1. Introduction
Light disturbance, characterized by visual phenomena such as glare, halos, starbursts, and decreased contrast sensitivity, significantly impacts visual performance, especially under low-light conditions when pupil dilates [
1]. Contrast sensitivity is crucial for discerning objects in low-light conditions, yet intense light sources can lead to the perception of dysphotopsias - visual disturbances around those lights [
2,
3,
4]. These disturbances are of paramount concern in the fields of visual sciences due to their profound effect on nighttime driving, reading ability in dim lighting and overall quality of life [
5,
6,
7].
Visual disturbances in low-lighting conditions can stem from various sources, including intraocular diffraction of light, ocular aberrations and uncorrected refractive errors [
3]. The pupil’s dilation in dim lighting exacerbates these effects, especially at night against a dark background, underscoring the importance of understanding and quantifying these disturbances accurately.
Along with diffraction and aberrations, light scattering is also a factor that contributes to the degradation of retinal image quality. Light scattering occurs in the cornea [
8] and especially in the lens of the human eye [
9]. The cornea is the first optical surface of the eye [
10] and has a significant and crucial role in the formation of the visual image. The curvature of the cornea contributes to approximately two-thirds of the eye's focusing ability. Consequently, any irregularities in its shape or changes in transparency can impact the quality of vision [
11,
12]. The crystalline lens, the second most powerful refractive element of the eye, is also responsible for adjusting its shape to refract light to be focused on the retina considering the focal distance (accommodation) [
13]. While scattering is usually minimal in young, healthy eyes, it is recognized to increase with age. This increase is attributed to changes in the structure of the lens, often associated with cataract formation. Consequently, these changes in the lens can alter its optical properties and lead to an increase in scattering [
14,
15]. In addition to ageing, ocular pathologies [
4,
16,
17], surgical procedures (such as refractive surgery and intraocular lens implantation) [
18,
19,
20], lenses for the control and progression of myopia [
21] and multifocal contact lenses [
22] among others, can increase ocular scattering and high-order aberrations, contributing to an increase in complaints of decreased contrast sensitivity and poor vision, particularly under dim light conditions [
23,
24,
25,
26,
27]. Night vision disorders can also become a limiting factor in individuals with corneal irregularities caused by ectasia (such as keratoconus) or after refractive surgery. Variations in the corneal shape will induce optical aberrations, specifically spherical aberration and coma, which can lead to image degradation, especially in low-light conditions [
1,
3,
17,
28].
The human eye functions as a complex optical system, where incoming light passes through different ocular media such as the cornea and the lens. In cases of very intense light or from highly intense sources, excessive dispersion may occur, leading to a glare sensation. This, in turn, can cause visual discomfort and a decrease in visual acuity [
29,
30].
There is growing interest in assessing visual function in low-light conditions and in the presence of light sources that induce glare, halos and other forms of light distortion, as documented and discussed in the literature [
1]. This concern is tied to the quest for methods to enhance visual performance in demanding situations, such as nighttime driving or in environments with fluctuating lighting conditions. The initial attempt to subjectively assess and understand the patient's perception of vision impairment at night and its impact on daily activities was through representations or drawings, as explained by Fan-Paul et al. [
1]. Visual function questionnaires have also been developed and aspects such as contrast sensitivity, photic phenomena such as halos around lights and glare, quality of night vision, impact on daily activities and general satisfaction with vision can be included in these questionnaires [
31,
32]. The Light Distortion Analyzer (LDA) represents a breakthrough in quantifying light disturbances in a clinical setting. Besides other devices that will be presented, that device is highlighted in the context of this review as it has been extensively used by the author’s team and other research groups for a very wide range of clinical applications spanning all fields of non-surgical and surgical visual correction, including but not limited to spectacle lenses, contact lenses, corneal refractive surgery, intra-ocular surgery applied for refractive error correction, presbyopia, myopia control, or cataract surgery. The device objectively assesses the size, location and regularity of optical phenomena like glare and halos, offering valuable metrics for clinicians and researchers [
33]. The following sections will explore this further, in addition to the concepts and definitions related to reduced visual quality in low lighting conditions either with or without the presence of bright light sources.
2. Concepts and Definitions
It is crucial to start by defining specific terms, considering the broad range of definitions and concepts that are sometimes inaccurately applied.
The term
light disturbance (LD) was first referred to in an editorial written by Klyce in 2007 [
34]. The concept refers to an optical effect in which light is deflected or distorted when passing through certain media, such as the atmosphere or other transparent media. This phenomenon often results from the formation of a ring of light around a luminous object [
34] and in the eye, it is an indicator of visual quality [
35,
36].
The term
night vision disturbance (NVD) is commonly used in the literature to describe the combined effects of glare disability and decreased contrast sensitivity on image degradation under scotopic or mesopic conditions. By grouping these two visual impairments under the term "night vision disturbance," researchers and clinicians can better understand and address the complex challenges individuals face when navigating/working under low-light environments. This term highlights the interconnected nature of glare disability and decreased contrast sensitivity, both of which can significantly impact an individual's ability to see clearly and safely in nighttime conditions. NVD can be defined based on the shape or size of the degradation that an intense light source produces subjectively and therefore, according to the description by Fan-Paul et al. [
1] the concept of "glare disability", "contrast sensitivity" and "image degradation" are grouped in the term [
37]. The most common “image degradations” are
halos and
starbursts. The following paragraphs define different photic phenomena that might be contained in the generic concept of light disturbances or NVD.
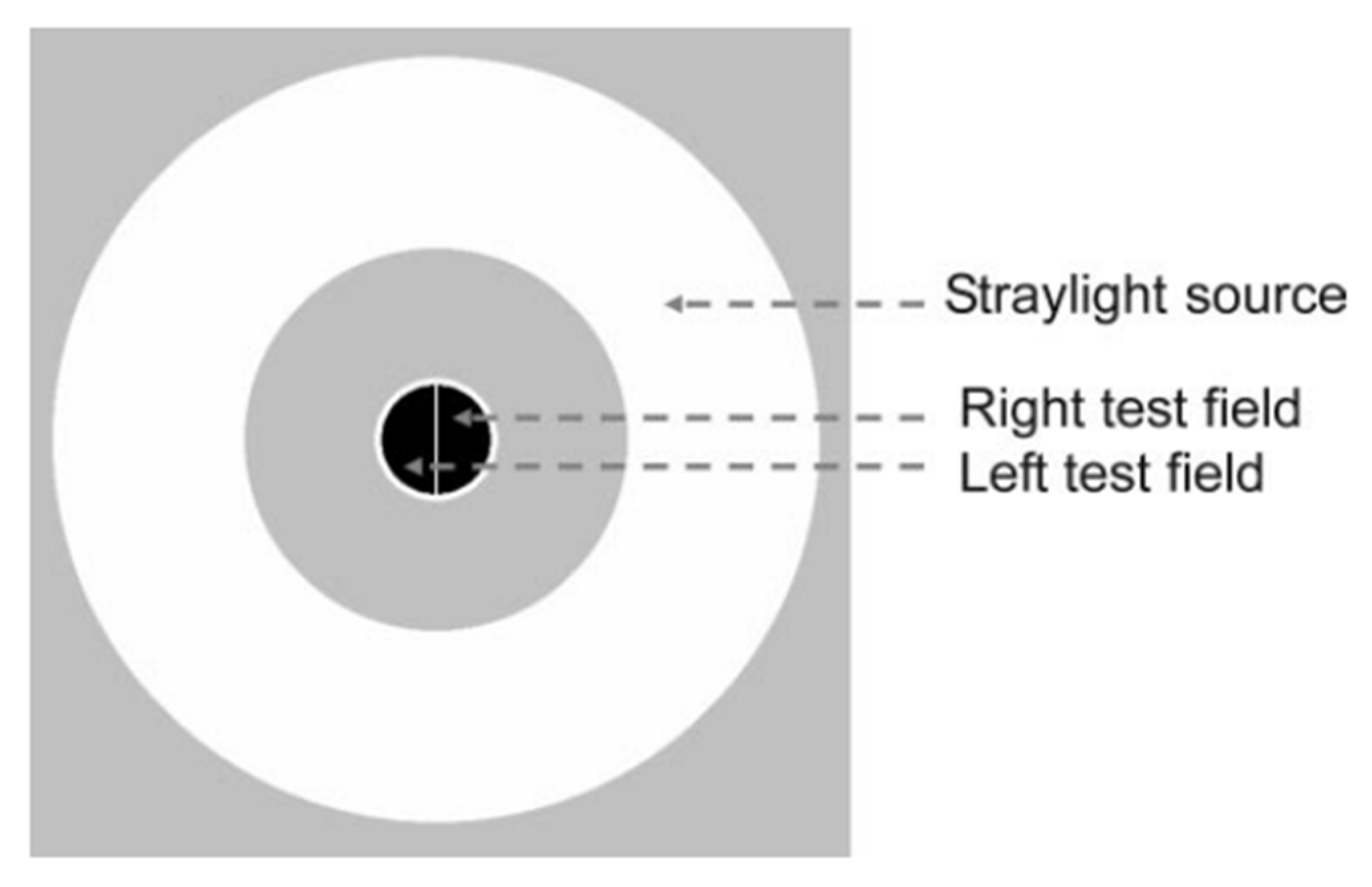
2.1. Glare Disability
Technically,
Glare refers to the physical term referring to a light source, while
Glare Disability is related to the subjective reduction of visual performance due to the glare source,
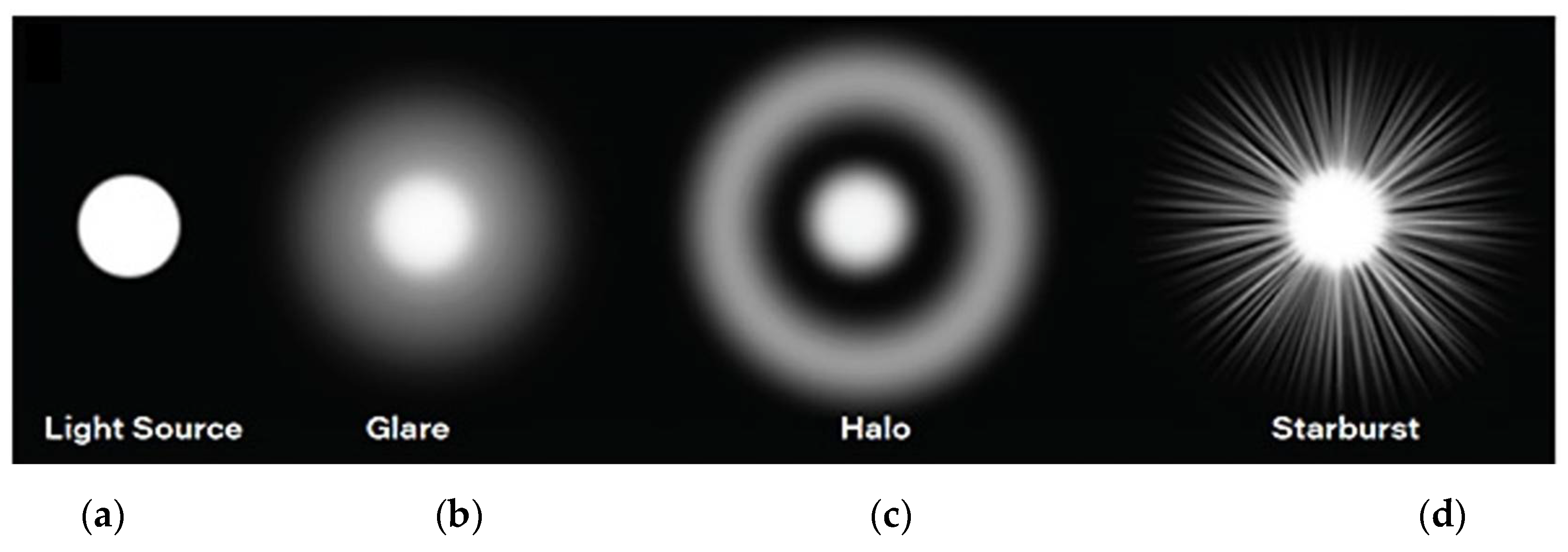
Figure 1 (
b) [
1]. Discomfort Glare, Disabling Glare and Blinding Glare have significant implications for traffic safety, among other conditions. Optically,
Glare Disability or Disabling Glare occurs when light from a source is scattered within the eye, causing a degradation in image formation on the retina and a reduction in its contrast, reducing the ability to perceive the original visual information [
1]. In practical terms, it can make it difficult for drivers to perceive important details such as traffic signs, pedestrians or other vehicles at night. In contrast to disabling glare,
Discomfort Glare does not lead to reducing of the ability to see the visual information. Instead, it results in feelings of discomfort and fatigue when exposed to intense light. This type of glare can be distracting and reduce the ability to focus and the reaction times [
12]. The term
Blinding Glare refers to the momentary loss of visual perception that persists even after the intense light exposure has ceased [
38].
2.2. Starburst
Starburst refers to a visual phenomenon where bright lights, such as headlights or streetlights, appear to radiate streaks or rays of light and can lead to visual discomfort and a decrease in visual acuity, especially in situations such as nighttime driving or exposure to bright lights. Rather than observing a single, well-defined point of light, individuals may perceive these streaks or rays extending outward from the light source,
Figure 1 (
d). The appearance of starbursts around light sources can vary among individuals and tends to be more prevalent in those with specific ocular conditions such as astigmatism, cataracts or that undergone ocular surgeries. In a surgical context, starbursts are often associated with a transient loss of transparency in the post-operative period [
39,
40]. In these instances, they appear to originate from the points where radial incision scars extend beyond the margins of the pupil [
39]. However, starburst is also reported even among patients who have not undergone surgical procedures, especially when they are wearing glasses or contact lenses that are under-corrected [
41,
42].
2.3. Halo
Halos refers to a visual phenomenon characterized by the perception of luminous circles or rings around light sources,
Figure 1 (
c). These circles or rings can appear as a result of light diffraction or scattering within the eye's optical system. Halos are directly related to sources of glare in the visual field and are often seen around bright lights, such as headlights or streetlights, and can be particularly noticeable in low-light conditions [
4]. Following refractive surgery, starbursts may appear as visual phenomena, but they are typically transient in nature. In contrast, halos can persist after the surgery as constant visual effects and the extent of the halo is related to the size of the pupil and the diameter of the ablation zone (the treated area). If the dilated pupil is beyond the treated zone, the light can diffract as it enters the eye, causing the halo effect around the light sources [
39,
40]. Even in early surgical procedures with small ablation zones, such as radial keratotomies, patients could experience halos around light sources, particularly in low-light conditions. This occurrence was due to the dispersion of light when the treated area was not precisely aligned with the pupil under low-light conditions, resulting in the formation of halos [
1,
40,
43].
