Submitted:
09 July 2024
Posted:
10 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
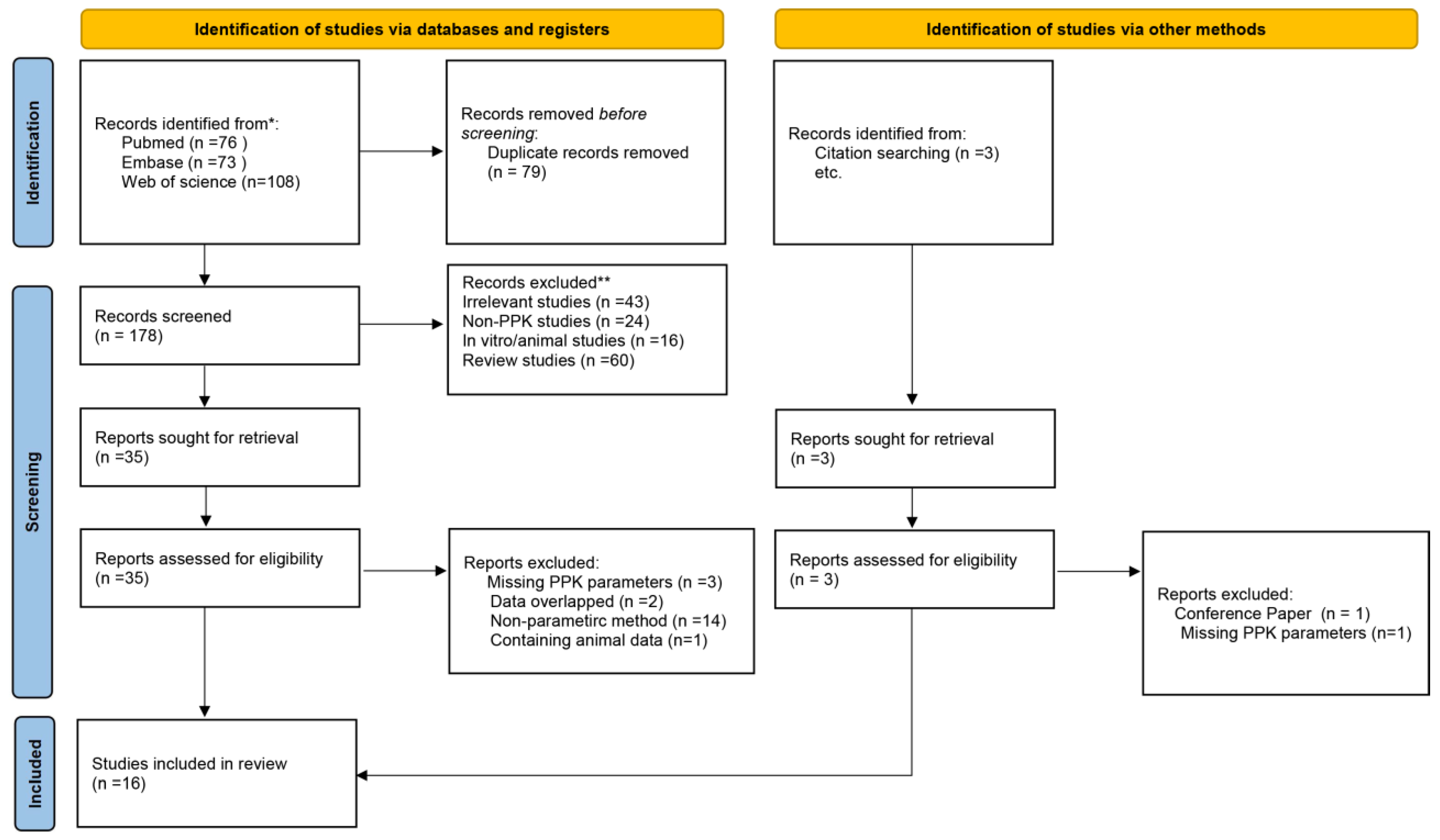
2.1. Study Identification
2.2. Data Extraction
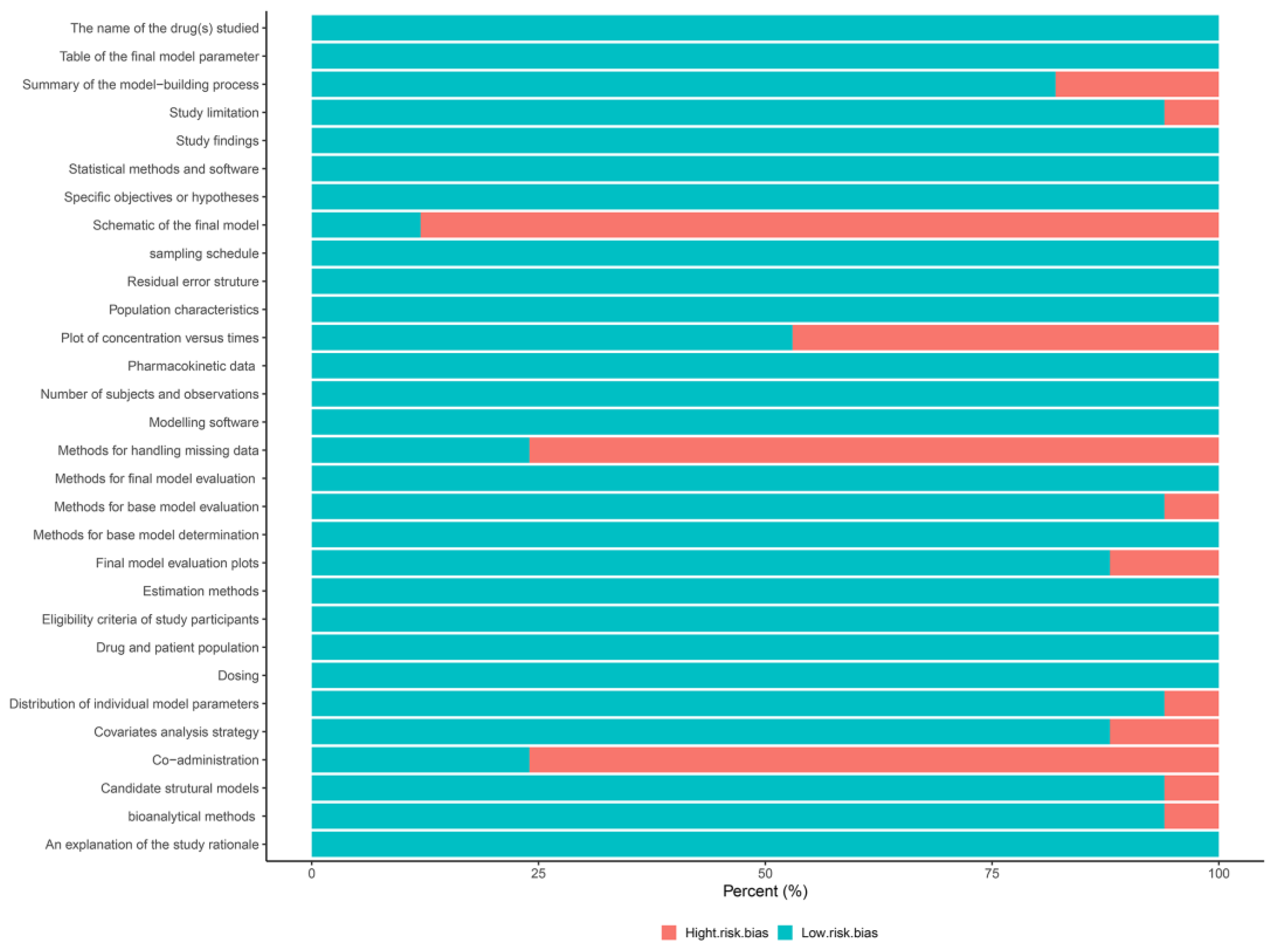
2.3. Literature Quality
2.4. Comparison of Studies
2.5. Monte Carlo Simulation for the Probability of Target Attainment
3. Results
3.1. Study Identification
3.2. Literature Quality
3.3. Study Comparison
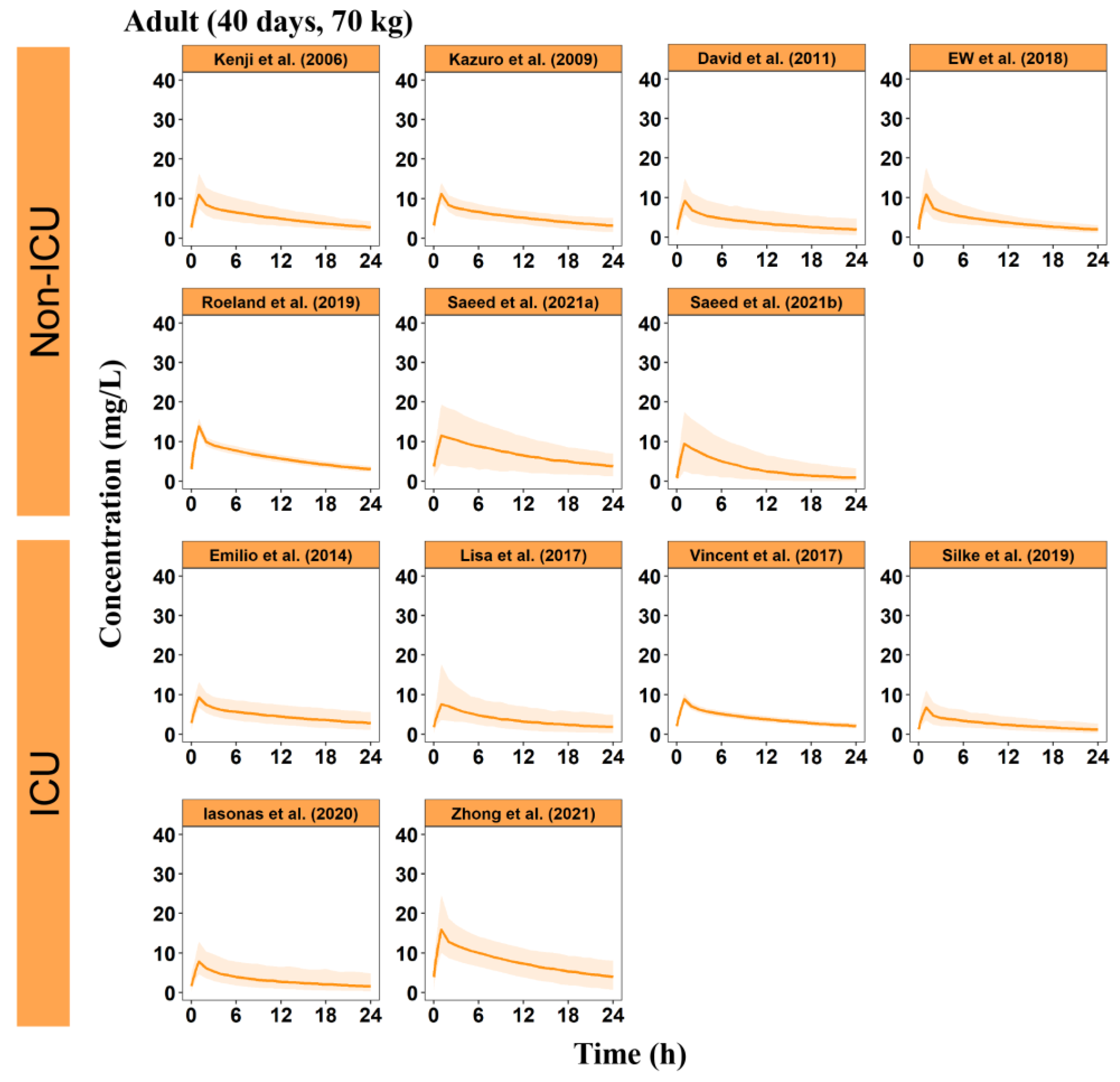
3.4. Visual Predictive Distributions
| Study (Year) | Study Type | Country /Race |
Study Population | No. of subjects (M/F) |
No. of Samples (Per Person) |
Age (Years) mean±sd median[range] |
Body weight (kg) mean±sd median[range] |
Dosing Regiments | Bioanalytical method [LLOQ, mg/L] |
|---|---|---|---|---|---|---|---|---|---|
| Kenji Tabata et al.(2006)[14] | Phase I, II, III | Japan | Healthy subjects Adult patients Pediatric patients |
82 97 19 |
1353(16.2) 395(4.1) 77(4) |
43.5[0.67-78.0]a 55[19–77]a 6.1±4.8[0.67-15]a |
62.8[45.1-80.6]a 50.3[28-76.4]a 22.0±14.0[7–48] |
2.5-150 mg 12.5-150 mg 1-6 mg/kg |
HPLC-FLD [0.05] |
| Kazuro Ikawa et al.(2009)[15] | prospective | Japan | adult haematology patients | 10(4/6) | 48(4.8) | 63.5+16.2 [30–79] | 55.4±10.3 [46.0–77.4] |
50–300 mg, single dose | HPLC-FLD [0.05] |
| P.B. Smith et al. (2009)[19] | Phase I | America | critically ill preterm neonates > 48 hours | 34(21/13) | NA(>5) | GTA: 26.65[23–39]c PCA: 30.45[26–39]c PTA:26.7[2–82]a |
1.185a [0.54-2.2] | 15 mg qd, 5 days 0.75 mg/kg, 1.5mg/kg, 3.0mg/kg, single dose |
HPLC-MS/MS [0.05] |
| David Andes et al.(2011)[7] | Phase III | North America, Europe, Brazil, India, Thailand, South Africa, Australia | invasive candidiasis or candidemia infectionn | 493(290/203) | NA | 55[13–89]b | 68[28–155]b | 100 -150 mg qd, 14-56 days | NA |
| Emilio Maseda et al. (2014)[27] | prospective | Spain | ICU patients | 10(8/2) | 280(28) | 72±8.2 73.5[54–83] |
69.6±6.3 70.0[61–80] |
100 mg qd | HPLC-UV [0.2] |
| William W. Hope et al. (2015)[18] | Phase I, II | America | treatment or prophylaxis against aspergillus spp. or Candida spp. | 229 | 1919(8.4) | 4 mo to <2 yrs: 1.0±0.4 2–5 yrs: 3.7 ±1.2 6–11 yrs: 9.0 ± 1.5 12–16 yrs: 14.5 ±1.5 |
4 mo to <2 yrs: 7.9 ± 1.7 2–5 yrs: 15.3 ± 4.4 6–11 yrs: 28.9 ±9.0 12–16 yrs: 54.4 ± 17.3 |
0.5, 1, 1.5, 2, 3, 4, 4.5 mg/kg qd | HPLC-FLD [0.05] |
| Lisa C. Martial et al. (2017)[10] | prospective | America | ICU patients | 20(8/12) | 356(17.8) | 68 [20–84] | 76.5 [50–134] | 100 mg qd | HPLC-UV [0.01] |
| Vincent Jullien et al. (2017)[11] | Phase III | France | ICU patients | 100(66/33) | 436(4.4) | 61.4[29.9–92.7] | 84.5[48–141] | 100 mg qd, 14 days | HPLC-FLD [0.2] |
| E. W. Muilwijk et al. (2018)[25] | Phase II | Netherlands | Adult haematology patients | 20(12/8) | ~340(17) | 59.5[38–68] | 86.6[53.5–110.1] | 300 mg q2w or 100 mg qd | HPLC-FLD [0.01] |
