1. Introduction
In most life situations, the senses cooperate with each other, providing consistent information about the world around us, which was of great importance for the survival of our species. For example, beautiful flowers usually smell pleasant, while things containing toxic substances in nature are usually bitter and repel us with their appearance. Often unconsciously, based on impressions provided by one sense, we “create” information to ourselves, completing the whole picture with information to which we do not have access at a given moment and which should be provided by other senses. For example, based on a telephone conversation (e.g. the timbre of the interlocutor's voice) we imagine his appearance. Cooperation in the field of sight-taste (which is the subject of the proposed manuscript) also seems to be very close - for example, red drinks suggest the taste of specific fruits like strawberries or cherries. Thanks to our experiences, we learn predictable connections between the appearance, smell and taste of food, as well as its nutritional value. These associations allow us to create an conceptual model of the foods we eat, which is often used in advertising food products.
Many studies, on for example taste-visual dissonance, have shown that the influence of the visual cortex on taste sensation is enormous [
1,
2,
3,
4,
5]. A large part of the volunteers incorrectly evaluate the taste of a given product based on (false) exemplary impressions. When they were drinking the yellow milk, they felt for example the taste of banana, and for the brown milk - chocolate, even though the milk they consumed did not have any additional taste. So we can say that we actually eat with our eyes and the appearance of the dish is undoubtedly important to us and cause the activation of gustatory cortex [
6].
Is the reverse true? Can we say that a taste stimulus can cause response in the visual cortex? When we eat or drink, do we unconsciously trigger visual impressions in our brain? There are few studies in the literature answering this question, and the only way to answer it today seems to be functional brain research such as fMRI or fNIRS.
fMRI is a well-known method and allows the examination of the entire brain, but it requires enormous equipment, space and personnel requirements, hence it is a relatively difficult and expensive test [
7,
8,
9,
10,
11]. A relatively new and interesting method of functional brain research is fNIRS - functional near infrared spectroscopy. This method, used in neurology and neurobiology, uses near-infrared spectroscopy for functional neuroimaging, including the study of the response of the cerebral cortex to taste stimuli [
12,
13]. Using fNIRS, brain activity is measured by hemodynamic responses related to neuronal behavior [
14]. This is a non-invasive test that allows you to determine, based on IR absorption by hemoglobin and oxyhemoglobin, which areas of the cerebral cortex are characterized by a greater demand for oxygen and nutrients after a specific stimulus [
15]. Although this very sophisticated method is sensitive to changes in oxyHb levels as low as 10-3 mmol/L (1 μM) its undoubted disadvantage is the possibility of examining only the cerebral cortex (up to 1.5 cm) and the inability to refer to the anatomy as precisely as in the case of fMRI [
15,
16].
The aim of the presented work is to investigate whether a taste stimulus, in this case the taste of bitter/coffee, also causes stimulation of the visual cortex in fNIRS study.
2. Materials and Methods
The study was conducted in accordance with the decision of the local bioethics committee. All 51 volunteers gave written informed consent, and were informed about the purpose of the study and its course.
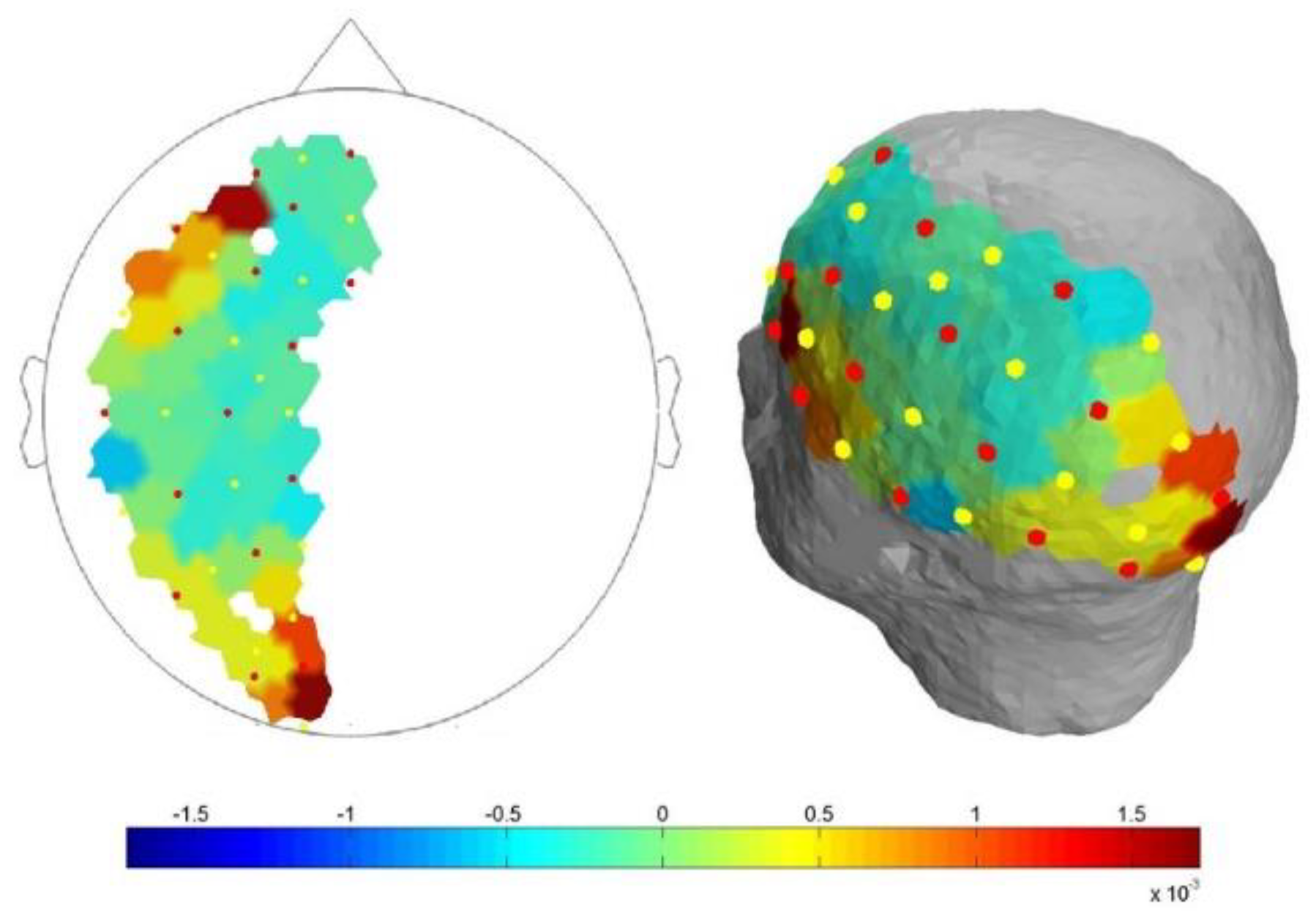
fNIRS spectroscope (NIRScout, NIRx Medical Technologies LLC, Glean Head, NY, USA) to record near-infrared brain signals was used. Light from eight dual-wavelength LED light sources (wavelengths 760 and 850 nm) was detected by avalanche photodiode (APD) optodes placed 3 cm from the emitters. Optodes were placed to cover the left hemisphere (mostly dominant hemisphere), as shown in
Figure 1.
