1. Introduction
Maternal glucose metabolism plays a crucial role in facilitating intrauterine growth. Gestational diabetes mellitus (GDM), a form of diabetes that develops during pregnancy, is characterized by elevated blood sugar levels. It affects approximately 7% of all pregnancies and poses increased risks for both the mother and the fetus. GDM is thought to arise from a combination of insulin resistance and impaired insulin secretion, resulting in hyperglycemia and metabolic abnormalities. The risk factors associated with GDM include obesity, advanced maternal age, a family history of diabetes, and certain ethnicities. GDM can lead to adverse outcomes, including preeclampsia, preterm birth, fetal macrosomia, and neonatal hypoglycemia, affecting the health of both the mother and the baby [
1,
2,
3,
4].
The Zebrafish (Danio rerio) is an important model organism extensively used for studying metabolic mechanism developmental biology [
5,
6] and genetics study [
7,
8,
9] due to its small size, optical transparency, easy treatment, low cost caring of the embryo. The zebrafish embryonic developmental and post embryonic zebrafish development are well established to further study of genetic changes that showed in the morphological changes [
10,
11]. Glucose plays a crucial role in the development of zebrafish embryos as it serves as a major energy source for cellular processes. Glucose is metabolized through several pathways in zebrafish embryos, including glycolysis, the pentose phosphate pathway, and the tricarboxylic acid (TCA) cycle. The metabolic profiling of zebrafish embryo development study showed the glucose played a vital role during early stage of zebrafish development [
12,
13,
14,
15,
16,
17].
Given the critical role of glucose in these metabolic pathways, alterations in glucose availability or metabolism can have significant impacts on zebrafish embryo development. Disruptions in glycolysis or the TCA cycle can lead to decreased ATP production, which can negatively impact cellular processes such as cell division and differentiation. Additionally, alterations in the pentose phosphate pathway can impact NADPH availability, which can impact biosynthesis and oxidative stress defense mechanisms [
16,
17].
The Wnt signaling pathway is an important regulator of embryonic development in many organisms, including zebrafish [
18,
19,
20,
21,
22]. This pathway is involved in a wide range of processes, such as cell fate specification, cell proliferation, differentiation, and tissue patterning [
23]. In zebrafish, the Wnt signaling pathway is particularly important in early embryonic development, where it plays a key role in the formation and patterning of the dorsal-ventral (DV) axis. Specifically, the Wnt pathway is involved in the specification of the organizer tissue, which is responsible for the induction of the embryonic axes and subsequent tissue patterning. During early embryonic development, the Wnt pathway is activated in the dorsal region of the embryo, where it induces the expression of organizer-specific genes, such as chordin and noggin. These organizer factors, in turn, inhibit the activity of bone morphogenetic proteins (BMPs) that are involved in ventral patterning, leading to the establishment of the DV axis. Furthermore, the Wnt pathway is also involved in cell proliferation and differentiation during embryonic development. For example, in the developing nervous system, Wnt signaling is required for neural progenitor proliferation, differentiation, and axon guidance [
21]. The Wnt signaling pathway plays a critical role in zebrafish embryonic development, regulating cell fate specification, tissue patterning, and differentiation. Alterations in this pathway can have significant impacts on embryonic development and can lead to various developmental abnormalities [
15,
16,
17,
18,
19].
With the development of sequencing technologies, genetic studies have been dramatically increased to discover the role of key genes and pathway networks during zebra fish development [
7,
8,
9]. Epigenetic studies reveal other key factors in early development including the epigenetic changes in maternal genes [
24,
25].
The mechanism of glucose effects on zebrafish development is still need more research to understand. In this study, we identified genes and pathways regulated under high glucose treatment using whole genome expression analysis of RNA in zebrafish embryos. Gene ontology analysis to identify functional groups of differentially expressed genes. Bioinformatic analyses of our data confirm the impact of high glucose condition that mimic the GMD on embryonic development
2. Results
2.1. Effect of Glucose on Zebrafish Embryo Development
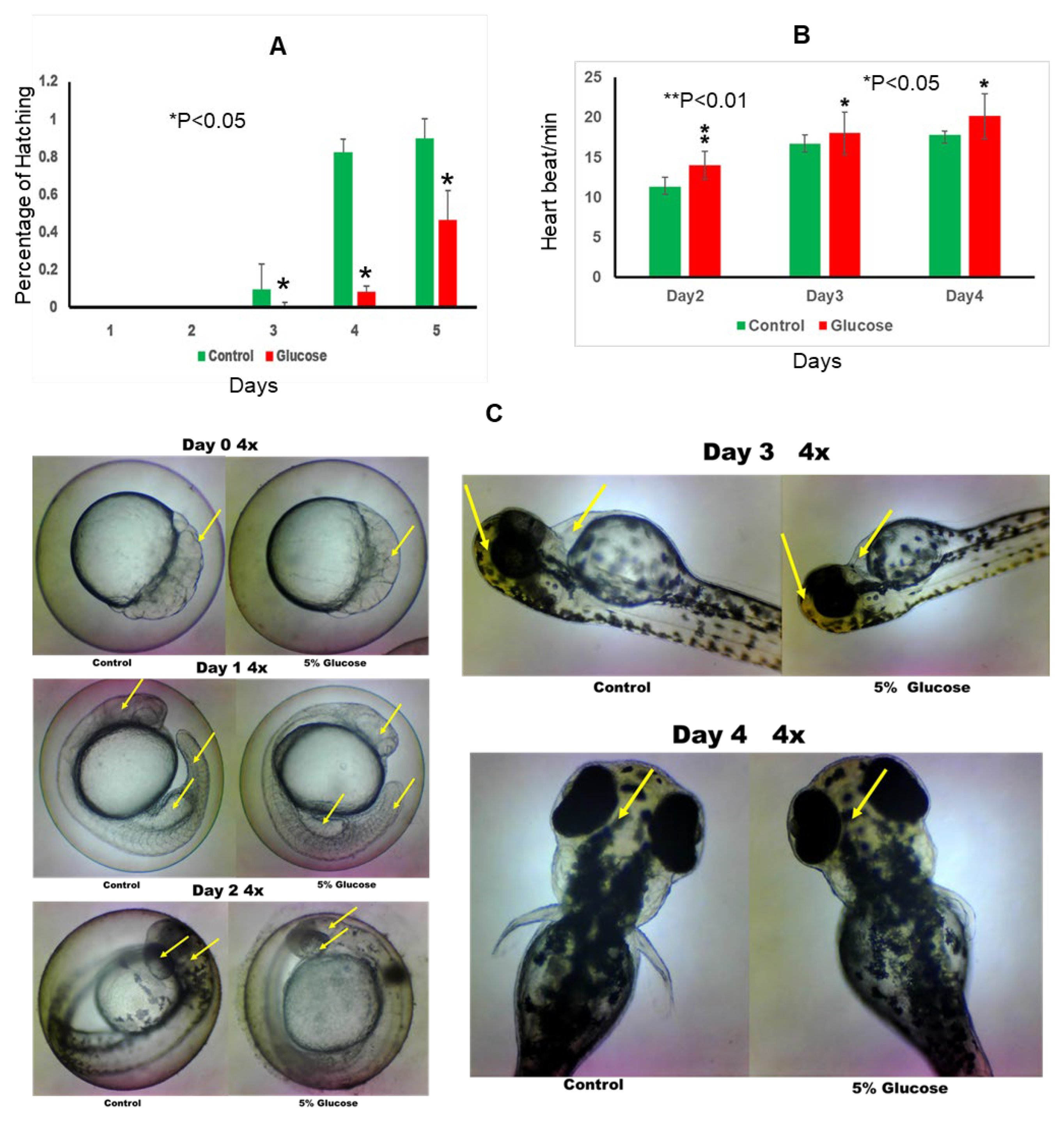
Glucose exposure significantly impacted the developmental timeline of zebrafish embryos. Notably, the hatch rate was delayed by approximately 24 hours in the glucose-exposed group compared to the control group, indicating a retardation in embryonic development associated with glucose exposure.(
Figure 1A)
Evaluation of heart rate revealed significant alterations induced by glucose exposure. On both day 2 and day 3 post-fertilization, embryos in the glucose group exhibited a faster heart rate compared to those in the control group. This difference was statistically significant, highlighting the potential influence of glucose on cardiovascular development in zebrafish embryos (
Figure 1B).