| Sharat Chandra et al. (2018)[17] | Phase I | America | HSCT patients and prophylaxis or treatment for fungal disease | 24(6/18) | 267(11.1) | 3.8[0.6-10.4] | 15.4[7.7-30.3] | 5 mg/kg, every 4 days | HPLC-UV [0.05] |
| Roeland E. Wasmann et al. (2019)[24] | Phase IV | Netherlands | Health volunteers (BMI 18.5–25) or obse adults (BMI ≥ 40) | 24(12/12) | ~240 (10) | 31 [22–56]d 51 [35–61]e 46 [24–54]f |
70.8 [61.5–81.5]d 156 [112–184]e 141 [126–180]f |
Morbidly obese subjects: 100 mg or 200mg Normal-weight subjects: 100 mg |
UPLC-FLD [0.01] |
| Silke Gastine et al. (2019)[28] | prospective | Germany | criticall ill adult patients | 36(24/12) | NA(≥9) | 65[22–84] | 94.5[49.9–162] | 100 mg qd | HPLC-FLD [0.1] |
| Zhong Shubai et al. (2019)[26] | prospective | China | Sepsis patients | 32(21/11) | 153(4.8) | 60.1 [23.0–89.0]a | 70.22a [55.0–90.0] | 100, 150, 200mg qd | HPLC-UV [0.2] |
| Iasonas Kapralos et al. (2020)[29] | prospective | Greece | critical ill patients | 14(7/7) | 210 (15) | 61±15 [31–83] | 85±22 [55–130] | 100mg qd | HPLC-FLD [0.059] |
| Saeed Alqahtani et al. (2021a)[30] | prospective | Saudi Arabia | noncancer patients | 9(6/3) | 63(7) | 51.1±19.1 | 69.8±15.7 | 100-150mg qd, two doses | HPLC-UV [0.1] |
| Saeed Alqahtani et al. (2021b)[30] | prospective | Saudi Arabia | cancer patients | 10(6/4) | 70(7) | 47.3±12.3 | 63.4±18.2 | 100mg qd, two doses | HPLC-UV [0.1] |
| Didi Bury et al. (2022)[16] | Phase IV | Netherlands | paediatric patients | 61(34/27) | ~420(>5) | 4.0[1.0–17] | 19.5[8.60–182] | 9 mg/kg (maximum 300 mg), twice-a-week | UPLC-FLD [0.01] |
| Study(Year) | Software/ Algorithm |
Compartment | Fixed effect Parameters | Between Subject Variability |
Residual Unexplained Variability |
Model Evaluation |
Model Application | |
|---|---|---|---|---|---|---|---|---|
| Kenji Tabata et al.(2006)[14] | NONMEM FOCE-I |
2 CMT zero-order input first-order elimination |
CL(ml/min) | 13.0+0.228×(BW-2.3)×FIX+0.0345×(PLT-21.6) (IF AGE≥16, FIX=0, IF AGE<16, FIX=1) |
23.80% | 11.00% | GOF; VPC | NA |
| V(L) | 11.2 | |||||||
| Vss(L) | 20.6 | |||||||
| Q(ml/min) | 96.5 | |||||||
| Kazuro Ikawa et al.(2009)[15] | NONMEM FOCE-I |
2 CMT zero-order input first-order elimination |
CL(L/h) | 0.762 | 15.40% | 0.642 mg/L | GOF, boostrap | Assessment of micafungin regimens based on PTA of fAUC24/MIC aganist Aspergillus |
| Vc(L) | 9.25 | 24.60% | ||||||
| Vp(L) | 8.86 | 71.80% | ||||||
| Q(L/h) | 7.02 | 0 FIXED | ||||||
| P Brian Smith et al. (2009)[19] | NONMEM | two compartment zero-order input first-order elimination |
CL(L/h) | 0.0365 | 48.80% | 29.20% | NA | NA |
| FOCE | V(L) | 0.507 | 48.80% | |||||
| Vss(L) | 1.6 | 48.80% | ||||||
| Q(L/h) | 0.0316 | / | ||||||
| David Andes et al.(2011)[7] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.05×(BW/65)0.258 | 36.00% | 19.30% | GOF | Explore the relationship between clinical and micrological response based on PTA for various Candida species. |
| FOCE-I | Vc(L) | 10.2 | 28.30% | |||||
| Vp(L) | 10.3 | 50.50% | ||||||