All measurements were recorded using NIRStar 15.0 (NIRx Medizintechnik GmbH, Berlin, Germany) software. Signal intensity was calibrated and verified for each channel before data collection, and a low-pass filter (0.2 Hz) was used for data collection. Participants were asked to remain in a sitting position and with their eyes closed throughout the study.
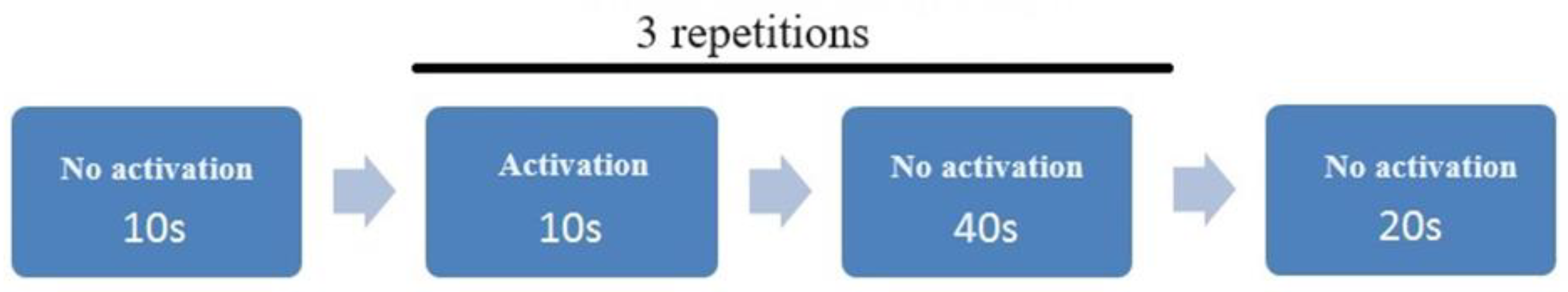
Four protocols were used to carry out the research project, every with the same scheme of study (
Figure 2). Protocol I, II, IV – activation with distillate water, coffee A and coffee B respectively. In all protocols on the sound signal the participants opened mouth and 0.5 ml (with the pipette) of substance was dropped (there were 3 repetitions). In protocol III the scheme of study was identical but there was no activation (reference measurement).
Study participants completed a short questionnaire in which, in addition to data on age, gender, height, laterality and weight, they assessed the bitterness of both coffee solutions and declared the amount of coffee they drank per day.
Data acquisition parameters were the same for every protocols. All data were analysed using MATLAB-based NIRSLab 15.0. The recordings were band-pass filtered, with a high cutoff frequency of 0.2 Hz and a low cut-off frequency of 0.01 Hz. The normal spectrum for oxyHb and deoxy-Hb used in the analyses was created in accordance with the manufacturer's recommendations. The final data for each participant was the average of three repetitions.
After preliminary visual analysis, the highest signals were recorded for two channels 14 and 48. First correspond to the location of the cortical taste centre, second to the visual cortex (see
Table 1). That’s way we analysed the data for 14 and 48 channel, whole cortical taste centre (channels 14, 21, 28, 29, and 30) and visual cortex (45, 46, 47, 48, and 49). fOLD v2.2 software was used to locate the optodes [
17].
Data regarding the difference between the maximum and minimum changes in oxyHb concentrations (ΔoxyHb) were collected and analysed (in summary 51 volunteers, 204 examinations, 9 996 records).
To assess the effect of the taste activation for water and coffee on the change in oxyHb concentration in the cortex, data obtained from protocols I, II and IV were compared with data from protocol III (reference). To evaluate the influence of water, coffe A and coffe B on oxyHb, data from protocol I, II and IV were compared.
Data distribution was assessed using the Shapiro-Wilk test. To investigate the differences between the groups for normally distributed data, the Student’s t-test was used; otherwise, the Mann-Whitney U-test was performed (Dell Inc. [2016]. Dell Statistica [data analysis software system], version 13. software.dell.com). Spearman tests were used for correlation analysis. Differences were considered statistically significant for p values < 0.05.
3. Results
The study involved 51 healthy volunteers (41 women, 10 men) with an median age of 19 years (18–21 years). In protocols, I, II and IV, characteristic reactions dependent on the level of blood oxygenation, defined as an increase in the concentration of oxyHb and a decrease in the level of deoxy-Hb for all analysed channels after stimulation, were observed. The increase of ΔoxyHb (difference between max and min oxyHb value) up to 15 s after the stimulus, for every protocols was analyzed. The statistical information on ΔoxyHb [μmol/l] values for all protocols, for male, female and all participants are shown in
Table 2,
Table 3 and
Table 4.
