Submitted:
11 July 2024
Posted:
11 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Results and Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Antimicrobial Resistance: Global Report on Surveillance; World Health Organization, 2014; ISBN 978-92-4-156474-8.
- Mann, A.; Nehra, K.; Rana, J.S.; Dahiya, T. Antibiotic Resistance in Agriculture: Perspectives on Upcoming Strategies to Overcome Upsurge in Resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Bahr, G.; González, L.J.; Vila, A.J. Metallo-β-Lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem. Rev. 2021, 121, 7957–8094. [Google Scholar] [CrossRef] [PubMed]
- Bush, K. The ABCD’s of β-Lactamase Nomenclature. J. Infect. Chemother. 2013, 19, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Brem, J.; Panduwawala, T.; Hansen, J.U.; Hewitt, J.; Liepins, E.; Donets, P.; Espina, L.; Farley, A.J.M.; Shubin, K.; Campillos, G.G.; et al. Imitation of β-Lactam Binding Enables Broad-Spectrum Metallo-β-Lactamase Inhibitors. Nat. Chem. 2022, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, O.K.; Hussein, W.M.; Vella, P.; Yusof, Y.; Sidjabat, H.E.; Schenk, G.; McGeary, R.P. Design, Synthesis, and in Vitro and Biological Evaluation of Potent Amino Acid-Derived Thiol Inhibitors of the Metallo-β-Lactamase IMP-1. Eur J Med Chem 2016, 114, 318–327. [Google Scholar] [CrossRef] [PubMed]
- McGeary, R.P.; Tan, D.T.C.; Selleck, C.; Monteiro Pedroso, M.; Sidjabat, H.E.; Schenk, G. Structure-Activity Relationship Study and Optimisation of 2-Aminopyrrole-1-Benzyl-4,5-Diphenyl-1H-Pyrrole-3-Carbonitrile as a Broad Spectrum Metallo-β-Lactamase Inhibitor. Eur J Med Chem 2017, 137, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C.; Fu, K.P. Clavulanic Acid, a Novel Inhibitor of β-Lactamases. Antimicrob. Agents Chemother. 1978, 14, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Krco, S.; Davis, S.J.; Joshi, P.; Wilson, L.A.; Monteiro Pedroso, M.; Douw, A.; Schofield, C.J.; Hugenholtz, P.; Schenk, G.; Morris, M.T. Structure, Function, and Evolution of Metallo-β-Lactamases from the B3 Subgroup—Emerging Targets to Combat Antibiotic Resistance. Front. Chem. