Submitted:
17 August 2024
Posted:
20 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Methods and Chemicals
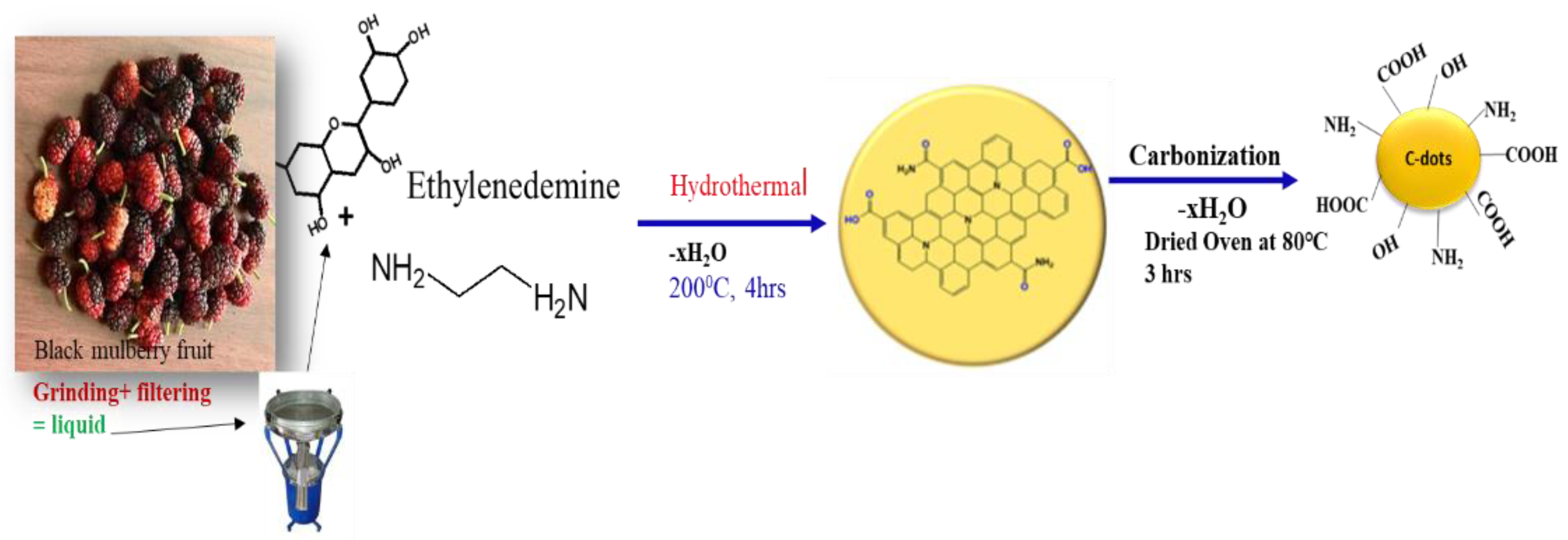
2.1. Extraction of Black Mulberry Fruits
2.2. Green Synthesis of C-Dots
2.3. Photocatalytic Degradation of Methylene Blue
3. Results and Discussion
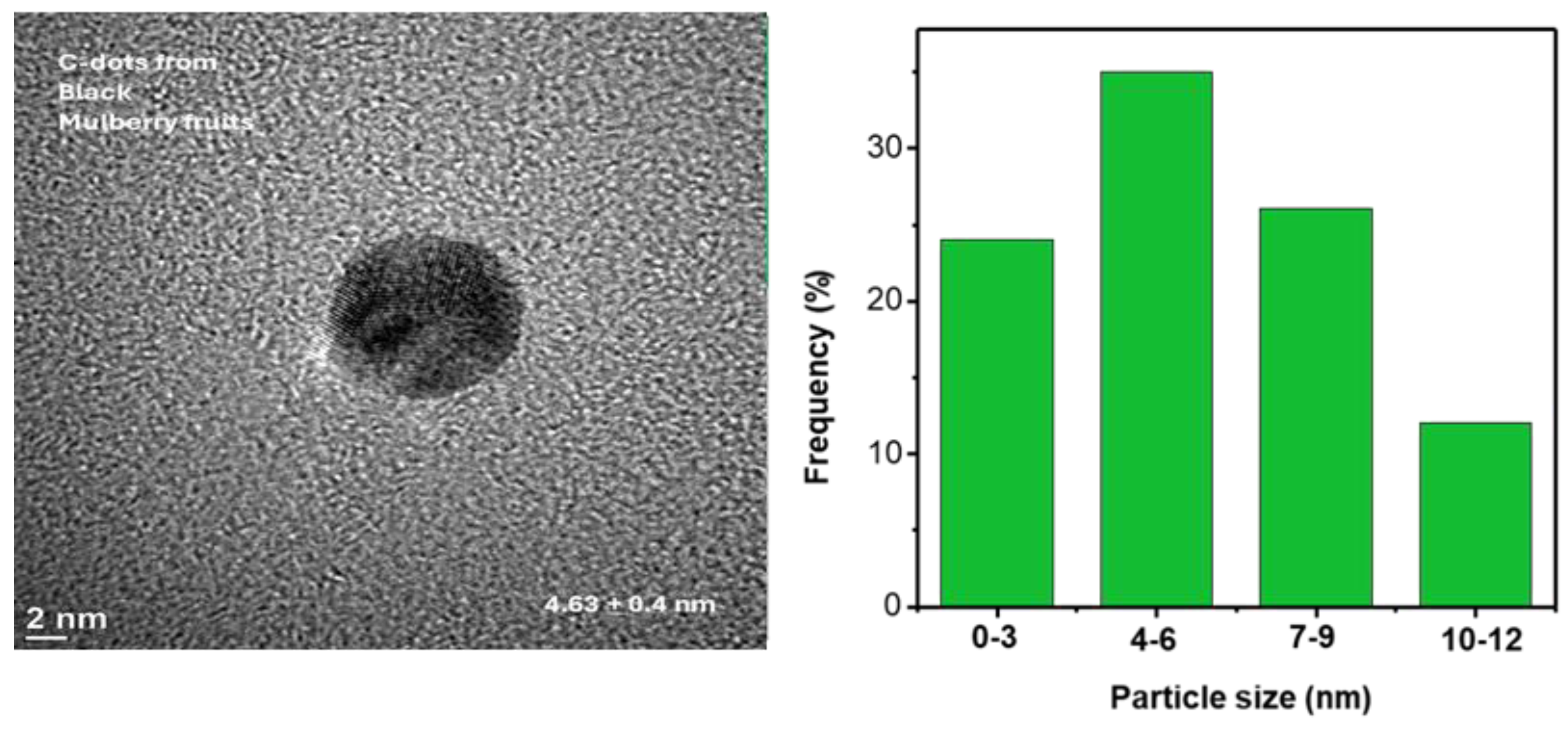
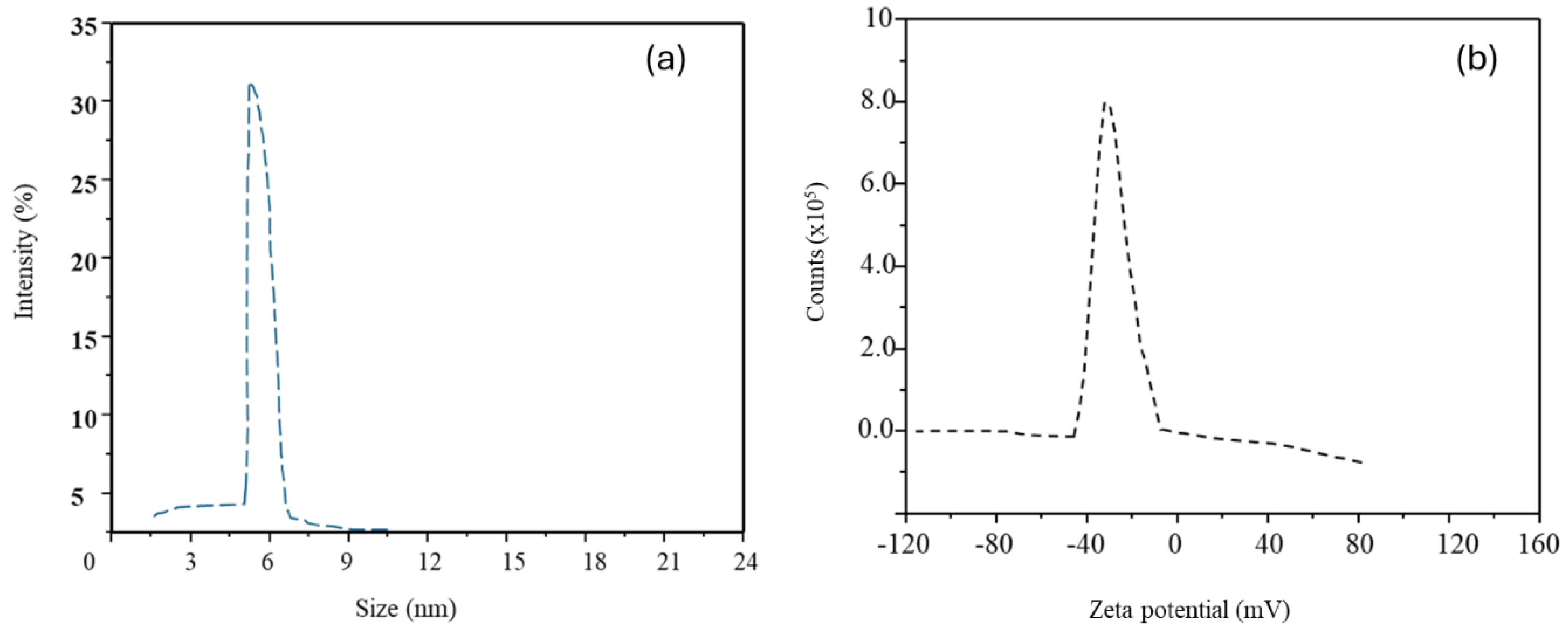
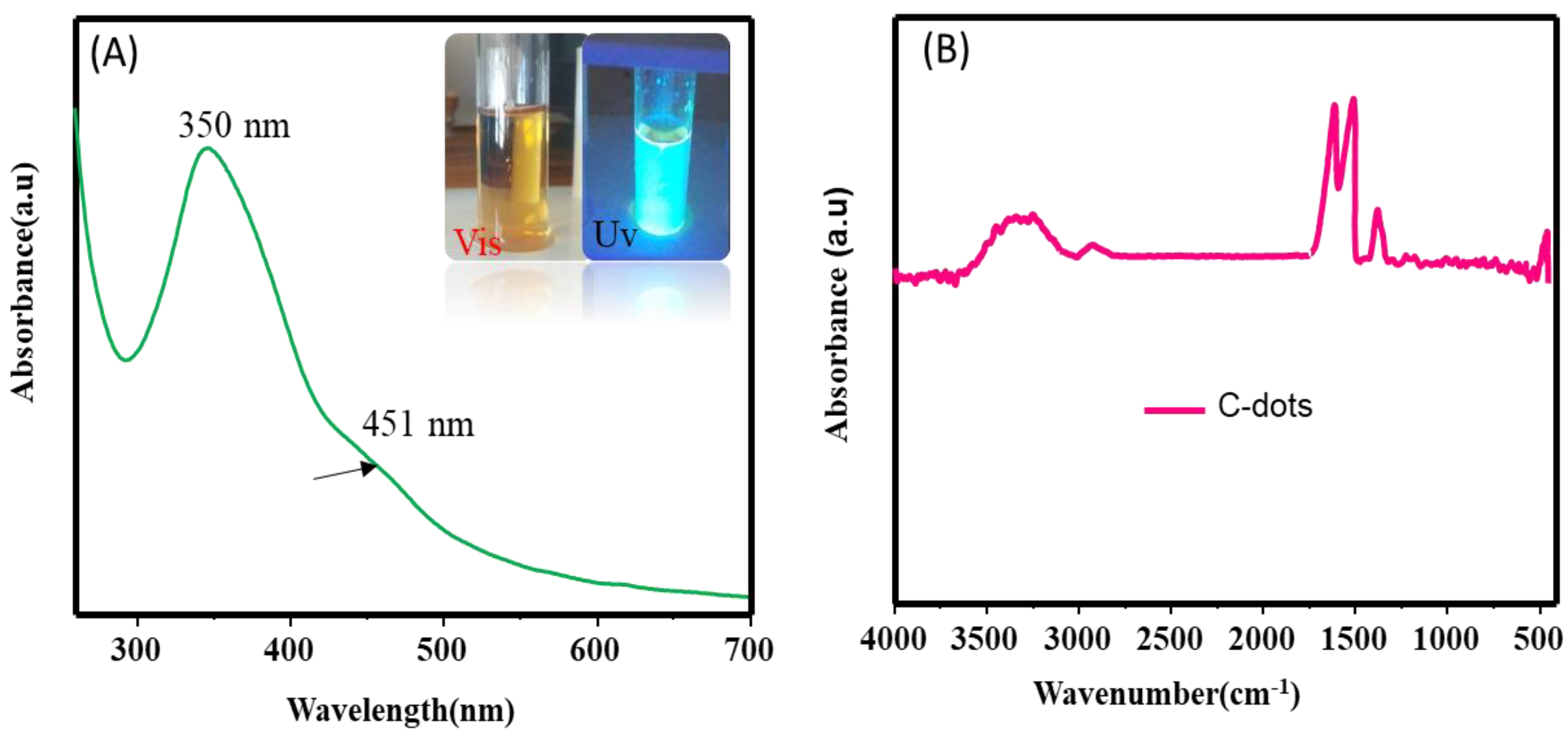
3.1. Characterization