Analysis of morphological parameters revealed distinct differences between the glucose-exposed and control groups. At 24 hours post-fertilization (hpf), embryos in the glucose group exhibited smaller brain, heart, and tail morphology compared to those in the control group. This suggests that glucose exposure may interfere with the normal growth and development of these vital organs during early embryogenesis (
Figure 1C).
The hatch rate with control and glucose groups
The heart rate with control and glucose groups
The morphology changes with control and glucose groups
2.2. RNA-seq Analysis Displayed Significant Gene Dysregulation in the High Glucose Treated Embryos
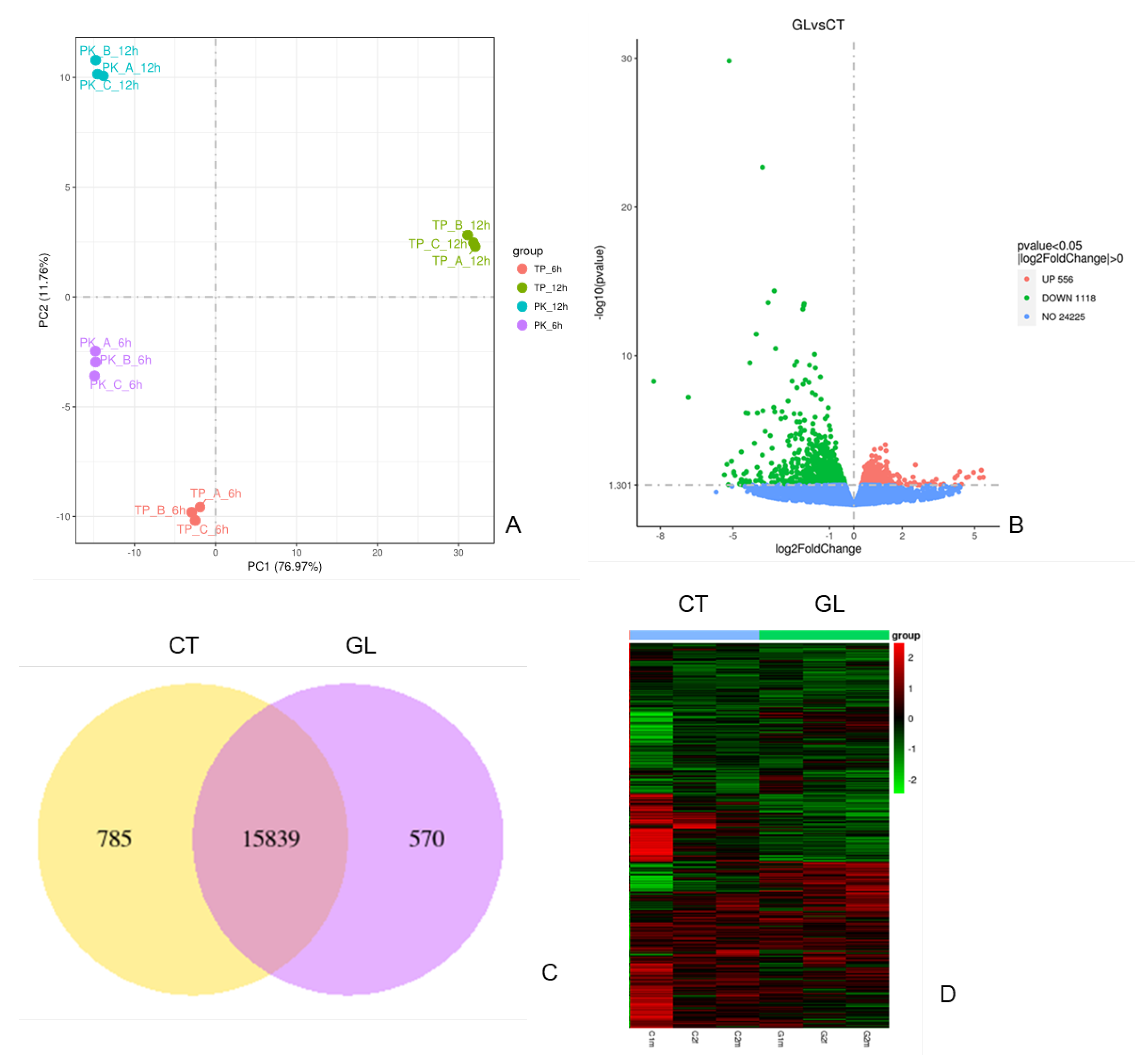
In this study, we investigated the global transcriptome changes in high glucose treated zebrafish embyos using RNAseq. Biological triplicates were performed for the RNA seq. Principle components analysis shows low variability among triplicates (
Figure 2A). With about 25K transcripts, data analysis identified 1674 (6.5%) differentially expressed genes (DEGs) with 556 upregulated and 1118 downregulated respectively (fold chang>2 and p<0.05). (
Figure 2B) Venn Diagram shows the overlap genes and unique genes in these two groups data. (
Figure 2C). Gene expression differs between the control and high glucose treatement group shown in
Figure 2D heatmap.
