Submitted:
11 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
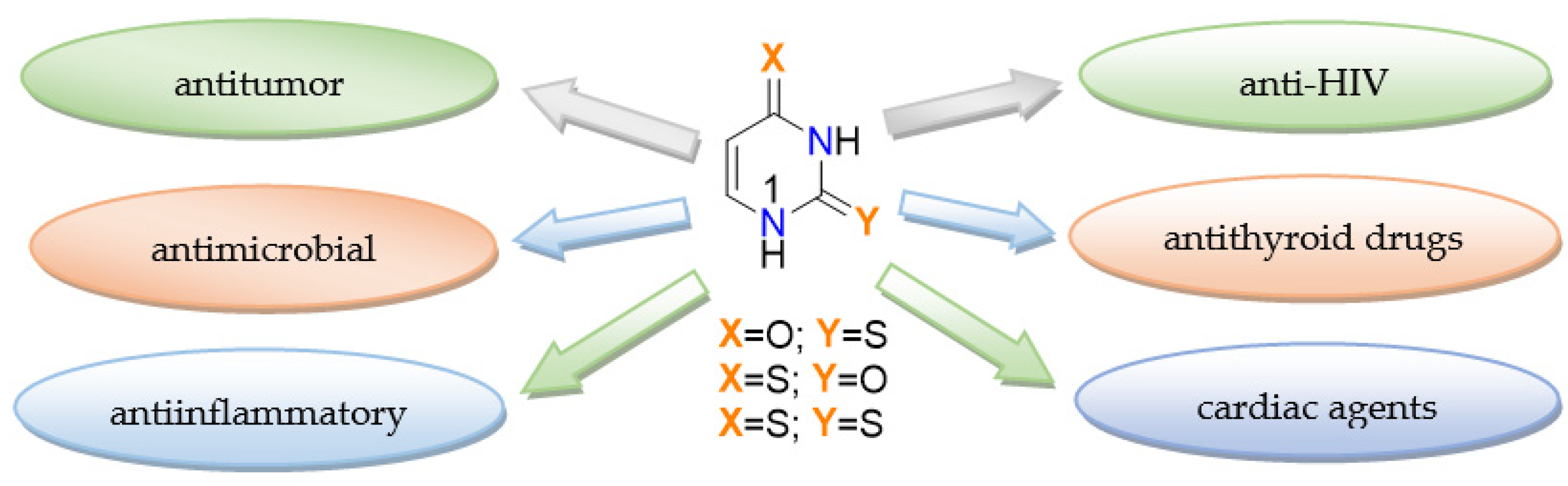
1. Introduction
2. Materials and Methods
2.1. Spectra Measurements
2.2. MP-AES Determination of Cu and Au and ICP-OES Determination of S in the Complexes
2.3. Synthesis of Cu(II) and Au(III) Complexes of 2,4-Dithiouracil (L) – General Procedure
2.3.1. Synthesis of Cu(II)L and Au(III)L
2.4. Spectral Data of the Free Ligand and Its Metal Complexes
2.5. Antimicrobial Assay
3. Results and Discussion
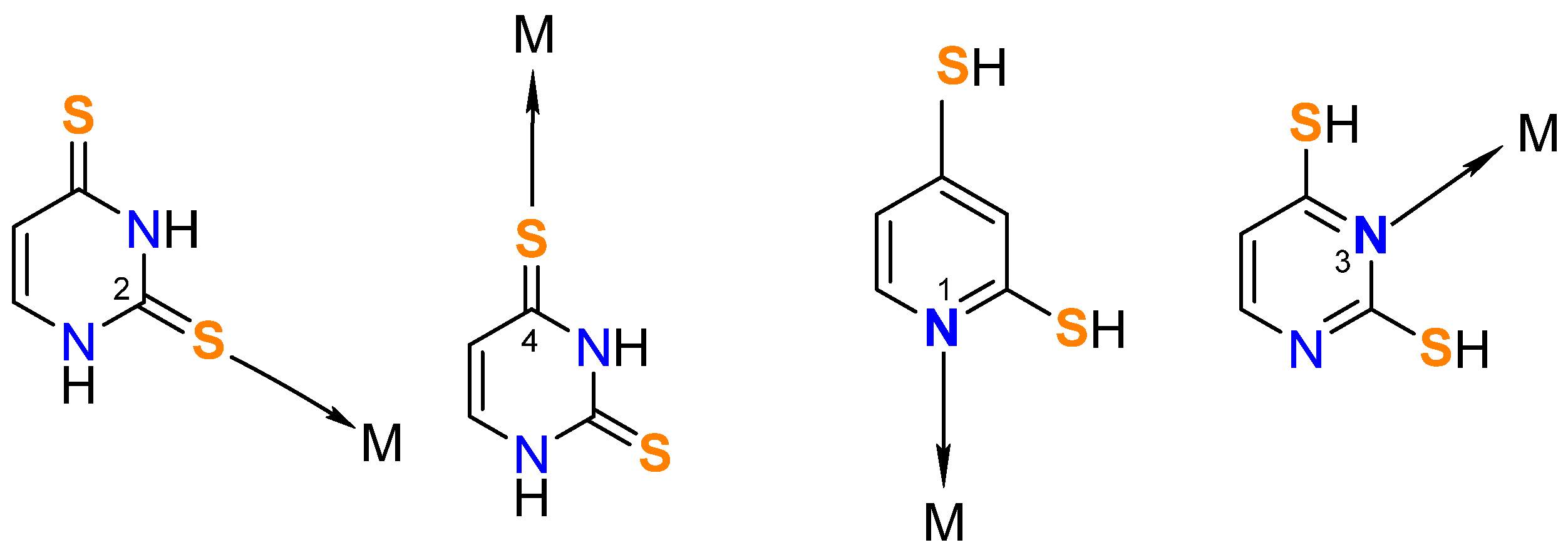
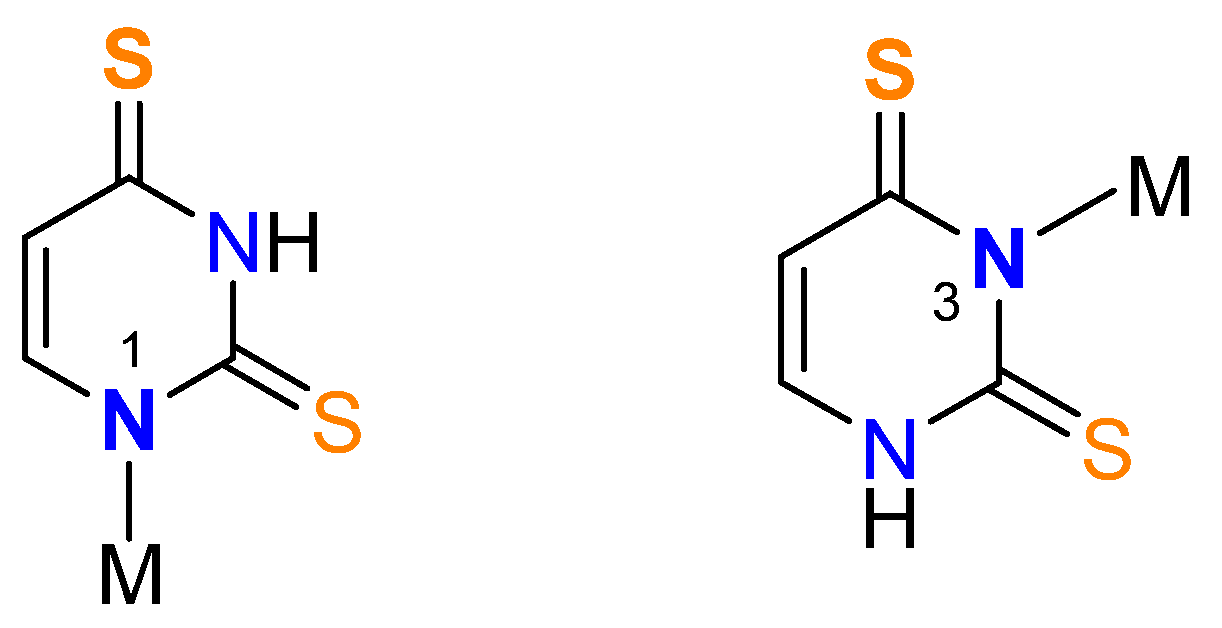
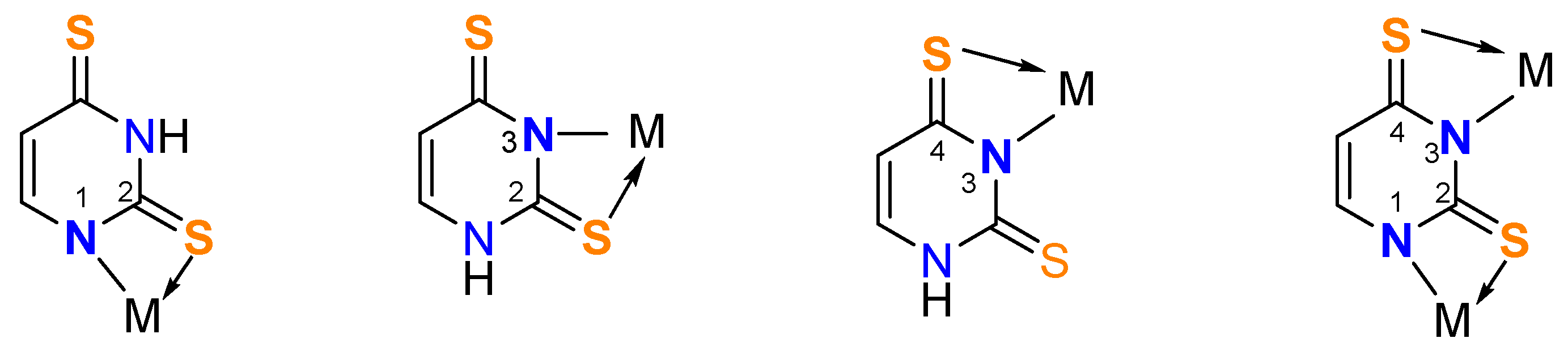
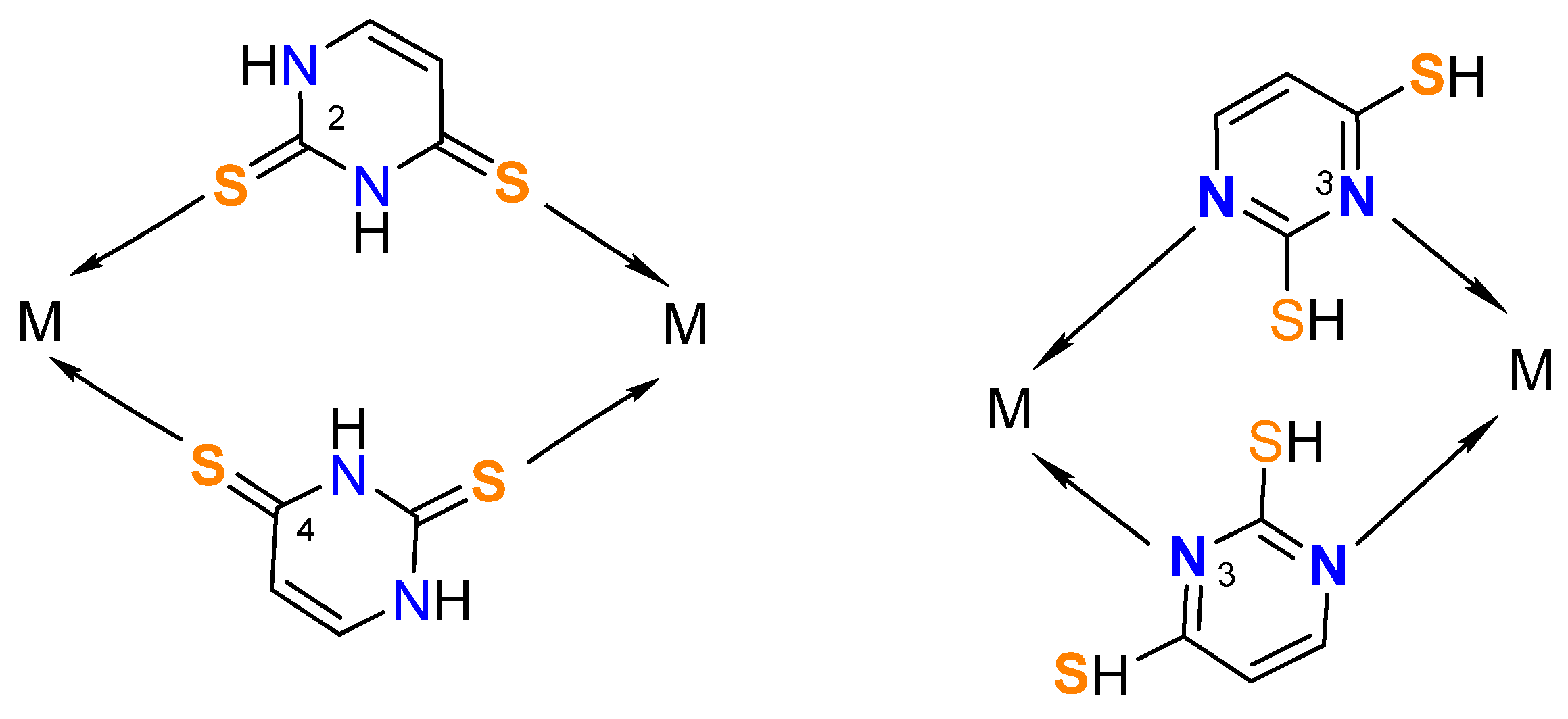
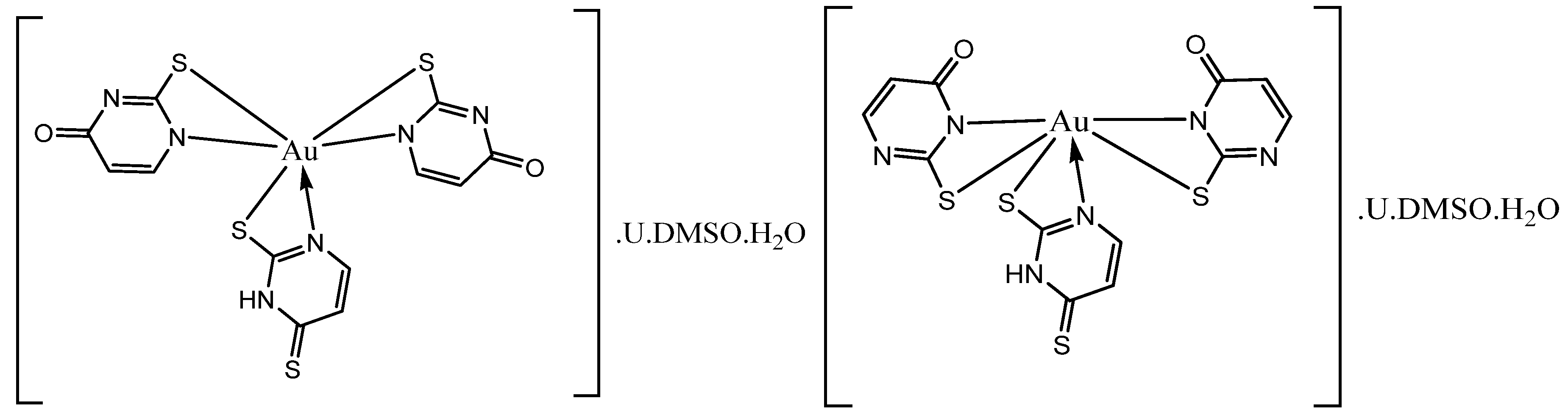
