Submitted:
10 July 2024
Posted:
12 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocols
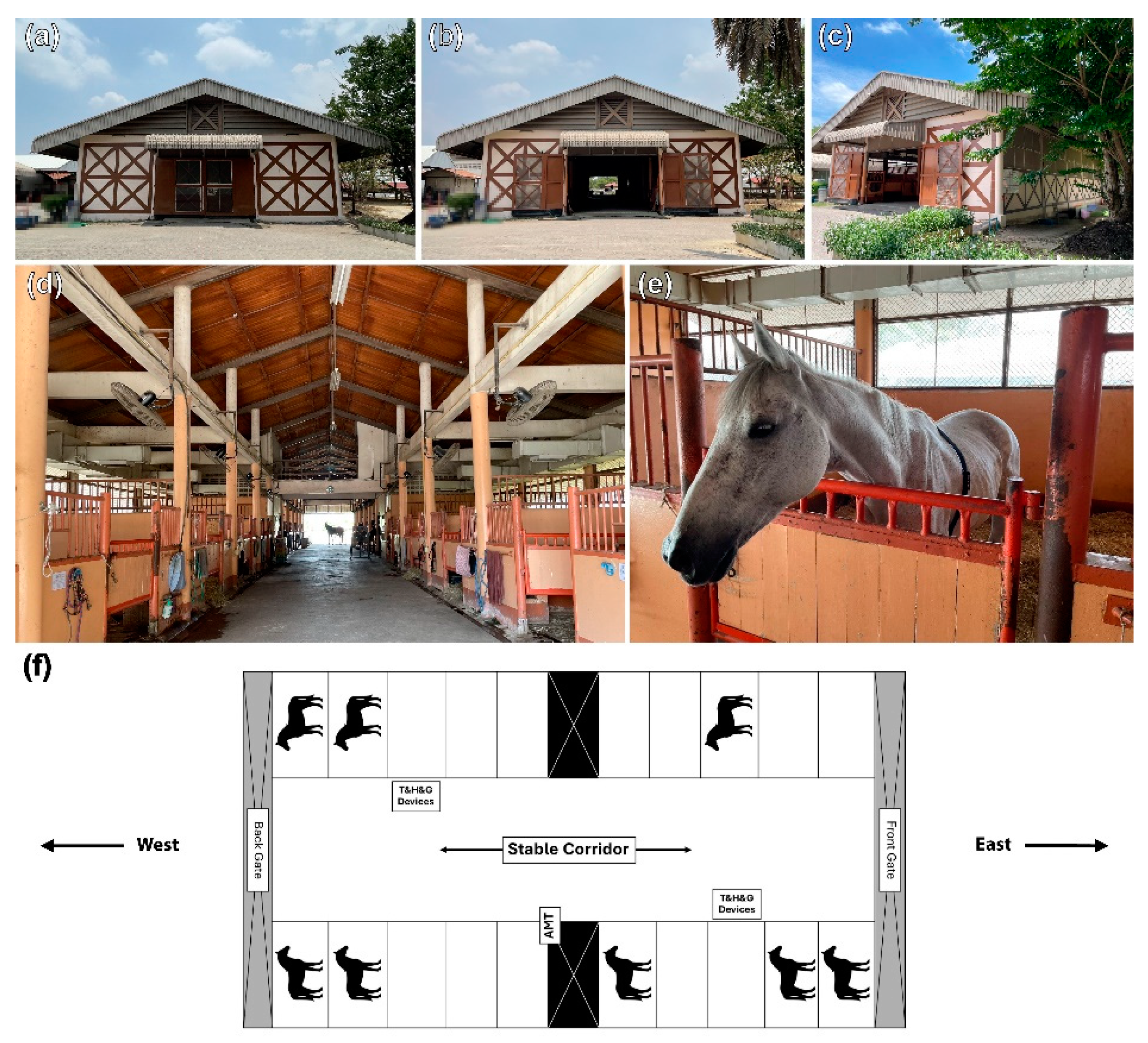
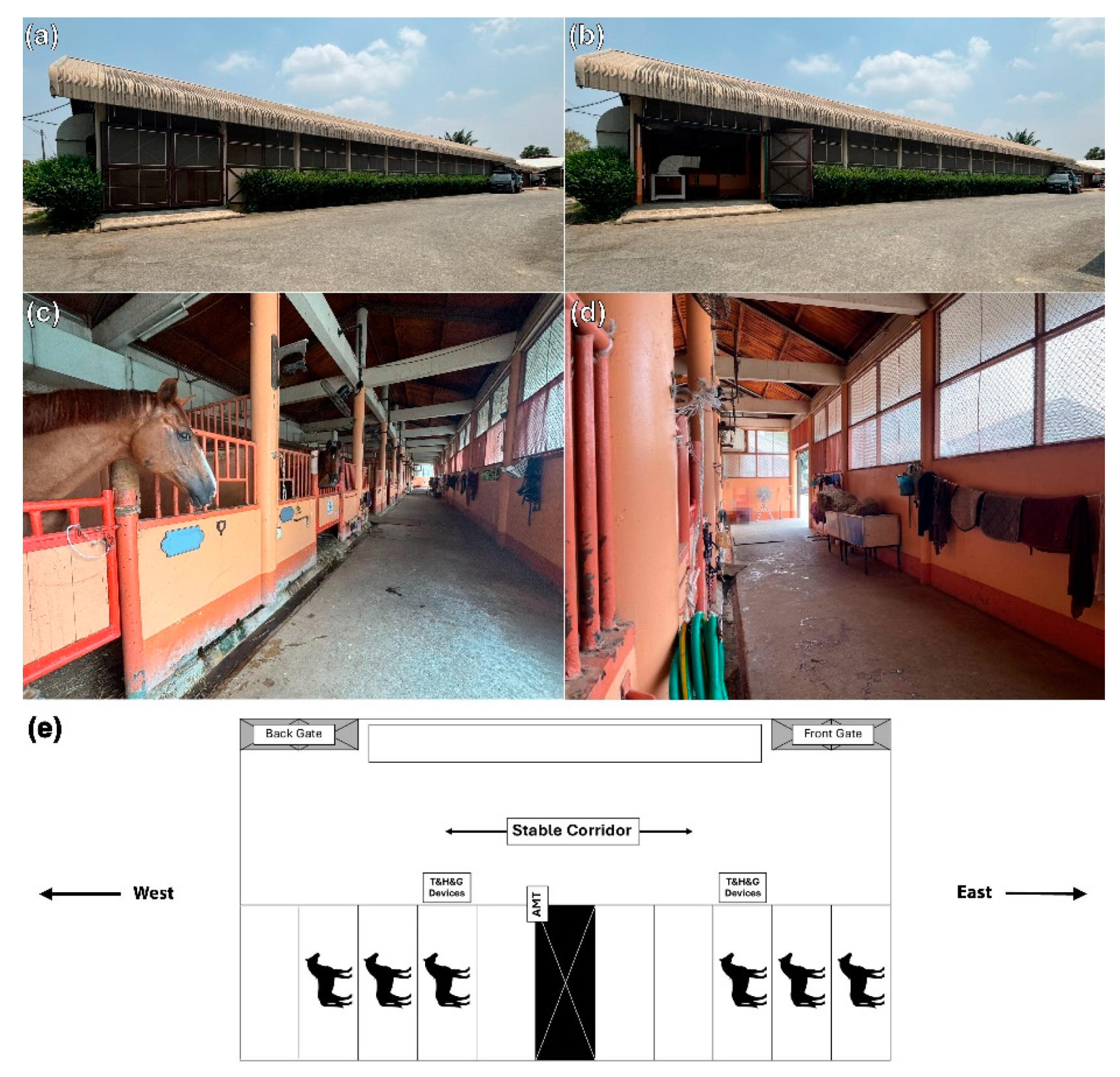
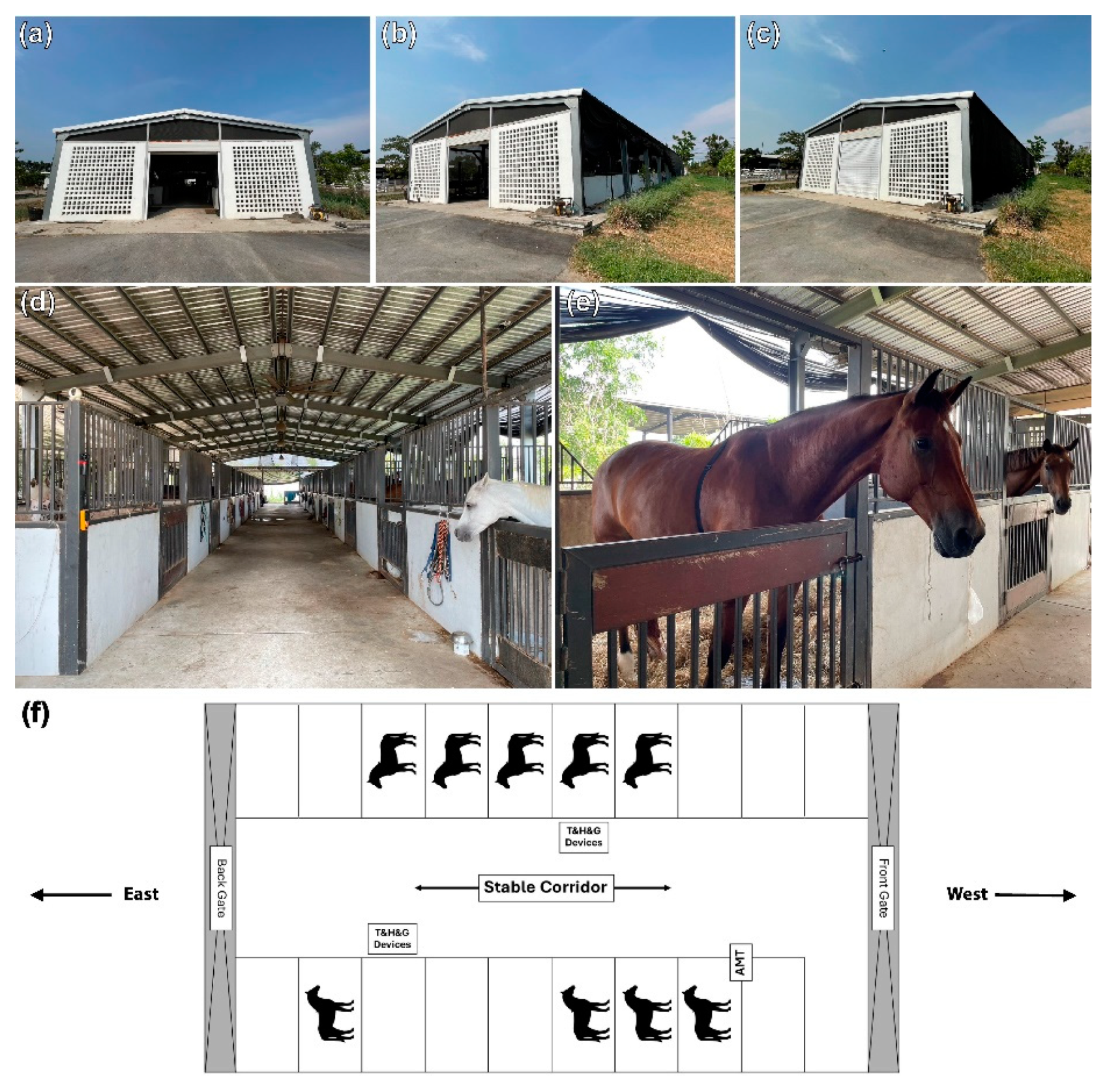
2.3. Stable Designs and Characteristics
2.4. Data Acquisition
2.4.1. Humidity and Air Temperature
2.4.2. Air Velocity and Noxious Gases
2.4.3. Heart Rate and Heart Rate Variability
2.5. Statistical Analysis
3. Results
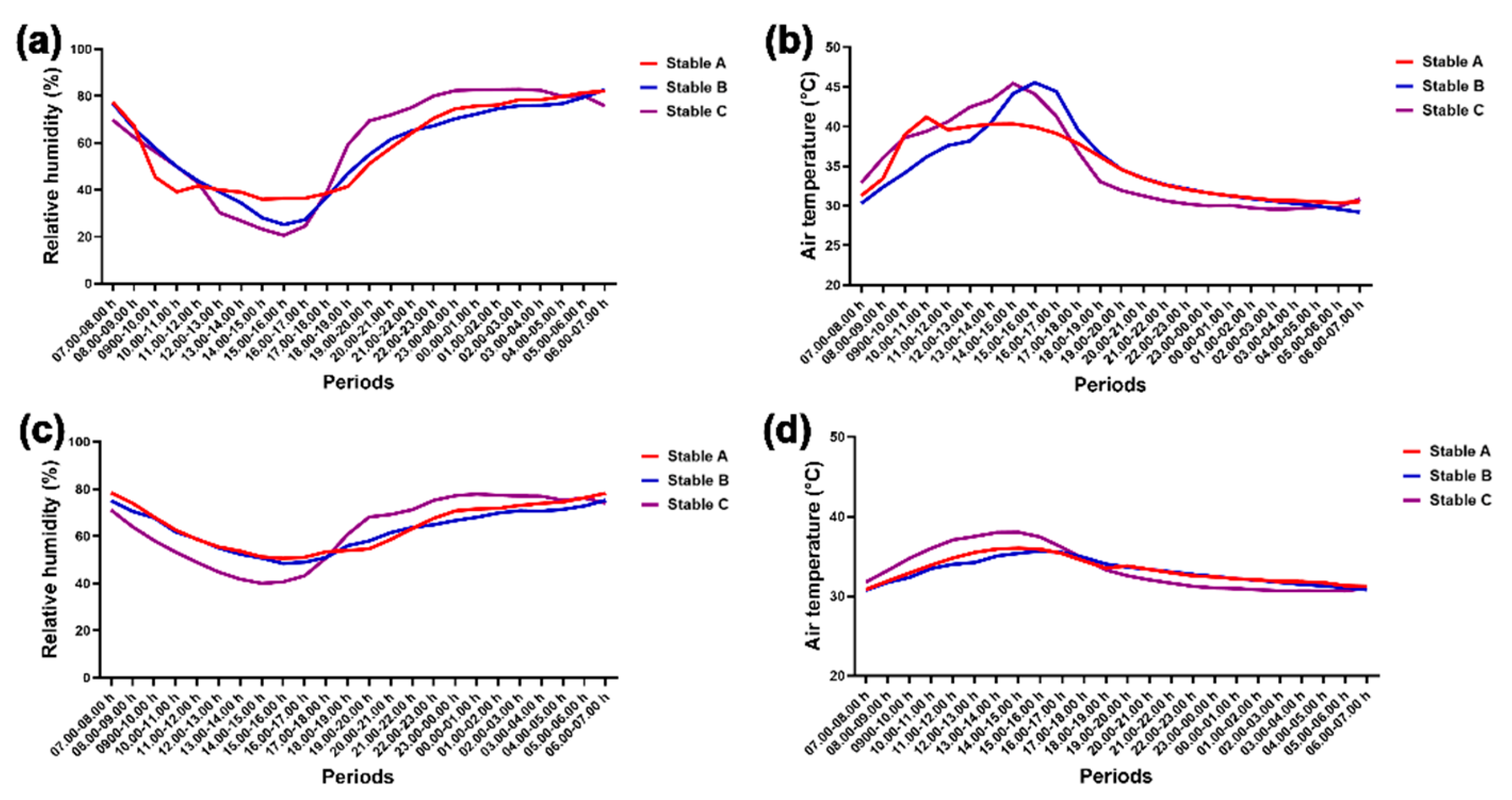
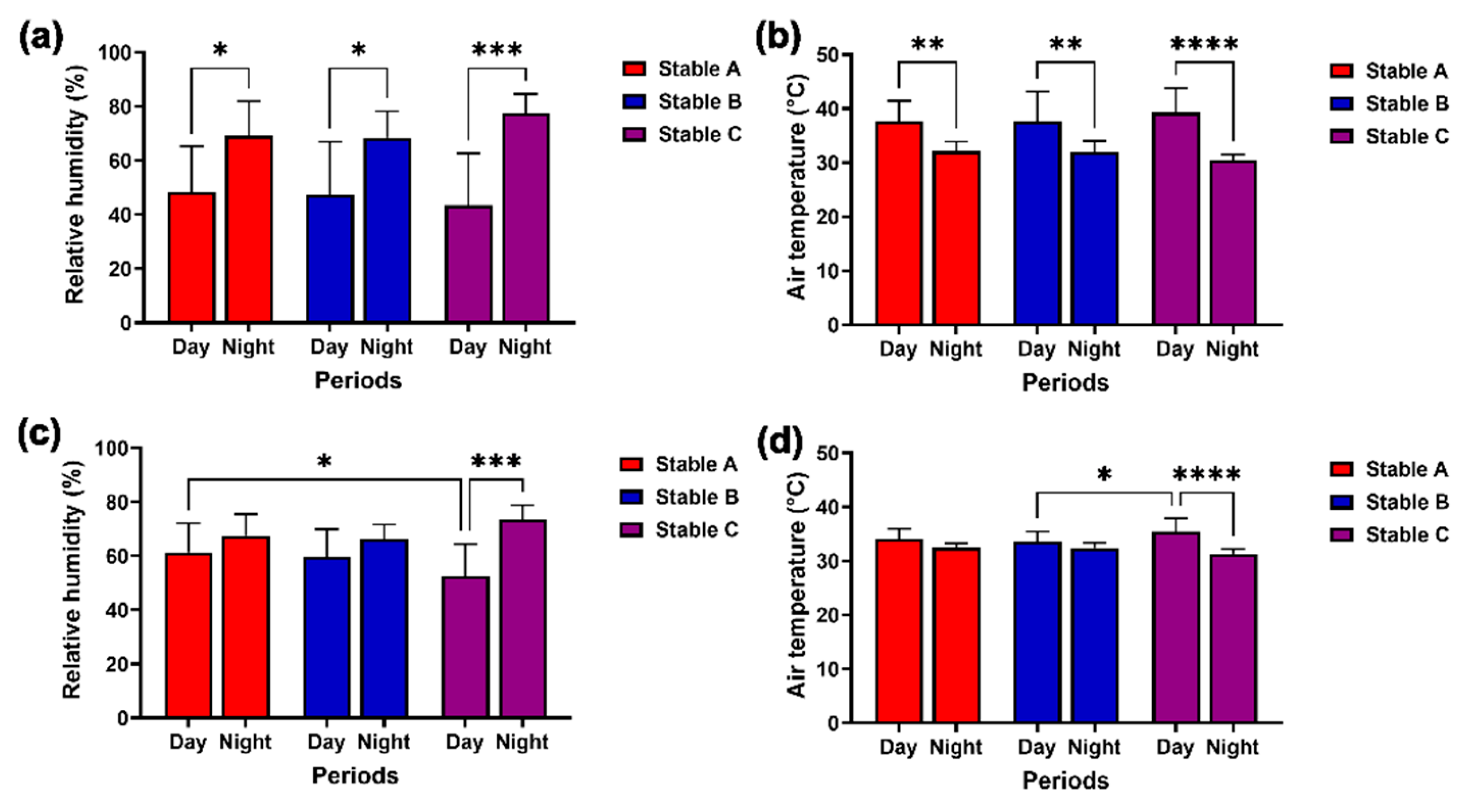
3.1. Relative Humidity and Air Temperature
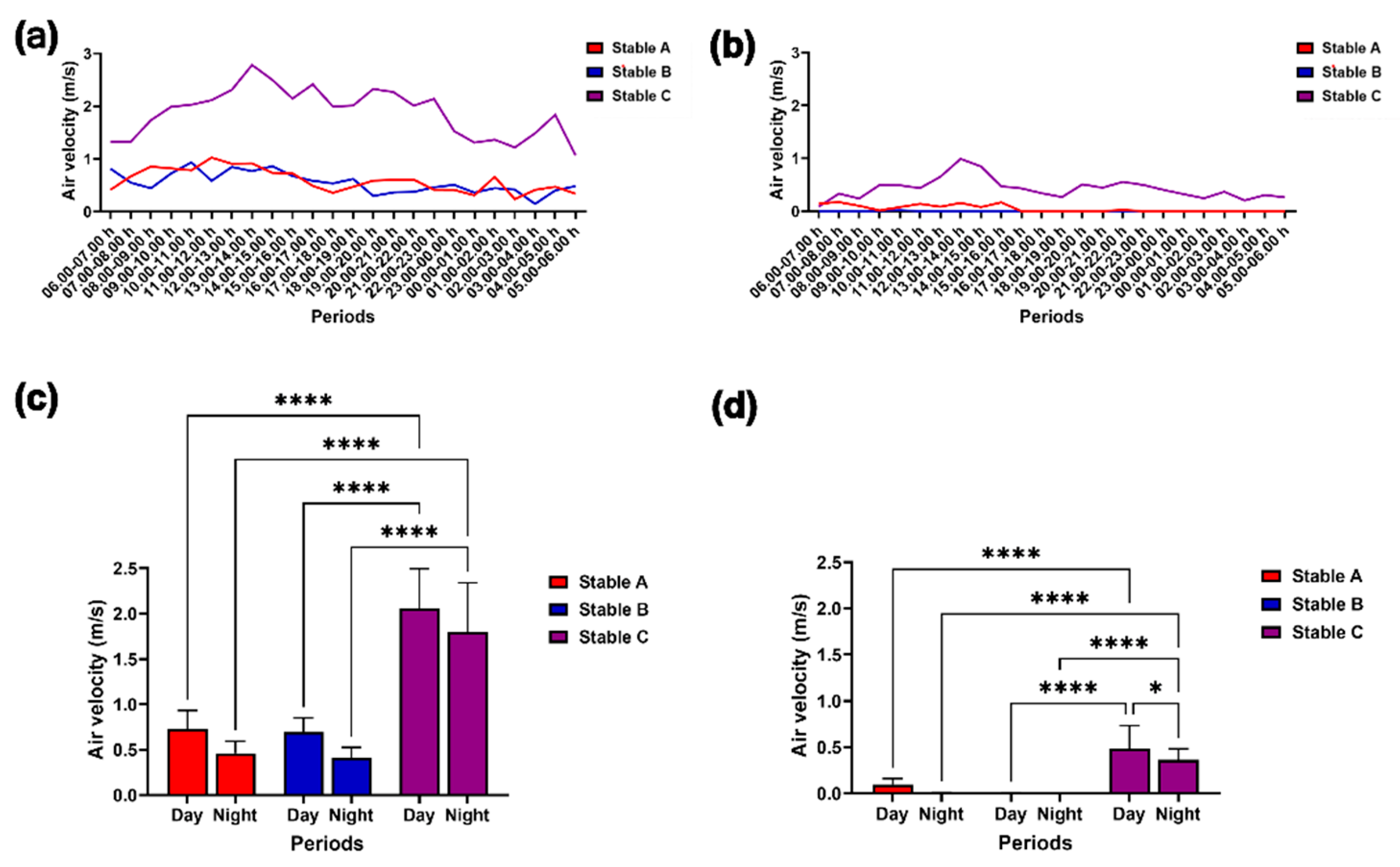
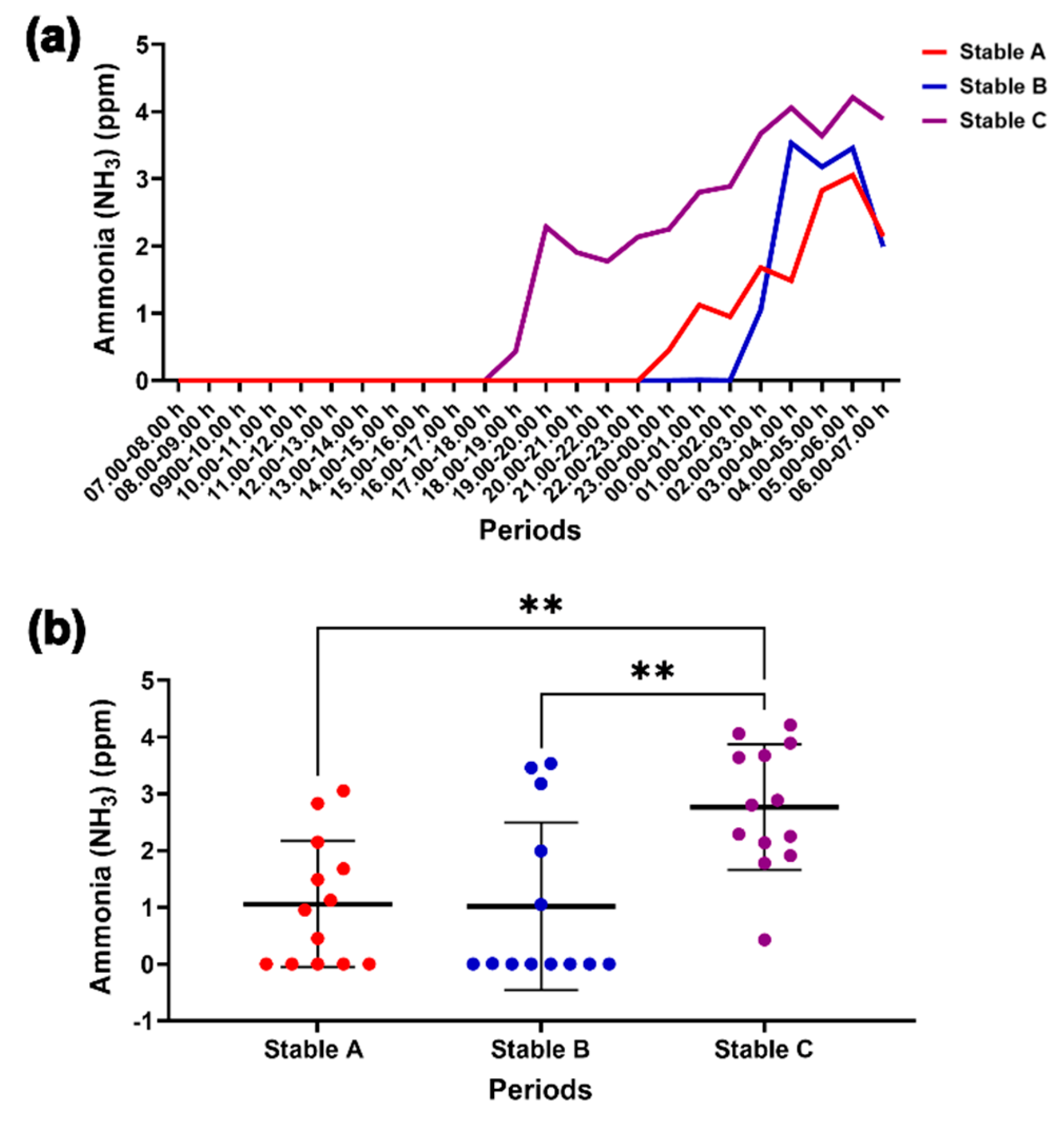
3.2. Air Velocity and Noxious Gases
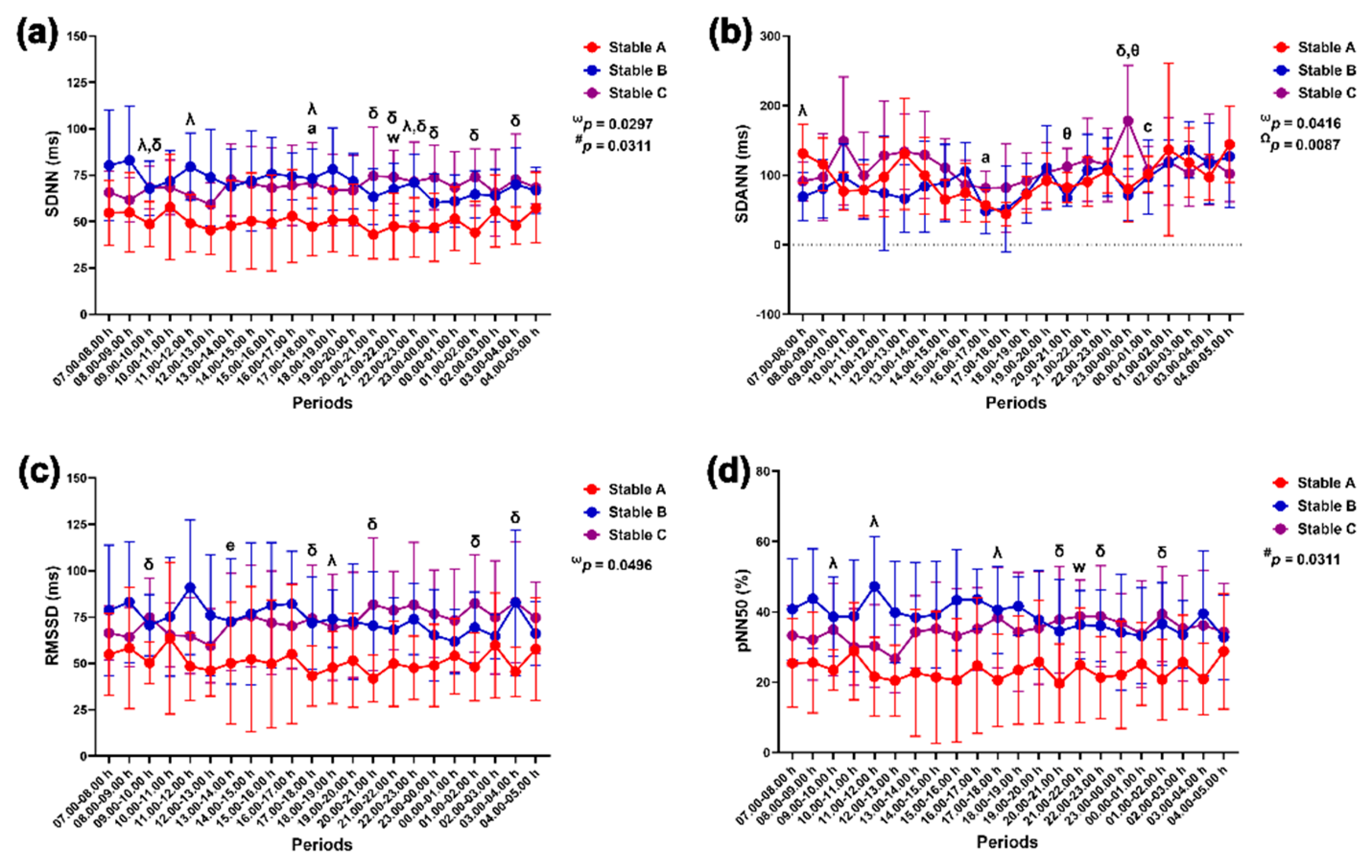
3.3. Heart Rate and Heart Rate Variability
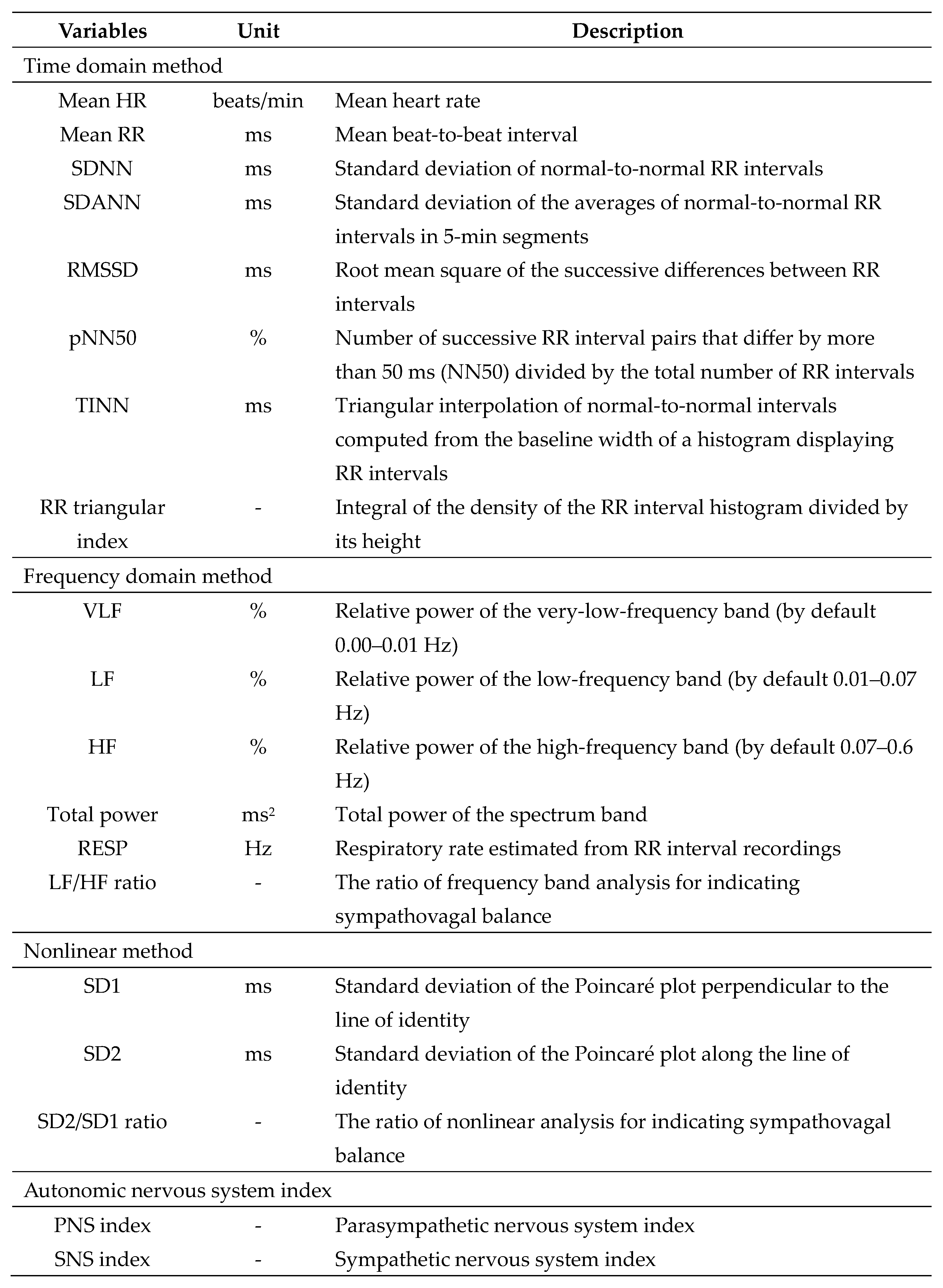
3.3.1. Time Domain Method
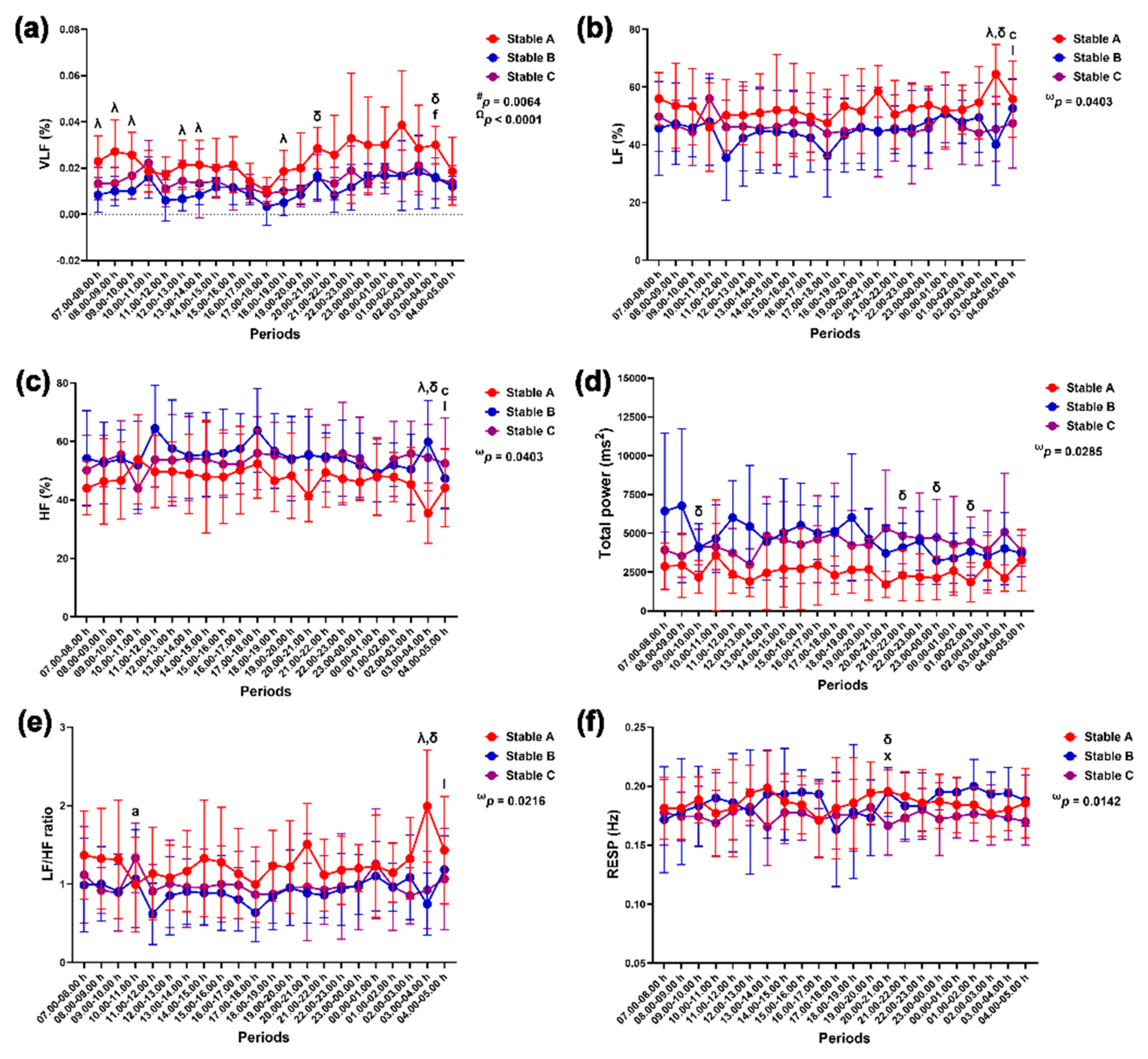
3.3.2. Frequency Domain Method
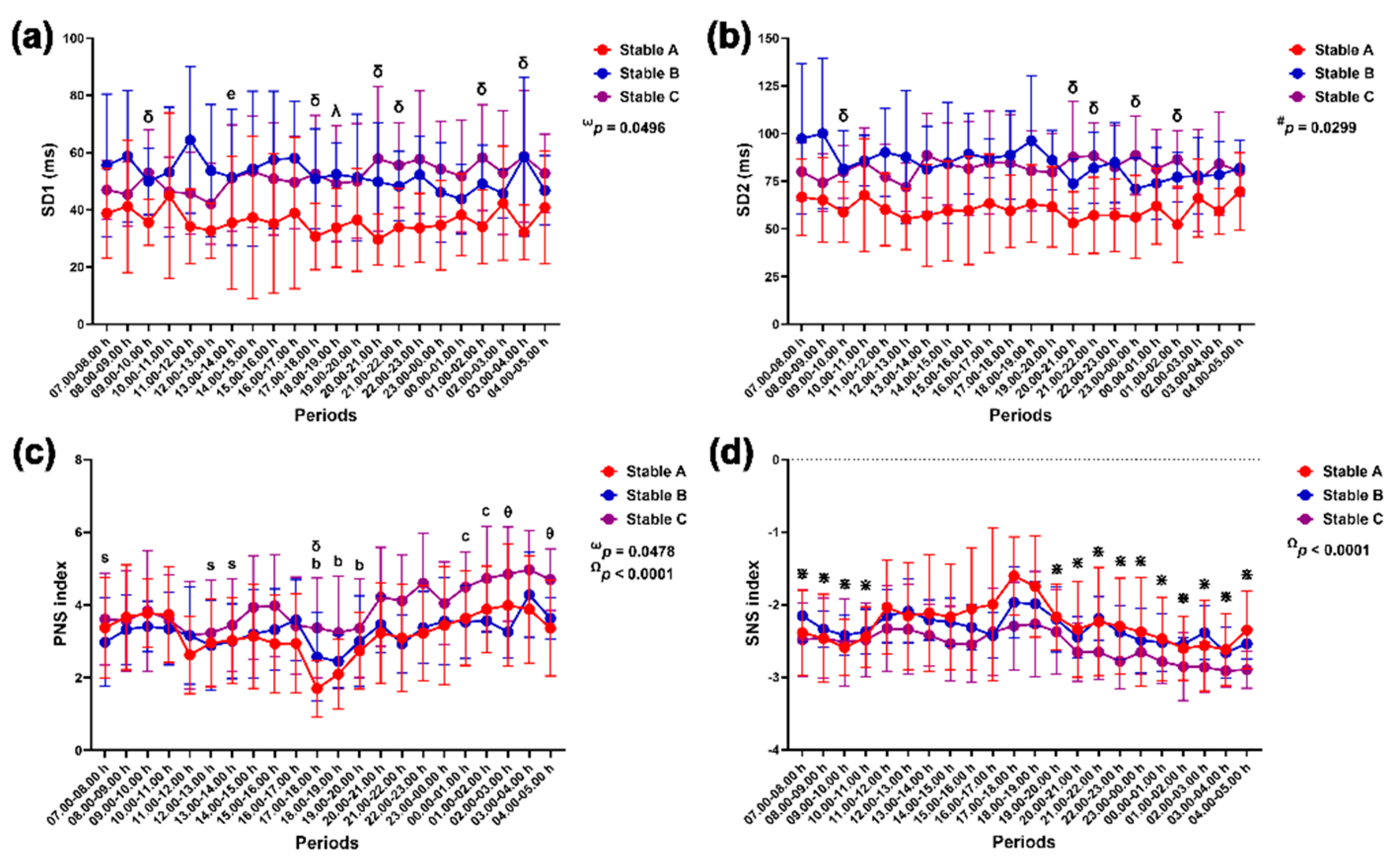
3.3.3. Nonlinear Method and Autonomic Nervous System Index
3.4. Correlation between HRV Variables and Internal Environment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Fraser, D. Animal behaviour, animal welfare and the scientific study of affect. Appl. Anim. Behav. Sci. 2009, 118, 108–117. [Google Scholar] [CrossRef]
