Submitted:
10 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Livestream Camera Observations
2.3. Statistical Methods
3. Results
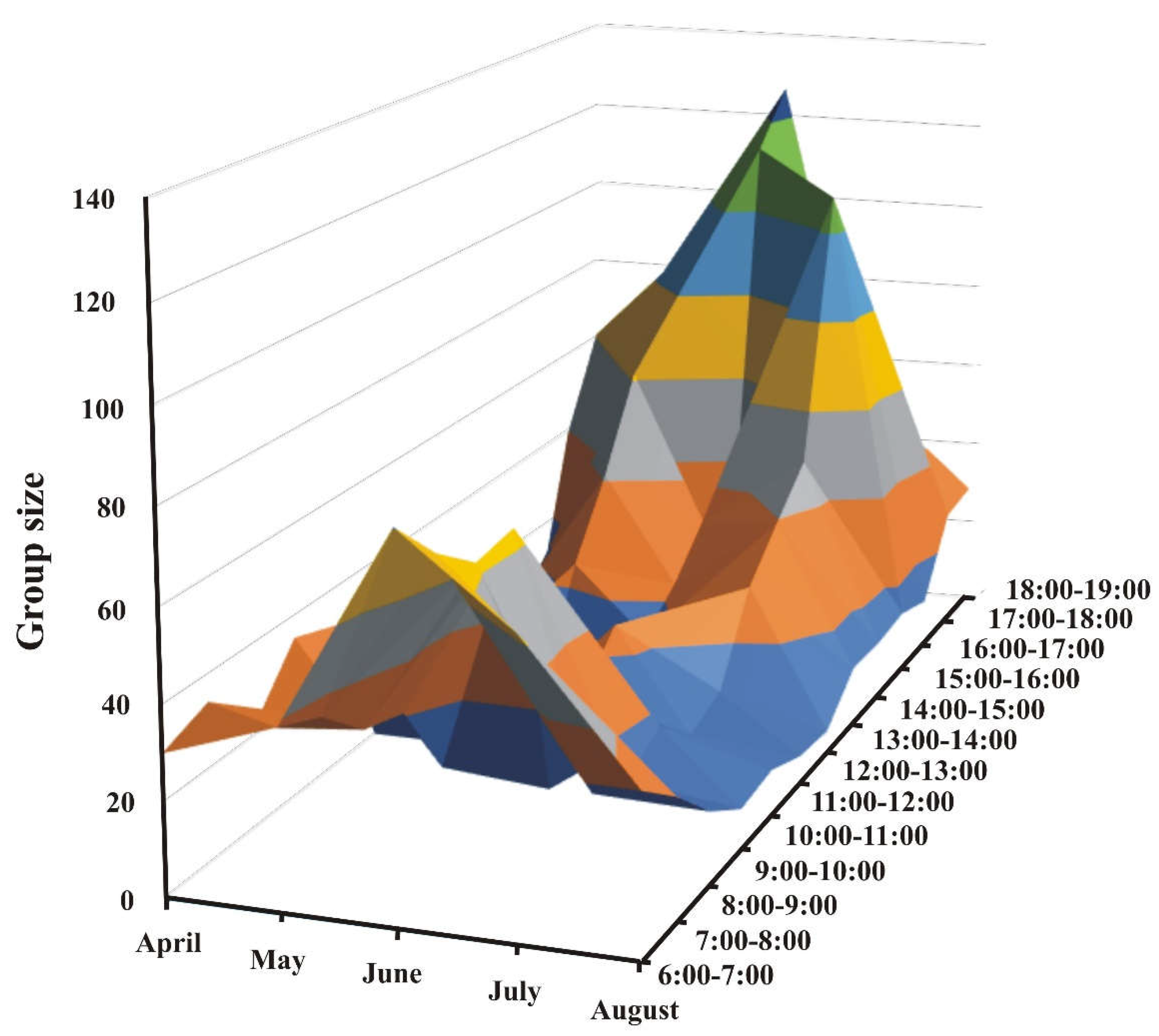
3.1. Spatiotemporal Group Size Dynamic
3.2. Group Sizes on the Plain and in the Scrub
3.3. Social Distance
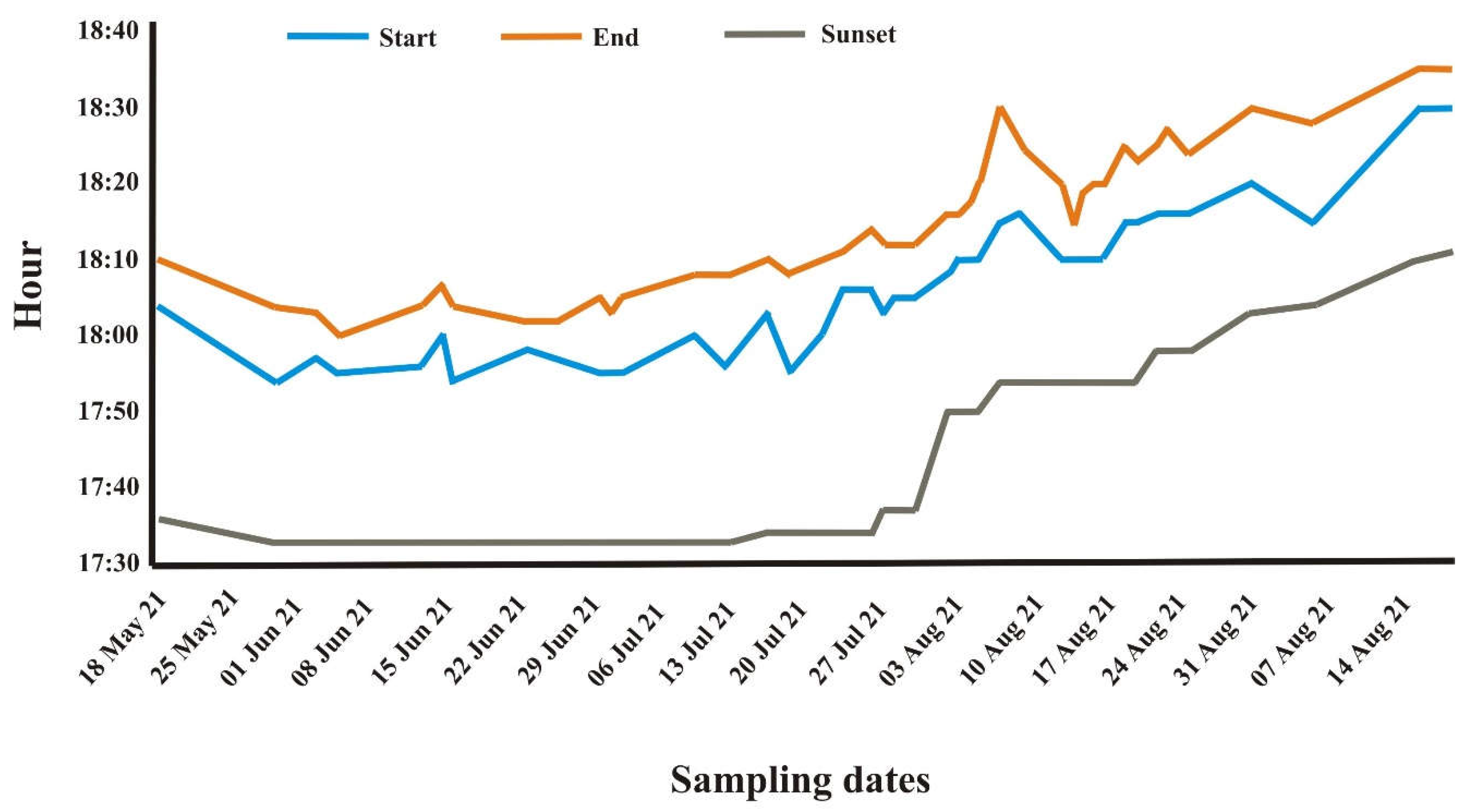
3.4. Roosting Behaviour
3.5. Surveillance Behaviour
3.6. Vocalisations
3.7. Interspecific association
3.8. Jackal-Targeted Guineafowl Groups
3.9. Guineafowl Response to Predatory Bird Attacks
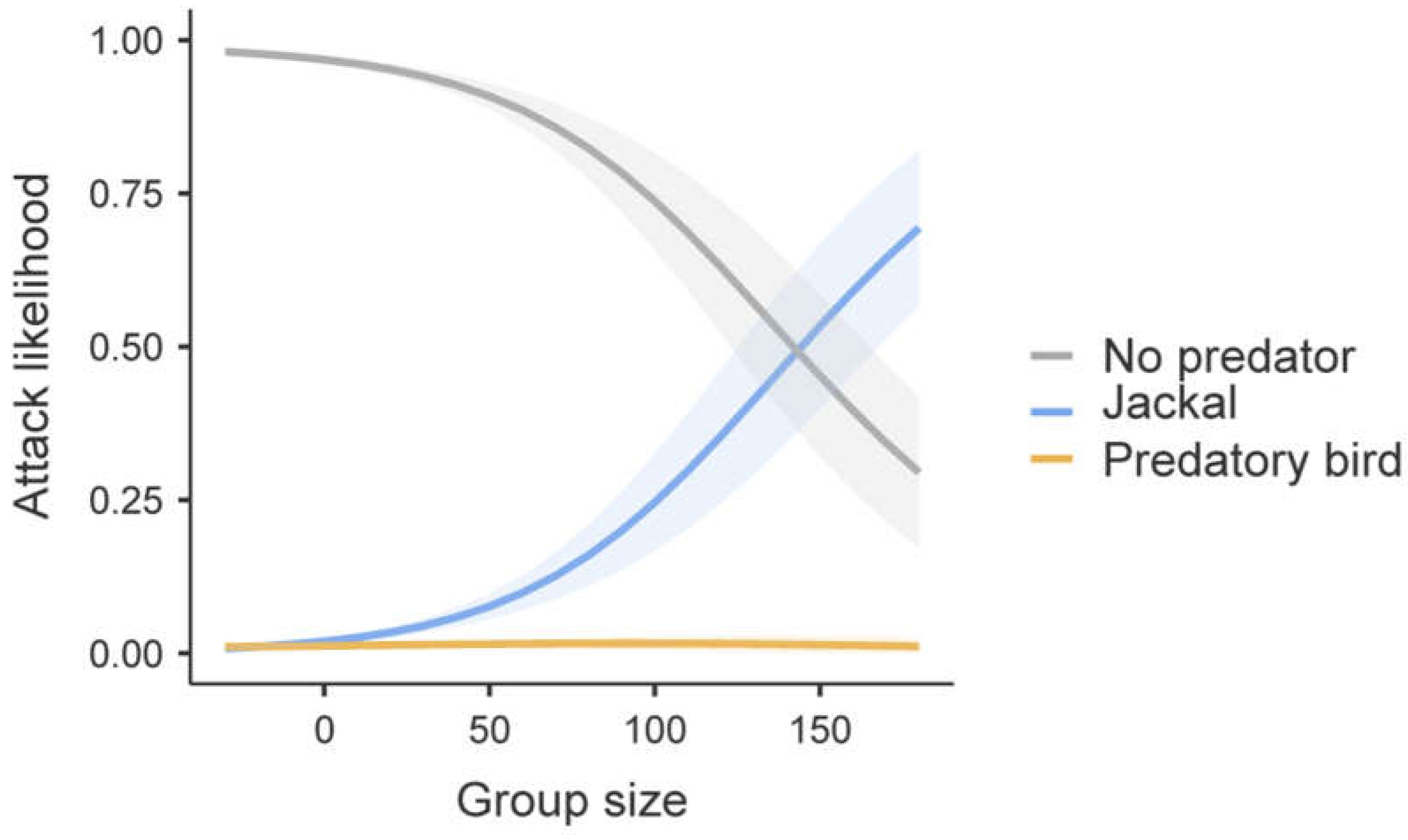
3.10. Group Size as Predictor of Predator Attack
3.11. Estimated Avoidance Time
4. Discussion
4.1. Habitat Use and Lethal Space
4.2. Terrestrial Predation
4.3. Aerial Predation
4.4. Surveillance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovette, I.J.; Fitzpatrick, J.W. Handbook of Bird Biology. Cornell Lab of Ornithology: Ithaca, NY, USA, 2016.
- Ling, H.; Mclvor, G.E.; van der Vaart, K.; Vaughan, R.T.; Thornton, A.; Ouellette, N.T. Local interactions and their group-level consequences in flocking jackdaws. Proc. R. Soc. B: Biol. Sci. 2019, 286, 20190865. [Google Scholar] [CrossRef]
- Storms, R.F.; Carere, C.; Zoratto, F.; Hemelrijk, C.K. Complex patterns of collective escape in starling flocks under predation. Behav. Ecol. Sociobiol. 2019, 73, 10. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, W. Predation in bird populations. J. Ornithol. 2010, 152, 251–263. [Google Scholar] [CrossRef]
- Crowe, T.M. Limitation of population in the helmeted guineafowl. S. Afr. J. Wildl. Res. 1978, 8, 117–126. [Google Scholar]
- Newton, I. Group Limitation in Birds. Academic Press: London, UK, 2003.