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, M.M.; Waite, D.W.; Melse, O.; Wilson, L.; Mitić, N.; McGeary, R.P.; Antes, I.; Guddat, L.W.; Hugenholtz, P.; Schenk, G. Broad Spectrum Antibiotic-Degrading Metallo-β-Lactamases Are Phylogenetically Diverse. Protein Cell 2020, 11, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, M.M.; Selleck, C.; Enculescu, C.; Harmer, J.R.; Mitić, N.; Craig, W.R.; Helweh, W.; Hugenholtz, P.; Tyson, G.W.; Tierney, D.L.; et al. Characterization of a Highly Efficient Antibiotic-Degrading Metallo-β-Lactamase Obtained from an Uncultured Member of a Permafrost Community†. Metallomics 2017, 9, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Vella, P.; Miraula, M.; Phelan, E.; Leung, E.W.W.; Ely, F.; Ollis, D.L.; McGeary, R.P.; Schenk, G.; Mitić, N. Identification and Characterization of an Unusual Metallo-β-Lactamase from Serratia Proteamaculans. JBIC J. Biol. Inorg. Chem. 2013, 18, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman Mark, A.; Bell, J.; Ritchie, B.; Pratt, R.; Ryley, H.; Walsh Timothy, R. Genetic and Biochemical Characterization of an Acquired Subgroup B3 Metallo-β-Lactamase Gene, blaAIM-1, and Its Unique Genetic Context in Pseudomonas Aeruginosa from Australia. Antimicrob. Agents Chemother. 2012, 56, 6154–6159. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, W.; Zhang, J.; Li, Y.; Zheng, P.; Zhang, H. Draft Genome Sequence of a Metallo-β-Lactamase (Bla(AIM-1))-Producing Klebsiella Pneumoniae ST1916 Isolated from a Patient with Chronic Diarrhoea. J Glob Antimicrob Resist 2019, 16, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Carfi, A.; Pares, S.; Duée, E.; Galleni, M.; Duez, C.; Frère, J.M.; Dideberg, O. The 3-D Structure of a Zinc Metallo-Beta-Lactamase from Bacillus Cereus Reveals a New Type of Protein Fold. Embo J 1995, 14, 4914–4921. [Google Scholar] [CrossRef] [PubMed]
- Dominski, Z. Nucleases of the Metallo-Beta-Lactamase Family and Their Role in DNA and RNA Metabolism. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Dominski, Z.; Carpousis, A.J.; Clouet-d’Orval, B. Emergence of the β-CASP Ribonucleases: Highly Conserved and Ubiquitous Metallo-Enzymes Involved in Messenger RNA Maturation and Degradation. Biochim. Biophys. Acta BBA - Gene Regul. Mech. 2013, 1829, 532–551. [Google Scholar] [CrossRef]