- Leme, D.P.; Parsekian, A.B.H.; Kanaan, V.; Hötzel, M.J. Management, health, and abnormal behaviors of horses: A survey in small equestrian centers in Brazil. J. Vet. Behav. 2014, 9, 114–118. [Google Scholar] [CrossRef]
- Pessoa, G.O.; Trigo, P.; Mesquita Neto, F.D.; Lacreta Junior, A.C.C.; Sousa, T.M.; Muniz, J.A.; Moura, R.S. Comparative well-being of horses kept under total or partial confinement prior to employment for mounted patrols. Appl. Anim. Behav. Sci. 2016, 184, 51–58. [Google Scholar] [CrossRef]
- Fraser, D. Understanding animal welfare. Acta Veterinaria Scandinavica 2008, 50, S1. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Dalla Costa, E.; Minero, M.; Briant, C. Does housing system affect horse welfare? The AWIN welfare assessment protocol applied to horses kept in an outdoor group-housing system: The ‘parcours’. Animal Welfare 2023, 32, e22. [Google Scholar] [CrossRef]
- Lesimple, C.; Reverchon-Billot, L.; Galloux, P.; Stomp, M.; Boichot, L.; Coste, C.; Henry, S.; Hausberger, M. Free movement: A key for welfare improvement in sport horses? Appl. Anim. Behav. Sci. 2020, 225, 104972. [Google Scholar] [CrossRef]
- Chaplin, S.J.; Gretgrix, L. Effect of housing conditions on activity and lying behaviour of horses. animal 2010, 4, 792–795. [Google Scholar] [CrossRef]