- Cresswell, W.; Quinn, J.L. Predicting the optimal prey group size from predator hunting behaviour. J. Anim. Ecol. 2011, 80, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C. Grouping and predation. In Encyclopedia of Evolutionary Psychological Science; Shackelford, T., Weekes-Shackelford, V., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- 9Cresswell,W. Flocking is an effective anti-predator strategy in redshanks, Tringa tetanus. Anim. Behav. 1994, 47, 433–442.
- 1Carere, C.; Montanino, S.; Moreschini, F.; Zoratto, F.; Chiarotti, F.; Santucci, D.; Alleva, E. Aerial flocking patterns of wintering starlings, Sturnus vulgaris, under different predation risk. Anim. Behav. 2009, 77, 101–107. [Google Scholar] [CrossRef]
- Morrell, L.J.; Ruxton, G.D.; James, R. Spatial positioning in the selfish herd. Behav. Ecol. 2011, 22, 16–22. [Google Scholar] [CrossRef]
- Hamilton, W.D. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Ancel, A.; Gilbert, C.; Poulin, N.; Beaulieu, M.; Thierry, B. New insights into the huddling dynamics of emperor penguins. Anim. Behav. 2015, 110, 91–98. [Google Scholar] [CrossRef]
- Colorado Zuluaga, G.L. Why animals come together, with the special case of mixed-species bird flocks. Rev. EIA. Esc. Ing. Antioq. 2013, 146, 999–1023. [Google Scholar]
- Clark, C.W.; Mangel, M. Foraging and Flocking Strategies: Information in an Uncertain Environment. Am. Nat. 1984, 123, 626–641. [Google Scholar] [CrossRef]
- Lima, S.L. Vigilance while feeding and its relation to the risk of predation. J. Theor. Biol. 1987, 124, 303–316. [Google Scholar] [CrossRef]
- Jackson, A.L.; Beauchamp, G.; Broom, M.; Ruxton, G.D. Evolution of anti-predator traits in response to a flexible targeting strategy by predators. Proc. R. Soc. B: Biol. Sci. 2006, 273, 1055–1062. [Google Scholar] [CrossRef]