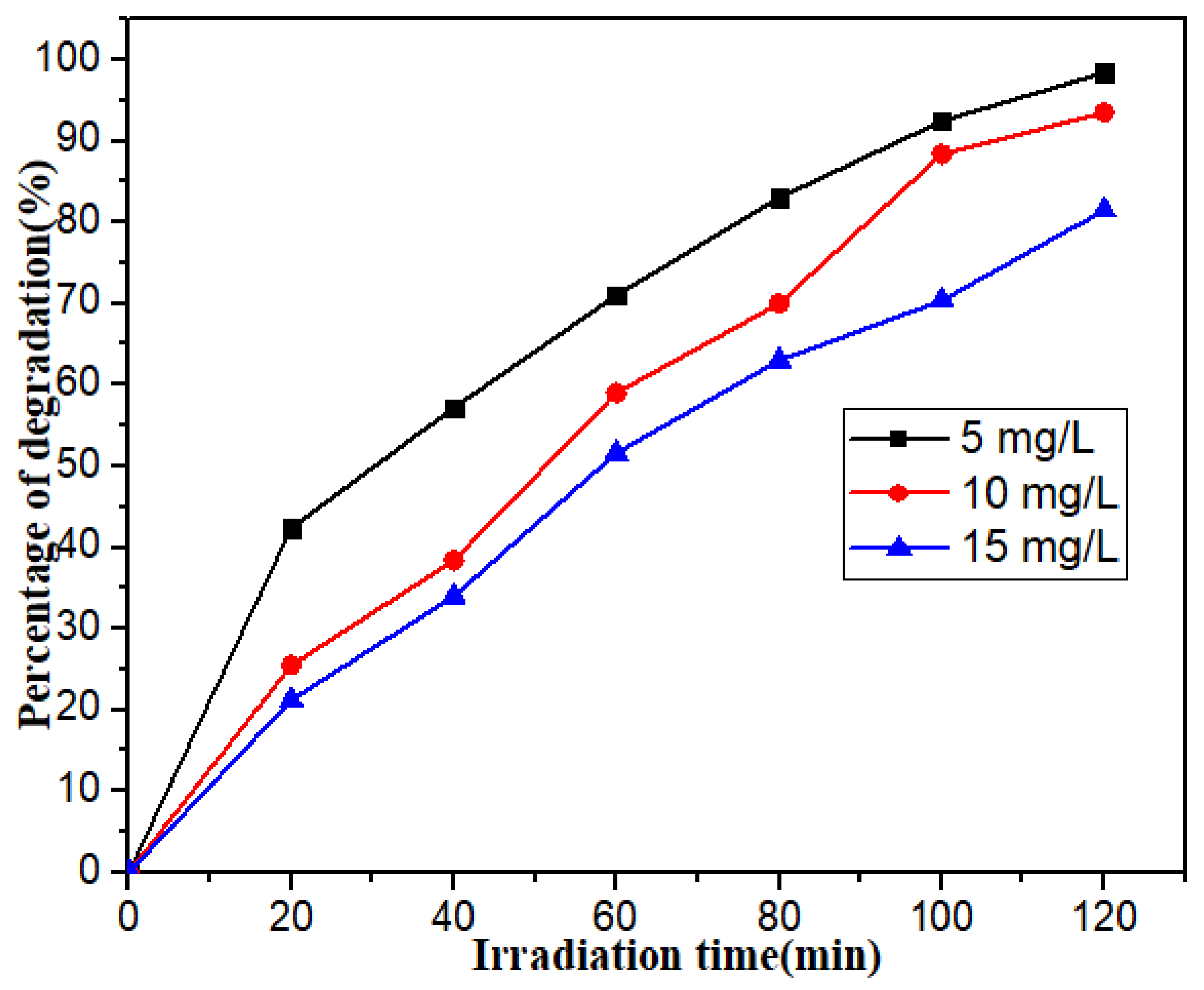
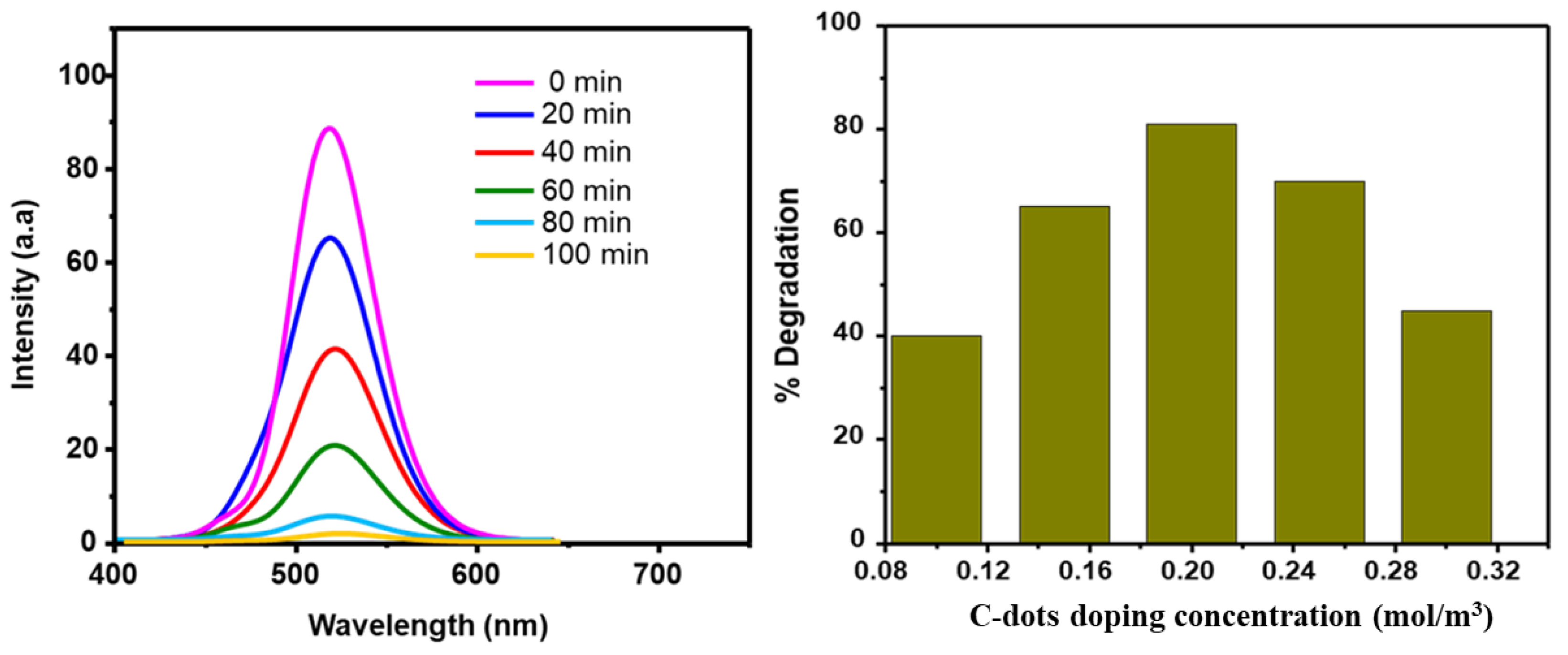
3.2. Photocatalytic Degradation Performance
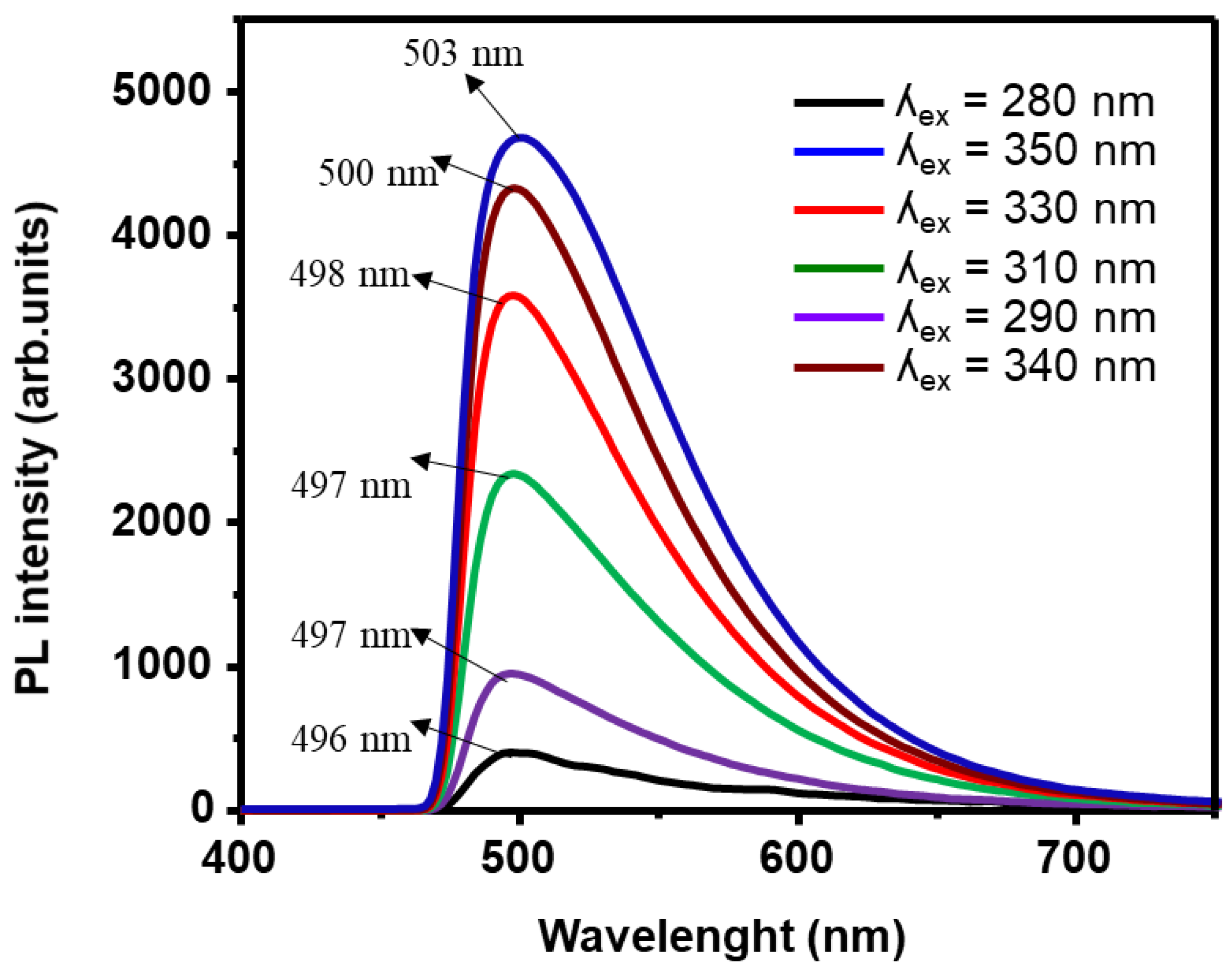
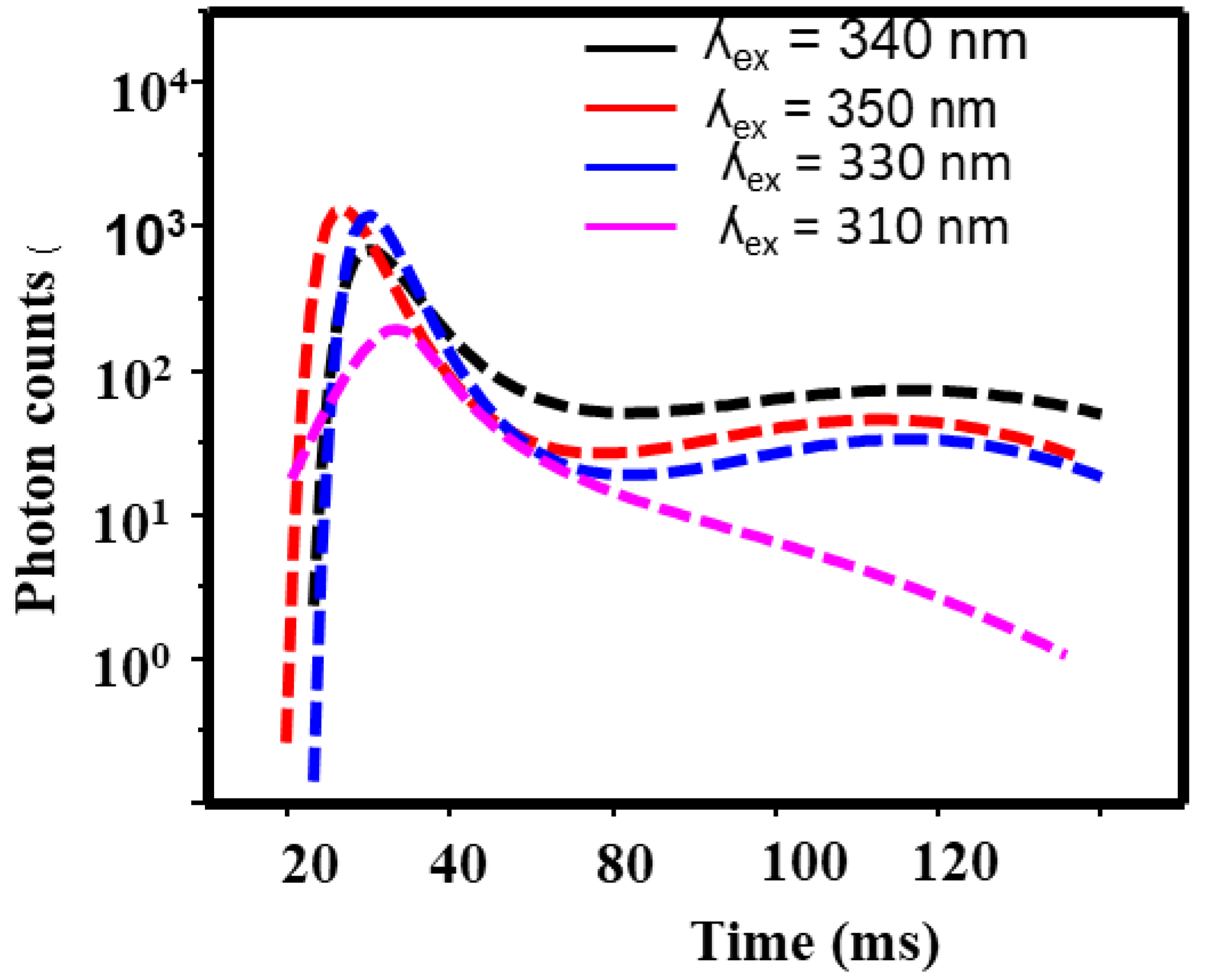
3.3. Photoluminsince Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Shen, Y.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Chemical Cleavage of Layered Carbon Nitride with Enhanced Photoluminescent Performances and Photoconduction. ACS Nano 2015, 9, 12480–12487. [CrossRef]