- Søndergaard, E.; Ladewig, J. Group housing exerts a positive effect on the behaviour of young horses during training. Appl. Anim. Behav. Sci. 2004, 87, 105–118. [Google Scholar] [CrossRef]
- Jansson, A.; Harris, P.A. A bibliometric review on nutrition of the exercising horse from 1970 to 2010. Comp. Exerc. Physiol. 2013, 9, 169–180. [Google Scholar] [CrossRef]
- Popescu, S.; Diugan, E.A.; Spinu, M. The interrelations of good welfare indicators assessed in working horses and their relationships with the type of work. Research in Veterinary Science 2014, 96, 406–414. [Google Scholar] [CrossRef]
- Lesimple, C.; Gautier, E.; Benhajali, H.; Rochais, C.; Lunel, C.; Bensaïd, S.; Khalloufi, A.; Henry, S.; Hausberger, M. Stall architecture influences horses’ behaviour and the prevalence and type of stereotypies. Appl. Anim. Behav. Sci. 2019, 219, 104833. [Google Scholar] [CrossRef]
- Hotchkiss, J.W.; Reid, S.W.J.; Christley, R.M. A survey of horse owners in Great Britain regarding horses in their care. Part 1: Horse demographic characteristics and management. Equine Vet. J. 2007, 39, 294–300. [Google Scholar] [CrossRef]