- Palmer, M.S.; Packer, C. Reactive anti-predator behavioral strategy shaped by predator characteristics. PLoS ONE 2021, 16, e0256147. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.J.; Evans, K.L. The effects of habitat structure on predation risk of birds in agricultural landscapes. Ibis 2004, 146, 210–220. [Google Scholar] [CrossRef]
- Tellería, J.L.; Virgós, E.; Carbonell, R.; Pérez-Tris, J.; Santos, J. Behavioural responses to changing landscapes: Flock structure and antipredator strategies of tits wintering in fragmented forests. OIKOS 2001, 95, 253–264. [Google Scholar] [CrossRef]
- Jovani, R.; Schielzeth, H.; Mavor, R.; Oro, D. Specificity of grouping behaviour: Comparing colony sizes for the same seabird species in distant populations. J. Avian Biol. 2012, 43, 397–402. [Google Scholar] [CrossRef]
- Ramírez-Santos, P.L.; Enríquez, P.; Raúl Vázquez-Pérez, J.; Rangel-Salazar, J.L. Bird behaviour during prey–predator interaction in a tropical forest in Mexico. In Owls; Mikkola, H (Ed.) IntechOpen, 2018. [CrossRef]
- Abdulwahab, U.A.; Osinubi, S.T.; Abalaka, J. Risk of predation: A critical force driving habitat quality perception and foraging behavior of granivorous birds in a Nigerian forest reserve. Avian Res. 2019, 10, 33. [Google Scholar] [CrossRef]
- Park, K.J.; Graham, J.; Calladine, J.; Wernham, C.W. Impacts of birds of prey on gamebirds in the UK: A review. Ibis 2008, 150, 9–26. [Google Scholar] [CrossRef]
- Berruti, A. The AGRED Guide to Gamebird Management in South Africa. African Gamebird Research Education and Development Trust, Johannesburg, South Africa, 2011.