PCA analysis within treatment groups
Volcano plots for gene changes. Upregulated genes are showed in red and downregulated genes as showing in green.
Gene differentially expressin in control and glucose groups
Heatmap showing gene expression intensity. Red showing higher experssion and green showing lower expression in RNA-seq levels.
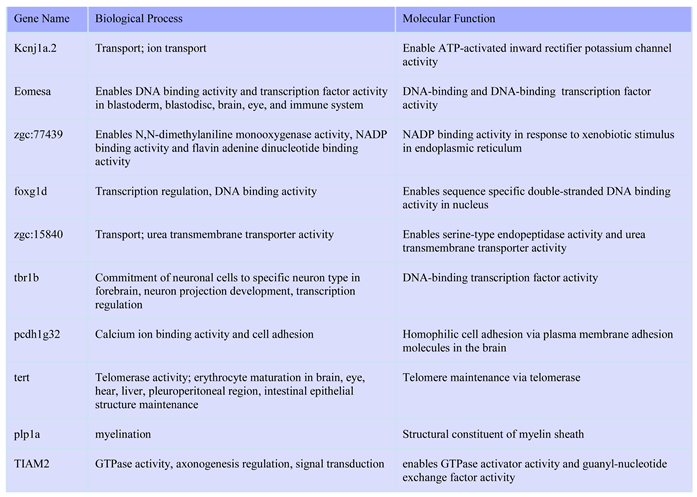
To determine changes in expresson levels of individual genes, we identified the top 10 most significant up and down regulated genes are listed in tables 1 and 2. The major GO biological precesses and molecular functions enriched from the top 10 upregulated genes included extracellular region part,extracellular space,DNA binding transcription factor activity,transcription regulator activity,protein dimerization activity,and NAD binding, and within the top 10 downregulated gnees, enriched processes and molecular functions included oxidation-reduction process,cofactor biosynthetic process,cofactor metabolic process,oxidoreductase activity,oxidoreductase activity, acting on the CH-NH2 group of donors,cofactor binding,oxidoreductase activity, acting on peroxide as acceptor,antioxidant activity,oxidoreductase activity, acting on the CH-NH2 group of donors, oxygen as acceptor.
Table 1.
Top 10 up-regulated genes in glucose treated zebrafish embryos in RNA Seq.
Table 1.
Top 10 up-regulated genes in glucose treated zebrafish embryos in RNA Seq.
Table 2.
Top 10 up-regulated genes in glucose treated zebrafish embryos in RNA Seq.
Table 2.
Top 10 up-regulated genes in glucose treated zebrafish embryos in RNA Seq.
| Gene` |
Biological Process |
Molecular Function |
| apoda.2 |
lipid metabolic process, response to reactive oxygen species, aging, lipid transport |
Enables lipid binding activity |
| lct |
Carbohydrate metabolic processes, hydrolase activity |
Hydrolyzes O-glycosyl compounds |
| tnfrsf11b |
Response to mechanical stimulus |
Tumor necrosis factor receptor; head and scale |
| opn5 |
Absorption of visible light |
Enables 11-cis retinal binding activity and all-trans retinal binding activity |
| rgra |
G protein-coupled receptor signaling, cellular response to light stimulus and phototransduction |
Enables G protein-coupled photoreceptor activity |
| zgc:153154 |
Small molecule binding in extracellular region |
Small molecule binding activity |
| cd74b |
Ear development and neuromast development |
MHC class II protein binding activity, cytokine receptor activity, macrophage migration inhibitory factor binding activity |
| zgc:153911 |
T cell receptor signaling, cytokine production regulation |
Signaling receptor binding activity in membrane |
| pah |
Cellular response to estrogen stimulus in structures incl. Digestive system, epidermis, eye, optic vesicle, and periderm |
Phenylalanine 4-monooxygenase activity |
| rasef |
GDP and GTP binding in cytosol and perinuclear region of cytoplasm |
Enables GDP and GTP binding activity |
2.3. Functional Analysis of DEGs Using Gene Ontology(GO)Analysis
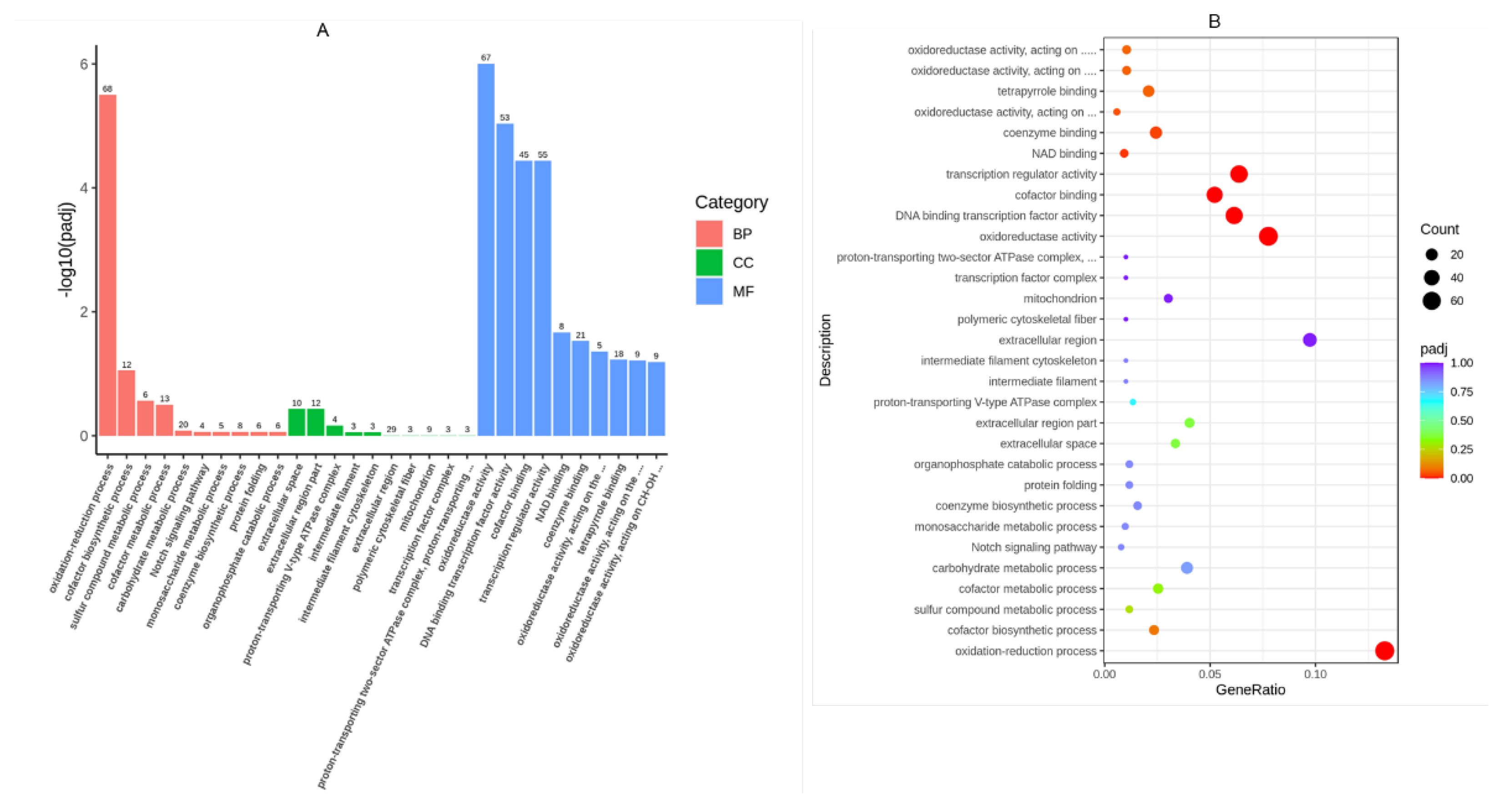
In order to identify the functional associations of the DEGs, Gene Ontology (GO) enrichment analysis was performed by the cluster Profiler R package, in which gene length bias was correct-ed. GO terms withcorrected Pvalue less than 0.05 were considered significantly en-riched by differentialexpressed genes. Aamong 410 categaries of GO analysis results, 6 categaries are significant. Two Molecular Function (MF) and four Cellular Component (CC) were identified from up regulated DEGs. Among 683 categaries of GO analysis results, 9 categaries are significant. 3 Biological process(BP) and 6 Molecular Function (MF) were identified from down regulated DEGs.