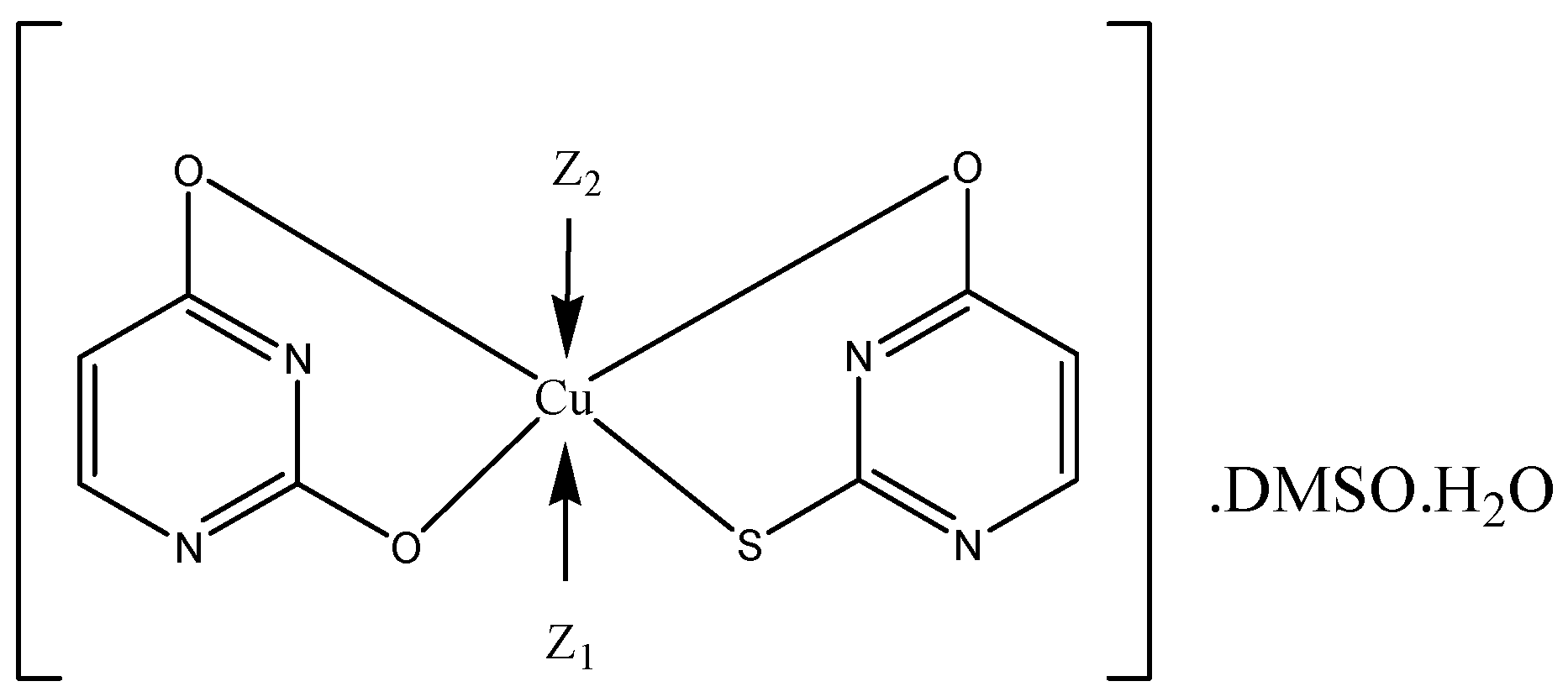
3.1. Synthesis of the Metal Complexes
3.2. Antimicrobial Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Yadav, R.A. Raman and IR studies and DFT calculations of the vibrational spectra of 2,4-Dithiouracil and its cation and anion. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 130, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ruckenbauer, M.; Mai, S.; Marquetand, P.; González, L. ; González, L. Photoelectron spectra of 2-thiouracil, 4-thiouracil, and 2,4-dithiouracil. J. Chem. Phys. 2016, 144, 074303. [Google Scholar] [CrossRef] [PubMed]