- Skead, C.J. A study of the Crowned Guineafowl Numida meleagris coronata Gurney. Ostrich 1962, 33, 51–65. [Google Scholar] [CrossRef]
- Rose, M. ; The guineafowl and the buzzard. Witwatersrand Bird Club. News Sheet 1967, 58, 7–8. [Google Scholar]
- Rose, M. Crowned guineafowl behaviour. Witwatersrand Bird Club. News Sheet 1966, 55, 2. [Google Scholar]
- Monty, K. Helmeted guineafowl spars with black sparrowhawk. Albatross 1983, 271, 4. [Google Scholar]
- Van Niekerk, J.H. Interflock movement in a population of Helmeted Guineafowl Numida meleagris at the Krugersdorp Game Reserve, Gauteng province, South Africa. Ostrich 2009, 80, 201–204. [Google Scholar] [CrossRef]
- Van Niekerk, J.H. Social organization of a flock of Helmeted Guineafowl (Numida meleagris) at the Krugersdorp Game Reserve, South Africa. Chin. Birds 2010, 1, 22–29. [Google Scholar] [CrossRef]
- Papageorgiou, D.; Christensen, D.C.; Gabriella, E.C.; Gall, J.A.; Klarevas-Irby, B.; Nyaguthii, B.; Couzin, I.D.; Farine, D.R. The multilevel society of a small-brained bird. Curr. Biol. 2019, 29, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, J.H. Notes on habitat use by guineafowl in the Krugersdorp Game Reserve, South Africa. S. Afr. J. Wildl. 2002, 2, 166–168. [Google Scholar]
- Viljoen, P.J. AGRED’s Gamebirds of South Africa. African Gamebird Research Education and Development Trust, Johannesburg, South Africa, 2005.
- Van Niekerk, J.H. Roosting requirements of Helmeted Guineafowl Numida meleagris on Highveld grain and livestock farms with alien tree groves, Gauteng province, South Africa. Ostrich 2019, 90, 37–40. [Google Scholar] [CrossRef]
- Papadopoulou, M.; Hildenbrandt, H.; Sankey, D.W.E.; Portugal, S.J.; Hemelrijk, C.K. Self-organization of collective escape in pigeon flock. PLoS Comput. Biol. 2022, 18, e1009772. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. (Eds.), The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria. 2006.
- Hayward, M.W.; Hayward, M.D. Waterhole use by African fauna. S. Afr. J. Wildl. 2012, 42, 117–127. [Google Scholar] [CrossRef]
- Magige, F.J. Flock-size effect on scanning behaviour of Maasai Ostrich Struthio camelus massaicus. Scopus 2017, 37, 38–41. [Google Scholar]
- Petrželková, K.J.; Zukal, J. Does a live barn owl (Tyto alba) affect emergence behavior of serotine bats (Eptesicus serotinus)? Acta Chiropt. 2003, 5, 177–184. [Google Scholar] [CrossRef]
- Kelm, D.H.; Langheld, M.; Nogueras, J.; Popa-Lisseanu, A.G.; Ibáñez, C. Continuous low-intensity predation by owls (Strix aluco) on bats (Nyctalus lasiopterus) in Spain and the potential effect on bat colony stability. R. Soc. Open Sci. 2023, 10, 230309. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, J.H. Some socio-biological features of Crowned Guineafowl in the Krugersdorp Game Reserve. Bokmakierie 1980, 32, 102–108. [Google Scholar]
- Van Niekerk, J.H. Vocal behavior of Crested Guineafowl (Guttera edouardi) based on visual and sound playback surveys in the Umhlanga Lagoon Nature Reserve, KwaZulu-Natal province, South Africa. Avian Res. 2015, 6, 13. [Google Scholar] [CrossRef]
- Weihs, D.; Webb, P.W. Optimal avoidance and evasion tactics in predator-prey interactions. J. Theor. Biol. 1984, 106, 189–206. [Google Scholar] [CrossRef]
- Bonter, D.N.; Zuckerberg, B.; Sedgwick, C.W.; Hochachka, W.M. Daily foraging patterns in free-living birds: Exploring the predation–starvation trade-off. Proc. Royal Soc. B. 2013, 280, 20123087. [Google Scholar] [CrossRef]
- Olson, R.S.; Haley, P.B.; Dyer, F.C.; Adami, C. Exploring the evolution of a trade-off between vigilance and foraging in group-living organisms. R. Soc. Open Sci. 2015, 2, 150135. [Google Scholar] [CrossRef]