Figure 3A shows the GO analysis for both up and down regulated DEGs. Both set of genes are involved Molecular function. Oxidatase reductive activity are the main meoleular funcion changes in down regulated genes. For the biological process, it mailly involved in reduction and oxidation and cofactor binding activities. In the up regulated genes, the main molecular function is on transcription and protein change level. For the cell component, it mainly focus on extracelullar components.
GO terms for significantly enriched Des based on molecular functions
Scatter plot showing top 30 enriched KEGG pathways in control and high glucose groups
2.4. KEGG Pathway Analysis
We analyzed the biological pathways using Kyoto Encyclopedia of Genes and Genomes (KEGG). KEGG is a database resource for understanding high-level functions andutilities of the biological system, such as the cell, the organism and the ecosystem, frommolecular-level information, especially large-scale molecular datasets generated by genomesequencing and other high-through put experimental technologies(
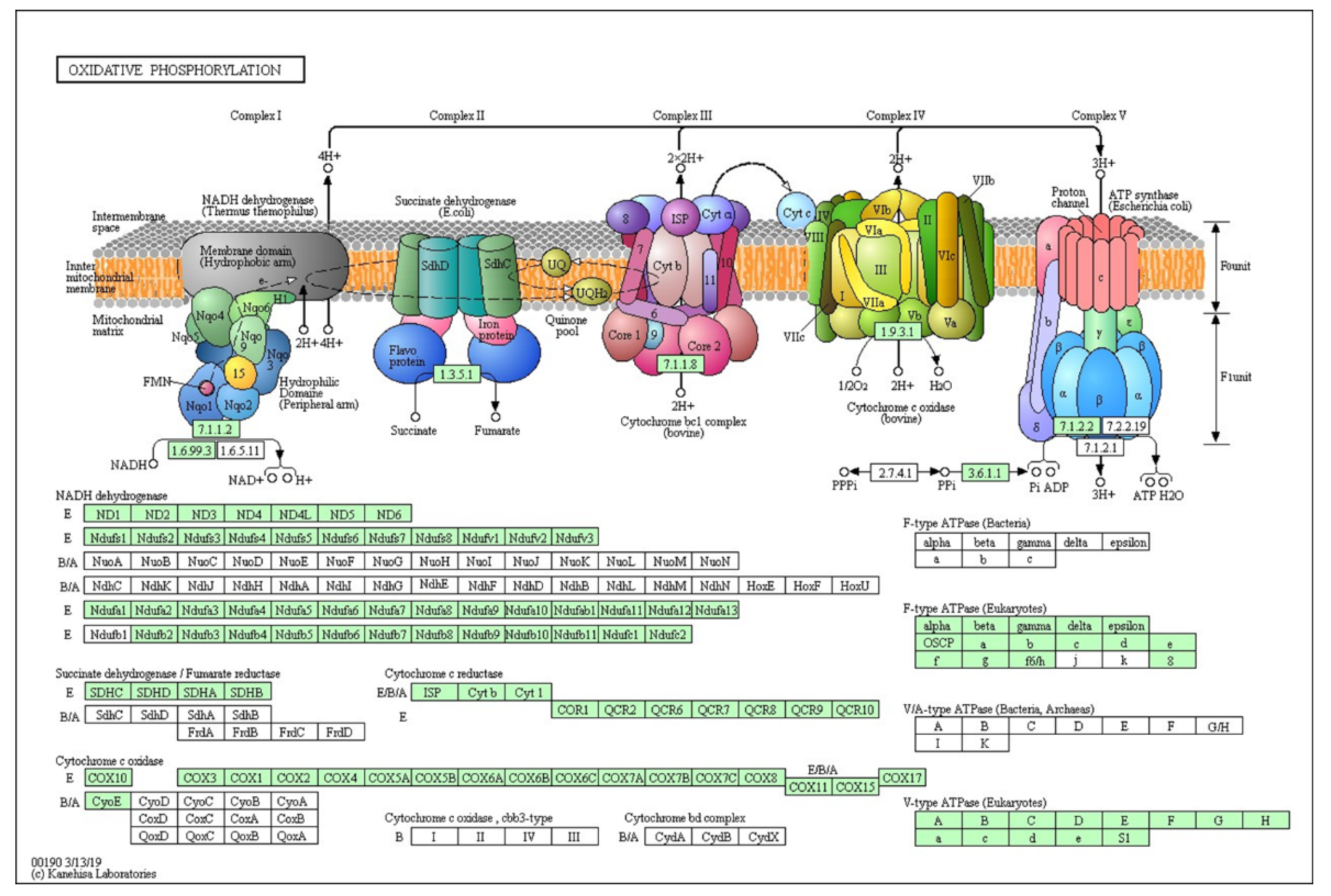
http://www.genome.jp/kegg/). 725 out of 1118 downregulated DEGs were found in KEGG.Where 137 pathways were involved at least one gene. The significant enrichment for pathways in KEGG was 8 pathways includingfive matebolism pathways and 1 p53 pathway and one and one PPAR pathway and one ABC transposters pathway. 236 genes out of 556 up regulated DEGs were found in KEGG where 96 pathway were involved at least one DEGs. The significant KEGG enrichment has Wnt and Notch signaling pathways. These pathways are highly consistent with the development of Zebrafish embryos and the metabolism system during development.