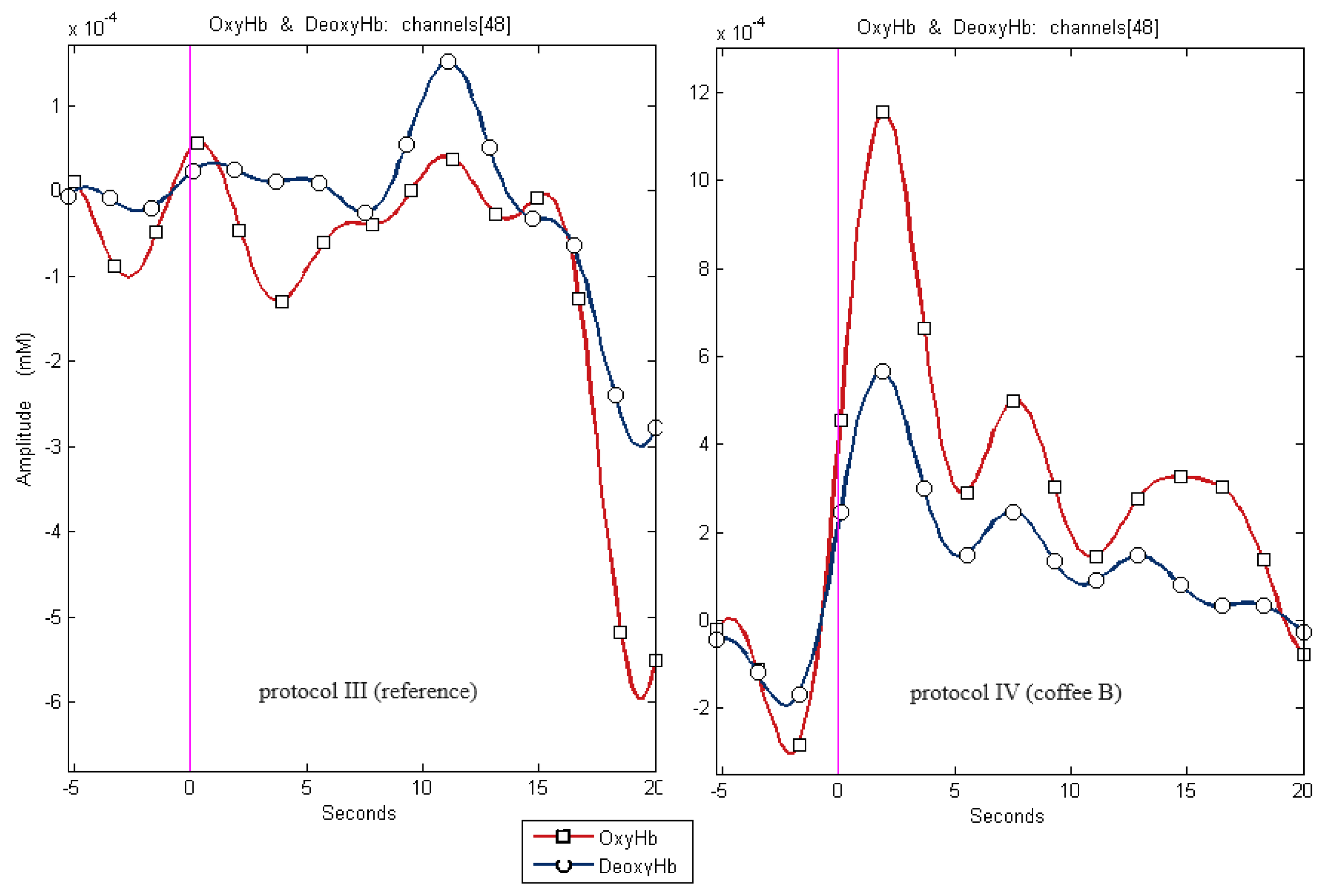
For some cases, fewer than 51 results were presented because some of the records were disqualified due to artifacts that made their evaluation impossible. Sample recordings for channel 48 for protocol IV and III shows
Figure 3.
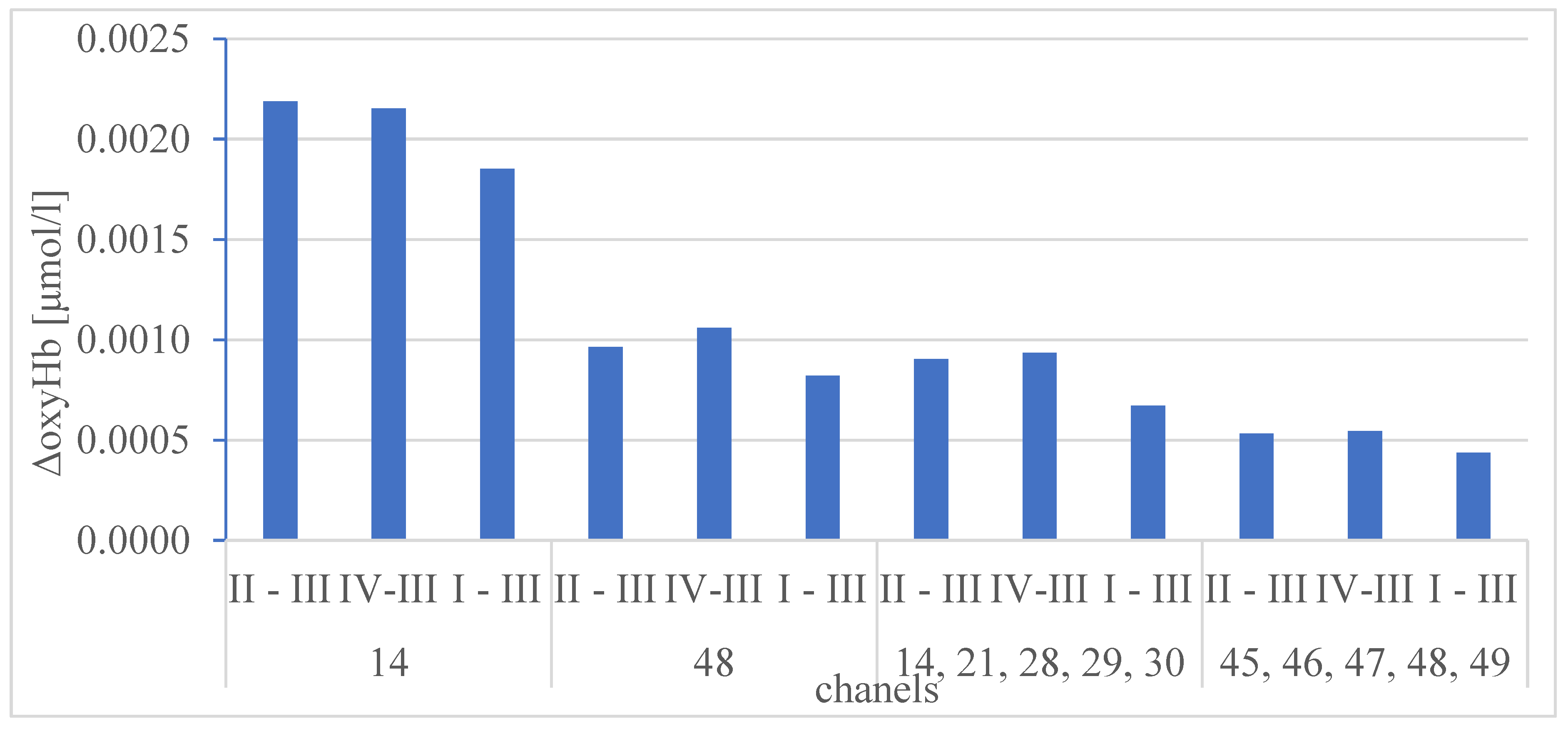
The results of the statistical comparison of medians ΔoxyHb for the protocols I, II, III and IV for all analysed channels (for all participants) are presented on
Figure 4 and in
Table 5 and
Table 6.