- Van Niekerk, J.H. Habitat preferences of Helmeted Guineafowl Numida meleagris in the Krugersdorp Game Reserve, Gauteng province, South Africa. Ostrich 2013, 84, 153–156. [Google Scholar] [CrossRef]
- Van Niekerk, J.H. Observations of Red-billed Spurfowl (Pternistis adspersus) in the arid Molopo Nature Reserve, North West Province, South Africa. Chin. Birds 2011, 2, 117–124. [Google Scholar] [CrossRef]
- Newton, I. Predation and limitation of bird numbers. In Current Ornithology; Power, D.M. (Ed.)., vol. 11. Springer, Boston, MA, USA,, 1993, pp 143–198. [CrossRef]
- Avery, G.; Avery, D.M.; Braine, S.; Loutit, R. Prey of coastal black-backed jackal Canis mesomelas (Mammalia: Canidae) in the Skeleton Coast Park, Namibia. J. Zool. 1987, 213, 81–94. [Google Scholar] [CrossRef]
- Alexander, R.D. The evolution of social behavior. Annu. Rev. Ecol. Syst. 1974, 5, 325–383. [Google Scholar] [CrossRef]
- Creswell, W. Non-lethal effects of predation in birds. Ibis 2008, 150, 3–17. [Google Scholar] [CrossRef]
- Kalb, N.; Randler, C. Behavioral responses to conspecific mobbing calls are predator-specific in great tits (Parus major). Ecol. Evol. 2019, 9, 9207–9213. [Google Scholar] [CrossRef] [PubMed]
- Grafton, R.N. Food of the black-backed jackal: A preliminary report. Afr. Zool. 1965, 1, 41–53. [Google Scholar] [CrossRef]
- Humphries, D.H. The Ecology of Black-Backed Jackal (Canis mesomelas) on Farmlands in the Midlands of KwaZulu-Natal, South Africa. Master of Science in the Discipline of Ecology, School of Life Sciences, College of Agriculture, Science and Engineering, University of KwaZulu-Natal, Pietermaritzburg Campus, 2014.
- Minnie, l.; Avenant, N.L.; Kamler, J.; Butler, H.; Parker, D.; Rouilly, M.; du Plessis, J.; Do Linh San, E. A conservation assessment of Canis mesomelas. In The Red List of Mammals of South Africa, Swaziland and Lesotho; Child, M.F., Roxburgh, l., Do Linh San, E., Raimondo, D., Daviesmostert, H.T. (Eds.); South African National Biodiversity Institute and Endangered Wildlife Trust, South Africa. 2016.
- Potts, G.R. Partridges. Collins. London, UK, 2013.
- Branch, W.R. Predation on Helmeted Guineafowl by Grey-footed Chacma Baboon in Mashatu Game Reserve, north east Botswana. Biodivers. Observations 2017, 8, 1–5. [Google Scholar]
- Woller, A.; Gonze, D. The Bird Circadian Clock: Insights from a Computational Model. J. Biol. Rhythms 2013, 28, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Couzin, I.; Krause, J. Self-organisation and collective behavior in vertebrates. Adv. Study Behav. 2003, 32, 1–75. [Google Scholar] [CrossRef]
- Sweet, K.A.; Sweet, B.P.; Gomes, D.G.E.; Francis, C.D.F.; Barber, J.R. Natural and anthropogenic noise increase vigilance and decrease foraging behaviors in song sparrows. Behav. Ecol. 2022, 33, 288–297. [Google Scholar] [CrossRef]

| Description | Suitable sub-samples |
|---|---|
| Calculation of summer group sizes | 13 |
| Calculation of mean group sizes at different times of the day | 174 |
| SD Calculation in groups at different times of the day | 381 |
| Calculation of mean monthly group sizes getting ready to roost | 82 |
| Calculation of time it takes for communal rooting group to ascend rooting tree | 42 |
| Calculation of time it takes for communal rooting group to descend rooting tree | 29 |
| Selected photographs of groups to conduct head-up counts (surveillance behaviour) | 190 |
| Observations revealing associations between guineafowl and Blue Wildebeest | 17 |
| Groups selected to correlate jackal/hour vs. mean group sizes of guineafowl | 393 |