- Pettinati, I.; Brem, J.; Lee, S.Y.; McHugh, P.J.; Schofield, C.J. The Chemical Biology of Human Metallo-β-Lactamase Fold Proteins. Trends Biochem Sci 2016, 41, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, G.A.W.; Li, Q.; Bruner, S.D.; Hanson, A.D. An Unusual Diphosphatase from the PhnP Family Cleaves Reactive FAD Photoproducts. Biochem. J. 2018, 475, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Castillo Villamizar Genis, A.; Funkner, K.; Nacke, H.; Foerster, K.; Daniel, R.; Sawers, G. Functional Metagenomics Reveals a New Catalytic Domain, the Metallo-β-Lactamase Superfamily Domain, Associated with Phytase Activity. mSphere 2019, 4, e00167-19. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Gahan, L.R.; Schenk, G.; Ollis, D.L. Altering the Substrate Specificity of Methyl Parathion Hydrolase with Directed Evolution. Arch Biochem Biophys 2015, 573, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, F.J.; Garces, F.; López-Estepa, M.; Aguilar, J.; Baldomà, L.; Coll, M.; Badia, J.; Vega, M.C. The UlaG Protein Family Defines Novel Structural and Functional Motifs Grafted on an Ancient RNase Fold. BMC Evol. Biol. 2011, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Miraula, M.; Whitaker, J.J.; Schenk, G.; Mitić, N. β-Lactam Antibiotic-Degrading Enzymes from Non-Pathogenic Marine Organisms: A Potential Threat to Human Health. J. Biol. Inorg. Chem. 2015, 20, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Au, S.X.; Dzulkifly, N.S.; Muhd Noor, N.D.; Matsumura, H.; Raja Abdul Rahman, R.N.Z.; Normi, Y.M. Dual Activity BLEG-1 from Bacillus Lehensis G1 Revealed Structural Resemblance to B3 Metallo-β-Lactamase and Glyoxalase II: An Insight into Its Enzyme Promiscuity and Evolutionary Divergence. Int. J. Mol. Sci. 2021, 22, 9377. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Normi, Y.M.; Leow, A.T.C.; Salleh, A.B.; Murad, A.M.A.; Mahadi, N.M.; Rahman, M.B.A. Danger Lurking in the “Unknowns”: Structure-to-Function Studies of Hypothetical Protein Bleg1_2437 from Bacillus Lehensis G1 Alkaliphile Revealed an Evolutionary Divergent B3 Metallo-Beta-Lactamase. J. Biochem. (Tokyo) 2017, 161, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, R.; Li, S.; Jiang, J. A Novel Hydrolytic Dehalogenase for the Chlorinated Aromatic Compound Chlorothalonil. J Bacteriol 2010, 192, 2737–2745. [Google Scholar] [CrossRef] [PubMed]
- Barbeyron, T.; Potin, P.; Richard, C.; Collin, O.; Kloareg, B. Arylsulphatase from Alteromonas Carrageenovora. Microbiol. Read. 1995, 141 ( Pt 11), 2897–2904. [Google Scholar] [CrossRef]