2.4. Ocular Scattering
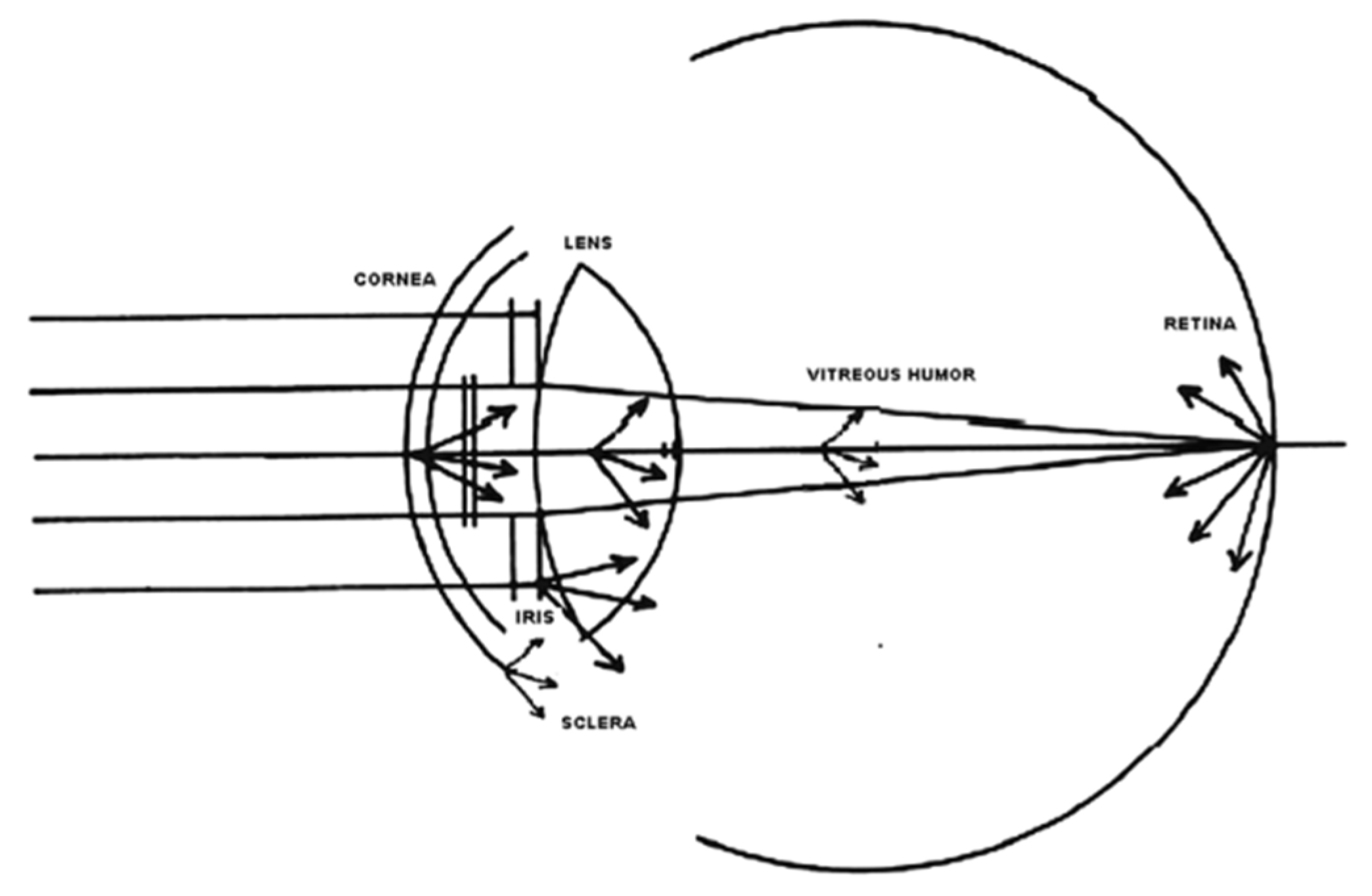
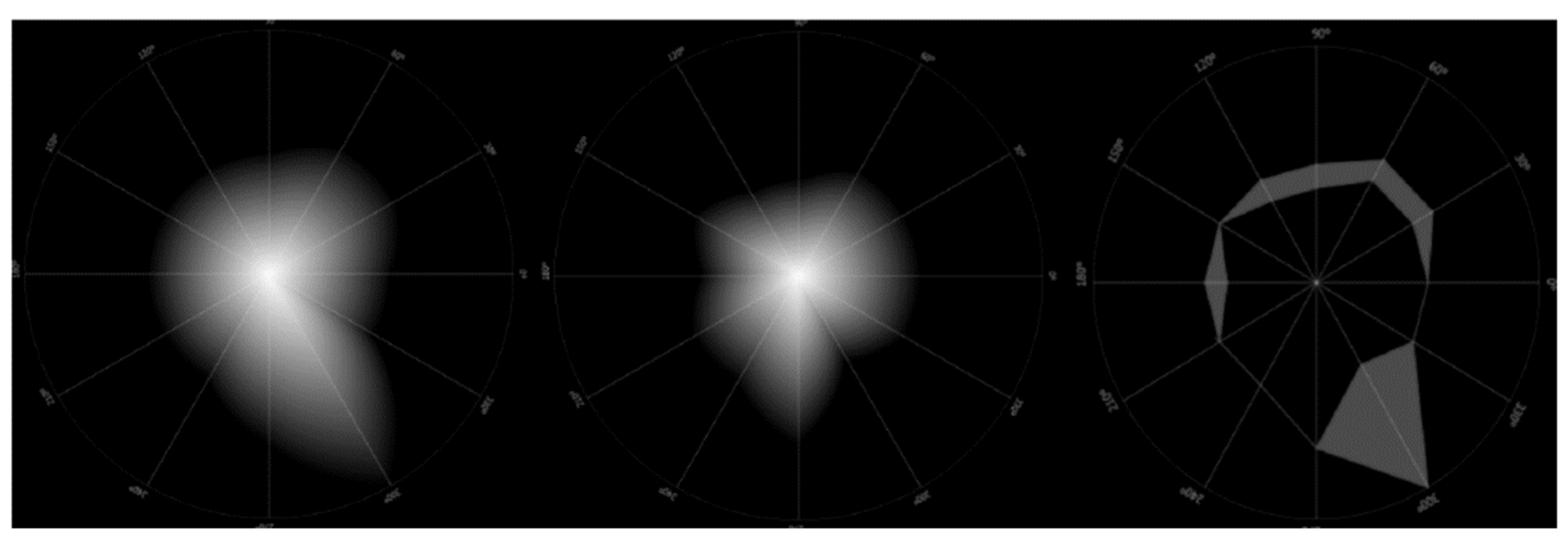
Ocular scattering is a complex optical phenomenon that affects image quality in the human eye. Its effects are compared to the effects of ocular aberrations, diffraction and variations in the refractive index. This degradation of the image occurs due to the deviation of light from the theoretical rectilinear path, resulting from the inhomogeneities or non-uniformities present in the optical medium through which the light passes. Ocular dispersion is due to a combination of optical phenomena, including diffraction, refraction and reflection. Small particles, foreign bodies, density fluctuations and the roughness of the surface of the different ocular optical elements are mentioned as non-uniformities that act as potential microscopic scatterers. These inhomogeneities can interact with light, causing dispersion and, consequently, image degradation. The cornea and lens are fundamental to image formation on the retina, but when their transparency is compromised, they can become significant sources of scattered light, affecting the retinal image quality. However, there are other sources of ocular scattering, such as the iris, sclera, vitreous humor and the retina itself, as shown in
Figure 2 [
4].
As previously mentioned, the retina is also regarded as a source of scattering, as the light that strikes is not entirely absorbed. Instead, a portion of the light is reflected to other areas of the retina, thereby contributing to intraocular scattering [
45,
46]. Measurement of ocular dispersion can be accomplished using optical methods such as the double-pass or Hartmann-Shack techniques. These approaches are objective, as they do not rely on the subjective responses of patients to assess the optical quality of the eye [
4]. Straylight, on the other hand, is the result of combined effects from light scattering within the optical media and diffuse reflectance from different layers of the fundus.[
47] Straylight is the combined effect of light scattering in the optical media and the diffuse reflectance from the various fundus layers [
47]. It refers to the light within the eye that scatters instead of following a direct path to the retina. It can also result from light entering the eye in oblique angles and can occur due to corneal irregularities, cataracts or other ocular conditions.
Understanding phenomena such as
dispersion, diffraction and
glare are crucial, especially for designing surgical procedures and developing visual corrections to compensate for changes in the optical quality of the eye. Many authors attempted to evaluate different devices and strategies for measuring the bothersome nature of NVD. However, there are some limitations about the methods and techniques used so far in measuring NVD. This includes lack of standardization, subjectivity, insufficient scientific validity of certain tests and difficulties in interpretation both for physicians and patients. Furthermore, many of the tests come with a high cost and do not correlate with the reported symptoms. While there are different methods available to quantify ocular scattering and other NVD, few clinical reports validate these systems for their use in clinical practice [
4]. The following sections will review some of the most commonly used techniques and devices to quantify, either objectively or subjectively, those phenomena.
3. Methods to measure Night Vision Disturbances
To objectively assess and understand the subjective symptoms of pre-and post-operative visual disturbances, various tests have been created. Initially, the tests were based on subjective surveys and questionnaires to quantify the type and level of visual disturbance. Since then, more formal methods have been introduced, including psychometric tests and more detailed rating scales, to increase rigor in quantifying these disorders [
1].
Table 1 provides a structured summary of various techniques employed in the assessment of NVD. It categorizes the tests based on whether they are subjective or objective and highlights the parameters measured by each one.
3.1. Night Vision Recording Chart
The first pictorial representations, illustrations or graphic schemes were created at an early stage to quantify photic phenomena. One of these tests was the Night Vision Recording Chart (NVRC) designed to quantify NVD such as halos and starburst [
48]. The test was designed to be conducted in a dimly lit environment to mimic conditions that exacerbate NVDs, unlike other tests with brightness sources that constrict the pupil, obtaining unrealistic results [
59]. This test involves projecting a small circle from a projector in a low-light environment, where patients are asked to reproduce what they see on a table adapted from an Amsler Grid.
Figure 3 (a) and (b) show a subjective perception of a starburst and a halo, respectively. However, this representation relies on personal illustration which introduces variability in the results, depending on individual drawing skills and perceptions. This subjectivity, while offering unique personal insights into the patient’s experience, poses challenges in standardization and comparability of outcomes across different individuals. This also introduces complexities for clinical studies or investigations that strive for more objective outcomes, particularly in the longitudinal monitoring of patients and/or the categorization of various treatments and surgical interventions on NVDs.
3.2. Simulators
As mentioned above, the visual experience and subjective perception of optical phenomena tend to vary considerably between subjects, especially when assessing the severity and discomfort of symptoms associated with NVDs. Various night vision simulators are available, offering individuals a platform to visually articulate their visual experiences at night. However, it is crucial to acknowledge that these simulators may be prone to patient biases, potentially leading to the overestimation or underestimation of the size of halos [
60].
The use of simulators or electronic media for studying these phenomena has not been extensively explored or addressed in comparison to other methods [
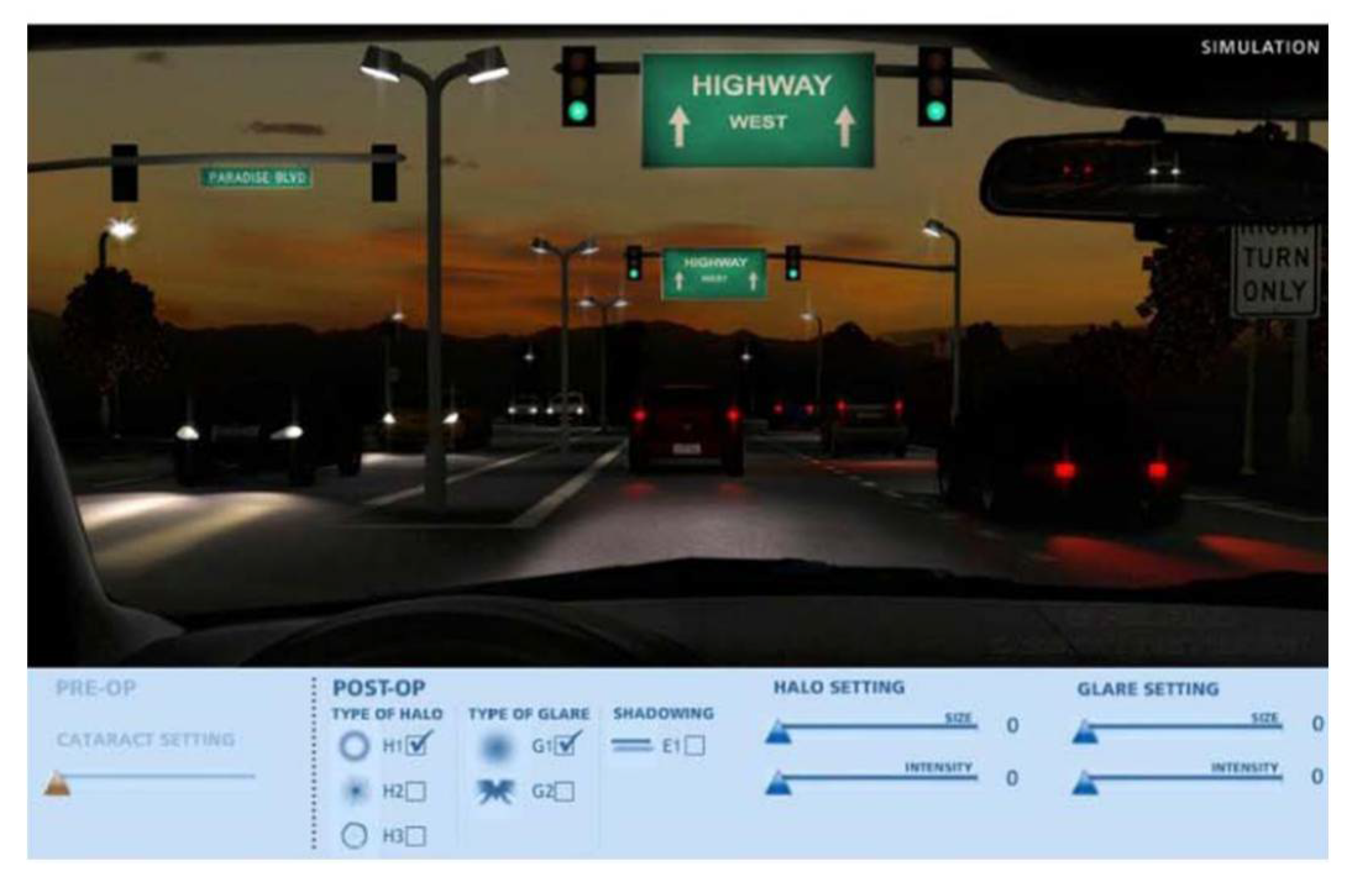
61]. While there are only a few studies on this particular approach, scientific literature often discusses the use of the Halo and Glare Simulator and the Vision Simulator in post-implantation evaluations of presbyopia-correcting IOLs. The
Halo and Glare Simulator (Eyeland-Design Network GmbH, Vreden, Germany) is a software that simulates a night driving scenario,
Figure 4 [
51]. This simulator allows patients to adjust the type, size and intensity of the halo, starburst and glare [
49].