Figure 3B shows the enriched pathways in up and down regulated DEGs.
97 out of 161 genes were down regulated in oxidative phosphorylation pathway (
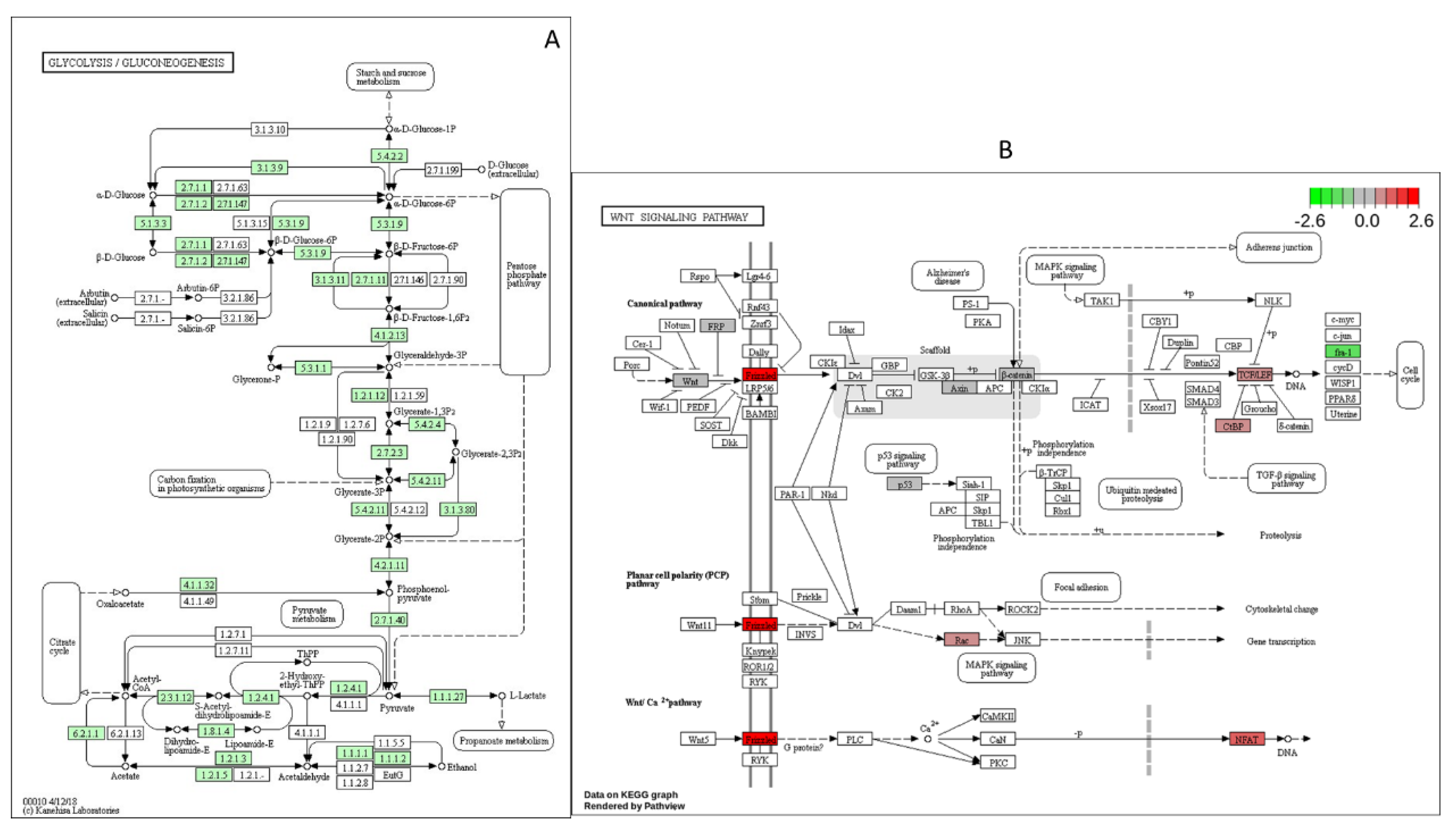
Figure 4). 35 out of 60 genes were down regulated in glycolysis/gluconeogeneisis pathway (
Figure 5A). These data are consistant with previou studies that glucose played vital role at metabolism during zebrafish embronic development. Frizzed was significatly up regulated while Fra1 was signicantly down regulated in Wnt/β-catenin pathway (
Figure 5B).
3. Discussion
In this study, we used 5% D-glucose that mimic the wide fluctuations in glycaemia experienced by human. Following high glucose treatment, zebrafish embryos exhibited the size change, the heart beats ration changes, hatch rates and survival rates changes. In human cases, the offspring born from mothers with GDM were more obese and conveyed more cardiovascular risks at the young ages [
20,
21].
Zebrafish embryos is great model to study hyper glycaemia related impact because they zebrafish embryos can be treated directly with glucose that by passed any limitations due to the placental-fetal barrier present in mammals [
22,
23].
The observed delays in hatch rate and alterations in morphological features, particularly in the brain, heart, and tail, suggest that glucose exposure exerts a disruptive effect on zebrafish embryonic development. These findings align with previous studies indicating the susceptibility of developing embryos to metabolic disturbances [
13]. The accelerated heart rate observed in the glucose-exposed group further underscores the impact of glucose on cardiovascular function during early development.
The mechanisms underlying these effects warrant further investigation. It is plausible that glucose-mediated alterations in metabolic pathways or signaling cascades may contribute to the observed developmental abnormalities. In the pathway analysis, we found the pathways related to the heart development were changed in the treatment group. Details of KEGG pathway will be discussed in the following discussion section. Additionally, elucidating the long-term consequences of glucose exposure on zebrafish embryogenesis and subsequent phenotypic outcomes would provide valuable insights into the potential risks associated with elevated glucose levels during critical stages of development.
Our findings highlight the importance of understanding the impact of metabolic factors such as glucose on embryonic development and underscore the potential relevance of zebrafish as a model organism for studying developmental disorders associated with metabolic dysregulation though Wnt signaling.
We performed RNA-seq analysis on high glucose treated vs. control zebrafish embryos in order to better understand the molecular level mechanism of glucose effect on embryonic development through the transcriptome and pathways analysis results. This research will provide the clue to further investigate the impact of glucose on GDM’s offspring health condition, diagnosis and treatments.