- Visser, E.K.; Neijenhuis, F.; de Graaf-Roelfsema, E.; Wesselink, H.G.M.; de Boer, J.; van Wijhe-Kiezebrink, M.C.; Engel, B.; van Reenen, C.G. Risk factors associated with health disorders in sport and leisure horses in the Netherlands1. Journal of Animal Science 2014, 92, 844–855. [Google Scholar] [CrossRef]
- Larsson, A.; Müller, C.E. Owner reported management, feeding and nutrition-related health problems in Arabian horses in Sweden. Livest. Sci. 2018, 215, 30–40. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Cripps, P.J.; French, N.P.; Green, L.E.; Nicol, C.J. Management factors associated with stereotypic and redirected behaviour in the Thoroughbred horse. Equine Vet. J. 1995, 27, 86–91. [Google Scholar] [CrossRef]
- Lesimple, C.; Poissonnet, A.; Hausberger, M. How to keep your horse safe? An epidemiological study about management practices. Appl. Anim. Behav. Sci. 2016, 181, 105–114. [Google Scholar] [CrossRef]
- Raaybymagle, P. , Ladewig, J. In The effect of box size on the lying behavior of horses. In Proceedings of the 38th International Congress of ISAE, ISAE, Helsinki; 2004. [Google Scholar]
- Raabymagle, P.; Ladewig, J. Lying behavior in horses in relation to box size. J. Equine Vet. Sci. 2006, 26, 11–17. [Google Scholar] [CrossRef]
- Internationale, F.É. Veterinary regulation. 2024, 128.