- Hagelueken, G.; Adams Thorsten, M.; Wiehlmann, L.; Widow, U.; Kolmar, H.; Tümmler, B.; Heinz Dirk, W.; Schubert, W.-D. The Crystal Structure of SdsA1, an Alkylsulfatase from Pseudomonas Aeruginosa, Defines a Third Class of Sulfatases. Proc. Natl. Acad. Sci. 2006, 103, 7631–7636. [Google Scholar] [CrossRef] [PubMed]
- Muok, A.R.; Deng, Y.; Gumerov, V.M.; Chong, J.E.; DeRosa, J.R.; Kurniyati, K.; Coleman, R.E.; Lancaster, K.M.; Li, C.; Zhulin, I.B.; et al. A Di-Iron Protein Recruited as an Fe[II] and Oxygen Sensor for Bacterial Chemotaxis Functions by Stabilizing an Iron-Peroxy Species. Proc. Natl. Acad. Sci. 2019, 116, 14955–14960. [Google Scholar] [CrossRef] [PubMed]
- Morán-Barrio, J.; Lisa, M.-N.; Larrieux, N.; Drusin, S.I.; Viale, A.M.; Moreno, D.M.; Buschiazzo, A.; Vila, A.J. Crystal Structure of the Metallo-β-Lactamase GOB in the Periplasmic Dizinc Form Reveals an Unusual Metal Site. Antimicrob. Agents Chemother. 2016, 60, 6013–6022. [Google Scholar] [CrossRef] [PubMed]
- Selleck, C.; Larrabee, J.A.; Harmer, J.; Guddat, L.W.; Mitić, N.; Helweh, W.; Ollis, D.L.; Craig, W.R.; Tierney, D.L.; Monteiro Pedroso, M.; et al. AIM-1: An Antibiotic-Degrading Metallohydrolase That Displays Mechanistic Flexibility. Chem. – Eur. J. 2016, 22, 17704–17714. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.A.; Knaven, E.G.; Morris, M.T.; Monteiro Pedroso, M.; Schofield, C.J.; Brück, T.B.; Boden, M.; Waite, D.W.; Hugenholtz, P.; Guddat, L.; et al. Kinetic and Structural Characterization of the First B3 Metallo-β-Lactamase with an Active-Site Glutamic Acid. Antimicrob. Agents Chemother. 2021, 65, e00936-21. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a New Metallo-β-Lactamase Gene, blaNDM-1, and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella Pneumoniae Sequence Type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Bebrone, C.; Anne, C.; Galleni, M.; Frère, J.-M.; Dideberg, O. A Metallo-β-Lactamase Enzyme in Action: Crystal Structures of the Monozinc Carbapenemase CphA and Its Complex with Biapenem. J. Mol. Biol. 2005, 345, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Diene, S.M.; Pinault, L.; Keshri, V.; Armstrong, N.; Khelaifia, S.; Chabrière, E.; Caetano-Anolles, G.; Colson, P.; La Scola, B.; Rolain, J.-M.; et al. Human Metallo-β-Lactamase Enzymes Degrade Penicillin. Sci Rep 2019, 9, 12173. [Google Scholar] [CrossRef] [PubMed]