- Xiao, L.; Wang, Y.; Huang, Y.; Wong, T.; Sun, H. Self-trapped exciton emission from carbon dots investigated by polarization anisotropy of photoluminescence and photoexcitation. Nanoscale 2017, 9, 12637–12646. [CrossRef]
- Li, Y.; Miao, P.; Zhou, W.; Gong, X.; Zhao, X. N-doped carbon-dots for luminescent solar concentrators. J. Mater. Chem. A 2017, 5, 21452–21459. [CrossRef]
- Hola, K., et al., Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today, 2014. 9(5): p. 590-603.
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [CrossRef]
- Luo, W.-K.; Zhang, L.-L.; Yang, Z.-Y.; Guo, X.-H.; Wu, Y.; Zhang, W.; Luo, J.-K.; Tang, T.; Wang, Y. Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing. J. Nanobiotechnology 2021, 19, 1–30. [CrossRef]
- Kanwal, A., et al., Recent advances in green carbon dots (2015–2022): synthesis, metal ion sensing, and biological applications. Beilstein Journal of Nanotechnology, 2022. 13(1): p. 1068-1107.
- Umar, M. and H.A. Aziz, Photocatalytic degradation of organic pollutants in water. Organic pollutants-monitoring, risk and treatment, 2013. 8: p. 196-197.
- Ajith, M.; Aswathi, M.; Priyadarshini, E.; Rajamani, P. Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresour. Technol. 2021, 342, 126000. [CrossRef]
- Anju, A., P. Ravi S, and S. Bechan, Water pollution with special reference to pesticide contamination in India. Journal of Water Resource and Protection, 2010. 2010.
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [CrossRef]
- Bhosale, T.T.; Kuldeep, A.R.; Pawar, S.J.; Shirke, B.S.; Garadkar, K.M. Photocatalytic degradation of methyl orange by Eu doped SnO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 18927–18935. [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [CrossRef]
- Barman, M.K.; Patra, A. Current status and prospects on chemical structure driven photoluminescence behaviour of carbon dots. J. Photochem. Photobiol. C: Photochem. Rev. 2018, 37, 1–22. [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [CrossRef]
- zkara, A., D. Akyıl, and M. Konuk, Pesticides, environmental pollution, and health, in Environmental health risk-hazardous factors to living species. 2016, IntechOpen.