- Shefter, E.; Mautner, H. G. The Crystal and Molecular Structure of 2,4-Dithiouracil. J. Am. Chem. Soc. 1967, 89, 1249–1253. [Google Scholar] [CrossRef]
- Leszczyiiski, J.; Lammertsma, K. 2,4-Dithiouracil tautomers: structures and energies. J. Phys. Chem., 1991, 95, 3128–3132. [Google Scholar] [CrossRef]
- Palafox, M. A.; Benial, A. M. F.; Rastogi, V. K. Biomolecules of 2-Thiouracil, 4-Thiouracil and 2,4-Dithiouracil: A DFT Study of the Hydration, Molecular Docking and Effect in DNA:RNA Microhelixes. Int. J. Mol. Sci., 2019, 20, 3477–3507. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H. C.; Villa, J. F. Copper(II) Complexes of 2-Thiocytosine and 2,4-Dithiouracil. Inorg. Chim. Acta, 1979, 34, L235–L237. [Google Scholar] [CrossRef]
- Lamsabhi, A. M.; Alcamí, M.; Mó, O.; Yáñez, M.; Tortajada, J. Association of Cu2+ with Uracil and Its Thio Derivatives: A Theoretical Study. Chem. Phys. Chem. 2004, 5, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Lamsabhi, A.M.; Alcamí, M.; Mó, O.; Yáñez, M. Gas-phase reactivity of uracil, 2-thiouracil, 4-thiouracil, and 2,4-dithiouracil towards the Cu+ cation: a DFT study. Chem. Phys. Chem. 2003, 4, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.K. , Alcolea Palafox, M., Singh, C., Gupta, S.L. 1999. Synthesis and characterisation of complex of Cu(II) with 5-carboxy-2-thiouracil. In: Greve, J., Puppels, G.J., Otto, C. (eds) Spectroscopy of Biological Molecules: New Directions. Springer, Dordrecht.