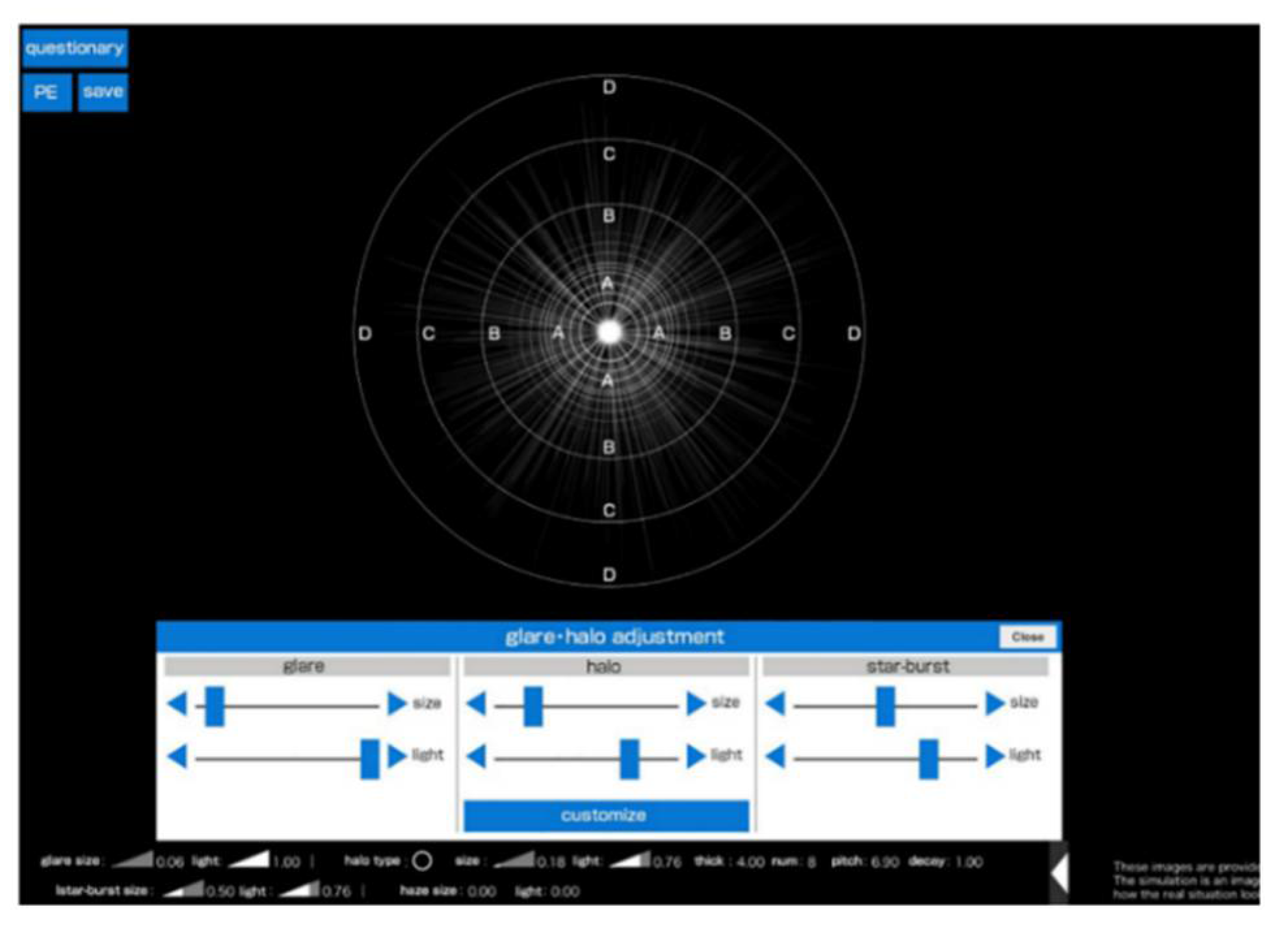
The
Vision Simulator exposes patients to light sources identical to halometers and subjectively reproduces the patient’s perception of the photic phenomena. It also allows you to adjust the size, intensity, width of the ring, interval and shape of the halo, as well as adjusting the size of the glare and halo and the length of the starburst’s radiant beam. In this case, the size and intensity of the phenomena are converted into numerical values, as shown in
Figure 5 [
61].
Nonetheless, the literature also highlights certain drawbacks associated with these simulators. Among the limitations is the difficulty in accurately evaluating the optical phenomena associated with different types of intraocular lenses (IOLs), mainly because of the inability to continuously adjust parameters such as ring width or the spacing between halos. Furthermore, the simulators' lack of capability to modify the size and intensity of starbursts independently is cited as a significant limitation. An additional point of concern is the reliance on the patient's memory rather than the direct observation of actual light sources, which could compromise the reliability and consistency of the findings [
61].
Recognizing the limitations of computer media in simulating night vision disturbances is essential, including the restricted range of intensities and contrasts, which might not accurately reflect real visual experiences and technological constraints that limit the replication of specific visual effects. Additionally, individual variations in visual perception can lead to discrepancies in how these simulations are experienced. Such limitations necessitate cautious use and validation of these tools, understanding that while they provide insights, their results need careful interpretation due to potential inconsistencies across different devices and environments. Despite these challenges, combining traditional methods like questionnaires with advanced simulators offers a more nuanced and interactive approach to studying visual phenomena [
50].
Several methods and instruments to quantify the halo phenomena through objective measurement of the size and shape of light distortion under nighttime lighting conditions, are detailed below.
3.3. Direct Compensation Method
The first publications regarding the Direct Compensation Method and the study of straylight by van den Berg back to the late 80’s [
62,
63]. This psychophysical method was designed to objectively quantify the phenomenon of light scattering and halo effects under nighttime conditions. This method operates on the principle of neutralizing the light scatter within the eye by introducing a compensatory light source. By meticulously adjusting this source until it offsets the scattered light perceived by the observer, researchers can accurately measure the extent of light distortion or straylight [
62].
Conventional straylight meter (CSLM) and computer-implemented straylight meter (NSLM)
The CSLM (Conventional) and NSLM (Computer-implemented) straylight meters operate under van der Berg’s principles for quantifying retinal straylight or light scattering within the eye. Both utilize the Direct Compensation Method to measure the intensity of straylight by compensating for the scattered light perceived by the observer. This is achieved by adjusting a light source in the device until the observer can no longer distinguish between the straylight and the background, allowing for precise measurement of the eye’s scattering properties. Although both (CSLM and NSLM) measure retinal straylight based on the principles outlined by van der Berg, they incorporate different technologies and operational features. The
CSLM (commonly referred to as
van den Berg Straylightmeter) is a small portable device that operates with a more manual or semi-automated approach, requiring direct interaction by the clinician for the adjustments and measurements [
64]. This necessitates a steeper learning curve or greater expertise to ensure accurate measurements. On the other hand, the
NSLM leverages computer technology to automate much of the process, enhancing efficiency, reducing potential for user error, and often providing a more user-friendly experience. It keeps the luminance constant in the central detection field and the button does not have a limit switch. This absence makes it possible to eliminate clues and avoid increases in fraud during measurement. It was designed to be used binocularly, where the patient looks at a computer screen instead of a test tube, which in turn makes the instrument more practical, facilitating the patient’s interaction with the device [
65]. In essence, both CSLM and NSLM serve similar purposes in the assessment of straylight and its impact on visual quality, with NSLM representing an advancement in the technology, offering benefits in terms of efficiency and accuracy [
1].
3.4. Compensation Comparison Method
The Compensation Comparison Method is a psychophysical method used in the assessment of straylight, crucial to understand visual disturbances like glare and reduced contrast sensitivity. It involves presenting the subject with a visual stimulus that included two separate components: one that remains constant and another that fluctuates in intensity,
Figure 6 [
25]. The key objective is to adjust the intensity of the fluctuating component until the observer perceives both components as equally bright, making the method capable of quantify the degree of light scatter affecting visual perception [
66].
An instrument that employs the Direct Compensation Method is the
C-Quant (cataract-quantifier) (Oculus Optikgeräte, Wetzlar-Dutenhofen, Germany) [
66]. The observer is asked to adjust the brightness of a flickering light source in a test field until it matches the brightness of the surrounding field, indirectly quantifying the impact of straylight [
52,
53].
3.5. Double-pass System
Double-pass systems are optical instruments used to evaluate the quality of the human eye, specifically by measuring the effects of scatter and aberrations on light as it passes through the eye [
67]. In practical terms, the name “double pass” refers to the path of light traveling twice through the optical media of the eye: once entering the eye to reach the retina and second time as it reflects off the retina and exists the eye. By analyzing the intensity and spread of the light in the captured image, known as Point Spread Function (PSF), researchers and clinicians can assess the eye’s optical quality. The PSF provides information on how different factors, such as lens opacity (cataracts) or corneal irregularities (ectasias, dry eye) affect light transition, influencing visual clarity and contrast sensitivity [
58,
68].
The Optical Quality Analysis System (
OQAS) (Visiometrics S.L. Tarrasa, Spain) is a diagnostic device [
67] that employs a double-pass technique to evaluate the optical quality of the eye by quantifying ocular media transparency and ocular aberrations [
55]. It provides the OSI (objective scatter index), the MTF (modulation transfer function) and SR (Strehl ratio). This approach allows for the detection of both symmetrical and asymmetrical aberrations, such as coma, which cannot be measured with a conventional double-pass system [
54]. This objective method has been useful for clinically assessing optical quality in patients undergoing refractive surgery and cataract surgery in the pre-and post-operative period, where part of the ocular media may have been affected [
55,
69,
70].
3.6. Night Vision Test
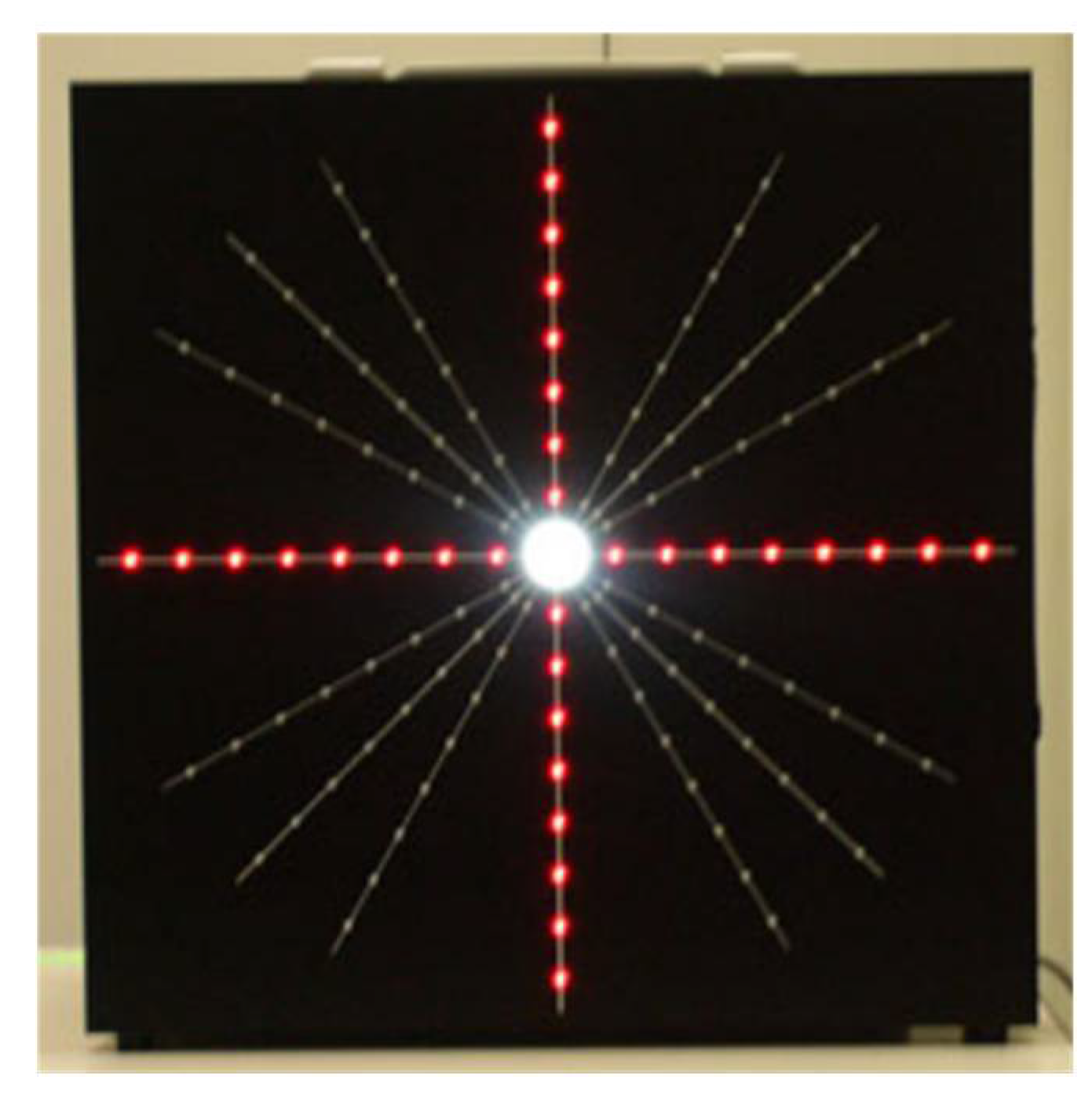
The Night Vision Test (NVT) is a method used to evaluate the impact of glare, halos and other NVD on visual performance [
56]. It consists of a blackboard equipped with an adjustable central light source, surrounded by red LED lights to measure the extent of the light scatter, as shown in
Figure 7. Participants observe the NVT board and use a laser pointer to outline the perceived shape of the light. From these outlines, a Glare Score is derived by measuring the distance from each point to the center, providing a quantifiable measure of the impact of glare [
56].
3.7. Starlights System
The Starlights System (v.1.0, Novosalud, Valencia, Spain) enables quantitative analysis of light distortion through the use of the Disturbance Index. The device contains a black screen with a central light (fixation stimulus) and is surrounded by more LEDs in 12 meridians. It calculates the percentage of the visual field obscured due to the central light source [
25]. The Starlight software is also mentioned as an additional tool for assessing the quality of scotopic and mesopic vision, especially in multifocal intraocular lens implantations [
69]. A study by Pieh et al. [
71] measured the diameters of halos using the computer program Glare & Halo (Fitzke FW and C Lohmann, Tomey AG) in patients implanted with multifocal intraocular lenses,
Figure 8.