- Burn, C.C.; Dennison, T.L.; Whay, H.R. Environmental and demographic risk factors for poor welfare in working horses, donkeys and mules in developing countries. Vet. J. 2010, 186, 385–392. [Google Scholar] [CrossRef]
- Nengomasha, E.; Jele, N.; Pearson, R. Morphological characteristics of working donkeys in south-western Zimbabwe. In Proceedings of the ATNESA Workshop: Improving Donkey Utilisation and Management; 1997; pp. 5–9. [Google Scholar]
- Demelash Biffa, D. Causes and factors associated with occurrence of external injuries in working equines in Ethiopia. Int. J. Appl. Res. Vet. Med. 2006, 4, 1–7. [Google Scholar]
- Morgan, K. Thermoneutral zone and critical temperatures of horses. J. Therm. Biol. 1998, 23, 59–61. [Google Scholar] [CrossRef]
- contributors, W. Tropical climate. Wikipedia, The Free Encyclopedia 2024, 1220081210.
- Oke, O.E.; Uyanga, V.A.; Iyasere, O.S.; Oke, F.O.; Majekodunmi, B.C.; Logunleko, M.O.; Abiona, J.A.; Nwosu, E.U.; Abioja, M.O.; Daramola, J.O.; et al. Environmental stress and livestock productivity in hot-humid tropics: Alleviation and future perspectives. J. Therm. Biol. 2021, 100, 103077. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.C.; Peripolli, V.; Amador, S.A.; Brandão, E.G.; Esteves, G.I.F.; Sousa, C.M.Z.; França, M.F.M.S.; Gonçalves, F.G.; Barbosa, F.A.; Montalvão, T.C.; et al. Physiological and thermographic response to heat stress in zebu cattle. Livest. Sci. 2015, 182, 83–92. [Google Scholar] [CrossRef]
- Lin, H.; Jiao, H.C.; Buyse, J.; Decuypere, E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006, 62, 71–86. [Google Scholar] [CrossRef]
- Pawar, S.S.; Sajjanar, B.; Lonkar, V.; Kurade, N.; Kadam, A.; Nirmal, A.; Brahmane, M.; Bal, S. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci 2016, 4, 332–341. [Google Scholar] [CrossRef]
- Daskalov, P.I.; Arvanitis, K.G.; Pasgianos, G.D.; Sigrimis, N.A. Non-linear Adaptive Temperature and Humidity Control in Animal Buildings. Biosys. Eng. 2006, 93, 1–24. [Google Scholar] [CrossRef]
- Balnave, D. Challenges of Accurately Defining the Nutrient Requirements of Heat-Stressed Poultry1. Poultry Science 2004, 83, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, A.; Daramola, J. Effects of a By-Pass Protein on Water Utilization by West African Dwarf Goats. J. Agric. Res. Dev. 2004, 3, 75–82. [Google Scholar] [CrossRef]
- Hernandez, A.; Galina, C.S.; Geffroy, M.; Jung, J.; Westin, R.; Berg, C. Cattle welfare aspects of production systems in the tropics. Anim. Prod. Sci. 2022, 62, 1203–1218. [Google Scholar] [CrossRef]