The results of the statistical comparison of medians ΔoxyHb for all channels, for all protocols (for all participants) are presented in
Table 7,
Table 8 and
Table 9.
Additionally, the correlations between the amount of consumed coffee (ACC), the bitterness assessments solutions (bitterness) and the signal height for individual channels were examined. The results of Spearman's rank correlation test are collected in
Table 10.
Differences in signal height between female and men, for all protocols and all channels, were also examined (Mann-Whitney U-test). The only statistically significant differences for ΔoxyHb were obtained for channel 14, for protocol I (water). Median/mean for female and male was respectively 0,00214/0,00226 and 0,00128/0,00134, with respect to reference protocol (I-III) 0,00200/0,00199 and 0,00108/0,00111 (see
Table 3 and
Table 4).
4. Discussion
Taste is a complex sense, indispensable for species to distinguish between harmful and nutritive foods [
17]. Because taste governs food acceptability, it has a critical role in human survival and, more in general, it can affect health conditions [
18].
Numerous works in the field of mapping the human brain cortex prove that the reception and analysis of stimuli, including gustatory ones, reaching us from various senses is not one-track. As a result of the conducted research, including functional brain research, it turns out that information about the external and internal world (our body) in the cerebral cortex is mixed [
19,
20,
21]. Until recently, it seemed that we knew perfectly well which pathways and to which places in the cerebral cortex signals from specific senses reach. It turns out, however, that it is not so obvious, hence it is still a current topic of research in the field of neurology, neurocognitive science and physiology. The topic of the presented work focuses on the taste stimulus. It would seem that this simple stimulus, a bitter taste, would induce hemodynamic activity only in the gustatory cortex too.
In our previous work on the hemodynamic response of the cerebral cortex after sour taste activation, we initially noticed that the gustatory cortex is not the only place in our cerebral cortex where a taste stimulus generates an increased hemodynamic response [
22]. Numerous publications also show the influence of the sense of sight on the perception of taste, which seems quite intuitive. However, the authors did not find any studies that would clearly confirm that a taste stimulus can also generate activity in the cerebral cortex in places that are generally considered to be responsible only for the reception of visual stimuli. Analyzing the available literature, we can draw the conclusion that both senses cooperate closely, so it seems highly probable.
After analyzing over 200 results of fNIRS records, we have undoubtedly confirmed that the use of a taste stimulus such as water or coffee with different concentrations each time caused a hemodynamic reaction in the gustatory cortex. We recorded signals for teste activation on channels 14, 21, 28- 30, which confirms the correctness of the choice of research method. What seems important, and somewhat surprising, is the fact that we also received high signals for the channels 45-49, which cover the visual cortex. This may indicate that when tasting various dishes and drinks, we involuntarily and unconsciously create a certain image of the food or drink we consume, perhaps associating it with specific places, situations or people.
The
Figure 4 shows the height of the ΔoxyHb which is confirmation of the correctness of the methodology protocol. The highest values were recorded for channels 14, 48, representing gustatory channels (14, 21, 28-30) and visual channels (45-49) respectively. The statistical analysis presented in
Table 5 and
Table 6 does not show any statistical differences between the protocols I, II and IV (different taste activation – water, coffee A and coffee B) for specific channels. The lack of difference in the hemodynamic response to the taste stimulus of pure water and coffee measured by the objective fNIRS method leads to the conclusion that this method is not suitable for assessing taste and cannot compete with the subjective declared assessment of the examined person.
There is statistically important lower signal for the reference protocol III (no activation) compared with taste activation what seems to be a confirmation of the correctness of the methodology. However there is some significant differences between level of signal ΔoxyHb for different channels what is presented in
Table 7,
Table 8 and
Table 9. What is the most significant, signal for channel 14 is always the highest one. When comparing the signal heights between the 48, gustatory and visual channels, they are not always statistically distinguishable, hence it can be concluded that the stimulation of these areas was repeatedly comparable. Based on the collected data, we can clearly state that the senses of sight and taste work closely together. Moreover, this cooperation is not one-sided - not only the appearance of the food (visual impression) affects the perception of taste what is quite precisely described in literature, but interestingly, the taste stimulus (in this study, the taste of coffee and water) can generate a hemodynamic response - activity in the visual cortex. This is surprising because there are no reports in the literature on the stimulation of the visual cortex by a taste stimulus. For example, for a bitter taste, many studies indicate as a place that is activated by a bitter stimulus: right precentral gyrus [
23,
24,
25,
26,
27,
28,
29], left amygdala [
24], right thalamus [
10,
24,
27], and there is no mention of the visual cortex. The research requires repetition on a larger group of subjects. It is also interesting how other flavors would affect the hemodynamic response of the cortex, which also requires further researches.