Among the KEGG pathways, in all regulated DEGs, there were 20 pathways are regulated in high glucose treatment group and 16 out of 20 are metabolism pathways including biosynthesis of amino acids, fatty acids and the metabolism by cytochrome p450. Four signaling pathways are related to development which are Notch signaling pathway, Wnt signaling pathway, FoxO pathway PPAR and p53 signaling pathway. One ABC transporters pathway. All these pathways play crucial function during embryo development.
Our investigation into the effects of high glucose levels on zebrafish embryo development revealed significant alterations in key metabolic pathways, Wnt singling pathway shedding light on the intricate relationship between glucose metabolism and embryonic development. Notably, our findings indicate pronounced downregulation of genes involved in glycolysis/gluconeogenesis and oxidative phosphorylation pathways in glucose-exposed embryos compared to controls.
Our findings are consistent with previous studies highlighting the pivotal role of glucose in modulating metabolic pathways during zebrafish embryonic development. High glucose levels have been shown to exert multifaceted effects on cellular metabolism, influencing various metabolic processes essential for embryogenesis. The observed downregulation of genes involved in glycolysis/gluconeogenesis and oxidative phosphorylation pathways corroborates the notion that glucose serves as a central regulator of metabolic homeostasis during critical stages of development.
There are 3 pathways that regulate the heart development are significantly regulated in our data. The vascular smooth muscle contraction, cardiac muscle contraction, adrenergic signaling in cardiomyocytes, and regulation of actin cytoskeleton pathways play important roles for heart normal functions. These changes indicate the high glucose impacts the heart function by changing the molecules level in these pathways (data not shown).
Wnt signaling plays a crucial role in zebrafish embryonic development, regulating different processes such as axis formation, neurogenesis, neural crest, organogenesis [
23,
24]. The Wnt signaling regulates the skin and scale development in zebrafish along with Hedgehog pathway [
25]. The Wnt signaling pathway is initiated by the binding of Wnt ligands to Frizzled receptors, leading to the activation of intracellular signaling cascades. One well-studied component of this pathway is β-catenin, which acts as a key transcriptional co-activator. Upon activation, β-catenin translocated to the nucleus, where it interacts with TCF/LEF transcription factors to regulate the expression of target genes involved in embryonic patterning and tissue differentiation. Wnt1,3,7,10 are the key molecules to stimulate the neural progenitor cells. Wnt8 and lef1 are the molecules that involved in posterior hypothalamic neural specific development [
26].
In our study, Wnt signaling, Notch and Hedgehog pathways are significantly changed in the EKGG enrichment pathways.
Figure 5B shows that Frizzled receptor and Fra1 are significantly regulated by high glucose treatment compare to the control group. The treatment group had speed up heart rates, abnormal body shape and that matches well with the function of Wnt signaling pathway function during zebrafish embryonic development.
Implications and Future Directions
The relationship between maternal genes modification under the high glucose level and fetal development gene changes is another way to explore the impact of high glucose level in mother to embryo development [
27,
28]. Further investigations are warranted to delineate the precise molecular mechanisms underlying these pathways in both maternal and offspring effects on embryonic development.
Conclusions
In conclusion, our study provides compelling evidence of the profound influence exerted by elevated glucose levels on the Wnt signaling pathway during zebrafish embryo development, as revealed by RNA sequencing (RNA-seq) analysis. The intricate interplay we observed between glucose levels and Wnt signaling underscores the complexity of molecular mechanisms governing embryonic development. This dysregulation not only sheds light on the immediate effects on early developmental processes but also raises intriguing questions about the long-term implications for offspring during subsequent developmental stages. Our findings contribute to a deeper understanding of the molecular pathways involved in glucose-mediated developmental changes, with potential implications for developmental biology and human health. Further investigations into the precise mechanisms by which high glucose levels disrupt Wnt signaling, as elucidated by RNA-seq, will be crucial for unraveling the broader impact on embryonic development and for identifying potential therapeutic interventions to mitigate adverse effects.
4. Materials and Methods
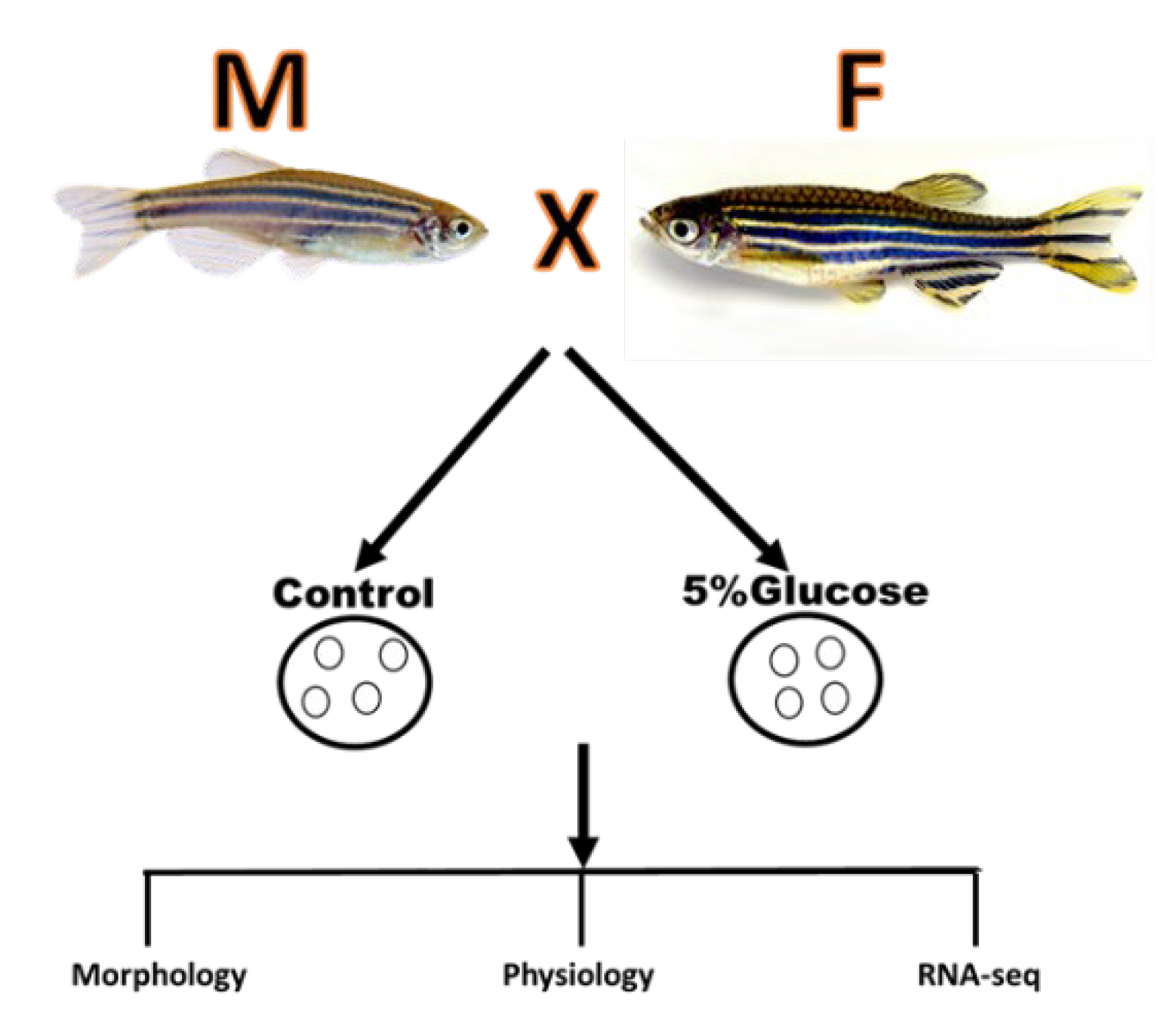
Figure 6.