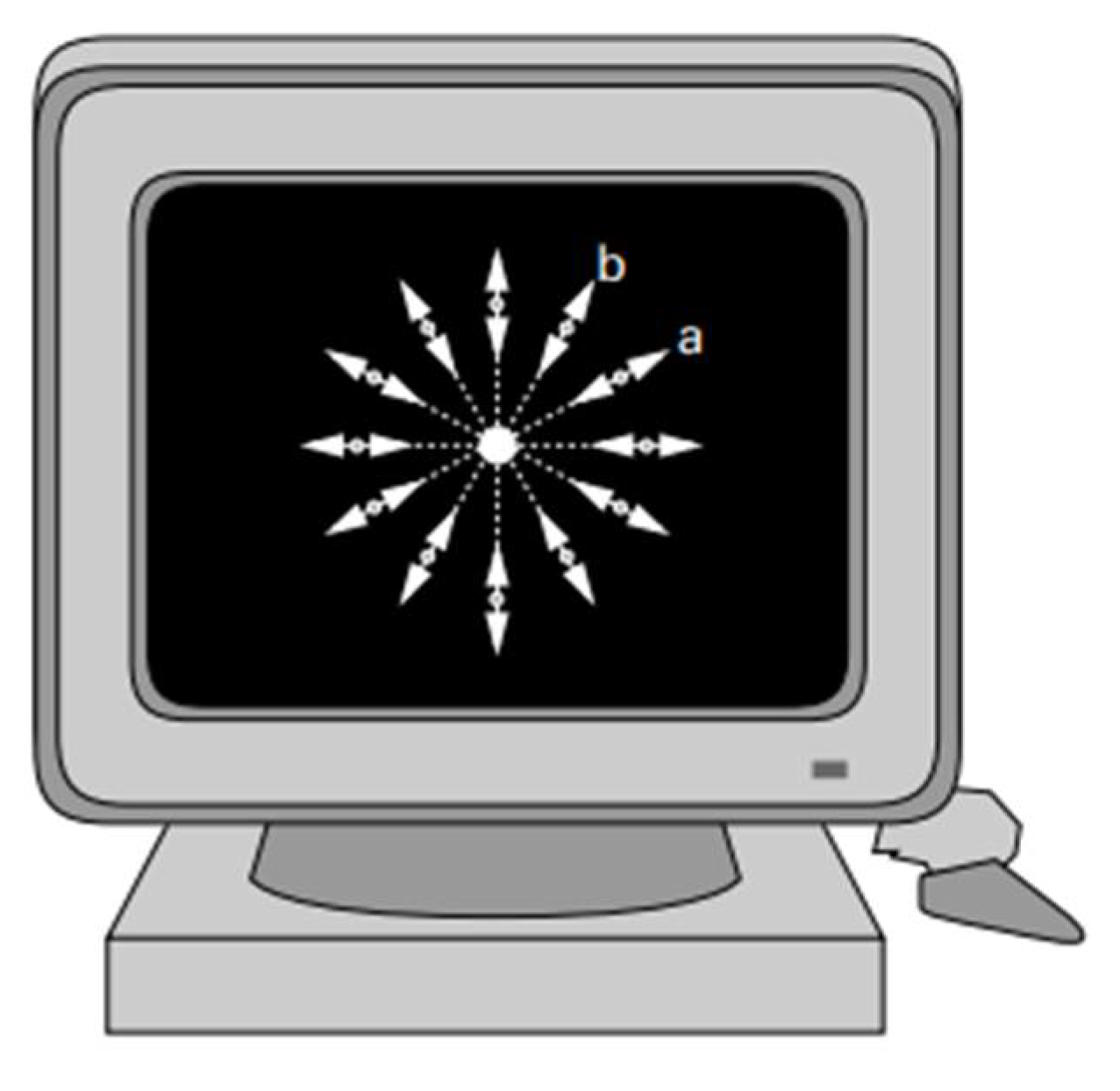
3.8. Gutiérrez Halometer
To assess the effect of halos on night vision, a psychophysical device called a Halometer was developed in 2003 [
37]. This device enables the precise assessment of the influence of halos on visual perception by generating a disturbance index. Briefly, the Halometer consists of two plates inside a methacrylate box. The front of the box has a black cover, also made of methacrylate, with several holes to allow light to escape from the light-emitting diodes (LEDs) located on the plate. The back has guides and holes to isolate the LEDs, and the electronic board is connected to the back of the plates, as shown in
Figure 9 (a). The subject must stand in front of the device and see a black screen, where the central light source (which also serves as a fixation point) is surrounded by a series of dots distributed over 12 radial lines. In turn, the device is connected to a computer which processes the data collected during the examination,
Figure 9 (b). The subject's task is to differentiate between the different peripheral light points and the central point [
37].
3.9. Vision Monitor (Metrovision)
The Vision Monitor (MonCv3; Metrovision, Pérenchies, France) is a commercial instrument particularly used to measure the size of a halo. It consists of two circular white light sources (LEDs) on each side to generate glare,
Figure 10 (a). Each glare source has a unique luminance of 200,000 candelas (cd)/m
2 and forms a visual angle (
) of 3,8º from the center of the monitor at a distance of 2.5 m from the observer,
Figure 10 (b) [
57].
3.10. Aston Halometer
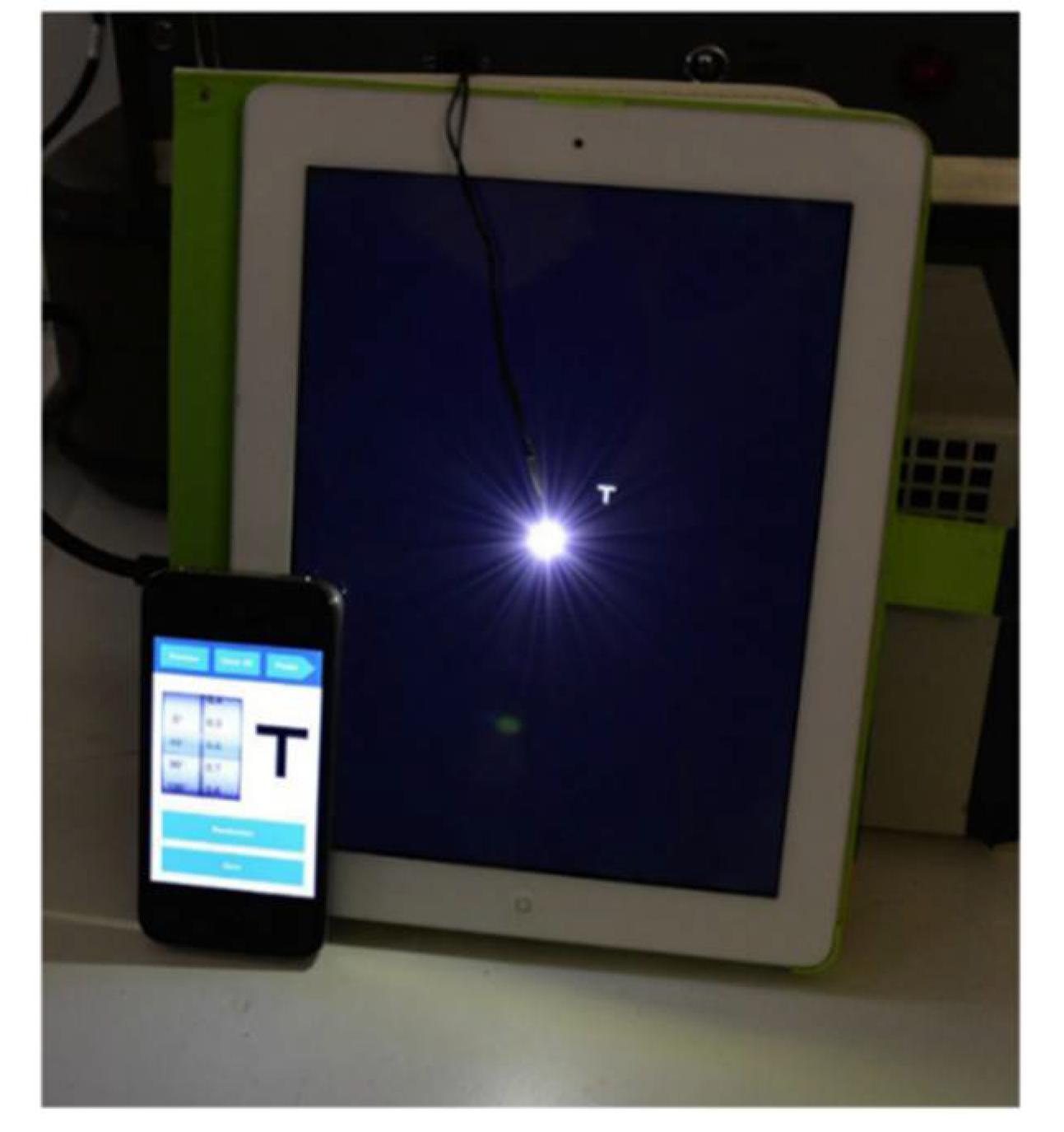
This halometer consists of a bright LED in the center and the test is carried out on a standard mobile tablet, which makes it possible to quantify and analyze the extent of dysphotopsias in different directions of vision,
Figure 11. The tablet with a central LED is placed 2 meters (m) away in a dark-lit room and the remote control is via Bluetooth. There is a 1-minute adaptation and the measurement of the eccentricity of the letters equivalent to 0.03 Logarithm of the Minimum Angle of Resolution (LogMAR) is achieved by moving the lenses themselves more eccentrically from the central LED brightness source in steps of 0.05 degrees (°) until they are first consistently recognized. The eccentricity is noted and this assessment is repeated in each of the 8 orientations, separated by 45°, to delineate the specific area of glare on a target caused by the halo, measured in degrees [
30].
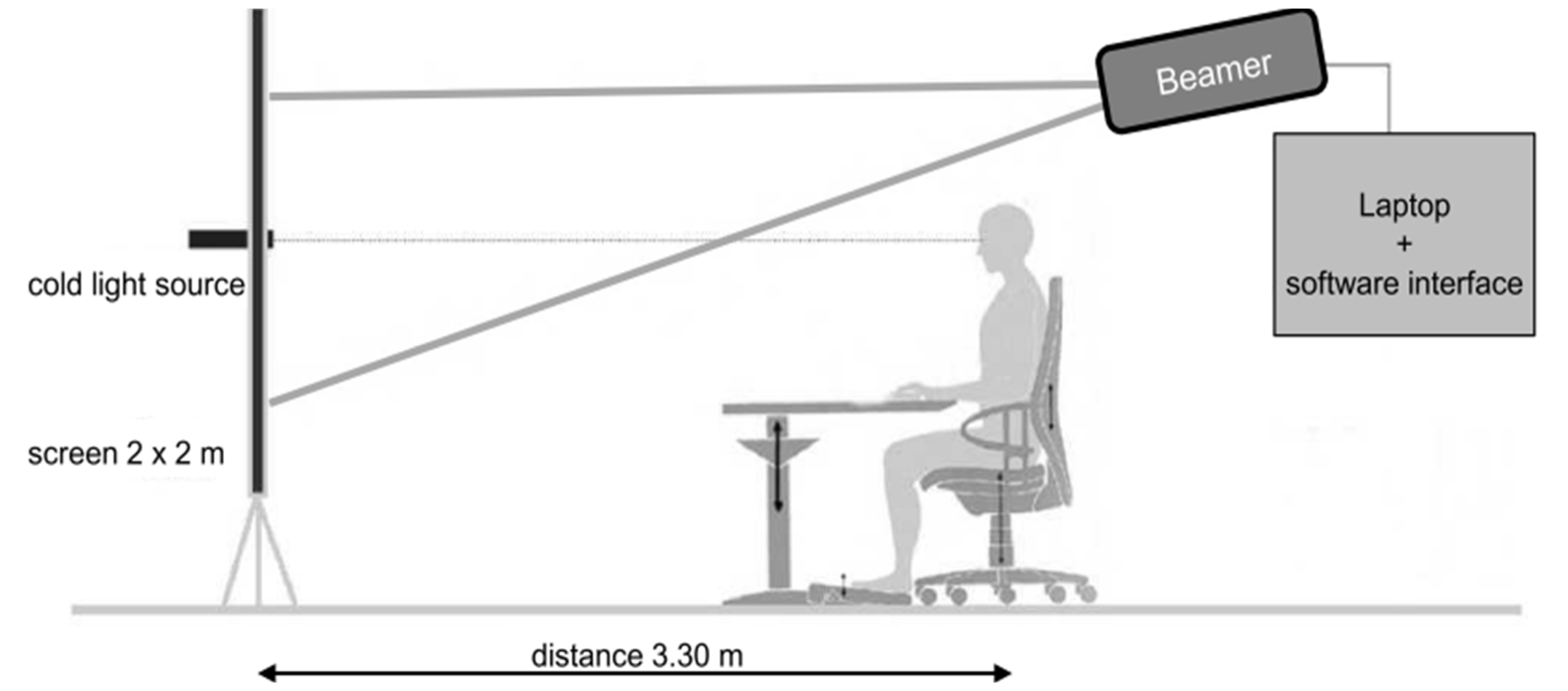
3.11. Rostock Glare Perimeter
This equipment is used to quantify the effects of dysphotopsias under realistic simulated conditions. This method is sensitive and useful in detecting and quantifying age-related glare differences in a healthy population and binocular summation,
Figure 12. It also aids in refractive correction procedures and intraocular lens design, and therefore has a potential use in assessing visual quality in patients undergoing refractive or cataract surgery [
29].
In short, the subject is placed 3.30 meters from a screen that integrates a central cold light source with 2 mm diameter optical fibers, as shown in
Figure 13. The software produced a black background (with a luminance of less than 0.01 cd/m
2 projected onto the screen by a projector) and a white marker (with an angular dimension of 0.09 degrees and a luminance of 22 cd/m
2) which moved gradually from the periphery to the center. The dot shifts direction unpredictably, moving through one of twelve possible paths. Subjects are required to verbally indicate when they can differentiate the marker's brightness from that of the light source. Upon making this distinction, the distance from the dot to the central light source is noted. Subsequently, the path of the dot's movement is randomly altered again [
29].