As a results (see
Table 10) of the analysis of the correlation between the subjective bitterness assessment solutions, and the signal ΔoxyHb height, it was observed that statically important correlation, although weak, occurs only for 14 and gustatory channels, only for coffee with a higher concentration. The lack of correlation for coffee A may be the result of the fact that respondents rated the level of bitterness of coffee at a similar level (less spread of results). No correlation between the subjective bitterness assessment solutions, and the signal ΔoxyHb height for visual channels seems logical - a visual image, e.g. a cup of coffee, will probably "appear" activating the visual cortex at a similar level, regardless of how strong the coffee we drink or what kind of taste stimulus we use in general. There was no statistically important correlation between the declared amount of consumed coffee and the signal ΔoxyHb height.
Based on research by other researchers, the perception of taste seems to be related to gender, which has not been fully proven in this paper [
23,
30,
31,
32,
33]. We recorded the only statistically significant difference between women and men for protocol I (water), where the ΔoxyHb signal was twice as high for women. This may indicate that women are more sensitive to taste stimuli, which is confirmed by other researchers. However, this relationship could not be observed for coffee. This could be probably the result of the small number of male representatives in the study. The stronger reaction to water might be due to the fact that water was the first stimulus used in the study and therefore caused higher cortical activation in women than in men. It was observed only for channel 14, the one that shows the greatest response in the taste cortex. More extensive research is required on this topic, with more male participants.
In conclusion, we can clearly state that the senses of sight and taste work closely together. Moreover, this cooperation is not one-sided - not only the visual activation affects the perception of taste, but interestingly, the taste stimulus can generate a hemodynamic response - activity in the visual cortex.
Author Contributions
Conceptualization, K.J. and W.P.; methodology, K.J. and W.P.; validation, K.J. and W.P.; formal analysis, K.J. and W.P.; investigation, K.J. and W.P.; data curation, K.J..; writing—original draft preparation, K.J., A.C-P., J.Z. and W.P.; writing—review and editing, K.J. and W.P.; visualization, K.J. and W.P.; supervision, K.J. and W.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Pomeranian Medical University (protocol code: KB-0012/12/2021, date of approval: 31.05.2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shankar, M.U.; Levitan, C.A.; Spence, C. Grape Expectations: The Role of Cognitive Influences in Color–Flavor Interactions. Consciousness and Cognition 2010, 19, 380–390. [Google Scholar] [CrossRef]
- Zampini, M.; Sanabria, D.; Phillips, N.; Spence, C. The Multisensory Perception of Flavor: Assessing the Influence of Color Cues on Flavor Discrimination Responses. Food Quality and Preference 2007, 18, 975–984. [Google Scholar] [CrossRef]
- Zampini, M.; Wantling, E.; Phillips, N.; Spence, C. Multisensory Flavor Perception: Assessing the Influence of Fruit Acids and Color Cues on the Perception of Fruit-Flavored Beverages. Food Quality and Preference 2008, 19, 335–343. [Google Scholar] [CrossRef]
- The effect of color on perceived flavor intensity and acceptance of foods by young adults and elderly adults – ProQuest. Available online: https://www.proquest.com/openview/5037eb29d98a95dd88c6b7b061de133f/1?pq-origsite=gscholar&cbl=49142 (accessed on 12 June 2024).
- Shankar, M.U.; Levitan, C.A.; Prescott, J.; Spence, C. The Influence of Color and Label Information on Flavor Perception. Chem. Percept. 2009, 2, 53–58. [Google Scholar] [CrossRef]
- Rosasco Silva, V. Influences of Spiciness and Visual Color Cue On Salty Taste Intensity Perception In Reduced-Sodium Cheese Dips. LSU Master’s Theses 2018. [CrossRef]
- fMRI Study of Taste Cortical Areas in Humans - Faurion - 1998 - Annals of the New York Academy of Sciences - Wiley Online Library. Available online: https://nyaspubs.onlinelibrary.wiley.com/doi/full/10.1111/j.1749-6632.1998.tb10623.x (accessed on 12 June 2024).
- Veldhuizen, M.G.; Gitelman, D.R.; Small, D.M. An fMRI Study of the Interactions Between the Attention and the Gustatory Networks. Chemosens Percept 2012, 5, 117–127. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, X.; Small, D.M.; Chen, H. Extreme Spicy Food Cravers Displayed Increased Brain Activity in Response to Pictures of Foods Containing Chili Peppers: An fMRI Study. Appetite 2019, 142, 104379. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Sadachi, H.; Nakamura, J.; Tonoike, M. Functional Magnetic Resonance Imaging Investigation of Brain Regions Associated with Astringency. Neurosci Res 2017, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Cerf-Ducastel, B.; Van de Moortele, P.F.; MacLeod, P.; Le Bihan, D.; Faurion, A. Interaction of Gustatory and Lingual Somatosensory Perceptions at the Cortical Level in the Human: A Functional Magnetic Resonance Imaging Study. Chem Senses 2001, 26, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Bembich, S.; Lanzara, C.; Clarici, A.; Demarini, S.; Tepper, B.J.; Gasparini, P.; Grasso, D.L. Individual Differences in Prefrontal Cortex Activity during Perception of Bitter Taste Using fNIRS Methodology. Chem Senses 2010, 35, 801–812. [Google Scholar] [CrossRef]
- Hu, C.; Kato, Y.; Luo, Z. An fNIRS Research on Prefrontal Cortex Activity Response to Pleasant Taste. JBBS 2013, 03, 617–623. [Google Scholar] [CrossRef]
- Csipo, T.; Mukli, P.; Lipecz, A.; Tarantini, S.; Bahadli, D.; Abdulhussein, O.; Owens, C.; Kiss, T.; Balasubramanian, P.; Nyúl-Tóth, Á.; et al. Assessment of Age-Related Decline of Neurovascular Coupling Responses by Functional near-Infrared Spectroscopy (fNIRS) in Humans. Geroscience 2019, 41, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, K.; Sękowska-Namiotko, A.; Pala, B.; Lietz-Kijak, D.; Gronwald, H.; Podraza, W. Searching for the Mechanism of Action of Extremely Low Frequency Electromagnetic Field—The Pilot fNIRS Research. International Journal of Environmental Research and Public Health 2022, 19, 4012. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, Y.; Ueji, K.; Yamamoto, T. Activity of Frontal Pole Cortex Reflecting Hedonic Tone of Food and Drink: fNIRS Study in Humans. Sci Rep 2018, 8, 16197. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.; Simon, S.A. Chemosensory Processing in the Taste-Reward Pathway. Flavour and Fragrance Journal 2011, 26, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Feeney, S.M.; Johnson, M.C.; Mortlock, D.J.; Peiris, H.V. First Observational Tests of Eternal Inflation. Phys. Rev. Lett. 2011, 107, 071301. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.H.F. Psychological Sweetening: Multisensory Interactions Shaping Sweet Taste Perception. doctoralThesis, 2024.