| Calculation of SD in groups in different parts of the site, drinking and huddling | 411 |
| Month | Number of days | Number of logins | Mins observed | Additional time/mins |
|---|---|---|---|---|
| Aug. 2020 | 4 | 36 | 180 | 0 |
| Sep. 2020 | 6 | 54 | 270 | 0 |
| Oct. 2020 | 16 | 144 | 720 | 0 |
| Nov. 2020 | 16 | 144 | 720 | 0 |
| Dec. 2020 | 6 | 54 | 270 | 0 |
| Jan. 2021 | 19 | 171 | 855 | 0 |
| Feb. 2021 | 18 | 162 | 810 | 0 |
| Mar. 2021 | 28 | 252 | 1260 | 0 |
| Apr. 2021 | 25 | 225 | 1125 | 0 |
| May 2021 | 26 | 234 | 1170 | 60 |
| Jun. 2021 | 20 | 180 | 900 | 40 |
| Jul. 2021 | 23 | 207 | 1035 | 155 |
| Aug. 2021 | 28 | 252 | 1260 | 245 |
| Total | 235 | 2115 | 10575 | 500 |
| Time | Mean group size | Std. D | Sample | Range |
| 07:00 | 15.61 | 16.77 | 18 | 2-55 |
| 09:00 | 6.35 | 4.42 | 39 | 1-22 |
| 16:00 | 12.08 | 11.55 | 67 | 2-80 |
| 18:00 | 20.16 | 19.86 | 50 | 3-90 |
| Pair | Difference | H-statistics | Critical value | p-value |
| 7:00 – 9:00 | 2.5 | 4.01 | 6.23 | 0.04 |
| 7:00-16:00 | 2.5 | 0.00 | 6.23 | 0.96 |
| 7:00-18:00 | 3.5 | 2.38 | 6.23 | 0.12 |
| 9:00-16:00 | 5 | 11.40 | 6.23 | 0.00 |
| 9:00-16:00 | 6 | 23.95 | 6.23 | 0 |
| 16:00-18:00 | 1 | 5.90 | 6.23 | 0.015 |
| Activity/Place | Mean social distance | Std. D | Sample | Range |
| Plain | 0.835 | 1.53 | 249 | - 0.280 - 11.550 |
| Scrub | 0.274 | 0.537 | 144 | - 0.277 - 3.080 |
| Huddling | 0.034 | 0.135 | 8 | - 0.194 - 0.210 |
| Drinking | 0.071 | 0.091 | 10 | - 0.021 - 0.190 |
| Pair | Difference | H-statistics | Critical value | p-value |
| Plain-scrub | 0.22 | 22.88 | 6.23 | 0.00 |
| Plain-huddling | 0.28 | 6.42 | 6.23 | 0.01 |
| Plain-drinking | 0.31 | 6.26 | 6.23 | 0.01 |
| Scrub-huddling | 0.06 | 0.90 | 6.23 | 0.34 |
| Scrub-drinking | 0.09 | 0.11 | 6.23 | 0.73 |
| Huddling-drinking | 0.03 | 0.95 | 6.23 | 0.32 |
| Time | Mean | Std. D | Sample | Range |
| 7:00-8:00 | 0.233 | 0.407 | 44 | - 0.24 - 1.61 |
| 8:00-9:00 | 0.382 | 0.594 | 53 | - 0.28 - 2.23 |
| 9:00-10:00 | 0.614 | 1.43 | 21 | - 0.233 - 6.3 |
| 10:00-11:00 | 0.653 | 0 | 10 | - 0.81 - 1.42 |
| 11:00-12:00 | 0.316 | 0.589 | 5 | - 0.142 - 1.14 |
| 12:00-13:00 | 0.408 | 0.54 | 9 | - 0.13 - 1.42 |
| 13:00-14:00 | 0.546 | 0.77 | 8 | - 0.58 - 2.28 |
| 14:00-15:00 | 1.09 | 2.06 | 14 | - 0.19 - 7.91 |
| 15:00-16:00 | 0.889 | 1.47 | 42 | - 0.21 - 6.25 |
| 16:00-17:00 | 0.806 | 1.07 | 73 | - 0.19 - 4.2 |
| 17:00-18:00 | 0.806 | 1.69 | 81 | - 0.24 - 11.55 |
| 18:00-19:00 | 0.439 | 0.72 | 21 | - 0.16 - 2.64 |
| Pair | Difference | H-statistics | Critical value | p-value |
| 6:00-7:00 vs 14:00-15:00 | 0.34 | 8.90 | 8.35 | 0.00 |
| 7:00-8:00 vs 14:00-15:00 | 0.28 | 5.08 | 8.35 | 0.02 |
| 7:00-8:00 vs 15:00 -16:00 | 0.18 | 6.77 | 8.35 | 0.00 |
| 8:00-9:00 vs 11:00 - 12:00 | 0.21 | 1.34 | 8.35 | 0.00 |
| 11:00-12:00 vs 12:00-13:00 | 0.22 | 4.17 | 8.35 | 0.04 |
| Month(s) | Mean | Std. D | Sample | Range |
|---|---|---|---|---|
| March | 60 | 45.82 | 3 | 10-100 |
| April | 47.5 | 21.1 | 10 | 20-80 |
| May | 80.53 | 45.57 | 15 | 30-160 |
| June | 109.21 | 43.36 | 14 | 29-200 |
| July | 91.31 | 39.38 | 16 | 16-140 |
| August | 53.37 | 19.12 | 24 | 30-100 |
| Pair | Difference | H-statistics | Critical value | p-value |
| March-April | 25 | 0.25 | 6.96 | 0.61 |
| March-May | 10 | 0.17 | 6.96 | 0.67 |
| March-June | 40 | 2.71 | 6.96 | 0.09 |
| March-July | 30 | 1.86 | 6.96 | 0.17 |
| March-August | 20 | 0.33 | 6.96 | 0.56 |
| April-May | 15 | 3.27 | 6.96 | 0.07 |
| April-June | 65 | 10.84 | 6.96 | 0.00 |
| April-July | 55 | 7.02 | 6.96 | 0.00 |
| April-August | 5 | 0.60 | 6.96 | 0.43 |
| May-June | 50 | 2.41 | 6.96 | 0.12 |