The schematic of experimental design on the effect of Glucose on zebrafish embryos.
Figure 6.
The schematic of experimental design on the effect of Glucose on zebrafish embryos.
Materials:
D-glucose(Modernist Pantry 5% w/v)was used in this study. Embryonic media, E3 was made from out lab from the standard protocol.
Animal Husbandry
Adult wild-type zebrafish were obtained from breeding facilities at Carolina Biological Supply Co. Fish maintenance, breeding conditions, and egg production were done according to internationally accepted standards protocol [
29]. Embryos were obtained from Carolina Biological Supply, USA. Adult raised in embryonic media (E3) under standard conditions at 28.5 °C with a 14 h light/10 h dark cycle, and the embryonic stages are according to standard procedures [
5].
Preparation of D-glucose application
Embryos are separated into 5 petri dishes with each petri dish containing 25 embryos. At the time of grouping completion, the embryos are ~3 hours post fertilization (hpf). Once all 100 embryos have been separated in 5 groups the collection water was removed and replaced with embryo fish water (1 teaspoon aquarium salt, then up to 1L RO water- validated for 800-900uS and pH 7.0-7.3). Embryos kept in incubator at 28ºC to wait until they are 5 hpf. Addition of D-Glucose & collection of embryos
Our different D-glucose concentrations were each added to their own dish of embryos, where all the previous embryo water was removed prior to addition of the D-glucose concentration. At this stage embryos are 5hpf. Embryos were incubated in the 28⁰C until they were 32hpf. Tricaine is used and placed into each of the 5 petri dishes in order to euthanize each embryo. After a few minutes each dish is taken under the Leica dissecting scope in order to measure the mortality rate in each group and scored for any occurring changes to the embryo. Once the mortality rate is noted, next was to remove the chorion of the surviving embryo with surgical grade forceps. Each of the treatment concentrations as well as the control will undergo the same process. The surviving embryos are placed into individual Eppendorf tubes and three drops of 4% PFA is added in order to conserve the embryos structure.
Total RNA Extraction
The control and glucose treated zebrafish embryos were collected for total RNA extraction. The experiment design includes 3 control samples and 3 high glucose treated samples. Total 6 RNA samples were extracted using Zymo RNA extraction kit(Irvine, CA) according to the manufacture’s protocol. Concentration of the RNA samples was measured by Nano drop 2000(DeNovix Inc.Wilmington, DE).
RNA seq
RNA seq was performed by experts at Novogene Corporation Inc.(West Coast: Sacramento, CA). The sample quality control, library preparation, Clustering and sequencing are in the supplemental methods.
RNA seq Data Analysis was performed by Novogene Corporation Inc.(West Coast: Sacramento, CA).
Briefly, Raw data (raw reads) of Fastq format were firstly processed through in-house per scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapter, reads containing ploy-N and low-quality reads from raw data. At the same time, Q20, Q30 and GC content the clean data were calculated. All the downstream analyses were based on the clean data with high quality.
Reads mapping to the reference genome
Reference genome and gene model annotation files were downloaded from genome website2directly. Index of the reference genome was built using Hisat2 v2.0.5 and paired-end clean reads were aligned to the reference genome using Hisat2 v2.0.5. We selected Hisat2 as the mapping tool for that Hisat2 can generate a database of splice junctions based on the Gene model annotation file and thus a better mapping result than other non-splice mapping tools.
Novel transcripts prediction
The mapped reads of each sample were assembled by StringTie (v1.3.3b) (Mihaela Pertea.et.al. 2015) in a reference-based approach. StringTie uses a novel network flow algorithm as well as an optional de novo assembly step to assemble and quantitate full-length transcripts representing multiple splice variants for each gene locus.
Quantification of gene expression level
FeatureCounts v1.5.0-p3 was used to count the reads numbers mapped to each gene. And then FPKM of each gene was calculated based on the length of the gene and reads count mapped to this gene. FPKM, expected number of Fragments Per Kilobase of transcript sequence per-Millions base pairs sequenced, considers the effect of sequencing depth and gene length for the reads count at the same time, and is currently the most commonly used method for estimating gene expression levels.
Differential expression analysis
(For DESeq2 with biological replicates) Differential expression analysis of two conditions/groups (two biological replicates per condition) was performed using the DESeq2R package (1.20.0). DESeq2 provide statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value <=0.05 found by DESeq2 were assigned as differentially expressed. (For edgeR without biological replicates) Prior to differential gene expression analysis, foreach sequenced library, the read counts were adjusted by edge R program package through one scaling normalized factor. Differential expression analysis of two conditions was performed using the edge R R package (3.22.5). The P values were adjusted using the Benjamini &Hochberg method. Corrected P-value of 0.05 and absolute foldchange of 2 were set as the3threshold for significantly differential expression.
GO and KEGG enrichment analysis of differentially expressed genes
Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by the cluster Profiler R package, in which gene length bias was corrected. GO terms with corrected P value less than 0.05 were considered significantly enriched by differential expressed genes. We used cluster Profiler R package to test the statistical enrichment of differential expression genes in KEGG pathways.
Gene Set Enrichment Analysis
Gene Set Enrichment Analysis (GSEA) is a computational approach to determine if a predefined Gene Set can show a significant consistent difference between two biologicals states. The genes were ranked according to the degree of differential expression in the two samples, and then the predefined Gene Set were tested to see if they were enriched at the top or bottom of the list. Gene set enrichment analysis can include subtle expression changes. We use the local version of the GSEA analysis tool
http://www.broadinstitute.org/gsea/index.jsp, GO, KEGG data set were used for GSEA independently.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. RNA-Seq raw data can be found at NCBI GEO website with ID GSE228661
Author Contributions
Conceptualization, R.T; methodology, R.T.; investigation, R.T.; resources, R.T.; data curation, E.T and J.H; writing—original draft preparation, R.T.; writing—review and editing, R.T., E.T. and J.H; visualization, J.H.; supervision, R.T.; project administration, R.T.; funding acquisition, R.T. All authors have read and agreed to the published version of the manuscript.”.