- Gehlen, H.; Faust, M.-D.; Grzeskowiak, R.M.; Trachsel, D.S. Association between Disease Severity, Heart Rate Variability (HRV) and Serum Cortisol Concentrations in Horses with Acute Abdominal Pain. Animals 2020, 10. [Google Scholar] [CrossRef]
- Huangsaksri, O.; Sanigavatee, K.; Poochipakorn, C.; Wonghanchao, T.; Yalong, M.; Thongcham, K.; Srirattanamongkol, C.; Pornkittiwattanakul, S.; Sittiananwong, T.; Ithisariyanont, B.; et al. Physiological stress responses in horses participating in novice endurance rides. Heliyon 2024, 10, e31874. [Google Scholar] [CrossRef]
- Gehlen, H.; Loschelder, J.; Merle, R.; Walther, M. Evaluation of Stress Response under a Standard Euthanasia Protocol in Horses Using Analysis of Heart Rate Variability. Animals 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanigavatee, K.; Poochipakorn, C.; Huangsaksri, O.; Wonghanchao, T.; Yalong, M.; Poungpuk, K.; Thanaudom, K.; Chanda, M. Hematological and physiological responses in polo ponies with different field-play positions during low-goal polo matches. PLoS One 2024, 19, e0303092. [Google Scholar] [CrossRef] [PubMed]
- contributors, W. Tropical savanna climate. Wikipedia, The Free Encyclopedia 2024, 1220733028.