| May-July | 40 | 0.45 | 6.96 | 0.49 |
| May-August | 10 | 2.67 | 6.96 | 0.10 |
| June-July | 10 | 0.93 | 6.96 | 0.33 |
| June-August | 60 | 14.14 | 6.96 | 0.00 |
| July-August | 50 | 8.95 | 6.96 | 0.00 |
| Fly up to roost | Fly down from trees | ||||||
|---|---|---|---|---|---|---|---|
| Star | End | Duration | No. of birds | Start | End | Duration | No. of birds |
| 18:04 | 18:10 | 6 | 50 | 06:31 | 06:35 | 4 | 70 |
| 17:54 | 18:04 | 10 | 120 | 06:30 | 06:35 | 5 | 80 |
| 17:57 | 18:03 | 6 | 120 | 06:32 | 06:35 | 3 | 100 |
| 17:55 | 18:00 | 5 | 100 | 06:36 | 06:38 | 2 | 100 |
| 17:54 | 18:03 | 7 | 130 | 06:38 | 06:40 | 2 | 100 |
| 17:56 | 18:04 | 8 | 130 | 06:38 | 06:40 | 2 | 50 |
| 18:00 | 18:07 | 7 | 100 | 06:40 | 06:44 | 4 | 75 |
| 17:54 | 18:04 | 10 | 100 | 06:38 | 06:43 | 5 | 100 |
| 17:58 | 18:02 | 4 | 120 | 06:39 | 06:42 | 3 | 100 |
| 17:57 | 18:05 | 8 | 70 | 06:40 | 06:44 | 4 | 100 |
| 17:53 | 18:03 | 10 | 100 | 06:44 | 06:46 | 2 | 100 |
| 17:55 | 18:03 | 8 | 100 | 06:42 | 06:44 | 2 | 110 |
| 17:57 | 18:05 | 8 | 140 | 06:43 | 06:55 | 2 | 120 |
| 18:00 | 18:08 | 10 | 100 | 06:40 | 06:42 | 2 | 100 |
| 17:58 | 18:08 | 12 | 100 | 06:42 | 06:44 | 2 | 130 |
| 18:03 | 18:10 | 7 | 50 | 06:39 | 06:42 | 3 | 70 |
| 17:55 | 18:08 | 13 | 120 | 06:37 | 06:40 | 3 | 100 |
| 18:00 | 18:10 | 10 | 110 | 06:36 | 06:38 | 2 | 100 |
| 18:06 | 18:11 | 5 | 90 | 06:35 | 06:37 | 2 | 120 |
| 18:06 | 18:14 | 8 | 50 | 06:32 | 06:34 | 2 | 80 |
| 18:03 | 18:12 | 9 | 100 | 06:38 | 06:39 | 1 | 80 |
| 18:05 | 18:12 | 7 | 100 | 06:34 | 06:35 | 1 | 80 |
| 18:05 | 18:12 | 7 | 70 | 06:36 | 06:38 | 2 | 80 |
| 18:08 | 18:16 | 8 | 120 | 06:31 | 06:33 | 2 | 40 |
| 18:10 | 18:16 | 6 | 110 | 06:29 | 06:30 | 1 | 40 |
| 18:10 | 18:17 | 7 | 130 | 06:21 | 06:22 | 1 | 40 |
| 18:10 | 18:20 | 10 | 100 | 06:10 | 06:14 | 4 | 45 |
| 18:15 | 18:30 | 15 | 90 | 06:10 | 06:11 | 1 | 40 |
| 18:16 | 18:25 | 9 | 70 | 06:00 | 06:02 | 2 | 46 |
| 18:10 | 18:20 | 10 | 65 | ||||
| 18:10 | 18:15 | 5 | 60 | ||||
| 18:10 | 18:19 | 9 | 70 | ||||
| 18:14 | 18:20 | 6 | 80 | ||||
| 18:10 | 18:20 | 10 | 40 | ||||
| 18:15 | 18:25 | 10 | 50 | ||||
| 18:15 | 18:23 | 8 | 50 | ||||
| 18:16 | 18:27 | 11 | 50 | ||||
| 18:16 | 18:24 | 8 | 50 | ||||
| 18:20 | 18:30 | 10 | 40 | ||||
| 18:15 | 18:28 | 9 | 40 | ||||
| 18:30 | 18:35 | 5 | 50 | ||||
| 18:30 | 18:35 | 5 | 50 | ||||
| Activity | Date | No. photos* | No. birds | Head-ups |
Group size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Counts | Ratios** | % | Mean | Range | Std. D | ||||
| Huddle | May-August 2021 | 56 | 2037 | 917 | 1:2.22 | 45.01 | 36.6 | 2-190 | 42.92 |
| Drinking | September 2020 - August 2021 | 33 | 547 | 220 | 1:2.45 | 40.21 | 16.63 | 2-190 | 32.9 |
| Foraging in scrub | August 2020-August 2021 | 29 | 241 | 13 | 1:18.53 | 5.39 | 8.31 | 2-40 | 8.28 |
| Foraging in open | August 2020-August 2021 | 72 | 991 | 11 | 1:90.09 | 1.1 | 13.76 | 2-55 | 12.42 |
| Totals | 190 | 3816 | 1161 | ||||||
| Date | Guineafowl | Blue Wildebeest |
|---|---|---|
| 9 August 2021 | 20 | 20 |
| 20 | 15 | |
| 10 August 2021 | 30 | 10 |
| 10 | 3 | |
| 15 | 20 | |
| 11 August 2021 | 25 | 24 |
| 5 | 20 | |
| 4 | 30 | |
| 13 August 2021 | 10 | 20 |
| 15 | 20 | |
| 19 August 2021 | 15 | 15 |
| 22 August 2021 | 10 | 9 |
| 24 August 2021 | 7 | 5 |
| 4 | 10 | |
| 27 August 2021 | 10 | 10 |
| Average | 13.33 | 15.4 |
| Ratio | 1 | 1.15 |
| Name | Observations | Predator attack strategy | Prey response |
|---|---|---|---|