- Campo, R.; Reinoso-Carvalho, F.; Rosato, P. Wine Experiences: A Review from a Multisensory Perspective. Applied Sciences 2021, 11, 4488. [Google Scholar] [CrossRef]
- Verhagen, J.V.; Engelen, L. The Neurocognitive Bases of Human Multimodal Food Perception: Sensory Integration. Neuroscience & Biobehavioral Reviews 2006, 30, 613–650. [Google Scholar] [CrossRef]
- Taste Dysfunction after COVID-19: Analysis with Functional near-Infrared Spectroscopy. Available online: https://otolaryngologypl.com/article/537423/en (accessed on 12 June 2024).
- Haase, L.; Green, E.; Murphy, C. Males and Females Show Differential Brain Activation to Taste When Hungry and Sated in Gustatory and Reward Areas. Appetite 2011, 57, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Haase, L.; Cerf-Ducastel, B.; Murphy, C. Cortical Activation in Response to Pure Taste Stimuli during the Physiological States of Hunger and Satiety. NeuroImage 2009, 44, 1008–1021. [Google Scholar] [CrossRef]
- Spetter, M.S.; Smeets, P.A.M.; De Graaf, C.; Viergever, M.A. Representation of Sweet and Salty Taste Intensity in the Brain. Chemical Senses 2010, 35, 831–840. [Google Scholar] [CrossRef]
- Green, E.; Murphy, C. Altered Processing of Sweet Taste in the Brain of Diet Soda Drinkers. Physiology & Behavior 2012, 107, 560–567. [Google Scholar] [CrossRef]
- Dalenberg, J.R.; Hoogeveen, H.R.; Renken, R.J.; Langers, D.R.M.; ter Horst, G.J. Functional Specialization of the Male Insula during Taste Perception. NeuroImage 2015, 119, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Riddell, C.D.; Gotts, S.J.; Martin, A. Taste Quality Representation in the Human Brain. J Neurosci 2020, 40, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Eldeghaidy, S.; Yang, Q.; Abualait, T.; Williamson, A.-M.; Hort, J.; Francis, S.T. Thermal Taster Status: Temperature Modulation of Cortical Response to Sweetness Perception. Physiology & Behavior 2021, 230, 113266. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Tregellas, J.R. Sex-Based Differences in the Behavioral and Neuronal Responses to Food. Physiol Behav 2010, 99, 538–543. [Google Scholar] [CrossRef]
- Ballantyne, K.N.; Ralf, A.; Aboukhalid, R.; Achakzai, N.M.; Anjos, M.J.; Ayub, Q.; Balažic, J.; Ballantyne, J.; Ballard, D.J.; Berger, B.; et al. Toward Male Individualization with Rapidly Mutating Y-Chromosomal Short Tandem Repeats. Human Mutation 2014, 35, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Van Langeveld, A.W.B.; Teo, P.S.; Vries, J.H.M. de; Feskens, E.J.M.; Graaf, C. de; Mars, M. Dietary Taste Patterns by Sex and Weight Status in the Netherlands. British Journal of Nutrition 2018, 119, 1195–1206. [Google Scholar] [CrossRef]
- Ponticorvo, S.; Prinster, A.; Cantone, E.; Di Salle, F.; Esposito, F.; Canna, A. Sex Differences in the Taste-Evoked Functional Connectivity Network. Chemical Senses 2022, 47, bjac015. [Google Scholar] [CrossRef]
Figure 1.
Diagram of optodes arrangement on the head surface.
Figure 1.
Diagram of optodes arrangement on the head surface.
Figure 2.
Scheme of study. Activation is understood as stimulation of the cerebral cortex with 0.5 ml of water, coffee A or coffee B or no stimulation for the protocol III (reference one).
Figure 2.
Scheme of study. Activation is understood as stimulation of the cerebral cortex with 0.5 ml of water, coffee A or coffee B or no stimulation for the protocol III (reference one).
Figure 3.
Sample recordings for channel 48 for protocol IV and III (reference)for channel 48.
Figure 3.
Sample recordings for channel 48 for protocol IV and III (reference)for channel 48.
Figure 4.
Difference between the median of ΔoxyHb for protocols I, II, IV and the median for reference protocol III, for all participants.
Figure 4.
Difference between the median of ΔoxyHb for protocols I, II, IV and the median for reference protocol III, for all participants.
Table 1.
The area of the cerebral cortex covered by particular groups of channels.
Table 1.
The area of the cerebral cortex covered by particular groups of channels.
| Channels |
|
| 14, 21, 28, 29, 30 |
Brodmann area 43,
the subcentral area
the postcentral gyrus
the inferior frontal gyrus (including the pars opercularis, pars triangularis, pars orbitalis) |
| 45, 46, 47, 48, 49 |
part of the primary visual cortex (the striate cortex; Brodmann area 17)
the secondary visual cortex (Brodmann area 18)
the tertiary visual cortex (Brodmann area 19) |
Table 2.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for all participants. Data were not normally distributed.