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. 2006, 15, 259–263. [CrossRef]
- Khedari, J.; Sangprajak, A.; Hirunlabh, J. Thailand climatic zones. Renew. Energy 2002, 25, 267–280. [Google Scholar] [CrossRef]
- Frippiat, T.; van Beckhoven, C.; Moyse, E.; Art, T. Accuracy of a heart rate monitor for calculating heart rate variability parameters in exercising horses. J. Equine Vet. Sci. 2021, 104, 103716. [Google Scholar] [CrossRef] [PubMed]
- Kapteijn, C.M.; Frippiat, T.; van Beckhoven, C.; van Lith, H.A.; Endenburg, N.; Vermetten, E.; Rodenburg, T.B. Measuring heart rate variability using a heart rate monitor in horses (Equus caballus) during groundwork. Front. vet. sci. 2022, 9, 939534. [Google Scholar] [CrossRef] [PubMed]
- Ille, N.; Erber, R.; Aurich, C.; Aurich, J. Comparison of heart rate and heart rate variability obtained by heart rate monitors and simultaneously recorded electrocardiogram signals in nonexercising horses. J. Vet. Behav. 2014, 9, 341–346. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126. [Google Scholar] [CrossRef]
- contributors, W. Köppen climate classification. Köppen climate classification 2024, ID: 1232275529.
- contributors, W. Tropics. Tropics 2024, ID: 1231146049.
- Sahu, D.; Mandal, D.K.; Hussain Dar, A.; Podder, M.; Gupta, A. Modification in housing system affects the behavior and welfare of dairy Jersey crossbred cows in different seasons. Biol Rhythm Res 2021, 52, 1303–1312. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livestock Production Science 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Wheeler, E.F. Ventilation. In Horse Stable and Riding Arena Design; John Wiley & Sons: 2008; pp. 57–80.
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. Journal of Dairy Science 2016, 99, 2438–2452. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, D.K.; Bhatt, N.; Sriranga, K.; Roy, S. Housing management for dairy animals under Indian tropical type of climatic conditions-a review. Vet. Res. Int 2020, 8, 94–99. [Google Scholar]
- Janczarek, I.; Wilk, I.; Wiśniewska, A.; Kusy, R.; Cikacz, K.; Frątczak, M.; Wójcik, P. Effect of air temperature and humidity in a stable on basic physiological parameters in horses. Animal Science and Genetics 2020, 16, 55–65. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S. Ammonia concentrations in a free-stall dairy barn. Annals of Animal Science 2014, 14, 153–166. [Google Scholar] [CrossRef]
- Fleming, K.; Hessel, E.F.; Van den Weghe, H.F.A. Gas and particle concentrations in horse stables with individual boxes as a function of the bedding material and the mucking regimen1. J. Anim. Sci. 2009, 87, 3805–3816. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska-Stenzel, A.; Sowińska, J.; Witkowska, D. Analysis of Noxious Gas Pollution in Horse Stable Air. J. Equine Vet. Sci. 2014, 34, 249–256. [Google Scholar] [CrossRef]
- Budzyńska, M. Stress Reactivity and Coping in Horse Adaptation to Environment. J. Equine Vet. Sci. 2014, 34, 935–941. [Google Scholar] [CrossRef]
- Park, S.-K.; Jung, H.-J.; Choi, Y.-L.; Kwon, O.-S.; Jung, Y.-H.; Cho, C.-I.; Yoon, M. The effect of living conditions on stress and behavior of horses. Journal of Animal Science and Technology 2013, 55, 325–330. [Google Scholar] [CrossRef]
- Auer, U.; Kelemen, Z.; Engl, V.; Jenner, F. Activity Time Budgets—A Potential Tool to Monitor Equine Welfare? Animals 2021, 11. [Google Scholar] [CrossRef]
- Esch, L.; Wöhr, C.; Erhard, M.; Krüger, K. Horses’ (Equus Caballus) Laterality, Stress Hormones, and Task Related Behavior in Innovative Problem-Solving. Animals 2019, 9. [Google Scholar] [CrossRef]
- Cayado, P.; MuÑOz-Escassi, B.; DomÍNguez, C.; Manley, W.; Olabarri, B.; De La Muela, M.S.; Castejon, F.; MaraÑOn, G.; Vara, E. Hormone response to training and competition in athletic horses. Equine Vet. J. 2006, 38, 274–278. [Google Scholar] [CrossRef]
- Scholler, D.; Zablotski, Y.; May, A. Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels. Animals 2023, 13. [Google Scholar] [CrossRef]
- Ishizaka, S.; Aurich, J.E.; Ille, N.; Aurich, C.; Nagel, C. Acute Physiological Stress Response of Horses to Different Potential Short-Term Stressors. J. Equine Vet. Sci. 2017, 54, 81–86. [Google Scholar] [CrossRef]
- Szabó, C.; Vizesi, Z.; Vincze, A. Heart Rate and Heart Rate Variability of Amateur Show Jumping Horses Competing on Different Levels. Animals 2021, 11, 693. [Google Scholar] [CrossRef] [PubMed]
- Wonghanchao, T.; Sanigavatee, K.; Poochipakorn, C.; Huangsaksri, O.; Yalong, M.; Poungpuk, K.; Thanaudom, K.; lertsakkongkul, P.; lappolpaibul, K.; Deethong, N.; et al. Impact of different cooling solutions on autonomic modulation in horses in a novice endurance ride. animal 2024, 18, 101114. [Google Scholar] [CrossRef]
- Poochipakorn, C.; Wonghanchao, T.; Huangsaksri, O.; Sanigavatee, K.; Joongpan, W.; Tongsangiam, P.; Charoenchanikran, P.; Chanda, M. Effect of Exercise in a Vector-Protected Arena for Preventing African Horse Sickness Transmission on Physiological, Biochemical, and Behavioral Variables of Horses. J. Equine Vet. Sci. 2023, 131, 104934. [Google Scholar] [CrossRef] [PubMed]
- Stucke, D.; Große Ruse, M.; Lebelt, D. Measuring heart rate variability in horses to investigate the autonomic nervous system activity – Pros and cons of different methods. Appl. Anim. Behav. Sci. 2015, 166, 1–10. [Google Scholar] [CrossRef]
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals — A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Frontiers in Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Moacir Fernandes de, G.; Michele Lima, G. Heart Rate Variability as a Marker of Homeostatic Level. In Autonomic Nervous System; Theodoros, A., Christos, N., Eds.; IntechOpen: Rijeka, 2022. [Google Scholar]
- Scopa, C.; Palagi, E.; Sighieri, C.; Baragli, P. Physiological outcomes of calming behaviors support the resilience hypothesis in horses. Sci. Rep. 2018, 8, 17501. [Google Scholar] [CrossRef]
- Hammond, A.; Sage, W.; Hezzell, M.; Smith, S.; Franklin, S.; Allen, K. Heart rate variability during high-speed treadmill exercise and recovery in Thoroughbred racehorses presented for poor performance. Equine Vet. J. 2023, 55, 727–737. [Google Scholar] [CrossRef]
- Lorello, O.; Ramseyer, A.; Burger, D.; Gerber, V.; Bruckmaier, R.M.; van der Kolk, J.H.; Navas de Solis, C. Repeated Measurements of Markers of Autonomic Tone Over a Training Season in Eventing Horses. J. Equine Vet. Sci. 2017, 53, 38–44. [Google Scholar] [CrossRef]
- Rietmann, T.R.; Stuart, A.E.A.; Bernasconi, P.; Stauffacher, M.; Auer, J.A.; Weishaupt, M.A. Assessment of mental stress in warmblood horses: heart rate variability in comparison to heart rate and selected behavioural parameters. Appl. Anim. Behav. Sci. 2004, 88, 121–136. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