| Q(L/h) | 6.59 | 84.50% | ||||||
| Emilio Maseda et al. (2014)[27] | NONMEM | two compartment zero-order input first-order elimination |
CL(L/h) | 0.88×(BW/70)0.75 | 20.20% | 1.30% | GOF, bootstrap, VPC |
Evaluate covariate effects; Describe PK in specific population |
| FOCE-I | 22.1% (IOV) | 0.36mg/L | ||||||
| Vc(L) | 12.5 | 8.30% | ||||||
| 28.1% (IOV) | ||||||||
| Vp(L) | 10 | 7.50% | ||||||
| 27.4% (IOV) | ||||||||
| Q(L/h) | 5.03 | / | ||||||
| William W. Hope et al. (2015)[18] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.356×(BW/21.5)0.787×(AST/50)-0.0601×(TBIL/12)-0.0492 | 28.90% | 17.69% | GOF, bootstrap | Evaluate covariate effects; Describe PK in specific population; Identify therapeutic micafungin regimens based on exposure camparible to adult. |
| FOCE-I | Vc(L) | 1.21 | 98.30% | 35.92%a | ||||
| 4.62 | 16.61% | 0.0666 mg/L | ||||||
| Q(L/h) | 5.54 | 123.20% | ||||||
| Lisa C. Martial et al. (2017)[10] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.1 | 40.10% | GOF, bootstrap, pcVPC |
Evaluate covariate effects; Opitimize dosing regimens based on PTA for various Candida species. |
|
| FOCE-I | Vc(L) | 17.6 | 73.20% | |||||
| Vp(L) | 3.63 | 37.0% (IOV) | ||||||
| Q(L/h) | 0.363 | / | ||||||
| Vincent Jullien et al. (2017)[11] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.34×(BW/84)0.59 × 1.14 (if ALB ≤25 g/L) × 0.75 (if SOFA ≥10) | 11.40% | 1.44% | GOF, bootstrap, VPC, NPDE |
Evaluate covariate effects; Analyze the PK/PD in specific population; Evaluate the PTA of current dosing regimen; Opitimize dosing regimens based on PK/PD model. |
| FOCE-I | Vc(L) | 11.8×(BW/84)0.61 × 1.14 (if ALB ≤25 g/L) | 37.81% | |||||
| Vp(L) | 7.68×(BW/84)0.67 × 1.14 (if ALB ≤25 g/L) | 15.00% | ||||||
| Q(L/h) | Q(L/h)=4.67 | 13.90% | ||||||
| EW Muilwijk et al. (2018)[25] | NONMEM | 3 CMT zero-order input first-order elimination |
CL(L/h) | 1.01×(FFM/57.18)0.75 | 21.30% | 7.71% | GOF, bootstrap, VPC |
Evaluated the PK rationale of extending the dosing interval in special population |
| FOCE-I | 9.78% (IOV) | 0.0878 mg/L | ||||||
| V1(L) | 6.26×(FFM/57.18)1 | 48.10% | ||||||
| V2(L) | 6.26×(FFM/57.18)1 | 48.10% | ||||||
| V3(L) | 6.26×(FFM/57.18)1 | 48.10% | ||||||
| 0.809b | ||||||||
| Q1(L/h) | 10.3×(FFM/57.18)0.75 | / | ||||||
| Q2(L/h) | 2.04×(FFM/57.18)0.75 | / | ||||||
| Sharat Chandra et al. (2018)[17] |
NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.78×(BW/70)0.75 | 20.50% | 18% | GOF, pcVPC, boostrap |
Describe PK in specific population; Evaluated the PK rationale of extending the dosing interval of micafungin. |
| FOCE-I | Vc(L) | 13.9×(BW/70) | 31.20% | 0.15mg/L | ||||
| Vp(L) | 5.9×(BW/70) | 0 | ||||||
| Q(L/h) | 1.1×(BW/70)0.75 | 78.30% | ||||||
| Roeland E. Wasmann et al. (2019)[24] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.690×(BW/70)0.74 | 8.10% | 5% | GOF, pcVPC, boostrap |
Evaluate covariate effects; Describe PK in specific population; Opitimize dosing regimens based on PTA in special populations. |