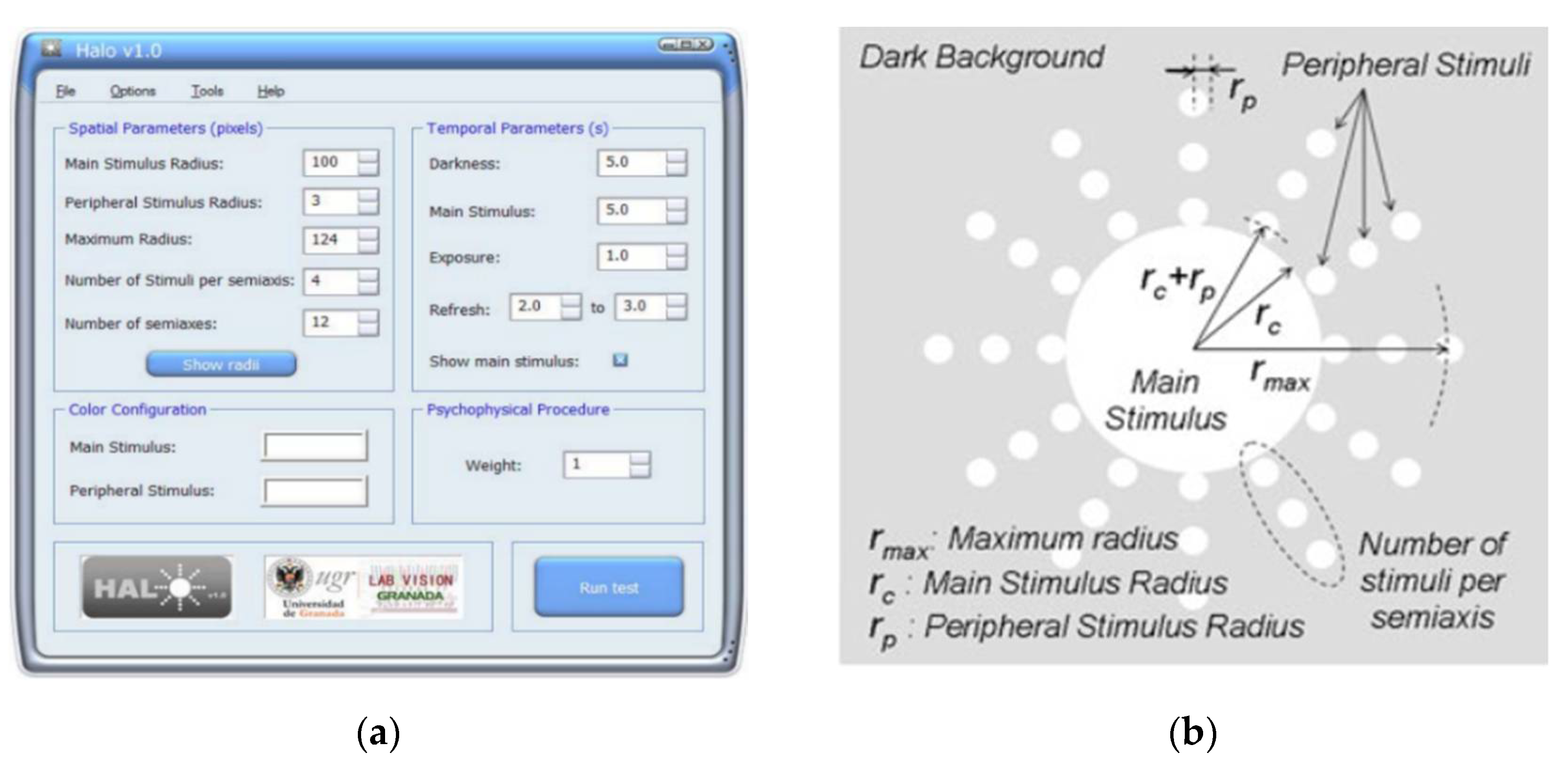
3.12. Halometer: Halo v1.0
The Halometer or Halo v1.0 (Laboratory of Vision Sciences and Applications, University of Granada, Granada, Spain) is a free software that quantifies visual disturbances perceived by detecting peripheral stimulus around a central main stimulus on the dark background of the monitor,
Figure 14 [
69].
Implementing such software in clinical practice results in several advantages, including the simplification of the process, the reliability of the results obtained and its widespread accessibility, which enables eye care professionals to utilize the software without incurring extra costs. Additionally, it boasts extensive clinical applications, namely the assessment of nocturnal visual performance, monitoring post-refractive surgery outcomes [
72,
73] and overseeing the management of ocular diseases [
74].
3.13. Light Distortion Analyzer
Recently, the Physics Center at the University of Minho in Braga, Portugal, has developed and introduced a device designed to quantify and characterize optical phenomena, including light distortion: the Light Distortion Analyzer (LDA). The radiometric characterization [
50] and its validation [
33] were already described in the literature. The LDA device focuses on characterizing the impact of optical aberrations such as halos, glare, and starbursts that individuals may experience under various lighting conditions, particularly in low-light or night-time environments. The LDA measures the size, shape, and regularity of these distortions, providing valuable metrics that can help in assessing the optical quality of the eye and the visual disturbances perceived by the patient [
33,
35].
The hardware consists of a black electronic board with a bright, high-intensity (3000 cd/m
2) central light source measuring 5 millimeters (mm) which acts as a source of glare/disturbance and is therefore responsible for the glare condition. On its periphery are 240 smaller, less intense LEDs (up to 6 cd/m
2) of 1 mm each. They are distributed over 24 semi-meridians with a minimum angular separation of 15°, covering an area of 10° at an examination distance of 2 m and act as stimulus for detecting the limits of glare at different points in the visual field. Their representation and distribution are shown in
Figure 15 (a).
Figure 15 (b) and (
c) show the central LED switched off and on at minimum intensity, respectively. The electronic board is connected to a central control device (laptop) and whenever the subject can identify the light from one of the peripheral LEDs, this feedback is transmitted via a remote response device (laptop mouse). The peripheral stimulus is presented around the central light source in different sequences and semi-meridians and at random times between 250 and 750 milliseconds (ms). Whenever the subject identifies the stimulus, the system presents the next semi-meridian and the procedure is repeated. In this test, three evaluations are carried out on each semi-meridian. The equipment then determines the average limit of light distortion [
33,
50].
The software evaluates different metrics during the scan:
Distortion Area (DA): this is the result of the sum of the areas of all the sectors formed between each pair of semi-meridians under analysis, in mm2.
Light Distortion Index (LDI): this is the main parameter and is calculated from the ratio between the area not seen by the subject and the total area explored and is expressed as a percentage. It is indicative of the area that is not visible due to the impairment of light distortion phenomena. Higher LDI values are understood as a lower ability to discriminate small stimulus surrounding the central light source and, therefore, the greater the light disturbance induced by the central light source.
Best Fit Circle Radius (BFCRad): corresponds to the radius of the circle that best fits the Distortion Area, whose value is equal to the average length of the disturbance along each semi-meridian under study, presented in mm.
Coordinates of the Best Fit Circle (XCoord e YCoord): these are the Cartesian coordinates of the center of the screen, in degrees.
Best Fit Circle Center Orientation (BFCOrient): angle of the BFC center from the origin of the coordinates, which corresponds to the center of the screen, in degrees.
BFC Irregularity (BFCIrreg): this is the sum of the deviations between the actual distortion area and the outer perimeter of the BFC along all semi-meridians. It is the sum of the positive and negative values depending on whether the distortion limit is inside or outside the perimeter of the BFC, in mm.
BFC Irreg Standard Deviation (BFCIrregSD): standard deviation of the BFC Irreg. It determines the degree of asymmetry of the distortion area limited from a perfectly circular shape, in mm. Higher values correspond to more irregular distortion [
33].
4. Advantages and Applications of LDA in Clinical Practice
The LDA offers practical advantages for clinical practice [
50] including its practicality, as the instrument is described as a self-contained physical device, eliminating the need for video display units, cathode ray tubes, flat screens, or multimedia data projectors. Such a feature has the potential to enhance consistency between examinations conducted in various settings, streamlining configuration and reducing reliance on additional components [
33]. The LDA device's capability to offer various metrics related to the size, location, and regularity of disturbances provides more comprehensive information on the current visual condition. This feature proves particularly useful in situations where the optical characteristics are asymmetrical or off-center. Other equipment measures light distortion only in one direction and extrapolate to all directions of the visual field [
33]. The ability to define different configurations allows the system to be adapted for a variety of applications, making it flexible and essential for clinical and scientific research [
33]. This psychophysical procedure allows scattering, that has a higher potential to degrade the retinal image, to be measured at large eccentricities [
50].
The sensitivity of LDA to assess light distortion has already been investigated and verified in previous studies (
Table 2 and
Table 3) with monofocal, bifocal and trifocal intraocular lenses [
35,
76,
77], changes in spherical aberration in healthy accommodated and non-accommodated eyes [
78], eyes undergoing orthokeratology [
79], presbyopes wearing monofocal and multifocal contact lenses [
22,
75] and pseudophakic individuals [
35,
77].
4.1. Ablative Refractive Surgery
Several studies have highlighted the primary key factors influencing the visual outcomes of refractive surgery, including the ablation zone size, variations in patient’s pupil diameters, and optical aberrations like spherical aberration and coma caused by corneal shape changes. These elements play crucial roles in determining visual quality and daily task performance [
1,
42,
59]. Under low-light conditions, pupil dilation exposes the transition and unoperated anterior corneal zones, leading to multiple retinal images and blurred vision due to differing dioptric powers. In bright light, blurred areas may cause halo formation, impairing the ability to see peripheral lights, particularly affecting night vision. This phenomenon significantly impacts visual acuity and quality after refractive surgery, highlighting the complex effects of pupil dynamics on post-surgical visual outcomes [
37].
An example of refractive surgery is SMILE (Small incision lenticule extraction). This surgery has visual and refractive results equivalent to LASIK (Laser in situ keratomileusis) surgery for cases of high myopia and astigmatism [
60]. A study carried out by Reinstein et al. [
60] in 2022 used LDA to objectively assess the extent of visual disturbances and monitor the performance of this surgical treatment. The quality of vision symptoms was also assessed using the Quality of Vision (QoV) questionnaire to investigate a possible correlation between the subjective results of the questionnaire and the results obtained with the LDA. The authors found a significant post-operative irregularity of light disturbance, indicating changes in the shape of the light disturbance, but not in its size. Moreover, a correlation was identified between the area of light disturbance after the surgery and the QoV questionnaire metrics.
4.2. Intraocular Lenses after Refractive Lens Exchange (Clear Lens Exchange) and Cataract Surgery
The perception of photic phenomena like halos and glare critically affects visual quality after multifocal intraocular lens (IOL) implantation, primarily due to light dispersion across the lens's various focal zones [
80]. This dispersion can significantly influence the patient's overall visual experience.
Table 2 summarizes the results obtained with LDA in various studies on intraocular lenses after refractive lens exchange and cataract surgery.
Table 2.
Results obtained with LDA after refractive lens exchange and cataract surgery.
Table 2.
Results obtained with LDA after refractive lens exchange and cataract surgery.
| Author (s) |
Intraocular Lens |
Number of patients |
Type of Surgery |
Outcomes |
| Brito et al. (2015) [35] |
Diffractive multifocal IOLs
AT Lisa 839M (trifocal) or 909MP (bifocal toric) IOL, the latter if corneal astigmatism was more than 0.75 D. Control group with a Tecnis ZCB00 1-piece monofocal IOL |
66 eyes of 34 patients.
Trifocal group comprised 33 eyes; bifocal toric group, 15 eyes; and the monofocal control group, 18 eyes |
Refractive lens exchange |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
| |
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
| Trifocal Group |
46.97 ± 17.27 |
29.29 ± 9.19 |
55.28 ± 10.03 |
43.84 ± 6.83 |
5.71 ± 3.15 |
4.75 ± 1.01 |
| Bifocal Toric Group |
53.57 ± 18.55 |
40.49 ± 12.00 |
58.89 ± 10.86 |
47.84 ± 11.04 |
7.25 ± 3.58 |
6.20 ± 1.73 |
| Monofocal Control Group |
23.94 ± 14.89 |
15.28 ± 6.87 |
38.14 ± 12.09 |
28.24 ± 8.01 |
4.36 ± 3.63 |
3.81 ± 1.18 |
| Escandón-García et al. (2021) [81] |
Diffractive trifocal lenses and EDoF.