Table 2.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for all participants. Data were not normally distributed.
| Channels |
Stimulation (protocol) |
n |
ΔoxyHb[μmol/l]
|
| Mdn |
Q1 |
Q3 |
| 14 |
water (I) |
51 |
0,00201 |
0,00090 |
0,00273 |
| 48 |
water (I) |
51 |
0,00118 |
0,00077 |
0,00226 |
| 14, 21, 28, 29, 30 |
water (I) |
51 |
0,00081 |
0,00048 |
0,00130 |
| 45, 46, 47, 48, 49 |
water (I) |
49 |
0,00067 |
0,00055 |
0,00117 |
| 14 |
coffee A (II) |
51 |
0,00235 |
0,00138 |
0,00311 |
| 48 |
coffee A (II) |
51 |
0,00132 |
0,00080 |
0,00276 |
| 14, 21, 28, 29, 30 |
coffee A (II) |
50 |
0,00104 |
0,00061 |
0,00162 |
| 45, 46, 47, 48, 49 |
coffee A (II) |
50 |
0,00076 |
0,00049 |
0,00118 |
| 14 |
coffee B (IV) |
50 |
0,00232 |
0,00138 |
0,00348 |
| 48 |
coffee B (IV) |
51 |
0,00142 |
0,00059 |
0,00247 |
| 14, 21, 28, 29, 30 |
coffee B (IV) |
50 |
0,00108 |
0,00060 |
0,00168 |
| 45, 46, 47, 48, 49 |
coffee B (IV) |
50 |
0,00077 |
0,00051 |
0,00150 |
| 14 |
reference (III) |
51 |
0,00016 |
0,00008 |
0,00028 |
| 48 |
reference (III) |
51 |
0,00036 |
0,00018 |
0,00067 |
| 14, 21, 28, 29, 30 |
reference (III) |
51 |
0,00014 |
0,00008 |
0,00021 |
| 45, 46, 47, 48, 49 |
reference (III) |
50 |
0,00023 |
0,00011 |
0,00038 |
Table 3.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Mn - mean, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for male participants. *Results for normally distributed data.
Table 3.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Mn - mean, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for male participants. *Results for normally distributed data.
| Channels |
Stimulation (protocol) |
n |
ΔoxyHb [μmol/l] |
| Mdn/Mn* |
Q1/Min* |
Q2/Max* |
| 14 |
water (I) |
10 |
0,00134* |
0,00042* |
0,00242* |
| 48 |
water (I) |
10 |
0,00108* |
0,00018* |
0,00271* |
| 14, 21, 28, 29, 30 |
water (I) |
10 |
0,00074* |
0,00016* |
0,00152* |
| 45, 46, 47, 48, 49 |
water (I) |
10 |
0,00077* |
0,00040* |
0,00147* |
| 14 |
coffee A (II) |
10 |
0,00177* |
0,00080* |
0,00323* |
| 48 |
coffee A (II) |
10 |
0,00121 |
0,00083 |
0,00136 |
| 14, 21, 28, 29, 30 |
coffee A (II) |
10 |
0,00063 |
0,00053 |
0,00085 |
| 45, 46, 47, 48, 49 |
coffee A (II) |
10 |
0,00092* |
0,00042* |
0,00199* |
| 14 |
coffee B (IV) |
10 |
0,00192* |
0,00082* |
0,00358* |
| 48 |
coffee B (IV) |
10 |
0,00139* |
0,00053* |
0,00286* |
| 14, 21, 28, 29, 30 |
coffee B (IV) |
9 |
0,00096* |
0,00028* |
0,00141* |
| 45, 46, 47, 48, 49 |
coffee B (IV) |
10 |
0,00084 |
0,00063 |
0,00125 |
| 14 |
reference (III) |
10 |
0,00023* |
0,00002* |
0,00053* |
| 48 |
reference (III) |
10 |
0,00046* |
0,00011* |
0,00102* |
| 14, 21, 28, 29, 30 |
reference (III) |
10 |
0,00021* |
0,00000* |
0,00060* |
| 45, 46, 47, 48, 49 |
reference (III) |
10 |
0,00022 |
0,00012 |
0,00038 |
Table 4.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Mn - mean, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for female participants. *Results for normally distributed data.
Table 4.
The statistical information on ΔoxyHb [μmol/l] for all protocols and all analysed channels, where n – number of cases, Mdn – median, Mn - mean, Min – minimum, Max – maximum, Q1- the 25th percentile, Q3 - 75th percentile, for female participants. *Results for normally distributed data.
| Channels |
Stimulation (protocol) |
n |
ΔoxyHb[μmol/l]
|
| Mdn/Mn* |
Q1/Min* |
Q2/Max* |
| 14 |
water (I) |
41 |
0,00214 |
0,00121 |
0,00279 |
| 48 |
water (I) |
41 |
0,00124 |
0,00086 |
0,00230 |
| 14, 21, 28, 29, 30 |
water (I) |
41 |
0,00094 |
0,00051 |
0,00138 |
| 45, 46, 47, 48, 49 |
water (I) |
39 |
0,00070 |
0,00054 |
0,00132 |
| 14 |
coffee A (II) |
41 |
0,00263* |
0,00017* |
0,00723* |
| 48 |
coffee A (II) |
41 |
0,00157 |
0,00076 |
0,00287 |
| 14, 21, 28, 29, 30 |
coffee A (II) |
40 |
0,00120 |
0,00074 |
0,00167 |
| 45, 46, 47, 48, 49 |
coffee A (II) |
40 |
0,00077 |
0,00047 |
0,00120 |
| 14 |
coffee B (IV) |
40 |
0,00259* |
0,00018* |
0,00727* |
| 48 |
coffee B (IV) |
41 |
0,00146 |
0,00059 |
0,00268 |
| 14, 21, 28, 29, 30 |
coffee B (IV) |
41 |
0,00115 |
0,00060 |
0,00188 |
| 45, 46, 47, 48, 49 |
coffee B (IV) |
40 |
0,00077 |
0,00046 |
0,00153 |
| 14 |
reference (III) |
41 |
0,00014 |
0,00008 |
0,00025 |
| 48 |
reference (III) |
41 |
0,00032 |
0,00017 |
0,00062 |
| 14, 21, 28, 29, 30 |
reference (III) |
41 |
0,00014 |
0,00008 |
0,00020 |
| 45, 46, 47, 48, 49 |
reference (III) |
40 |
0,00023 |
0,00011 |
0,00036 |
Table 5.