| FOCE-I | Vc(L) | 5.84×(BW/70)1.17 | 12.80% | |||||
| Vp(L) | 6.96×(BW/70)0.71 | / | ||||||
| Q(L/h) | 7.15 | / | ||||||
| Silke Gastine et al. (2019)[28] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.56×0.789 (IF TBIL >4 mg/dL) | 48.90% | 0.26% | GOF, VPC | Evaluate covariate effects; Describe PK in specific population; Evaluate the efficacy of dosing regimen. |
| FOCE-I | Vc(L) | 16.2×0.692 (IF SOFA>10) | 70% | |||||
| Vp(L) | 13.8 | / | ||||||
| Q(L/h) | 14.4 | / | ||||||
| Iasonas Kapralos et al. (2020)[29] |
NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.31 | 19.00% | 14.90% | GOF, boostrap, pcVPC |
Analyze the PK/PD in specific population; Evaluate and optimize dosage regimens. |
| FOCE-I | 45% (IOV) | |||||||
| Vc(L) | 14.2 | 18.00% | ||||||
| 27% (IOV) | ||||||||
| Vp(L) | 12.6 | 51.00% | ||||||
| Q(L/h) | 2.89 | 63.00% | ||||||
| Zhong Shubai et al. (2021)[26] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.76×e((ALT/43)x(-0.268)) | 24.10% | 1.06mg/L | GOF, VPC, Boostrap, NPDE |
Evaluate covariate effects; Evaluated the PK rationale of extending the dosing interval of micafungin. |
| FOCE-I | Vc(L) | 6.7 | 52.80% | |||||
| Vp(L) | 10.2×e(θx(-1.08)) (SOFA score <10, θ=0; SOFA score≥10, θ=1) | 78.87% | ||||||
| Q(L/h) | 4.72 | / | ||||||
| Saeed Alqahtani et al. (2021a)[30] |
Monolix | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.6 | 11.80% | 38.70% | GOF, pcVPC | Describe PK of micafungin; Analyze the PK/PD in specific population; Evaluate the PTA of different dosing regimens within cancaer or within non-cancer populatons. |
| SAEM | Vc(L) | 12 | 7.60% | 0.42mg/L | ||||
| Vp(L) | 2.77 | 20.40% | ||||||
| Q(L/h) | 0.188 | 32.10% | ||||||
| Saeed Alqahtani et al. (2021b)[30] |
Monolix | 2 CMT zero-order input first-order elimination |
CL(L/h) | 1.2 | 34.10% | 45.82% | GOF, pcVPC | Describe PK of micafungin; Analyze the PK/PD in specific population; Evaluate the PTA of different dosing regimens within cancaer or within non-cancer populatons. |
| SAEM | Vc(L) | 10.7 | 7.60% | 0.47mg/L | ||||
| Vp(L) | 3.5 | 36.80% | ||||||
| Q(L/h) | 0.144 | 32.20% | ||||||
| Didi Bury et al. (2022)[16] | NONMEM | 2 CMT zero-order input first-order elimination |
CL(L/h) | 0.678×(FFM/57.19)0.75 | 24.90% | 9% | GOF, pcVPC | Evaluated the PK rationale of extending the dosing interval in special population. |
| FOCE | 10.1% (IOV) | |||||||
| Vc(L) | 7.91×(FFM/57.19) | 34.30% | ||||||
| 87.3%c | ||||||||
| Vp(L) | 9.01×(FFM/57.19) | / | ||||||
| Q(L/h) | 3.50×(FFM/57.19)0.75 | 70.60% |
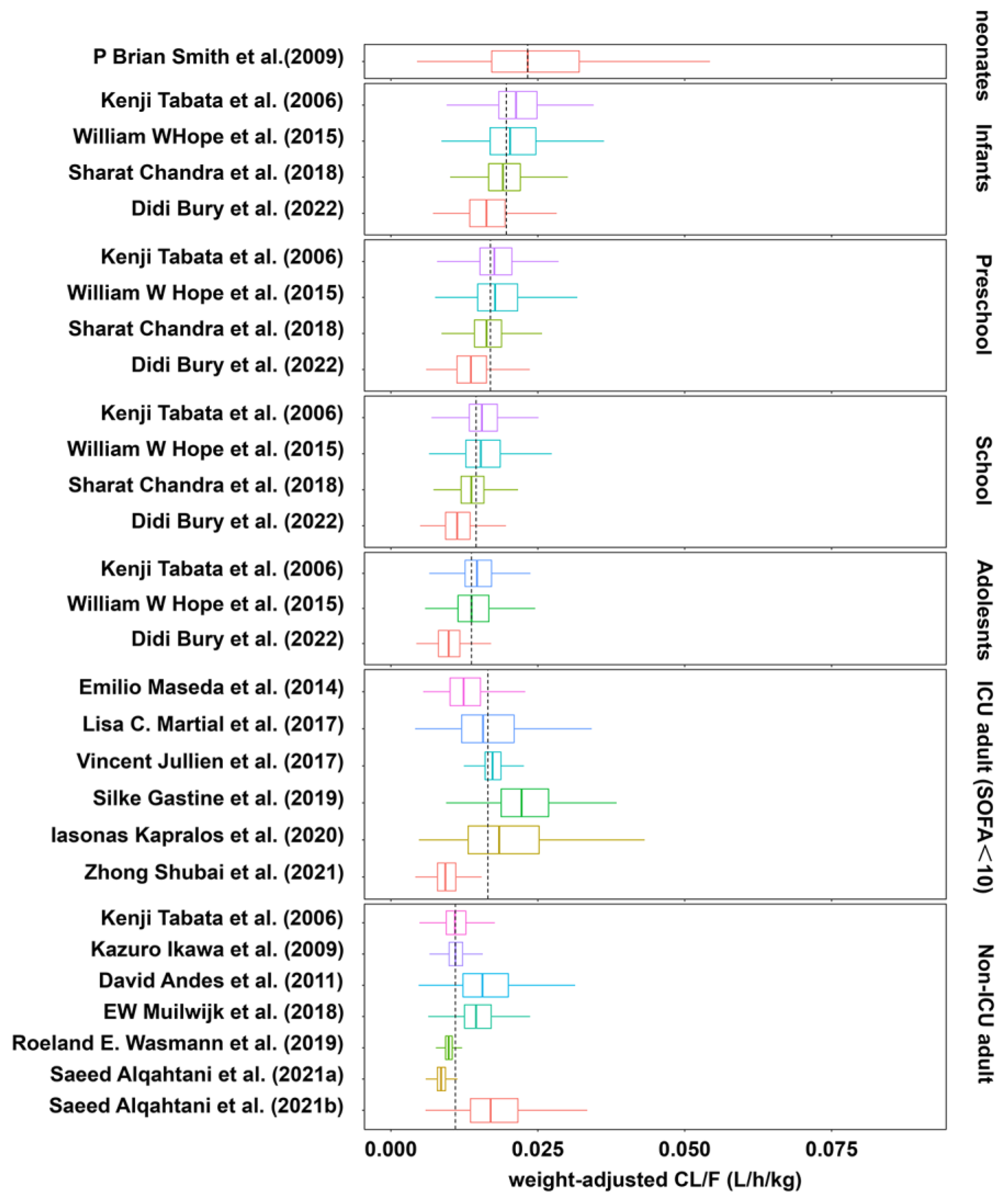
3.5. Pharmacokinetic Parameters
3.6. Covariate Effect on Pharmacokinetic Parameters
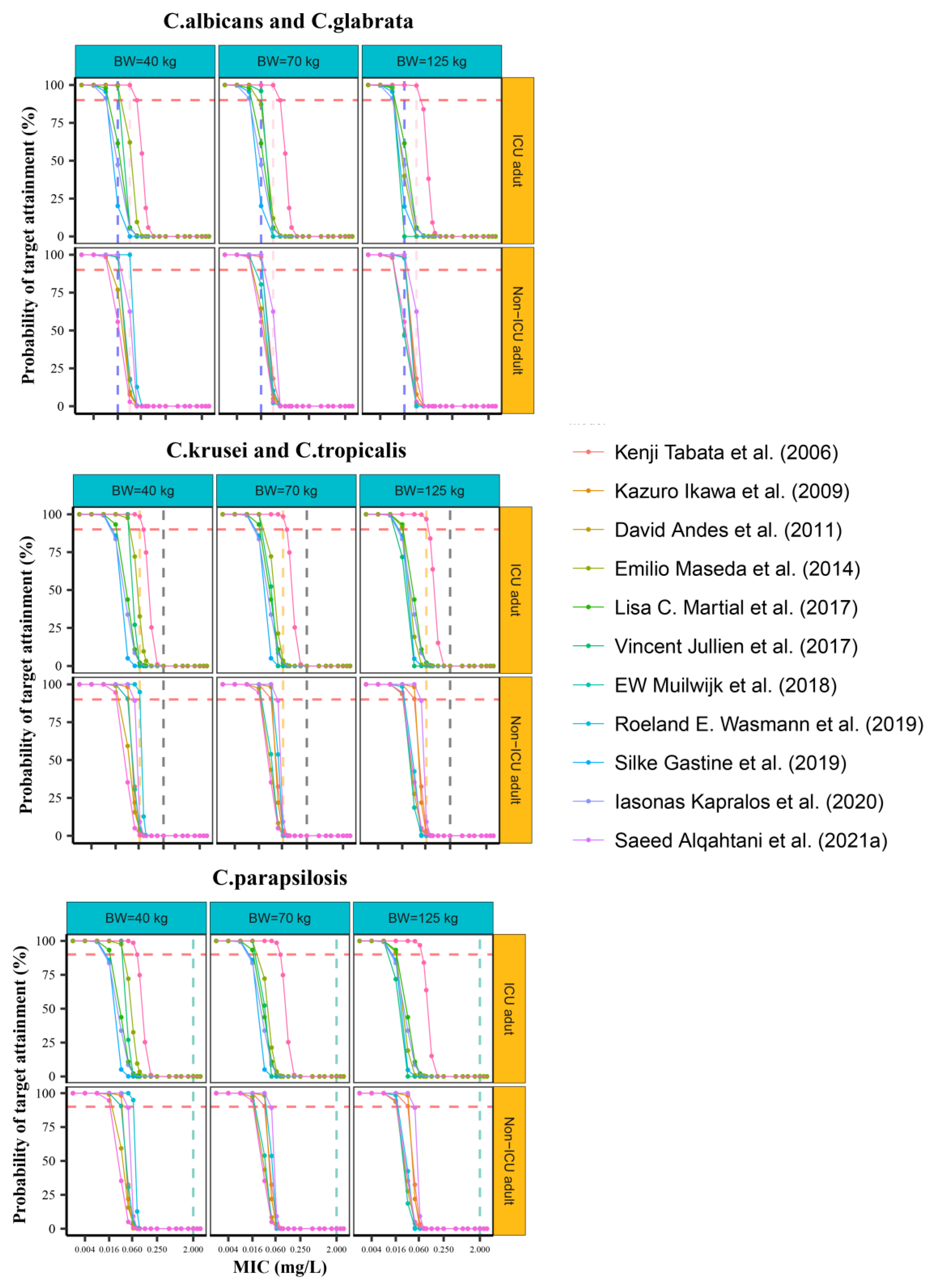
