [CrossRef]
- Siafaka PI, Özcan Bülbül E, Okur ME, Karantas ID, Üstündağ Okur N. The Application of Nanogels as Efficient Drug Delivery Platforms for Dermal/Transdermal Delivery. Gels. 2023;9(9):753. [CrossRef]
- Manimaran V, Nivetha RP, Tamilanban T, et al. Nanogels as novel drug nanocarriers for CNS drug delivery. Front Mol Biosci. 2023;10. [CrossRef]
- Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics. 2020;12(3):290. [CrossRef]
- Sun M, He L, Fan Z, Tang R, Du J. Effective treatment of drug-resistant lung cancer via a nanogel capable of reactivating cisplatin and enhancing early apoptosis. Biomaterials. 2020;257:120252. [CrossRef]
- Li X, Ouyang Z, Li H, et al. Dendrimer-decorated nanogels: Efficient nanocarriers for biodistribution in vivo and chemotherapy of ovarian carcinoma. Bioact Mater. 2021;6(10):3244-3253. [CrossRef]
- She D, Huang H, Li J, Peng S, Wang H, Yu X. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem Eng J. 2021;408:127359. [CrossRef]
- Lu Y, Jia D, Ma X, et al. Reduction-Responsive Chemo-Capsule-Based Prodrug Nanogel for Synergistic Treatment of Tumor Chemotherapy. ACS Appl Mater Interfaces. 2021;13(7):8940-8951. [CrossRef]
- Pooresmaeil M, Namazi H, Salehi R. Dual anticancer drug delivery of D-galactose-functionalized stimuli-responsive nanogels for targeted therapy of the liver hepatocellular carcinoma. Eur Polym J. 2022;167:111061. [CrossRef]
- Zhang Y, Wang F, Li M, et al. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv Sci. 2018;5(5). [CrossRef]
- Cao Y, Mao Z, He Y, et al. Extremely Small Iron Oxide Nanoparticle-Encapsulated Nanogels as a Glutathione-Responsive T 1 Contrast Agent for Tumor-Targeted Magnetic Resonance Imaging. ACS Appl Mater Interfaces. 2020;12(24):26973-26981. [CrossRef]
- Feng Z, Li Q, Wang W, et al. Superhydrophilic fluorinated polymer and nanogel for high-performance 19F magnetic resonance imaging. Biomaterials. 2020;256:120184. [CrossRef]
- Kimura A, Jo J, Yoshida F, et al. Ultra-small size gelatin nanogel as a blood brain barrier impermeable contrast agent for magnetic resonance imaging. Acta Biomater. 2021;125:290-299. [CrossRef]
- Zhu J, Sun W, Zhang J, et al. Facile Formation of Gold-Nanoparticle-Loaded γ-Polyglutamic Acid Nanogels for Tumor Computed Tomography Imaging. Bioconjug Chem. 2017;28(11):2692-2697. [CrossRef]
- Sun W, Zhang J, Zhang C, et al. A unique nanogel-based platform for enhanced dual mode tumor MR/CT imaging. J Mater Chem B. 2018;6(29):4835-4842. [CrossRef]
- Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and Osteoclasts in Bone Remodeling and Inflammation. Curr Drug Target -Inflammation Allergy. 2005;4(3):325-328. [CrossRef]
- Shimoda A, Chen Y, Akiyoshi K. Nanogel containing electrospun nanofibers as a platform for stable loading of proteins. RSC Adv. 2016;6(47):40811-40817. [CrossRef]
- Zhang Q, Chen X, Geng S, et al. Nanogel-based scaffolds fabricated for bone regeneration with mesoporous bioactive glass and strontium: In vitro and in vivo characterization. J Biomed Mater Res Part A. 2017;105(4):1175-1183. [CrossRef]
- Fan Y, Lüchow M, Zhang Y, et al. Nanogel Encapsulated Hydrogels As Advanced Wound Dressings for the Controlled Delivery of Antibiotics. Adv Funct Mater. 2021;31(7). [CrossRef]
- Zhang S, Li Y, Qiu X, et al. Incorporating redox-sensitive nanogels into bioabsorbable nanofibrous membrane to acquire ROS-balance capacity for skin regeneration. Bioact Mater. 2021;6(10):3461-3472. [CrossRef]
- El-Feky GS, El-Banna ST, El-Bahy GS, Abdelrazek EM, Kamal M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr Polym. 2017;177:194-202. [CrossRef]
- Zhang J, Xue S, Zhu X, et al. Emerging chitin nanogels/rectorite nanocomposites for safe and effective hemorrhage control. J Mater Chem B. 2019;7(33):5096-5103. [CrossRef]
- Tang J, Cui X, Caranasos TG, et al. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano. 2017;11(10):9738-9749. [CrossRef]
- Zhang L, Liu G, Lv K, et al. Surface-Anchored Nanogel Coating Endows Stem Cells with Stress Resistance and Reparative Potency via Turning Down the Cytokine-Receptor Binding Pathways. Adv Sci. 2021;8(3). [CrossRef]
- Dang Y, Gao N, Niu H, Guan Y, Fan Z, Guan J. Targeted Delivery of a Matrix Metalloproteinases-2 Specific Inhibitor Using Multifunctional Nanogels to Attenuate Ischemic Skeletal Muscle Degeneration and Promote Revascularization. ACS Appl Mater Interfaces. 2021;13(5):5907-5918. [CrossRef]
- Wang Y, Ma B, Liu K, Luo R, Wang Y. A multi-in-one strategy with glucose-triggered long-term antithrombogenicity and sequentially enhanced endothelialization for biological valve leaflets. Biomaterials. 2021;275:120981. [CrossRef]
- Tehrani SF, Bharadwaj P, Leblond Chain J, Roullin VG. Purification processes of polymeric nanoparticles: How to improve their clinical translation? J Control Release. 2023;360:591-612. [CrossRef]
- Khongkhunthian S, Sastraruji T, Klayraung S, Okonogi S. Efficacy of anesthetic rice nanogel on pain reduction in human oral cavity. Drug Discov Ther. 2018;12(1):31-36. [CrossRef]
- Yue Qiao P. Gold-silver-cuprous Oxide (Au-Ag-Cu2O) Composite Nanogel Combined With Photothermal Therapy in the Treatment of Severe Drug-resistant Microbial Keratitis.
- Shaimaa I Omar M. Periocular Rejuvenation by Topical Hyaluronic Acid Nano Particles.
- Todaro B, Ottalagana E, Luin S, Santi M. Targeting Peptides: The New Generation of Targeted Drug Delivery Systems. Pharmaceutics. 2023;15(6):1648. [CrossRef]
- Rosa E, Diaferia C, Gallo E, Morelli G, Accardo A. Stable formulations of peptide-based nanogels. Molecules. 2020;25(15):3455. [CrossRef]
- Neamtu I, Ghilan A, Rusu AG, Nita LE, Chiriac VM, Chiriac AP. Design and applications of polymer-like peptides in biomedical nanogels. Expert Opin Drug Deliv. 2024;21(5):713-734. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).