- Diene, S.M.; Pinault, L.; Armstrong, N.; Azza, S.; Keshri, V.; Khelaifia, S.; Chabrière, E.; Caetano-Anolles, G.; Rolain, J.-M.; Pontarotti, P.; et al. Dual RNase and β-Lactamase Activity of a Single Enzyme Encoded in Archaea. Life 2020, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Takahashi, M.; Jeon, J.H.; Kang, L.-W.; Seki, M.; Park, K.S.; Hong, M.-K.; Park, Y.S.; Kim, T.Y.; Karim, A.M.; et al. Dual Activity of PNGM-1 Pinpoints the Evolutionary Origin of Subclass B3 Metallo-β-Lactamases: A Molecular and Evolutionary Study. Emerg. Microbes Infect. 2019, 8, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Miraula, M.; Schenk, G.; Mitić, N. Promiscuous Metallo-β-Lactamases: MIM-1 and MIM-2 May Play an Essential Role in Quorum Sensing Networks. J. Inorg. Biochem. 2016, 162, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, P.; Kobus, S.; Gertzen, C.G.W.; Hoeppner, A.; Holzscheck, N.; Strunk, C.H.; Huber, H.; Jaeger, K.-E.; Gohlke, H.; Kovacic, F.; et al. A Promiscuous Ancestral Enzyme’s Structure Unveils Protein Variable Regions of the Highly Diverse Metallo-β-Lactamase Family. Commun. Biol. 2021, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Pinault, L.; Azza, S.; Armstrong, N.; Chabriere, E.; La Scola, B.; Pontarotti, P.; Raoult, D. A Protein of the Metallo-Hydrolase/Oxidoreductase Superfamily with Both Beta-Lactamase and Ribonuclease Activity Is Linked with Translation in Giant Viruses. Sci. Rep. 2020, 10, 21685. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Hong, M.-K.; Jeon, J.W.; Kim, J.H.; Jeon, J.H.; Lee, J.H.; Kim, T.Y.; Karim, A.M.; Malik, S.K.; Kang, L.-W.; et al. The Novel Metallo-β-Lactamase PNGM-1 from a Deep-Sea Sediment Metagenome: Crystallization and X-Ray Crystallographic Analysis. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2018, 74, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.Y.; Colquhoun, J.M.; Perl, A.L.; Chamakura, K.R.; Kuty Everett, G.F. Complete Genome of Bacillus Subtilis Myophage Grass. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Willms, I.M.; Hoppert, M.; Hertel, R. Characterization of Bacillus Subtilis Viruses vB_BsuM-Goe2 and vB_BsuM-Goe3. Viruses 2017, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.J.; Wein, T.; Lu, A.; Morehouse, B.R.; Schnabel, J.; Leavitt, A.; Yirmiya, E.; Sorek, R.; Kranzusch, P.J. Phage Anti-CBASS and Anti-Pycsar Nucleases Subvert Bacterial Immunity. Nature 2022, 605, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Melamed, S.; Millman, A.; Shulman, G.; Oppenheimer-Shaanan, Y.; Kacen, A.; Doron, S.; Amitai, G.; Sorek, R. Cyclic GMP–AMP Signalling Protects Bacteria against Viral Infection. Nature 2019, 574, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Tal, N.; Morehouse, B.R.; Millman, A.; Stokar-Avihail, A.; Avraham, C.; Fedorenko, T.; Yirmiya, E.; Herbst, E.; Brandis, A.; Mehlman, T.; et al. Cyclic CMP and Cyclic UMP Mediate Bacterial Immunity against Phages. Cell 2021, 184, 5728–5739.e16. [Google Scholar] [CrossRef]
- Lee, S.Y.; Brem, J.; Pettinati, I.; Claridge, T.D.W.; Gileadi, O.; Schofield, C.J.; McHugh, P.J. Cephalosporins Inhibit Human Metallo β-Lactamase Fold DNA Repair Nucleases SNM1A and SNM1B/Apollo. Chem. Commun. 2016, 52, 6727–6730. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-β-Lactamase (NDM): A Threat to Public Health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Takahashi, M.; Jeon, J.H.; Kang, L.-W.; Seki, M.; Park, K.S.; Hong, M.-K.; Park, Y.S.; Kim, T.Y.; Karim, A.M.; et al. Dual Activity of PNGM-1, a Metallo-β-Lactamase and tRNase Z, Pinpoints the Evolutionary Origin of Subclass B3 Metallo-β-Lactamases. bioRxiv 2019, 575373. [Google Scholar] [CrossRef]
- Malgapo, M.I.P.; Safadi, J.M.; Linder, M.E. Metallo-β-Lactamase Domain-Containing Protein 2 Is S-Palmitoylated and Exhibits Acyl-CoA Hydrolase Activity. J. Biol. Chem. 2021, 296, 100106. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Rozewicki, J.; Li, S.; Amada, K.M.; Standley, D.M.; Katoh, K. MAFFT-DASH: Integrated Protein Sequence and Structural Alignment. Nucleic Acids Res. 2019, 47, W5–W10. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, C.; Perilli, M.; Marcoccia, F.; Piccirilli, A.; Pellegrini, C.; Colapietro, M.; Sabatini, A.; Celenza, G.; Kerff, F.; Amicosante, G.; et al. Kinetic Studies on CphA Mutants Reveal the Role of the P158-P172 Loop in Activity versus Carbapenems. Antimicrob. Agents Chemother. 2016, 60, 3123–3126. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, L.E.; Izougarhane, Y.; Lassaux, P.; Selevsek, N.; Liénard, B.M.R.; Poirel, L.; Kupper, M.B.; Hoffmann, K.M.; Frère, J.-M.; Galleni, M.; et al. Broad Antibiotic Resistance Profile of the Subclass B3 Metallo-β-Lactamase GOB-1, a Di-Zinc Enzyme. FEBS J. 2011, 278, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Segatore, B.; Massidda, O.; Satta, G.; Setacci, D.; Amicosante, G. High Specificity of cphA-Encoded Metallo-Beta-Lactamase from Aeromonas Hydrophila AE036 for Carbapenems and Its Contribution to Beta-Lactam Resistance. Antimicrob. Agents Chemother. 