- Singh U., P. , Singh S., Singh S. M., Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil. Metal-Based Drugs 1998, 5, 35–39. [Google Scholar] [CrossRef]
- Papazoglou, I.; Cox, P.J.; Hatzidimitriou, A.G.; Kokotidou, C.; Choli-Papadopoulou, T.; Aslanidis, P. Copper(I) halide complexes of 5-carbethoxy-2-thiouracil: Synthesis, structure and in vitro cytotoxicity. Eur. J. Med. Chem. 2014, 78, 383–391. [Google Scholar] [CrossRef]
- Worachartcheewan, A.; Pingaew, R.; Lekcharoen, D.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, Antioxidant and Antimicrobial Activities of Metal Complexes of 2-thiouracil-hydroxyquinoline Derivatives Lett. Drug Des. Discov. 2018, 15, 602–611. [Google Scholar] [CrossRef]
- Shaikh, M. N.; Al-Maythalony, B. A.; Wazeer, M. I. M.; Isab, A. A. Complexations of 2-thiouracil and 2,4-dithiouracil with Cd(SeCN)2 and Hg(SeCN)2: NMR and anti-bacterial activity studies. Spectroscopy, 2011, 25, 187–195. [Google Scholar] [CrossRef]
- Cookson, P.D.; Tiekink, E.R.T.; Whitehouse, M.W. Phosphinegold(I) complexes Containing the Purine-6-thiolate Anion, and their Antiart hritic Activity. Aust. J. Chem. 1994, 47, 577–586. [Google Scholar] [CrossRef]
- Gimeno, M.C.; Laguna, A. Some recent highlights in gold chemistry. Gold Bull 2003, 36, 83–92. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Y. Conformations of 2-thiouracil in the aqueous solution and its adsorption behavior on the gold substrates explored by DFT calculations and experimental methods, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 134, 399–405. [CrossRef]
- Lorenzana-Vázquez, G.; Pavel, I.; Meléndez, E. Gold Nanoparticles Functionalized with 2-Thiouracil for Antiproliferative and Photothermal Therapies in Breast Cancer Cells. Molecules 2023, 28, 4453. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreira, V.; Herrera, R. P.; Gimeno, M. C. Anticancer properties of gold complexes with biologically relevant ligands. Pure Appl. Chem, 2018. [Google Scholar] [CrossRef]
- Vicente, J.; Chicote, S. M.-T.; Gonzalez-Herrero, P.; Jones P., G. Complexes with S-Donor Ligands. Part 2. Synthesis of Anionic Bis(thiolato)gold(I) Complexes. Crystal Structure of [N(PPh3)2][Au(SR)2] (R = benzoxazol-2-yl)+. J. Chem. Soc. Dalton Trans. 1994; 3183–3187. [Google Scholar] [CrossRef]
- Seifert, T. P.; Naina, V. R.; Feuerstein, T. J.; Knöfel, N. D.; Roesk P., W. Molecular gold strings: aurophilicity, luminescence and structure–property correlations. Nanoscale 2020, 12, 20065–20088. [Google Scholar] [CrossRef]