- Chauhan, D.S.; Quraishi, M.A.; Verma, C. Carbon nanodots: recent advances in synthesis and applications. Carbon Lett. 2022, 32, 1603–1629. [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [CrossRef]
- Cailotto, S.; Massari, D.; Gigli, M.; Campalani, C.; Bonini, M.; You, S.; Vomiero, A.; Selva, M.; Perosa, A.; Crestini, C. N-Doped Carbon Dot Hydrogels from Brewing Waste for Photocatalytic Wastewater Treatment. ACS Omega 2022, 7, 4052–4061. [CrossRef]
- Patial, S.; Sonu; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; Chaukura, N.; Selvasembian, R. A Review on Carbon Quantum Dots Modified g-C3N4-Based Photocatalysts and Potential Application in Wastewater Treatment. Appl. Sci. 2022, 12, 11286. [CrossRef]
- Gautam, S.; Agrawal, H.; Thakur, M.; Akbari, A.; Sharda, H.; Kaur, R.; Amini, M. Metal oxides and metal organic frameworks for the photocatalytic degradation: A review. J. Environ. Chem. Eng. 2020, 8, 103726. [CrossRef]
- Zhang, X.; Kamali, M.; Zhang, S.; Yu, X.; Appels, L.; Cabooter, D.; Dewil, R. Photo-assisted (waste)water treatment technologies — A scientometric-based critical review. Desalination 2022, 538. [CrossRef]
- Gudimella, K.K.; Appidi, T.; Wu, H.-F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand bath assisted green synthesis of carbon dots from citrus fruit peels for free radical scavenging and cell imaging. Colloids Surfaces B: Biointerfaces 2021, 197, 111362. [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 57, 14543–14578. [CrossRef]
- El-Shafey, A.M., Carbon dots: Discovery, structure, fluorescent properties, and applications. Green Processing and Synthesis, 2021. 10(1): p. 134-156.
- Nemera, D.J.; Etefa, H.F.; Kumar, V.; Dejene, F.B. Hybridization of nickel oxide nanoparticles with carbon dots and its application for antibacterial activities. Luminescence 2022, 37, 965–970. [CrossRef]
- Manzoor, S.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Srivastava, S.; Bashir, I.; Khan, S.A. Carbon dots applications for development of sustainable technologies for food safety: A comprehensive review. Appl. Food Res. 2023, 3, 100263. [CrossRef]
- Shanker, U.; Hussain, C.M.; Rani, M. Green Functionalized Nanomaterials for Environmental Applications; Elsevier: Amsterdam, NX, Netherlands, 2021; ISBN: .
- Etefa, H.F.; Nemera, D.J.; Dejene, F.B. Green Synthesis of Nickel Oxide NPs Incorporating Carbon Dots for Antimicrobial Activities. ACS Omega 2023, 8, 38418–38425. [CrossRef]
- Etefa, H.F.; Kumar, V.; Dejene, F.B.; Efa, M.T.; Jule, L.T. Modification of Flexible Electrodes for P-Type (Nickel Oxide) Dye-Sensitized Solar Cell Performance Based on the Cellulose Nanofiber Film. ACS Omega 2023, 8, 15249–15258. [CrossRef]
- Alzahrani, E.A.; Nabi, A.; Kamli, M.R.; Albukhari, S.M.; Althabaiti, S.A.; Al-Harbi, S.A.; Khan, I.; Malik, M.A. Facile Green Synthesis of ZnO NPs and Plasmonic Ag-Supported ZnO Nanocomposite for Photocatalytic Degradation of Methylene Blue. Water 2023, 15, 384. [CrossRef]
- Peng, G.; Chou, N.-N.; Lin, Y.-S.; Yang, C.-F.; Meen, T.-H. Comparison of the Degradation Effect of Methylene Blue for ZnO Nanorods Synthesized on Silicon and Indium Tin Oxide Substrates. Materials 2023, 16, 4275. [CrossRef]
- Etefa, H.F.; Imae, T.; Yanagida, M. Enhanced Photosensitization by Carbon Dots Co-adsorbing with Dye on p-Type Semiconductor (Nickel Oxide) Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 18596–18608. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