The results of the statistical comparison of medians ΔoxyHb for the protocols I, II, III and IV for channel 14 and channels 14, 21, 28, 29, 30, for all participants.
Table 5.
The results of the statistical comparison of medians ΔoxyHb for the protocols I, II, III and IV for channel 14 and channels 14, 21, 28, 29, 30, for all participants.
| |
p-values for |
| Channel 14 |
Channels 14, 21, 28, 29, and 30 |
| vs |
protocols |
vs |
protocols |
| III |
I |
II |
IV |
III |
I |
II |
IV |
| Protocols |
III |
|
<0,05 |
<0,05 |
<0,05 |
III |
|
<0,05 |
<0,05 |
<0,05 |
| I |
<0,05 |
|
0,12 |
0,16 |
I |
<0,05 |
|
0,12 |
0,18 |
| II |
<0,05 |
0,12 |
|
0,98 |
II |
<0,05 |
0,12 |
|
0,87 |
| IV |
<0,05 |
0,16 |
0,98 |
|
IV |
<0,05 |
0,18 |
0,87 |
|
Table 6.
The results of the statistical comparison of medians ΔoxyHb for the protocols I, II, III and IV for channel 48 and channels 45, 46, 47, 48, and 49, for all participants.
Table 6.
The results of the statistical comparison of medians ΔoxyHb for the protocols I, II, III and IV for channel 48 and channels 45, 46, 47, 48, and 49, for all participants.
| |
p-values for |
| Channel 48 |
Channels 45, 46, 47, 48, and 49 |
| vs |
Protocols |
vs |
Protocols |
| III |
I |
II |
IV |
III |
I |
II |
IV |
| Protocols |
III |
|
<0,05 |
<0,05 |
<0,05 |
III |
|
<0,05 |
<0,05 |
<0,05 |
| I |
<0,05 |
|
0,50 |
0,74 |
I |
<0,05 |
|
0,99 |
0,69 |
| II |
<0,05 |
0,50 |
|
0,80 |
II |
<0,05 |
0,99 |
|
0,68 |
| IV |
<0,05 |
0,74 |
0,80 |
|
IV |
<0,05 |
0,69 |
0,68 |
|
Table 7.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol I-III, for all participants.
Table 7.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol I-III, for all participants.
| p-values for protocol I - III |
| vs |
Channels |
| 14 |
48 |
14, 21, 28-30 |
45-49 |
| Channels |
14 |
|
<0,05 |
<0,05 |
<0,05 |
| 48 |
<0,05 |
|
<0,05 |
<0,05 |
| 14, 21, 28-30 |
<0,05 |
<0,05 |
|
0,11 |
| 45-49 |
<0,05 |
<0,05 |
0,11 |
|
Table 8.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol II-III, for all participants.
Table 8.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol II-III, for all participants.
| p-values for protocol II - III |
| vs |
Channels |
| 14 |
48 |
14, 21, 28-30 |
45-49 |
| Channels |
14 |
|
<0,05 |
<0,05 |
<0,05 |
| 48 |
<0,05 |
|
0,65 |
<0,05 |
| 14, 21, 28-30 |
<0,05 |
0,65 |
|
<0,05 |
| 45-49 |
<0,05 |
<0,05 |
<0,05 |
|
Table 9.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol IV-III, for all participants.
Table 9.
The results of the statistical comparison of medians ΔoxyHb, for all analysed channels for protocol IV-III, for all participants.
| p-values for protocol IV - III |
| vs |
Channels |
| 14 |
48 |
14, 21, 28-30 |
45-49 |
| Channels |
14 |
|
<0,05 |
<0,05 |
<0,05 |
| 48 |
<0,05 |
|
0,44 |
<0,05 |
| 14, 21, 28-30 |
<0,05 |
0,44 |
|
0,06 |
| 45-49 |
<0,05 |
<0,05 |
0,06 |
|
Table 10.
The correlations between the amount of consumed coffee, the bitterness assessments solutions and the signal height for individual channels. Data were not normally distributed. R - Spearman correlation coefficient, ACC - the amount of consumed coffee, bitterness - the bitterness assessments solutions.
Table 10.
The correlations between the amount of consumed coffee, the bitterness assessments solutions and the signal height for individual channels. Data were not normally distributed. R - Spearman correlation coefficient, ACC - the amount of consumed coffee, bitterness - the bitterness assessments solutions.
| |
Variable pairs |
|
|
Bitterness& ΔoxyHb for channels:
|
R |
p value |
| Coffee (protocol) |
A (II) |
14 |
0,1 |
0,45 |
| 48 |
-0,1 |
0,70 |
| 14, 21, 28, 29, 30 |
-0,1 |
0,74 |
| 45, 46, 47, 48, 49 |
-0,1 |
0,39 |
| B (IV) |
14 |
0,4 |
<0,05 |
| 48 |
0,2 |
0,19 |
| 14, 21, 28, 29, 30 |
0,3 |
<0,05 |
| 45, 46, 47, 48, 49 |
0,1 |
0,47 |
| |
ACC & ΔoxyHb for channels: |
R |
p value |
| Coffee (protocol) |
A (II) |
14 |
-0,2 |
0,18 |
| 48 |
0,2 |
0,16 |
| 14, 21, 28, 29, 30 |
0,004 |
0,97 |
| 45, 46, 47, 48, 49 |
0,2 |
0,13 |
| B (IV) |
14 |
-0,2 |
0,18 |
| 48 |
0,02 |
0,90 |
| 14, 21, 28, 29, 30 |
-0,2 |
0,14 |
| 45, 46, 47, 48, 49 |
0,03 |
0,86 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).