| Bombshell in the scrub | 3 | Jackals approached a group of guineafowl in pairs, chasing them until they scattered in all directions. | After that, some birds flew to the tops of nearby trees, making a loud “chit chit chirr” call. This distracted one predator while another searched for guineafowl hidden in the grass. The birds in the tree attracted the jackals, which allowed the hidden birds in the grass to fly up into the trees. |
| Surprise attack | 4 | Lone jackals were seen approaching a group of six to ten guineafowl in a non-threatening manner on the plain, lying 5 to 10 meters away from the group. When a bird turned its back on the jackal, it would dash towards it to catch it. | The guineafowl responded by running towards each other first, then all together 10 to 20 meters away, producing a loud “chit chit chirr” call before continuing to feed. |
| The challenge | 4 | A pair of jackals dashed into a group, stopping near the birds and waiting to catch one. | The birds gathered in a dense group, confronting predators by mobbing them and emitting loud calls. The jackals stood motionless for 10 minutes, appearing confused, before walking away as the birds continued foraging. |
| Herding | 20 | Groups were chased by a jackal at the launch pad, where they gathered from different directions before roosting. | The guineafowl responded by forming a close-knit group of about 20 to 30 birds and running short distances, staying close to the launching pad, just ahead of the jackal for several minutes before flying into the roosting trees. |
| Eavesdropping | 4 | This type of hunting occurred when the groups of guineafowl in the scrub were first disturbed or chased by other animals perceived to be hazardous, such as lions and hyenas. The threatening animals caused the guineafowl to flush out, and the jackal quickly appeared on the scene to pursue the hidden birds in the grass cover. | Like the first response above. Some birds distracted predators by making loud calls, allowing others to escape into trees. |
| Time of day | Jackal counts | Jackal/hour | Guineafowl/group (mean) | Std. D | Range | Groups | Hours |
|---|---|---|---|---|---|---|---|
| 6:00-7:00 | 18 | 1.81 | 40.91 | 31.85 | 9-135 | 28 | 9.93 |
| 7:00-8:00 | 4 | 0.42 | 30.58 | 30.21 | 1-100 | 43 | 9.93 |
| 8:00-9:00 | 3 | 0.30 | 21.17 | 19.82 | 5-100 | 41 | 9.93 |
| 9:00-10:00 | 4 | 0.40 | 24.1 | 18.2 | 2-85 | 19 | 9.93 |
| 10:00-11:00 | 0 | 0 | 12.54 | 11.82 | 5-35 | 12 | 9.93 |
| 11:00-12:00 | 0 | 0 | 7.63 | 4.65 | 5 - 15 | 12 | 9.93 |
| 12:00-13:00 | 1 | 0.1 | 13.78 | 11.8 | 6-43 | 13 | 9.93 |
| 13:00-14:00 | 4 | 0.40 | 19.58 | 17.29 | 7-80 | 20 | 9.93 |
| 14:00-15:00 | 4 | 0.40 | 21.9 | 19.06 | 5-90 | 35 | 9.93 |
| 15:00-16:00 | 10 | 1 | 34.02 | 17.63 | 3-90 | 40 | 9.93 |
| 16:00-17:00 | 35 | 3.52 | 28.5 | 25.31 | 7-130 | 62 | 9.93 |
| 17:00-18:00 | 30 | 3.02 | 39.92 | 33.5 | 20-150 | 68 | 9.93 |
| Code | Parameters | Black-backed Jackal | Predatory birds | Totals | Statistical test |
|---|---|---|---|---|---|
| A | Total webcam observation minutes during winter (plus ad-hoc time added for predator incidents observations) | 4375 plus 208 | 4375 plus 105 | - | - |
| B | Foraging time available per day in min | 360 | 360 | - | - |
| C | Number of attacks recorded | 26 | 14 | 39 | - |
| D | Avoidance attempts recorded | 22 | 12 | 34 | χ2= 3.102, p = 0.07 |
| E | Jackal and predatory birds noted on the plain | 113 | 14 | 127 | χ2= 77.17, p = 0. 00 |
| F | Mean avoidance time per attack in min | 15.13 | 14.66 | - | H= 0.32811, n =35; p = 0.56 |
| G | Avoidance time: The mean avoidance time x the number of attacks = (F x D) (min) | 332.99 | 175.92 | 508.91 | |
| H | Estimated minutes lost foraging per day = B/(A/C) x F | 30.90 | 16.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