FineVision Pod F and AcrySof IQ PanOptix
(TFNT00) (trifocal). TECNIS Symfony model ZXR00 (EDoF lenses) |
30 eyes of 17 patients. 9 patients with trifocal lenses and 8 patients with EDoF lenses |
Refractive lens exchange |
|
LDI (%) |
|
|
BFCIrreg (mm) |
BFCIrregSD (mm) |
| |
Monocular |
|
|
Monocular |
Monocular |
|
| Before Surgery |
31.46 ± 14.54 |
|
|
0.91 ± 1.72 |
5.67 ± 3.71 |
|
| 1 Month After |
39.26 ± 13.33 |
|
|
1.43 ± 2.53 |
7.54 ± 6.27 |
|
| 3 Months After |
37.38 ± 15.07 |
|
|
1.06 ± 1.31 |
5.96 ± 2.83 |
|
| Salgado-Borges et al. (2015) [77] |
Bilaterally implanted with an aspheric monofocal IOL |
18 patients |
Cataract Surgery |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
|
| |
Monocular |
Monocular |
Monocular |
Monocular |
|
| 5 to 8 Months After Surgery |
23.00 ± 23.20 |
39.00 ± 14.98 |
0.45 ± 0.72 |
6.16 ± 4.80 |
|
| Escandón-García et al. (2018) [76] |
Bilaterally implanted with diffractive trifocal IOLs and one EDoF. FineVision Pod F and AcrySof IQ PanOptix
(TFNT00) (trifocal). TECNIS Symfony model ZXR00 (EDoF lenses) |
45 patients
23 FineVision
7 PanOptix
15 Symfony |
Cataract Surgery |
|
LDI (%) |
|
|
|
|
| |
Monocular |
|
|
|
|
| Symfony (EDoF) |
34.6 ± 16.0 |
|
|
|
|
| |
|
|
|
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
| |
|
|
|
|
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
|
| Alió et al. (2018) [82] |
Diffractive trifocal IOLs AcrySof IQ PanOptix™
(TFNT00) (trifocal) |
52 eyes of 26 bilateral patients |
Cataract Surgery |
6 Months After |
36.8 ± 18.5 |
23.81 ± 11.6 |
47.11 ± 11.11 |
39.05 ± 9.24 |
0.44 ± 0.32 |
0.20 ± 0.17 |
|
| Escandón-García et al. (2021) [83] |
Bilaterally implanted with multifocal IOLs
FineVision Pod F and AcrySof IQ PanOptix
(TFNT00) (trifocal). TECNIS Symfony model ZXR00 (EDoF lenses) |
57 patients
38 patients were implanted with trifocal lenses (19 FineVision and 7 PanOptix) and 19 patients with Symfony |
Cataract extraction or refractive lens exchange |
|
LDI (%) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
|
| |
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
|
| 1 Month After |
31.46 ± 14.54 |
28.97 ± 13.28 |
0.91 ± 1.72 |
0.46 ± 0.66 |
7.48 ± 6.44 |
4.17 ± 2.64 |
|
| 3 Months After |
39.26 ± 13.33 |
27.77 ± 12.09 |
1.43 ± 2.53 |
0.48 ± 0.80 |
6.91 ± 5.90 |
4.30 ± 4.17 |
|
| 6 Months After |
37.38 ± 15.07 |
27.58 ± 9.32 |
1.06 ± 1.31 |
0.62 ± 0.72 |
6.40 ± 4.18 |
4.90 ± 4.80 |
|
| Oliveira et al. (2020) [80] |
Bilateral implantation of a diffractive trifocal intraocular lens (FineVision Micro F) |
24 eyes of 12 patients |
Cataract Surgery |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
| |
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
|
| 60 Months After |
32.88 ± 18.37 |
23.34 ± 16.04 |
45.21 ± 12.70 |
39.39 ± 12.57 |
0.54 ± 0.60 |
0.39 ± 0.23 |
|
| Fernández et al. (2021) [18] |
Trifocal IOL AT Lisa Tri 839MP |
62 patients |
Cataract Surgery |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
| |
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
|
| 6 Years After |
18.82 ± 7.25 |
15.64 ± 8.41 |
34.79 ± 6.89 |
30.98 ± 6.35 |
0.44 ± 0.38 |
0.45 ± 0.35 |
|
| Vargas et al. (2020) [84] |
LENTIS Mplus IOL |
40 eyes of 20 patients |
Refractive lens exchange |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
| |
+1.50 Add |
+3.00 Add |
Binocular |
+1.50 Add |
+3.00 Add |
Binocular |
+1.50 Add |
+3.00 Add |
Binocular |
|
| 12 Months After |
20.58 ± 7.74 |
26.5 ± 18.88 |
15.24 ± 8.53 |
36.46 ± 6.60 |
40.2 ± 13.29 |
30.8 ± 9.01 |
1.00 ± 0.95 |
0.70 ± 1.11 |
0.52 ± 0.75 |
|
| Lajara-Blesa et al. (2023) [85] |
Implantation (multifocal/EDoF) aspheric diffractive IOLs. Artis Symbiose Mid IOL in the distance-dominant eye and the Artis Symbiose Plus IOL in the contralateral eye |
23 patients |
Cataract Surgery |
|
LDI (%) |
|
|
|
|
|
|
|
| |
Mid IOL (Intermediate distances) |
Plus IOL (Near distances) |
Binocular |
|
|
|
|
|
|
|
| |
12.57 ± 6.61 |
14.99 ± 5.70 |
10.36 ± 4.42 |
|
|
|
|
|
|
|
| Fernández et al. (2023) [86] |
Patients implanted with the trifocal Q-Flex M 640PM or Liberty 677MY |
58 patients |
Cataract Surgery or Refractive lens exchange |
|
LDI (%) |
|
|
|
BFCIrreg (mm) |
|
| |
Monocular |
|
|
|
Monocular |
|
| Chord-IOLa
|
0.08 |
|
|
|
0.37 |
|
| Horizontal chord IOL |
0.32 |
|
|
|
-0.12 |
|
| Vertical chord IOL |
0.02 |
|
|
|
0.11 |
|
| Chord-mub
|
0.16 |
|
|
|
0.13 |
|
| Horizontal chord mu |
-0.15 |
|
|
|
-0.05 |
|
| Vertical chord mu |
-0.20 |
|
|
|
-0.15 |
|
| Chord-alphac
|
-0.19 |
|
|
|
0.07 |
|
| Horizontal chord alpha |
0.20 |
|
|
|
-0.04 |
|
| Vertical chord alpha |
-0.02 |
|
|
|
-0.01 |
|
LDA has been used in several studies to investigate the visual performance of multifocal IOLs after refractive surgery or cataract surgery and how different types of lenses can influence visual perception [
18,
35,
76,
77,
80,
81,
82,
83,
84,
85]. Some studies have compared the performance of different lens designs and concluded that monofocal IOLs tend to cause less light disturbance than multifocal IOLs [
35,
76]. Other studies have found an increase in light distortion after surgery, followed by a partial recovery in the first month after implantation (coinciding with an improvement in the subjective quality of vision in low-light conditions) [
81,
82,
83] and a reduction in light distortion in binocular conditions, as shown in
Figure 16 [
18,
35,
80,
82,
83]. More recent studies have analyzed the behavior of IOLs over the long term, highlighting a possible adaptation to dysphotopsias over time, but emphasizing the need for further research to better understand these phenomena [
18,
80]. Other studies have explored the performance of IOLs with different optical designs and found that the addition of multifocality can increase the size of the light disturbance, although patient satisfaction with these lenses is generally high [
83,
84]. And more recently, a study by Fernandéz et al. [
86] evaluated, for the first time, the influence and impact that the ocular axes (chord-mu and chord-alpha) and the centering of a multifocal IOL can have on the light distortion index (LDI) and concluded that the perceived LDI after lens implantation is particularly related to the orientation of the lens, although future studies in this direction are recommended.
4.3. Applications on Contact Lens
4.3.1. Scleral Lenses
Individuals presenting with corneal irregularities, such as primary corneal ectasias frequently experience significant visual degradations due to high-order aberrations [
17], especially noted under low lighting conditions when the pupil dilates [
1,
42]. Among possible visual rehabilitations for these patients, scleral lenses pose as an excellent visual compensation method to improve visual quality through masking high-order aberrations from anterior corneal surface. A study published in 2020 evaluated the size and shape of light disturbances before and after scleral lens wear in patients with corneal irregularities and patients with regular corneas and concluded that scleral lenses wear reduced the LDI and BFCIrreg in both groups of patients [
87].
4.3.2. Orthokeratology
Orthokeratology is a non-surgical visual compensation method that uses specially designed gas-permeable contact lenses to temporarily reshape the cornea and correct refractive errors. Because of the treatment zone diameter, this can affect visual quality under low-light conditions when compared to spectacles, however these phenomena are more noticeable at the start of treatment but tend to diminish over time [
79]. Previous studies have shown that this change in visual quality depends on the size of the pupil and the value of the refractive error to be corrected [
88,
89]. A number of studies have emerged along these lines, one example being the study by Santolaria-Sanz et al. [
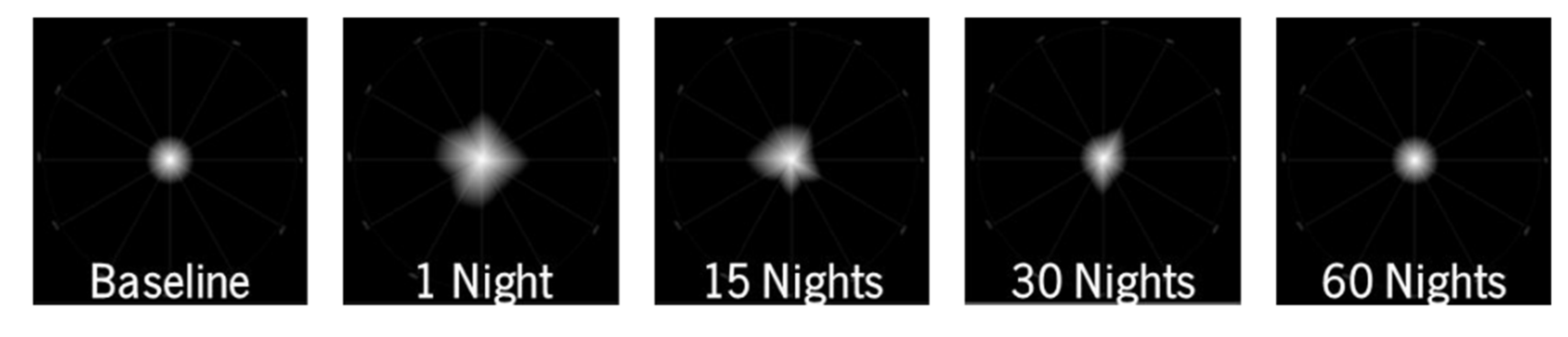
79] which aimed to analyze high-order aberrations and light distortion in patients undergoing orthokeratology of over 1 year. Different quantitative metrics were obtained from the LDA, and the authors found that the light distortion experienced by the patients worsened on the first night but reduced after the first month of treatment and remained stable in the long term. This is in line with another study by Pereira-da-Mota et al. [
90] who found that there was a transient increase in light distortion followed by a reduction in the first 30 nights of orthokeratology treatment,
Figure 17.
4.3.3. Contact lenses for presbyopia and myopia control
The use of contact lenses for the management of presbyopia and myopia control has been an area of significant interest in optometry and visual sciences. These lenses, considering their special designs incorporating multifocality, have implications for light disturbance. Dysphotopsias are common in multifocal contact lenses fittings because simultaneous vision of images focused on different distances from the focal plane will cause this kind of symptoms and photic phenomena [
22].
Table 3 summarizes the results obtained with LDA in various studies on contact lenses for presbyopia and myopia control and the effect of defocus and spectacle lenses for myopia control.
Table 3.
Results obtained with LDA in studies on contact lenses for presbyopia and myopia control and the effect of defocus and spectacle lenses for myopia control.
Table 3.
Results obtained with LDA in studies on contact lenses for presbyopia and myopia control and the effect of defocus and spectacle lenses for myopia control.
| Author (s) |
Lens |
Number of patients |
Type of Condition |
Outcomes |
| Fernandes et al. (2018) [22] |
Two modalities of contact lens wear: Biofinity multifocal and monovision |
20 patients |
Presbyopia |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
|
| |
Binocular |
Dominant
(Biofinity Multifocal) |
Binocular |
Dominant
(Biofinity Multifocal) |
Non-Dominant
(Biofinity Monovision) |
Dominant
(Biofinity Multifocal) |
|
|
| 1 Day Versus 15 Days |
−0.38 ± 0.20 |
−1.47 ± 0.65 |
−0.71 ± 0.38 |
−1.86 ± 0.78 |
−1.83 ± 0.91 |
−0.61 ± 0.36 |
|
|
| Ruiz-Pomeda et al. (2019) [91] |
Dual Focus (DF) MiSight contact lenses for myopia control compared with single vision spectacles (SV) |
74 children
41 in the DF group and 33 in the SV group |
Moderate myopia (-0.75 to -4.00 D) and astigmatism (<−1.00 D) |
|
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
| |
|
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
Monocular |
Binocular |
| DF Group |
Baseline |
8.81 ± 11.95 |
5.22 ± 2.11 |
22.12 ± 9.73 |
18.44 ± 3.83 |
0.61 ± 0.72 |
0.51 ± 0.51 |
4.06 ± 2.21 |
3.41 ± 2.13 |
| 12 Months |
14.90 ± 7.37 |
10.87 ± 4.97 |
30.64 ± 7.40 |
26.35 ± 6.01 |
0.87 ± 0.91 |
0.47 ± 0.44 |
6.37 ± 3.11 |
4.96 ± 2.60 |
| 24 Months |
12.02 ± 6.15 |
8.70 ± 3.87 |
27.29 ± 7.21 |
23.57 ± 5.37 |
0.84 ± 1.29 |
0.47 ± 0.49 |
4.53 ± 1.45 |
4.09 ± 2.06 |
| SV Group |
Baseline |
6.43 ± 2.74 |
5.57 ± 3.07 |
20.52 ± 4.58 |
19.28 ± 4.50 |
0.47 ± 0.49 |
0.37 ± 0.31 |
3.75 ± 1.74 |
3.30 ± 2.15 |
| 12 Months |
5.67 ± 2.93 |
5.18 ± 2.16 |
18.69 ± 4.58 |
19.28 ± 4.50 |
0.37 ± 0.35 |
0.44 ± 0.50 |
3.15 ± 2.02 |
3.25 ± 2.57 |
| 24 Months |
5.44 ± 2.31 |
4.74 ± 1.44 |
18.43 ± 3.34 |
17.48 ± 2.47 |
0.32 ± 0.26 |
0.39 ± 0.29 |
2.97 ± 1.52 |
2.56 ± 1.44 |
| García-Marqués et al. (2020) [92] |
Compare Dual Focus (DF) MiSight with single vision (SV) |
28 patients Were binocularly fitted with either a DF or a SV |
Healthy myopic between 18 and 32 years of age with astigmatism of ≤0.75 D |
|
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
|
|
| |
|
Monocular |
Monocular |
Monocular |
Monocular |
|
|
| DF Group |
25 Minutes After Contact Lens Insertion |
12.97 |
29.19 |
0.74 |
4.00 |
|
|
| SV Group |
5.77 |
19.65 |
0.30 |
3.30 |
|
|
| Martins et al. (2020) [93] |
3 Multifocal test lenses and 1 monofocal control lens in random order |
30 right eyes
Control Lens; Lens 1 – Center distance and Lens 2 and 3 – Center-near |
Young-adult myopic subjects |
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
|
|
| |
Monocular |
Monocular |
Monocular |
|
|
| Control Lens |
4.65 ± 2.25 |
0.42 ± 0.35 |
2.83 ± 1.57 |
|
|
| Lens 1 |
7.95 ± 3.54 |
0.43 ± 0.48 |
3.54 ± 1.37 |
|
|
| Lens 2 |
5.79 ± 3.27 |
0.43 ± 0.31 |
3.13 ± 1.34 |
|
|
| Lens 3 |
5.69 ± 3.10 |
0.62 ± 0.92 |
2.97 ± 1.76 |
|
|
| García-Marqués et al. (2022) [36] |
Two dual focus (DF) having different inner zone diameters
|
28 Subjects
Were then binocularly fitted with the DF, with only the sensorial dominant eye being assessed. Lenses were had inner zone diameters of either 2.1 mm (S design) or 4.0 mm (M design). |
Healthy myopic between 18 and 32 years of age with astigmatism of ≤0.75 D |
|
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
|
|
| |
|
Monocular |
Monocular |
Monocular |
Monocular |
|
|
S
(Zone diameter 2.1 mm) |
25 Minutes After Contact Lens Insertion |
16.83 |
33.30 |
0.71 |
4.86 |
|
|
M
(Zone diameter 4.0 mm) |
13.65 |
30.00 |
0.79 |
4.78 |
|
|
| |
|
|
|
|
LDI (%) |
BFCRad (mm) |
BFCIrreg (mm) |
BFCIrregSD (mm) |
|
|
|
| Silva-Leite et al. (2023) [21] |
Control measurements with monofocal lenses, followed by the same examinations with the perifocal lenses |
17 patients
|
Myopic young adults |
Monofocal lenses |
11.60 ± 6.42 |
26.85 ± 7.37 |
0.53 ± 0.48 |
4.00 ± 1.01 |
|
|
|
| Perifocal lenses |
10.88 ± 6.10 |
26.04 ± 7.02 |
0.69 ± 0.57 |
4.07 ± 1.69 |
|
|
|
LDA has also been used in studies with presbyopic patients, as well as in studies evaluating multifocal lens designs for myopia control [
21,
22,
36,
91,
92,
93]. A study investigated the optical performance concerning light distortion among presbyopic individuals wearing multifocal contact lenses in comparison to monofocal lenses. The study revealed that LDI escalated with multifocal lenses wear, especially evident with larger pupil sizes. Additionally, it was observed that the configuration of the distortion pattern correlated with the shape of the pupil [
75]. Contrarily, other studies have reported a superior performance of multifocal lenses regarding light distortion [
22,
94].