3.7. Analysis of Probability of Target Attainment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, S. Micafungin: A sulfated echinocandin. The Journal of antibiotics 2009, 62, 27–35. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clinical microbiology and infection : The official publication of the European Society of Clinical Microbiology and Infectious Diseases 2012, 18 (Suppl. S7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases : An official publication of the Infectious Diseases Society of America 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Leroux, S.; Jacqz-Aigrain, E.; Elie, V.; Legrand, F.; Barin-Le Guellec, C.; Aurich, B.; Biran, V.; Dusang, B.; Goudjil, S.; Coopman, S.; et al. Pharmacokinetics and safety of fluconazole and micafungin in neonates with systemic candidiasis: A randomized, open-label clinical trial. British journal of clinical pharmacology 2018, 84, 1989–1999. [Google Scholar] [CrossRef]
- Auriti, C.; Falcone, M.; Ronchetti, M.P.; Goffredo, B.M.; Cairoli, S.; Crisafulli, R.; Piersigilli, F.; Corsetti, T.; Dotta, A.; Pai, M.P. High-Dose Micafungin for Preterm Neonates and Infants with Invasive and Central Nervous System Candidiasis. Antimicrobial agents and chemotherapy 2016, 60, 7333–7339. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Ambrose, P.G.; Hammel, J.P.; Van Wart, S.A.; Iyer, V.; Reynolds, D.K.; Buell, D.N.; Kovanda, L.L.; Bhavnani, S.M. Use of Pharmacokinetic-Pharmacodynamic Analyses To Optimize Therapy with the Systemic Antifungal Micafungin for Invasive Candidiasis or Candidemia. Antimicrobial agents and chemotherapy 2011, 55, 2113–2121. [Google Scholar] [CrossRef]
- Andes, D. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrobial agents and chemotherapy 2003, 47, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Diekema, D.J.; Pfaller, M.A.; Marchillo, K.; Bohrmueller, J. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrobial agents and chemotherapy 2008, 52, 3497–3503. [Google Scholar] [CrossRef]
- Martial, L.C.; Ter Heine, R.; Schouten, J.A.; Hunfeld, N.G.; van Leeuwen, H.J.; Verweij, P.E.; de Lange, D.W.; Pickkers, P.; Brüggemann, R.J. Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients. Clinical pharmacokinetics 2017, 56, 1197–1206. [Google Scholar] [CrossRef]
- Jullien, V.; Azoulay, E.; Schwebel, C.; Le Saux, T.; Charles, P.E.; Cornet, M.; Souweine, B.; Klouche, K.; Jaber, S.; Trouillet, J.-L.; et al. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. Journal of Antimicrobial Chemotherapy 2017, 72, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.H.; Foster, D.R.; Hardy, B.; et al. Reporting Guidelines for Clinical Pharmacokinetic Studies: The ClinPK Statement. Clinical pharmacokinetics 2015, 54, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, K.M.; McLeay, S.C.; Barras, M.A.; Green, B. Reporting a population pharmacokinetic-pharmacodynamic study: A journal’s perspective. Clinical pharmacokinetics 2014, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Katashima, M.; Kawamura, A.; Kaibara, A.; Tanigawara, Y. Population pharmacokinetic analysis of micafungin in Japanese