1993, 37, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Bebrone, C.; Anne, C.; De Vriendt, K.; Devreese, B.; Rossolini, G.M.; Van Beeumen, J.; Frère, J.-M.; Galleni, M. Dramatic Broadening of the Substrate Profile of the Aeromonas Hydrophila CphA Metallo-β-Lactamase by Site-Directed Mutagenesis*. J. Biol. Chem. 2005, 280, 28195–28202. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.V.; Huang, W.; LaRocco, M.; Palzkill, T. Characterization of TEM-1 Beta-Lactamase Mutants from Positions 238 to 241 with Increased Catalytic Efficiency for Ceftazidime. J. Biol. Chem. 1994, 269, 23444–23450. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.-Y.; Doucet, N.; Pelletier, J.N. High Tolerance to Simultaneous Active-Site Mutations in TEM-1 β-Lactamase: Distinct Mutational Paths Provide More Generalized β-Lactam Recognition. Protein Sci. 2009, 18, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Héritier, C.; Tolün, V.; Nordmann, P. Emergence of Oxacillinase-Mediated Resistance to Imipenem in Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Robin, F.; Delmas, J.; Machado, E.; Bouchon, B.; Peixe, L.; Bonnet, R. Characterization of the Novel CMT Enzyme TEM-154. Antimicrob. Agents Chemother. 2011, 55, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Marcoccia, F.; Leiros, H.-K.S.; Aschi, M.; Amicosante, G.; Perilli, M. Exploring the Role of L209 Residue in the Active Site of NDM-1 a Metallo-β-Lactamase | PLOS ONE. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0189686 (accessed on 1 July 2024).
- Chiou, J.; Cheng, Q.; Shum, P.T.; Wong, M.H.; Chan, E.W.; Chen, S. Structural and Functional Characterization of OXA-48: Insight into Mechanism and Structural Basis of Substrate Recognition and Specificity. Int. J. Mol. Sci. 2021, 22, 11480. [Google Scholar] [CrossRef] [PubMed]
- Mammeri, H.; Galleni, M.; Nordmann, P. Role of the Ser-287-Asn Replacement in the Hydrolysis Spectrum Extension of AmpC β-Lactamases in Escherichia Coli. Antimicrob. Agents Chemother. 2009, 53, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, A.; Cornaglia, G.; Nikaido, H. Contributions of the AmpC β-Lactamase and the AcrAB Multidrug Efflux System in Intrinsic Resistance of Escherichia Coli K-12 to β-Lactams. Antimicrob. Agents Chemother. 2000, 44, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, F.; Petit, A.; Labia, R.; Maveyraud, L.; Samama, J.-P.; Masson, J.-M. Site-Directed Mutagenesis of β-Lactamase TEM-1. Eur. J. Biochem. 1993, 217, 939–946. [Google Scholar] [CrossRef] [PubMed]