Studies investigating multifocal lenses for myopia control have demonstrated that dual-focus lenses can initially heighten the perception of light distortion, particularly when used monocularly. However, this effect tends to diminish over time, and significant attenuation occurs when using the lenses binocularly [
91]. García-Marqués et al. [
92]and Martins et al. [
93] also compared dual-focus and monofocal lenses in young people with astigmatism and concluded that dual-focus lenses induce more significant light disturbance compared to monofocal lenses. Similarly, another study examining dual-focus lenses with varying optical zone diameters found that lenses with the smallest optical zone diameter resulted in greater light disturbance [
36]. In 2023 Silva-Leite et al. [
21] studied the effect of peripheral defocus induced by perifocal lenses for the control of myopia and found that there were no significant differences in light disturbance with perifocal lenses. This observation could be attributed to the likelihood that the central area of the lens was sufficiently large for patients not to perceive increased glare compared to monofocal lenses. This conclusion aligns with findings from the study conducted by García-Marqués et al. [
92].
4.3.4. Changes in Tear Film
Dry eye disease is a common eye condition that can affect the quality of vision and ocular comfort [
94]. It is important to consider how dry eye disease can influence light dispersion and, consequently, visual quality. If there is a significant increase in light dispersion due to dry eye, this can compromise the quality of overall vision, resulting in symptoms such as halos, starbursts and visual discomfort. For example, an objective study carried out by Diaz-Valle et al. [
94] revealed a notable rise in light scatter in cases of mild to moderate dry eye. Another study by Talens-Estarelles et al. [
95] assessed changes in optical quality and tear film in computer users and concluded that various aspects of visual function and quality of vision worsened over a day of computer use. These changes were accompanied by increased symptoms of dry eye and changes in the tear film, which may have increased light scattering, causing greater disturbance of the central light source in the LDA.
4.3.5. Orthokeratology
A 2016 study by Macedo-de-Araújo et al. [
78] evaluated the effect of induced spherical aberration on light distortion. The study included pupil measurements under natural conditions and after pupil dilation and compared light distortion parameters obtained in the LDA with and without cycloplegia. They concluded that pupil dilation and cycloplegia increased the size of light distortion in healthy eyes, that positive spherical aberration induces more distortion than negative spherical aberration and, finally, that accommodation and pupil dilation can compensate for the degradation of optical quality induced by spherical aberration. Later, in 2019 Amorim-de-Sousa et al. [
28] carried out a study to assess the impact of different levels of positive and negative defocus on luminous disturbance measurements and analyzed how high-order aberrations and topographic quality parameters could influence the perception of photic phenomena. Light distortion was assessed with the LDA in natural accommodative and cycloplegic conditions with a positive and negative induced defocus of 1.00D and they concluded that both positive and negative induced defocus (uncorrected refractive errors) significantly increased the size of the luminous distortion, but not its irregularity index and that spherical aberration was associated with the size of the distortion, while coma and total aberration were associated with the irregularity of the distortion. In 2023 Martino et al. [
96] similarly concluded that under various conditions of optical degradation, including spherical and cylindrical aberrations, and with meticulous control over pupil size, binocular summation was evident. This observation suggests the influence of neural factors. Thus, it implies that apart from optical attributes, there exists a neural component or adaptation mechanism at play to enhance binocular visual perception.
5. Conclusion
Visual disturbances contribute to the degradation of visual quality, particularly at night when the pupils are dilated, which can considerably impact daily activities. Various subjective and objective methods have been developed for assessing and quantifying visual disturbances caused by light scattering. The Light Distortion Analyzer (LDA), a device that has been introduced and validated to objectively quantify light distortion in a clinical context, provides metrics on the size, location and regularity of optical phenomena, making it a practical tool for assessing visual disturbances. The LDA’s role in quantifying light disturbance has provided a valuable tool for assessing visual quality, contributing to a better understanding of the optical phenomena associated with various ophthalmic interventions: ablative refractive surgery, intraocular lens implantation, contact lens fitting among others. Surgical interventions have been shown to influence/ increase light disturbance perception, underscoring the importance of careful patient selection and preoperative planning. Moreover, the contact lens fitting in presbyopic patients or for myopia control purposes presents a nuanced understanding of how these optical aids can affect light disturbance, offering insights into the potential neural adaptation over time, and binocular summation to the dysphotopsias. This research not only enhances our comprehension of light distortion and its impact on visual quality but also opens new paths for future investigations aimed at optimizing visual outcomes in low-light conditions. Those include but are not limited to longitudinal studies on the neural adaptation mechanism (understanding these temporal dynamics could help in designing CLs or IOLs that minimize the adaptation periods and enhance patient comfort and satisfaction), or development of standardized assessment protocols to measure NVDs and create protocols to evaluate drivers, among other research opportunities.
Author Contributions
Conceptualization, R.M.A. and J.M.G.M.; methodology, R.M.A. and J.M.G.M; investigation, R.S.A.C; writing—original draft preparation, R.S.A.C.; writing—review and editing, R.S.A.C, R.M.A and J.M.G.M; supervision, R.M.A. and J.M.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Binarytarget Lda, Braga, Portugal.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors R.M.A. and J.M.G.M. declare that they have competing interests with the development of the LDA device.
References
- Fan-Paul, N.I.; Li, J.; Miller, J.S.; Florakis, G.J. Night Vision Disturbances after Corneal Refractive Surgery. Surv Ophthalmol 2002, 47, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Artal, P. Optics of the Eye and Its Impact in Vision: A Tutorial. Adv Opt Photonics 2014, 6, 340. [Google Scholar] [CrossRef]
- Marcos, S. Image Quality of the Human Eye, Vol.43, No. 2. In International Ophthalmology Clinics; 2003; pp. 43–62.
- Piñero, D.P.; Ortiz, D.; Alio, J.L. Ocular Scattering. Optometry and Vision Science 2010, 87, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A.; Turner, P.L. Retinal Injuries from Light: Mechanisms, Hazards, and Prevention. In Retina; 2006; pp. 1857–1870.
- Mainster, M.A.; Turner, P.L. Glare’s Causes, Consequences, and Clinical Challenges after a Century of Ophthalmic Study. Am J Ophthalmol 2012, 153, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Thibos, L.N. Principles of Hartmann-Shack Aberrometry. Journal of Refractive Surgery 2000, 16, S563–S565. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.W.; Farrell, R.A. Light Scattering in the Cornea. J Opt Soc Am 1969, 59, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Thomas J.T.P. van den, Berg; Spekreijse, H. Light Scattering Model for Donor Lenses as a Function of Depth. Vision Res 1999, 39, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.F.; Khorrami-Nejad, M.; Hamidirad, M. Posterior Corneal Astigmatism: A Review Article. Clin Optom (Auckl) 2019, 11, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T. [The Cornea: Stasis and Dynamics]. Nippon Ganka Gakkai Zasshi 2008, 112, 179–212. [Google Scholar] [PubMed]
- Spadea, L.; Maraone, G.; Verboschi, F.; Vingolo, E.M.; Tognetto, D. Effect of Corneal Light Scatter on Vision: A Review of the Literature. Int J Ophthalmol 2016, 9, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-Related Changes in the Kinetics of Human Lenses: Prevention of the Cataract. Int J Ophthalmol 2016, 9, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.L.; Opalecky, D.; Bettelheim, F.A. Light Scattering of Normal Human Lens. II. Age Dependence of the Light Scattering Parameters. Exp Eye Res 1981, 33, 603–614. [Google Scholar] [CrossRef]
- Thomas J.T.P. van den, Berg; van Rijn, L.J.; Kaper-Bongers, R.; Vonhoff, D.J.; Völker-Dieben, H.J.; Grabner, G.; Nischler, C.; Emesz, M.; Wilhelm, H.; Gamer, D.; et al. Disability Glare in the Aging Eye. Assessment and Impact on Driving. J Optom 2009, 2, 112–118. [Google Scholar] [CrossRef]
- Artal, P.; Benito, A.; Pérez, G.M.; Alcón, E.; de Casas, Á.; Pujol, J.; Marín, J.M. An Objective Scatter Index Based on Double-Pass Retinal Images of a Point Source to Classify Cataracts. PLoS One 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jinabhai, A.; O’Donnell, C.; Radhakrishnan, H.; Nourrit, V. Forward Light Scatter and Contrast Sensitivity in Keratoconic Patients. Contact Lens and Anterior Eye 2012, 35, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Rodríguez-Vallejo, M.; Martínez, J.; Burguera, N.; Piñero, D.P. Long-Term Efficacy, Visual Performance and Patient Reported Outcomes with a Trifocal Intraocular Lens: A Six-Year Follow-Up. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Lyu, I.J.; Choi, S.H.; Chung, E.S.; Chung, T.Y. Risk Factors Associated With Night Vision Disturbances After Phakic Intraocular Lens Implantation. Am J Ophthalmol 2014, 157, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Bona, A.; Lorente-Velázquez, A.; Collar, C.V.; Nieto-Bona, P.; Mesa, A.G. Intraocular Straylight and Corneal Morphology Six Months after LASIK. Curr Eye Res 2010, 35, 212–219. [Google Scholar] [CrossRef]
- Silva-Leite, S.; Amorim-de-Sousa, A.; Queirós, A.; González-Méijome, J.M.; Fernandes, P. Peripheral Refraction and Visual Function of Novel Perifocal Ophthalmic Lens for the Control of Myopia Progression. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Amorim-de-Sousa, A.; Queirós, A.; Escandón-Garcia, S.; McAlinden, C.; González-Méijome, J.M. Light Disturbance with Multifocal Contact Lens and Monovision for Presbyopia. Contact Lens and Anterior Eye 2018, 41, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Anera, R.G.; Villa, C.; Jiménez, J.R.; Gutierrez, R. Effect of LASIK and Contact Lens Corneal Refractive Therapy on Higher Order Aberrations and Contrast Sensitivity Function. J Refract Surg 2009, 25, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Minasyan, H.; Richer, S.P. Cataract Halos: A Driving Hazard in Aging Populations. Implication of the Halometer DG Test for Assessment of Intraocular Light Scatter. Appl Ergon 2009, 40, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Cerviño, A.; Villa-Collar, C.; Gonzalez-Meijome, J.M.; Ferrer-Blasco, T.; García-Lázaro, S. Retinal Straylight and Light Distortion Phenomena in Normal and Post-LASIK Eyes. Graefe’s Archive for Clinical and Experimental Ophthalmology 2011, 249, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Santolaria Sanz, E.; Cerviño, A.; Queirós, A.; Villa-Collar, C.; Lopes-Ferreira, D.; González-Méijome, J.M. Short-Term Changes in Light Distortion in Orthokeratology Subjects. Biomed Res Int 2015, 2015, 278425. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Gutiérrez, R.; Jiménez, J.R.; González-Méijome, J.M. Night Vision Disturbances after Successful LASIK Surgery. British Journal of Ophthalmology 2007, 91, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Amorim-de-Sousa, A.; Macedo-De-Araújo, R.; Fernandes, P.; Queirós, A.; González-Méijome, J.M. Impact of Defocus and High-Order Aberrations on Light Disturbance Measurements. J Ophthalmol 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Meikies, D.; van der Mooren, M.; Terwee, T.; Guthoff, R.F.; Stachs, O. Rostock Glare Perimeter: A Distinctive Method for Quantification of Glare. Optometry and Vision Science 2013, 90, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Buckhurst, P.J.; Naroo, S.A.; Davies, L.N.; Shah, S.; Buckhurst, H.; Kingsnorth, A.; Drew, T.; Wolffsohn, J.S. Tablet App Halometer for the Assessment of Dysphotopsia. J Cataract Refract Surg 2015, 41, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-Item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001, 119, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, C.; Pesudovs, K.; Moore, J.E. The Development of an Instrument to Measure Quality of Vision: The Quality of Vision (QoV) Questionnaire. Invest Ophthalmol Vis Sci 2010, 51, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Neves, H.; Macedo-de-Araújo, R.; Rico-del-Viejo, L.; da-Silva, A.C.; Queirós, A.; González-Méijome, J.M. Validation of a Method to Measure Light Distortion Surrounding a Source of Glare. J Biomed Opt 2015, 20, 075002. [Google Scholar] [CrossRef] [PubMed]
- Klyce, S.D. Night Vision Disturbances after Refractive Surgery: Haloes Are Not Just for Angels. British Journal of Ophthalmology 2007, 91, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Brito, P.; Salgado-Borges, J.; Neves, H.; Gonzalez-Meijome, J.; Monteiro, M. Light-Distortion Analysis as a Possible Indicator of Visual Quality after Refractive Lens Exchange with Diffractive Multifocal Intraocular Lenses. J Cataract Refract Surg 2015, 41, 613–622. [Google Scholar] [CrossRef] [PubMed]
- García-Marqués, J.V.; Macedo-De-Araújo, R.J.; McAlinden, C.; Faria-Ribeiro, M.; Cerviño, A.; González-Méijome, J.M. Short-Term Tear Film Stability, Optical Quality and Visual Performance in Two Dual-Focus Contact Lenses for Myopia Control with Different Optical Designs. Ophthalmic and Physiological Optics 2022, 42, 1062–1073. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Jiménez, J.R.; Villa, C.; Valverde, J.A.; Anera, R.G. Simple Device for Quantifying the Influence of Halos after Lasik Surgery. J Biomed Opt 2003, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. The Charles F. Prentice Award Lecture 1990: Specific Tests and Specific Blindnesses: Keys, Locks, and Parallel Processing. Optom Vis Sci 1991, 68, 489–512. [Google Scholar] [CrossRef] [PubMed]
- O’Brart, D.P.; Lohmann, C.P.; Fitzke, F.W.; Klonos, G.; Corbett, M.C.; Kerr-Muir, M.G.; Marshall, J. Disturbances in Night Vision after Excimer Laser Photorefractive Keratectomy. Eye (Lond) 1994, 8 (Pt 1) Pt 1, 46–51. [Google Scholar] [CrossRef]
- O’Brart, D.P.; Lohmann, C.P.; Fitzke, F.W.; Smith, S.E.; Kerr-Muir, M.G.; Marshall, J. Night Vision after Excimer Laser Photorefractive Keratectomy: Haze and Halos. Eur J Ophthalmol 1994, 4, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jewelewicz, D.A.; Evans, R.; Chen, R.; Trokel, S.; Florakis, G.J. Evaluation of Night Vision Disturbances in Contact Lens Wearers. CLAO J 1998, 24, 107–110. [Google Scholar] [PubMed]
- Oliver, K.M.; Hemenger, R.P.; Corbett, M.C.; O’Brart, D.P.; Verma, S.; Marshall, J.; Tomlinson, A. Corneal Optical Aberrations Induced by Photorefractive Keratectomy. J Refract Surg 1997, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Rajan, M.S.; O’Brart, D.; Jaycock, P.; Marshall, J. Effects of Ablation Diameter on Long-Term Refractive Stability and Corneal Transparency after Photorefractive Keratectomy. Ophthalmology 2006, 113, 1798–1806. [Google Scholar] [CrossRef]
- Chang, D.H.; Huggins, L.K. Review of Optometry. 2018, pp. 48–51.