Funding
“We would like to thank Southwest Baptist University faculty development committee for issuing this grant to R.T.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Organization for Research Review Board (RRB) No. 21 according to the regulations of Public Health Service (PHS), the Animal Welfare Act (AWA) and IACUC.
Informed Consent Statement
Not applicable.
Data Availability Statement
We would like to share the data published in this article.
Acknowledgments
We would like to thank Novogene company for conducting the RNA seq and seq data analysis.
Conflicts of Interest
The authors declare no conflict of interest
References
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022, 46, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Juan J, Yang H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int J Environ Res Public Health. 2020, 18, 17–24.
- Szmuilowicz ED, Josefson JL, Metzger BE. Gestational Diabetes Mellitus. Endocrinol Metab Clin North Am. 2019 Sep;48(3):479-493. Epub 2019 Jun 18. [CrossRef] [PubMed]
- Pérez-Pérez A, Vilariño-García T, Guadix P, Dueñas JL, Sánchez-Margalet V. Leptin and metabolic. [CrossRef] [PubMed]
- Veldman, M. , Lin, S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatr Res 64, 470–476 (2008). [CrossRef]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008 May 8;2(5):497-507. PMID: 18462699; PMCID: PMC2683414. [CrossRef]
- Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, Kiang A, Schultz S, Epstein JA, Toyama R, Dawid IB. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001 Dec;11(12):1979-87. [CrossRef] [PubMed]
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004 Aug 31;101(35):12792-7. Epub 2004 Jul 15. [CrossRef] [PubMed]
- White RJ, Collins JE, Sealy IM, Wali N, Dooley CM, Digby Z, Stemple DL, Murphy DN, Billis K, Hourlier T, Füllgrabe A, Davis MP, Enright AJ, Busch-Nentwich EM. A high-resolution mRNA expression time course of embryonic development in zebrafish. Elife. 2017 Nov 16;6: e30860. [CrossRef] [PubMed]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995 Jul;203(3):253-310. [CrossRef] [PubMed]
- Parichy, D.M. , Elizondo, M.R., Mills, M.G., Gordon, T.N. and Engeszer, R.E. (2009), Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn., 238: 2975-3015. [CrossRef]
- Elo B, Villano CM, Govorko D, White LA. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J Mol Endocrinol. 2007 Apr;38(4):433-40. [CrossRef] [PubMed]
- Singh A, Castillo HA, Brown J, Kaslin J, Dwyer KM, Gibert Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci Rep. 2019 Mar 11;9(1):4121. [CrossRef] [PubMed]
- Dhillon SS, Torell F, Donten M, Lundstedt-Enkel K, Bennett K, Rännar S, Trygg J, Lundstedt T. Metabolic profiling of zebrafish embryo development from blastula period to early larval stages. PLoS ONE. 2019 May 14;14(5):e0213661. [CrossRef] [PubMed]
- Bridget Konadu, Carol K. Cox, Michael R. Garrett, Yann Gibert,Excess glucose or fat differentially affects metabolism and appetite-related gene expression during zebrafish embryogenesis,iScience,Volume 26, Issue 7,2023,107063,ISSN 2589-0042,.
- Elo, B., Villano, C. M., Govorko, D., & White, L. A. (2007). Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. Journal of Molecular Endocrinology, 38(4), 433-440. Retrieved Mar 13, 2024. [CrossRef]
- Nishio S, Gibert Y, Berekelya L, Bernard L, Brunet F, Guillot E, Le Bail JC, Sánchez JA, Galzin AM, Triqueneaux G, Laudet V. Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol Endocrinol. 2012 Aug;26(8):1316-26. Epub 2012 Jun 14. PMID: 22700585; PMCID: PMC5416984. [CrossRef]
- Westphal M, Panza P, Kastenhuber E, Wehrle J, Driever W. Wnt/β-catenin signaling promotes neurogenesis in the diencephalospinal dopaminergic system of embryonic zebrafish. Sci Rep. 2022 Jan 19;12(1):1030.PMID: 35046434; PMCID: PMC8770493. [CrossRef]
- Yin A, Korzh S, Winata CL, Korzh V, Gong Z. Wnt signaling is required for early development of zebrafish swimbladder. PLoS ONE. 2011 Mar 30;6(3):e18431. [CrossRef] [PubMed]
- Aman AJ, Fulbright AN, Parichy DM. Wnt/β-catenin regulates an ancient signaling network during zebrafish scale development. Elife. 2018 Jul 17;7:e37001. [CrossRef] [PubMed]
- Sutton G, Kelsh RN, Scholpp S. Review: The Role of Wnt/β-Catenin Signalling in Neural Crest Development in Zebrafish. Front Cell Dev Biol. 2021 Nov 29; 9:782445. [CrossRef] [PubMed]
- Wang X, Lee JE, Dorsky RI. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish. 2009 Mar;6(1):49-58. [CrossRef] [PubMed]
- Akieda Y, Ogamino S, Furuie H, Ishitani S, Akiyoshi R, Nogami J, Masuda T, Shimizu N, Ohkawa Y, Ishitani T. Cell competition corrects noisy Wnt morphogen gradients to achieve robust patterning in the zebrafish embryo. Nat Commun. 2019 Oct 17;10(1):4710. [CrossRef] [PubMed]
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000 May 4;405(6782):76-81. [CrossRef] [PubMed]
- Aman AJ, Fulbright AN, Parichy DM. Wnt/β-catenin regulates an ancient signaling network during zebrafish scale development. Elife. 2018 Jul 17;7: e37001. [CrossRef] [PubMed]
- Wang X, Lee JE, Dorsky RI. Identification of Wnt-responsive cells in the zebrafish hypothalamus. Zebrafish. 2009 Mar;6(1):49-58. [CrossRef] [PubMed]
- Graham JM Hickey, Candice L Wike, Xichen Nie, Yixuan Guo, Mengyao Tan, Patrick J Murphy, Bradley R Cairns (2022) Establishment of developmental gene silencing by ordered polycomb complex recruitment in early zebrafish embryos eLife 11: e67738.
- Abrams EW, Mullins MC. Early zebrafish development: it’s in the maternal genes. Curr Opin Genet Dev. 2009 Aug;19(4):396-403. Epub 2009 Jul 14. [CrossRef] [PubMed]
- Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C Toxicol Pharmacol. 2009 Mar;149(2):196-209. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).