| 1 | Both systems resulted in the production of soluble, pure enzyme. However, preliminary assays indicated that only the MBP-tagged enzymes displayed significant β-lactamase activity and were hence used for more detailed catalytic characterisation. Any attempts to remove the MBP tag by proteolytic cleavage resulted in precipitation of the viral MBL proteins. Unless otherwise specified, the terms ApycGoe3 and ApycGrass correspond to the MBP-tagged constructs. |
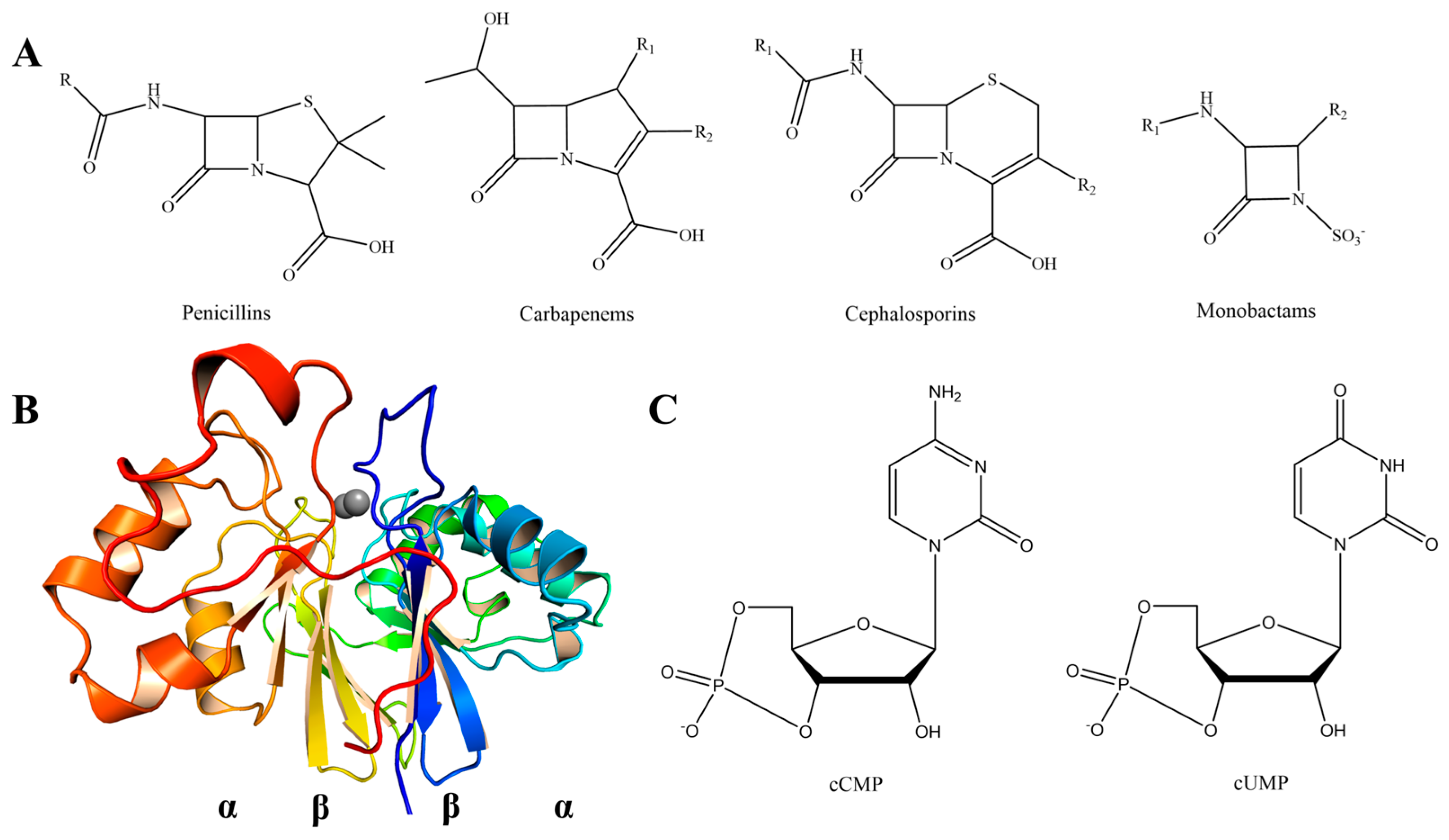
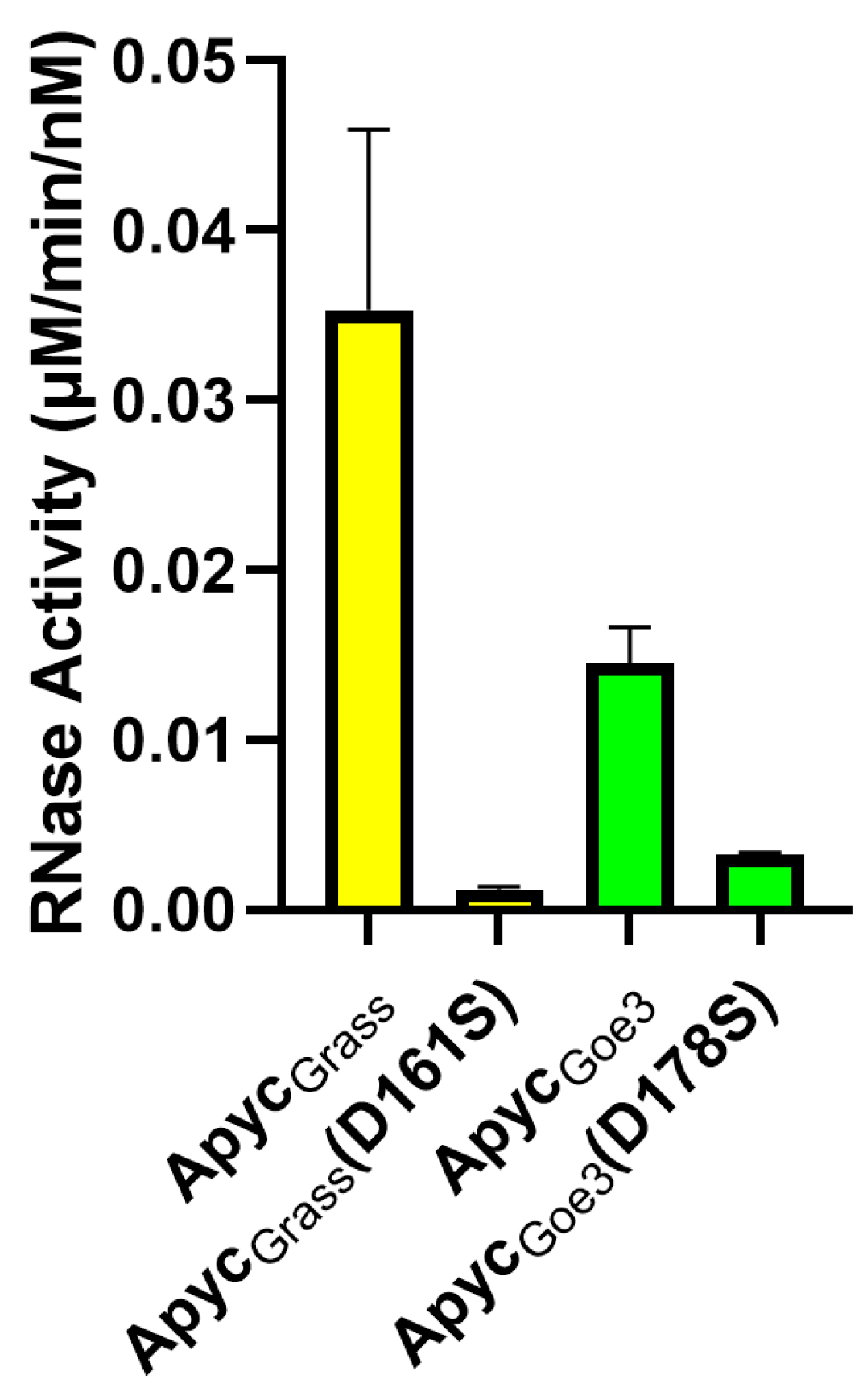
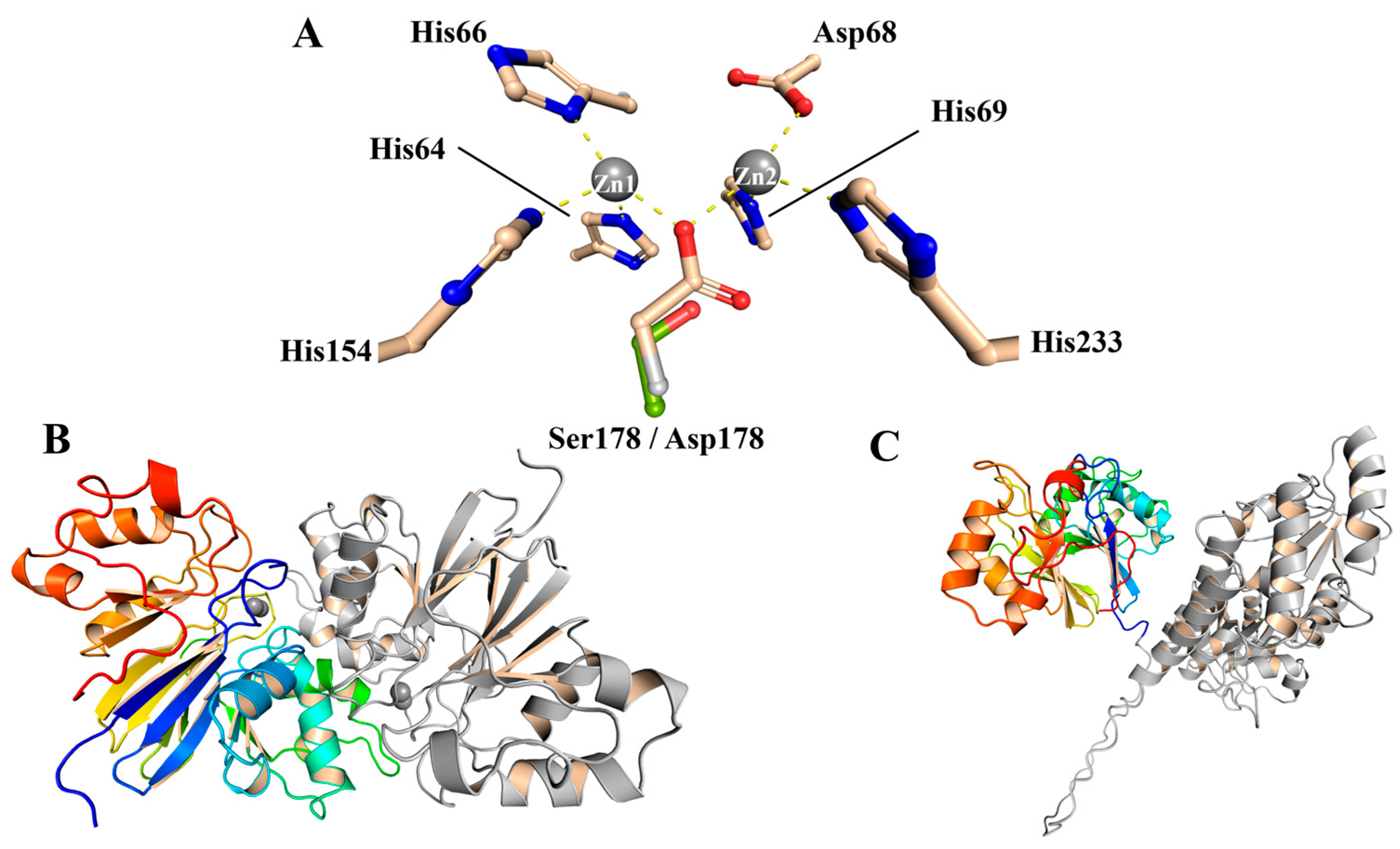
| ApycGoe3 | ApycGoe3 (D178S) | ApycGrass | ApycGrass (D161S) | PNGM-1a | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | kcat | KM | kcat/KM | kcat | KM | kcat/KM | kcat | KM | kcat/KM | kcat | KM | kcat/KM | kcat | KM | kcat/KM | ||||||||||||
| Penicillins | |||||||||||||||||||||||||||
| Penicillin G | 0.24 | 230 | 1.04 | 0.45 | 751 | 0.60 | 0.67 | 231 | 2.90 | 0.48 | 180 | 2.70 | 7.5x10-2 | 16 | 4.7 | ||||||||||||
| Ampicillin | 1.8 | 663 | 2.67 | 0.72 | 526 | 1.37 | 0.54 | 418 | 1.29 | 0.39 | 891 | 0.44 | 2.7x10-2 | 15 | 1.8 | ||||||||||||
| Carbenicillin | 0.32 | 205 | 1.56 | 0.13 | 157 | 0.83 | 0.15 | 330 | 0.45 | 0.24 | 462 | 0.52 | - | - | - | ||||||||||||
| Carbapenems | |||||||||||||||||||||||||||
| Meropenem | 0.22 | 215 | 1.02 | 0.43 | 160 | 2.69 | 1.1 | 98 | 11.2 | 4.4 | 287 | 15.3 | 8.0x10-4 | 2 | 0.42 | ||||||||||||
| Imipenem | 0.27 | 436 | 0.62 | - | - | - | 3.1 | 200 | 15.5 | 4.2 | 253 | 16.6 | 1.1x10-3 | 2 | 0.55 | ||||||||||||
| Cephalosporins | |||||||||||||||||||||||||||
| Cefuroxime | 8.6x10-3 | 18 | 0.48 | 8.3x10-3 | 3.5 | 2.37 | 5.3x10-3 | 44 | 0.12 | 6.0x10-3 | 142 | 0.042 | - | - | - | ||||||||||||
| Cephalothin | 3.0x10-3 | 19 | 0.16 | 7.0x10-3 | 23 | 0.30 | 5.6x10-3 | 51 | 0.11 | 1.3x10-3 | 54 | 0.024 | 0.13 | 62 | 2.1 | ||||||||||||
| Monobactams | |||||||||||||||||||||||||||
| Aztreonam | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | N.H. | - | - | - | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