patients with fungal infections. Drug metabolism and pharmacokinetics 2006, 21, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, K.; Nomura, K.; Morikawa, N.; Ikeda, K.; Taniwaki, M. Assessment of micafungin regimens by pharmacokinetic-pharmacodynamic analysis: A dosing strategy for Aspergillus infections. Journal of Antimicrobial Chemotherapy 2009, 64, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.; Wolfs, T.F.W.; Ter Heine, R.; Muilwijk, E.W.; Tissing, W.J.E.; Brüggemann, R.J. Pharmacokinetic evaluation of twice-a-week micafungin for prophylaxis of invasive fungal disease in children with acute lymphoblastic leukaemia: A prospective observational cohort study. The Journal of antimicrobial chemotherapy 2022, 77, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fukuda, T.; Mizuno, K.; Davies, S.M.; Teusink-Cross, A.; Tarin, R.; Marsh, R.A.; Vinks, A.A.; Mehta, P.A. Micafungin antifungal prophylaxis in children undergoing HSCT: Can we give higher doses, less frequently? A pharmacokinetic study. Journal of Antimicrobial Chemotherapy 2018, 73, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Kaibara, A.; Roy, M.; Arrieta, A.; Azie, N.; Kovanda, L.L.; Benjamin, D.K., Jr. Population Pharmacokinetics of Micafungin and Its Metabolites M1 and M5 in Children and Adolescents. Antimicrobial agents and chemotherapy 2015, 59, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.B.; Walsh, T.J.; Hope, W.; Arrieta, A.; Takada, A.; Kovanda, L.L.; Kearns, G.L.; Kaufman, D.; Sawamoto, T.; Buell, D.N.; et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. The Pediatric infectious disease journal 2009, 28, 412–415. [Google Scholar] [CrossRef]
- Duffull, S.B.; Wright, D.F. What do we learn from repeated population analyses? British journal of clinical pharmacology 2015, 79, 40–47. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, C.Y.; Zhu, X.; Jiao, Z. Population Pharmacokinetics of Levetiracetam: A Systematic Review. Clinical pharmacokinetics 2021, 60, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N. Comparison of EUCAST and CLSI broth microdilution methods for the susceptibility testing of 10 systemically active antifungal agents when tested against Candida spp. Diagnostic microbiology and infectious disease 2014, 79, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimicrobial agents and chemotherapy 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Wasmann, R.E.; Smit, C.; ter Heine, R.; Koele, S.E.; van Dongen, E.P.H.; Wiezer, R.M.J.; Burger, D.M.; Knibbe, C.A.J.; Bruggemann, R.J.M. Pharmacokinetics and probability of target attainment for micafungin in normal-weight and morbidly obese adults. Journal of Antimicrobial Chemotherapy 2019, 74, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Muilwijk, E.W.; Maertens, J.A.; van der Velden, W.J.F.M.; ter Heine, R.; Colbers, A.; Burger, D.M.; Andes, D.; Theunissen, K.; Blijlevens, N.M.A.; Bruggemann, R.J.M. Pharmacokinetics of extended dose intervals of micafungin in haematology patients: Optimizing antifungal prophylaxis. Journal of Antimicrobial Chemotherapy 2018, 73, 3095–3101. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhu, X.; Zhao, L.; Song, Y.; Yu, J.; Zheng, Z.; Zang, B. Optimization of Micafungin Dosage for Chinese Patients with Sepsis in the Intensive Care Unit Based on a Population Pharmacokinetic-Pharmacodynamic Analysis. Pharmaceutical research 2021, 38, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Maseda, E.; Grau, S.; Villagran, M.J.; Hernandez-Gancedo, C.; Lopez-Tofino, A.; Roberts, J.A.; Aguilar, L.; Luque, S.; Sevillano, D.; Gimenez, M.J.; et al. Micafungin pharmacokinetic/pharmacodynamic adequacy for the treatment of invasive candidiasis in critically ill patients on continuous venovenous haemofiltration. The Journal of antimicrobial chemotherapy 2014, 69, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Gastine, S.; Lanckohr, C.; Blessou, M.; Horn, D.; Fobker, M.; Bause, D.; Hempel, G.; Ellger, B. Pharmacokinetics of Micafungin in Critically Ill Patients. Scientific reports 2019, 9, 17741. [Google Scholar] [CrossRef]
- Kapralos, I.; Mainas, E.; Neroutsos, E.; Apostolidi, S.; Siopi, M.; Apostolopoulou, O.; Dimopoulos, G.; Sambatakou, H.; Valsami, G.; Meletiadis, J.; et al. Population pharmacokinetics of micafungin over repeated doses in critically ill patients: A need for a loading dose? Journal of Pharmacy and Pharmacology 2020, 72, 1750–1760. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alfarhan, A.; Alsultan, A.; Alsarhani, E.; Alsubaie, A.; Asiri, Y. Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics-Basel 2021, 10, 1363. [Google Scholar] [CrossRef]
- Seibel, N.L.; Schwartz, C.; Arrieta, A.; Flynn, P.; Shad, A.; Albano, E.; Keirns, J.; Lau, W.M.; Facklam, D.P.; Buell, D.N.; et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrobial agents and chemotherapy 2005, 49, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Maseda, E.; Grau, S.; Luque, S.; Castillo-Mafla, M.P.; Suarez-de-la-Rica, A.; Montero-Feijoo, A.; Salgado, P.; Gimenez, M.J.; Garcia-Bernedo, C.A.; Gilsanz, F.; et al. Population pharmacokinetics/pharmacodynamics of micafungin against Candida species in obese, critically ill, and morbidly obese critically ill patients. Critical care (London, England) 2018, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.G.; Swancutt, M.A.; Gumbo, T. Fractal geometry and the pharmacometrics of micafungin in overweight, obese, and extremely obese people. Antimicrobial agents and chemotherapy 2011, 55, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Wasmann, R.E.; Muilwijk, E.W.; Burger, D.M.; Verweij, P.E.; Knibbe, C.A.; Bruggemann, R.J. Clinical Pharmacokinetics and Pharmacodynamics of Micafungin. Clinical pharmacokinetics 2018, 57, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Ohno, K.; Shimamura, T.; Furukawatodo, H. Optimal prophylactic dosage and disposition of micafungin in living donor liver recipients. Clinical transplantation 2004, 18, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Aoyama, T.; Matsumoto, Y.; Ogawa, F.; Yamazaki, H.; Kikuti, A.; Yamamoto, Y. Pharmacokinetics of antifungal agent micafungin in critically ill patients receiving continuous hemodialysis filtration. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 2007, 127, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol Aspects Med 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Trainor, G.L. The importance of plasma protein binding in drug discovery. Expert Opin Drug Discov 2007, 2, 51–64. [Google Scholar] [CrossRef]
- Hebert, M.F.; Smith, H.E.; Marbury, T.C.; Swan, S.K.; Smith, W.B.; Townsend, R.W.; Buell, D.; Keirns, J.; Bekersky, I. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol 2005, 45, 1145–1152. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hoener, B.A. Changes in plasma protein binding have little clinical relevance. Clinical pharmacology and therapeutics 2002, 71, 115–121. [Google Scholar] [CrossRef]
- Guo, F.; Yang, Y.; Kang, Y.; Zang, B.; Cui, W.; Qin, B.; Qin, Y.; Fang, Q.; Qin, T.; Jiang, D.; et al. Invasive candidiasis in intensive care units in China: A multicentre prospective observational study. The Journal of antimicrobial chemotherapy 2013, 68, 1660–1668. [Google Scholar] [CrossRef]
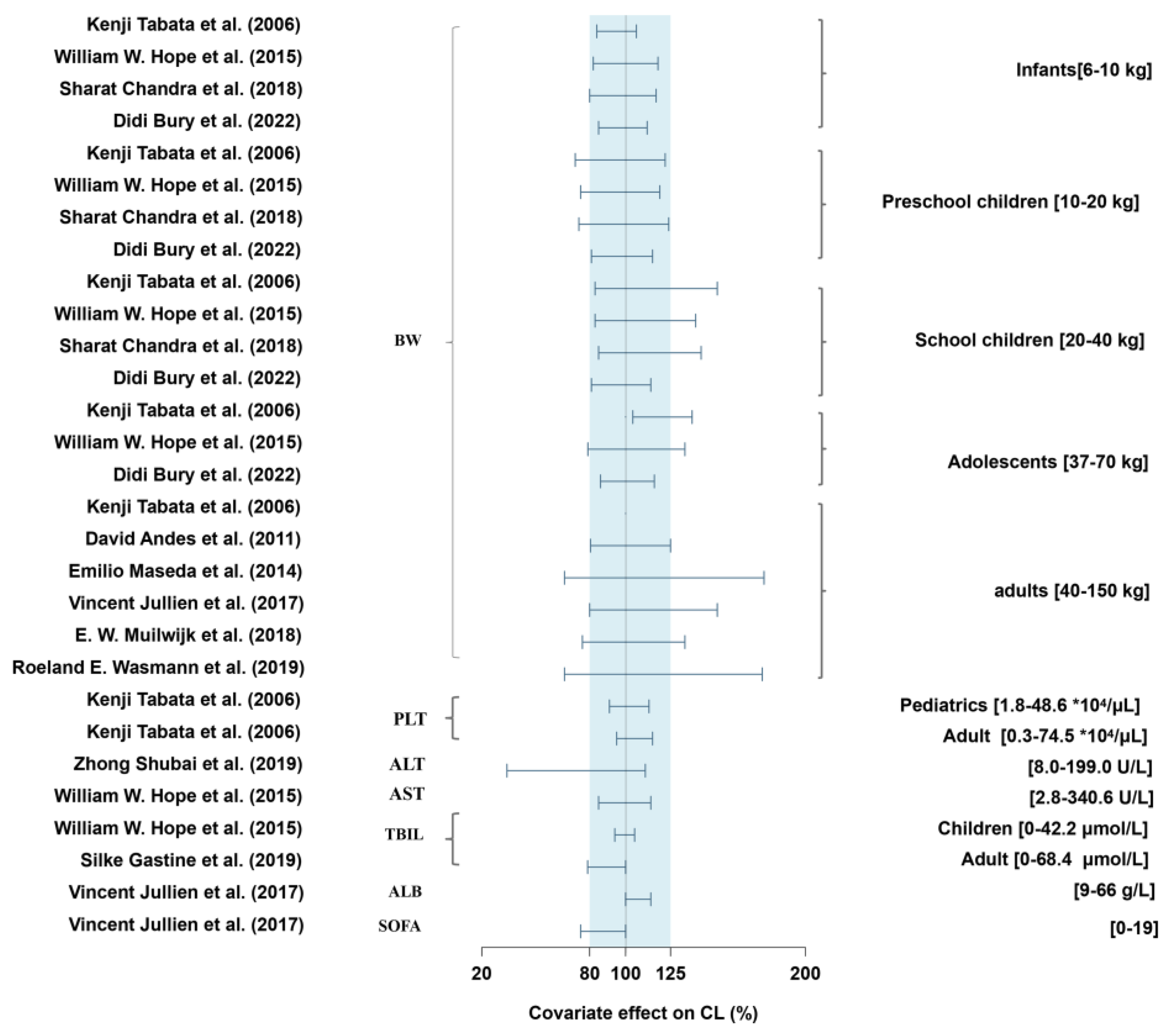
| Neonates | Infants | Preschool | School | adolescent | ICU adults (SOFA≥10) |
ICU adults (SOFA<10) |
Non-ICU adults | |
|---|---|---|---|---|---|---|---|---|
| BW-adjusted CLa (mL/h/kg) | 23.2 | 19.22 (2.17)** | 16.31 (1.91)* | 13.94 (1.95) | 12.73 (2.56) | 15.85 (4.92) | 16.47 (6.07) | 11.88 (3.03) |
| BW-adjusted CLb (mL/h/kg) | 16.70 (3.78) | 19.92 (2.60)* | ||||||
| CLa (L/h) | 0.03 | 0.16 (0.01)*** | 0.24 (0.03)*** | 0.42 (0.06)** | 0.64 (0.13) | 1.07 (0.34) | 1.10 (0.35) | 0.83 (0.21) |
| CLb (L/h) | 1.17 (0.26) | 1.20 (0.25)* | ||||||
| V1a (L) | 0.49 | 1.43 (0.48)*** | 2.24 (0.95)*** | 3.95 (2.20)** | 4.97 (3.65) | 12.67 (3.77) | 12.29 (4.20) | 8.58 (2.60) |
| V1b (L) | 13.82 (2.81)* | 14.56 (3.16)* | ||||||
| AUC24a (mg*h/L) | 162.53 | 103.96 (60.46) | 123.77 (16.10) | 145.68 (22.41) | 161.85 (36.28) | 128.19 (98.11) | 93.73 (31.87) | 126.01 (31.69) |
| AUC24b (mg*h/L) | 88.55 (19.40) | 85.72 (19.00)* |
| 50% | 60% | 70% | 80% | |||||
|---|---|---|---|---|---|---|---|---|
| ICU (mg/d) |
Non-ICU (mg/d) |
ICU (mg/d) |
Non-ICU (mg/d) |
ICU (mg/d) |
Non-ICU (mg/d) |
ICU (mg/d) |
Non-ICU (mg/d) | |
| C. albican | 150 | 100 | 150 | 100 | 150 | 150 | 200 | 150 |
| C. glabrata | 250 | 200 | 300 | 250 | 300 | 250 | > 300 | 300 |
| C. krusei | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 |
| C. tropicalis | 250 | 200 | > 300 | 200 | > 300 | 300 | > 300 | > 300 |
| C. parapsilosis | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 | > 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