- Lusty, J.R.; Chan, H.S.O.; Peeling, J. The synthesis and characterisation of dithiouracil complexes of rodium(III), irdium(III), palladium(II) and platinum(II). Transition Met. Chem. 1983, 8, 343–345. [Google Scholar] [CrossRef]
- Vetter, C.; Kaluderovic, G. N.; Paschke, R.; Kluge, R.; Schmidt, J.; Steinborn, D. Synthesis, characterization and in vitro cytotoxicity studies of platinum(IV) complexes with thiouracil ligands. Inorg.Chim. Acta 2010, 363, 2452–2460. [Google Scholar] [CrossRef]
- Crestoni, M. E.; Corinti, D.; Chiavarino, B.; Fornarini, S.; Scuderi, D.; Salpin, J.-Y. Insights into Cisplatin Binding to Uracil and Thiouracils from IRMPD Spectroscopy and Tandem Mass Spectrometry. J. Am. Soc. for Mass Spectrometry, 2020, 31, 946–960. [Google Scholar]
- Kamalakannan, P.; Venkappayya, D.; Balasubramanian, T. A new antimetabolite, 5-morpholinomethyl-2-thiouracil—spectral properties, thermal profiles, antibacterial, antifungal and antitumour studies of some of its metal chelates. J. Chem. Soc. Dalton Trans. 2002, 3381–3391. [Google Scholar] [CrossRef]
- Marinova, P.; Tsoneva, S.; Frenkeva, M.; Blazheva, D.; Slavchev, A.; Penchev, P. New Cu(II), Pd(II) and Au(III) complexes with 2-thiouracil: Synthesis, Characteration and Antibacterial Studies, Russ. J. Gen. Chem. 2022, 92, 1578–1584. [Google Scholar] [CrossRef]
- Marinova, P.; Hristov, M.; Tsoneva, S.; Burdzhiev, N.; Blazheva, D.; Slavchev, A.; Varbanova, E.; Penchev, P. Synthesis, Characterization and Antibacterial Studies of new Cu(II) and Pd(II) complexes with 6-methyl-2-thiouracil and 6-propyl-2-thiouracil, Appl. Sci. 2023, 13, 13150. [Google Scholar] [CrossRef]
- Abou-Melha, K. S. A Series of Nano-sized Metal ion-thiouracil Complexes, tem, Spectral, γ- irradiation, Molecular Modeling and Biological Studies. Orient. J. Chem. 2015, 31, 1897–1913. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Youns, M.M.; Ahmed N., M. Synthesis, antimicrobial, antioxidant activities of novel 6-aryl- 5-cyano thiouracil derivatives. Eur. J. Med. Chem, 2013. [Google Scholar]
- Masoud, M. S.; Soayed, A. A.; El-Husseiny A., F. Coordination modes, spectral, thermal and biological evaluation of hetero-metal copper containing 2-thiouracil complexes. Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy 2012, 99, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Singh U., P. , Singh S., Singh S. M., Synthesis, characterization and antitumour activity of metal complexes of 5-carboxy-2-thiouracil. Metal-Based Drugs 1998, 5, 35–39. [Google Scholar] [CrossRef]