- van den Berg, T.J.T.P.; IJspeert, J.K.; de Waard, P.W. Dependence of Intraocular Straylight on Pigmentation and Light Transmission through the Ocular Wall. Vision Res 1991, 31, 1361–1367. [Google Scholar] [CrossRef]
- van den Berg, T.J.T.P. Analysis of Intraocular Straylight, Especially in Relation to Age. Optom Vis Sci 1995, 72, 52–59. [Google Scholar] [CrossRef]
- Ginis, H.S.; Perez, G.M.; Bueno, J.M.; Pennos, A.; Artal, P. Wavelength Dependence of the Ocular Straylight. Invest Ophthalmol Vis Sci 2013, 54, 3702–3708. [Google Scholar] [CrossRef]
- Florakis, G.J.; Jewelewicz, D.A.; Michelsen, H.E.; Trokel, S.L. Evaluation of Night Vision Disturbances. J Refract Corneal Surg 1994, 10, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, T.; Suryakumar, R. Measures of Visual Disturbance in Patients Receiving Extended Depth-of-Focus or Trifocal Intraocular Lenses. J Cataract Refract Surg 2021, 47, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Linhares, J.M.M.; Neves, H.; Lopes-Ferreira, D.; Faria-Ribeiro, M.; Peixoto-De-Matos, S.C.; Gonzalez-Meijome, J.M. Radiometric Characterization of a Novel LED Array System for Visual Assessment. J Mod Opt 2013, 60, 1136–1144. [Google Scholar] [CrossRef]
- Naggar, F. El; Alnassar, A.; Tarib, I.; Detlev R H Breyer; Gerl, M. ; Kretz, F.T. Enhancing Intermediate Vision in Different Working Distances with a Novel Enhanced Depth of Focus Intraocular Lens (EDOF). EC Ophthalmol 2018, 9, 94–99. [Google Scholar]
- Drum, B.; Calogero, D.; Rorer, E. Assessment of Visual Performance in the Evaluation of New Medical Products. Drug Discov Today Technol 2007, 4, 55–61. [Google Scholar] [CrossRef]
- Guber, I.; Bachmann, L.M.; Guber, J.; Bochmann, F.; Lange, A.P.; Thiel, M.A. Reproducibility of Straylight Measurement by C-Quant for Assessment of Retinal Straylight Using the Compensation Comparison Method. Graefe’s Archive for Clinical and Experimental Ophthalmology 2011, 249, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Artal, P.; Green, D.G.; Iglesias, I.; López-Gil, N. Double-Pass Measurements of the Retinal-Image Quality with Unequal Entrance and Exit Pupil Sizes and the Reversibility of the Eye’s Optical System. Journal of the Optical Society of America A 1995, 12, 2358. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, M.; Peris, E.; Pujol, J.; Borras, R.; Arjona, M. Intra- and Intersession Repeatability of a Double-Pass Instrument. Optometry and Vision Science 2010, 87, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Hasegawa, A.; Hara, S.; Horai, R.; Yoshida, Y.; Nakamura, T.; Dogru, M.; Ichikawa, K. Quantitative Evaluation of Night Vision and Correlation of Refractive and Topographical Parameters with Glare after Orthokeratology. Graefe’s Archive for Clinical and Experimental Ophthalmology 2011, 249, 1519–1526. [Google Scholar] [CrossRef]
- Puell, M.C.; Pérez-Carrasco, M.J.; Barrio, A.; Antona, B.; Palomo-Alvarez, C. Normal Values for the Size of a Halo Produced by a Glare Source. Journal of Refractive Surgery 2013, 29, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.; Pérez, G.M.; Mirabet, S.; Vilaseca, M.; Pujol, J.; Marín, J.M.; Artal, P. Objective Optical Assessment of Tear-Film Quality Dynamics in Normal and Mildly Symptomatic Dry Eyes. J Cataract Refract Surg 2011, 37, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Wachler, B.S.B.; Durrie, D.S.; Assil, K.K.; Krueger, R.R. Improvement of Visual Function with Glare Testing after Photorefractive Keratectomy and Radial Keratotomy. Am J Ophthalmol 1999, 128, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; González-Méijome, J.M.; Vida, R.S.; Gupta, R. Changes in Light Disturbance Analyzer Evaluation in SMILE for High Myopia and Astigmatism. Journal of Refractive Surgery 2022, 38, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ukai, Y.; Okemoto, H.; Seki, Y.; Nakatsugawa, Y.; Kawasaki, A.; Shibata, T.; Mito, T.; Kubo, E.; Sasaki, H. Quantitative Assessment of Photic Phenomena in the Presbyopia-Correcting Intraocular Lens. PLoS One 2021, 16, 1–11. [Google Scholar] [CrossRef]
- van den Berg, T.J.T.P. Importance of Pathological Intraocular Light Scatter for Visual Disability. Doc Ophthalmol 1986, 61, 327–333. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, T.J.T.P. Measurement of the Straylight Function of the Eye in Cataract and Other Optical Media Disturbances by Means of a Direct Compensation Method. Invest Ophthalmol Vis Sci 1987, 28, 397. [Google Scholar]
- van den Berg, T.J.T.P.; Ijspeert, J.K. Clinical Assessment of Intraocular Stray Light. Appl Opt 1992, 31, 3694. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, L.J.; Nischler, C.; Gamer, D.; Franssen, L.; De Wit, G.; Kaper, R.; Vonhoff, D.; Grabner, G.; Wilhelm, H.; Völker-Dieben, H.J.; et al. Measurement of Stray Light and Glare: Comparison of Nyktotest, Mesotest, Stray Light Meter, and Computer Implemented Stray Light Meter. British Journal of Ophthalmology 2005, 89, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Franssen, L.; Coppens, J.E.; van den Berg, T.J.T.P. Compensation Comparison Method for Assessment of Retinal Straylight. Invest Ophthalmol Vis Sci 2006, 47, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Roda, J.A.; Vilaseca, M.; Ondategui, J.C.; Giner, A.; Burgos, F.J.; Cardona, G.; Pujol, J. Optical Quality and Intraocular Scattering in a Healthy Young Population. Clin Exp Optom 2011, 94, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Thibos, L.N.; Bradley, A.; Himebaugh, N.; Liu, H. Forward Light Scatter Analysis of the Eye in a Spatially-Resolved Double-Pass Optical System. Opt Express 2011, 19, 7417. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.J.; Ortiz, C.; Pozo, A.M.; Anera, R.G.; Soler, M. A Visual Test Based on a Freeware Software for Quantifying and Displaying Night-Vision Disturbances: Study in Subjects after Alcohol Consumption. Theoretical Biology and Medical Modelling 2014, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Garcin, T.; Grivet, D.; Thuret, G.; Gain, P. Using Optical Quality Analysis System for Predicting Surgical Parameters in Age-Related Cataract Patients. PLoS One 2020, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pieh, S.; Lackner, B.; Hanselmayer, G.; Zöhrer, R.; Sticker, M.; Weghaupt, H.; Fercher, A.; Skorpik, C. Halo Size under Distance and near Conditions in Refractive Multifocal Intraocular Lenses. British Journal of Ophthalmology 2001, 85, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, A.; Anera, R.G.; Villa, C.; Jiménez del Barco, L.; Gutierrez, R. Visual Quality after Monovision Correction by Laser in Situ Keratomileusis in Presbyopic Patients. J Cataract Refract Surg 2011, 37, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Anera, R.G.; Castro, J.J.; Jiménez, J.R.; Villa, C.; Alarcón, A. Optical Quality and Visual Discrimination Capacity after Myopic LASIK with a Standard and Aspheric Ablation Profile. J Refract Surg 2011, 27, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.J.; Jiménez, J.R.; Ortiz, C.; Alarcón, A.; Anera, R.G. New Testing Software for Quantifying Discrimination Capacity in Subjects with Ocular Pathologies. J Biomed Opt 2011, 16, 015001. [Google Scholar] [CrossRef] [PubMed]
- Monsálvez-Romín, D.; González-Méijome, J.M.; Esteve-Taboada, J.J.; García-Lázaro, S.; Cerviño, A. Light Distortion of Soft Multifocal Contact Lenses with Different Pupil Size and Shape. Contact Lens and Anterior Eye 2020, 43, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Escandón-García, S.; Ribeiro, F.J.; McAlinden, C.; Queirós, A.; González-Méijome, J.M. Through-Focus Vision Performance and Light Disturbances of 3 New Intraocular Lenses for Presbyopia Correction. J Ophthalmol 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Borges, J.; Dias, L.; Costa, J. Light Distortion and Ocular Scattering with Glistening and Aberration-Free Pseudophakic IOL : A Pilot Study. Journal of Emmetropia 2015, 6, 127–132. [Google Scholar]
- Macedo-de-Araújo, R.; Ferreira-Neves, H.; Rico-del-Viejo, L.; Peixoto-de-Matos, S.C.; González-Méijome, J.M. Light Distortion and Spherical Aberration for the Accommodating and Nonaccommodating Eye. J Biomed Opt 2016, 21, 075003. [Google Scholar] [CrossRef] [PubMed]
- Santolaria-Sanz, E.; Cerviño, A.; González-Méijome, J.M. Corneal Aberrations, Contrast Sensitivity, and Light Distortion in Orthokeratology Patients: 1-Year Results. J Ophthalmol 2016, 2016, 8453462. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; Vargas, V.; Plaza-Puche, A.B.; Alió, J.L. Long-Term Results of a Diffractive Trifocal Intraocular Lens: Visual, Aberrometric and Patient Satisfaction Results. Eur J Ophthalmol 2020, 30, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Escandón-García, S.; Ribeiro, F.; McAlinden, C.; González Méijome, J.M. Light Distortion as an Indicator of Adaptation to Multifocality after Refractive Lens Exchange (RLE). Biomed J Sci Tech Res 2021, 37, 29583–29591. [Google Scholar] [CrossRef]
- Alió, J.L.; Plaza-Puche, A.B.; Alió del Barrio, J.L.; Amat-Peral, P.; Ortuño, V.; Yébana, P.; Al-Shymali, O.; Vega-Estrada, A. Clinical Outcomes with a Diffractive Trifocal Intraocular Lens. Eur J Ophthalmol 2018, 28, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Escandón-García, S.; Ribeiro, F.; McAlinden, C.; González-Méijome, J.M. Attenuation of Light Disturbances and Subjective Complains After 6 Months with New Generation Multifocal IOLs. Biomed J Sci Tech Res 2021, 37, 29097–29106. [Google Scholar] [CrossRef]
- Vargas, V.; Ferreira, R.; Del Barrio, J.L.A.; Alió, J.L. Visual Outcomes, Patient Satisfaction, and Light Distortion Analysis after Blended Implantation of Rotationally Asymmetric Multifocal Intraocular Lenses. Journal of Refractive Surgery 2020, 36, 796–803. [Google Scholar] [CrossRef]
- Lajara-Blesa, J.; Rodríguez-Izquierdo, M.Á.; Vallés-San-Leandro, L.; Jutley, G.; de los Remedios Ortega-García, M.; Zapata-Díaz, J.F. Standard Clinical Outcomes, Light Distortion, Stereopsis, and a Quality-of-Life Assessment of a New Binocular System of Complementary IOLs. Journal of Refractive Surgery 2023, 39, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Burguera, N.; Rocha-de-Lossada, C.; Rachwani-Anil, R.; Rodríguez-Vallejo, M. Influence of a Multifocal Intraocular Lens Centration and Eye Angles on Light Distortion and Ocular Scatter Index. Graefe’s Archive for Clinical and Experimental Ophthalmology 2023, 261, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Macedo-de-Araújo, R.J.; Faria-Ribeiro, M.; McAllinden, C.; Van der Worp, E.; González-Méijome, J.M. Optical Quality and Visual Performance for One Year in a Sample of Scleral Lens Wearers. Optometry and Vision Science 2020, 97, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Barbero, S.; Cano, D.; Dorronsoro, C.; Marcos, S. Myopic versus Hyperopic Eyes: Axial Length, Corneal Shape and Optical Aberrations. J Vis 2004, 4, 288–298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, K.; Jin, Y.; Niu, Y.; Zuo, T. Changes of Higher Order Aberration with Various Pupil Sizes in the Myopic Eye. J Refract Surg 2003, 19. [Google Scholar] [CrossRef] [PubMed]
- Pereira-da-Mota, A.F.; Costa, J.; Amorim-de-sousa, A.; González-Méijome, J.M.; Queirós, A. The Impact of Overnight Orthokeratology on Accommodative Response in Myopic Subjects. J Clin Med 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pomeda, A.; Fernandes, P.; Amorim-de-Sousa, A.; González-Méijome, J.M.; Prieto-Garrido, F.L.; Pérez-Sánchez, B.; Villa-Collar, C. Light Disturbance Analysis in the Controlled Randomized Clinical Trial MiSight ® Assessment Study Spain (MASS). Contact Lens and Anterior Eye 2019, 42, 200–205. [Google Scholar] [CrossRef]
- García-Marqués, J.V.; Macedo-De-Araújo, R.J.; Cerviño, A.; García-Lázaro, S.; McAlinden, C.; González-Méijome, J.M. Comparison of Short-Term Light Disturbance, Optical and Visual Performance Outcomes between a Myopia Control Contact Lens and a Single-Vision Contact Lens. Ophthalmic and Physiological Optics 2020, 40, 718–727. [Google Scholar] [CrossRef]
- Martins, C.; Amorim-de-Sousa, A.; Faria-Ribeiro, M.; Pauné, J.; González-Méijome, J.M.; Queirós, A. Visual Performance and High-Order Aberrations with Different Contact Lens Prototypes with Potential for Myopia Control. Curr Eye Res 2020, 45, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Valle, D.; Arriola-Villalobos, P.; García-Vidal, S.E.; Sánchez-Pulgarín, M.; Borrego Sanz, L.; Gegúndez-Fernández, J.A.; Benitez-Del-Castillo, J.M. Effect of Lubricating Eyedrops on Ocular Light Scattering as a Measure of Vision Quality in Patients with Dry Eye. J Cataract Refract Surg 2012, 38, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Talens-Estarelles, C.; Mechó-García, M.; McAlinden, C.; Cerviño, A.; García-Lázaro, S.; González-Méijome, J.M. Changes in Visual Function and Optical and Tear Film Quality in Computer Users. Ophthalmic and Physiological Optics 2023, 43, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Pereira-da-Mota, A.F.; Amorim-de-Sousa, A.; Castro-Torres, J.J.; González-Méijome, J.M. Pupil Size Effect on Binocular Summation for Visual Acuity and Light Disturbance. Int Ophthalmol 2023, 43, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).