- Tyagi, S.; Singh, S. M.; Gencaslan, S.; Sheldrick, W. S.; Singh, U. P. Metal-5-fluorouracil-histamine complexes: solution, structural, and antitumor studies. Metal Based Drugs 2002, 8, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Suman, A. Synthesis, spectroscopic characterization and biological application of copper complex of 5-carbethoxy-2-thiouracil. J. Drug Deliv. Ther. 2020, 10, 145–148. [Google Scholar] [CrossRef]
- Illán-Cabeza, N. A.; García-García, A. R.; Moreno-Carretero, M. N.; Martínez-Martos, J. M.; Ramírez-Expósito M., J. Synthesis, characterization and antiproliferative behavior of tricarbonyl complexes of rhenium(I) with some 6-amino-5-nitrosouracil derivatives: Crystal structure of fac-[ReCl(CO)3(DANU-N5,O4)] (DANU = 6-amino-1,3-dimethyl-5-nitrosouracil). J. Inorg. Biochem. 2005, 99, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Marinova, P. E.; Tamahkyarova, K. D. Synthesis and Biological Activities of Some Metal Complexes of 2-Thiouracil and Its Derivatives: A Review. Compounds 2024, 4, 186–213. [Google Scholar] [CrossRef]
- Christoph Steinbeck, Stefan Kuhn, NMRShiftDB – compound identification and structure elucidation support through a free community-built web database. Phytochemistry, 2004, 65, 2711–2717. [CrossRef]
- A. Novakov, B. S. Orlinson and M. B. Navrotskii, Desulfurization of 2-Thioxo-1,2,3,4-tetrahydropyrimidin-4-ones with oxiranes and 2-Haloacetonitriles. Russian Journal of Organic Chemistry. 2005, 41, 607–609. [Google Scholar] [CrossRef]
- Marinova, P.; Burdzhiev, N.; Blazheva, D.; Slavchev, A. Synthesis and Antibacterial Studies of a New Au(III) Complex with 6-Methyl-2-Thioxo-2,3-Dihydropyrimidin-4(1H)-One. Molbank 2024, 2024, M1827. [Google Scholar] [CrossRef]
- Shareena Dasari, T.P.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold(I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. 2015, 4, 199–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Dasari, T.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 3, 286–327. [Google Scholar] [CrossRef]
- Ghosh, P.; Mukhopadhyay, T. K.; Sarkar, A.R. Interaction of Divalent Metal Ions with Uracil III. Complexes of MnII, FeII, CoII, NiII and CuII with Uracil Acting as Bidentate Ligand, Transition Met. Chem. 1984, 9, 46–48. [Google Scholar] [CrossRef]
| complexes | Colour | Yield (%) | Melting point (°C) | Solubility |
|---|---|---|---|---|
| L | yellow | 279-281 | soluble in DMSO | |
| Cu(II)L | yellow-green | 56 | >350 ᵒC | limited solubility in DMSO, C6H12 and DMF; and insoluble in EtOH, H2O, THF, EtOAc. |
| Au(III)L | beige | 52 | >350 ᵒC | limited solubility in DMSO and DMF; insoluble in H2O, THF, EtOH, EtOAc and C6H12. |
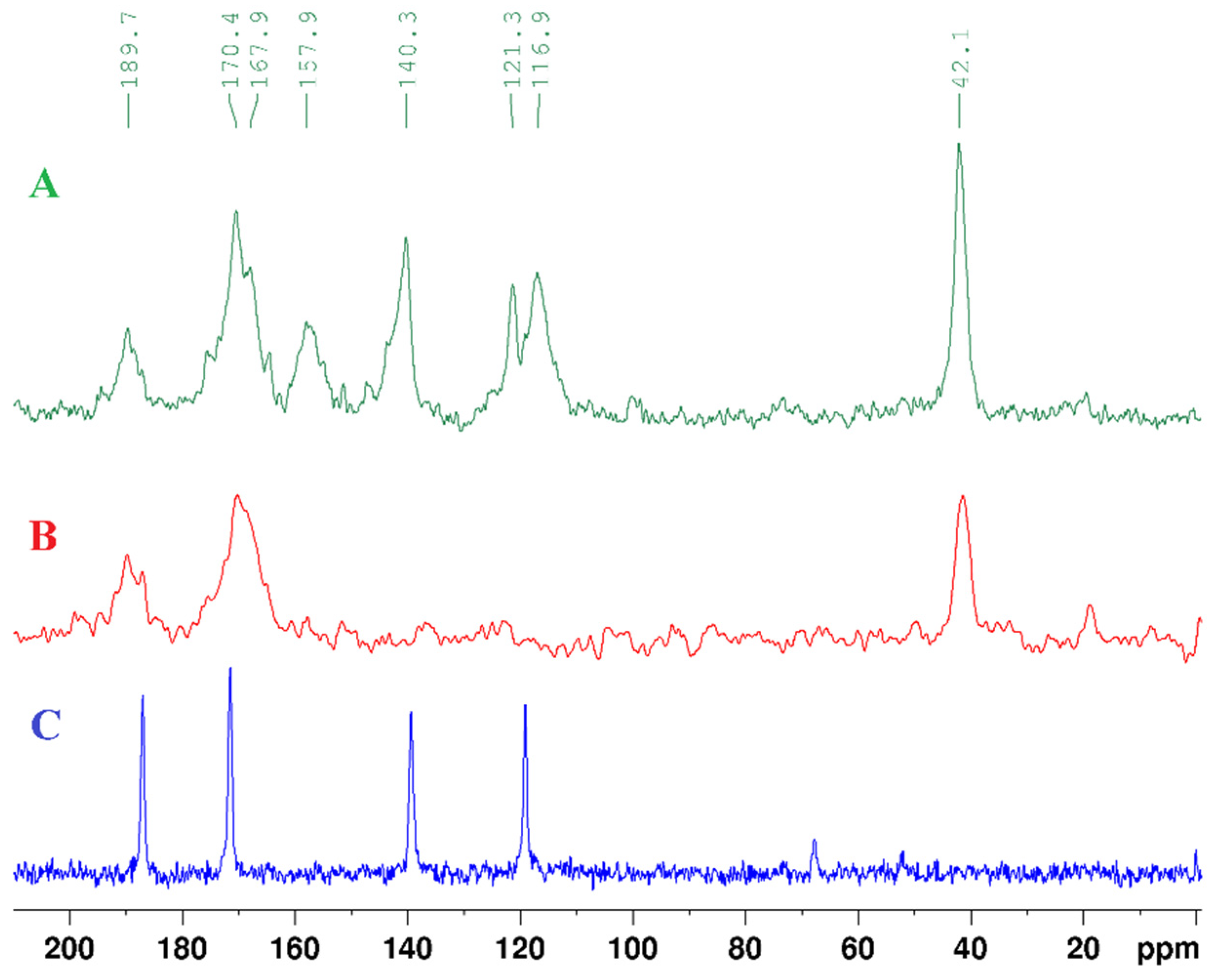
| Atom | δ (13C) ppm | DEPT-135 | δ (1H) ppm | Multiplicity (J, Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|---|---|
| 1 (NH) | 12.90 | s | ||||
| 2 (C=S) | 172.87 | C | ||||
| 3 (NH) | 13.64 | s | ||||
| 4 (C=S) | 187.81 | C | ||||
| 5 | 117.16 | CH | 6.50 | d (7.1) | 6 | 4, 6 |
| 6 | 136.69 | CH | 7.27 | d (7.1) | 5 | 2, 4, 5 |
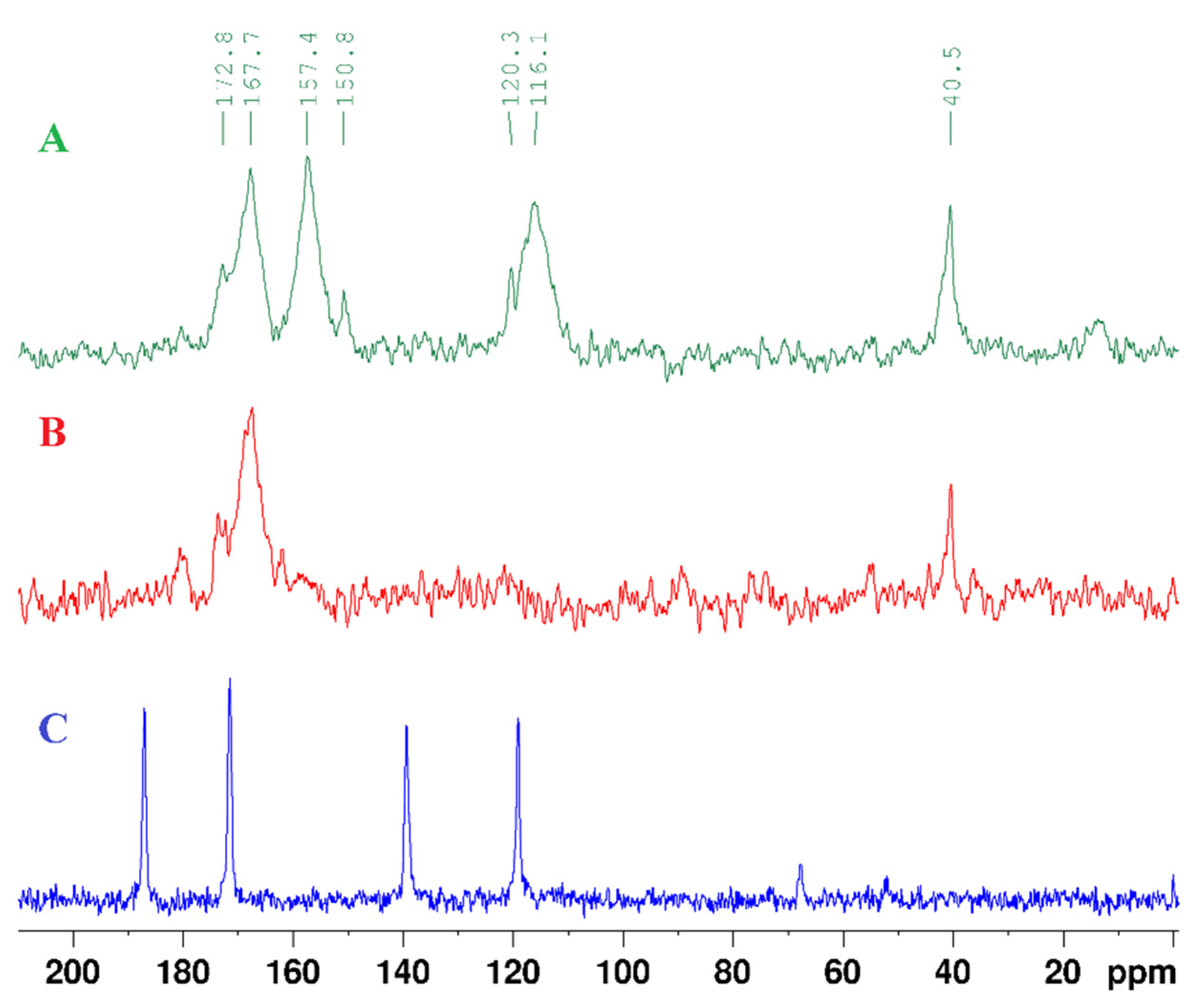
| Atom | 2,4-DTub | 2-Tub | Ub | 2,4-DTu.(2-Tu)2.Auc |
|---|---|---|---|---|
| 1 | 12.88, s | 12.27, s | 10.81, s | 14.13, s (2,4-DTu) |
| 2 | ||||
| 3 | 13.62, s | 12.43, s | 11.00, s | - |
| 4 | ||||
| 5 | 6.51, d(6.7 Hz) | 5.81, d(7.50 Hz) | 5.44, m | 7.10, d(6.8 Hz), (2,4-DTu) 7.39, m, (2-Tu) 7.39, m, (2-Tu) |
| 6 | 7.26, t(6.2 Hz) | 7.39, m | 7.39, m | 7.76, d(6.7 Hz), (2,4-DTu) 8.31, d(4.7 Hz), (2-Tu) 8.31, d(4.7 Hz), (2-Tu) |
| metal complex | composition* | Formula | Moleculаr weight | W(M)% calc./exp. |
W(S)% calc./exp. |
|---|---|---|---|---|---|
| Au(III)L | [2,4-DTu.(2-Tu)2.Au].U.5DMSO.5H2O | C26H51N8O14S9Au | 1185.28 g/mol | 16.62/16.7±1.5 | 24.35/25.2 ± 2.1 |
| Au(III)LCu(II)L | [2,4-DTu.(2-Tu)2.Au].U.2-Tu. 2,4-DTu.2DMSO.3H2O [2-Tu.U.Cu]. 4DMSO.4H2O |
C28H37N12O10S9Au C16H36N4O11S5Cu |
1187.22 g/mol 684.35 g/mol |
16.59/16.7±1.5 9.29/9.23±0.65 |
24.31/25.2±2.1 23.43/21.9 ± 1.8 |
| Atom | 2-Tu b | U b | 2-Tu.U.Cu c |
|---|---|---|---|
| 1 | 12.26, s | 10.80, s | |
| 2 | |||
| 3 | 12.43, s | 11.00, s | |
| 4 | |||
| 5 | 5.81, d(7.3 Hz) | 5.45, d(8.2 Hz) | 7.28, d (5.5 Hz) (U) 7.40 d, m (2-Tu) |
| 6 | 7.38d, m | 7.38 d, m | 8.33, m (U) 8.33, m (2-Tu) |
| Test microorganisms | DMSO | 2,4-DTu | Cu(II)L | Au(III)L | |||
|---|---|---|---|---|---|---|---|
| Inhibition zone, mm | |||||||
| Staphylococcus aureus ATCC 25923 | - | 14 | 15 | 19* | |||
| Escherichia coli ATCC 8739 | - | 14 | 15 | 14 | |||
| Eterococcus faecalis ATCC 19433 | - | 12 | 13 | 16 | |||
| Salmonella enterica ssp. enterica ser. Enetritidis ATCC 13076 | - | 14 | 15 | 15 | |||
| Pseudomonas aeruginosa ATCC 9027 | - | 13 | 12 | 15 | |||
| Proteus vulgaris G | - | 12* | 12* | 14* | |||
| Bacillus subtilis ATCC 6633 | - | 11 | 11 | 13 | |||
| Bacillus cereus ATCC 11778 | - | 11 | 11 | 14* | |||
| Listeria monocytogenes ATCC 8787 | - | 12 | 12 | 14 | |||
| Klebsiella pneumoniae ATCC 13883 | - | 12* | 11* | 15 | |||
| Candida albicans ATCC 10231 | - | 10 | 11* | 12 | |||
| Saccharomyces cerevisiae | - | 11 | 10 | 12 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





